Non-Thermal O2 Plasma Efficacy on C. albicans and Its Effect on Denture Base Resin Color
Abstract
Featured Application
Abstract
1. Introduction
2. Material and Methods
2.1. Samples Preparation
2.1.1. Microbiological Assay
- 55 × 25 mm glass microscope slides (Ghäasel, Rogo-Sampaic, Wissous, France) (n = 27) were sterilized by high-pressure saturated steam at 134 °C for 18 min.
- 55 × 25 × 1.8 mm polymethyl methacrylate (PMMA) resin samples (ProBase®Hot 36 P-V, Ivoclar Vivadent, Saint-Jorioz, France) (n = 27) were prepared following the manufacturer’s instructions, cleaned by exposure to 70% alcohol and by mechanical agitation in distilled water with a soft sonication for 5 min, then dried.
2.1.2. Visual and Spectrophotometry Assay
2.2. Efficacy of Non-Thermal O2 Plasma against C. albicans
2.2.1. Candida albicans Growth Conditions
2.2.2. Sterilization by Non-Thermal O2 Plasma
2.2.3. Counts on Sabouraud Dextrose Agar
2.2.4. Scanning Electron Microscopy
2.3. Non-Thermal O2 Plasma Effects on Resin Color
2.3.1. Visual Inspection
2.3.2. Spectrophotometry
2.4. Statistical Analysis
3. Results
3.1. Efficacy of Non-Thermal O2 Plasma against C. albicans
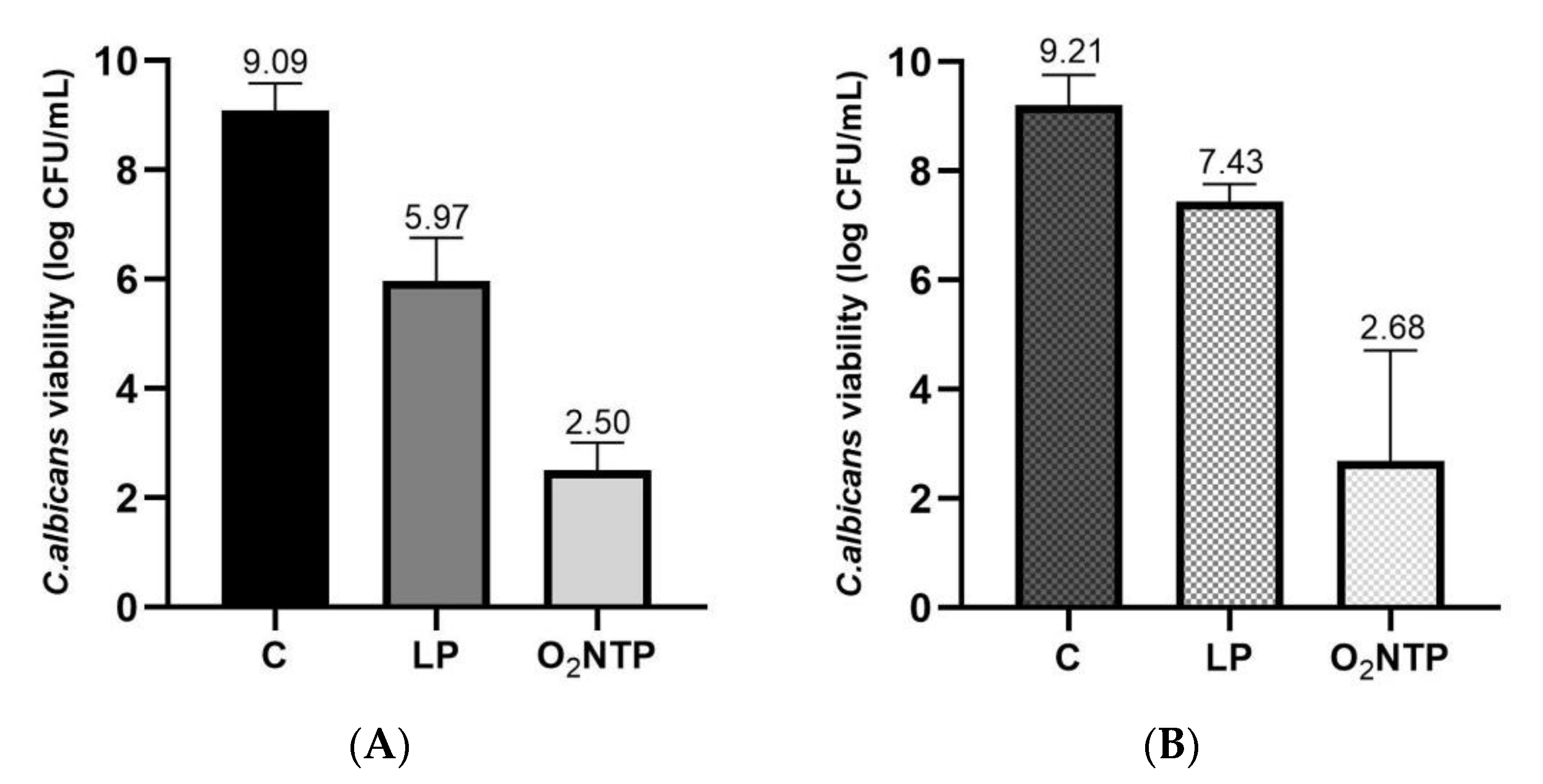
3.1.1. Low-Pressure Effect on C. albicans Viability
3.1.2. Efficacy of O2 NTP Treatment on C. albicans Viability
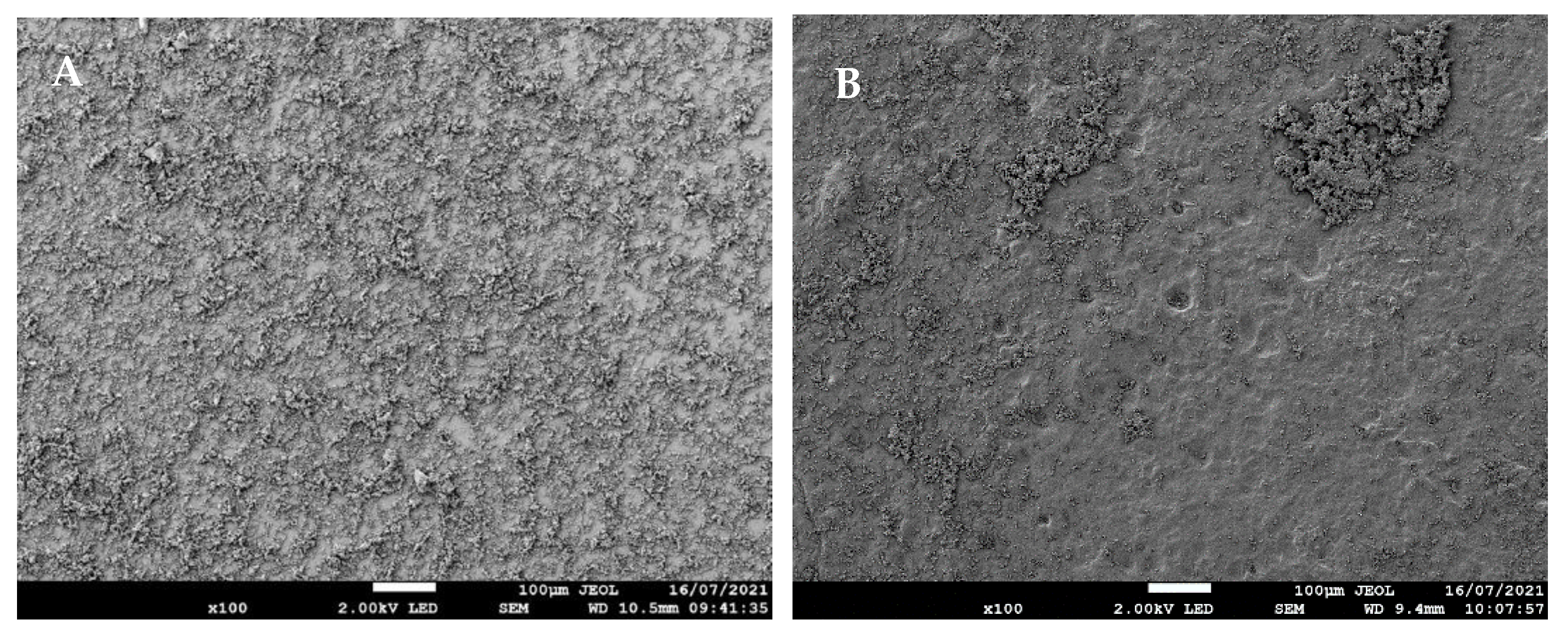
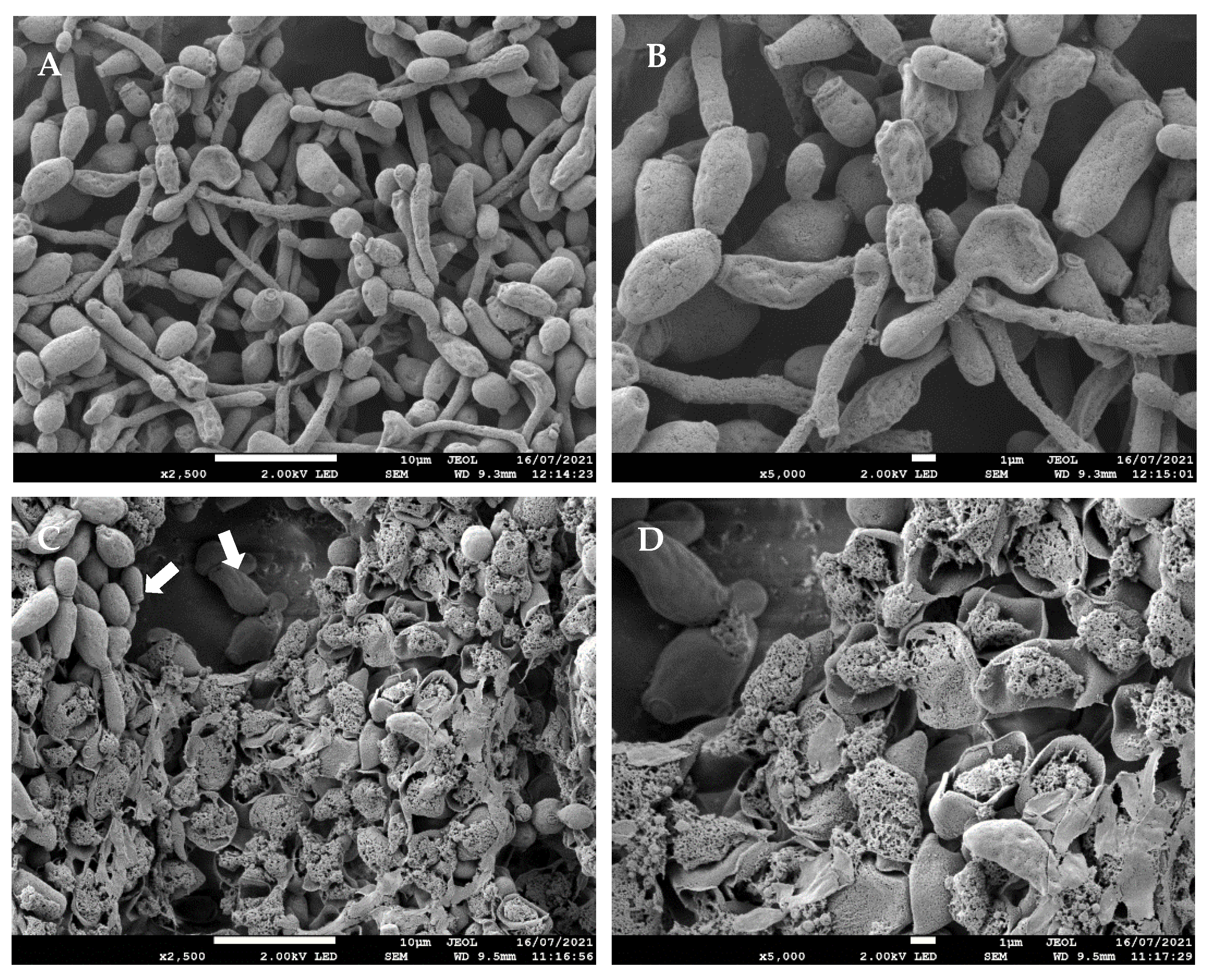
3.1.3. Effects of O2 NTP Treatment on C. albicans Morphology Determined by SEM
3.2. Low-Pressure and O2 NTP Treatments Effect on the Resin COLOR
3.2.1. Visual Aspect
3.2.2. Spectrophotometry
4. Discussion
5. Conclusions
6. Patents/Brevets
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mosaddad, S.A.; Tahmasebi, E.; Yazdanian, A.; Rezvani, M.B.; Seifalian, A.; Yazdanian, M.; Tebyanian, H. Oral Microbial Biofilms: An Update. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2005–2019. [Google Scholar] [CrossRef]
- Millsop, J.W.; Fazel, N. Oral Candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef]
- Salerno, C.; Pascale, M.; Contaldo, M.; Esposito, V.; Busciolano, M.; Milillo, L.; Guida, A.; Petruzzi, M.; Serpico, R. Candida-Associated Denture Stomatitis. Med. Oral. Patol. Oral. Cir. Bucal. 2011, 16, e139–e143. [Google Scholar] [CrossRef] [PubMed]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef]
- Susewind, S.; Lang, R.; Hahnel, S. Biofilm Formation and Candida Albicans Morphology on the Surface of Denture Base Materials. Mycoses 2015, 58, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Ikawa, S.; Kitano, K.; Maeda, N. A Proposal of Remedies for Oral Diseases Caused by Candida: A Mini Review. Front. Microbiol. 2018, 9, 1522. [Google Scholar] [CrossRef]
- Gendreau, L.; Loewy, Z.G. Epidemiology and Etiology of Denture Stomatitis: Denture Stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Hilgert, J.B.; Giordani, J.M.D.A.; de Souza, R.F.; Wendland, E.M.D.R.; D’Avila, O.P.; Hugo, F.N. Interventions for the Management of Denture Stomatitis: A Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2016, 64, 2539–2545. [Google Scholar] [CrossRef]
- Webb, B.C.; Thomas, C.J.; Willcox, M.D.P.; Harty, D.W.S.; Knox, K.W. Candida-Associated Denture Stoma Titis. Aetiology and Management: A Review. Part 2. Oral Diseases Caused by Candida Species. Aust. Dent. J. 1998, 43, 160–166. [Google Scholar] [CrossRef]
- Yarborough, A.; Cooper, L.; Duqum, I.; Mendonça, G.; McGraw, K.; Stoner, L. Evidence Regarding the Treatment of Denture Stomatitis: Treatment of Denture Stomatitis. J. Prosthodont. 2016, 25, 288–301. [Google Scholar] [CrossRef]
- Passariello, C.; Di Nardo, F.; Polimeni, A.; Di Nardo, D.; Testarelli, L. Probiotic Streptococcus Salivarius Reduces Symptoms of Denture Stomatitis and Oral Colonization by Candida Albicans. Appl. Sci. 2020, 10, 3002. [Google Scholar] [CrossRef]
- Emami, E.; Kabawat, M.; Rompre, P.H.; Feine, J.S. Linking Evidence to Treatment for Denture Stomatitis: A Meta-Analysis of Randomized Controlled Trials. J. Dent. 2014, 42, 99–106. [Google Scholar] [CrossRef]
- Spettel, K.; Barousch, W.; Makristathis, A.; Zeller, I.; Nehr, M.; Selitsch, B.; Lackner, M.; Rath, P.-M.; Steinmann, J.; Willinger, B. Analysis of Antifungal Resistance Genes in Candida Albicans and Candida Glabrata Using next Generation Sequencing. PLoS ONE 2019, 14, e0210397. [Google Scholar] [CrossRef]
- Pereira, R.; Santos Fontenelle, R.O.; Brito, E.H.S.; Morais, S.M. Biofilm of Candida Albicans: Formation, Regulation and Resistance. J. Appl. Microbiol. 2021, 131, 11–22. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to Azoles and Echinocandins Worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Felton, D.; Cooper, L.; Duqum, I.; Minsley, G.; Guckes, A.; Haug, S.; Meredith, P.; Solie, C.; Avery, D.; Deal Chandler, N. Evidence-Based Guidelines for the Care and Maintenance of Complete Dentures: A Publication of the American College of Prosthodontists. J. Prosthodont. 2011, 20, S1–S12. [Google Scholar] [CrossRef]
- Bartlett, D.; Carter, N.; de Baat, C.; Duyck, J.; Goffin, G.; Müller, F.; Kawai, Y. White Paper on Optimal Care and Maintenance of Full Dentures for Oral and General Health; 2018. Available online: https://www.dentalhealth.org/denturecareguidelines (accessed on 21 August 2021).
- Madeira, P.L.B.; Carvalho, L.T.; Paschoal, M.A.B.; de Sousa, E.M.; Moffa, E.B.; dos Santos da Silva, M.A.; de Jesus Rodolfo Tavarez, R.; Gonçalves, L.M. In Vitro Effects of Lemongrass Extract on Candida Albicans Biofilms, Human Cells Viability, and Denture Surface. Front. Cell. Infect. Microbiol. 2016, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Peracini, A.; Machado Andrade, I.; Oliveira, V.C.; Macedo, A.P.; Silva-Lovato, C.H.; Oliveira Pagnano, V.; Watanabe, E.; Oliveira, P.H.F. Antimicrobial Action and Long-Term Effect of Overnight Denture Cleansers. Am. J. Dent. 2017, 30, 101–108. [Google Scholar]
- Kurt, A.; Erkose-Genc, G.; Uzun, M.; Sarı, T.; Isik-Ozkol, G. The Effect of Cleaning Solutions on a Denture Base Material: Elimination of Candida Albicans and Alteration of Physical Properties: Effect of Cleaning Solutions on Denture Properties. J. Prosthodont. 2018, 27, 577–583. [Google Scholar] [CrossRef]
- Zago, P.M.W.; dos Santos Castelo Branco, S.J.; de Albuquerque Bogéa Fecury, L.; Carvalho, L.T.; Rocha, C.Q.; Madeira, P.L.B.; de Sousa, E.M.; de Siqueira, F.S.F.; Paschoal, M.A.B.; Diniz, R.S.; et al. Anti-Biofilm Action of Chenopodium Ambrosioides Extract, Cytotoxic Potential and Effects on Acrylic Denture Surface. Front. Microbiol. 2019, 10, 1724. [Google Scholar] [CrossRef]
- Rocha, G.D.S.R.; Neves Duarte, T.; de Oliveira, C.G.; Nampo, F.K.; de Paula, R.S. Chemical Cleaning Methods for Prostheses Colonized by Candida Spp.: A Systematic Review. J. Prosthet. Dent. 2020, 124, 653–658. [Google Scholar] [CrossRef]
- Savabi, O.; Attar, K.; Nejatidanesh, F.; Goroohi, H.; Badrian, H. Effect of Different Chemical Disinfectants on the Flexural Strength of Heat-Polymerized Acrylic Resins. Eur. J. Prosthodont. Restor. Dent. 2013, 21, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Shah, D.; Chauhan, C.; Doshi, P.; Kumar, A. Evaluation of Flexural Strength and Color Stability of Different Denture Base Materials Including Flexible Material after Using Different Denture Cleansers. J. Indian Prosthodont. Soc. 2015, 15, 367–373. [Google Scholar] [CrossRef]
- Durkan, R.; Ayaz, E.A.; Bagis, B.; Gurbuz, A.; Ozturk, N.; Korkmaz, F.M. Comparative Effects of Denture Cleansers on Physical Properties of Polyamide and Polymethyl Methacrylate Base Polymers. Dent. Mater. J. 2013, 32, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Ozyilmaz, O.Y.; Akin, C. Effect of Cleansers on Denture Base Resins’ Structural Properties. J. Appl. Biomater. Funct. Mater. 2019, 17, 228080001982779. [Google Scholar] [CrossRef] [PubMed]
- Paranhos, H.D.F.O.; Peracini, A.; Pisani, M.X.; Oliveira, V.D.C.; de Souza, R.F.; Silva-Lovato, C.H. Color Stability, Surface Roughness and Flexural Strength of an Acrylic Resin Submitted to Simulated Overnight Immersion in Denture Cleansers. Braz. Dent. J. 2013, 24, 152–156. [Google Scholar] [CrossRef]
- Carvalho, C.F.; Vanderlei, A.D.; Marocho, S.M.S.; Pereira, S.M.B.; Nogueira, L.; Paes-Junior, T.J.A. Effect of Disinfectant Solutions on a Denture Base Acrylic Resin. Acta Odontol. Latinoam. 2012, 25, 6. [Google Scholar]
- Fernandes, F.H.C.N.; Orsi, I.A.; Villabona, C.A. Effects of the Peracetic Acid and Sodium Hypochlorite on the Colour Stability and Surface Roughness of the Denture Base Acrylic Resins Polymerised by Microwave and Water Bath Methods: Effects of the Peracetic Acid and Sodium Hypochlorite on Denture Base Acrylic Resins. Gerodontology 2013, 30, 18–25. [Google Scholar] [CrossRef]
- Fergus, C.; Santos, M.; Soo, S.; Petridis, H. The Effect of Different Chemical Intra-Oral Prostheses Cleansers on the Surface Properties of Parylene-Coated PMMA. Dent. Mater. J. 2017, 36, 129–134. [Google Scholar] [CrossRef][Green Version]
- Moon, A.; Powers, J.M.; Kiat-amnuay, S. Color Stability of Denture Teeth and Acrylic Base Resin Subjected Daily to Various Consumer Cleansers: Color Stability of Denture Teeth and Resin Exposed to Cleansers. J. Esthet. Restor. Dent. 2014, 26, 247–255. [Google Scholar] [CrossRef][Green Version]
- Lohitha, K.; Prakash, M.; Gopinadh, A.; Sai Sankar, A.; Sandeep, C.; Sreedevi, B. Color Stability of Heat-Cure Acrylic Resin Subjected to Simulated Short-Term Immersion in Fast-Acting Denture Cleansers. Ann. Med. Health Sci. Res. 2016, 6, 291–295. [Google Scholar] [CrossRef]
- Senna, P.M.; Jose Da Silva, W.; Faot, F.; Antoninha Del Bel Cury, A. Microwave Disinfection: Cumulative Effect of Different Power Levels on Physical Properties of Denture Base Resins. J. Prosthodont. 2011, 20, 606–612. [Google Scholar] [CrossRef]
- Polychronakis, N.; Polyzois, G.; Lagouvardos, P.; Andreopoulos, A.; Ngo, H.C. Long-Term Microwaving of Denture Base Materials: Effects on Dimensional, Color and Translucency Stability. J. Appl. Oral. Sci. 2018, 26, e20170536. [Google Scholar] [CrossRef] [PubMed]
- Binns, R.; Li, W.; Wu, C.D.; Campbell, S.; Knoernschild, K.; Yang, B. Effect of Ultraviolet Radiation on Candida Albicans Biofilm on Poly(Methylmethacrylate) Resin. J. Prosthodont. 2020, 29, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Alkawareek, M.Y.; Algwari, Q.T.; Laverty, G.; Gorman, S.P.; Graham, W.G.; O’Connell, D.; Gilmore, B.F. Eradication of Pseudomonas Aeruginosa Biofilms by Atmospheric Pressure Non-Thermal Plasma. PLoS ONE 2012, 7, e44289. [Google Scholar] [CrossRef]
- Ben Belgacem, Z.; Carré, G.; Boudifa, M.; Charpentier, E.; Cawe, B.; Gellé, M.P. Effectiveness of Non-Thermal O 2 –N 2 Plasma on P. Aeruginosa Multilayer Biofilms Cultured on Hydroxyapatite. IRBM 2016, 37, 68–74. [Google Scholar] [CrossRef]
- Ben Belgacem, Z.; Carré, G.; Charpentier, E.; Le-Bras, F.; Maho, T.; Robert, E.; Pouvesle, J.-M.; Polidor, F.; Gangloff, S.C.; Boudifa, M.; et al. Innovative Non-Thermal Plasma Disinfection Process inside Sealed Bags: Assessment of Bactericidal and Sporicidal Effectiveness in Regard to Current Sterilization Norms. PLoS ONE 2017, 12, e0180183. [Google Scholar] [CrossRef]
- Filipić, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetič, M.; Dobnik, D. Cold Plasma, a New Hope in the Field of Virus Inactivation. Trends Biotechnol. 2020, 38, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, G.; Moser, D.; Müller, S.; Pfister, W.; Sculean, A.; Eick, S. The Antimicrobial Effect of Cold Atmospheric Plasma against Dental Pathogens—A Systematic Review of In-Vitro Studies. Antibiotics 2021, 10, 211. [Google Scholar] [CrossRef] [PubMed]
- Moisan, M.; Barbeau, J.; Moreau, S.; Pelletier, J.; Tabrizian, M.; Yahia, L. Low-Temperature Sterilization Using Gas Plasmas: A Review of the Experiments and an Analysis of the Inactivation Mechanisms. Int. J. Pharm. 2001, 226, 1–21. [Google Scholar] [CrossRef]
- Moreau, M.; Orange, N.; Feuilloley, M.G.J. Non-Thermal Plasma Technologies: New Tools for Bio-Decontamination. Biotechnol. Adv. 2008, 26, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Koban, I.; Matthes, R.; Hübner, N.-O.; Welk, A.; Meisel, P.; Holtfreter, B.; Sietmann, R.; Kindel, E.; Weltmann, K.-D.; Kramer, A.; et al. Treatment of Candida Albicans Biofilms with Low-Temperature Plasma Induced by Dielectric Barrier Discharge and Atmospheric Pressure Plasma Jet. New J. Phys. 2010, 12, 073039. [Google Scholar] [CrossRef]
- Fiebrandt, M.; Lackmann, J.-W.; Stapelmann, K. From Patent to Product? 50 Years of Low-Pressure Plasma Sterilization. Plasma Process. Polym. 2018, 15, 1800139. [Google Scholar] [CrossRef]
- Popot, J.M.; Gelle, M.P. Device for Cold Plasma Sterilization of an Object, Such as a Medical Device, Particularly an Implant, and Method Using This. Device. Patent WO/2012/038669, 23 March 2012. [Google Scholar]
- Carré, G.; Charpentier, E.; Audonnet, S.; Terryn, C.; Boudifa, M.; Doliwa, C.; Belgacem, Z.B.; Gangloff, S.C.; Gelle, M.-P. Contribution of Fluorescence Techniques in Determining the Efficiency of the Non-Thermal Plasma Treatment. Front. Microbiol. 2018, 9, 2171. [Google Scholar] [CrossRef]
- Ren, J.; Lin, H.; Huang, Q.; Liang, Q.; Zheng, G. Color Difference Threshold Determination for Acrylic Denture Base Resins. BME 2015, 26, S35–S43. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Lin, H.; Huang, Q.; Zheng, G. Determining Color Difference Thresholds in Denture Base Acrylic Resin. J. Prosthet. Dent. 2015, 114, 702–708. [Google Scholar] [CrossRef]
- Lee, H.-E.; Li, C.-Y.; Chang, H.-W.; Yang, Y.-H.; Wu, J.-H. Effects of Different Denture Cleaning Methods to Remove Candida Albicans from Acrylic Resin Denture Based Material. J. Dent. Sci. 2011, 6, 216–220. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M.C.R. Promising Alternative Therapeutics for Oral Candidiasis. CMC 2019, 26, 2515–2528. [Google Scholar] [CrossRef]
- Uludamar, A.; Özkan, Y.K.; Kadir, T.; Ceyhan, I. In Vivo Efficacy of Alkaline Peroxide Tablets and Mouthwashes on Candida Albicans in Patients with Denture Stomatitis. J. Appl. Oral Sci. 2010, 18, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Matthes, R.; Jablonowski, L.; Koban, I.; Quade, A.; Hübner, N.-O.; Schlueter, R.; Weltmann, K.-D.; von Woedtke, T.; Kramer, A.; Kocher, T. In Vitro Treatment of Candida Albicans Biofilms on Denture Base Material with Volume Dielectric Barrier Discharge Plasma (VDBD) Compared with Common Chemical Antiseptics. Clin. Oral. Investig. 2015, 19, 2319–2326. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.M.; Sun, P.P.; Pan, H.; Ye, G.P.; Sun, K.; Zhang, J.; Pan, J.; Fang, J. Inactivation of Candida Albicans Biofilms on Polymethyl Methacrylate and Enhancement of the Drug Susceptibility by Cold Ar/O2 Plasma Jet. Plasma Chem. Plasma Process. 2016, 36, 383–396. [Google Scholar] [CrossRef]
- Halfmann, H.; Denis, B.; Bibinov, N.; Wunderlich, J.; Awakowicz, P. Identification of the Most Efficient VUV/UV Radiation for Plasma Based Inactivation of Bacillus Atrophaeus Spores. J. Phys. D Appl. Phys. 2007, 40, 5907–5911. [Google Scholar] [CrossRef]
- Lackmann, J.-W.; Schneider, S.; Edengeiser, E.; Jarzina, F.; Brinckmann, S.; Steinborn, E.; Havenith, M.; Benedikt, J.; Bandow, J.E. Photons and Particles Emitted from Cold Atmospheric-Pressure Plasma Inactivate Bacteria and Biomolecules Independently and Synergistically. J. R. Soc. Interface 2013, 10, 20130591. [Google Scholar] [CrossRef]
- Lackmann, J.-W.; Bandow, J.E. Inactivation of Microbes and Macromolecules by Atmospheric-Pressure Plasma Jets. Appl. Microbiol. Biotechnol. 2014, 98, 6205–6213. [Google Scholar] [CrossRef]
- Suchodolski, J.; Muraszko, J.; Bernat, P.; Krasowska, A. A Crucial Role for Ergosterol in Plasma Membrane Composition, Localisation, and Activity of Cdr1p and H+-ATPase in Candida Albicans. Microorganisms 2019, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Shaghaghi, F.; Noormohammadi, Z.; Atyabi, S.-M.; Razzaghi-Abyaneh, M. Inhibitory Effects of Cold Atmospheric Plasma on the Growth, Virulence Factors and HSP90 Gene Expression in Candida Albicans. Arch. Biochem. Biophys. 2021, 700, 108772. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Yadav, B.; Roopesh, M.S.; Jo, C. Cold Plasma for Effective Fungal and Mycotoxin Control in Foods: Mechanisms, Inactivation Effects, and Applications: Cold Plasma for Effective Funga. Compr. Rev. Food Sci. Food Saf. 2019, 18, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Yodmongkol, S.; Chantarachindawong, R.; Thaweboon, S.; Thaweboon, B.; Amornsakchai, T.; Srikhirin, T. The Effects of Silane-SiO2 Nanocomposite Films on Candida Albicans Adhesion and the Surface and Physical Properties of Acrylic Resin Denture Base Material. J. Prosthet. Dent. 2014, 112, 1530–1538. [Google Scholar] [CrossRef]
- Handorf, O.; Weihe, T.; Bekeschus, S.; Graf, A.C.; Schnabel, U.; Riedel, K.; Ehlbeck, J. Nonthermal Plasma Jet Treatment Negatively Affects the Viability and Structure of Candida Albicans SC5314 Biofilms. Appl. Environ. Microbiol. 2018, 84, e01163-18. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, J.; Zhang, Z.; Ma, J.; Ma, R.; Zhao, Y.; Sun, Q.; Qian, S.; Zhang, H.; Ding, L.; et al. Inactivation Effects of Non-Thermal Atmospheric-Pressure Helium Plasma Jet on Staphylococcus Aureus Biofilms: Inactivation Effects of He APPJ on S. Aureus Biofilms. Plasma Process. Polym. 2015, 12, 827–835. [Google Scholar] [CrossRef]
- Golda, J.; Biskup, B.; Layes, V.; Winzer, T.; Benedikt, J. Vacuum Ultraviolet Spectroscopy of Cold Atmospheric Pressure Plasma Jets. Plasma Process. Polym. 2020, 17, 1900216. [Google Scholar] [CrossRef]

| Group | Treatment | Number of Samples | |
|---|---|---|---|
| C | Control | Untreated samples (resin natural aging) | n = 12 |
| LP | Low-pressure | 120 min | n = 12 |
| P1 | O2 NTP | 120 min | n = 12 |
| P6 | O2 NTP | 120 min × 6 (between each treatment, the samples were immerged in distilled water for 48 h) | n = 12 |
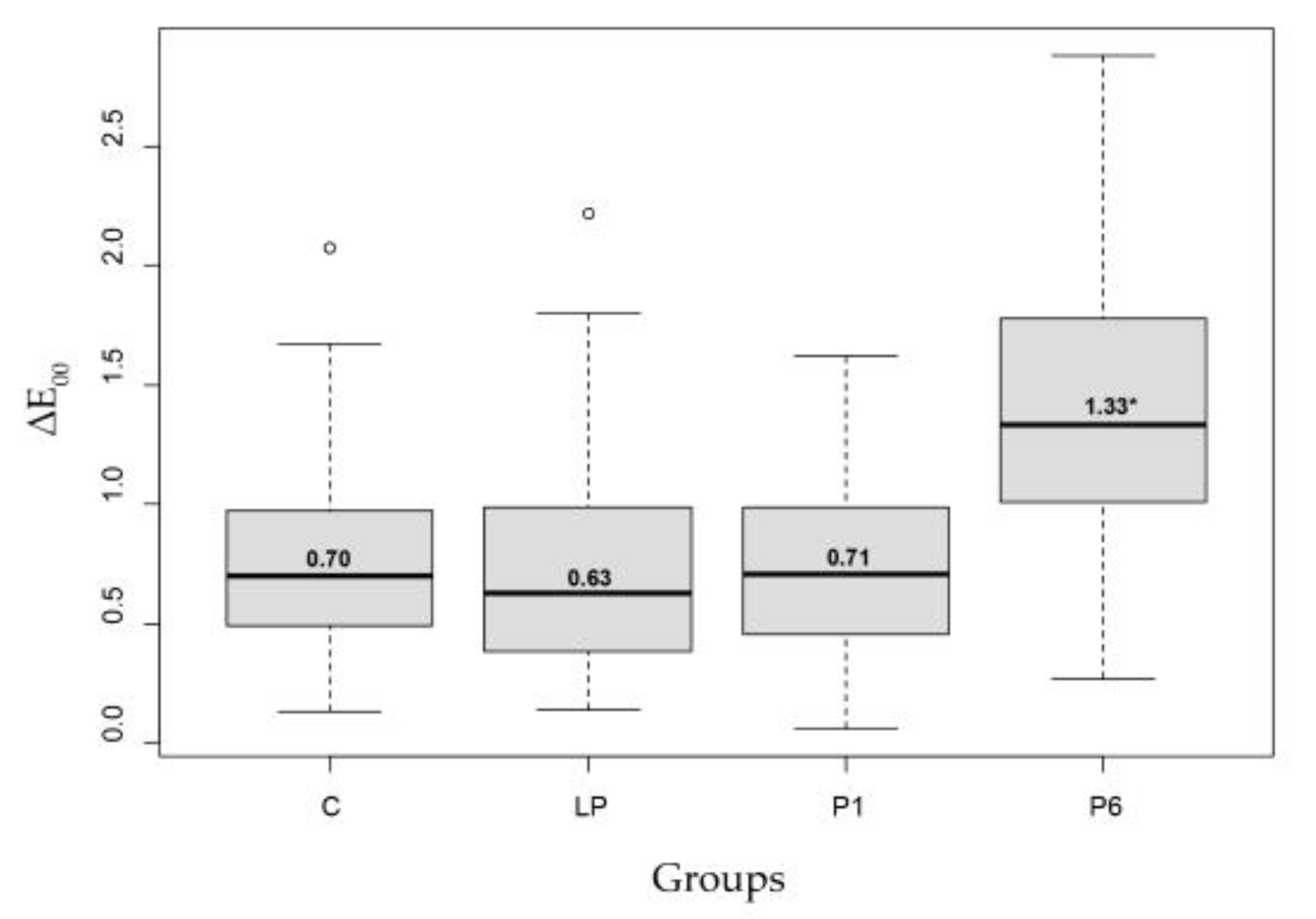
| ∆E00 | Min | 1st Qu. | Median | Mean | 3st Qu. | Max |
|---|---|---|---|---|---|---|
| C | 0.13 | 0.49 | 0.70 | 0.75 | 0.97 | 2.07 |
| LP | 0.14 | 0.38 | 0.63 | 0.73 | 0.99 | 2.22 |
| P1 | 0.06 | 0.45 | 0.71 | 0.75 | 0.98 | 1.62 |
| P6 | 0.27 | 1.01 | 1.33 * | 1.40 | 1.77 | 2.88 |
| L* | a* | b* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Measurement | Median (±SD) | min | max | Median (±SD) | min | max | Median (±SD) | min | max |
| C | initial | 34.77 (±0.98) | 32.43 | 37.29 | 8.36 (±0.65) | 6.85 | 10.05 | 1.55 (±0.40) | 0.78 | 2.96 |
| at 3 weeks | 35.10 (±1.10) | 32.81 | 37.55 | 8.25 (±0.66) | 6.65 | 9.73 | 1.46 (±0.44) | 0.63 | 2.98 | |
| LP | before | 34.26 (±0.82) | 32.51 | 36.44 | 8.22 (±0.60) | 7.13 | 10.04 | 1.39 (±0.28) | 0.58 | 2.29 |
| after | 34.97 (±0.79) | 33.08 | 38.08 | 8.12 (±0.56) | 6.87 | 9.58 | 1.32 (±0.26) | 0.72 | 2.26 | |
| P1 | before | 32.74 (±1.07) | 30.55 | 35.75 | 8.03 (±0.59) | 6.02 | 9.97 | 0.91 (±0.22) | 0.29 | 1.62 |
| after | 33.58 (±1.23) | 31.06 | 36.99 | 7.98 (±0.61) | 6.22 | 9.71 | 0.82 (±0.29) | 0.00 | 1.51 | |
| P6 | before | 32.95 (±1.02) | 30.70 | 36.03 | 8.23 (±0.60) | 7.07 | 9.49 | 0.98 (±0.30) | 0.38 | 1.78 |
| after | 35.31 (±1.27) * | 31.75 | 39.94 | 8.80 (±0.65) * | 7.13 | 10.31 | 1.45 (±0.43) * | 0.33 | 2.48 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maillet, C.; Odof, S.; Meuret, M.; Le Bras, F.; Velard, F.; Gelle, M.-P. Non-Thermal O2 Plasma Efficacy on C. albicans and Its Effect on Denture Base Resin Color. Appl. Sci. 2021, 11, 10367. https://doi.org/10.3390/app112110367
Maillet C, Odof S, Meuret M, Le Bras F, Velard F, Gelle M-P. Non-Thermal O2 Plasma Efficacy on C. albicans and Its Effect on Denture Base Resin Color. Applied Sciences. 2021; 11(21):10367. https://doi.org/10.3390/app112110367
Chicago/Turabian StyleMaillet, Christina, Serge Odof, Mikaël Meuret, Florian Le Bras, Frédéric Velard, and Marie-Paule Gelle. 2021. "Non-Thermal O2 Plasma Efficacy on C. albicans and Its Effect on Denture Base Resin Color" Applied Sciences 11, no. 21: 10367. https://doi.org/10.3390/app112110367
APA StyleMaillet, C., Odof, S., Meuret, M., Le Bras, F., Velard, F., & Gelle, M.-P. (2021). Non-Thermal O2 Plasma Efficacy on C. albicans and Its Effect on Denture Base Resin Color. Applied Sciences, 11(21), 10367. https://doi.org/10.3390/app112110367







