Abstract
Spirulina (formerly Arthrospira) maxima (SP) is a cyanobacterium reported to have great nutritional and pharmacological potential. The objective of this study was to evaluate the protective properties of SP against ethanol-induced toxicity. Male Wistar rats were used in the study and subjected to a 70% partial hepatectomy (PH); they were then divided into five groups. During the experiment, animals in two groups drank an aqueous solution of ethanol (EtOH) (40%, v/v). Additionally, they were administered an SP extract daily at a dose of 200 mg/kg body weight intragastrically. To explore possible mechanisms of action, we examined antioxidant defense enzymes, as well as serum biochemical parameters and histopathological changes in the liver. SP administration normalized elevated glutathione reductase (GR), glutathione (GSH), and superoxide dismutase (SOD) levels, in addition to increased catalase (CAT) and glutathione peroxidase (GPX) enzymes. Alterations in biochemical parameters were observed in the groups with PH treated with EtOH associated with a reduction in cholesterol and albumin levels, while glucose and triglyceride levels increased. The histological study supported the protective activity of SP, reducing apoptosis, necrosis, and congestion in the liver. Our findings demonstrated a protective effect of SP against EtOH that is related to less inflammation, a lesser antioxidant effect, and less free radical scavenging activity.
1. Introduction
Spirulina (formerly Arthrospira) maxima (SP) is a filamentous cyanobacterium found growing in alkaline water bodies and that has been consumed by humans for thousands of years without side effects. It is easily digested and absorbed by the human body and has been reported to have great nutritional and pharmacological potential [,,,]. SP contains several macro- and micronutrients, such as a high protein content (including all essential amino acids), vitamins (B12, E, C, K, and pro-vitamin A), beta carotene, gamma linolenic acid, and different active substances, such as phycocyanin, phenolic compounds, and trace elements. It is also a considerable source of sulfolipids and glycolipids; with regard to mineral content, calcium, iron, magnesium, manganese, zinc, and potassium are highlighted [,,,]. Recent in vivo and in vitro studies showed that SP has excellent antiviral, anticancer, anti-inflammatory, and antioxidant effects, and properties attributed to it include being neuroprotective and hepatoprotective. In addition, it possesses an immunomodulatory effect and is effective in treating hyperglycemia, hyperlipidemia, among other metabolic dysfunctions [,,,,,].
Liver diseases comprise an important public health problem due to their high morbidity and mortality []. The liver is the human body’s main organ of metabolism and excretion and is susceptible to various pathologies, the most frequent of these being acute and chronic hepatitis, cirrhosis, and hepatocarcinoma. Excessive alcohol consumption is one of the main causes of liver disease worldwide, encompassing different lesions, ranging from steatosis in various stages to cirrhosis with the subsequent risk of hepatocellular carcinoma. The main risk factors associated with the evolution of this disease are amount of alcohol ingested, type of alcohol, gender, obesity, genetic factors, and gene polymorphisms [].
Oxidative stress is the result of an alteration between prooxidants and antioxidants, causing the excessive production of reactive oxygen species (ROS), which play an essential role in pathological changes in the liver, particularly in cases of alcoholic and toxic liver diseases []. The main functions of antioxidative defenses are suppressors of the generation of ROS, with the scavenging of these, in addition to repairing and promoting the reconstitution of damage []. There are numerous antioxidant mechanisms, including low-molecular-weight non-enzymatic molecules and enzymes. Glutathione reductase (GR), superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) are the enzymes that provide cellular protection against the damage caused by free radicals and ROS. However, this protection may not be efficient when there is an overproduction of ROS; thus, different dietary antioxidants may contribute to achieving a protective mechanism. Partial hepatectomy (PH) induced liver regeneration and enzyme release, which have been described in detail in the literature in recent years. Altered levels of serum and liver enzymes have been reported in induced liver injury [,]. Likewise, alterations have been reported in the serum levels of cholesterol, glucose, triglycerides, and bilirubin, and these are the most specific markers for detecting liver damage [].
Currently, treatment options for the different hepatopathologies are limited; hence, there is a need to have different treatment schemes []. Therapeutic strategies are based on changes in lifestyle, such as exercising and consuming a balanced diet. Different studies have reported different phytotherapeutic agents that may have anti-hepatitis activity or that may act against liver-associated diseases. A large number of plants and phytopharmaceuticals worldwide have been employed for this purpose [,]: the antioxidant activities of Spirulina were demonstrated in a large number of preclinical studies [,,].
In this study, the protective properties of SP on ethanol (EtOH)-induced toxicity were evaluated by examining the effects on antioxidant enzyme activity in partial post-hepatectomy liver regeneration in male Wistar rats. Additionally, the effects of SP were analyzed on serum parameters, and histopathological changes in the liver of rats were also determined.
2. Materials and Methods
2.1. Animal Preparation
Male Wistar rats (Rattus norvegicus albinus) weighing 200–250 g were housed individually in cages in an air-conditioned room, which was maintained at a temperature of approximately 23 ± 2 °C and on a 12:12-h light:dark cycle and with a relative humidity around 50%. All animals remained in an acclimatization period of 5 days before the start of the experiment and had free access to food (Rodent Laboratory Chow 5001; PMI, Richmond, IN, USA) and drinking water. At the end of the experimental period (7 days), all rats were decapitated after being previously anesthetized with pentobarbital sodium (40 mg/kg BW). All procedures were approved by the Institutional Animal Care and Use Committee of the Autonomous University of Hidalgo State, Mexico, with approval number CICUAL/F011/2021. In addition, all procedures were performed according to the Official Mexican Guidelines for Laboratory Animal Use and Care (NOM-062-ZOO-1999).
2.2. Experimental Design
The animals were grouped (n = 6 for each experimental group) randomly in the following five groups: (1) control group (sham); (2) group with partial hepatectomy (PH); (3) group with PH plus intragastric (i.g.) administration of ethanol (PH-EtOH); (4) group receiving a hepatectomy and Spirulina (PH-SP); and (5) group receiving a hepatectomy, SP, and EtOH (PH-SP-EtOH). The rats in all groups received food and water throughout the treatment period. The four remaining experimental groups were subjected to a 70% PH, for which the animals were anesthetized with ethyl ether. The procedure for removal of two-thirds of the liver utilizing a technique known as a PH was performed as described [,,,] for the PH-induced test group (without administration). The PH group with EtOH received an i.g. dose of 1.5 g/kg body weight (BW) of EtOH (solution at 40% in isotonic saline solution), this equivalent to blood alcohol values between 75 and 150 mg/dL, which have been reported as capable of inhibiting the liver regenerative process [,] in positive control group. The positive control group, PH with Spirulina (SP), received an i.g. dose of 200 mg/kg body weight (BW). For the PH group with EtOH + SP, the alga Spirulina (200 mg/kg BW) was administered after 30 min of the single i.g. administration of EtOH (1.5 g kg BW, i.g.). All treatments from the EtOH solution and SP were administered daily for 7 days. The SP sample employed in this study was from a bulk production batch (SDW-9714) of standardized quality, supplied by Alimentos Esenciales para la Humanidad, S.A. de C.V., Mexico City, purchased from Sigma, St. Louis, MO, USA.
2.3. Determination of Body and Liver Weights
The BW of each rat from a different experimental group was measured initially and during the experiment. In addition, a liver-mass gain assessment and regeneration were determined by calculating the liver restitution weight. Finally, the number of deaths observed in the groups was analyzed [].
2.4. Preparation of Serum and Liver Samples
Plasma and serum were separated in a clinical centrifuge and were stored at −70 °C until use. The liver was isolated, weighed, rapidly placed in cold phosphate-buffered saline solution (PBS) solution with a phosphate tampon, with pH 7.5), and washed to eliminate the blood. The liver was placed in nine volumes of cold buffer (sucrose 0.25 mol/L, TRIS 10 mmol/L, EGTA 0.3 mmol/L, and bovine serum albumin [BSA] 0.2%, pH 7.4). The liver was homogenized employing a homogenizer with a piston-type driver with a Teflon tip. The homogenate was divided into aliquots and frozen at −70 °C until its later use. The total concentration of the protein of the homogenate was determined by the method of Bradford [] utilizing the BSA solution as a standard.
2.5. Assay of Hepatic Enzymes
2.5.1. Measurement of Catalase
Catalase (CAT) (Enzyme Commission number (EC) 1.11.1.6) activity was determined spectrophotometrically at absorbance at 540 nm by the Cayman Chemical Catalase Assay kit (Cayman Chemical Co., Inc., Ann Arbor, MI, USA), which utilizes the peroxidatic function of CAT for the determination of enzyme activity []; the result is reported in nmol/min/mg protein.
2.5.2. Measurement of Superoxide Dismutase
Superdismutase oxide SOD (EC 1.15.1.1) activity was determined by the colorimetric measurement of formazan crystals at 450 nm carried out with the commercial Superoxide Dismutase Assay kit (Cayman Chemical Co., Inc.) [], reporting results in U/min/mg/protein.
2.5.3. Measurement of Glutathione
Glutathione (GSH) (EC 1.1.1.284) activity was assayed with the commercial Glutathione Assay kit (Cayman Chemical Co., Inc.) []. Measurement of the absorbance of TNB at 405 nm gives an accurate estimate of GSH in the sample. GSH activity for each sample was determined by interpolation on a linear regression curve of the TNB standard and is reported in µM/mg/protein.
2.5.4. Measurement of Glutathione Reductase
Glutathione reductase (GR) (EC 1.6.4.2) activity was determined by the Cayman Chemical Glutathione Reductase Assay kit (Cayman Chemical Co., Inc.). The oxidation of NADPH to NADP+ is accompanied by a decrease in absorbance at 340 nm. Since GR is present at rate limiting concentrations, the rate of decrease in the A340 is directly proportional to the GR activity in the sample and reporting results in nmol/min/mg protein [].
2.5.5. Measurement of Glutathione Peroxidase
Glutathione peroxidase (GPx) (EC 1.11.1.9) activity was measured according to the Cayman Chemical Glutathione Peroxidase Assay kit (Cayman Chemical Co, Inc.). Oxidized glutathione (GSSG) produced upon reduction of an organic hydroperoxide by GPX is recycled to its reduced state by GR and NADPH. The oxidation of NADPH to NADP+ is accompanied by a decrease in absorbance at 340 nm. The rate of decrease in the A340 is directly proportional to the GPX activity in the sample []. The results are expressed in nmol/min/mg protein.
2.6. Serum Biochemical Parameters
At the end of the experimental period, a sample of blood was obtained from each rat and centrifuged at 1300× g for 10 min. Serum was separated for the assessment of cholesterol, glucose, triacylglycerides, and albumin, which were evaluated by spectrophotometric techniques (Spectrophotometer VE-5100uv) using diagnostic kits (Spinreact de México, S.A. de C.V.) following the instructions provided by the manufacturer. The results are reported in mg/dL, except for albumin, which is reported in g/dL.
2.7. Liver Histology
Hepatic samples from each group were utilized for the light microscopy. Samples were fixed with formaldehyde (10% in isotonic solution), embedded in wax, and stained with hematoxylin–eosin. Biopsy specimens were coded and read blindly without knowledge of the other data by independent observers at two different laboratories (J.A.M.-G. and J.B.-R.). The criteria used to analyze the morphological abnormalities were as follows: apoptosis, necrosis, and congestion (−absent, + mild, ++ moderate, and +++ severe) [].
2.8. Statistical Analysis
Results are expressed as mean values and the corresponding standard errors ± the standard error of the mean (SEM). A comparison was made between groups using analysis of variance (ANOVA) and for multiple comparison tests with a post hoc Tukey test (when significant differences were detected), according to data distribution. employing SPSS statistical software version 22.0 for Windows (SPPS, Chicago, IL, USA). A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Effect of Ethanol and SP on the Survival and Parameters of Liver Regeneration
The effect of EtOH and SP was observed in BW gain, liver regeneration, and mortality.
Regarding weight gain, all the experimental groups were below the control group, finding significant differences (p < 0.05); it was observed that the PH-EtOH group, at the end of the experiment, had a weight loss of 39.59% (−22.48 g), being significant (p < 0.05) with respect to the control and the other groups analyzed. The PH-EtOH and PH-SP groups presented a significant decrease (p < 0.05) in the restitution of liver mass compared to the PH group (Table 1).

Table 1.
Effect of the administration of EtOH and SP on body weight and survival in rats, and restitution of liver mass. Values are expressed as the mean ± standard error of the mean (SEM) in each experimental group (n = 6).
For the groups treated with SP, a gain in BW was observed, obtaining values higher than those of the PH group. In addition, the PH-EtOH-SP group demonstrated a restoration of weight gain in the liver (71.08%), obtaining values closest to those of the PH group (83.64%). Administration of EtOH reflected a marked mortality rate (16.6%) in the PH-EtOH group, this being statistically significant (p < 0.05) compared to the control group. In contrast, SP administration suppressed mortality compared to the previously mentioned group.
3.2. Effect of EtOH and SP on Histological Indicators (Congestion, Steatosis, Inflammation, Apoptosis, and Necrosis)
It has been observed that, after hepatic resection, different changes occur in the structural and functional characteristics of hepatocytes. The analysis of the histological indicators for the PH-EtOH group revealed an increase in apoptosis, necrosis, and congestion, corroborating the presence of inflammation and steatosis in the histological sections of these rats (Table 2, Figure 1); however, for the rats treated with SP, it can be observed that the histopathological study showed a decrease in the degree of said histological alterations (Figure 1).

Table 2.
Histological indicators. Liver lesion parameters in each experimental group treated (n = 6).
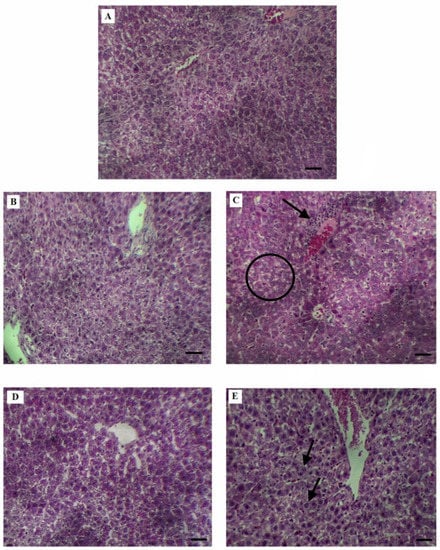
Figure 1.
Histological profiles of liver cells. (A) Depicts the control group in which the liver histology is normal; (B) moderate congestion in group PH; (C) PH-EtOH, microvesicular steatosis (circle), inflammation (arrow), apoptosis, necrosis, and congestion; (D) moderate congestion in PH with SP group; (E) PH-EtOH-SP, decrease in apoptosis, necrosis and microvesicular steatosis, mitotic images (arrow). (A–E): bar = 15 µm.
3.3. Effects of Treatment with EtOH and SP on Serum Concentrations of Cholesterol, Triacylglycerides, Glucose, and Albumin
The effects of treatment with EtOH and SP on concentrations of serum metabolites can be observed in Table 3.

Table 3.
Serum metabolites in the control group and partial hepatectomy, ethanol, and Spirulina (formerly Arthrospira)-treated animals.
The hepatectomy (the PH group) and the hepatotoxicity of animals treated with EtOH (PH-EtOH and PH-EtOH-SP) were associated with a significant (p < 0.05) reduction in cholesterol levels compared to the control, while, in the animals in the PH-SP group, the cholesterol concentration exhibited an increase (44.30 mg/dL) similar to the level of the control group. As can be observed in Table 3, only in the PH-EtOH group was there a significant (p < 0.05) increase in triglyceride concentration (179.90 mg/dL) compared to all experimental groups, with no significant differences found in the remaining three groups, compared to the control.
Serum glucose concentrations for the groups: PH, PH-EtOH, and PH-SP presented a significant increase (p < 0.05) with respect to the control group. Additionally, the increase in blood glucose in the groups: PH and PH-EtOH also showed significant differences with respect to the animals treated with SP and EtOH (PH-EtOH-SP), a group that restored glucose levels (129.68 ± 1.4 mg/dL) to similar values of the control (116.92 ± 2.0 mg/dL). Hepatectomy (PH) and the administration of EtOH (PH-EtOH) showed significant decreases (p < 0.05) in albumin with respect to the values of the control group (3.54 ± 0.10 g/dL) and the PH-EtOH-SP group (3.61 ± 0.17 g/dL).
However, it was observed that, in the animals treated with SP, that is, those in the PH-SP and PH-EtOH-SP groups, these presented a tendency toward normalizing the albumin values (Table 3).
3.4. Effect of SP on the Antioxidant Biomarkers Concentration in Rats with PH and Treatment with EtOH
In the present study, we determined the activities of the antioxidant enzymes CAT, SOD, GPX, and GR, in addition to performing the quantification of GSH (Table 4).

Table 4.
Activity of antioxidant enzymes in the liver of rats treated with EtOH and SP. Values are mean ± SEM. Number (n) of animals per group = 6.
The results for CAT enzyme indicate that, in the PH group, there is a significant decrease (p < 0.05) with respect to the normal values of the control (19.5463 ± 0.8 nmol/min/mg protein). Similarly, in the PH-EtOH group, there was a significant reduction of 54.09% in hepatic CAT (10.5729 nmol/min/mg protein). Regarding the results obtained from SOD, the PH group presented the lowest concentrations (1.1548 ± 0.9 U/min/mg/protein), its values being significant with the rest of the experimental groups. In contrast, in the PH-EtOH group, a significant increase in SOD values (7.2067 U/min/mg/protein) occurred compared to the control group and PH-EtOH-SP group. The CAT and SOD activities in the SP-treated groups were restored to near-normal values.
In addition, the GSH activity in the liver presented in the animals of the group with PH treated with EtOH decreased significantly (p < 0.05) with respect to the control group PH and PH-SP-EtOH.
As demonstrated for GSH in the groups treated with SP, the GSH activity was restored to a level practically equal to that of the control group.
Likewise, the quantification of GR was lower for this same experimental group (PH-EtOH), showing significant differences (p < 0.05) with respect to all the experimental groups. In contrast, the groups administered with SP (PH-SP and PH-SP-EtOH) presented a significant increase compared to the values of the control (1.0513 ± 0.16 nmol/min/mg protein) and the PH group (1.4209 ± 0.17 nmol/ min/mg protein).
The GPX activity for PH was significantly (p < 0.05) lower than the control. In the PH-EtOH group (0.6236 ± 0.1 nmol/min/mg protein), it was statistically significant compared with all the groups studied. Likewise, a significant difference (p < 0.05) was found between the PH-SP group and the GPX activity of the control animals. It should be noted that the PH-EtOH-SP group presented the tendency to restore normal GPX activity (7.1698 ± 0.8 nmol/min/mg protein).
4. Discussion
One of the mechanisms associated with alcoholic liver disease and excessive alcohol consumption is malnutrition, manifesting from poor dietary intake and mainly due to the presence of anorexia and alterations in smell and taste. The underlying mechanisms include disorders in the digestion and absorption of different food products, as well as an increase in the metabolism and excretion of nutrients. Nutritional deficiencies cause a decrease in body mass [,].
The results obtained are consistent with the latter, revealing a significant decrease in the BW of animals in the PH group treated with EtOH, which is also related to a decrease in the restitution of liver mass in this group compared to the control group (Table 1). These findings can be explained in part by malnutrition in the animals. Protein and nutritional energy deficiency, as well as micronutrient depletion, are clinical concerns in alcoholic liver disease. In addition, malnutrition is associated with high rates of complications and mortality. It has been described that an HP of 70% and the administration of EtOH alter the restoration of the original weight of the liver [] due to the excessive production of free radicals, giving rise to a decrease or inhibition in the proliferative process of this organ [], associated with marked mortality due to the EtOH mechanism of damage in the liver, which was mainly reflected in the PH-EtOH group.
It is noteworthy that the groups treated with SP demonstrated an increase in BW gain (g), surpassing the PH and PH-EtOH groups. The data obtained in this parameter provide conclusive support of the protective effect of SP against EtOH toxicity, mainly because this alga possesses a very important nutritional value, with a high content of amino acids, proteins, vitamins, minerals, and antioxidants, coupled with its bioavailability, that is, its great capacity to be digested and absorbed [,,]. The latter facilitated the increase in BW and a null mortality rate in the groups treated with SP.
Physiologically and clinically, it is important to determine the mechanisms necessary to restore or increase hepatocellular regenerative capacity. The liver possesses a remarkable ability to regenerate after surgical resection (PH) []. However, the administration of ethanol in the PH-EtOH group during liver regeneration resulted in the presence of apoptosis, necrosis, and congestion, in addition to the observation of microvesicular steatosis and moderate inflammation (Table 2, Figure 1). It is of note that, when SP was administered, microvascular accumulation of fat droplets and inflammation decreased. These changes were associated with those reported on the different SP hepatoprotective agents. Hepatoprotection by phycocyanin-C in vivo is associated with the biotransformation of xenobiotics into reactive intermediates, reducing the rate of their biotransformation into toxic species in addition to eliminating the production of reactive metabolites [,]. The latter is consistent with these experimental groups treated with SP, which presented less cell death with respect to the group treated with EtOH.
The liver plays a very important role as the metabolic center of the body. Serum biomarkers have been evaluated primarily for their ability to determine liver injury, including cholesterol, triglycerides, glucose, and albumin.
Cholesterol is the basic component of the cell membrane. It is a precursor to steroid hormones, as well as forming several different particles with lipoproteins.
It has been suggested that the administration of EtOH induces greater synthesis of fatty acids, as well as lower release of hepatic lipoproteins, aspects that could be associated with the decline in serum cholesterol. This was consistent with our work on decreased cholesterol values (24.97 mg/dL) in PH rats treated with EtOH [].
In addition, the hepatotoxic effect of EtOH administration was evidenced by a significant increase (p < 0.05) in triglycerides in the PH-EtOH group. This is consistent with what has been reported due to the presence of cellular alterations by different lipid metabolic disorders, which induce a worsening of hepatic steatosis, observed in the histological sections of these animals. In addition, these alterations predispose to a stage characterized by oxidative stress [,].
The administration of SP in the PH-SP and PH-EtOH-SP groups improved serum cholesterol concentrations (Table 3). These results coincided with those of several clinical trials in humans and in different animal models that consistently demonstrated the activity of SP as totally or partially normalizing the levels of total serum cholesterol and of the LDL and VLDL fractions [,,,]. In addition, the effects of SP restored the concentration of triglycerides (Table 3), obtaining similar values to the animals of the control group and the PH group. It is reasonable to assume that the potential hepatoprotective role of SP may be associated with the high content of dietary fiber, inducing a lipid-lowering action, in addition to its antioxidant components, such as phycocyanin-C, which plays an important role in cholesterol homeostasis. It has also been suggested that SP stimulates B-oxidation at the mitochondrial and peroximal levels, reducing triglycerides [,]. Therefore, the results of the present study showed that animals treated with SP were able to restore EtOH-induced changes in the cholesterol and triglyceride levels of the liver.
The alteration in the significant increase (177.63 mg/dL) of glucose in blood observed in rats treated with EtOH, with respect to the control group, confirmed that there is a metabolic disorder characterized by hyperglycemia, which implies excessive glucose production and decreased utilization by tissues. From the results obtained, it is interesting to observe that SP (PH-EtOH-SP) decreases the concentration of glucose in blood.
It has been reported that the SP cyanobacterium has the ability to act similarly to insulin and to stimulate the pancreatic β cells, increasing the production of said hormone and reducing blood glucose levels. It should be noted that, in the groups treated with SP, there is a tendency to restore the normal level of glucose in the blood, suggesting that this alga has a hypoglycemic effect, which coincides with different reported investigations [,,,]. These findings suggest the beneficial effect of SP supplementation in controlling blood glucose levels in humans and in animal models.
Albumin, the most abundant protein in serum, possesses antioxidant properties, and a decrease in this protein can result in an attenuation in the regulation of oxidative stress in cells []. These were reflected in the PH and PH-EtOH groups, where the level of albumin was lowest compared to the control and PH-EtOH-SP groups. This hepatoprotective effect was attributed to its blue tetrapyrrole chromophore (phycocyanin C), the main antioxidant protein of the SP alga, which has the ability to eliminate alkoxyl-, hydroxyl-, and peroxyl-free radicals, coupled with a decrease in nitrite production and inhibiting hepatic microsomal lipid peroxidation [,]. Different bioactives of phycocyanin tend to bind with high affinity to human serum albumin, increasing its thermal and proteolytic stability. In addition, phycocyanin improved its bioavailability and antioxidant activity [,,].
Because it is a multifunctional organ, the liver participates importantly in the neutralization of different harmful compounds, such as ethanol and its metabolites, which are involved in oxidative stress of different liver diseases. Therefore, it is of great interest to study bioactive substances, such as SP, that contribute to reducing the excessive amount and the effect of free radicals.
Therefore, there is an alteration in the enzyme activity in PH due to a release of damaged cells or cells with alterations in permeability [], which agrees with our data regarding the significant decrease in CAT in the PH group with respect to the values of the control. The reduced activity of CAT may be determined as a result of the toxic effects of free radicals produced in the transformation of ethanol; moreover, it may be determined by the direct effect of acetaldehyde resulting from the oxidation of ethanol and an intensification of lipid oxidation processes, as well as from tissue damage [,,,]. This is consistent with the data obtained from the level of this enzyme in the PH-EtOH group, which was statistically significant (p < 0.05) with respect to the control group.
In our study, we observed that, in the PH-SP and PH-SP-EtOH groups, CAT activity was restored (Table 4). Therefore, the administration of bioactive substances, such as SP, which possesses antioxidant properties, can comprise an effective therapeutic agent for preventing and treating EtOH hepatotoxicity and for significantly reducing free radicals, thus improving liver function [,].
The administration of xenobiotics (EtOH) produces high toxicity, altering the hepatic regeneration induced by HP; consequently, an increase in antioxidant mechanisms is originated as a defense system against an excess of free radicals [].
This corroborates that the levels of these rats (PH-EtOH group) were statistically (p < 0.05) higher for SOD, a mechanism by which activity would be compensated for by balancing the antioxidant system, exerting a protective effect.
In addition, hyperglycemia was observed in the PH-EtOH group. It is documented that exposure to high concentrations of blood glucose can generate an increase in ROS, causing the activation of the first antioxidant barriers of the cells, among which we find SOD [,].
The significant decrease in SOD in the PH group with respect to all the experimental groups could be due to the different mechanisms during liver regeneration induced by PH. On the other hand, for the groups with SP, there was a tendency to restore SOD activity, consistent with previously cited studies. These results could be attributed to the ability of Spirulina to maintain the structural integrity of the hepatocellular membrane after eliminating the free radicals generated during intoxication by different toxic substances [,,].
Glutathione participates in the elimination of free radicals and is a cofactor of the following antioxidant enzymes: glutathione peroxidase, glutathione reductase, and glutathione transferase. It participates in the regeneration of other antioxidants, such as vitamins C and E, and also influences the repair of proteins and lipids of the cell membrane and DNA [,,].
Studies on the hepatotoxicity of ethanol have reported that alcohol intoxication can reduce intracellular glutathione levels, weakening antioxidant defense mechanisms, which gives rise to oxidative damage in hepatocytes [,], consistent with what we can observe in Table 4, where there is a reduction in GSH in our animals treated with ethanol. Simultaneously, the GPX GR enzymes from the PH-EtOH group had the lowest levels, being statistically significant (p < 0.05). The depletion or inactivation of this antioxidant mechanism may be related to ROS, whose excessive amounts are formed during the course of ethanol transformation [,].
However, in the groups treated with SP, there is total recovery in GSH activity compared to the control. Simultaneously, the tendency to restore GR and GPX activity was also observed.
According to several authors, it can be considered that our results on the enzymatic regulation of GR, GPX, and GSH in the PH-SP and PH-ETOH-SP groups was related to the high amounts of phenolic compounds and ω-3 and ω-6 fatty acids available in SP; these substances possess a large number of double bonds in their molecular structure that are responsible for antioxidant effects [,].
5. Conclusions
In this study, it was found that exposure to EtOH alters hepatic regeneration, reflected in a decrease in body weight gain and poor hepatic restitution against PH; consequently, significant mortality was demonstrated. In addition to the above, histologically, cell damage caused by the hepatotoxicity of EtOH was presented, as well as an alteration in the biochemical parameters and the enzyme activity analyzed.
Treatment with SP in the face of EtOH toxicity contributed to the regulation of the biochemical parameters analyzed in plasma. In addition, the use of SP is suggested to improve the activity of different antioxidant enzymes and, therefore, reduce oxidative stress in liver damage caused by EtOH. The hepatoprotective effects of SP on liver regeneration may be due to the activity of phycocyanin as the main antioxidant component contributing to the elimination of free radicals.
Author Contributions
Conceptualization, A.P.-J., J.A.M.-G. and G.A.C.-C.; data curation, C.B.-A. and J.A.S.-C.; formal analysis, M.A.M.-V. and A.P.-M.; investigation, A.P.-J., J.A.M.-G. and G.A.C.-C.; methodology, A.P.-J. and J.A.M.-G.; supervision, J.L.A.-F. and G.A.C.-C.; writing—original draft, A.P.-J. and J.A.M.-G.; writing review and editing, A.P.-J., J.A.M.-G. and G.A.C.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SIP-ESM funding number: 20161980, the National Polytechnic Institute, México.
Institutional Review Board Statement
Institutional Animal Care and Use Committee of the Autonomous University of Hidalgo State, Mexico, with approval number CICUAL/F011/2021.
Data Availability Statement
Not applicable.
Acknowledgments
We give thanks for the histological review to: J Badillo R and for review of the writing to: Margaret E. Reynolds A.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abdel-Daim, M.M.; Abuzead, S.M.M.; Halawa, S.M. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS ONE 2013, 8, e72991. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.A. Microalgae as food and supplement. Crit. Rev. Food Sci. Nutr. 1991, 30, 555–573. [Google Scholar] [CrossRef]
- Belay, A. Spirulina (Arthrospira): Production and quality assurance. In Spirulina in Human Nutrition and Health; Gershwin, M.E., Belay, A., Eds.; CRC Press: New York, NY, USA, 2008; pp. 1–25. [Google Scholar] [CrossRef]
- Gorban, E.N.; Kuprash, L.P.; Gorban, N.E. Spirulina: Perspektivy ispol’zovaniia v meditsine [Spirulina: Perspectives of the application in medicine]. Likar. Sprav. 2003, 7, 100–110. (In Russian) [Google Scholar]
- Kameshwari, V.; Selvaraj, S.; Sundaramoorthy, S. Single Cell Protein Spirulina—A Nutrient Treasure. Res. J. Pharmacol. Pharmacodyn. 2020, 12, 49–54. [Google Scholar] [CrossRef]
- Anvar, A.; Nowruzi, B. Bioactive properties of spirulina: A review. Microb. Bioact. 2021, 4, 134–142. [Google Scholar] [CrossRef]
- Hossein, S.M.; Khosravi-Darani, K.; Mozafari, M.R. Nutritional and medical applications of Spirulina microalga. Mini-Rev. Med. Chem. 2013, 13, 1231–1237. [Google Scholar] [CrossRef]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Prabhu, S.; Vijayakumar, S.; Praseetha, P. Cyanobacterial metabolites as novel drug candidates in corona viral therapies: A review. Chronic Dis. Transl. Med. 2022, 1–12. [Google Scholar] [CrossRef]
- Mittal, A.; Kumar, P.V.; Banerjee, S.; Rao, A.R.; Kumar, A. Modulatory potential of Spirulina fusiformis on carcinogen metabolizing enzymes in Swiss albino mice. Phytother. Res. 1999, 13, 111–114. [Google Scholar] [CrossRef]
- Braune, S.; Krüger-Genge, A.; Kammerer, S.; Jung, F.; Küpper, J.H. Phycocyanin from Arthrospira platensis as Potential Anti-Cancer Drug: Review of In Vitro and In Vivo Studies. Life 2021, 11, 91. [Google Scholar] [CrossRef]
- Kasbi-Chadli, F.; Coué, M.; Aguesse, A.; Grit, I.; Souque, T.; Ferchaud-Roucher, V.; Ouguerram, K. Spirulina liquid extract prevents metabolic disturbances and improves liver sphingolipids profile in hamster fed a high-fat diet. Eur. J. Nutr. 2021, 60, 4483–4494. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Juárez, A.; Chamorro, G.; Alva-Sánchez, C.; Paniagua, N.; Pacheco-Rosado, J. Neuroprotective effect of Arthrospira (Spirulina) platensis against kainic acid-neuronal death. Pharm. Biol. 2016, 54, 1408–1412. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, O.; Katoh, T.; Okuwaki, Y. Enhancement of antibody production in mice by dietary Spirulina platensis. J. Nutr. Sci. Vitaminol. 1994, 40, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.R.; Rosso, N.; Bedogni, G.; Tiribelli, C.; Bellentani, S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018, 38, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.M.; Reinus, J.F. Prevalence and natural history of alcoholic liver disease. Clin. Liver Dis. 2012, 16, 659–666. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.; Chen, F.; Li, D.; Zhao, Y. MiR-214 promotes the alcohol-induced oxidative stress via down-regulation of glutathione reductase and cytochrome P450 in liver cells. Alcohol. Clin. Exp. Res. 2014, 38, 68–77. [Google Scholar] [CrossRef]
- Tiwari, A. Imbalance in antioxidant defence and human diseases: Multiple approach of natural antioxidants therapy. Curr. Sci. 2001, 81, 1179–1187. Available online: https://www.researchgate.net/journal/Current-Science-0011-3891 (accessed on 10 July 2022).
- Zimmerman, H.J.; Kodera, Y.; West, M. Rate of increase in plasma levels of cytoplasmic and mitochondrial enzymes in experimental carbon tetrachloride hepatotoxicity. J. Lab. Clin. Med. 1965, 66, 315–323. [Google Scholar] [CrossRef]
- Dinman, B.D.; Bernstein, I.A. Acute carbon tetrachloride hepatotoxicity. V. Enzymatic activity and structural concomitants during the regenerative phase. Arch. Environ. Health 1968, 16, 777–784. [Google Scholar] [CrossRef]
- Badrick, T.; Turner, P. Review and recommendations for the component tests in the liver function test profile. Indian J. Clin. Biochem. 2016, 31, 21–29. [Google Scholar] [CrossRef][Green Version]
- Osna, N.A.; Donohue, T.M.; Kharbanda, K.K. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Res. Curr. Rev. 2017, 38, 147–161. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/pmc5513682/ (accessed on 10 July 2022).
- Torres-González, L.; Waksman-de Torres, N.; Pérez-Meseguer, J.; Muñoz-Espinosa, L.; Salazar-Aranda, R.; Cordero Pérez, P. Review of plants with hepatoprotective activity evaluated in México. Med. Univ. 2014, 16, 78–86. Available online: www.elsevier.es/en-revista-medicina-universitaria-304-articulo-review-plants-with-hepatoprotective-activity-X1665579614366029 (accessed on 10 July 2022).
- Madrigal-Santillan, E.; Madrigal-Bujaidar, E.; Alvarez-Gonzalez, I. Review of natural products with hepatoprotective effects. World J. Gastroenterol. 2014, 20, 14787–14804. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.S.; Cintra, R.G.; Barros, S.B.; Mancini- Filho, J. Antioxidant activity of the microalga Spirulina maxima. Braz. J. Med. Biol. Res. 1998, 31, 1075–1079. [Google Scholar] [CrossRef]
- Dartsch, P.C. Antioxidant potential of selected Spirulina platensis preparations. Phytother. Res. 2008, 22, 627–633. [Google Scholar] [CrossRef]
- Martínez-Galero, E.; Pérez-Pastén, R.; Perez-Juarez, A.; Fabila-Castillo, L.; Gutiérrez-Salmeán, G.; Chamorro, G. Preclinical antitoxic properties of Spirulina (Arthrospira). Pharm. Biol. 2016, 54, 1345–1353. [Google Scholar] [CrossRef]
- Higgins, G.M.; Anderson, R.M. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. Lab. Med. 1931, 12, 186–202. [Google Scholar]
- Panis, Y.; McMullan, D.M.; Emond, J.C. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery 1997, 121, 142–149. [Google Scholar] [CrossRef]
- Morales-González, J.A.; Gutiérrez-Salinas, J.; Hernández-Muñoz, R. Pharmacokinetics of the ethanol bioavailability in the regenerating rat liver induced by partial hepatectomy. Alcohol. Clin. Exp. Res. 1998, 22, 1557–1563. [Google Scholar] [CrossRef]
- Chen, Y.; Hata, T.; Rehman, F.; Kang, L.; Yang, L.; Kim, B.Y.S.; Nguyen, J.H. Visualization of hepatocellular regeneration in mice after partial hepatectomy. J. Surg. Res. 2019, 235, 494–500. [Google Scholar] [CrossRef]
- Lamas-Paz, A.; Hao, F.; Nelson, L.J.; Vázquez, M.T.; Canals, S.; Gómez Del Moral, M.; Martínez-Naves, E.; Nevzorova, Y.A.; Cubero, F.J. Alcoholic liver disease: Utility of animal models. World J. Gastroenterol. 2018, 24, 5063–5075. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Du, Y.C.; Zeng, T.A. Mini-review of the rodent models for alcoholic liver disease: Shortcomings, application, and future prospects. Toxicol. Res. 2021, 10, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Orrego, H.; Crossley, I.; Saldivia, V.; Medline, A.; Varghese, G.; Israel, Y. Long-term ethanol administration and short- and long-term liver regeneration after partial hepatectomy. J. Lab. Clin. Med. 1981, 97, 221–230. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation ofmicrogram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Johansson, L.H.; Borg, L.A.H. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Malström, B.; Andreasson, L.; Reinhammer, B. The Enzymes; Boyer, P., Ed.; Academic Press: New York, NY, USA, 1975; Volume 3. [Google Scholar]
- Baker, M.A.; Cerniglia, G.J.; Zaman, A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem. 1990, 190, 360–365. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar] [CrossRef]
- Forstrom, J.W.; Zakowski, J.J.; Tappel, A.L. Identification of the catalytic site of rat liver glutathione peroxidase as selenocysteine. Biochemistry 1978, 17, 2639–2644. [Google Scholar] [CrossRef]
- Morales-González, J.A.; Gutiérrez-Salinas, J.; Yáñez, L.; VillagómezRico, C.; Badillo-Romero, J.; Hernández-Muñoz, R. Morphological and biochemical effects of a low ethanol dose on rat liver regeneration: Role of route and timing of administration. Dig. Dis. Sci. 1999, 44, 1963–1974. [Google Scholar] [CrossRef]
- Lieber, C.S. Alcohol: Its metabolism and interaction with nutrients. Annu. Rev. Nutr. 2000, 20, 395–430. [Google Scholar] [CrossRef]
- Rossi, R.E.; Conte, D.; Massironi, S. Diagnosis and treatment of nutritional deficiencies in alcoholic liver disease: Overview of available evidence and open issues. Dig. Liver Dis. 2015, 47, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Minicis, S.; Brenner, D.A. Oxidative stress in alcoholic liver disease: Role of NADPH oxidase complex. J. Gastroenterol. Hepatol. 2008, 23, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Vadiraja, B.B.; Gaikwad, N.W.; Madyastha, K.M. Hepatoprotective effect of C-phycocyanin: Protection for carbon tetrachloride and R-(+)-pulegone-mediated hepatotoxicity in rats. Biochem. Biophys. Res. Commun. 1998, 249, 428–431. [Google Scholar] [CrossRef]
- Vadiraja, B.B.; Madyastha, K.M. C-Phycocyanin: A potent peroxyl radical scavenger in vivo and in vitro. Biochem. Biophys. Res. Commun. 2000, 275, 20–25. [Google Scholar] [CrossRef]
- Parikh, P.; Mani, U.; Iyer, U. Role of Spirulina in the Control of Glycemia and Lipidemia in Type 2 Diabetes Mellitus. J. Med. Food 2001, 4, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Mani, U.V.; Desai, S.; Iyer, U. Studies on the Long-Term Effect of Spirulina Supplementation on Serum Lipid Profile and Glycated Proteins in NIDDM Patients. J. Nutraceuticals Funct. Med. Foods 2000, 2, 25–32. [Google Scholar] [CrossRef]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxidative Med. Cell. Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef]
- Colla, L.M.; Muccillo-Baisch, A.L.; Costa, J.A.V. Spirulina platensis effects on the levels of total cholesterol, HDL and triacylglycerols in rabbits fed with a hypercholesterolemic diet. Braz. Arch. Biol. Technol. 2008, 51, 405–411. [Google Scholar] [CrossRef]
- Lee, E.H.; Park, J.E.; Choi, Y.J.; Huh, K.B.; Kim, W.Y. A randomized study to establish the effects of Spirulina in type 2 diabetes mellitus patients. Nutr. Res. Pract. 2008, 2, 295–300. [Google Scholar] [CrossRef]
- Ansari, P.; Hannan, J.; Azam, S.; Flatt, P.; Abdel-Wahab, Y. Effects of Spirulina platensis on insulin secretion, DPP-IV activity and both carbohydrate digestion and absorption indicate potential as an adjunctive therapy for diabetes. Br. J. Nutr. 2020, 124, 1021–1034. [Google Scholar] [CrossRef]
- Senthil, N.; Balu1, P.M.; Murugesan, K. Antihyperglycemic effect of spirulina, insulin and Morinda citrifolia against streptozotocin induced diabetic rats. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 537–559. [Google Scholar]
- Oettl, K.; Stauber, R.E. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br. J. Pharmacol. 2007, 15, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Minic, S.; Stanic-Vucinic, D.; Radomirovic, M.; Radibratovic, M.; Milcic, M.; Nikolic, M.; Cirkovic-Velickovic, T. Characterization and effects of binding of food-derived bioactive phycocyanobilin to bovine serum albumin. Food Chem. 2017, 239, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Bae, C.S.; Ahn, T. Albumin infusion ameliorates liver injury in streptozotocin-induced diabetic rats. Vet. Med. 2022, 67, 245–256. [Google Scholar] [CrossRef]
- Radibratovic, M.; Minic, S.; Stanic-Vucinic, D.; Nikolic, M.; Milcic, M.; Cirkovic-Velickovic, T. Stabilization of Human Serum Albumin by the Binding of Phycocyanobilin, a Bioactive Chromophore of Blue-Green Alga Spirulina: Molecular Dynamics and Experimental Study. PLoS ONE 2016, 11, e0167973. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem. 2015, 15, 556–567. [Google Scholar] [CrossRef]
- Bindu, M.P.; Annamalai, P.T. Combined effect of alcohol and cigarette smoke on lipid peroxidation and antioxidant status in rats. Indian J. Biochem. Biophys. 2004, 41, 40–44. [Google Scholar]
- Tahir, M.; Sultana, S. Chrysin modulates ethanol metabolism in Wistar rats: A promising role against organ toxicities. Alcohol Alcohol. 2011, 46, 383–392. [Google Scholar] [CrossRef]
- Popovic, M.; Janicijevic-Hudomal, S.; Kaurinovic, B.; Rasic, J.; Trivic, S. Antioxidant effects of some drugs on ethanol-induced ulcers. Molecules 2009, 14, 816–826. [Google Scholar] [CrossRef]
- Guemouri, L.; Artur, Y.; Herbeth, B.; Jeandel, C.; Cuny, G.; Siest, G. Biological variability of superoxide dismutase, glutathione peroxidase, and catalase in blood. Clin. Chem. 1991, 37, 1932–1937. [Google Scholar] [CrossRef]
- Radic, I.; Mijovic, M.; Tatalovic, N.; Mitic, M.; Lukic, V.; Joksimovic, B.; Petrovic, Z.; Ristic, S.; Velickovic, S.; Nestorovic, V.; et al. Protective effects of whey on rat liver damage induced by chronic alcohol intake. Hum. Exp. Toxicol. 2019, 38, 632–645. [Google Scholar] [CrossRef]
- Arauz, J.; Ramos-Tovar, E.; Muriel, P. Redox state and methods to evaluate oxidative stress in liver damage: From bench to bedside. Ann. Hepatol. 2016, 15, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Ethanol and liver: Recent insights into the mechanisms of ethanol-induced fatty liver. World J. Gastroenterol. 2014, 20, 14672–14685. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Vasudevan, D.M. Alcohol-induced oxidative stress. Life Sci. 2007, 81, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Deleve, S.M.; Kaplowitz, N. Importance and regulation of hepatic glutathione. Semin. Liver Dis. 1990, 10, 251–256. [Google Scholar] [CrossRef]
- Zima, T.; Fialova, L.; Mestek, O.; Janebova, M.; Crkovska, J.; Malbohan, I.; Stipek, S.; Mikulikova, L.; Popov, P. Oxidative stress, metabolism of ethanol and alcohol-related diseases. J. Biomed. Sci. 2001, 8, 59–70. [Google Scholar] [CrossRef]
- Bishnoi, M.; Chopra, K. Antioxidant Profile of Spirulina: A Blue-Green Microalga. In Spirulina in Human Nutrition and Health; CRC Press: Boca Raton, FL, USA, 2007; pp. 101–118. [Google Scholar] [CrossRef]
- Al-Dhabi, N.; Valan-Arasu, M. Quantification of Phytochemicals from Commercial Spirulina Products and Their Antioxidant Activities. Evid. Based Complement. Altern. Med. 2016, 2016, 7631864. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).