1. Introduction
The use of electric fields to increase the permeability of biological membranes to molecular species is known as electroporation [
1]. One of the most commonly accepted explanation about the mechanism underlying electroporation is that due to the presence of a high-amplitude electric field in proximity of the membrane, the transmembrane potential changes, causing repulsion and dislocation of the phospholipids, which contributes to the development of pore formation in the membrane and makes it partially permeable, allowing the transport of molecules [
1]. As it is possible to achieve membrane permeabilization for every cell or tissue type, electroporation could enable a wide range of applications, including delivery of chemotherapeutic drugs [
2,
3], immunotherapy of cancer and neurodegenerative diseases [
4], and genetic transfection, e.g., for RNA and DNA vaccination [
5,
6].
Due to the wide range of possible applications, many different electroporation protocols and stimulation strategies have been developed to improve the efficacy of the treatment. However, all electroporation protocols require direct contact between the electrodes and the tissues, suffering from the drawbacks common to all invasive procedures and making it difficult to use the technique for a broad range of treatments. Moreover, the application of electric pulses can show other drawbacks, such as the presence of electro-chemical reactions in the electrode–electrolyte interfaces [
7] or the possible electrical breakdown between the electrodes [
8].
An alternative approach to achieving membrane permeabilization is the use of time-varying magnetic fields (Pulsed Electromagnetic Fields (PEMF)) [
9,
10,
11,
12,
13,
14]. This is a completely contactless technique, thus avoiding the use of electrodes, with many advantages such as lower costs and less patient discomfort, allowing the use of the techniques in a wide range of treatments. Studies using PEMF in vitro [
9,
10,
11,
12,
13] and in vivo [
14,
15,
16,
17] have demonstrated that PEMF allows permeabilizing the cell membrane, but the efficiency is much lower than that achieved by the conventional electroporation approach. This is because the amplitude of the electric field induced by PEMF in proximity of the cell membrane is almost two orders of magnitude lower that the one obtained by classical electroporation techniques [
18,
19].
A possible strategy to overcome this issue is to exploit the conductive properties of metal nanoparticles—such NPs, acting as nanoelectrodes, would enhance the induced electric field locally, i.e., close to the membrane, thus potentially enabling cell permeabilization. It was recently proved that the addition of highly conductive gold NPs (Au NPs) in conventional electroporation protocol local enhanced the induced electric field, leading to significant cellular permeabilization [
18]. Thanks to the local enhancement of the electric field, the presence of Au NPs was found to also ameliorate the complete coverage of the sample in terms of uniformity and intensity of the electric field [
20]. Moreover, the presence of Au NPs should cause membrane permeabilization over nanoscale regions without affecting the entire membrane, thus avoiding collateral damage. Improvements of conventional electroporation protocols by addition of Au NPs has been already observed experimentally in vitro by showing increased mammalian cells transfection [
21,
22] and efficiency in anticancer therapy [
22]. Moreover, two recent studies aimed to obtain a better comprehension of the phenomenon [
23,
24] by the numerical assessment of the local enhancement of the electric field due to Au NPs using different conventional electroporation protocols.
Regarding the use of Au NPs in contactless permeabilization by time-varying magnetic fields, a first in vitro attempt was described in [
18], assuming that the Au NPs could act as nanoelectrodes, locally enhancing the electric field amplitude to the levels known to allow permeabilizing the cell membrane in conventional electroporation protocols. The results described in [
18] showed that the combined use of Au NPs and PEMF enhanced the permeabilization of both CHO cells and Gram-negative bacteria, with no effect on survival. However, apart from these experimental findings, no numerical assessment and model of the nanoparticles placed in time-varying electromagnetic fields was described in the literature.
This study focused on filling this research gap: using a computational approach, the numerical assessment of the electric field enhancement by using Au NPs placed in time-varying electromagnetic fields was carried out. The experimental set-up described in [
18] was modelled, and the influence of the presence of Au NPs on the transmembrane potential were investigated. To account for the uncertainty about the positions, the geometry, and the aggregation of Au NPs around the cell membrane, different conditions including spherical NPs, cubic NPs, and aggregated of NPs were simulated. Using a spherical cell model and a pore dynamics model, the influence of the position and shape of the Au NPs, as well as the distance from the HI-PEMF source on the pore opening mechanism was assessed.
Such an assessment, quantifying amplitude and localization of the enhancement of the electric field due to the presence of the Au NPs, could help in giving a better comprehension about the mechanisms, still not completely understood, underlying cell membrane permeabilization by time-varying magnetic fields. This contribution could potentially enable the use of a contact-less cell permeabilization technique offering important improvements compared to the existing protocols based on conventional electroporation.
3. Results
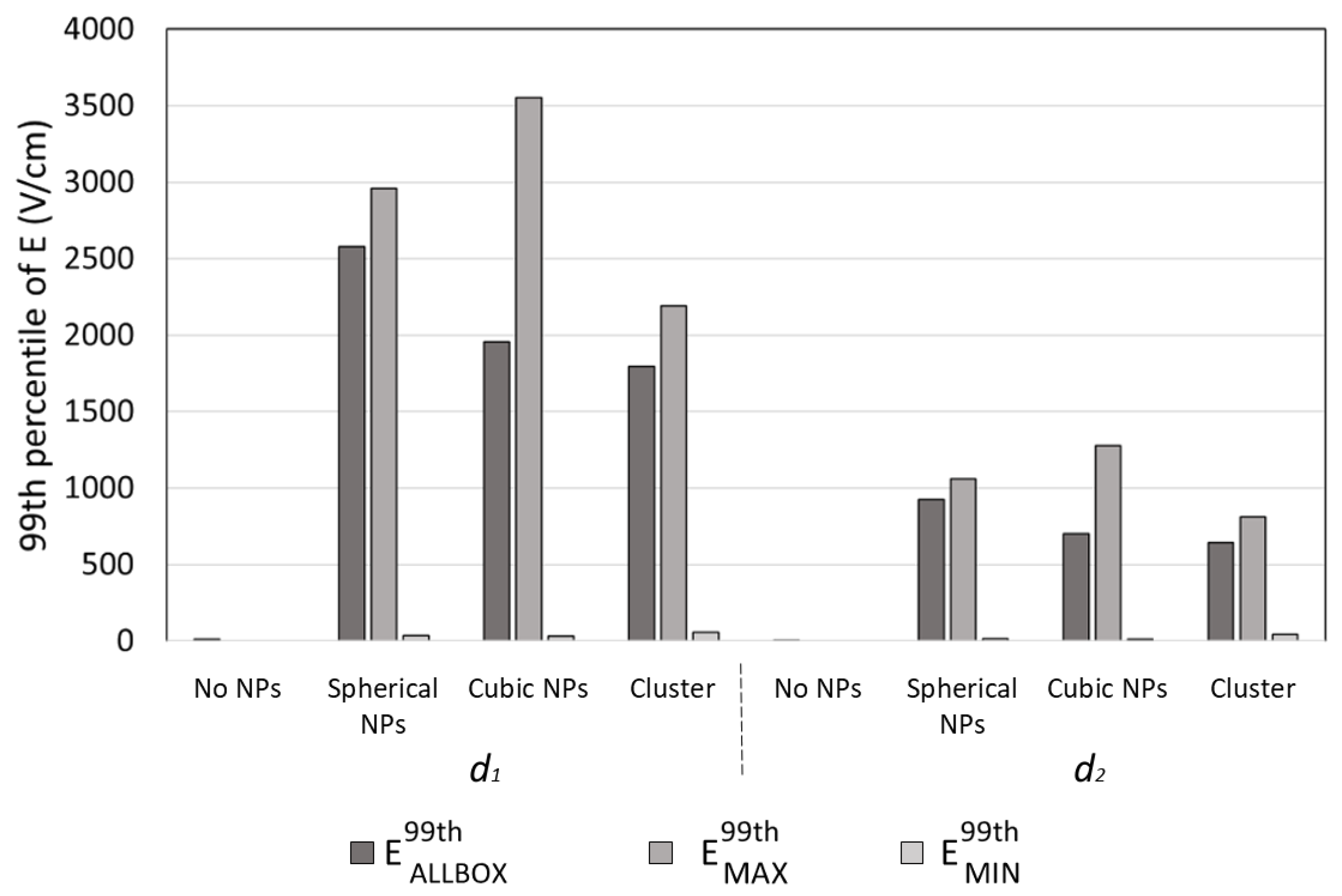
Figure 3 shows the 99th percentiles of the electric field module obtained for two different distances (
d1 and
d2) of the cell from the coil for the three different Au NP configurations considered, and, for a comparison, when no Au NP was used. As a first finding, a huge enhancement of the induced electric field was observed for both
d1 and
d2. For
d1, the observed values of
E99thAllBox were equal to 2580, 1955, and 1797 V/cm for the 24 spherical NPs, for the 24 cubic NPs, and for the 40 spherical NPs grouped in eight clusters, respectively, while when no Au NP was used, the 99th percentile of the module of the electric field was equal to 12.22 V/cm. When considering the enhancement in the neighboring of each NPs, a great difference was observed between NPs placed in different positions: in the spherical NP configuration, the maximum value of the 99th percentiles of the module of the electric field calculated around each NP,
E99thMAX, was equal to 2959.2 V/cm, while the minimum
E99thMIN was equal to 36.938 V/cm. A higher difference was observed for the cubic NP configuration, were
E99thMAX and
E99thMIN were found to be equal to 3553.8 V/cm and 34.3 V/cm. Finally, when considering clusters of NPs,
E99thMAX and
E99thMIN were found to be equal to 1796.6 V/cm and 58.95 V/cm. For
d2, as expected, lower values were observed, but the presence of the Au NPs still caused a significant enhancement of the electric field: the observed values of
E99thAllBox were equal to 924.26, 701.38, and 644.16V/cm for the 24 spherical NPs, for the 24 cubic NPs, and for the 40 spherical NPs grouped in eight clusters, respectively, while when no Au NP was used,
E99thAllBox was equal to 4.38 V/cm. Moreover, for
d2, a great difference was observed in the local enhancement of the electric field among NPs placed in different positions:
E99thMAX and
E99thMIN were found to be equal to 1059.9 and 13.786 V/cm for the spherical NP configuration, to 1276.5 and 12.25 V/cm for the cubic NP configuration, and to 811.4 and 42.5 V/cm for the 40 spherical NPs grouped in eight clusters.
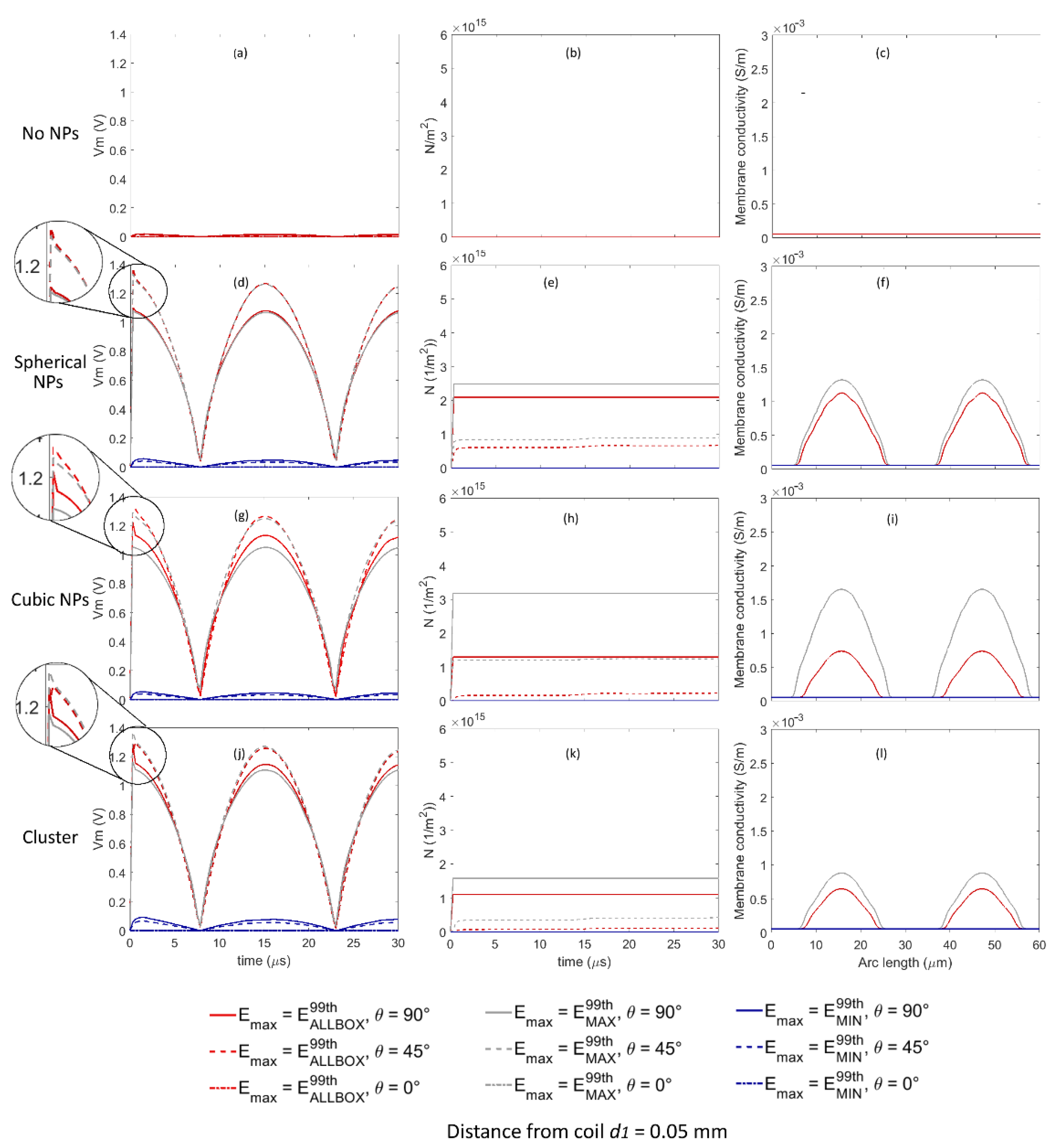
Figure 4 shows time evolution of the transmembrane voltage
Vm and pore density
N in three points of the cell membrane corresponding to θ equal to 0°, 45° and 90° (see
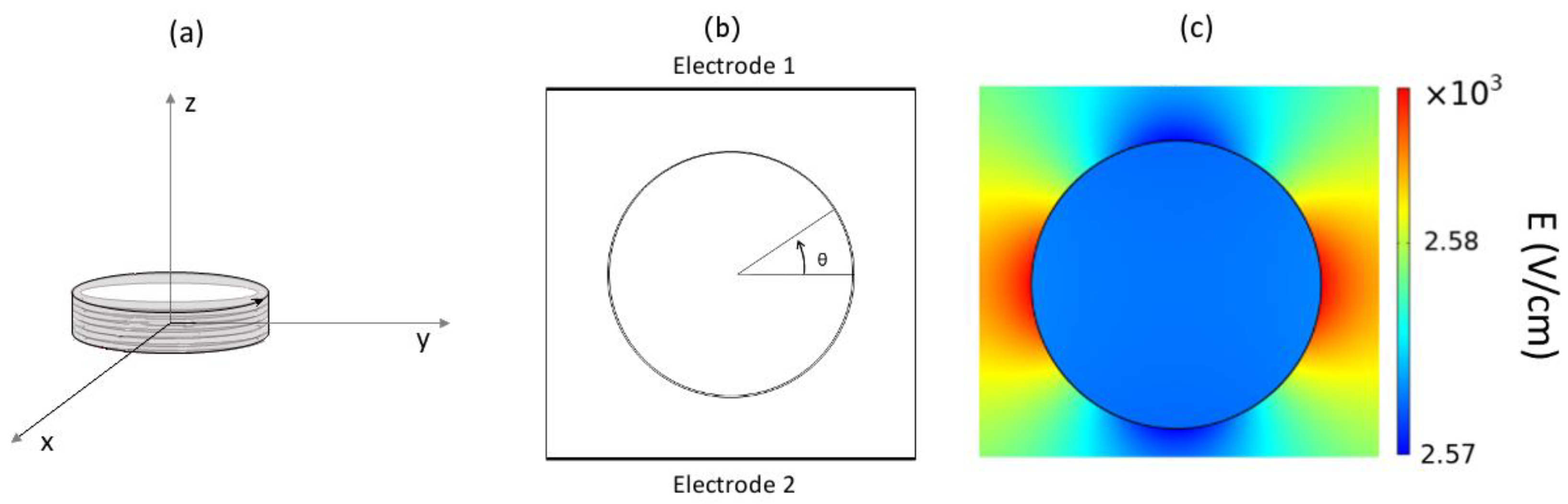
Figure 1b), and the variation in the conductivity along the cell membrane for
t equal to 15 µs when considering the amplitude of the external applied electric field equal to
E99thAllBox,
E99thMAX and
E99thMIN for all the Au NP configurations when the distance between the cell and the coil was equal to
d1. An enlarged detail of the time evolution of the transmembrane voltage
Vm is reported in
Figure 4d,g,j, to highlight the effect of the pore dynamics on
Vm. When no Au NP was considered, the transmembrane voltage
Vm was not high enough to allow the pore opening in any point of the cell membrane (see
Figure 4a–c).
For the spherical Au NP configuration (
Figure 4d–f), when the amplitude of the external applied electric field was set to
E99thAllBox (red lines) and to
E99thMAX (gray lines), the time evolution of
Vm was almost identical—in both cases, higher than the critical threshold assumed to enable pore opening for θ equal to 45° (dashed lines) and 90° (continuous lines). The corresponding time evolutions of the pore density
N are shown in
Figure 4e. As expected, the pore opening caused a sudden decrease in the time evolution of
Vm (see the enlarged detail of
Figure 4d). The membrane conductivity (
Figure 4f) showed an important increase from the starting value of 1.26 × 10
−7 to the maximum values equal to 1.12 × 10
−3 S/m and 1.32 × 10
−3 S/m, for
E99thAllBox (red lines) and
E99thMAX (gray lines), respectively, at arch lengths equal to 15.69 and 47.2 µm, corresponding to θ equal to 90° and 270°, i.e., at cell poles, while no increase was observed at arc length corresponding to θ equal to 0° and 180°. In addition to the significant increase corresponding to cell poles, membrane conductivity showed values higher than the starting value along a wide part of the cell membrane, corresponding to θ values rising from 32° to 148° and from 212° to 328° for both
E99thAllBox (red lines) and
E99thMAX (gray lines), respectively, meaning that a large part of the cell membrane was permeabilized. When the amplitude of the external applied electric field was set equal to
E99thMIN, i.e., to the minimum among the 99th percentiles values obtained in the neighborhood of the NPs, no pore opening was enabled at any point along the cell membrane.
For the cubic Au NP configuration (
Figure 4g–i), the time evolution of
Vm was slightly different when the amplitude of the external applied electric field was set equal to
E99thAllBox (red lines) and to
E99thMAX (gray lines). In both cases,
Vm was high enough to enable pore opening both for θ equal to 45° (dashed lines) and 90° (continuous lines), with higher pore density values for
E99thMAX than for
E99thAllBox (
Figure 4h), and a corresponding decrease in the time evolution of
Vm (enlarged detail of
Figure 4g), with a sharper shape for θ equal to 90° (continuous lines) than for θ equal to 45° (dashed lines). The membrane conductivity (
Figure 4i) showed an important increase from the starting value of 1.26 × 10
−7 to the maximum values equal to 0.74 × 10
−3 S/m and 1.65 × 10
−3 S/m, for
E99thAllBox (red lines) and
E99thMAX (gray lines), respectively, at arch lengths equal to 15.69 and 47.2 µm, corresponding to θ equal to 90° and 270°, i.e., at cell poles. Even if no increase was observed for θ equal to 0° and 180°, the variation in the conductivity along the cell arc length indicated that a large portion of the membrane, corresponding to θ rising from 27° to 153° and from 207° to 333° for
E99thMAX (gray lines), and to θ rising from 41° to 139° and from 229° to 311° for
E99thAllBox (red lines), was interested by pores opening. When the amplitude of the external applied electric field was set equal to
E99thMIN, no pore opening was enabled at any point along the cell membrane.
For the spherical Au NPs grouped in clusters (
Figure 4j–l), results were similar to those obtained for the spherical NPs (
Figure 4d–f), with a similar time evolution of
Vm for
E99thAllBox to
E99thMAX: in both cases,
Vm was high enough to enable pore opening both for θ equal to 45° (dashed lines) and 90° (continuous lines), with higher pore density values for
E99thMAX than for
E99thAllBox (
Figure 4k), and a corresponding decrease in the time evolution of
Vm (enlarged detail of
Figure 4j), with a sharper shape for θ equal to 90° (continuous lines) than for θ equal to 45° (dashed lines). The membrane conductivity (
Figure 4l) showed an increase from the starting value of 1.26 × 10
−7 to the maximum values equal to 0.64 × 10
−3 S/m and 0.89 × 10
−3 S/m for
E99thAllBox (red lines) and
E99thMAX (gray lines), respectively, in correspondence of θ equal to 90° and 270°. Even if no increase was observed for θ equal to 0° and 180°, the variation in the conductivity along the cell arc length indicated that a large portion of the membrane, corresponding to θ rising from 36° to 144° and from 224° to 316° for
E99thMAX (gray lines), and to θ rising from 45° to 135° and from 225° to 315° for
E99thAllBox (red lines), was interested by pores opening. When the amplitude of the external applied electric field was set equal to
E99thMIN, no pore opening was enabled at any point along the cell membrane.
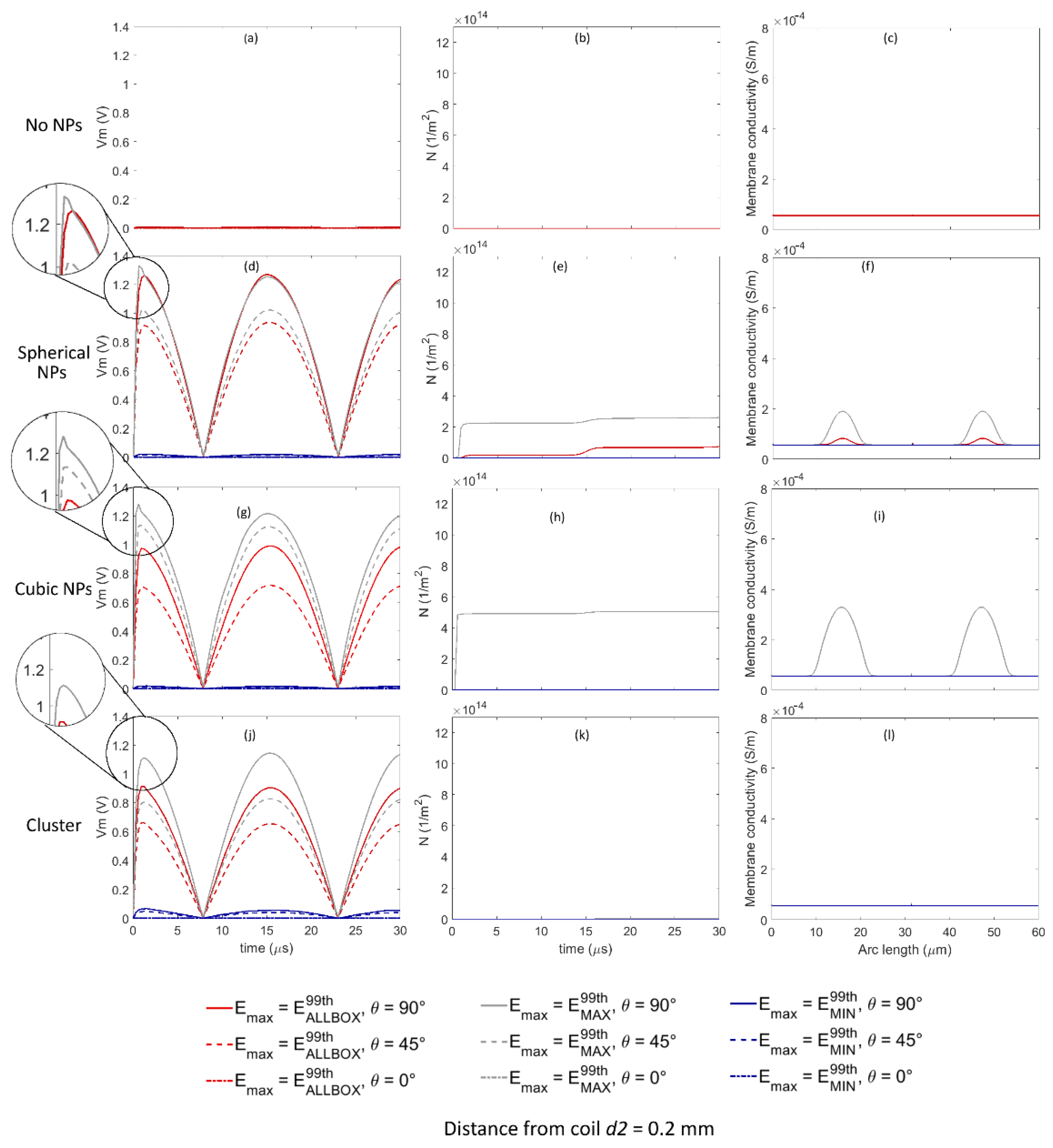
Analogously to
Figure 4 and
Figure 5 shows the time evolution of the transmembrane voltage
Vm, the pore density
N, and the variation in the conductivity along the cell membrane for
t equal to 15 µs when considering the amplitude of the external applied electric field equal to
E99thAllBox,
E99thMAX, and
E99thMIN for all the Au NP configurations when the distance between the cell and the coil was equal to
d2. An enlarged detail of the time evolution of the transmembrane voltage
Vm is reported in
Figure 5d,g,j, to highlight the possible effect of the pore dynamics on
Vm. Similarly to previous findings about
d1, also for
d2, when no Au NP was considered (
Figure 5a–c), the transmembrane voltage
Vm was not high enough to allow the pore opening at any point on the cell membrane.
When considering the spherical Au NP configuration (
Figure 5d–f), only when the amplitude of the external applied electric field was set equal to
E99thMAX, the
Vm values were high enough to enable pore opening for θ equal to 90° (continuous gray line), while when the amplitude of the external applied electric field was set equal to
E99thAllBox,
Vm values were comparable to the critical threshold for pore opening for θ equal to 90°, enabling a slow increase in pore density (
Figure 5e). This behavior is highlighted in the enlarged detail of
Figure 5d: a slight decrease in
Vm due to pore opening was observed only for
E99thMAX and for θ equal to 90° (continuous gray line). The membrane conductivity (
Figure 5f) showed an increase from the starting value of 1.26 × 10
−7 to the maximum values equal to 8.2 × 10
−5 S/m and 0.19 × 10
−3 S/m for
E99thAllBox (red lines) and
E99thMAX (gray lines), respectively, at arch lengths equal to 15.69 and 47.2 µm, corresponding to θ equal to 90° and 270°, i.e., at cell poles. The variation in the conductivity along the cell arc indicated that the portion of the membrane corresponding to θ rising from 65° to 115° and from 245° to 295° for
E99thMAX (gray lines), and to θ rising from 75° to 105° and from 255° to 285° for
E99thAllBox (red lines), was interested by pores opening. In all the remaining conditions, no pore opening was observed.
Moreover, for the cubic Au NP configuration (
Figure 5g–i), only when the amplitude of the external applied electric field was set equal to
E99thMAX, the
Vm values were high enough to enable pore opening for θ equal to 90° (continuous gray line), while for θ equal to 45°,
Vm values were comparable to the critical threshold for pore opening enabling a slow increase in pore density (
Figure 5h). This behavior is highlighted in the enlarged detail of
Figure 5g: a slight decrease in
Vm due to pore opening was observed only for
E99thMAX and for θ equal to 90° (continuous gray line), while no decrease was observed in the other cases. The membrane conductivity for
E99thMAX (
Figure 5f) showed an increase comparable to the values observed for
d1, ranging from the starting value of 1.26 × 10
−7 to the maximum value equal to 0.33 × 10
−3 S/m at arch lengths equal to 15.69 and 47.2 µm, corresponding to θ equal to 90° and 270°, i.e., at cell poles. The variation in the conductivity along the cell arc indicated that the portion of the membrane corresponding to θ rising from 53° to 127° and from 233° to 307° was interested by pores opening. In all the remaining conditions, no pore opening was observed.
Finally, when considering the spherical Au NPs grouped in clusters (
Figure 5j–l), for none of the simulated conditions of position of the membrane was the pore opening mechanism enabled.
4. Discussion
This study focused on using a computational approach for the assessment of the electric field enhancement by using Au NPs in time-varying electromagnetic field cell membrane permeabilization, estimating the influence of the presence of Au NPs on transmembrane potential and on the pore opening dynamics. The simulated conditions were based on the in vitro experimental set-up described by [
18]. To account for variability and uncertainty about geometries and relative placement and aggregations of the Au NPs, three different configurations were considered: spherical Au NPs equally spaced around the cell; cubic Au NPs, for accounting for the possible edge effect, equally spaced around the cell; and spherical Au NPs grouped in clusters.
The experimental approach consisted of two steps: first, the electric field induced by the time-varying magnetic field and enhanced by different geometries of Au NPs was evaluated. As the enhancement of the electric field was greatly dependent on the position of the Au NPs with respect to the source, three values were considered: E99thAllBox, corresponding to the 99th percentile of the electric field calculated on a cube containing the Au NPs, and E99thMAX and E99thMIN, corresponding to maximum and minimum values, respectively, among those obtained calculating the 99th percentiles of the electric field in 100 nm spherical neighborhood around each Au NP.
The results showed that when the cell and the Au NPs are placed very near to the coil, at distance d1 = 0.05 mm, and when they are slightly further, at d2 = 0.2 mm, a great enhancement of the electric field was obtained thanks to the presence of the NPs. When considering the E99thAllBox, the maximum increase due to the presence of the NPs was equal to about 21,000% for both d1 and d2 and was observed when simulating spherical NPs equally spaced around the cell. Comparing E99thAllBox to E99thMAX and E99thMIN, i.e., comparing the 99th percentile of the electric field obtained in the cube containing the Au NPs and the values obtained very near to the NPs, showed high variability. The maximum difference between E99thMAX and E99thAllBox was observed for the cubic NPs, as the E99thMAX was 0.817 and 0.819 times greater than E99thAllBox for d1 and d2, respectively, confirming previous findings about the edge effect and meaning that if a cubic Au NP was placed very near to the cell membrane, that specific point of the membrane would experience a very high electric field, thus enhancing the probability of pores opening. On the other hand, the maximum difference between E99thMIN and E99thAllBox was observed for the spherical NPs equally spaced around the cell, as between E99thAllBox and E99thMIN, a decrement was observed almost equal to 98% for both d1 and d2.
While all these findings describe the enhancement of the electric field obtained when each Au NP is placed so far from the others that is not influenced by their presence, the influence of the relative positions of NPs placed at a short distance was simulated by considering 40 NPs grouped in eight homogeneous clusters. Interestingly, although the number of simulated NPs was higher, i.e., 40 NPs in the cluster configuration vs. 24 NPs in the equally spaced configuration, the
E99thAllBox,
E99thMAX, and
E99thMIN values in the cluster configuration were lower than those observed for the equally spaced spherical NPs. Although a direct comparison with values observed in literature is not possible, as no other study has focused on the use of NPs when considering time-varying magnetic fields for cell permeabilization, a similar result was observed in [
24], where the authors found that if using Au NPs during electroporation protocol, the electric field enhancement was much higher for isolated NPs than for highly aggregated NPs.
All the considered configurations involved single NPs and grouped NPs equally spaced around and placed at very short distance, equal to 20 nm, from the cell membrane. To assess the influence of the distance from the cell membrane, two additional simulations considering the 24 spherical NPs placed at distances equal to 80 nm and 120 nm from the cell membrane were carried out. The results showed that, as expected, the E field values found along the cell membrane decreased for greater distances: maximum E values obtained for distances of 80 nm and 140 nm were equal to 30% and 11%, respectively, of the value obtained for a distance equal to 20 nm. Similarly, mean E values for distances of 80 nm and 140 nm were found to be equal to 37% and 21%, respectively, of the value obtained for a distance equal to 20 nm. Of course, a drastic decrease in the amplitude of the electric field in proximity of the cell membrane would result in a decreased probability of pore opening. These results, well expected, confirmed that the proximity of the Au NPs to the cell membrane could play a key role in the local enhancement of the electric field, and thus in the probability of enable pore opening.
The second step in the experimental approach consisted of time-dependent simulation of the transmembrane potential and of the pore opening dynamic when a spherical cell was stimulated by the electric field induced by the time-varying magnetic field and enhanced by the presence of the Au NPs.
As a first finding, when no NPs were considered, the electric field values induced by the time-varying magnetic field were not high enough to cause the transmembrane potential
Vm to overcome the critical threshold for pore opening, thus not enabling the cell membrane permeabilization, even if the cell was located at a small distance from the coil. This finding, well expected, was coherent with results by our previous study [
19] and by other modelling studies [
28,
29], in which the authors found that with half-sinusoidal signals with frequencies lower than 500 MHz and amplitude equal to 5.5 T, the maximum
Vm value was far below the critical value for pore opening.
When considering the enhancement due to the presence of NPs, the results were completely different: both for cells placed a distance of
d1 and
d2 from the coil, the presence of NPs enabled the cell permeabilization. As expected, a great difference in the transmembrane potential
Vm and in the pore density was observed if considering
E99thAllBox,
E99thMAX, and
E99thMIN. When the cell was placed in
d1, considering both
E99thAllBox and
E99thMAX, the cell permeabilization in all the considered NP configurations was obtained preferentially at cell poles, while a wider area of permeabilization was obtained mainly when considering
E99thMAX, i.e., when considering a very localized enhancement of the electric field, thus hypothesizing a very short distance between the cell membrane and Au NPs. This is coherent with findings of [
20], in which the authors found that considering Au NPs during electroporation protocols shows strong increases in
Vm with the presence of multiple NP clusters, demonstrating that enhanced pore opening could be possible even over sites far away from the poles, with proper distance between NPs cluster and cell membrane.
Finally, when the cell was placed in d2, even if cell permeabilization was not achieved in all the considered NP configurations and not in all points of the membrane, Au NPs played a fundamental role in enabling pore opening in conditions in which, without them, it would be completely impossible to reach permeabilization.
Our findings—although not directly comparable with previously reported literature, as to our knowledge, no previous modeling study focused on the use of NPs when considering time-varying magnetic fields for cell permeabilization—were roughly in line with observations reported in studies about the use of Au NPs in electroporation protocols [
20,
21,
22,
23,
24]. Moreover, our findings confirmed experimental findings reported in [
18], in which authors found that conductive gold nanoparticles can significantly potentiate the permeabilizing effect of time-varying magnetic fields, with an increase in permeabilization from 1.57% with no NPs, to 10.99% using 20 nm Au NPs.
In conclusion, our study showed that the combined use of Au NPs and time-varying magnetic fields can significantly improve the permeabilization of cell membranes.
As the electric field induced in the cell suspension is not uniform, also due to the presence of nanoparticles in proximity of the membrane, the approach of applying an almost uniform electric field distribution to assess the pore dynamics used in this study could represent an important simplification of the phenomenon. However, this approach, well established and already used in previous studies dealing with computational evaluation of pore dynamics influenced by electric fields distributions with [
25] or without (see, e.g., [
27]) the presence of nanoparticles near the cell membrane, allowed reducing the computational effort due to the presence of both nano-scale and millimeter-scale structures, while preserving the representativeness of the results.
The preliminary findings obtained in this study were encouraging and showed that the variability of NP geometries and configurations in proximity of the cell membrane had a strong influence on pore opening mechanisms. Future investigations should address all these aspects, and, due to the intrinsic variability and uncertainty characterizing a real in vitro experimental set, a stochastic dosimetry [
30] approach, i.e., an innovative approach combining computational bioelectromagnetic and stochastic methods, could be used to obtain a realistic representation of the phenomena under study.
Moreover, in future studies, it would be interesting to investigate how different types of NPs, such as magnetic ones, could influence the permeabilization of the cell membrane, and to take into account not only the enhancement of the electric field, but also the role that different types of NPs could play in terms of the mechanical stress on the cell membrane locally induced by the magnetic and electric fields, which are hypothesized to play an important role in the permeabilization mechanism [
19].