Abstract
Protease is the main enzyme of detergent. Through the combination of different proteases and the combination of protease and detergent additives, it can adapt to different washing conditions to improve the washing effect. In this experiment, whiteness determination, microscope scanning, Fourier transform infrared spectroscopy, and X-ray photoelectron spectroscopy were used to detect the whiteness values of the cloth pieces before and after washing, as well as the stain residue between the fibers on the surface of the cloth pieces. The protease detergent formula with better decontamination and anti-deposition effects was selected. The combination of alkaline protease, keratinase, and trypsin was cost-effective in removing stains. Polyacrylamide gel electrophoresis showed that the molecular weight of the protein significantly changed after adding the enzyme preparation during washing, and the molecular weight of the protein was directly proportional to protein redeposition. The composite protease had a better comprehensive decontamination effect, and when compatible with suitable surfactants, anti-redeposition agents, and water-softening agents, the compound protease detergent exhibited a stronger decontamination ability than commercial detergents.
1. Introduction
Protease is added to detergents to decompose stains during laundry [,]. The protease breaks the polypeptide chain, the macromolecular protein is broken down into small molecule polypeptides or amino acids, and it is peeled from the fabric under the action of surfactant and external force []. The earliest protease used for washing was trypsin, which only needs a small amount to achieve good washing results. However, trypsin is mainly extracted from animal materials [,], and the raw materials and processes have certain limitations. Thus, it has been gradually replaced with alkaline protease, which can be produced by large-scale fermentation. Current enzyme-added detergents only add alkaline protease and exhibit a single washing effect. With the large-scale production of multiple varieties of proteases, the production cost of proteases has been reduced. With the improvement of living conditions, people’s requirements for washing have increased, and new types of protein stain washing solutions are needed.
In 1963, Novozymes introduced protease, which led to a revolution in the industrial enzyme market and began a rapid expansion of detergent enzyme products. Currently, enzymes for detergents already account for 40% of all industrial enzymes. In the European and American markets, enzyme detergents already account for 80% of the detergent market, and almost all detergents are enzyme detergents in Japan. The current trend of enzymatic detergent research and development is extended from single protease to multiple enzymes, such as lipase, amylase, cellulase, mannanase, peroxidase, laccase, etc., and from a single type of stain to a comprehensive washing for multiple stains []. Improving the storage stability of proteases in detergents and washing stability under high/low temperature and high alkaline/acidic conditions have also become new research hotspots. In order to meet the diverse functional requirements of the consumer market for detergents, Novozymes has developed a hybrid liquid detergent Medley solution. The enzymes used in the new Medley solution maintain stability and washing performance at high water content. Alkaline proteases have become key ingredients in detergent formulations []. With the emergence of new strains, researchers have found that other proteases can be added to detergents, such as keratinase []. The current problems of protease detergents are the compatibility of protease with washing auxiliaries and the stability of protease at different temperatures and pH [,].
The effect of trypsin [], keratinase [,], and alkaline protease [] on cleaning is obvious. The compound washing effect is better after the protease is proportioned. Detergents are added with different ingredients [], such as surfactants [], water-softening agents [], anti-redeposition agents [], softening agents [,], and stabilizers, to improve the washing efficiency. We selected 30 common detergent auxiliaries, matched them with protease, and compared the washing effects of different compound protease detergents, and the optimal formula was obtained. The washing power of the composite protease detergent was better than those of several common commercial detergents [].
2. Materials and Methods
2.1. Materials
Selected proteases included alkaline protease (5.0 × 105 U/g), weakly alkaline protease (4.8 × 105 U/g), neutral protease (1.5 × 105 U/g), acid protease (8.0 × 105 U/g), trypsin (4.0 × 103 U/g), aminopeptidase (6.0 × 104 U/g), flavourzyme (5.0 × 105 U/g), and keratinase (1.0 × 105 U/g) in food-grade solid powder form from Lonct Enzymes Co., Ltd. (Linyi, China). Protease activity is expressed as protease activity units. At 40 °C and a certain pH, the amount of enzyme required for protease to hydrolyze casein to produce 1 μg of tyrosine per minute is one unit of enzyme activity. The pH of the reaction conditions for measuring the enzymatic activity of alkaline proteases, weakly basic proteases, aminopeptidases, and keratinases was 10.5. The pH of the reaction conditions for measuring the enzymatic activity of neutral proteases, trypsin, and flavored proteases was 7.5. The pH of the reaction conditions for measuring the enzymatic activity of acidic proteases was 3.0.
Detergent builders included polyethoxylated fatty alcohols, sodium ethoxy alkyl sulfate, dodecylbenzenesulfonic acid, triethanolamine, anhydrous sodium citrate, SNS-80, each in industrial-grade (≥90%) from Research Institute of Daily Chemical Industry (Taiyuan, China). 2-morpholineethanesulfonic acid (MES) in molecular biology grade (>99%) from Coolaber. Fatty acid methyl ester ethoxylate (FMEE), alkyl glycoside (APG), layered sodium disilicate (SKS-6) from Yousuo Chemical Technology Co., Ltd. (Linyi, China), modified oil ethoxylate (SOE) from Junxin Chemical Technology Co., Ltd. (Guangzhou, China), tea saponin from Zhongye Biotechnology Co., Ltd. (Lishui, China), sodium alginate, hyaluronic acid from Boxbio Science & Technology Co., Ltd. (Beijing, China), all of the above are industrial grade (≥90%). Sodium carboxymethyl cellulose (CMC), polyvinyl alcohol type 1799 (PVA), polyethylene glycol 6000 (PEG), polyaspartic acid (PASP), silicon dioxide (SiO2), ethylenediaminetetraacetic acid tetrasodium salt (EDTA), disodium maleate were used in chemically pure forms (≥99.5%) from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Hydroxypropyl methylcellulose sodium (HPMC), hydroxyethyl cellulose sodium, and sodium polyacrylate were used in food-grade (>99%) from Best Food Additives Co., Ltd. (Zhengzhou, China). 4A zeolite was industrial-grade (≥90%) from Runfeng Synthetic Technology Co., Ltd. (Nantong, China). Sodium tartrate and sodium gluconate in food-grade (>99%) were acquired from Gukang Biological Engineering Co., Ltd. (Jinan, China). Sodium laurate in industrial-grade (≥90%) was from Longhui Chemical Co., Ltd. (Jinan, China). α-cyclodextrin, β-cyclodextrin, and γ-cyclodextrin were used in food-grade (>99%) from Youlezi Food Ingredients Co., Ltd. (Shanghai, China). Sulfobutyl-β-cyclodextrin (Captisol), methyl-β-cyclodextrin, and 2-hydroxypropyl-β-cyclodextrin were analytical reagents (≥99.9%) from Aladdin (Shanghai, China). In the experiment, the materials used to simulate protein stains were eggs and carbon powder. Blood used was porcine anticoagulated whole blood. Double distilled water was used in the experiments.
2.2. Preparation of Soiled Cloth
The protein stain was prepared as follows: Weigh 2.4 g of gum arabic powder and dissolve it with a little water, add 1.6 g of carbon black powder and grind for about 2 min. Transfer this carbon black stain to 120 mL of aqueous solution containing 13.8 g of whole milk powder, add another 120 mL of distilled water, homogenize with an emulsifier at 4000–5000 r/min for 30 min, then slowly add 120 mL of aqueous solution containing 25 g of egg liquid (egg white: yolk = 3:2) and continue to homogenize for 1 h.
White cotton cloth was cut into circular pieces of ~6 cm. When preparing the protein fouling cloth, the protein stain solution was heated to 40 °C and filtered. Then, 200 μL was added dropwise onto the white cotton cloth, soaked, pressed, and then dried. The same method was followed when preparing the dirty blood cloth.
2.3. Washing Procedure
The preparation method of the basal detergent is as follows. Add 4% polyethoxylated fatty alcohol, 2% ethoxylated alkyl sulfate, 8% dodecylbenzene sulfonic acid, 0.5% triethanolamine, and 0.5% anhydrous sodium citrate in a volume of water, stir to dissolve, and use sodium hydroxide solution to adjust the pH of the solution to 8.5–9.0.
The water used during washing was hard water (250 mg/kg), and the molar ratio of Ca2+ to Mg2+ was 6:4. The configuration method is as follows: weigh 1.67 g CaCl2 and 2.04 g MgCl2·6H2O, add water to make 10.0 L, which is 250 mg/kg hard water.
Then, 400 U/g of protease was added to the basal detergent and mixed well to prepare a 0.2% solution. A 100 mL aliquot of the solution was added to an Erlenmeyer flask, and a cloth piece was added. Various proteases have their maximum enzyme activity at 50–60 °C. Thus, 50 °C was selected as the reaction temperature [,]. The rotating speed was maintained at 150 r/min at 50 °C and washed for 50 min. Each piece of cloth was rinsed, dehydrated, and then dried.
2.4. Characterization of the Cleaning Effect
A whiteness tester was used to detect the whiteness reflectance value of the cloth surface before and after washing at the wavelength of 457 nm. We took two points on the front and back sides of the cloth piece before washing or after washing and measured the whiteness value. The average value of the four measurements is the whiteness value of the cloth piece. The stain residues on the surface and inside of the fiber before and after washing were observed with a super depth-of-field microscope (Leica DVM6A, Chongqing, China) at magnifications of 200× and 500× []. The state of the fibers before and after washing the cloth and the stain residues between the fibers were observed via scanning electron microscopy (SEM, Phenom pure plus, Shanghai, China) at a voltage of 10 kV [,]. The magnifications were 410×, 430×, and 440×. Fourier transform infrared spectroscopy (ATR-FTIR, Nicolet10, Waltham, MA, USA) was performed before and after washing of the fabric sheet [,]. The wavenumber range of residual functional groups was 500–4000 cm−1. Energy dispersion analysis was performed using X-ray photoelectron spectrometry (XPS, ESCALABXi+, Waltham, MA, USA) [].
2.5. Detection of Stain Protein Molecular Weight
Eighty units of enzyme activities of alkaline protease, keratinase, and 4 U enzyme activities of trypsin were added to the protein-contaminated liquid to verify the decomposing effect of the proteases on protein stains. The reaction was carried out at 50 °C for 50 min, and the reaction solution was analyzed by SDS–PAGE [,]. Eighty units of alkaline protease, keratinase, and trypsin were added to the blood to verify the decomposing effect of the proteases on bloodstains. The reaction was carried out at 50 °C for 10–50 min, and the reaction solution was analyzed by SDS–PAGE. The electrophoresis gel was prepared using the Meilun protein gel kit. The sample was added with 80 μL Tricine-SDS-PAGE loading buffer (5×), boiled for 5 min, centrifuged to obtain the supernatant, and then loaded with 10–15 μL for each sample. Electrophoresis was performed after adding the electrophoresis buffer to the electrophoresis system.
2.6. Evaluation of the Effect of Blood Stains Redeposition
5 mL of 4% blood dilution solution was prepared; added with 80 U of alkaline protease, keratinase, and trypsin; and then shaken in a water bath at 50 °C for 10, 20, 30, 40, and 50 min. The reaction solution was boiled for 3 min, and then protein gel electrophoresis was performed. Double-distilled water (5 mL) was added to the reaction solution, in which the white cotton cloth was immersed and allowed to stand for 12 h at 30 °C. The cloth was rinsed with water and dried, and then the extent of surface deposition and the relationship between the extent of protein deposition on the surface of the cloth and the molecular weight of the protein were observed.
2.7. Optimization of Protease Washing Performance
Alkaline protease and keratinase were mixed in the detergent at different ratios, and the total enzyme activity of each experiment was set to 80 U. In the experiment, the enzyme activity ratios of alkaline protease and keratinase were 20 U:60 U, 40 U:40 U, 30 U:50 U, 60 U:20 U, and 10 U:70 U. On the basis of adding 80 U protease activity per 0.2 g detergent, the enzyme activity of trypsin was added as 5 U, 10 U, 15 U, 20 U successively, and the remaining enzyme activity was supplemented to 80 U by alkaline protease and keratinase. The selected detergent auxiliaries were added to the basal detergent, and the addition amount was 1% of the mass of basal detergent. One or two additives, enzymes, and basal detergents with the best cleaning effect were selected from the surfactants, anti-deposition agents, water softeners, and cyclodextrins for subsequent experiments. The optimized formula was compared with four common commercial detergents in terms of washing effect.
3. Results and Discussion
3.1. Washing Performance Test of Different Proteases
The washing effect of different proteases on protein fouling cloths and dirty blood cloths was analyzed. Saleem et al. [] reported the capability of protease to digest and convert the insoluble form of egg white and blood clot into their soluble forms. Bersic et al. [] reported that adding protease to detergent has a better washing effect. Through the above washing method, different proteases were used to clean protein-fouling cloths and blood-dirty cloths under the conditions of 80 U, 50 °C, and 50 min (Table 1). In the washing of protein stains, the highest reflectivity was obtained when trypsin was added, equivalent to 1.3 times upon the addition of alkaline protease, followed by keratinase. In the washing of bloodstains, the highest reflectivity was obtained when trypsin was added, equivalent to 1.6 times upon the addition of alkaline protease, followed by keratinase. Neutral protease and aminopeptidase have the worst washing effects. In washing blood-stained cloths with protease, some proteases could not completely decompose hemoglobin on the cloth piece, which caused hemoglobin to be retained by the fibers on the surface of the cloth piece and redeposited on the surface of the cotton cloth. Paul et al. [] reported that crude keratinase could effectively remove blood and egg yolk stains and can be added to detergent products as a washing aid. In addition, the sewage used for washing does not pollute water resources. Emran et al. [] reported that alkaline protease is the most commonly used protease in detergents because of its good thermal stability and compatibility with detergents. However, continuing research and development on detergents is important to find proteases with better effects. Commercial keratinase and trypsin have become promising choices. As a protease added to detergents, commercial keratinase is a more promising option. Trypsin has better detergent effects but is limited by price and production conditions and can be added to special detergents as appropriate.

Table 1.
Washing performance of protease on protein-fouling and blood-dirty cloths.
The stain residue on the surface and interior of the protein fouling cloth and blood-dirty cloth before cleaning and after protease washing was observed under an ultra-depth-of-field microscope and a desktop scanning electron microscope (Figure 1). A large number of stains were attached to the fiber surface and between the fibers before washing, and the amount of residual stains on the surface and between the fibers of the experimental group after washing with enzymes was significantly reduced, and the fibers were arranged loosely and smoothly. The cleaning effect of the cloth after washing with trypsin and keratinase was better than that after washing with alkaline protease.

Figure 1.
Surface image of protein-fouling cloth. (a) Unwashed protein-fouling cloth (500×). (b) Protein-fouling cloth after washing with alkaline protease (500×). (c) Protein-fouling cloth after washing with keratinase (500×). (d) Protein-fouling cloth after washing with trypsin (500×). Surface image of blood-dirty cloth (e) Unwashed blood-dirty cloth (500×). (f) Blood-dirty cloth after washing with alkaline protease (500×). (g) Blood-dirty cloth after washing with keratinase (500×). (h) Blood-dirty cloth after washing with trypsin (500×). SEM images of protein-fouling cloth before and after washing. (i) Unwashed protein-fouling cloth (420×). (j) Protein-fouling cloth after washing with alkaline protease (420×). (k) Protein-fouling cloth after washing with keratinase (420×). (l) Protein-fouling cloth after washing with trypsin (420×). (m) Blood-dirty cloth after washing with keratinase (440×). (n) Blood-dirty cloth after washing with trypsin (410×).
The total reflectance of the protein-fouling cloth and blood-dirty cloth before and after washing with different proteases was analyzed using FTIR in the wavenumber range of 500–4000 cm−1. McCutcheon et al. [] reported that the special spectral characteristics of protein detected by FTIR can reflect the residual protein stains on the surface of the fabric and the interior of the fabric fiber. ATR-FTIR analysis before washing the dirty cloth showed significant infrared absorption peaks at 1640 and 1526 cm−1, which represent the two infrared absorption peaks in the protein-peptide bond C=O stretching vibration absorption peaks and β-sheet conformation amide III band characteristic absorption band (Figure 2). The area of absorption peaks at 1640 and 1526 cm−1 of the stained cloth washed with enzymes was significantly reduced, indicating that protein stains were separated from the cloth after being decomposed by enzymes. Alkaline protease, keratinase, and trypsin can all decompose proteins to different degrees, but trypsin and keratinase were better than alkaline protease in decomposing proteins.
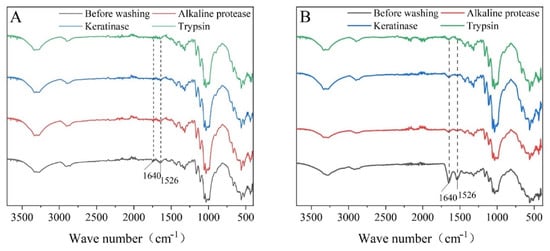
Figure 2.
Infrared spectra of dirty cloth before and after washing with different proteases. (A) Protein-fouling cloth. (B) Blooddirty cloth.
Elemental analysis on the cloth was performed using XPS before and after washing with alkaline protease, keratinase, and trypsin (Figure 3). The protein-fouling cloth and blood-dirty cloth before washing showed obvious peaks at 398–400 eV, indicating that they contain N elements. After washing with protease detergent, the peaks of N elements were significantly reduced. This result indicates that the protein on the surface of the dirty cloth was decomposed and removed by the proteases.
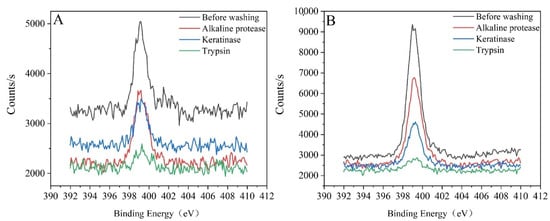
Figure 3.
X-ray photoelectron spectroscopy of dirty cloth before and after washing with different proteases. (A) Protein-fouling cloth. (B) Blood-dirty cloth.
3.2. Evaluation of the Ability of Protease to Resist Stain Redeposition
The reaction solution was analyzed by SDS–PAGE (Figure 4), the molecular weight of alkaline protease was approximately 30 kDa, and the molecular weight of keratinase was approximately less than 15 kDa. After the reaction of protein stain and alkaline protease, the molecular weight of the protein was concentrated in 50–80 kDa, and a small part of the molecular weight was concentrated in 25–30 kDa. After the protein stain liquid was hydrolyzed by keratinase, only a small amount of protein had a molecular weight of 30 kDa. This result indicates that the protein-decomposing effect of keratinase is significantly better than that of alkaline protease. The 4 U enzyme activity of trypsin was reacted with the protein stain solution. The molecular weight of the protein after the reaction was less than 25–35 kDa. The amount of trypsin added was much smaller than those of alkaline protease and keratinase, but the protein stains were decomposed effectively. A comparison of the SDS-PAGE results with the application results showed that the protease with a better washing effect could break down protein stains from large molecules into small molecules, and the stains were easier to remove under the action of detergents.
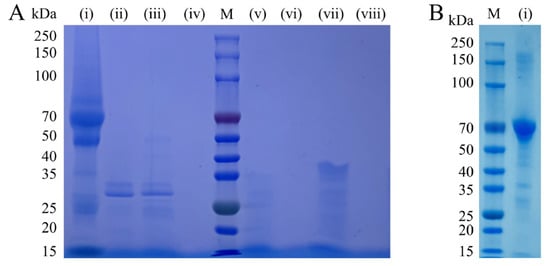
Figure 4.
SDS-PAGE result graph. (A) M is Marker. (i) Protein stain and alkaline protease. (ii) Alkaline protease and double-distilled water. (iii) Protein stain and keratinase. (iv) Keratinase and double-distilled water. (v) Protein stain and alkaline protease and keratinase. (vi) Alkaline protease and keratinase and double-distilled water. (vii) Protein stain and trypsin. (viii) Trypsin and double-distilled water. (B) M is Marker. (i) Protein stain.
The bloodstains on the surface of the cloth treated with alkaline protease, keratinase, and trypsin were compared (Figure 5). Protein molecular weights after treatment with alkaline protease were 70 and 25 kDa. Bloodstains were deposited on the surface of the cloth, but the deposit was relatively reduced as the enzymatic hydrolysis time was prolonged. Bloodstains were analyzed after treatment with keratinase. The protein molecular weights were mainly 50, 40, and 25 kDa. The bloodstains were deposited in the cloth piece. As the enzymatic hydrolysis time was prolonged, the deposition gradually decreased. Compared with the alkaline protease, there were less bloodstains deposited after the keratinase hydrolysis. The solution was treated with trypsin precipitation, seen from the electrophoresis pattern analysis, after 10 min, 20 min, and 30 min reactions. The protein molecular weights were mainly 50 kDa, 22 kDa, 15 kDa, 10 kDa, and macromolecular protein gradually reduced; after 40 min and 50 min reactions, protein molecular weight was mainly concentrated below 10 kDa. Blood from the cloth sheet deposition of view was observed, and the trypsin-treated blood was not deposited onto the fabric sheet again.
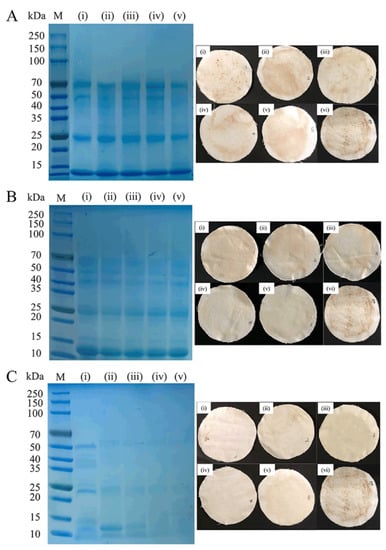
Figure 5.
Deposition effect after the blood is decomposed by proteases. (A) Alkaline protease. (B) Keratinase. (C) Trypsin. (i) Reaction 10 min. (ii) Reaction decomposition 20 min. (iii) Reaction 30 min. (iv) Reaction 40 min. (v) Reaction 50 min. (vi) No added protease.
3.3. Alkaline Protease and Detergent Auxiliary Washing Effect Test
The effect of detergents mixed with different detergent auxiliaries and alkaline protease ratios to clean protein-soiled cloth and blood-dirty cloth was tested (Table 2 and Table 3). The evaluation criterion is whether the decontamination effect of the mixed detergent is better than that of only the alkaline protease detergent. Better washing surfactants include sodium alginate, FMEE, SNS-80, hyaluronic acid, APG [], SOE, MES, and tea saponin. Water-softening agents with a better washing effect are 4A zeolite [,], sodium gluconate, sodium tartrate, and sodium laurate. The ones that inhibit the effect of alkaline protease are SKS-6, SiO2, and EDTA. Experimental results show that in the washing process of using alkaline protease detergent, whether it is for protein or bloodstains, it can be mixed with water-softening agent sodium gluconate, surfactant sodium alginate, FMEE, hyaluronic acid, APG, MES, and tea saponin [,]. In addition, bloodstain washing can be mixed with anti-deposition agent HPMC, sodium hydroxyethyl cellulose [], sodium polyacrylate, and PVA. SOE, SiO2, CMC, disodium maleate, PASP, PEG, EDTA, and EDTA exert inhibitory effects on the protein decomposing effect of alkaline protease.

Table 2.
Washing effect of different surfactants and water-softening agents mixed with alkaline protease on dirty cloth.

Table 3.
Washing effect of anti-redeposition agent mixed with alkaline protease on blood dirty cloth.
Adding cyclodextrin to the detergent can help remove blood stains. A variety of cyclodextrins [] mixed with alkaline protease can be used to wash blood-dirty cloth. Results showed that the addition of α-cyclodextrin and β-cyclodextrin to alkaline protease detergent was better than that of γ-cyclodextrin. The solubility of α-cyclodextrin and β-cyclodextrin was not good (Table 3), and 2-hydroxypropyl-β-cyclodextrin showed better water solubility than β-cyclodextrin. Adding sulfobutyl-β-cyclodextrin to the alkaline protease detergent demonstrated a better washing effect, but it is currently mainly used in the medical field and is expensive.
3.4. Trypsin, Keratinase, and Detergent Auxiliaries Washing Effect Test
The cleaning effect of a single alkaline protease is limited. The compatibility of detergent auxiliaries suitable for alkaline protease with keratinase and trypsin must be tested to improve protease compounding and compatibility with detergent auxiliaries. The protein-fouling and blood-dirty cloths were washed after mixing the detergent auxiliaries and trypsin (Table 4 (a)). The evaluation criterion is whether the washing effect of the mixed detergent is better than that of the detergent only added with trypsin. Results showed that the washing effect of the mixed detergent was better than that of only trypsin detergent. The effect of washing a blood-dirty cloth with mixed detergent was not much different from that of only adding trypsin detergent. Adding a detergent to trypsin detergent exerted no obvious effect on washing blood-dirty cloths.

Table 4.
Washing effect of detergent auxiliaries mixed with trypsin or keratinase on dirty cloths.
The protein-fouling and blood-dirty cloths were washed after mixing the detergent auxiliaries and keratinase (Table 4 (b)). The evaluation standard is whether the cleaning effect of the detergent after mixing is better than that of using only the keratinase detergent. For washing protein stains, it can be mixed with sodium alginate, sodium gluconate, hyaluronic acid, and 4A zeolite. For washing bloodstains, it can be mixed with sodium hydroxyethyl cellulose and CMC, followed by sodium polyacrylate, sodium alginate, hyaluronic acid, and HPMC.
3.5. Compound Protease Washing Test
The washing effect of alkaline protease alone is limited. Niyonzima et al. [] reported that using multiple enzymes to work together in the washing process can decompose and remove stains efficiently. In this experiment, a compound detergent formula composed of alkaline protease, keratinase, and trypsin was prepared, and the total enzyme activity of each group of experiments was set to 80 U. In the experiment, the mixing ratios of alkaline protease and keratinase were 2:6, 4:4, 3:5, 6:2, and 1:7. Stain removal showed a slight difference when alkaline protease and keratinase were added in different proportions to the detergent during washing protein-soiled cloths. When the detergent alkaline protease was mixed with keratinase at a ratio of 1:7, the effect was greatest in washing soiled cloths with blood (Table 5).

Table 5.
Cleaning effect of protease compound on protein-fouling cloth and blood-dirty cloth.
The detergent added with trypsin exerted obvious washing effects on protein stains. The addition amount of trypsin ranged from 5 U to 20 U. Each group to add 80 U enzyme activities of protease, wherein the proportion of alkaline protease and keratinase added in the detergent was set to 1:7. Four experimental groups had 5, 10, 15, and 20 U of trypsin added. When the amount of trypsin added in the compound detergent was 10 U, the effect of washing the protein-soiled cloth improved. When the addition amount of the composite detergent trypsin was 5 U, the washing effect with bloodstains became more pronounced (Table 6).

Table 6.
Protease liquid detergent sample composition and ratio (%).
3.6. Comparison of the Effect of Detergent Products
Liquid detergents usually have surfactants, enzymes, water-softening agents, and anti-redeposition agents added to improve their effect []. The current source of trypsin is mainly extracted from animal tissues, which is relatively expensive. When the ratio of alkaline protease and keratinase was set to 1:7, the washing effect can be close to the effect of using only trypsin. Therefore, alkaline protease and keratinase were selected for the compound experiment. One or two detergent auxiliaries, enzymes, and basal detergents were combined to improve washing effects in the above experiments. Four liquid detergents with the highest sales volume on the e-commerce platform and the mixed protease detergent samples were selected to compare the effects and verify the decontamination performance of the composite detergent. The whiteness value of the protein-fouling cloth washed with formulas A, B, C, and D were significantly higher than the whiteness value of the protein-fouling cloth after washing with the selected four commercial detergents. The whiteness value of the blood-dirty cloths washed with formula B, C, and D were slightly higher than that of samples 1 and 4 and was basically the same as that of samples 2 and 3. The whiteness value of the blood-dirty cloths washed with formula A, B, C, and D were slightly higher than the whiteness value of the -cloths after washing with the selected four commercial detergents. Formula A exhibited stronger washing effects and detergency than commercial detergents (Table 7, Figure 6).

Table 7.
Cleaning effect of protease liquid detergent samples and commercial liquid detergents on dirty cloth.
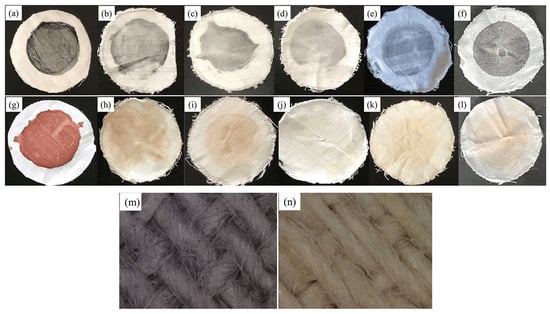
Figure 6.
Surface image of protein-fouling cloth. (a) Unwashed protein-fouling cloth. (b) Protein-fouling cloth after alkaline protease washing. (c) Protein-fouling cloth after keratinase washing. (d) Protein-fouling cloth after trypsin washing. (e) Protein-fouling cloth after complex enzyme detergent. (f) Protein-fouling cloth after commercial detergent. Surface image of blood dirty cloth. (g) Unwashed blood-dirty cloth. (h) Blood-dirty cloth after alkaline protease washing. (i) Blood-dirty cloth after keratinase washing. (j) Blood-dirty cloth after trypsin washing. (k) Blood-dirty cloth after complex enzyme detergent. (l) Blood-dirty cloth after commercial detergent. Microscopic images of protein stains after washing protein stains with protease liquid detergent. (m) Protein-fouling cloth after washing (500×). (n) Blood-dirty cloth after washing (500×).
4. Conclusions
Adding protease to detergents can remove protein stains. The washing performance of different proteases was tested through the protein-fouling cloth model, and the dirty cloth before and after washing was characterized and analyzed. The compound enzyme of the protease selected in the experiment was more efficient in removing protein stains than a single enzyme. Protein stains were disintegrated and peeled off, and stain redeposition resistance was effective. By step-by-step matching with the protease, washing aids such as sodium alginate, sodium gluconate, and sodium carboxymethyl cellulose were selected to further improve the washing effect of the composite protease. Formula A exhibited superior washing performance over common commercial detergents and, therefore, can provide new product solutions for the expansion and development of washing products.
Author Contributions
Conceptualization and methodology: W.Z. and J.X.; validation: W.Z. and J.W.; data analysis: M.Z.; investigation: H.Y.; writing—original draft preparation: W.Z. and J.W.; writing—review & editing: W.Z. and J.X.; funding acquisition: J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Major Science and Technology Innovation Project in Shandong Province (2019JZZY011001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All generated and analyzed data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mukherjee, A.K.; Adhikari, H.; Rai, S.K. Production of alkaline protease by a thermophilic Bacillus subtilis under solid-state fermentation (SSF) condition using Imperata cylindrica grass and potato peel as low-cost medium: Characterization and application of enzyme in detergent formulation. Biochem. Eng. J. 2008, 39, 353–361. [Google Scholar] [CrossRef]
- Vojcic, L.; Pitzler, C.; Korfer, G.; Jakob, F.; Ronny, M.; Maurer, K.H.; Schwaneberg, U. Advances in protease engineering for laundry detergents. New Biotechnol. 2015, 32, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Sukumaran, V.; Sen, S.S.; Oviya, M.; Banu, B.N.; Jena, P.K. Purification and partial characterization of a detergent and oxidizing agent stable alkaline protease from a newly isolated Bacillus subtilis VSG-4 of tropical soil. J. Microbiol. 2011, 49, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghanayem, A.A.; Joseph, B. Current prospective in using cold-active enzymes as eco-friendly detergent additive. Appl. Microbiol. Biotechnol. 2020, 104, 2871–2882. [Google Scholar] [CrossRef] [PubMed]
- Esposito, T.S.; Marcuschi, M.; Amaral, I.P.; Carvalho, L.B.; Bezerra, R.S. Trypsin from the processing waste of the lane snapper (Lutjanus synagris) and its compatibility with oxidants, surfactants and commercial detergents. J. Agric. Food Chem. 2010, 58, 6433–6439. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mangla, J.; Singh, S. Evaluation of Aspergillus fumigatus NTCC1222 as a source of enzymes for detergent industry. Resour. Environ. Sustain. 2021, 5, 100030. [Google Scholar] [CrossRef]
- Rai, S.K.; Konwarh, R.; Mukherjee, A.K. Purification, characterization and biotechnological application of an alkaline β-keratinase produced by Bacillus subtilis RM-01 in solid-state fermentation using chicken-feather as substrate. Biochem. Eng. J. 2009, 45, 218–225. [Google Scholar] [CrossRef]
- Rai, S.K.; Mukherjee, A.K. Optimization of production of an oxidant and detergent-stable alkaline β-keratinase from Brevibacillus sp. strain AS-S10-II: Application of enzyme in laundry detergent formulations and in leather industry. Biochem. Eng. J. 2011, 54, 47–56. [Google Scholar] [CrossRef]
- Rai, S.K.; Roy, J.K.; Mukherjee, A.K. Characterisation of a detergent-stable alkaline protease from a novel thermophilic strain Paenibacillus tezpurensis sp. nov. AS-S24-II. Appl. Microbiol. Biotechnol. 2010, 85, 1437–1450. [Google Scholar] [CrossRef]
- Manni, L.; Jellouli, K.; Ghorbel-Bellaaj, O.; Agrebi, R.; Haddar, A.; Sellami-Kamoun, A.; Nasri, M. An oxidant- and solvent-stable protease produced by Bacillus cereus SV1: Application in the deproteinization of shrimp wastes and as a laundry detergent additive. Appl. Biochem. Biotechnol. 2010, 160, 2308–2321. [Google Scholar] [CrossRef]
- Ribeiro Cardoso dos Santos, D.M.; Victor dos Santos, C.W.; Barros de Souza, C.; Sarmento de Albuquerque, F.; Marcos dos Santos Oliveira, J.; Vieira Pereira, H.J. Trypsin purified from Coryphaena hippurus (common dolphinfish): Purification, characterization, and application in commercial detergents. Biocatal. Agric. Biotechnol. 2020, 25, 101584. [Google Scholar] [CrossRef]
- Paul, T.; Jana, A.; Das, A.; Mandal, A.; Halder, S.K.; Das Mohapatra, P.K.; pati, B.R.; Chandra Mondal, K. Smart cleaning-in-place process through crude keratinase: An eco-friendly cleaning techniques towards dairy industries. J. Clean. Prod. 2014, 76, 140–153. [Google Scholar] [CrossRef]
- Paul, T.; Das, A.; Mandal, A.; Halder, S.K.; Jana, A.; Maity, C.; DasMohapatra, P.K.; Pati, B.R.; Mondal, K.C. An efficient cloth cleaning properties of a crude keratinase combined with detergent: Towards industrial viewpoint. J. Clean. Prod. 2014, 66, 672–684. [Google Scholar] [CrossRef]
- Niyonzima, F.N.; More, S. Purification and properties of detergent-compatible extracellular alkaline protease from Scopulariopsis spp. Prep. Biochem. Biotechnol. 2014, 44, 738–759. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Almeida, F.C.G.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Soares da Silva, R.d.C.F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Yu, H.; Lim, J. Synthesis of environment friendly nonionic surfactants from sugar base and characterization of interfacial properties for detergent application. J. Ind. Eng. Chem. 2016, 38, 157–166. [Google Scholar] [CrossRef]
- Li, Y.; Ma, S.; Fang, X.; Wu, C.; Chen, H.; Zhang, W.; Cao, M.; Liu, J. Water hardness effect on the association and adsorption of cationic cellulose derivative/anionic surfactant mixtures for fabric softener application. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127031. [Google Scholar] [CrossRef]
- Sutanto, S.; van Roosmalen, M.J.E.; Witkamp, G.J. Redeposition in CO2 textile dry cleaning. J. Supercrit. Fluids 2013, 81, 183–192. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Christov, N.; Cristobal, G.; Bourgaux, C.; Heux, L.; Boucenna, I.; Berret, J.F. Design of eco-friendly fabric softeners: Structure, rheology and interaction with cellulose nanocrystals. J. Colloid Interface Sci. 2018, 525, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Zambrano, M.C.; Pawlak, J.J.; Daystar, J.; Ankeny, M.; Venditti, R.A. Impact of dyes and finishes on the microfibers released on the laundering of cotton knitted fabrics. Environ. Pollut. 2021, 272, 115998. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Li, W.; Li, X.; Lian, X. Optimizing Detergent Formulation with Enzymes. J. Surfactants Deterg. 2014, 17, 1059–1067. [Google Scholar] [CrossRef]
- Grbavcic, S.; Bezbradica, D.; Izrael-Zivkovic, L.; Avramovic, N.; Milosavic, N.; Karadzic, I.; Knezevic-Jugovic, Z. Production of lipase and protease from an indigenous Pseudomonas aeruginosa strain and their evaluation as detergent additives: Compatibility study with detergent ingredients and washing performance. Bioresour. Technol. 2011, 102, 11226–11233. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, R.; Jayappriyan, K.R.; Rengasamy, R. Purification and characterization of a protease produced by Bacillus megaterium RRM2: Application in detergent and dehairing industries. J. Basic Microbiol. 2011, 51, 614–624. [Google Scholar] [CrossRef]
- Khan, M.F.; Kundu, D.; Hazra, C.; Patra, S. A strategic approach of enzyme engineering by attribute ranking and enzyme immobilization on zinc oxide nanoparticles to attain thermostability in mesophilic Bacillus subtilis lipase for detergent formulation. Int. J. Biol. Macromol. 2019, 136, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Kalak, T.; Gąsior, K.; Wieczorek, D.; Cierpiszewski, R. Improvement of washing properties of liquid laundry detergents by modification with N-hexadecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate sulfobetaine. Text. Res. J. 2020, 91, 115–129. [Google Scholar] [CrossRef]
- McCutcheon, J.N.; Trimboli, A.R.; Pearl, M.R.; Brooke, H.; Myrick, M.L.; Morgan, S.L. Diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) detection limits for blood on fabric: Orientation and coating uniformity effects. Sci. Justice 2021, 61, 603–616. [Google Scholar] [CrossRef]
- Gruian, C.; Vanea, E.; Simon, S.; Simon, V. FTIR and XPS studies of protein adsorption onto functionalized bioactive glass. Biochim. Biophys. Acta 2012, 1824, 873–881. [Google Scholar] [CrossRef]
- Rekik, H.; Zarai Jaouadi, N.; Gargouri, F.; Bejar, W.; Frikha, F.; Jmal, N.; Bejar, S.; Jaouadi, B. Production, purification and biochemical characterization of a novel detergent-stable serine alkaline protease from Bacillus safensis strain RH12. Int. J. Biol. Macromol. 2019, 121, 1227–1239. [Google Scholar] [CrossRef]
- Saleem, M.; Rehman, A.; Yasmin, R.; Munir, B. Biochemical analysis and investigation on the prospective applications of alkaline protease from a Bacillus cereus strain. Mol. Biol. Rep. 2012, 39, 6399–6408. [Google Scholar] [CrossRef]
- Bersi, G.; Vallés, D.; Penna, F.; Cantera, A.M.; Barberis, S. Valorization of fruit by-products of Bromelia antiacantha Bertol.: Protease obtaining and its potential as additive for laundry detergents. Biocatal. Agric. Biotechnol. 2019, 18, 101099. [Google Scholar] [CrossRef]
- Emran, M.A.; Ismail, S.A.; Hashem, A.M. Production of detergent stable thermophilic alkaline protease by Bacillus licheniformis ALW1. Biocatal. Agric. Biotechnol. 2020, 26, 101631. [Google Scholar] [CrossRef]
- Herrera-Márquez, O.; Fernández-Serrano, M.; Pilamala, M.; Jácome, M.B.; Luzón, G. Stability studies of an amylase and a protease for cleaning processes in the food industry. Food Bioprod. Process. 2019, 117, 64–73. [Google Scholar] [CrossRef]
- Koohsaryan, E.; Anbia, M.; Maghsoodlu, M. Application of zeolites as non-phosphate detergent builders: A review. J. Environ. Chem. Eng. 2020, 8, 104287. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Horn, M.B.; Ferret, L.S.; Azevedo, C.M.N.; Pires, M. Integrated synthesis of zeolites 4A and Na–P1 using coal fly ash for application in the formulation of detergents and swine wastewater treatment. J. Hazard. Mater. 2015, 287, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Yang, C.; Zhao, Y.; Jia, Y.; Chen, L.; Zhou, C.; Yong, Q. Promoting enzymatic saccharification of organosolv-pretreated poplar sawdust by saponin-rich tea seed waste. Bioprocess. Biosyst. Eng. 2020, 43, 1999–2007. [Google Scholar] [CrossRef]
- Jian, H.L.; Liao, X.X.; Zhu, L.W.; Zhang, W.M.; Jiang, J.X. Synergism and foaming properties in binary mixtures of a biosurfactant derived from Camellia oleifera Abel and synthetic surfactants. J. Colloid Interface Sci. 2011, 359, 487–492. [Google Scholar] [CrossRef]
- Lopez, C.G.; Richtering, W. Oscillatory rheology of carboxymethyl cellulose gels: Influence of concentration and pH. Carbohydr. Polym. 2021, 267, 118117. [Google Scholar] [CrossRef]
- Sheng, Y.; Xu, X.; Jiang, W.; Song, Y.; Gan, S.; Zou, H. Application of Oxidized Cornstarch as a Nonphosphoric Detergent Builder. J. Surfactants Deterg. 2012, 15, 393–398. [Google Scholar] [CrossRef]
- Niyonzima, F.N.; More, S.S. Coproduction of detergent compatible bacterial enzymes and stain removal evaluation. J. Basic Microbiol. 2015, 55, 1149–1158. [Google Scholar] [CrossRef]
- Cheng, K.C.; Khoo, Z.S.; Lo, N.W.; Tan, W.J.; Chemmangattuvalappil, N.G. Design and performance optimisation of detergent product containing binary mixture of anionic-nonionic surfactants. Heliyon 2020, 6, e03861. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).