Featured Application
Helium cold atmospheric plasma jet is safe to oral cells’ DNA in applications from 1 to 7 min.
Abstract
The effects of helium cold atmospheric pressure plasma (He-CAPP) jet on Porphyromonas gingivalis (HW24D-1) biofilm, on human gingival fibroblasts (HGF) and human gingival keratinocytes (OBA-9) were assessed. Standardized suspension of P. gingivalis was obtained, and biofilms were grown anaerobically for 48 h. After exposition to He-CAPP, the biofilm viability was evaluated by XTT assay. HGF were grown at 37 °C, in an CO2 chamber in DMEM, while OBA-9 cells were cultured in keratinocyte serum-free medium. After 24 h, plates were exposed to He-CAPP for 1 to 7 min. Plasma was generated using a commercial AC power supply with amplitude modulated signal (voltage amplitude of 20 kVp-p, frequency of 31.0 kHz and duty cycle of 22%). The corresponding discharge power was 0.6W at He flow rate of 1 L/min. DNA damage was accessed by static cytometry. Data were analyzed by GraphPad Prism (p < 0.05). Significant reductions in P. gingivalis viability in relation to non-treated groups were detected (p < 0.0001), directly proportional to exposure time. Treated groups were slightly aneuploid after 5- and 7-min treatment in HGF, and for 3 min in OBA-9 cells, with 1.2 DNA index mean. Helium cold atmospheric pressure plasma jet showed inhibitory effect on P. gingivalis mature biofilm and was not genotoxic for epithelial gingival cells and human oral fibroblasts.
1. Introduction
The biomedical application of cold atmospheric pressure plasmas (CAPP) has grown in the past decade, being studied for diverse purposes, such as application as a biomaterial and medical equipment decontaminant, wound treatment, cancer treatment, and biofilm and inflammation control [1,2].
Applying energy to a neutral gas can cause it to become electrically conductive, capable of emitting electromagnetic radiation and very sensitive to externally applied fields. The gas in such a state is known as plasma (physical plasma) and is composed by molecules, ions, and electrons that produce UV (ultra violet), reactive oxygen species/reactive nitrogen species (ROS/RNS) and can operate at body temperature [3,4,5]. Its efficiency against microorganisms seems to be driven mostly by hydroxyl and oxygen groups, better produced in devices working with atmospheric gas and, together with the other physical components (UV, heat, electromagnetical field), can vary according to the device’s parameters [1,2,6].
Plasma can be generated by different devices and discharge principles. Among them, plasma torch and plasma jet (cold atmospheric pressure plasma jet) produce a plasma plume and operate mostly with argon and helium or a mixture of gases. Other configurations, such as dielectric barrier discharge (DBD), floating electrode dielectric barrier discharge (FE-DBD), and surface micro-barrier discharge (SMD) devices can operate with air [7]. This variability, added to the different possibilities of parameters combinations for the reactor itself and the different types of cells and surfaces applied, makes it hard to compare results and requires repetitive safety tests in the studies.
Reactive oxygen species (ROS) can be found intra or extracellularly in superoxide form (O2−), peroxide (H2O2) or hydroxyl radical (OH−) and, together with reactive nitrogen species (RNS) interact with intracellular amino acids influencing in processes like cell aging, degeneration, and inflammatory damage or, in low concentration, be protective against oxidative stress, microbial agents, and induce mitogenic response [8,9]. Super production of ROS can trigger tumor suppressor gene and oncogene mutation [9]. The variation on plasma dose can either stimulate, inhibit, or cause DNA damage that cannot be repaired by cell cycle repair mechanisms [7].
The oxidative stress, together with a local dysbiosis, is responsible for tissue destruction on periodontal disease. Hyperactivated polymorphonuclear can over-release ROS that can kill invading pathogens but also be cytotoxic to host cells, leading to proinflammatory mechanisms, and inducing osteoclastogenesis [10]. A therapy that could not only inactivate periodontal pathogens but additionally balance the oxidative stress, reducing inflammation without cell damage, would be desirable for periodontitis.
The literature has shown a positive plasma effect on P. gingivalis (ATCC 33277) planktonic cells [11], and monospecies biofilm grown for 15 days [12]. We have previously shown that CAPP treatment was effective against planktonic growth of P. gingivalis (ATCC 33277) and dual-species biofilm (Porphyromonas gingivalis + Streptococcus gordonii) from 1- to 7-min treatment with no cytotoxic effects (in vitro) or tissue damage (in vivo) [13]. With these favorable results, we were intrigued with the plasma effect on oral cells’ DNA and the response of a more virulent strain of P. gingivalis (HW24D-1) in a single species biofilm.
The DNA damage can be accessed by different assays, from micronucleus to comet assays, detection of phosphorylated histone H2AX, or even static cytometry [7,14].
Static cytometry, or image DNA cytometry is an internationally recognized evaluation method for neoplastic transformation of oral cells, and cervical cancer diagnosis that evaluate Feulgen’ stained cells in an image software [15,16]. The image analysis systems give an integrated optical density (IOD) of a cell population through calibrated filters that capture the nucleus staining and calculate an optical density concordant with the DNA content [17].
Based on the successful antimicrobial effect against P. gingivalis, and the crescent literature on plasma and dentistry, it became important to evaluate the safety of our set up in oral cells, especially those involved on the periodontium. Epithelial gingival cells (OBA-9) and human oral fibroblasts (HGF) were chosen for this evaluation. The effects of helium cold atmospheric plasma (He-CAPP) jet exposure for 1 to 7 min on the cell DNA content were evaluated by the static cytometry assay.
2. Materials and Methods
2.1. Plasma Jet
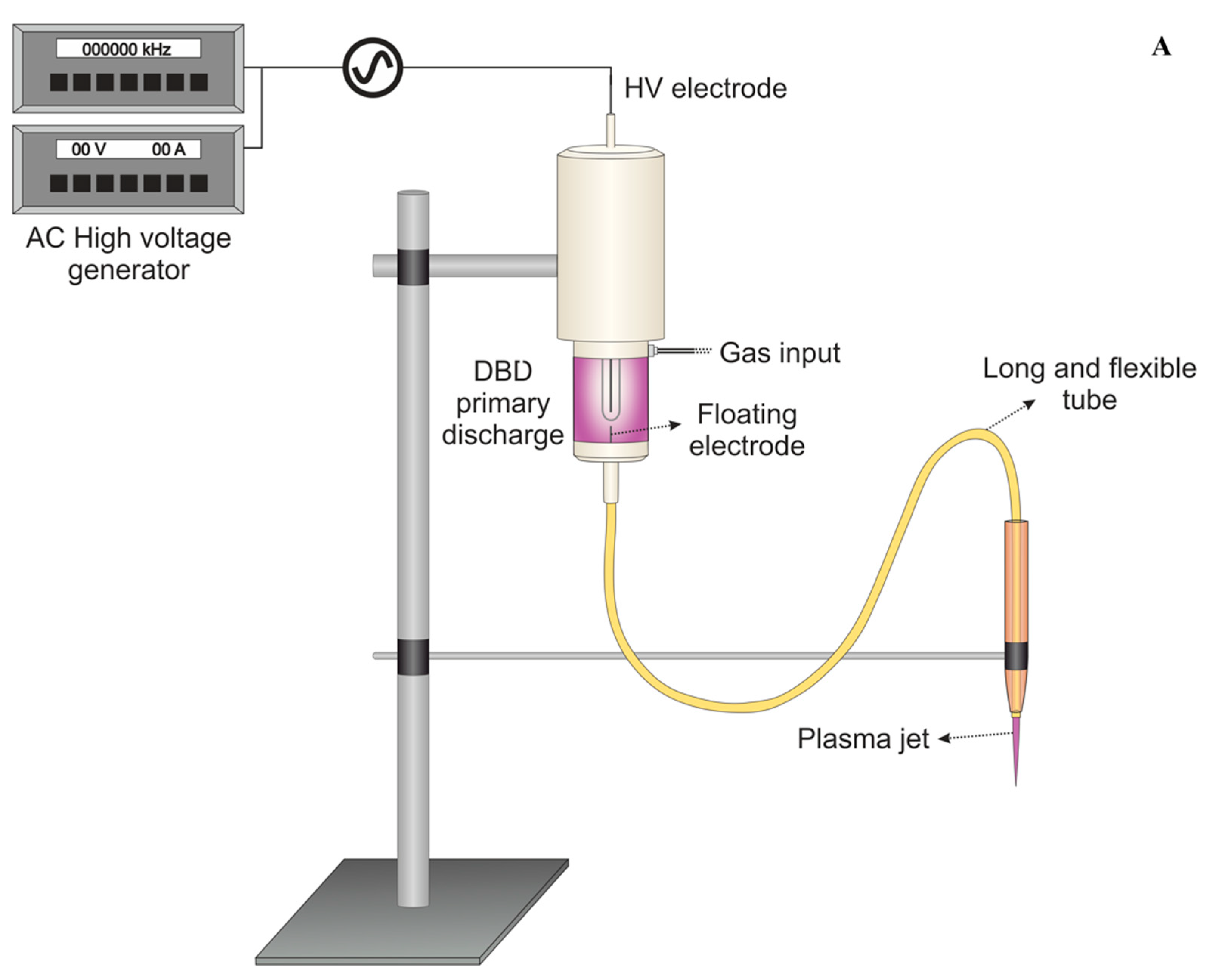
A flexible plasma jet was used in this study and the experimental setup was described previously [13,18]. The system consists of a 1.0-m-long flexible polyurethane tube (10 Fr/Ch, KangarooTM Nasogastric Feeding Tube, CardinalHealth, Dublin, OH, USA) with an inner diameter of 2.5 mm connected to a primary DBD-type plasma jet. In the primary region, an encapsulated high voltage pin electrode is connected to a Minipuls4 AC power supply (GBS Elektronik GmbH, Radeberg, Germany). The pin electrode is mounted inside a Delrin syringe-like enclosure, and the nasogastric tube is attached to the syringe’s exit nozzle. A floating thin copper wire penetrates the primary region and passes through the flexible tube, terminating a few millimeters before the tube tip. The primary reactor was fed with helium (He, 99.5% purity) at a flow rate of 1.0 standard liters per minute (slm). Since the wells have very small size (0.5 cm), higher gas flow rates can induce intense gas turbulence inside the well actually carrying the reactive species outside or even detach the biofilm from the well bottom. Therefore, the He flow rate was kept low at 1 slm, also aiming to avoid excessive samples evaporation.
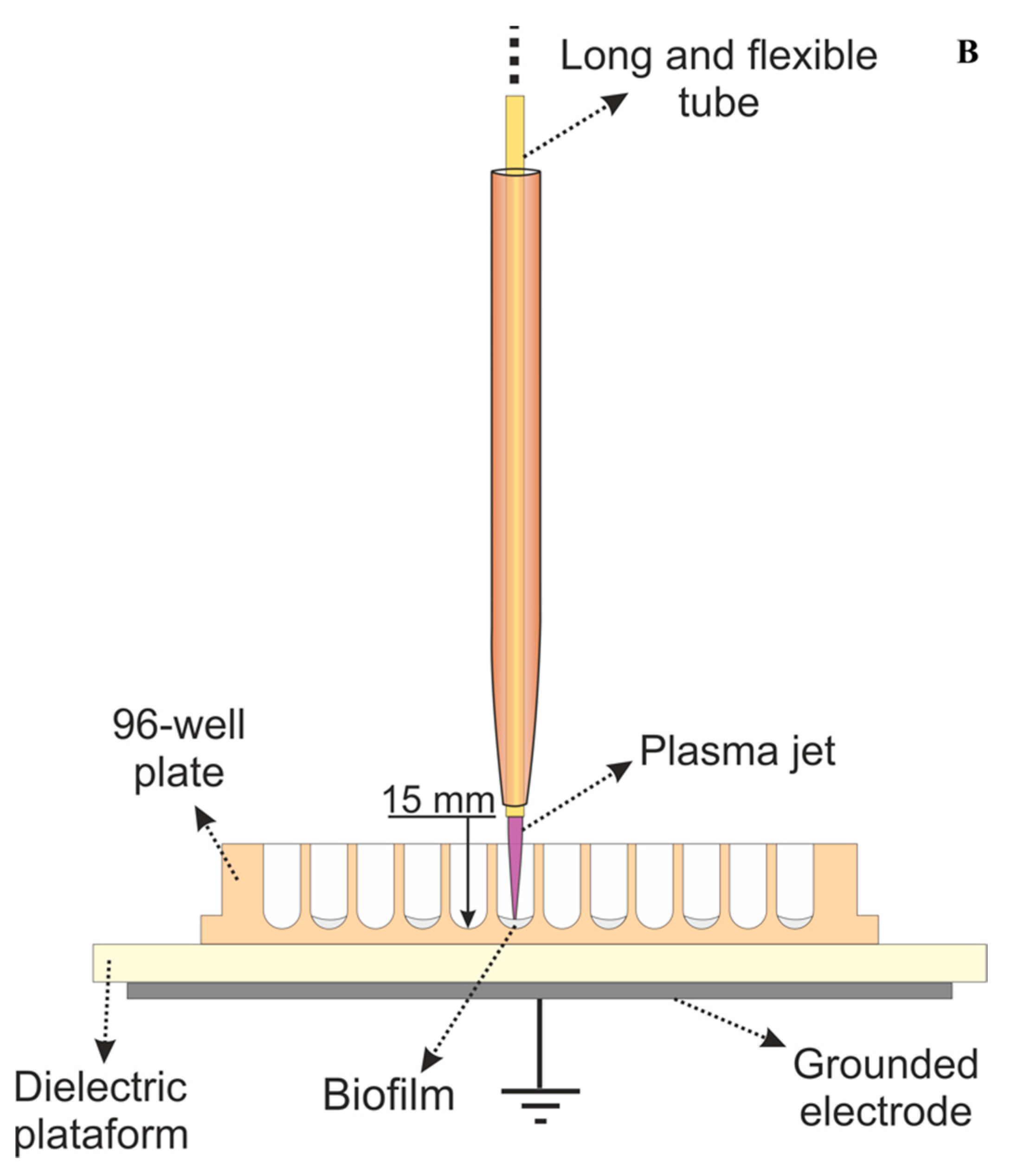
The gas is injected close to the electrodes (primary reactor) and passes through the tube. When high voltage is applied, a discharge is generated around the high-voltage electrode. The floating electrode acquires a certain potential from the discharge leading to the formation of a small plasma plume (cm-long) that is launched into ambient air through the tube exit. The flexibility of the polyurethane tube facilitates the manipulation of the remote plasma jet, allowing it to be held by hand and directed to targets of difficult access. A schematic setup of the flexible plasma jet used in this study is presented in Figure 1A, while in Figure 1B, a detailed scheme of plate treatment can be seen.
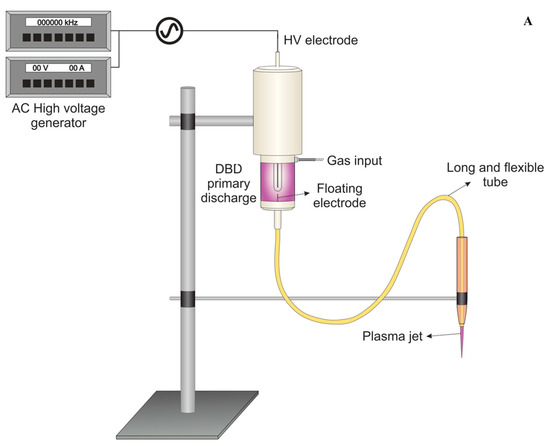
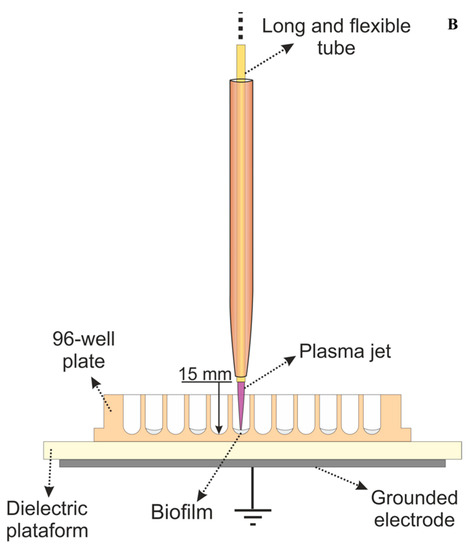
Figure 1.
(A) Schematic setup of the plasma jet with flexible plastic long tube. (B) Scheme of plasma application in a 96-well plate during the experiments.
The plasma jet was powered by a voltage signal of 10 kV amplitude with frequency of 31 kHz using burst mode (voltage amplitude modulation) with a duty cycle of 22%. Those parameters resulted in a plasma plume with mean discharge power of 0.6 W. For the experiments, the distance between the tube exit and the surface to be treated was set to 15 mm. In previous work, this plasma jet operating with similar parameters was reported, and the temperature at the target region was shown to reach around 37 °C after 5 min of plasma application [19]. For the study reported here, a lower applied voltage is used, and therefore, a temperature lower than the previously reported (lower than 37 °C) is expected.
2.2. Effect of He-CAPP on Mono Species Biofilm of P. gingivalis HW24D-1 (XTT Assay)
P. gingivalis HW24D-1 strain, which is a fimbriated and most virulent strain of P. gingivalis, was able to form a thick monospecies biofilm and chosen to be tested through XTT assay. XTT Reagent (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide), which is a tetrazolium-based compound, is sensitive to cellular redox potential and in the presence of actively respiring cells converts from a water-soluble compound to an orange-colored formazan product. Biofilm of P. gingivalis (HW24D-1) was performed as follows; a suspension of P. gingivalis (λ = 560, DO = 0.5 ± 0.02) in Brain Heart Infusion (BHI) broth supplemented with hemin (5 μg/mL), menadione (1 μg/mL) and 1% yeast extract, was obtained through spectrophotometry. Biofilms were grown for 48 h in anaerobiosis jar at 37 °C, washed with physiologic solution and treated with He-CAPP for 1, 3, 5 and 7 min, or not treated. Then, 40 μL of XTT (1 mg/mL) solution in PBS, 2 μL of 0.7 mg/mL menadione solution in ethanol, and 158 μL of phosphate buffered saline (PBS) were added per well in a 96-well plate. Plates were incubated in aluminum foil in a 5% CO2 chamber for 3 h, and then they were read by spectrophotometer at 494 nm wavelengths, in a plate reader (Bio-Tek, Synergy HT). Experiments were performed in triplicate for three times (n = 9). Data was analyzed By Kolmogorov-Smirnov normalization test, one-way ANOVA, and Tukey’s post-test using GraphPad Prism software. Significance level was set at 5%.
2.3. Cell Culture
Primary human gingival fibroblast cell line (HGF) was obtained from the Bank of cells of Rio de Janeiro (BCRJ code 0089) and cultivated in Dulbecco’s Modified Eagle Medium (DMEM) with 10% fetal bovine serum (Gibco) and supplemented with 1% penicillin/streptomycin (Gibco). Keratinocytes (OBA-9) were gently donated by Prof. Marcia Pinto Alves Mayer (Department of Microbiology, Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil), and cultivated in Keratinocyte Serum-Free (KSF) medium (Gibco) supplemented with Defined KSF medium Growth Supplement, gentamicin (1/1000), penicillin/streptomycin (1/100), and amphotericin B (1/1000). Cells were kept in humidified chamber with 5% CO2 at 37 °C.
2.4. Genotoxicity of He-CAPP on Epithelial Oral Cells
The genotoxicity was assessed by static cytometry, evaluating the cell ploidy [20]. Four thousand fibroblasts (FGH) and 150 thousand keratinocytes (OBA-9) were seeded per well, in a 96-well plate, in triplicate and stored for 24 h at 37 °C in a CO2, in their respective medium. Then, cells were washed in 100 μL of Hanks’ Balanced Salt solution (HBSS) and 50 μL of HBSS were added per well for the plasma treatment (1, 3, 5 and 7 min, or no treatment). After, residual HBSS was aspirated, 200 μL of culture medium was added, and plates were kept in a 5% CO2 atmosphere for 24 h, when cells were washed, released with trypsin, fixed in 4% formaldehyde, and transferred to glass slides. They were stained by Feulgen method, that hydrolase cells through 5N hydrochloric acid (HCl) and stains cells with Schiff’s reagent for 90 min at 4 °C.
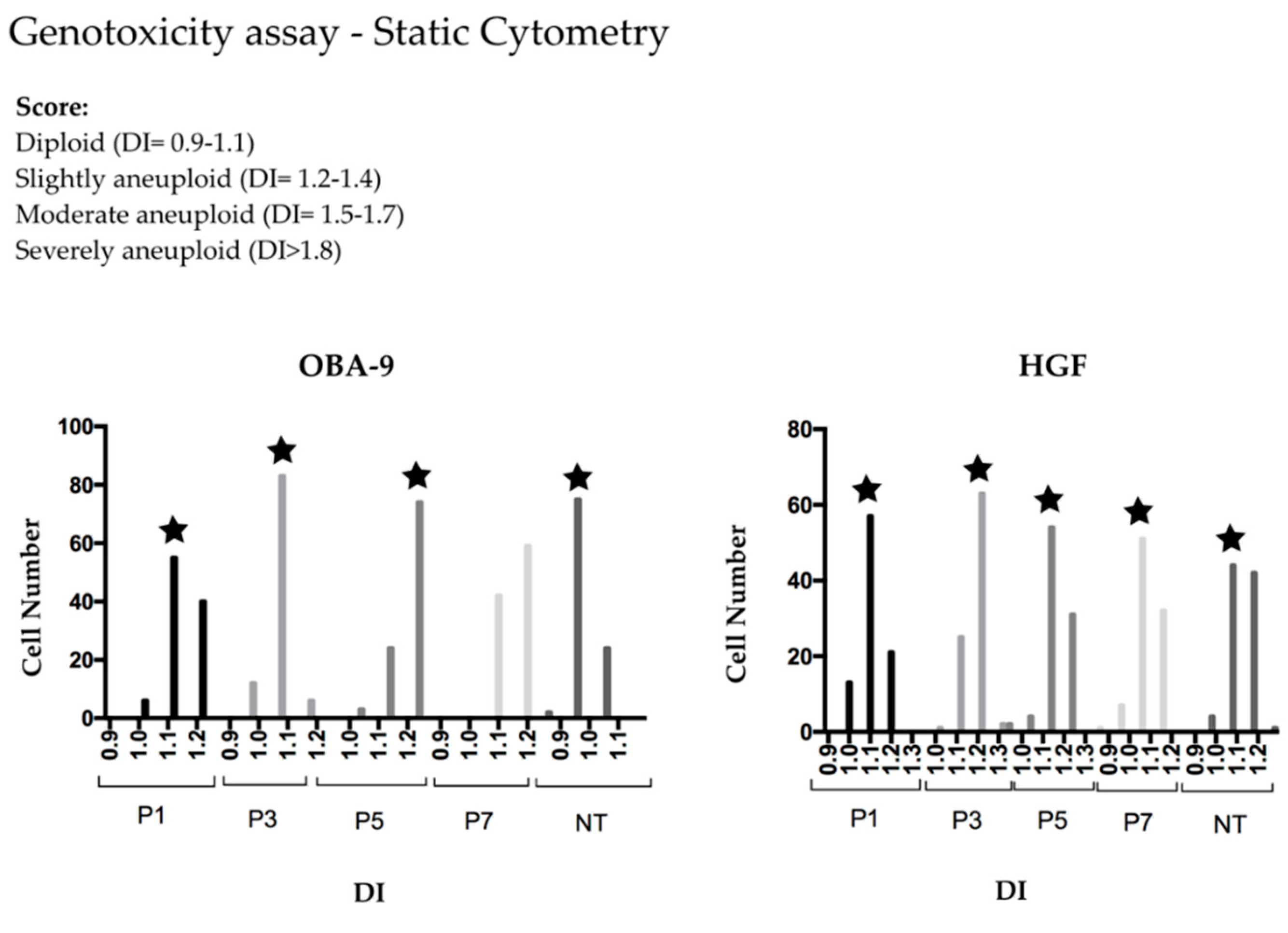
Integrated optical density (IOD), which is equivalent but not identical to DNA content, was obtained in the cell’s nucleus by static cytometry in an image analysis system (Carl Zeiss). Images were digitalized using an Olympus QColor3 camera with a 400× magnitude and analyzed with KS 400 software (Carl Zeiss, Oberkochen, Germany) in black and white using a specific filter for 570 nm wavelength. Later, DNA index (DI) was obtained by dividing IOD obtained from each of 90 fibroblasts and 100 keratinocytes by the mean of IOD from 20 lymphocytes used as diploid control. DI was classified as diploid (DI = 0.9–1.1), slightly aneuploid (DI = 1.2–1.4), moderated aneuploid (DI = 1.5–1.7), and severely aneuploid (DI > 1.8), based on Lima et al. [21].
Frequency histograms were built using DI values and the number of times they appear, dispersion was analyzed by D’Agostino-Pearson’s test followed by Kruskal-Wallis test, and significance level was 5%.
3. Results
3.1. Effect of He-CAPP on Mono Species Biofilm of P. gingivalis HW24D-1 (XTT Assay)
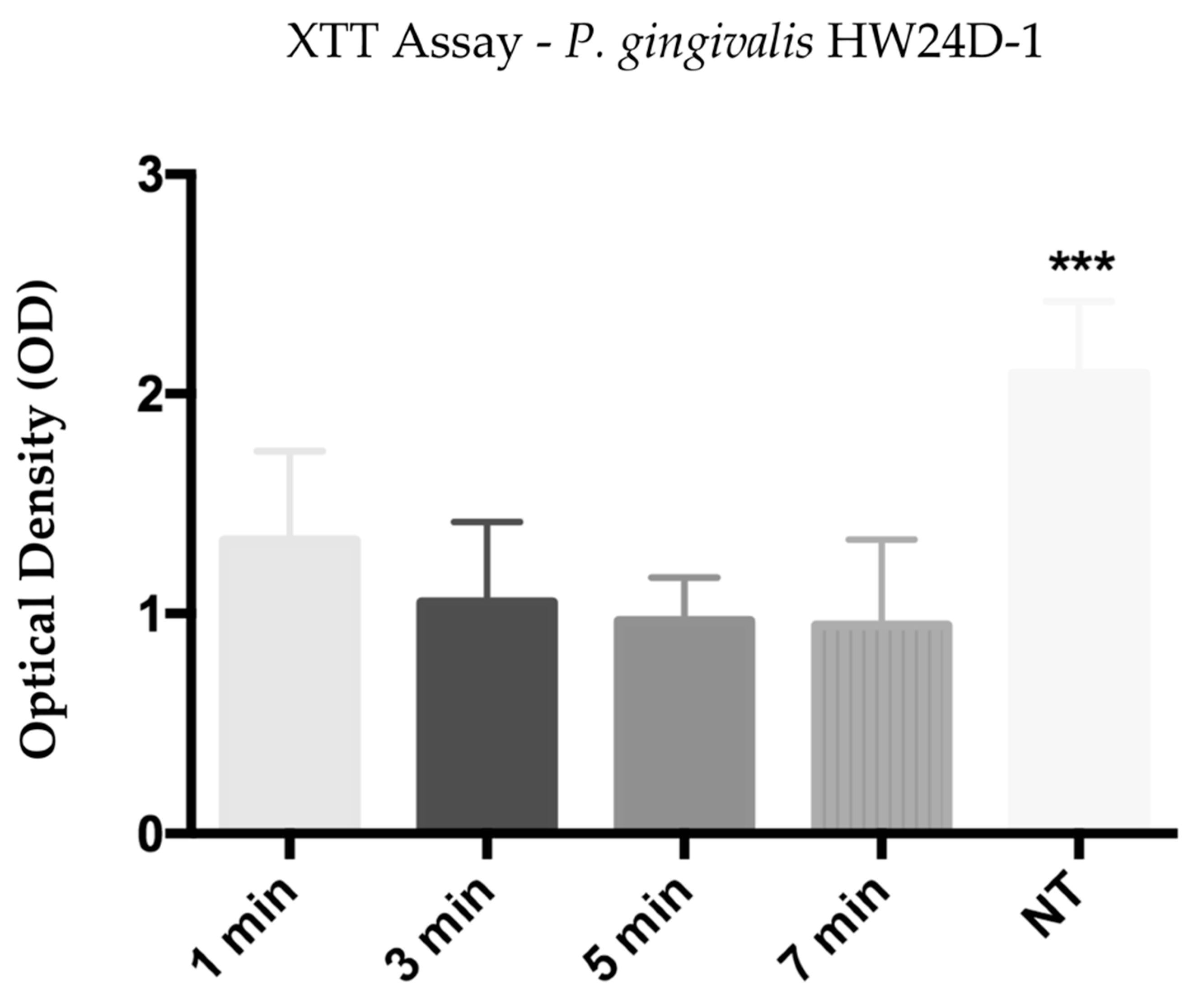
Optical density resulting from P. gingivalis HW24D-1 biofilm reading after the calorimetric XTT assay, showed that groups treated for 1, 3, 5, and 7 min with He-CAPP jet had bacterial cell viability reduced, accordingly with the time of He-CAPP exposure. All treated groups had a significant reduction when compared with the non-treated (NT) (p < 0.0001) group, while the reduction was not significant among treated groups. Mean values and standard deviations (SD) are shown in Figure 2.
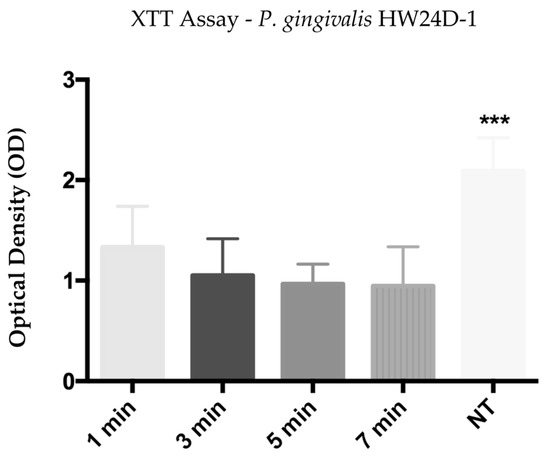
Figure 2.
Mean and standard deviation of the optical density (OD) obtained from XTT assay of P. gingivalis HW24D-1 monospecies mature biofilm exposed to He-CAPP for 1, 3, 5, and 7 min. NT—not-treated, (***) p < 0.0001.
3.2. Genotoxicity of He-CAPP on Epithelial Oral Cells
Treated groups were slightly aneuploid when He-CAPP was applied for 5 and 7 min in OBA-9, and for 3 min in HGF cells. HGF non-treated cells and HGF treated cells had similar DNA index for all treatment times. The DNA index mean was 1.2 in all slightly aneuploid groups (Table 1 and Table 2), which is the cut edge between diploid and slightly aneuploid score. There was no significant difference of DI among the analyzed groups for both cells (Figure 3).

Table 1.
Mean and standard deviation (SD) of DNA index (DI) obtained for OBA-9 cells exposed to He-CAPP for 1, 3, 5, and 7 min (P1 to P7).

Table 2.
Mean and Standard Deviation (SD) of DNA index (DI) obtained for HGF cells exposed to He-CAPP for 1, 3, 5, and 7 min (P1 to P7).
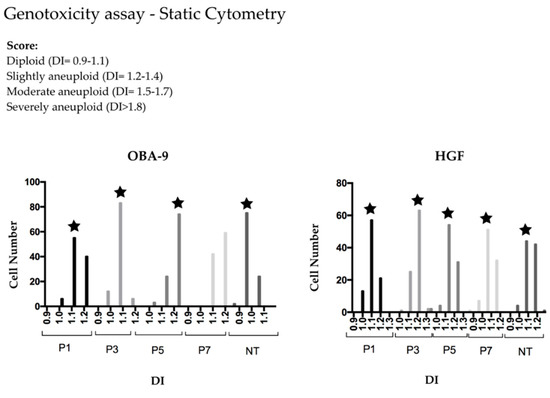
Figure 3.
Frequency histogram of the obtained DNA index (DI) for OBA-9 and HGF cells. P#—minutes of plasma treatment; NT—not-treated; star represents the DI peak in each group.
4. Discussion
In the last decades, CAPP has been proven as an antimicrobial agent, capable of reducing bacterial and fungal cell viability in mono and multispecies biofilms [13,14,22]. In addition, plasma can ameliorate tissue microenvironment with anti-inflammatory and anabolic effects on diverse human cells [23,24]. There is evidence that plasma can inactivate malignant cells from diverse cancer types [25,26,27,28]. All this favorable evidence led to an increase in the literature on CAPP in medical science and the development of different sources, and set ups, including commercial and experimental devices.
The excited species generated in this plasma jet were previously reported with similar parameters [18]. The optical emission spectrum (OES) presented mostly excited nitrogen bands (), ionized nitrogen species (), produced by helium metastable species, and few helium lines. Excited oxygen species such as OH and atomic O were also present. OES measurements confirm the presence of RNS and ROS that are produced in the plume when plasma interacts with the surrounding ambient air. The spread area of ROS in starch-iodine-agar plates for this plasma jet operating with similar parameters was also investigated in a former study [29]. ROS can diffuse radially outward from the plasma plume where long-lived species, such as ozone, can reach further away or act on the surface for longer time. These reactive species (ROS/RNS) combined with other plasma agents, such as UV radiation, charged particles and helium metastable species can act synergistically leading to microbicide effects.
Only a few studies on plasma and periodontal disease or periodontal disease-related biofilms can be found in the literature. P. gingivalis is the most virulent bacterial species associated with periodontal disease development [30,31]. It is a Gram-negative anaerobe bacterium that can lead to dysbiosis and changes in host immune response through virulence mechanisms, such as fimbriae, lipopolysaccharides, capsules, and gingipains (Arg- and Lys-specific proteases) [30,32]. Fimbriae helps P. gingivalis on biofilm formation, aggregation to oral bacteria and bacterial adhesion to host molecules [30].
P. gingivalis HW24D-1 possess FimA type 2, a gene that encodes a fimbriae protein type that is strongly associated with periodontal disease patients and seems to play a pivotal role as a periodontopathogen [31]. This genotype was found to be the most prevalent in sites of periodontitis in a Brazilian population, and can be more virulent, associated with severe periodontitis [33]. A recent study showed a strong relationship between FimA types II and IV and periodontitis, and that the presence of FimA type II increases the colonization of P. gingivalis in deep periodontal pockets [32]. There is a lack of studies in the literature about the effect of CAPP jet in FimA type 2 Porphyromonas models.
In our study, an experimental setup previously described [34] was used. It worked with He, and a 1.0-m-long flexible polyurethane tube was used for remote generation of the jet. Its antimicrobial potential was previously described against P. gingivalis (ATCC 33277) dual biofilm [13], but we were intrigued about its effect in a more virulent P. gingivalis strain, capable of forming a thick single species biofilm in 48 h (in vitro) and involved in the progression of periodontitis. We detected that He-CAPP jet was capable of reducing cell viability of P. gingivalis HW24D-1 in a time-dependent manner in single species biofilms with significant difference between treated groups and the not-treated group. The results are in accordance with our previous experiments and previous studies in the literature treating P. gingivalis biofilm with CAPP, although the virulence of literature’s strains and biofilm maturation stages were different [12,35], which prevents comparison.
As P. gingivalis HW24D-1 is a strain associated with deep pockets in chronic periodontitis, and in a translational study, more than one application may be necessary in an unknown application period, in vivo, the safety of our setup in oral cells became necessary. Epithelial gingival cells (OBA-9) and human oral fibroblasts (HGF) were then chosen to be tested in 1 to 7 min of CAPP exposure to evaluate the safety of the treatment to the cell DNA content using the static cytometry assay.
Static cytometry, also known as DNA image cytometry in Feulgen-stained cells, is an internationally accepted method for oral cell dysplasia and cervical cancer diagnosis for more than a decade [15,16]. Image analysis systems classify a cell population integrated optical density (IOD) through calibrated filters that help with cell nucleus selection and capture the optical density proportional to the DNA content [17]. Optical density is selected and automatically calculated in each field through a specific algorithmic function. Commonly, 100 to 300 cell nuclei are selected, and inflammatory or stroma cells are used as a diploid control [17]. In this study, we selected 90 HGF and 100 OBA-9 cells, based on the cell type yield in culture. The diploid control cell was the lymphocyte based on studies in dentistry [15,20,21], although the IOD mean from oral keratinocytes was 0.64, and lymphocytes was 0.65.
Our results showed slight aneuploidy for OBA-9 cells in 5 and 7 min of He-CAPP treatment (DI mean = 1.2 ± 0.05) and HGF in 3 min. There was no significance in the ID value distribution among the groups for both cell types (OBA-9 p = 0.993; HGF p = 0.996). These results cannot be solely considered as discussed in Lima et al. [21]. Based on the cut edge DI between euploid (DI = 0.9–1.1) and slightly aneuploid (DI = 1.2–1.4), and in the results previously presented for our group regarding cytotoxicity and histological safety of the plasma jet in the used parameters [13], we can conclude that the plasma device and conditions used in this study are safe for oral human cells.
Analyzing the dispersion histograms, we can observe that in all groups, there is a small percentage of cells classified as slightly aneuploid, which is in accordance with clinical trials of studying smokers [20,21], in which inside the healthy control, there was always a small percentage of aneuploid cells.
Plasma genotoxic evaluation has been previously evaluated in different cell types and devices, and different assays, such as micronucleus (MN), comet assay, and hypoxanthine phosphoribosyltransferase (HPRT1) assay, indicate no mutagenicity of the treated cells [4,36,37,38]. Unfortunately, the static cytometry has been used for CAPP safety evaluation in only one study [14] in which plasma treatment was not genotoxic to fibroblast-like cells from mice. In the study of Oliveira et al. [14], the same periods of treatment as the present study, but different setup parameters, were adopted. DI obtained from treated cells corresponded to slight aneuploid scores for all treatment times, including the non-treated group, with a mean of 1.27, the same obtained after 7 min-treatment, which led authors to conclude that CAPP was non-cytotoxic, as well as in the present study.
Although static cytometry is an internationally accepted method for oral cell dysplasia and cervical cancer diagnosis, the limited number of studies using this methodology for genotoxicity evaluation of CAPP application makes it difficult to compare our study with others in the literature. Future assays using the same conditions and periods of treatments will confirm our results. In addition, the use of culture cells limits the number of analyzed cells. Clinical trials in which exfoliative cytology could be applied would improve the translation of results.
Our study shows that CAPP is effective against a P. gingivalis virulent strain, commonly dominant in patients with chronic periodontitis, in different countries, with evidence of cell safety application in oral cells. In addition, the 5-min application was optimal, with good antimicrobial properties and cell safe results in this study. Plus, it is a well tolerable period of treatment for patients in dentistry and was successfully used in vivo in our previous study in CAPP and periodontal disease [13]. These results encourage clinical trials using approved medical plasma devices for evaluation of CAPP application as an adjuvant to periodontal traditional therapy in patients with chronic periodontal disease.
5. Conclusions
Helium cold atmospheric pressure plasma jet showed an inhibitory effect on the cell viability of the virulent P. gingivalis HW24D-1 strain, in a 2-day mature biofilm. The study findings reflect the importance of CAPP as an adjuvant therapy for periodontitis since the HW24D-1 strain possesses FimA type 2, a gene that encodes a fimbriae protein type that is strongly associated to periodontal disease in chronic patients.
Furthermore, CAPP application was not genotoxic for the evaluated oral cells (keratinocytes and fibroblasts) in periods from 1 to 7 min. CAPP was set by a voltage signal of 10 kV amplitude with a frequency of 31 kHz using burst mode (voltage amplitude modulation) with a duty cycle of 22%, plasma plume discharge power of 0.6 W, distance of 15 mm (between the tube exit and the surface to be treated), and temperature at the target region around 37 °C after 5 min of plasma application.
The final CAPP conditions can be reproduced in future studies as safe for oral cells with an optimal application time of 5 min, a well tolerable period of treatment for patients in dentistry which encourages future clinical trials in patients with deep pockets and chronic periodontal disease.
Author Contributions
Conceptualization, G.d.M.G.L., C.F.L.C. and C.Y.K.-I.; methodology, G.d.M.G.L., A.C.B., T.M.C.N., C.F.L.C., M.V.C., M.P.A.M., K.G.K. and C.Y.K.-I.; validation, G.d.M.G.L., A.C.B., T.M.C.N., M.V.C., K.G.K. and C.Y.K.-I.; formal analysis, G.d.M.G.L., K.G.K. and C.Y.K.-I.; investigation, G.d.M.G.L., A.C.B., T.M.C.N., C.A.V.d.S., M.V.C., K.G.K. and C.Y.K.-I.; resources, C.Y.K.-I.; data curation, G.d.M.G.L.; writing—original draft preparation, G.d.M.G.L. and C.Y.K.-I.; writing—review and editing, G.d.M.G.L., A.C.B., T.M.C.N., K.G.K. and C.Y.K.-I.; visualization, G.d.M.G.L. and C.Y.K.-I.; supervision, C.Y.K.-I.; project administration, C.Y.K.-I.; funding acquisition, C.Y.K.-I. and K.G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPESP (2015/03470-3; 2016/07196-6).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Clelia Aparecida Paiva, laboratory technician from Genoma Lab, ICT-Unesp, for valuable help with experiment support and personal dedication to our team.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Laroussi, M.F.L. Evaluation of the roles of reactive species, heat and UV radiation in the inactivation of bacterial cells by air plasma at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Kolb, J.F.; Mohamed, A.-A.H.; Price, R.O.; Swanson, R.J.; Bowman, A.; Chiavarini, R.L.; Stacey, M.; Schoenbach, K.H. Cold atmospheric pressure air plasma jet for medical applications. Appl. Phys. Lett. 2008, 92, 241501. [Google Scholar] [CrossRef] [Green Version]
- McCombs, G.B.; Darby, M.L. New discoveries and directions for medical, dental and dental hygiene research: Low temperature atmospheric pressure plasma. Int. J. Dent. Hyg. 2010, 8, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Schmidt, A.; Kramer, A.; Metelmann, H.R.; Adler, F.; von Woedtke, T.; Niessner, F.; Weltmann, K.D.; Wende, K. High throughput image cytometry micronucleus assay to investigate the presence or absence of mutagenic effects of cold physical plasma. Environ. Mol. Mutagen. 2018, 59, 268–277. [Google Scholar] [CrossRef]
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxidative Med. Cell Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, D.; Bourke, P. Safety implications of plasma-induced effects in living cells—A review of in vitro and in vivo findings. Biol. Chem. 2018, 400, 3–17. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Chandel, N.S. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Sczepanik, F.S.C.; Grossi, M.L.; Casati, M.; Goldberg, M.; Glogauer, M.; Fine, N.; Tenenbaum, H.C. Periodontitis is an inflammatory disease of oxidative stress: We should treat it that way. Periodontology 2020, 84, 45–68. [Google Scholar] [CrossRef]
- Mahasneh, A.; Darby, M.; Tolle, S.L.; Hynes, W.; Laroussi, M.; Karakas, E. Inactivation of Porphyromonas gingivalis by Low-Temperature Atmospheric Pressure Plasma. Plasma Med. 2011, 1, 191–204. [Google Scholar] [CrossRef]
- Liu, D.; Xiong, Z.; Du, T.; Zhou, X.; Cao, Y.; Lu, X. Bacterial-killing effect of atmospheric pressure non-equilibrium plasma jet and oral mucosa response. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.D.M.G.; Borges, A.C.; Nishime, T.M.C.; Santana-Melo, G.D.F.; Kostov, K.G.; Mayer, M.P.A.; Koga-Ito, C.Y. Cold Atmospheric Plasma Jet as a Possible Adjuvant Therapy for Periodontal Disease. Molecules 2021, 26, 5590. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.A.C.D.; Lima, G.D.M.G.; Nishime, T.M.C.; Gontijo, A.V.L.; Menezes, B.R.C.D.; Caliari, M.V.; Kostov, K.G.; Koga-Ito, C.Y. Inhibitory Effect of Cold Atmospheric Plasma on Chronic Wound-Related Multispecies Biofilms. Appl. Sci. 2021, 11, 5441. [Google Scholar] [CrossRef]
- Chitturi, R.T.; Nirmal, R.M.; Sunil, P.M.; Devy, A.S.; Reddy, B.V.R. Evaluation of ploidy status using DNA-image cytometry of exfoliated mucosal cells in oral lichen planus. J. Cytol. 2014, 31, 131–135. [Google Scholar] [CrossRef]
- Wong, O.G.; Ho, M.W.; Tsun, O.K.; Ng, A.K.; Tsui, E.Y.; Chow, J.N.; Ip, P.P.-C.; Cheung, A.N.Y. An automated quantitative DNA image cytometry system detects abnormal cells in cervical cytology with high sensitivity. Cytopathology 2018, 29, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Tolmachoff, T.; Marchevsky, A.M. DNA Content Analysis (“Ploidy”) by Image Analysis: Clinical Applications and Comparison with Flow Cytometry. In Image Analysis: A Primer for Pathologists; Marchevsky, A.M., Bartels, P.H., Eds.; Raven Press: New York, NY, USA, 1994; pp. 263–309. [Google Scholar]
- Kostov, K.G.; Machida, M.; Prysiazhnyi, V.; Honda, R.Y. Transfer of a cold atmospheric pressure plasma jet through a long flexible plastic tube. Plasma Sources Sci. Technol. 2015, 24, 25038. [Google Scholar] [CrossRef]
- Borges, A.C.; de Morais Gouvêa Lima, G.; Nishime, T.M.C.; Gontijo, A.V.L.; Kostov, K.G.; Koga-Ito, C.Y. Amplitude-modulated cold atmospheric pressure plasma jet for treatment of oral candidiasis: In vivo study. PLoS ONE 2018, 13, e0199832. [Google Scholar] [CrossRef]
- Souto, G.R.; Caliari, M.; Lins, C.E.C.; De Aguiar, M.C.F.; De Abreu, M.H.N.G.; Mesquita, R.A. Tobacco use increase the number of aneuploid nuclei in the clinically healthy oral epithelium. J. Oral Pathol. Med. 2010, 39, 605–610. [Google Scholar] [CrossRef]
- Lima, C.F.; Alves, M.G.O.; Carvalho, B.F.D.C.; de Lima, T.A.; Coutinho-Camillo, C.M.; Soares, F.A.; Scholz, J.; Almeida, J.D. Is DNA ploidy related to smoking? J. Oral. Pathol. Med. 2017, 46, 961–966. [Google Scholar] [CrossRef]
- Borges, A.C.; Castaldelli Nishime, T.M.; Kostov, K.G.; de Morais Gouvêa Lima, G.; Lacerda Gontijo, A.V.; de Carvalho, J.N.M.M.; Yzumi Honda, R.; Yumi Koga-Ito, C. Cold atmospheric pressure plasma jet modulates Candida albicans virulence traits. Clin. Plasma Med. 2017, 7–8, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Eggers, B.; Marciniak, J.; Memmert, S.; Kramer, F.J.; Deschner, J.; Nokhbehsaim, M. The beneficial effect of cold atmospheric plasma on parameters of molecules and cell function involved in wound healing in human osteoblast-like cells in vitro. Odontology 2020, 108, 607–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, J.S.; Kim, Y.H.; Choi, E.H.; Kim, C.K.; Kim, K.N.; Kim, K.M. Non-thermal atmospheric pressure plasma increased mRNA expression of growth factors in human gingival fibroblasts. Clin. Oral. Investig. 2016, 20, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef] [Green Version]
- Schuster, M.; Seebauer, C.; Rutkowski, R.; Hauschild, A.; Podmelle, F.; Metelmann, C.; Metelmann, B.; von Woedtke, T.; Hasse, S.; Weltmann, K.-D.; et al. Visible tumor surface response to physical plasma and apoptotic cell kill in head and neck cancer. J. Cranio-Maxillofac. Surg. 2016, 44, 1445–1452. [Google Scholar] [CrossRef]
- Schneider, C.; Arndt, S.; Zimmermann, J.L.; Li, Y.; Karrer, S.; Bosserhoff, A.-K. Cold atmospheric plasma treatment inhibits growth in colorectal cancer cells. Biol. Chem. 2018, 400, 111–122. [Google Scholar] [CrossRef]
- Gümbel, D.; Bekeschus, S.; Gelbrich, N.; Napp, M.; Ekkernkamp, A.; Kramer, A.; Stope, M.B. Cold Atmospheric Plasma in the Treatment of Osteosarcoma. Int. J. Mol. Sci. 2017, 18, 2004. [Google Scholar] [CrossRef] [Green Version]
- Nishime, T.M.C.; Wagner, R.; Kostov, G.K. Study of Modified Area of Polymer Samples Exposed to a He Atmospheric Pressure Plasma Jet Using Different Treatment Conditions. Polymers 2020, 12, 1028. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Nagano, K. Porphyromonas gingivalis FimA and Mfa1 fimbriae: Current insights on localization, function, biogenesis, and genotype. Jpn. Dent. Sci. Rev. 2021, 57, 190–200. [Google Scholar] [CrossRef]
- Asai, Y.; Yasuda, K.; Ohyama, Y.; Ogawa, T. Genetic variation of a fimbrial protein from Porphyromonas gingivalis and its distribution in patients with periodontal diseases. Microbiol Res. 2005, 160, 257–263. [Google Scholar] [CrossRef]
- Kugaji, M.; Muddapur, U.; Bhat, K.; Joshi, V.; Manubolu, M.; Pathakoti, K.; Peram, M.R.; Kumbar, V. Variation in the Occurrence of. Int. J. Environ. Res. Public Health. 2020, 17, 1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Missailidis, C.G.; Umeda, J.E.; Ota-Tsuzuki, C.; Anzai, D.; Mayer, M.P.A. Distribution of fimA genotypes of Porphyromonas gingivalis in subjects with various periodontal conditions. Oral Microbiol. Immunol. 2004, 19, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.G.; Nishime, T.M.C.; Machida, M.; Borges, A.C.; Prysiazhnyi, V.; Koga-Ito, C.Y. Study of Cold Atmospheric Plasma Jet at the End of Flexible Plastic Tube for Microbial Decontamination. Plasma Process. Polym. 2015, 12, 1383–1391. [Google Scholar] [CrossRef]
- Xiong, Z.; Du, T.X.; Lu, Y.C.; Pan, Y. How deep can plasma penetrate into a biofilm? Appl. Phys. Lett. 2011, 98, 221503. [Google Scholar] [CrossRef]
- Boxhammer, V.; Li, Y.F.; Köritzer, J.; Shimizu, T.; Maisch, T.; Thomas, H.M.; Schlegel, J.; Morfill, G.E.; Zimmermann, J.L. Investigation of the mutagenic potential of cold atmospheric plasma at bactericidal dosages. Mutat. Res. 2013, 753, 23–28. [Google Scholar] [CrossRef]
- Kluge, S.; Bekeschus, S.; Bender, C.; Benkhai, H.; Sckell, A.; Below, H.; Stope, M.B.; Kramer, A. Investigating the Mutagenicity of a Cold Argon-Plasma Jet in an HET-MN Model. PLoS ONE 2016, 11, e0160667. [Google Scholar] [CrossRef] [Green Version]
- Wende, K.; Bekeschus, S.; Schmidt, A.; Jatsch, L.; Hasse, S.; Weltmann, K.D.; Masur, K.; von Woedtke, T. Risk assessment of a cold argon plasma jet in respect to its mutagenicity. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 798-799, 48–54. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).