RETRACTED: Biomaterial-Modified Magnetic Nanoparticles γ-Fe2O3, Fe3O4 Used to Improve the Efficiency of Hyperthermia of Tumors in HepG2 Model
Abstract
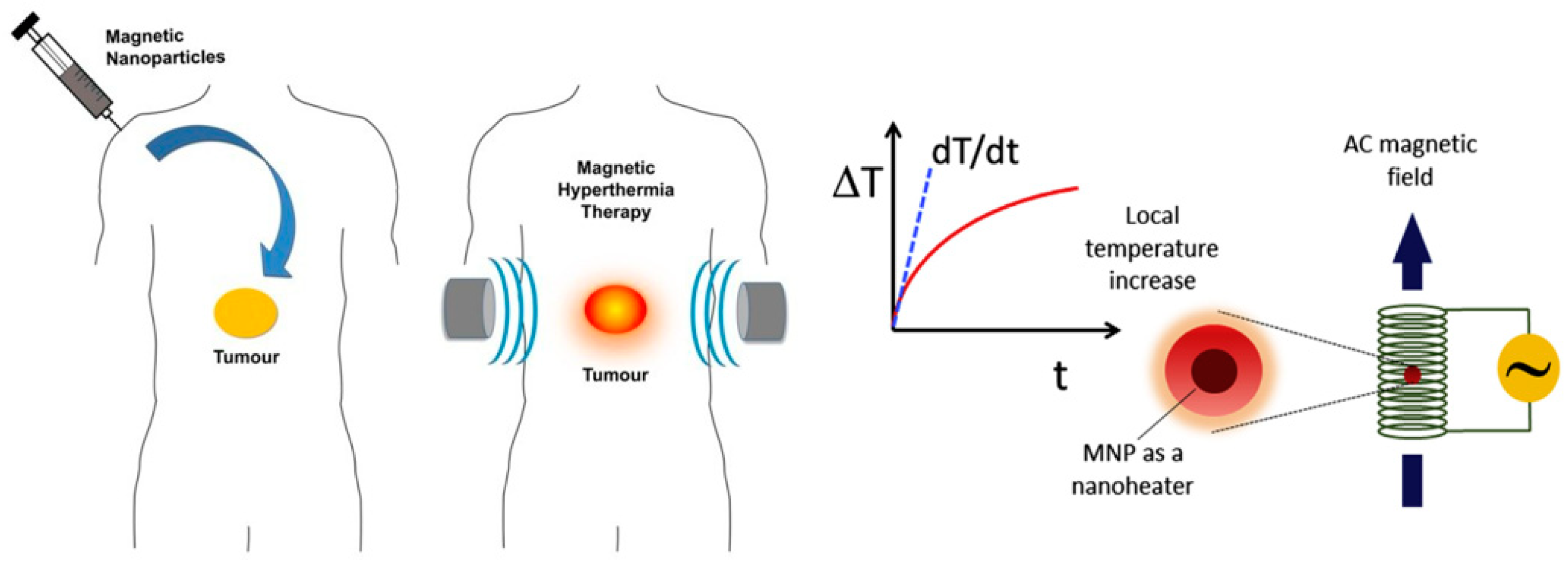
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Theory and Method
2.2.1. Preparation Procedure of WPI
2.2.2. Preparation of WPI-SNPs
2.2.3. ADM Loading of WPI-SNPs
2.2.4. HepG2 Tumor Inhibition
3. Experiment and Results
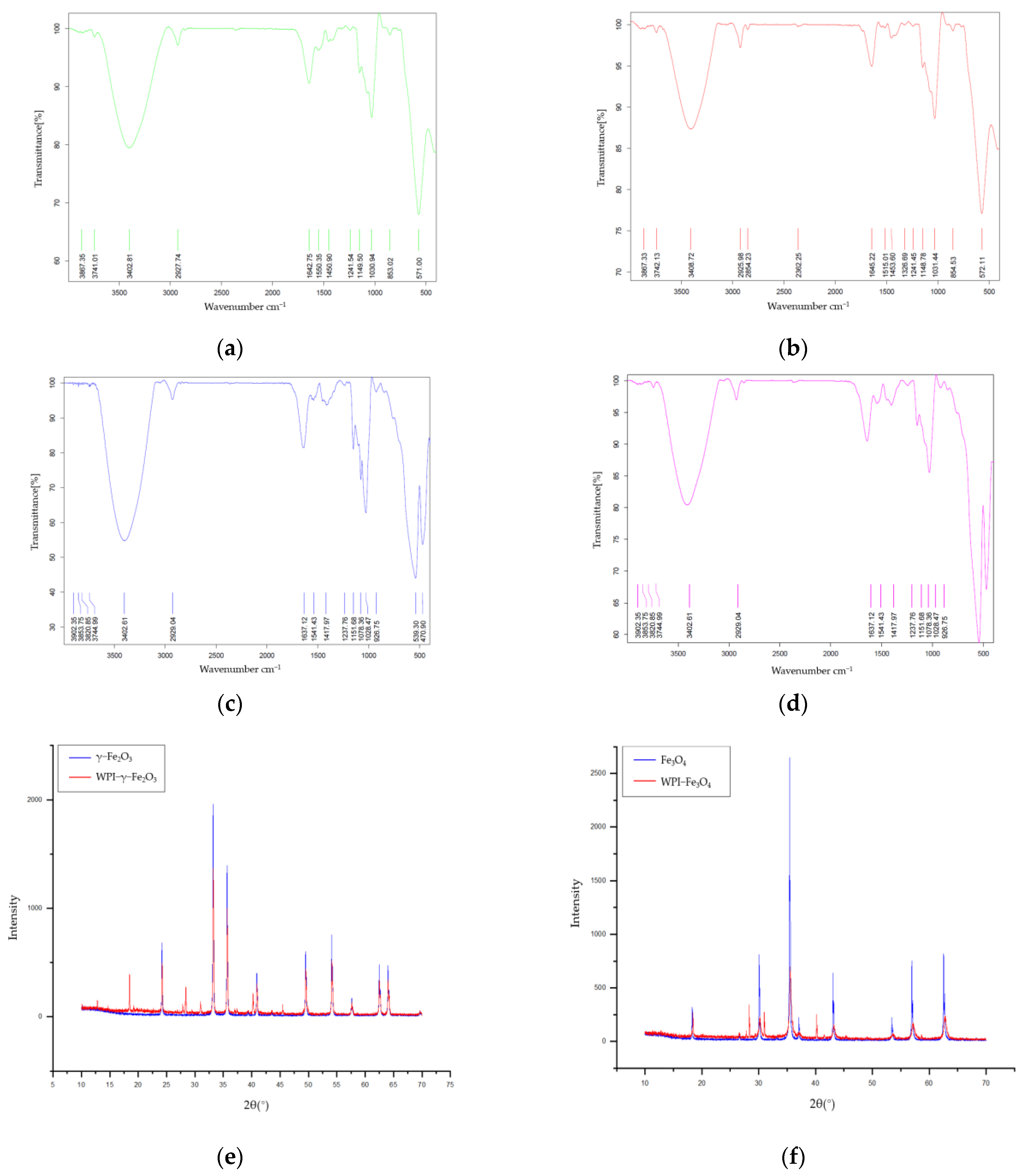
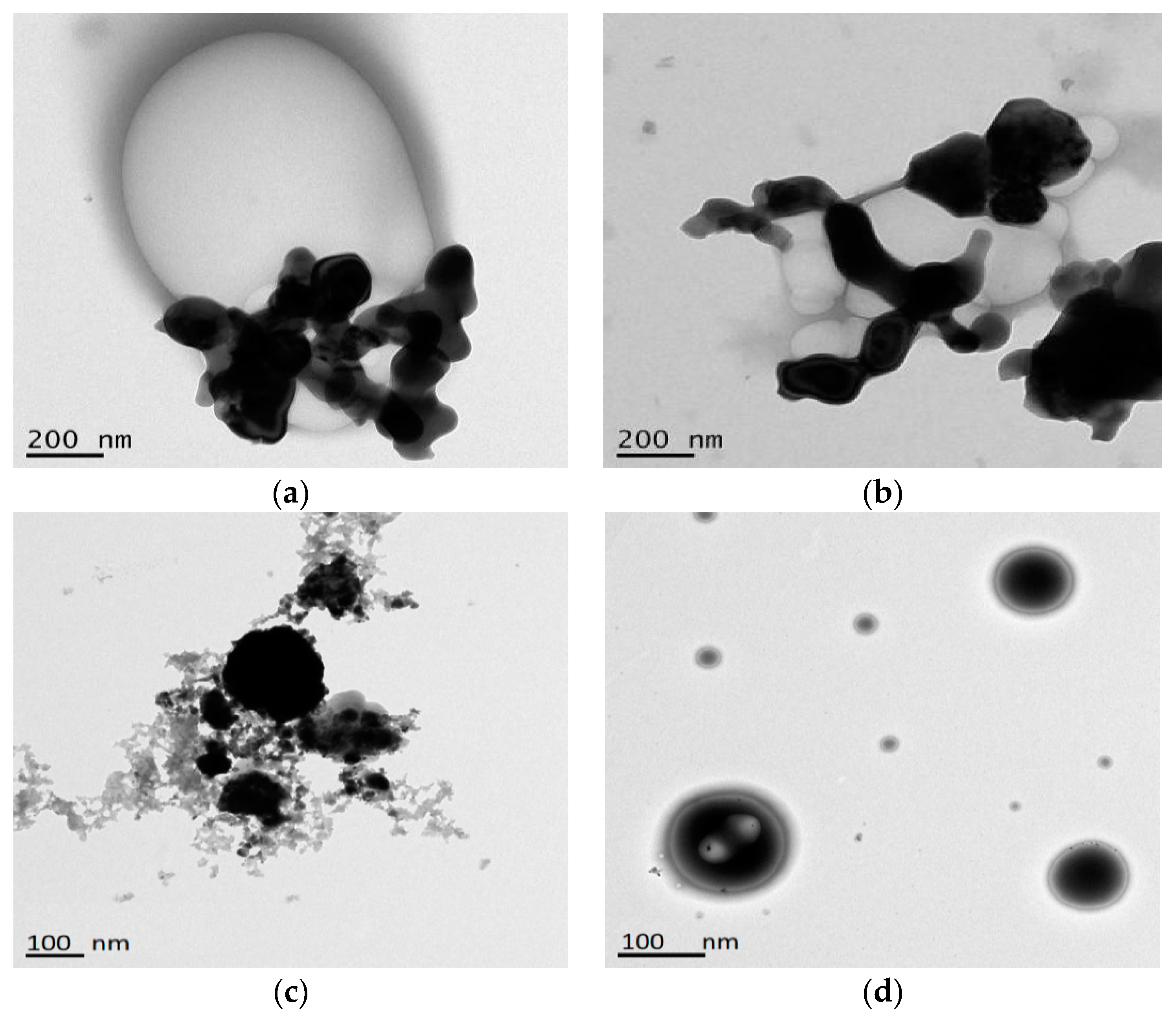
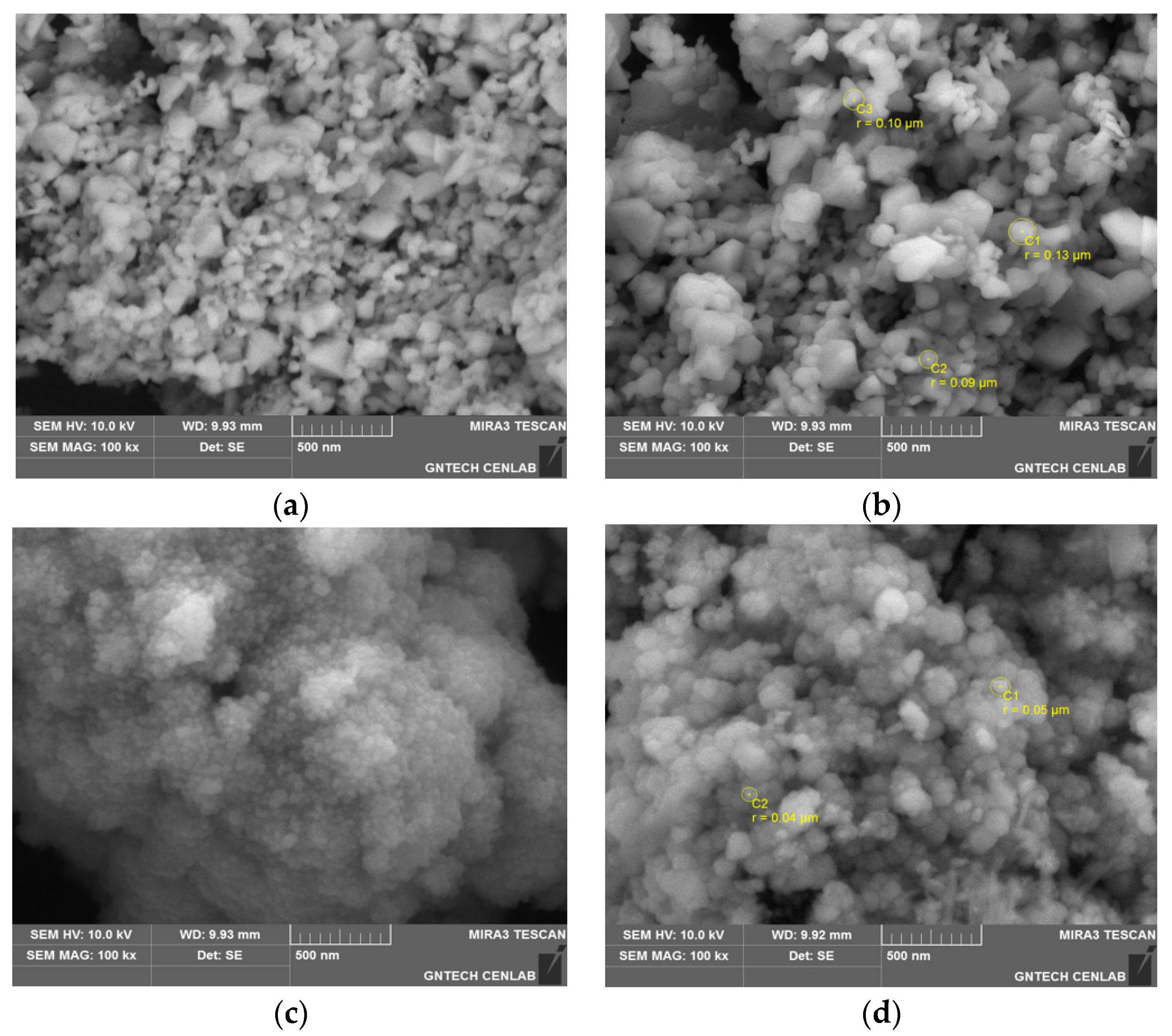
3.1. Characterization of WPI-SNPs
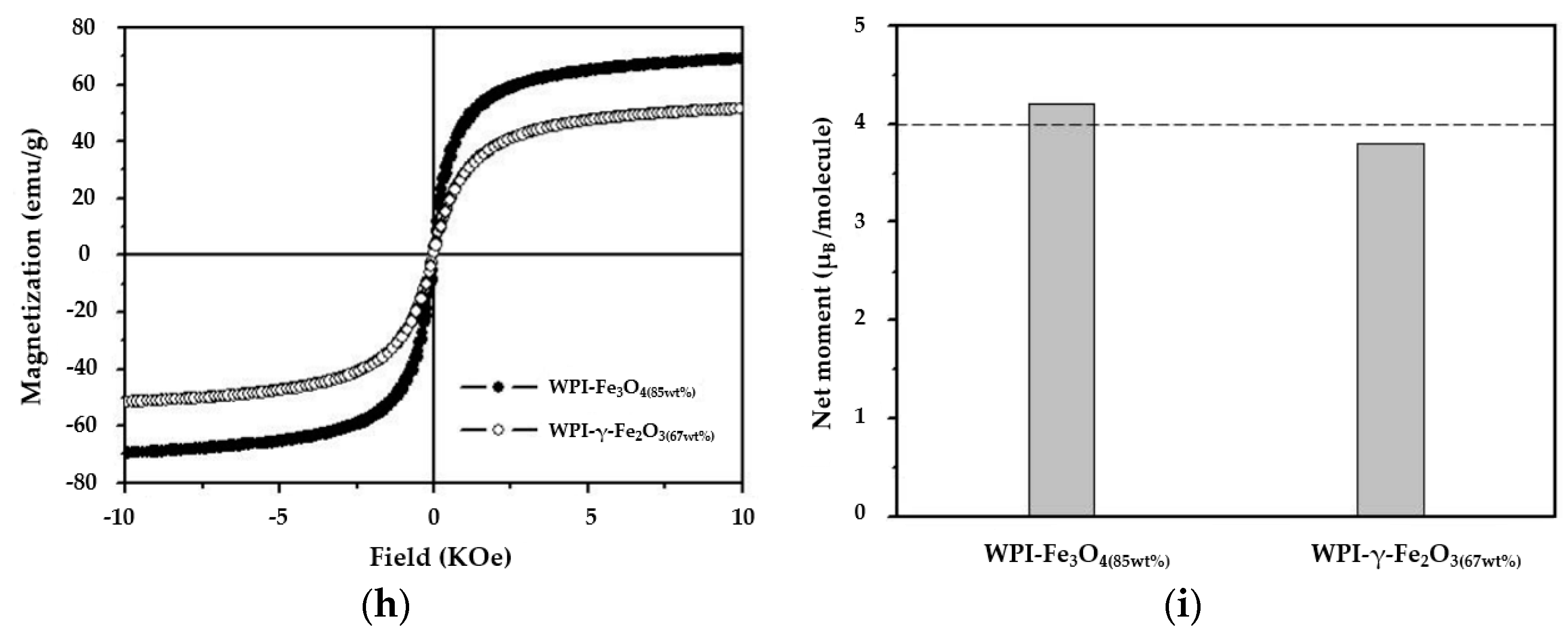
3.2. Structural of WPI-SNPs
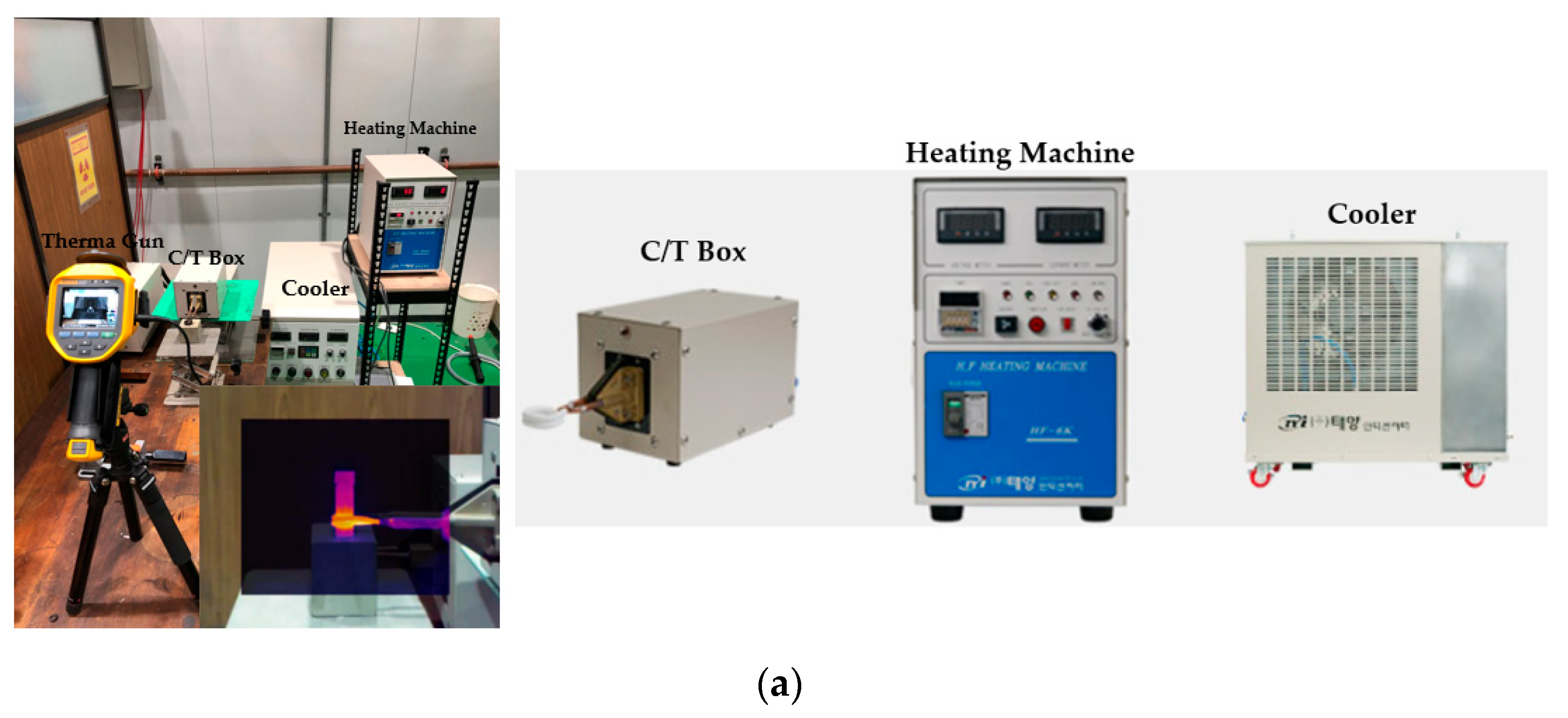
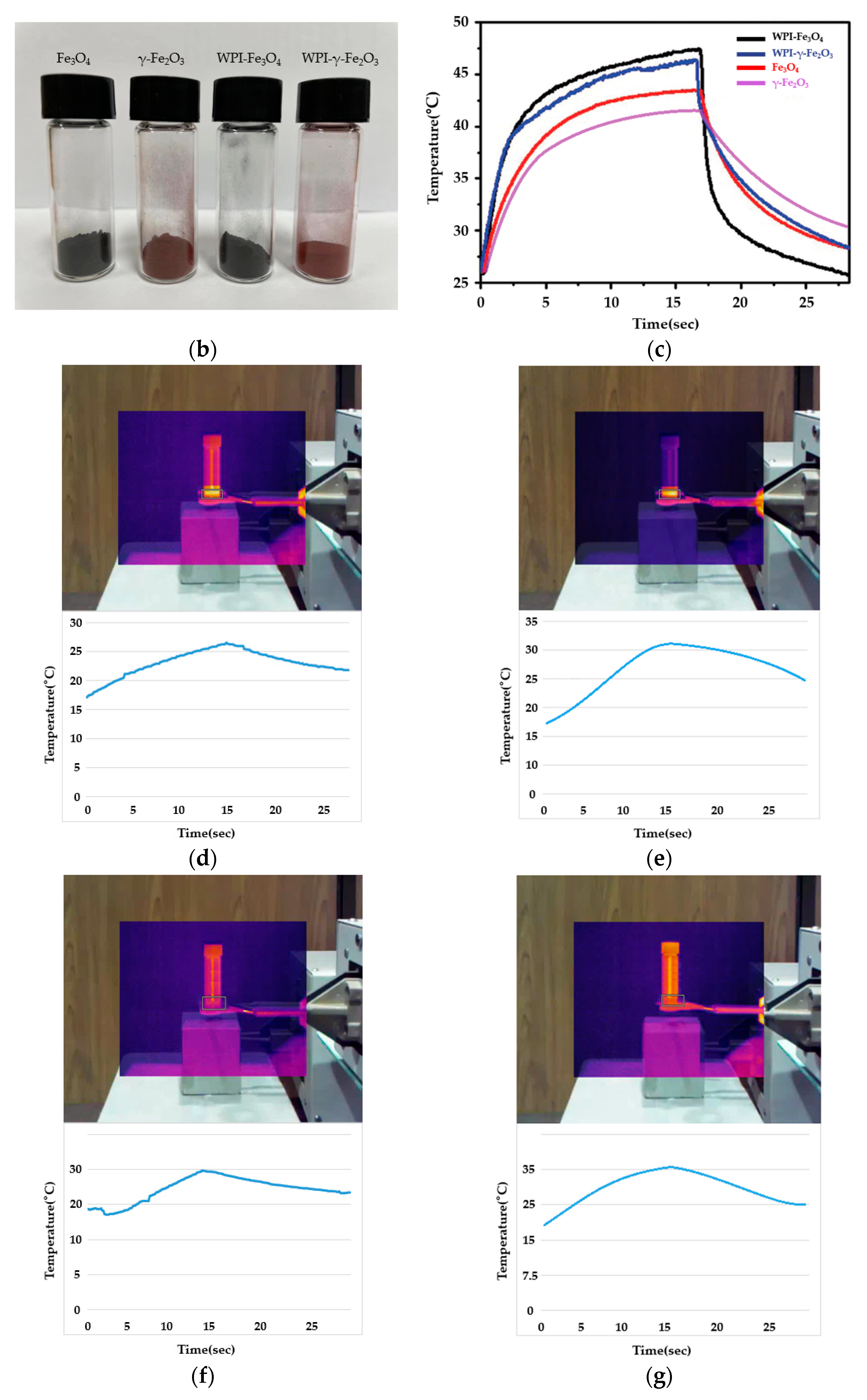
3.3. Heating Properties of WPI-SNPs
3.4. In Vitro Release of WPI-SNPs
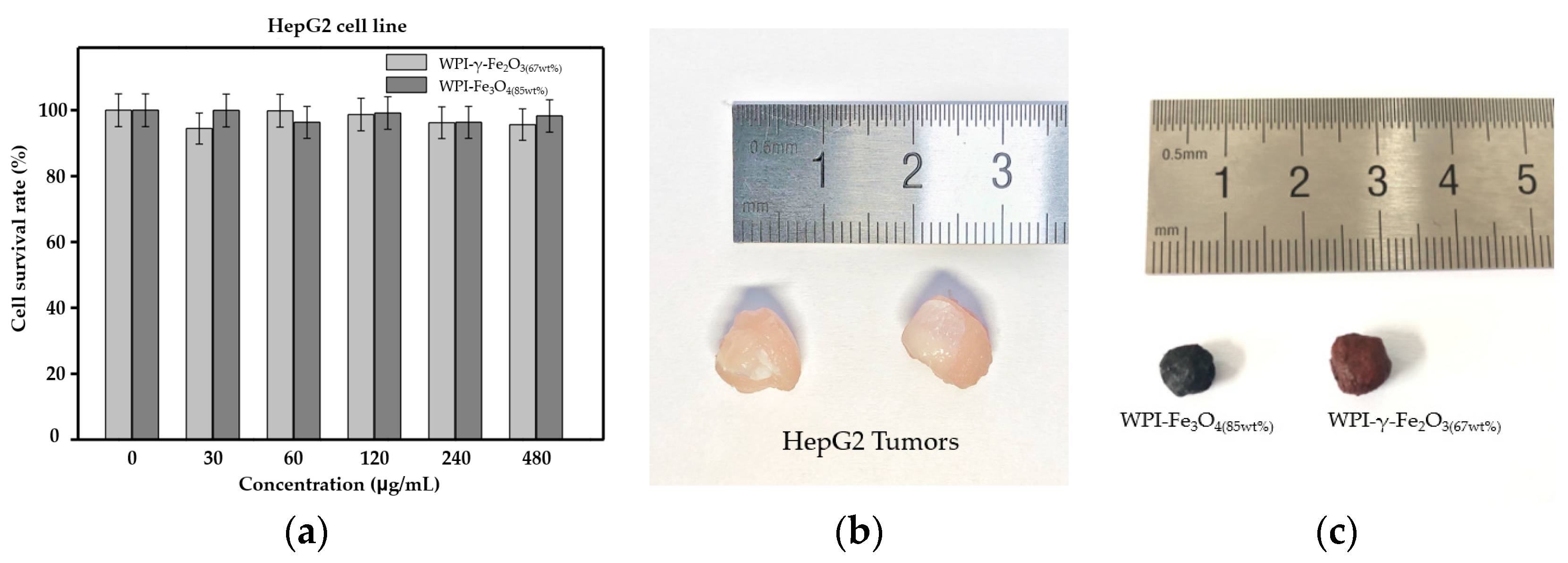
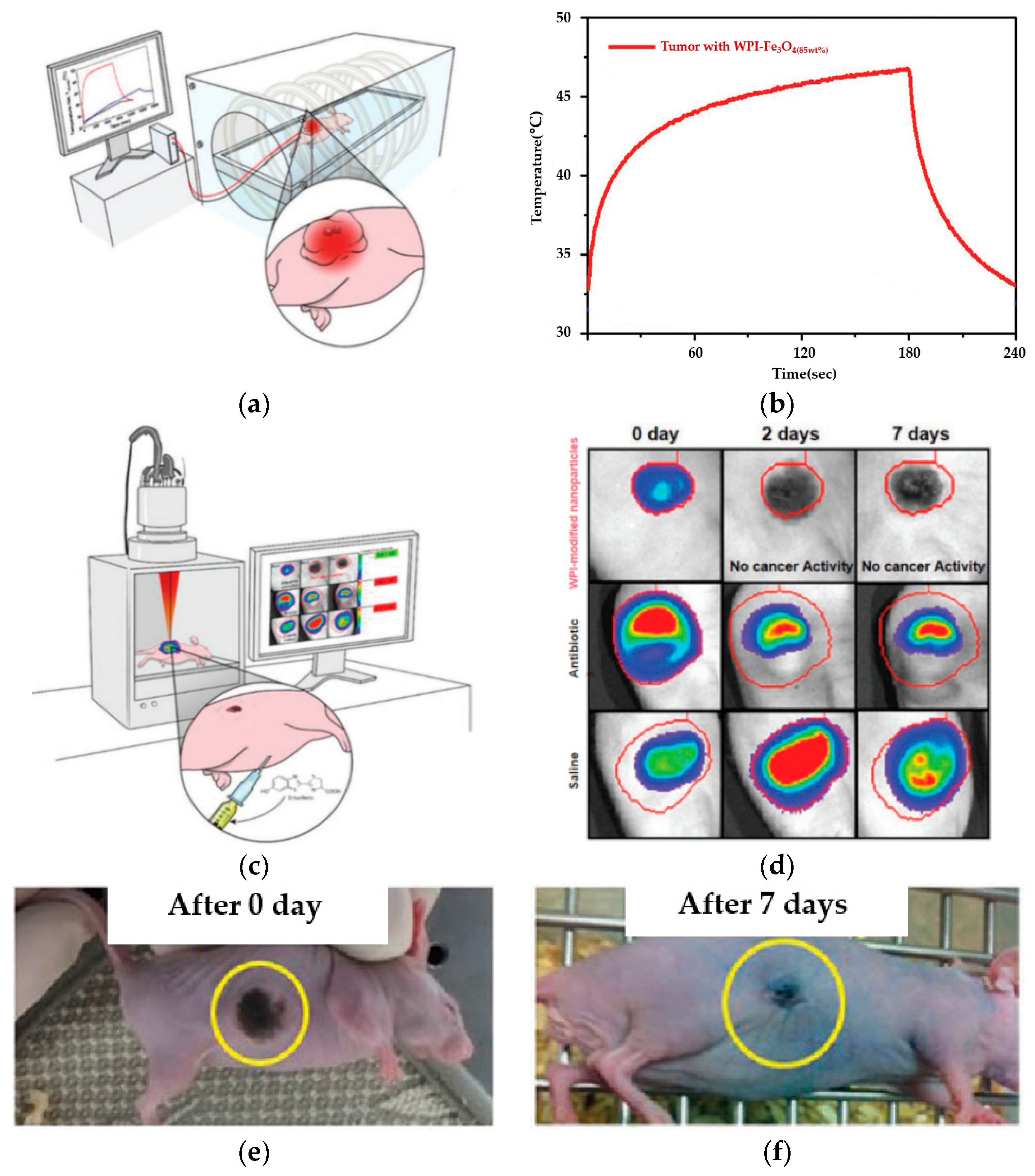
3.5. In Vivo Magnetic Hyperthermia
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemine, O.M. Magnetic Hyperthermia Therapy Using Hybrid Magnetic Nanostructures; Chapter 7; Elsevier: Riyadh, Saudi Arabia, 2019; pp. 125–138. [Google Scholar]
- Rivas, J.; Kolen’ko, Y.V.; Banobre-lopez, M. Magnetic Nanocolloids; Chapter 3; Elsevier: Braga, Portugal, 2016; pp. 75–129. [Google Scholar]
- Jeun, M.; Kim, Y.J.; Park, K.H.; Paek, S.H.; Bae, S. Physical Contribution of Néel and Brown Relaxation to Interpreting Intracellular Hyperthermia Characteristics Using Superparamagnetic Nanofluids. J. Nanosci. Nanotechnol. 2013, 13, 5719–5725. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I.; Hergt, R.; Kaiser, W.A. Use of magnetic nanoparticle heating in the treatment of breast cancer. IEE Proc.-Nanobiotechnol. 2005, 152, 33–39. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Roder, M. Effects of size distribution on hysteresis losses of magnetic nanoparticles for hyperthermia. J. Phys. Condens. Matter 2008, 20, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, K.; Lee, H.Y.; Xu, C.; Hsu, A.R.; Peng, S.; Chen, S.; Sun, S. Ultra-Small c(RGDyK)-Coated Fe3O4 Nanoparticles and Their Specific Targeting to Integrin avB3-rich Tumor Cells. J. Am. Chem. Soc. 2008, 130, 7542–7543. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shen, C.; Hou, Y.; Gao, H.; Sun, S. Oleylamine as Both Reducing Agent and Stabilizer in a Facile Synthesis of Magnetite Nanoparticles. Chem. Mater. 2009, 21, 1778–1780. [Google Scholar] [CrossRef]
- Cui, X.J.; Dong, L.L.; Zhong, S.L.; Shi, C.; Sun, Y.X.; Chen, P. Sonochemical fabrication of folic acid functionalized multistimuli-responsive magnetic graphene oxide-based nanocapsules for targeted drug delivery. Chem. Eng. J. 2017, 326, 839–848. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Deng, C.; Meng, F.H.; Yu, L.; Zhong, Z.Y. Redox-sensitive and intrinsically fuorescent photoclick hyaluronic acid nanogels for traceable and targeted delivery of cytochrome c to breast tumor in mice. ACS Appl. Mater. Interfaces 2016, 8, 21155–21162. [Google Scholar] [CrossRef]
- Kale, S.S.; Burga, R.A.; Sweeney, E.E.; Zun, Z.; Sze, R.W.; Tuesca, A.; Subramony, A.; Fernandes, R. Composite iron oxide-Prussian blue nanoparticles for magnetically guided T1-weighted magnetic resonance imaging and photothermal therapy of tumors. Int. J. Nanomed. 2017, 12, 6413–6418. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, G.; Sudame, A.; Luthra, T.; Saini, K.; Maity, D. Functionalized Hydrophilic Superparamagnetic Iron Oxide Nanoparticles for Magnetic Fluid Hyperthermia Application in Liver Cancer Treatment. ACS Omega 2018, 3, 3991–4005. [Google Scholar] [CrossRef]
- Ji, H.; Zhou, S.; Fu, Y.; Wang, Y.; Mi, J.; Lu, T.; Wang, X.; Lü, C. Size-controllable preparation and antibacterial mechanism of thermo-responsive copolymer-stabilized silver nanoparticles with high antimicrobial activity. Mater. Sci. Eng. C. 2020, 110, 110735. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wu, Y.; Wang, H.; Han, H. Synergistic antibacterial effects of curcumin modified silver nanoparticles through ROS-mediated pathways. Mater. Sci. Eng. C. 2019, 99, 255–263. [Google Scholar] [CrossRef]
- Hajizadeh, H.; Peighambardoust, S.J.; Peighambardoust, S.H.; Peressini, D. Physical, mechanical, and antibacterial characteristics of bio-nanocomposite films loaded with Ag-modified SiO2 and TiO2 nanoparticles. J. Food Sci. 2020, 85, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Scholz, R.; Maier-Hauff, K.; Johannsen, M.; Wust, P.; Nadobny, J.; Schirra, H.; Schmidt, H.; Loening, E.; Felix, R.; et al. Presentation of a new magnetic field therapy system for the treatment of human solid tumors with magnetic fluid hyperthermia. J. Magn. Magn. 2001, 225, 118–126. [Google Scholar] [CrossRef]
- Kandasamy, G.; Sudame, A.; Bhati, P.; Chakrabarty, A.; Kale, S.N.; Maity, D. Systematic magnetic fluid hyperthermia studies of carboxyl functionalized hydrophilic superparamagnetic iron oxide nanoparticles based ferrofluids. J. Colloid Interface Sci. 2018, 514, 534–543. [Google Scholar] [CrossRef]
- Saeedi, M.; Vahidi, O.; Bonakdar, S. Synthesis and characterization of glycyrrhizic acid coated iron oxide nanoparticles for hyperthermia applications. Mater. Sci. Eng. C. Mater. Biol. Appl. 2017, 77, 1060–1067. [Google Scholar] [CrossRef]
- Lahiri, B.B.; Ranoo, S.; Philip, J. Magnetic hyperthermia study in water based magnetic fluids containing TMAOH coated Fe3O4 using infrared thermography. Infrared Phys. Technol. 2017, 80, 71–82. [Google Scholar] [CrossRef]
- Oenbrink, G.; Jurgenlimke, P.; Gabel, D. Accumulation of porphyrins in cells: Influence of hydrophobicity aggregation and protein binding. Photochem. Photobiol. 1988, 48, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Martinkova, P.; Brtnicky, M.; Kynicky, J.; Pohanka, M. Iron Oxide Nanoparticles: Innovative Tool in Cancer Diagnosis and Therapy. Adv. Healthc. Mater. 2017, 7, 1700932. [Google Scholar] [CrossRef] [PubMed]
- Basini, M.; Guerrini, A.; Cobianchi, M.; Orsini, F.; Bettega, D.; Avolio, M.; Innocenti, C.; Sangregorio, C.; Lascialfari, A.; Arosio, P. Tailoring the magnetic core of organic-coated iron oxides nanoparticles to influencetheir contrast efficiency for Magnetic Resonance Imaging. J. Alloys Compd. 2019, 770, 58–66. [Google Scholar] [CrossRef]
- Peddis, D.; Cannas, C.; Musinu, A.; Ardu, A.; Orrù, F.; Fiorani, D.; Laureti, S.; Rinaldi, D.; Muscas, G.; Concas, G.; et al. Beyond the Effect of Particle Size: Influence of CoFe2O4 Nanoparticle Arrangements on Magnetic Properties. Chem. Mater. 2013, 25, 2005–2013. [Google Scholar] [CrossRef]
- Omelyanchik, A.; Salvador, M.; D’Orazio, F.; Mameli, V.; Cannas, C.; Fiorani, D.; Musinu, A.; Rivas, M.; Rodionova, V.; Varvaro, G.; et al. Magnetocrystalline and Surface Anisotropy in CoFe2O4 Nanoparticles. Nanomaterials 2020, 10, 1288. [Google Scholar] [CrossRef]
- Xie, H.; Zhu, Y.; Jiang, W.; Zhou, Q.; Yang, H.; Gu, N.; Zhang, Y.; Xu, H.; Xu, H.; Yang, X. Lactoferrin-conjugated superparamagnetic iron oxide nanoparticles as a specific MRI contrast agent for detection of brain glioma in vivo. Biomaterials 2011, 32, 495–502. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef]
- Ho, D.; Sun, X.L.; Sun, S.H. Monodisperse magnetic nanoparticles for theranostic applications. Accounts Chem. Res. 2011, 44, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hemat. 2002, 43, 33–56. [Google Scholar] [CrossRef]
| FTIR | XRD | TEM | SEM | Induction Heater |
|---|---|---|---|---|
| Spectral range: 7800~100 cm−1 | Measuring circle diameter: 600 mm | LaB6, high-voltage range: 20 to 120 kV | Resolution: 0.5 nm at 15 kV with BD; 0.8 nm at 1 kV with BD; 1.0 nm at 15 kV in LowVac | 380 H × 230 W × 385 L |
| Resolution: 0.09 cm−1 | Max. usable angular range: −60~+158 | Point resolution: 0.34 nm | Landing energy: 20 eV~30 keV | Power dissipation: 6 KW |
| Scan speed: 65 scan/sec | X-Ray Generator: 60 kV, 2.2 kW | Line resolution: 0.20 nm | EDS resolution: 127 eV at Mn Ka, ta 130,000 cps | Frequency: 100 KHz–600 KHz |
| S/N ratio: 55,000:1 | Maximum Rotating Speed: 120 rpm | Gatan US1000 digital CCD camera | High-vacuum heating stage to 1100 °C/ EDS compatibility to 500 °C | Power supply: single-phase 220 VAC, 50/60 Hz |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Lee, S. RETRACTED: Biomaterial-Modified Magnetic Nanoparticles γ-Fe2O3, Fe3O4 Used to Improve the Efficiency of Hyperthermia of Tumors in HepG2 Model. Appl. Sci. 2021, 11, 2017. https://doi.org/10.3390/app11052017
Zhao S, Lee S. RETRACTED: Biomaterial-Modified Magnetic Nanoparticles γ-Fe2O3, Fe3O4 Used to Improve the Efficiency of Hyperthermia of Tumors in HepG2 Model. Applied Sciences. 2021; 11(5):2017. https://doi.org/10.3390/app11052017
Chicago/Turabian StyleZhao, Shang, and Seoksoon Lee. 2021. "RETRACTED: Biomaterial-Modified Magnetic Nanoparticles γ-Fe2O3, Fe3O4 Used to Improve the Efficiency of Hyperthermia of Tumors in HepG2 Model" Applied Sciences 11, no. 5: 2017. https://doi.org/10.3390/app11052017
APA StyleZhao, S., & Lee, S. (2021). RETRACTED: Biomaterial-Modified Magnetic Nanoparticles γ-Fe2O3, Fe3O4 Used to Improve the Efficiency of Hyperthermia of Tumors in HepG2 Model. Applied Sciences, 11(5), 2017. https://doi.org/10.3390/app11052017