Featured Application
Formaldehyde monitoring has become mandatory for a large class of plants. Currently, the experience of the methods to be used and the values present is insufficient. This type of activity allows for the creation of and validation of effective and efficient methods for monitoring this compound.
Abstract
Formaldehyde (H−CHO) is a chemical compound extremely common in many industrial productions. However, in 2004, it was reclassified as carcinogenic (H350) and mutagenic (H341). Therefore, stringent limitations on emissions were implemented; among them, the lowest limit (3 mg/m3) was adopted by some Italian Local Competent Authorities. Up to now, no European-validated method for emission control was available, and for this reason, a specific working group (WG 40) has been created in the framework of the European Committee for Standardization Technical Committees 264 (CEN TC 264) to publish a qualified method for the quantification of Formaldehyde emissions from stationary sources (i.e., power stations, incinerators, petrochemicals, and industrial plants that uses combustion for their energetic purposes). Some preliminary trial tests were conducted to evaluate (1) the sampling protocol, and (2) the analytical technique. From a measurement perspective, two methods were selected: EPA 323—VDI 3862-6 and VDI 3862-2. Every new method prepared by CEN shall be verified before publication in the field and in real conditions to verify its metrological properties (i.e., precision, biases, reproducibility, and repeatability), costs and the training needs for involved personnel. With this aim, two measuring campaigns were conducted, and some important conclusions emerged concerning the H−CHO sampling procedure. Due to high water levels normally present, condensation during sampling is critical and can cause unpredictable errors; wet traps (impingers) give good responses. The sampling in pure water appeared unstable, but using an H2SO4 solution solved this issue, thus being recommended.
1. Introduction
Formaldehyde (H−CHO) belongs to the class of carbonyls. It is the simplest aldehyde (R-CHO) and the most present carbonyl compound in the atmosphere [1,2,3].
Its sources are either natural or anthropogenic. Atmospheric H−CHO can be classified as primary (coming directly from emissions) or secondary (formed in the atmosphere). Primary emissions can result from fossil fuel combustion [4,5], biomass burning [6], industrial activities [7], and vegetation [8,9].
In the atmosphere, formaldehyde can be formed from the oxidation of CH4 and of many other hydrocarbons [10], or from the methoxy radical under tropospheric conditions with O2:
CH3O + O2 → H−CHO + HO2
Formaldehyde undergoes two main reactions in the atmosphere, reaction with OH radicals and photolysis [10,11].
H−CHO was widely used for its preservative and antibacterial properties [12]. However, formaldehyde-based chemistry is still used in a wide range of industries, such as chemicals, manufacturing, cosmetics, and textiles [13,14], and nowadays it is mainly used in the production of industrial resins [15,16].
In the field of energy production from biogas, formaldehyde has its own importance as it is released during the combustion of wood (e.g., for domestic heating), and of its derivatives, such as wood chips [17], pellets or similar fuels [18,19,20]. Also, as H−CHO is present in many products in common use, such as food, drugs, furniture, etc. [21,22], its presence in urban waste cannot be neglected, so emissions from incinerators shall be monitored as there is no guarantee that it is completely destroyed during the combustion process.
The interest in formaldehyde in the field of biomass emissions is related to the increasing production of biomethane; during the combustion of methane using alternative engines to produce electricity and heat, H−CHO is synthesized according to the following reaction:
CH4 + O2 → H−CHO + H2O
In this reaction, part of the methane is transformed into H−CHO and not into CO2 and H2O; the average concentration of H−CHO is between 5 and 50 mg/Nm3 [23,24].
Formaldehyde is an important pollutant in urban atmospheres, and due to its widespread use, toxicity, and volatility, this compound represents a significant hazard to human health [25].
Due to its widespread use, toxicity, and volatility, formaldehyde represents a significant hazard to human health. In 2004, the International Agency for Research on Cancer (IARC) reclassified formaldehyde into Group 1-”carcinogenic to humans” [26].
In Italy, due to Dlgs 152/2006 (Italian Legislative Decree nr 152/2006), the formaldehyde limit at emissions from combustion engines was 20 mg/Nm3. After, in 2014, due to the modification of Part 3 of Annex VI of the EC Regulation 1272/2008, formaldehyde was classified as ‘may cause cancer’ (risk phase H350) and ‘suspected of causing genetic defects’ (H341) [27] and its emission limit in Italy was reduced to 5 mg/Nm3 and, for some specific applications, 3 mg/Nm3.
Compared to the past, when formaldehyde was not considered a dangerous chemical compound, the actual stringent limitation at emissions requires a robust analytical protocol aimed at performing systematical, accurate and reproducible environmental measurements, which was not performed in the past.
Thus, there still is no European method validated for its determination of emissions. For this reason, the process for the definition of a standardized method has been started in the European Committee for Standardization (CEN) context. It is detailed hereinafter in the present work.
This work is the first of a series that shows research conducted by the authors to qualify robust monitoring methods for determining H−CHO in emissions from stationary sources, i.e., power plants, incinerators, chemical or petrochemical units, etc.
2. Materials and Methods
2.1. CEN TC 264 WG 40 “Emissions—Formaldehyde”
To develop an accurate and reproducible analytic protocol for formaldehyde quantification in emission, CEN activated in 2016 the “TC 264 WG 40 Measurement of formaldehyde emissions” working group.
Several techniques for the detection of formaldehyde are available [28]. These methods include spectrophotometry [29], gas chromatography [30], high-performance liquid chromatography [31] and ion chromatography [32].
In the past several intercomparison experiments have been performed on the measurement of formaldehyde [33]. The general conclusions in these intercomparisons are that H−CHO measurements at low-ppb levels are still problematic and that further validation work is needed [34,35,36,37,38,39].
The working group, having examined existing methods, developed the first draft of a suitable methodology that needed to be verified and validated by measurements achieved in real plants by WG participants, as well as using emission simulation laboratories called bench loops [40,41].
The chosen methods for formaldehyde quantification are based on two different analytical principles: spectrophotometric method (EPA 323) [42] using various chromophore compounds, and high-performance liquid chromatography [43] using the reaction of formaldehyde with the DNPH (2,4-dinitrophenylhydrazine), the main characteristics of which are showed in Table 1.

Table 1.
Characteristics of the method EPA 323—VDI 3862-6 and method VDI 3862-2.
To choose the best candidate methods (or to approve both, if possible), it was necessary to compare the two methods to evaluate:
- Reproducibility
- Repeatability
- Sampling procedures
- Safety aspects
2.2. Experimental Investigations
To investigate methods characteristics, two field campaigns were programmed on a real biomass plant that produces biogas (biomethane) from anaerobic fermentation of organic waste; biogas is then burned using an alternative engine to produce electricity and heat. The selected plant is considered state-of-the-art and implements the best available techniques for emission control, i.e. a thermal oxidizer and a quality management system.
Each campaign was conducted by two independent laboratories:
- Agenzia Provinciale Protezione Ambiente (APPA) Trento Laboratory, Trento (TN), Italy;
- Ricerca sul Sistema Energetico (RSE) Laboratory, Milan (MI), Italy.
Chemical analyses were conducted by APPA Laboratory and LabAnalysys s.r.l., Italy.
Each laboratory implemented independent measurements using different analytical methods: DNPH (VDI 3862-2) and Acetylacetone (EPA 323—VDI 3862-6).
Samplings lasted 30 min, in line with the CEN indication to harmonize all the sampling methods for emissions to allow measurements on a half-hourly basis.
The use of hazardous reagents in the field was minimized to reduce the risks associated with experimental activities and left the most critical chemical manipulations for the laboratory.
2.3. Sampling System
The sampling was carried out following the procedures described in the CEN/TS 17638:2021 [44], implementing flue gas extraction and capturing it on H−CHO using impingers loaded with absorbing solution (Figure 1). Solutions were then stabilized and analyzed in the laboratory.
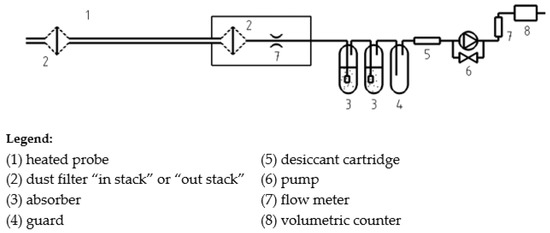
Figure 1.
Scheme of integral low-flow sampling.
Regarding the sampling technique implemented, the APPA laboratory used an unheated Teflon tube, air-cooled impingers and dry gas meter for volume determination.
RSE used a heated line up to the impingers, water-cooled impingers, and mass flow meters.
The ‘cold’ capture line used by APPA is very simple and light and allows for good cleaning and control of contaminations, and this is its main advantage. Its main flaw, however, is that, not being heated, it allows the sample condensation with the formation of liquid droplets that capture part of the analyte. For this reason, at the end of the sampling activity, it is necessary to do a recovery wash of the whole sampling line with distilled water to collect all the droplets present: added water (from 20 up to 100 mL) flows in the final absorption solution, diluting it (the final volume is from 100 up to 200 mL). In this way, however, the method’s sensitivity decreases, and it is no longer possible to determine the water content of the stack gas.
Conversely, the hot line (more complex and heavier) used by RSE does not alter the gas samples and limits condensation phenomena out of the bubbler; in this way, the volume of the absorbing solution is reduced (about 50 mL) with an increased sensibility, and it is possible to trace the humidity of the fumes as it results in a weight increase of the solution. This feature, although not strictly necessary for sampling, nevertheless constitutes an aid for checking the quality of sampling. On the other hand, the sampling line is heavier and requires power and special controllers, and it is necessary to use water-cooled impingers to dissipate the thermal power absorbed by impingers.
In principle, the choice of cold line and uncooled traps offers the greatest simplicity and the lowest cost associated with sampling but does not allow an accurate determination of the humidity of the fumes and offers a lower capture efficiency. The choice, on the other hand, of heated line and cooled traps requires a greater complexity of the sampling systems but allows an accurate assessment of the humidity of the fumes and the quantity of analyte captured, with a great improvement in the accuracy of the method.
2.4. Instruments
The Formaldehyde was determined by Lab. APPA Trento using two methods: VDI 3862-2) and Acetylacetone (EPA 323—VDI 3862-6) (detailed information can be found in supplementary material).
For the VDI 3862-2 method, an HPLC system HP 1100 (Hewlett-Packard, Waldbronn, Germany) equipped with a Restek Allure AK column 5 μm (200 × 4.6 mm) was used.
An elution gradient of ultrapure water/acetonitrile was used with a constant flow rate of 1 mL/min. The column was both kept at 20 °C.
For the EPA 323—VDI 3862-6 method, a Jasco V-630 (Jasco, Easton, MD, USA) UV-VIS spectrophotometer was used; the solutions were analyzed at 412 nm.
Lab. RSE used the VDI 3862-2 method. For the analysis, a Shimadzu Nexera XR (Shimadzu, Kyoto, Japan) HPLC system equipped with a Supelcosil LC-18 column 5 μm (250 × 4.6 mm) was used. An elution gradient of ultrapure water/acetonitrile was used with a constant flow rate of 0.45 mL/min. The column was kept at 25 °C.
2.5. Protocol Description
During the First Campaign, 14 sets of 3 simultaneous samplings were done, described in Table 2 and Table 3:

Table 2.
Characteristics of the sampling method during the First Campaign.

Table 3.
Description of samples during the First Campaign.
The same laboratories that worked on the First Campaign also performed the second one using the protocol described in Table 4 and Table 5:

Table 4.
Characteristics of the sampling method during the Second Campaign.

Table 5.
Description of samples during the Second Campaign.
3. Results and Discussion
Statistical experimental data is elaborated using a linear regression approach [45].
In which the two sets of results (from method A and method B) are compared. This simple model is widely diffuse and commonly used. Furthermore, confidence and prediction bands are evaluated from the provisions of ISO 10155:95 [45].
3.1. Sampling
Water contents and H−CHO concentrations measured by RSE in the first campaign are shown in Table 6, accompanied by the analyzes carried out with the HPLC method (Figure 2).

Table 6.
Humidity and H−CHO obtained by Lab. RSE. during First Campaign.
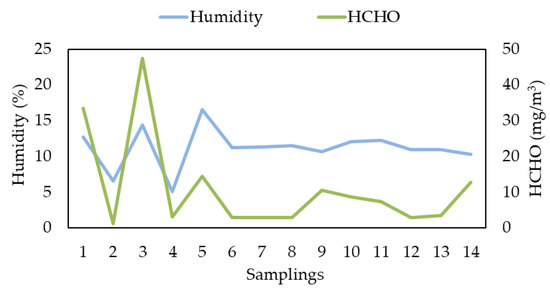
Figure 2.
Relation between measured humidity and H−CHO concentrations obtained by Lab. RSE during First Campaign.
The expected humidity of the flue gases was 10–12%, the value obtained in sampling from 6 onwards. Values from 1 to 5 are not compatible with expected values and must be related to a malfunction of the sampling procedure; it was verified that, due to a malfunction of the heated line, there was a cold (unheated) point in which condensation occurred accumulating liquid droplets that, from time to time, ended up flowing into the trap.
The most important effect is that the formaldehyde content found in the laboratory has the same behavior, as this compound is very soluble and was trapped in the cold point of the line and ended up flowing with the condensate.
It should be noted that such verification can be used as a quality assurance indicator. The first 5 samplings can be considered invalid for any legal purpose of the measurement, as they would significantly alter the results obtained.
The malfunction of the heated line during the second campaign has been resolved, as demonstrated by the values obtained (Table 7; Figure 3).

Table 7.
Humidity and H−CHO obtained by Lab. RSE. during Second Campaign.
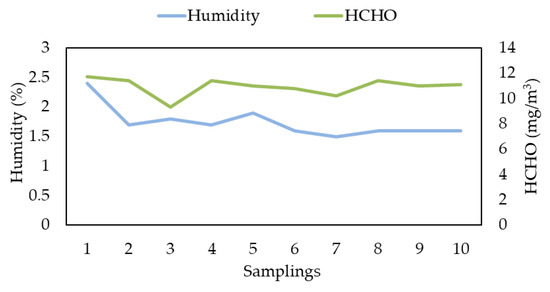
Figure 3.
Relation between measured humidity and H−CHO concentrations obtained by Lab. RSE during Second Campaign.
3.2. Analysis
In Figure 4, three chromatograms obtained by HPLC are shown:
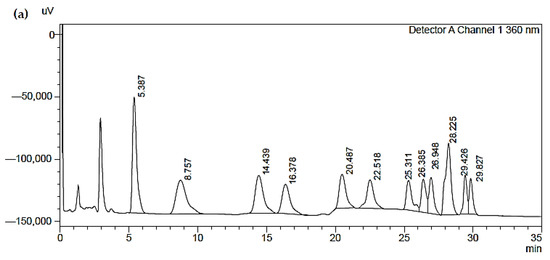
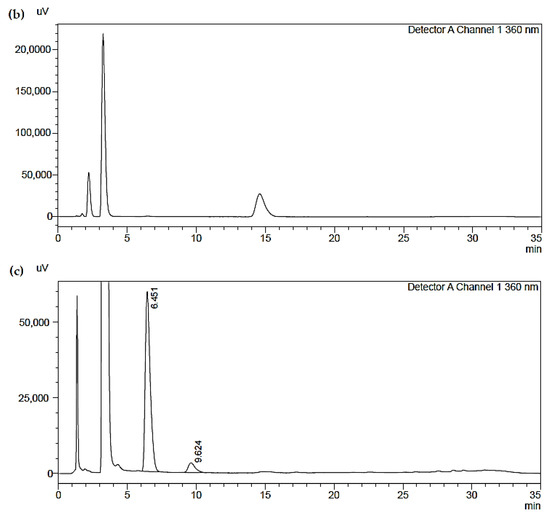
Figure 4.
(a) Chromatogram of a reference standard used; (b) Chromatogram of a blank sample; (c) Chromatogram of a blank sample of one of the analyzed samples.
- The first (a) represents a reference standard used (the concentration values are guaranteed by the sample supplier). The peaks of the chromatograms are described in Table 8;
 Table 8. Peak table of the reference standard.
Table 8. Peak table of the reference standard. - The second (b) represents a blank sample, which shows the substantial suitability of the solutions used (the blank samples are real analytical samples not exposed to the fumes but which have been treated exactly like the others);
- The third (c) represents one of the analyzed samples: the sample contains only formaldehyde (H−CHO and acetaldehyde (CH3CHO), compatible with the analyzed analytes. The chromatogram results with a good baseline and very well identified peaks, which guarantees good accuracy of results, demonstrating the validity of the method.
All the results of the sampling carried out by each of the laboratories during the First and Second Campaigns are reported in detail in Table 9, Table 10 and Table 11.

Table 9.
Concentration of H−CHO analyzed during the First Campaign.

Table 10.
Concentration of H−CHO analyzed by Lab. RSE during the First Campaign.

Table 11.
The results obtained by Lab. APPA Trento (sampling #1 and #2) from sampling in acid solution, washing, and analysis with HPLC are reported. The addition of DNPH was made directly at the end of the sampling.
The values of samples obtained by Lab. APPA Trento are shown in Table 11.
Lab. APPA Trento also analyzed the samples obtained from Lab. RSE to verify the reproducibility of the 2 laboratories. In Table 12, the comparison is shown.

Table 12.
Comparison between the analyses carried out with the VDI 3862-2 method conducted by Lab. APPA Trento on the solutions sampled by both during Second Campaign.
Lab. RSE conducted 10 samplings simultaneously with those at Lab. APPA Trento. The results are reported in Table 13, where the main values obtained are reported. The samples are those immediately complexed and analyzed 5 days after collection.

Table 13.
Values of the sampling conducted by the Lab. RSE with acid solution and analysis by HPLC; the solutions were complexed the same evening as the sampling of the Second Campaign.
To evaluate the stability of the samples, each solution collected was divided into 5 aliquots:
- The first was analyzed by Lab. APPA Trento;
- The second complexed immediately and was analyzed after 5 days;
- The third complexed immediately and was analyzed after 15 days;
- The fourth complexed after 5 days and was analyzed after 5 days;
- The fifth complexed after 5 days and was analyzed after 15 days.
The results obtained are shown in Table 14.

Table 14.
Results of repeated analyzes on samples collected by Lab. RSE during Second Campaign.
The comparison conducted by Lab. APPA Trento in First Campaign, which analyzed the samples collected by Lab. RSE, both according to the EPA 323 and VDI 3862-2 methods, shows a good correlation (Y = 1.0888 × [mg/L]—0.2666 [mg/L]) which seems to show an excellent correspondence between the methods. It is reported in Figure 5.
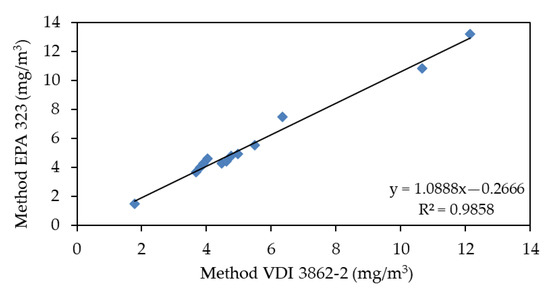
Figure 5.
Correlation between EPA 323 and VDI 3862-2 analysis of samples taken by Lab. APPA Trento during First Campaign.
The same comparison was made between the values determined by Lab. APPA Trento and by Lab. RSE with the VDI 3862-2 method, made on the same samples, is instead shown in Figure 6.
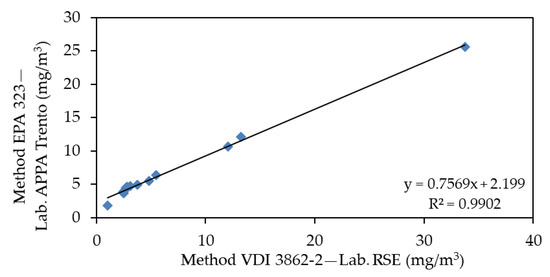
Figure 6.
Correlation of same samples according to the VDI 3862-2 analyzed by Lab. APPA Trento and Lab. RSE during First Campaign.
In this case, a difference emerges between the values obtained. The first and second correlation lines have an R2 close to 1, which suggests a reproducibility problem between the standards of the two laboratories that must be further investigated.
In the tests conducted during the second campaign, comparing the analytical techniques shows a worse result (Figure 7). The two techniques have provided significantly different values, 3.40 ± 0.45 mg/m3 for the VDI 3862-2 method and 2.88 ± 0.92 mg/m3 for the one according to EPA 323—VDI 3862-6.
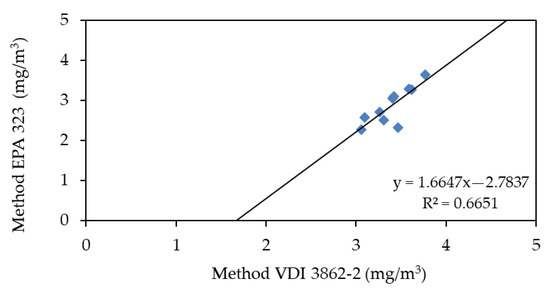
Figure 7.
Correlation between EPA 323 and VDI 3862-2 analysis of samples taken by Lab. A during Second Campaign.
The smallness of the values collected does not allow us to understand what the possible cause is, although it can be concluded that it must be deterministic, given the good correlation of the results; possible reasons could be:
- An objective error in carrying out the analyzes;
- A modification of the plant conditions, which has led to the formation of an interferent.
3.3. Performance of the Methods
To compare the two sampling methods, the correlation between the values obtained by the two different methods was calculated.
Figure 8 shows the linear correlation between the analyses conducted with the colorimetric and HPLC method during the First Campaign (the best set of tests obtained), together with the confidence and prediction bands obtained through the provisions of the ISO 10155:95 [45].
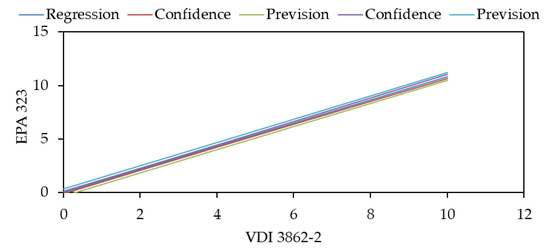
Figure 8.
Linear correlation between EPA 323 analysis from Lab. APPA Trento and VDI 3862-2 analysis from Lab. RSE.
Figure 9 shows the values of the confidence and prediction bands only.
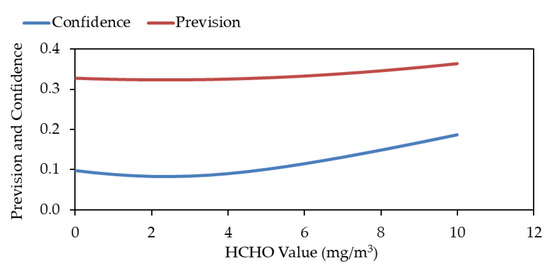
Figure 9.
Confidence and prevision bands of the correlation between EPA 323 analysis from Lab. APPA Trento and VDI 3862-2 analysis from Lab. RSE.
It must be remembered that the conclusions that can be drawn are partial since the ‘bias’ concerning the true value is unknown; thus, the uncertainty of the methods cannot be fully assessed.
The data show, however, that the predicted values for the forecast band are, in the 0–10 mg/m3 range, below 0.4 mg/m3 (Figure 8 and Figure 9); considering that for other compounds (like HCl, for example), the corresponding values are around 1.5/2.0 mg/m3, it’s possible to assume that such results are very satisfactory and would suggest an uncertainty of the analytical methods not exceeding 1.0–1.5 mg/m3.
These values, which can only be demonstrated through tests conducted on experimental loops, where the true value is known, are state-of-the-art for organic emissions sampling but are still quite high compared to the limit values assumed for H−CHO of 3.0 mg/m3, requiring further analysis.
4. Conclusions
The determination of formaldehyde in emissions is critical for many reasons. Condensation during sampling can absorb formaldehyde in an unpredictable way, which must be absolutely avoided. For this reason, the use of heated probes is recommended. Wet capture using impingers shows very good results. Even if the solubility of H−CHO is very good in water, its stability in pure water is critical, so using a solution of H2SO4 (at least) 0.01 N is recommended.
If the measurement method VDI 3862-2 is used, the solution must be treated, within 24 h of sampling, with DNPH and kept at least 48 h before analysis, which can be done in the following 15 days if the samples are stored at 4 °C.
The relationship between EPA 323 methods and VDI 3862-2 is good (considering the values shown in Figure 7), but it is necessary to carry out new evaluations to better evaluate reproducibility and repeatability.
Due to the danger of underestimation related to the high solubility of H−CHO and its instability once in the sampling solution, real emissions from industrial plants could be underestimated. For this reason, on the one hand, a more accurate assessment of the legal technical framework of this compound is necessary, and on the other, an experimental survey of the real emissions of the industrial world for objective quantification of H−CHO releases into the environment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app121910150/s1, Table S1: Elution gradient of HPLC analysis by Lab. APPA Trento; Table S2: Elution gradient of HPLC analysis by Lab. RSE; Table S3: Analytical values obtained by Lab. APPA Trento and Lab. RSE using different methods during first campaign; Table S4: Analytical values obtained by Lab. APPA Trento and Lab. RSE using different methods during second campaign; Figure S1: Comparison between the values measured during the first campaign; Figure S2: Comparison between the values measured during the second campaign.
Author Contributions
Conceptualization, D.C. and A.M.C.; methodology, D.C. and A.M.C.; software, D.C. and A.M.C.; validation, D.C. and A.M.C.; formal analysis, D.C., G.C. and S.M.; data curation, D.C., A.M.C., L.F., G.C. and C.D.; writing—original draft preparation, D.C. and A.M.C.; writing—review and editing, L.F and E.B.; supervision, D.C. and E.B.; project administration, D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been financed by the Research Fund for the Italian Electrical System with the Decree of 16 April 2018.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Li, M.; Shao, M.; Li, L.Y.; Lu, S.H.; Chen, W.T.; Wang, C. Quantifying the ambient formaldehyde sources utilizing tracers. Chinese Chem. Lett. 2014, 25, 1489–1491. [Google Scholar] [CrossRef]
- Parrish, D.D.; Ryerson, T.B.; Mellqvist, J.; Johansson, J.; Fried, A.; Richter, D.; Walega, J.G.; Washenfelder, R.A.; de Gouw, J.A.; Peischl, J.; et al. Primary and secondary sources of formaldehyde in urban atmospheres: Houston Texas region. Atmos. Chem. Phys. 2012, 12, 3273–3288. [Google Scholar] [CrossRef]
- Lei, W.; Zavala, M.; de Foy, B.; Volkamer, R.; Molina, M.J.; Molina, L.T. Impact of primary formaldehyde on air pollution in the Mexico City Metropolitan Area. Atmos. Chem. Phys. 2009, 9, 2607–2618. [Google Scholar] [CrossRef]
- Corrêa, S.M.; Arbilla, G. Formaldehyde and acetaldehyde associated with the use of natural gas as a fuel for light vehicles. Atmos. Environ. 2005, 39, 4513–4518. [Google Scholar] [CrossRef]
- Ho, S.S.H.; Ho, K.F.; Lee, S.C.; Cheng, Y.; Yu, J.Z.; Lam, K.M.; Feng, N.S.Y.; Huang, Y. Carbonyl emissions from vehicular exhausts sources in Hong Kong. J. Air Waste Manag. Assoc. 2012, 62, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Ho, K.F.; Chan, L.Y.; Zielinska, B.; Chow, J.C. Polycyclic Aromatic Hydrocarbons (pahs) and Carbonyl Compounds in Urban Atmosphere of Hong Kong. Atmos. Environ. 2001, 35, 5949–5960. [Google Scholar] [CrossRef]
- Guven, B.B.; Olaguer, E.P. Ambient formaldehyde source attribution in Houston during TexAQS II and TRAMP. Atmos. Environ. 2011, 45, 4272–4280. [Google Scholar] [CrossRef]
- Kaiser, J.; Wolfe, G.M.; Bohn, B.; Broch, S.; Fuchs, H.; Ganzeveld, L.N.; Gomm, S.; Häseler, R.; Hofzumahaus, A.; Holland, F.; et al. Evidence for an unidentified non-photochemical ground-level source of formaldehyde in the Po Valley with potential implications for ozone production. Atmos. Chem. Phys. 2015, 15, 1289–1298. [Google Scholar] [CrossRef]
- Seco, R.; Peñuelas, J.; Filella, I. Short-chain oxygenated VOCs: Emission and uptake by plants and atmospheric sources, sinks, and concentrations. Atmos. Environ. 2007, 41, 2477–2499. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. ATMOSPHERIC From Air Pollution to Climate Change, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2006. [Google Scholar]
- Vlasenko, A.; MacDonald, A.M.; Sjostedt, S.J.; Abbatt, J.P.D. Formaldehyde measurements by Proton transfer reaction—Mass spectrometry (PTR-MS): Correction for humidity effects. Atmos. Meas. Tech. 2010, 3, 1055–1062. [Google Scholar] [CrossRef]
- Duan, Y.; He, K.; Zhang, G.; Hu, J. Photoresponsive micelles enabling codelivery of nitric oxide and formaldehyde for combinatorial antibacterial applications. Biomacromolecules 2021, 22, 2160–2170. [Google Scholar] [CrossRef] [PubMed]
- Birkett, N.; Al-Zoughool, M.; Bird, M.; Baan, R.A.; Zielinski, J.; Krewski, D. Overview of biological mechanisms of human carcinogens. J. Toxicol. Environ. Health Part B Crit. Rev. 2019, 22, 288–359. [Google Scholar] [CrossRef]
- Brandão, P.F.; Ramos, R.M.; Rodrigues, J.A. GDME-based methodology for the determination of free formaldehyde in cosmetics and hygiene products containing formaldehyde releasers. Anal. Bioanal. Chem. 2018, 410, 6873–6880. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Bai, Y.; Duong, A.; Smith, M.T.; Li, L.; Zhang, L. Formaldehyde in China: Production, consumption, exposure levels, and health effects. Environ. Int. 2009, 35, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-D.; Kim, J.-W. Dynamic Mechanical Analysis of Urea-Formaldehyde Resin Adhesives with Different Formaldehyde-To-Urea Molar Ratios. J. Appl. Polym. Sci. 2008, 108, 2045–2051. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Y.; Wei, W. Formaldehyde and VOC emissions at different manufacturing stages of wood-based panels. Build. Environ. 2012, 47, 197–204. [Google Scholar] [CrossRef]
- Lui, K.H.; Ho, S.S.H.; Louie, P.K.; Chan, C.; Lee, S.; Hu, D.; Chan, P.; Lee, J.C.W.; Ho, K. Seasonal behavior of carbonyls and source characterization of formaldehyde (HCHO) in ambient air. Atmos. Environ. 2017, 152, 51–60. [Google Scholar] [CrossRef]
- Carlier, P.; Hannachi, H.; Mouvier, G. The chemistry of carbonyl compounds in the atmosphere-A review. Atmos. Environ. 1986, 20, 2079–2099. [Google Scholar] [CrossRef]
- Cheng, Y.; Lee, S.; Huang, Y.; Ho, K.; Ho, S.; Yau, P.; Louie, P.; Zhang, R. Diurnal and seasonal trends of carbonyl compounds in roadside, urban, and suburban environment of Hong Kong. Atmos. Environ. 2014, 89, 43–51. [Google Scholar] [CrossRef]
- Fappiano, L.; Carriera, F.; Iannone, A.; Notardonato, I.; Avino, P. A Review on Recent Sensing Methods for Determining Formaldehyde in Agri-Food Chain: A Comparison with the Conventional Analytical Approaches. Foods 2022, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cui, X.; Fang, G. Rapid determination of formaldehyde and sulfur dioxide in food products and Chinese herbals. Food Chem. 2007, 103, 1487–1493. [Google Scholar] [CrossRef]
- Ahrenfeldt, J.; Jensen, T.K.; Henriksen, U.; Schramm, J. Investigation of continuous gas engine CHP operation on biomass producer gas. SAE Tech. Pap. 2005, 114, 1464–1475. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, S.; Liu, F.; Liu, J.; Zhu, Z.; Li, G. Formaldehyde and Methanol Emissions from a Methanol/Gasoline-Fueled Spark-Ignition (SI) Engine. Energy Fuels 2009, 23, 3313–3318. [Google Scholar] [CrossRef]
- Villanueva, F.; Lara, S.; Amo-Salas, M.; Cabañas, B.; Martín, P.; Salgado, S. Investigation of formaldehyde and other carbonyls in a small urban atmosphere using passive samplers. A comprehensive data analysis. Microchem. J. 2021, 167, 106270. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Monographs on the Evaluation of Carcinogenic Risks to Humans; WHO: Lyon, France, 2006; Volume 88.
- European Commission Regulation. Part 3 of Annex VI of the EC Regulation 1272/2008; European Commission: Brussel, Belgium, 2014.
- Panda, N.; Kim, M.; Aoki, N.; Zhou, Z.; Shimosaka, T.; Kim, Y.; Lee, S.; Kim, D. Validation of primary formaldehyde gas standards prepared by dynamic thermogravimetry through a tri-national comparison of gaseous formaldehyde amount fraction. Accredit. Qual. Assur. 2016, 21, 295–304. [Google Scholar] [CrossRef]
- Hanoune, B.; LeBris, T.; Allou, L.; Marchand, C.; le Calvé, S. Formaldehyde measurements in libraries: Comparison between infrared diode laser spectroscopy and a DNPH-derivatization method. Atmos. Environ. 2006, 40, 5768–5775. [Google Scholar] [CrossRef]
- Dumas, T. Determination of formaldehyde in air by gas chromatography. J. Chromatogr. A 1982, 247, 289–295. [Google Scholar] [CrossRef]
- Mann, B.; Grayeski, M.L. New chemiluminescent derivatizing agent for the analysis of aldehydes and ketones by high-performance liquid chromatography with peroxyoxalate chemiluminescence. J. Chromatogr. A 1987, 386, 149–158. [Google Scholar] [CrossRef]
- Lorrain, J.M.; Fortune, C.R.; Dellinger, B. Sampling and Ion Chromatographic Determination of Formaldehyde and Acetaldehyde. Anal. Chem. 1981, 53, 1302–1305. [Google Scholar] [CrossRef]
- Wisthaler, A.; Apel, E.C.; Bossmeyer, J.; Hansel, A.; Junkermann, W.; Koppmann, R.; Meier, R.; Müller, K.; Solomon, S.J.; Steinbrecher, R.; et al. Technical Note: Intercomparison of formaldehyde measurements at the atmosphere simulation chamber SAPHIR. Atmos. Chem. Phys. 2008, 8, 2189–2200. [Google Scholar] [CrossRef]
- Hak, C.; Pundt, I.; Trick, S.; Kern, C.; Platt, U.; Dommen, J.; Ordóñez, C.; Prévôt, A.S.H.; Junkermann, W.; Astorga-Lloréns, C.; et al. Intercomparison of four different in-situ techniques for ambient formaldehyde measurements in urban air. Atmos. Chem. Phys. 2005, 5, 2881–2900. [Google Scholar] [CrossRef]
- Salthammer, T.; Mentese, S. Comparison of analytical techniques for the determination of aldehydes in test chambers. Chemosphere 2008, 73, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Giesen, R.; Schripp, T.; Markewitz, D.; Meyer, B.; Schwab, H.; Uhde, E.; Salthammer, T. Comparison of Methods for the Determination of Formaldehyde in Air. Anal. Lett. 2016, 49, 1613–1621. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.J. Comparison of standard methods and gas chromatography method in determination of formaldehyde emission from MDF bonded with formaldehyde-based resins. Bioresour. Technol. 2005, 96, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Risholm-Sundman, M.; Larsen, A.; Vestin, E.; Weibull, A. Formaldehyde emission-Comparison of different standard methods. Atmos. Environ. 2007, 41, 3193–3202. [Google Scholar] [CrossRef]
- Kleindienst, T.E.; Corse, E.W.; Blanchard, F.T.; Lonneman, W.A. Evaluation of the performance of DNPH-coated silica gel and C18 cartridges in the measurement of formaldehyde in the presence and absence of ozone. Environ. Sci. Technol. 1998, 32, 124–130. [Google Scholar] [CrossRef]
- Cipriano, D.; Cefalì, A.M.; Allegrini, M. Experimenting with odour proficiency tests implementation using synthetic bench loops. Atmosphere 2021, 12, 761. [Google Scholar] [CrossRef]
- Cipriano, D.; Fialdini, L. Definition of reference values in synthetic emission monitoring bench loops. Accredit. Qual. Assur. 2018, 23, 125–132. [Google Scholar] [CrossRef]
- EPA. Method 323—Measurement of Formaldehyde Emissions from Natural Gas; U.S. Government Publishing Office: Washington, DC, USA, 2020.
- VDI 3862; Gaseous Emission Measurement; Measurement of Aliphatic and Aromatic Aldehydes and Ketones by DNPH Method. Verlag des Vereins Deutscher Ingenieure: Berlin, Germany, 2000.
- CEN/TS 17638:2021; Stationary Source Emissions—Manual Method for the Determination of the Mass Concentration of Formaldehyde—Reference Method. European Commission: Brussel, Belgium, 2021.
- ISO 10155; Stationary Source Emission—Automated Monitoring of Mass Concentrations of Particles—Performance Characteristics, Test Methods and Specifications. ISO: Geneva, Switzerland, 1995.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).