Evolution of Fusarium Head Blight Management in Wheat: Scientific Perspectives on Biological Control Agents and Crop Genotypes Protocooperation
Abstract
:1. Introduction
2. Fusarium Head Blight (FHB)
2.1. FHB and Crops
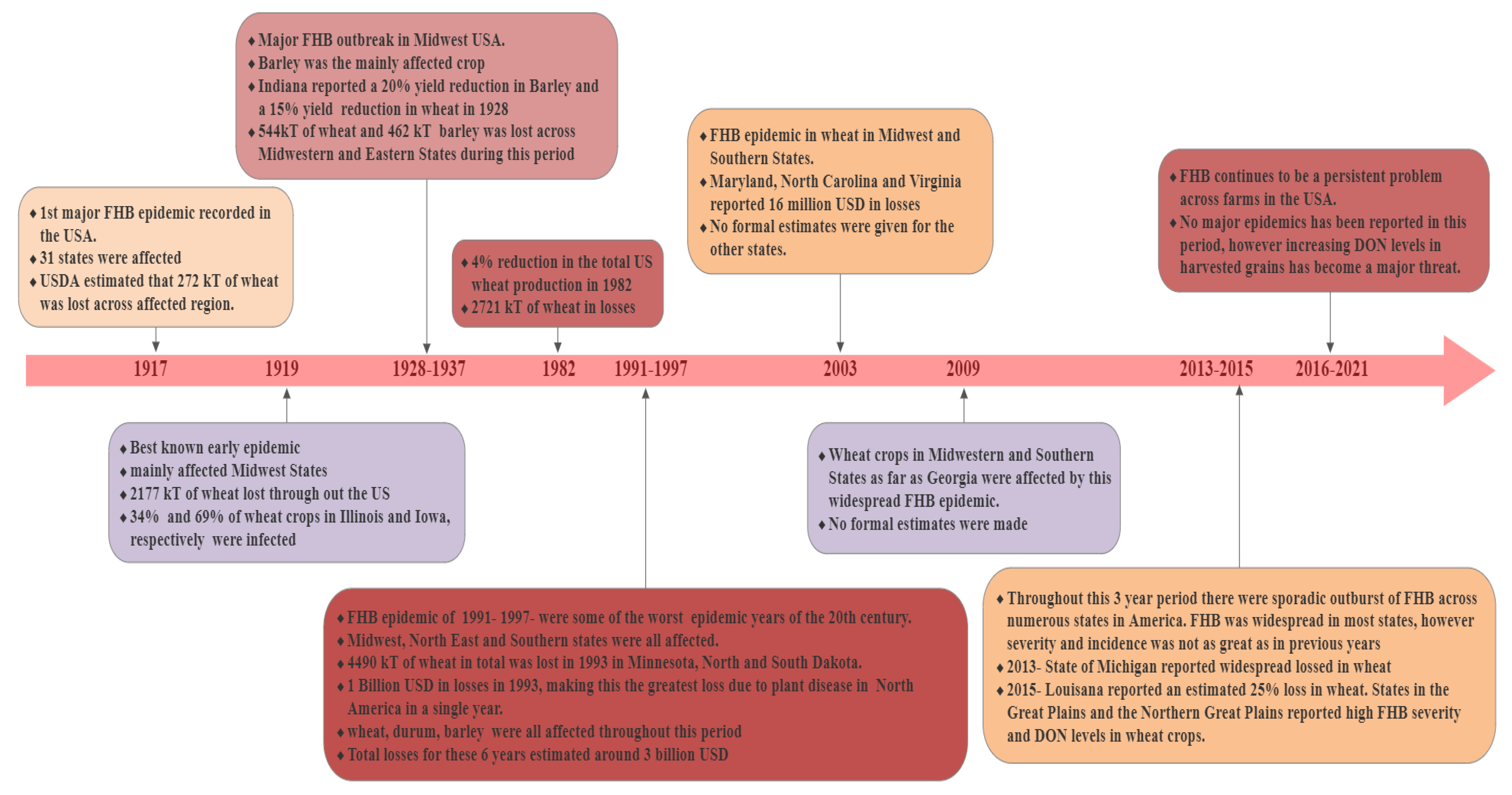
2.2. FHB in the US
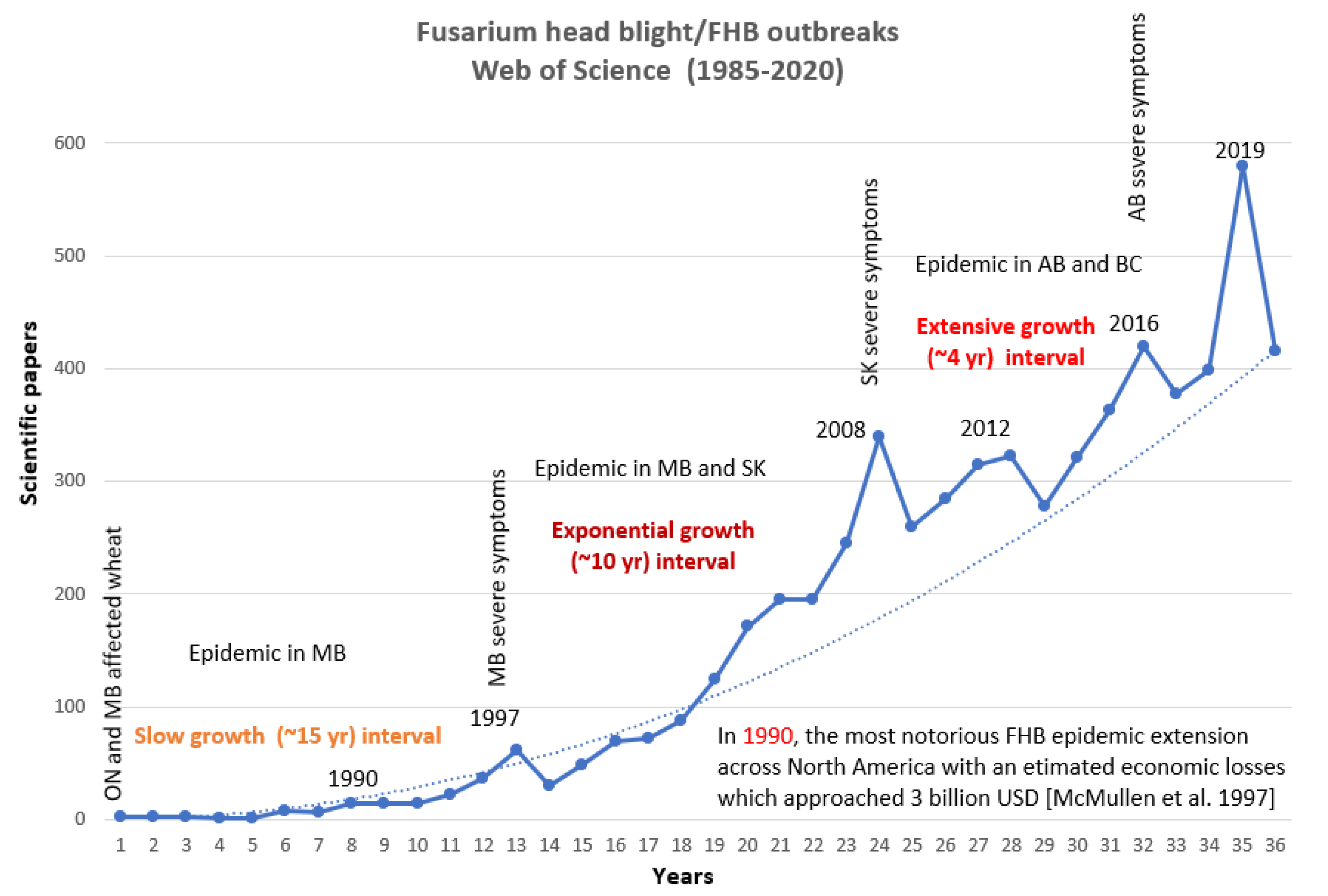
2.3. FHB in Canada
3. Mycotoxigenic Fusarium Pathogens
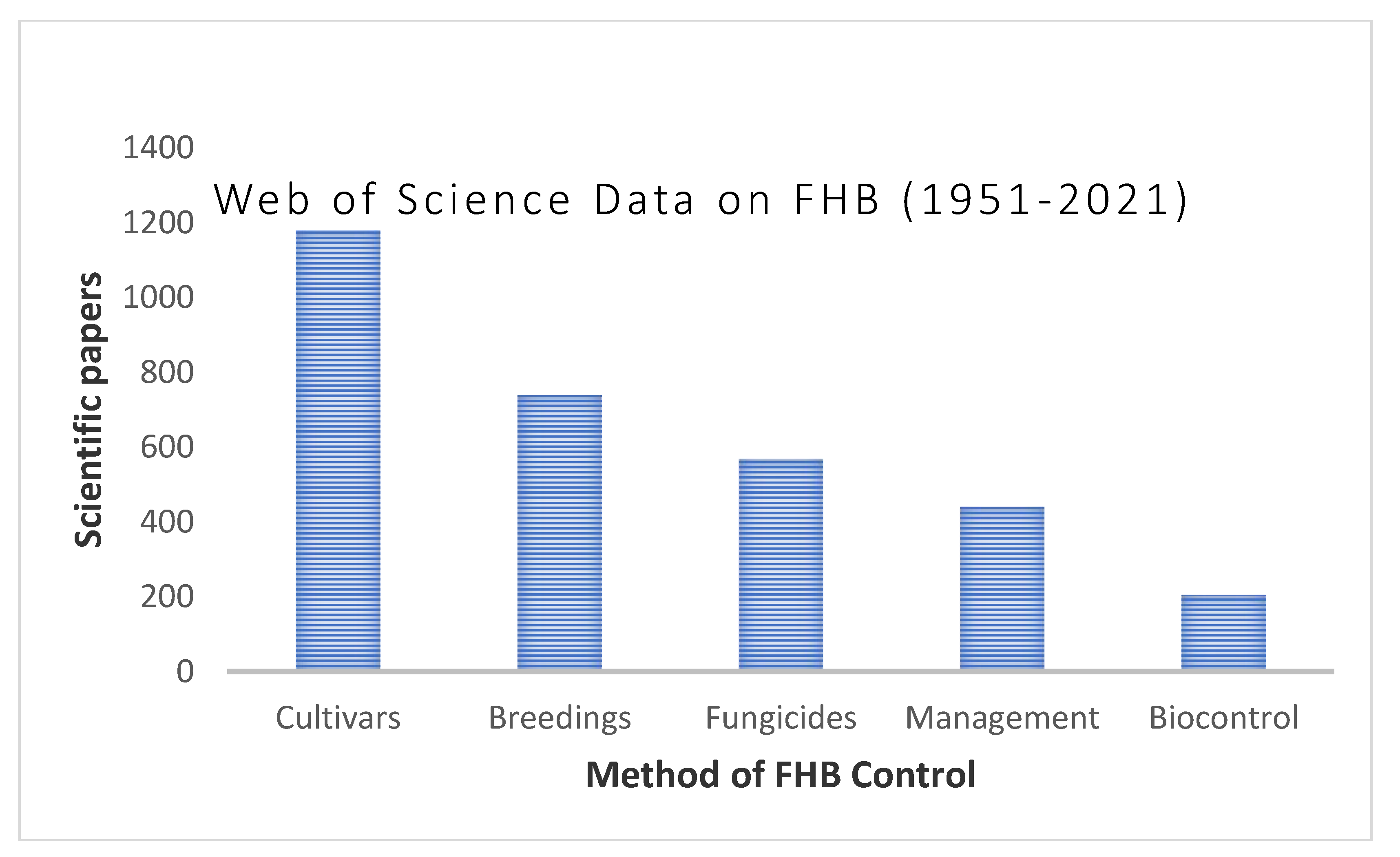
4. FHB Management
4.1. Chemical and Physical Control
4.2. Host Resistance
4.3. Conventional Breeding
4.4. Resistance in Common Wheat
4.5. Resistance in Durum Wheat
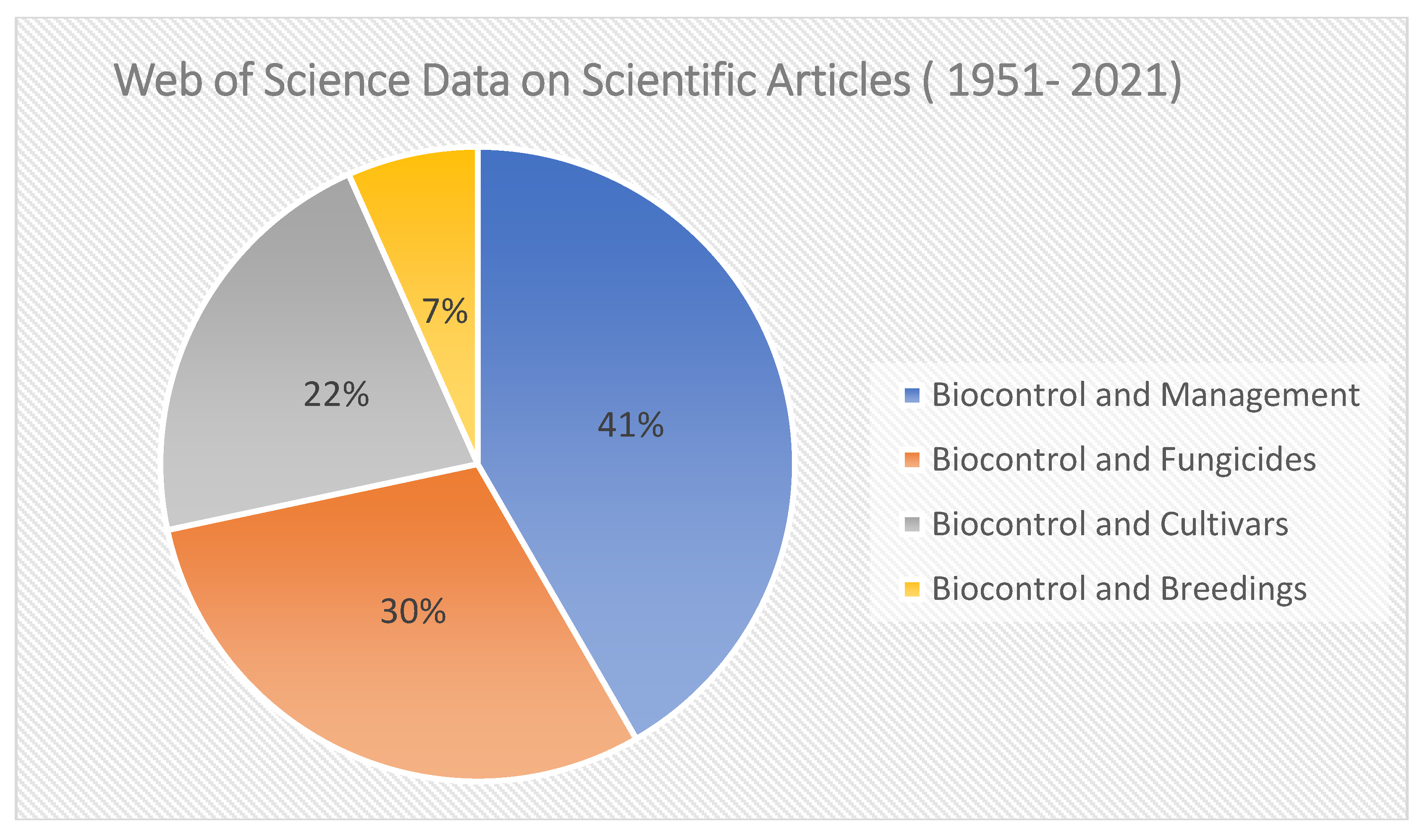
4.6. Biocontrol
4.7. Potential of Biocontrol Agent (BCA)-Resistant Crop Genotype (RCG) Protocooperation
5. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Voss-Fels, K.P.; Qian, L.; Gabur, I.; Obermeier, C.; Hickey, L.T.; Werner, C.R.; Kontowski, S.; Frisch, M.; Friedt, W.; Snowdon, R.J.; et al. Genetic insights into underground responses to Fusarium graminearum infection in wheat. Sci. Rep. 2018, 8, 13153. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Kistler, H.; Tacke, B.K.; Casper, H.H. Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc. Natl. Acad. Sci. USA 2000, 97, 7905–7910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, K.; Ward, T.J.; Aberra, D.; Kistler, H.C.; Aoki, T.; Orwig, N.; Kimura, M.; Bjørnstad, Å.; Klemsdal, S.S. Multilocus genotyping and molecular phylogenetics resolve a novel head blight pathogen within the Fusarium graminearum species complex from Ethiopia. Fungal Genet. Biol. 2008, 45, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Summerell, B.A.; Laurence, M.H.; Liew, E.C.Y.; Leslie, J.F. Biogeography and phylogeography of Fusarium: A review. Fungal Divers. 2010, 44, 3–13. [Google Scholar] [CrossRef]
- Ward, T.J.; Clear, R.M.; Rooney, A.; O’Donnell, K.; Gaba, D.; Patrick, S.; Starkey, D.E.; Gilbert, J.; Geiser, D.M.; Nowicki, T.W. An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America. Fungal Genet. Biol. 2008, 45, 473–484. [Google Scholar] [CrossRef]
- Nešić, K.; Habschied, K.; Mastanjević, K. Possibilities for the Biological Control of Mycotoxins in Food and Feed. Toxins 2021, 13, 198. [Google Scholar] [CrossRef]
- Shah, D.A.; De Wolf, E.D.; Paul, P.A.; Madden, L.V. Accuracy in the prediction of disease epidemics when ensembling simple but highly correlated models. PLoS Comput. Biol. 2021, 17, e1008831. [Google Scholar] [CrossRef]
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef] [Green Version]
- Hucl, P.; Briggs, C.; Graf, R.; Chibbar, R.N. Genetic Gains in Agronomic and Selected End-Use Quality Traits over a Century of Plant Breeding of Canada Western Red Spring Wheat. Cereal Chem. J. 2015, 92, 537–543. [Google Scholar] [CrossRef]
- Steiner, B.; Michel, S.; Maccaferri, M.; Lemmens, M.; Tuberosa, R.; Buerstmayr, H. Exploring and exploiting the genetic variation of Fusarium head blight resistance for genomic-assisted breeding in the elite durum wheat gene pool. Theor. Appl. Genet. 2019, 132, 969–988. [Google Scholar] [CrossRef] [Green Version]
- Sari, E.; Knox, R.E.; Ruan, Y.; Henriquez, M.A.; Kumar, S.; Burt, A.J.; Cuthbert, R.D.; Konkin, D.J.; Walkowiak, S.; Campbell, H.L.; et al. Historic recombination in a durum wheat breeding panel enables high-resolution mapping of Fusarium head blight resistance quantitative trait loci. Sci. Rep. 2020, 10, 7567. [Google Scholar] [CrossRef]
- Bollina, V.; Kumaraswamy, G.K.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S.; Faubert, D.; Hamzehzarghani, H. Mass spectrometry-based metabolomics application to identify quantitative resistance-related metabolites in barley against Fusarium head blight. Mol. Plant Pathol. 2010, 11, 769–782. [Google Scholar] [CrossRef]
- Cuperlovic-Culf, M.; Wang, L.; Forseille, L.; Boyle, K.; Merkley, N.; Burton, I.; Fobert, P.R. Metabolic Biomarker Panels of Response to Fusarium Head Blight Infection in Different Wheat Varieties. PLoS ONE 2016, 11, e20592. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Liu, Z.; Surendra, A.; Pan, Y.; Li, Y.; Zaharia, L.I.; Ouellet, T.; Fobert, P.R. Integrated transcriptome and hormone profiling highlight the role of multiple phytohormone pathways in wheat resistance against fusarium head blight. PLoS ONE 2018, 13, e0207036. [Google Scholar] [CrossRef]
- Maccaferri, M.; Harris, N.S.; Twardziok, S.O.; Pasam, R.K.; Gundlach, H.; Spannagl, M.; Ormanbekova, D.; Lux, T.; Prade, V.M.; Milner, S.G.; et al. Durum wheat genome highlights past domestication signatures and future improvement targets. Nat. Genet. 2019, 51, 885–895. [Google Scholar] [CrossRef] [Green Version]
- Walkowiak, S.; Gao, L.; Monat, C.; Haberer, G.; Kassa, M.T.; Brinton, J.; Ramirez-Gonzalez, R.H.; Kolodziej, M.C.; Delorean, E.; Thambugala, D.; et al. Multiple wheat genomes reveal global variation in modern breeding. Nat. Cell Biol. 2020, 588, 277–283. [Google Scholar] [CrossRef]
- Krattinger, S.; Keller, B. Molecular genetics and evolution of disease resistance in cereals. New Phytol. 2016, 212, 320–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wang, J.; Yang, N.; Wen, Z.; Sun, X.; Chai, Y.; Ma, Z. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 2018, 9, 3429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Lahlali, R.; Karunakaran, C.; Vujanovic, V. Specific Mycoparasite-Fusarium Graminearum Molecular Signatures in Germinating Seeds Disabled Fusarium Head Blight Pathogen’s Infection. Int. J. Mol. Sci. 2021, 22, 2461. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shi, Q.; Yuan, J.; Wang, M.; Wang, J.; Wang, C.; Zhang, J.; Fu, S.; Su, H.; Liu, Y.; et al. Alien chromatin but not Fhb7 confers Fusarium head blight resistance in wheat breeding. bioRxiv 2021. [Google Scholar] [CrossRef]
- Rampersad, S.N. Pathogenomics and Management of Fusarium Diseases in Plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, A.C.; Castroverde, C.D.; He, S.Y. Plant–Pathogen Warfare under Changing Climate Conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight from a Microbiome Perspective. Front. Microbiol. 2021, 12, 628373. [Google Scholar] [CrossRef]
- Smith, W.G. Diseases of Field and Garden Crops: Chiefly Such as Caused by Fungi; Macmillian: London, UK, 1884; pp. 208–213. [Google Scholar]
- Bal, G. Scab of Wheat: Prospects For Control. Plant Dis. 1994, 78, 760–766. [Google Scholar] [CrossRef]
- Champeil, A.; Doré, T.; Fourbet, J.F. Fusarium head blight: Epidemiological origin of the effects of cultural prac-tices on head blight attacks and the production of mycotoxins by Fusarium in wheat grains. Plant Sci. 2004, 166, 1389–1415. [Google Scholar] [CrossRef]
- McMullen, M.; Jones, R.; Gallenberg, D. Scab of Wheat and Barley: A Re-emerging Disease of Devastating Impact. Plant Dis. 1997, 81, 1340–1348. [Google Scholar] [CrossRef] [Green Version]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A Unified Effort to Fight an Enemy of Wheat and Barley: Fusarium Head Blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef] [Green Version]
- Moschini, R.C.; Fortugno, C. Predicting wheat head blight incidence using models based on meteorological factors in Pergamino, Argentina. Eur. J. Plant Pathol. 1996, 102, 211–218. [Google Scholar] [CrossRef]
- Snijders, C.H.A. Genetic variation for resistance to Fusarium head blight in bread wheat. Euphytica 1990, 50, 171–179. [Google Scholar] [CrossRef]
- Stack, R.W. Return of an Old Problem: Fusarium Head Blight of Small Grains. Plant Health Prog. 2000, 1, 19. [Google Scholar] [CrossRef]
- Stack, R.W. History of Fusarium Head Blight with emphasis on North America. In Fusarium Head Blight of Wheat and Barley; Leonard, K.J., Bushnell, W.R., Eds.; APS Press: St. Paul, MN, USA, 2003; pp. 1–34. [Google Scholar]
- Tekauz, A. History of FHB research in (western) Canada. In Proceedings of the 9th Canadian Workshop on Fusarium Head Blight, Winnipeg, MB, Canada, 22 November 2018; Available online: https://static1.squarespace.com/static/56be29e022482ec146a7c5b8/t/5bfed9768985834c7e47a1fb/1543428484162/Plenary+C+Tekauz.pdf (accessed on 18 March 2021).
- Clear, R.; Patrick, S.; Mycology, Grain Research Laboratory, Canadian Grain Commission. Fusarium Head Blight in Western Canada. 2018. Available online: https://grainscanada.gc.ca/en/grain-research/scientific-reports/fhb-western/fhb-1.html (accessed on 20 November 2020).
- Khan, M.K.; Pandey, A.; Athar, T.; Athar, T.; Choudhary, S.; Deval, R.; Gezgin, S.; Hamurcu, M.; Topal, A.; Atmaca, E.; et al. Fusarium head blight in wheat: Contemporary status and molecular ap-proaches. Biotech 2020, 10, 172. [Google Scholar] [CrossRef]
- Fernando, W.D.; Oghenekaro, A.O.; Tucker, J.R.; Badea, A. Building on a foundation: Advances in epidemiology, resistance breeding, and forecasting research for reducing the impact of fusarium head blight in wheat and barley. Can. J. Plant Pathol. 2021, 43, 495–526. [Google Scholar] [CrossRef]
- Starkey, D.E.; Ward, T.J.; Aoki, T.; Gale, L.R.; Kistler, H.; Geiser, D.M.; Suga, H.; Tóth, B.; Varga, J.; O’Donnell, K. Global molecular surveillance reveals novel Fusarium head blight species and trichothecene toxin diversity. Fungal Genet. Biol. 2007, 44, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Yli-Mattila, T.; Gagkaeva, T.; Ward, T.J.; Aoki, T.; Kistler, H.C.; O’Donnell, K. A novel Asian clade within the Fusarium graminearum species complex includes a newly discovered cereal head blight pathogen from the Russian Far East. Mycologia 2009, 101, 841–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarver, B.A.; Ward, T.J.; Gale, L.R.; Broz, K.; Kistler, H.C.; Aoki, T.; Nicholson, P.; Carter, J.; O’Donnell, K. Novel Fusarium head blight pathogens from Nepal and Louisiana revealed by multilocus genealogical concordance. Fungal Genet. Biol. 2011, 48, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.J.; Bielawski, J.; Kistler, H.; Sullivan, E.; O’Donnell, K. Ancestral polymorphism and adaptive evolution in the trichothecene mycotoxin gene cluster of phytopathogenic Fusarium. Proc. Natl. Acad. Sci. USA 2002, 99, 9278–9283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Suga, H.; Karugia, G.W.; Ward, T.; Gale, L.R.; Tomimura, K.; Nakajima, T.; Miyasaka, A.; Koizumi, S.; Kageyama, K.; Hyakumachi, M. Molecular Characterization of the Fusarium graminearum Species Complex in Japan. Phytopathology 2008, 98, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.-B.; Sun, J.-T.; Yu, M.-Z.; Xu, J.-H.; Shi, J.-R. Temporal dynamics, population characterization and mycotoxins accumulation of Fusarium graminearum in Eastern China. Sci. Rep. 2016, 6, 36350. [Google Scholar] [CrossRef]
- Talas, F.; Longin, F.; Miedaner, T. Sources of resistance to Fusarium head blight within Syrian durum wheat landraces. Plant Breed. 2011, 130, 398–400. [Google Scholar] [CrossRef]
- Wagacha, J.M.; Steiner, U.; Dehne, H.-W.; Zuehlke, S.; Spiteller, M.; Muthomi, J.; Oerke, E.-C. Diversity in myco-toxins and fungal species infecting wheat in Nakuru District, Kenya. J. Phytopathol. 2010, 158, 527–535. [Google Scholar] [CrossRef]
- Bai, G.; Shaner, G. Management and resistance in wheat and barley to fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef]
- Legrand, F.; Picot, A.; Díaz, J.F.C.; Chen, W.; Le Floch, G. Challenges facing the biological control strategies for the management of Fusarium Head Blight of cereals caused by F. graminearum. Biol. Control 2017, 113, 26–38. [Google Scholar] [CrossRef]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases-A field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar] [CrossRef]
- Gilbert, J.; Tekauz, A. Strategies for management of fusarium head blight (FHB) in cereals. Prairie Soils Crop J. 2011, 4, 97–104. Available online: https://prairiesoilsandcrops.ca/articles/volume-4-11-screen.pdf (accessed on 22 March 2021).
- Statistics Canada (STC) and Agriculture and AgriFood Canada. Canada: Historical Supply and Disposition, Crops 2017–2018 to 2018–2019. Available online: https://aimis-simia.agr.gc.ca/rp/index-eng.cfm?action=pR&r=244&lang=EN (accessed on 27 March 2021).
- Windels, C.E. Economic and Social Impacts of Fusarium Head Blight: Changing Farms and Rural Communities in the Northern Great Plains. Phytopathology 2000, 90, 17–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, R.; Urban, M.; Hammond-Kosack, M.C.U.; Hassani-Pak, K.; Hammond-Kosack, K.E. The completed genome sequence of the pathogenic ascomycete fungus Fusarium graminearum. BMC Genom. 2015, 16, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Hao, Y.; Mergoum, M.; Bai, G.; Humphreys, G.; Cloutier, S.; Xia, X.; He, Z. Breeding wheat for resistance to Fusarium head blight in the Global North: China, USA, and Canada. Crop J. 2019, 7, 730–738. [Google Scholar] [CrossRef]
- Wegulo, S.N.; Baenziger, P.S.; Nopsa, J.F.H.; Bockus, W.W.; Hallen-Adams, H. Management of Fusarium head blight of wheat and barley. Crop Prot. 2015, 73, 100–107. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef]
- Aboukhaddour, R.; Fetch, T.; McCallum, B.D.; Harding, M.W.; Beres, B.L.; Graf, R.J. Wheat diseases on the prairies: A Canadian story. Plant Pathol. 2020, 69, 418–432. [Google Scholar] [CrossRef]
- Clear, R.; Patrick, S. Fusarium head blight pathogens isolated from fusarium-damaged kernels of wheat in western Canada, 1993 to 1998. Can. J. Plant Pathol. 2000, 22, 51–60. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Roscoe, M.; Trelka, R.; Gaba, D.; Chan, J.M.; Patrick, S.K.; Sulyok, M.; Krska, R.; McKendry, T.; Gräfenhan, T. Fusarium Damage in Small Cereal Grains from Western Canada. 2. Occurrence of Fusarium Toxins and Their Source Organisms in Durum Wheat Harvested in 2010. J. Agric. Food Chem. 2013, 61, 5438–5448. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Blagden, R.; Chan, J.; Gaba, D.; McKendry, T.; Pleskach, K.; Roscoe, M. Fusarium and Alternaria mycotoxins present in Canadian wheat and durum harvest samples. Can. J. Plant Pathol. 2019, 41, 403–414. [Google Scholar] [CrossRef]
- Web of Science Database. Available online: https://apps-webofknowledge-com.cyber.usask.ca/ (accessed on 4 May 2021).
- Miller, J.D. Epidemology of Fusarium ear diseases. In Mycotoxins in Grain: Compounds other than Aflatoxins; Mil-ler, J.D., Trenholm, H.L., Eds.; Eagan Press: St. Paul, MN, USA, 1994; pp. 19–36. [Google Scholar]
- Dean, R.; van Kan, J.; Pretorius, Z.A.; Hammond-Kosack, K.; Di Pietro, A.; Spanu, P.; Rudd, J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dweba, C.; Figlan, S.; Shimelis, H.; Motaung, T.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Kim, S.H.; Vujanovic, V. Relationship between mycoparasites lifestyles and biocontrol behaviors against Fusarium spp. and mycotoxins production. Appl. Microbiol. Biotechnol. 2016, 100, 5257–5272. [Google Scholar] [CrossRef]
- Desjardins, A.E. Fusarium mycotoxins: Chemistry, genetics, and biology. Int. J. Food Microbiol. 2006, 119, 47–50. [Google Scholar] [CrossRef]
- Canadian Phytopathological Society. Canadian Plant Disease Survey. 1923. Available online: phytopath.ca/publication/cpds (accessed on 12 January 2021).
- Canadian Grain Commission. Fusarium Head Blight in Western Canada. Available online: https://grainscanada.gc.ca/en/grain-research/scientific-reports/fhb-western/fhb-2.html (accessed on 12 January 2021).
- Jin, F.; Bai, G.; Zhang, D.; Dong, Y.; Ma, L.; Bockus, W.; Dowell, F. Fusarium-Damaged Kernels and Deoxynivalenol in Fusarium-Infected U.S. Winter Wheat. Phytopathology 2014, 104, 472–478. [Google Scholar] [CrossRef] [Green Version]
- Schaafsma, A.W.; Hooker, D. Climatic models to predict occurrence of Fusarium toxins in wheat and maize. Int. J. Food Microbiol. 2007, 119, 116–125. [Google Scholar] [CrossRef]
- Amarasinghe, C.C.; Fernando, W.G.D. Comparative Analysis of Deoxynivalenol Biosynthesis Related Gene Expression among Different Chemotypes of Fusarium graminearum in Spring Wheat. Front. Microbiol. 2016, 7, 1229. [Google Scholar] [CrossRef]
- Azam, M.S.; Yu, D.; Wu, A. Enzymes for degradation of Fusarium mycotoxins. In Food Safety & Mycotoxins; Wu, A., Ed.; Springer: Singapore, 2019; pp. 113–135. [Google Scholar] [CrossRef]
- Gilbert, J.; Haber, S. Overview of some recent research developments in fusarium head blight of wheat. Can. J. Plant Pathol. 2013, 35, 149–174. [Google Scholar] [CrossRef]
- Dawson, A. Fusarium Concerns Reach New Heights. In The Western Producer—SaskSeed Guide 2017; Western Producer: Saskatoon, SK, Canada; pp. 22–24. Available online: https://static.producer.com/wp-content/uploads/2017/01/2017_SK_Seed_Guide.pdf (accessed on 16 April 2021).
- Ji, C.; Fan, Y.; Zhao, L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.N.; Cotnoir, P.-A.; Leroux, T.; Laprade, R.; Schwartz, J.-L. A Canadian national survey on the public perception of biological control. BioControl 2010, 55, 445–454. [Google Scholar] [CrossRef]
- Paulitz, T.C.; Bélanger, R.R. Biologicalcontrol ingreenhousesystems. Annu. Rev. Phytopathol. 2001, 39, 103–133. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Fernando, W.G.D. Epidemiology and biological control of Gibberella zeae/Fusarium graminearum. Can. J. Plant Pathol. 2014, 26, 464–472. [Google Scholar] [CrossRef]
- Miller, J.D.; Young, J.C.; Sampson, D.R. Deoxynivalenol and Fusarium Head Blight Resistance in Spring Cereals. J. Phytopathol. 1985, 113, 359–367. [Google Scholar] [CrossRef]
- Shaner, G.E. Epidemiology of Fusarium head blight of small grain cereals in North America. In Fusarium Head Blight of Wheat and Barley; Leonard, K.J., Bushnell, W.R., Eds.; APS Press: St. Paul, MN, USA, 2003; pp. 84–119. [Google Scholar]
- Wollenberg, R.D.; Taft, M.H.; Giese, S.; Thiel, C.; Balázs, Z.; Giese, H.; Manstein, D.J.; Sondergaard, T.E. Phenamacril is a reversible and noncompetitive inhibitor of Fusarium class I myosin. J. Biol. Chem. 2019, 294, 1328–1337. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.-P.; Qu, X.-P.; Mao, X.-W.; Kuang, J.; Duan, Y.-B.; Song, X.-S.; Wang, J.-X.; Chen, C.-J.; Zhou, M.-G. Resistance mechanism of Fusarium fujikuroi to phenamacril in the field. Pest Manag. Sci. 2018, 74, 607–616. [Google Scholar] [CrossRef]
- Hou, Y.-P.; Mao, X.-W.; Wang, J.-X.; Zhan, S.-W.; Zhou, M.-G. Sensitivity of Fusarium asiaticum to a novel succinate dehydrogenase inhibitor fungicide pydiflumetofen. Crop Prot. 2017, 96, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Nagelkirk, M.; Chilvers, M. Managing Fusarium Head Blight in Winter Wheat. MSU—Michigan Wheat Program and the U.S. Wheat and Barley Head Scab Initiative. 2020. Available online: https://www.canr.msu.edu/wheat/disease/FHB%20fact%20sheet%202020%20revised%20MN_MC_Final%202.pdf. (accessed on 18 March 2021).
- Zhang, Y.-J.; Yu, J.-J.; Zhang, X.; Cheng, C.-J.; Wang, J.-X.; Hollomon, D.W.; Fan, P.-S.; Zhou, M.-G. Effect of Carbendazim Resistance on Trichothecene Production and Aggressiveness of Fusarium graminearum. Mol. Plant Microbe Interact. 2009, 22, 1143–1150. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chen, X.; Jiang, J.; Hamada, M.S.; Yin, Y.; Ma, Z. Detection and dynamics of different carbendazim-resistance conferring β-tubulin variants of Gibberella zeae collected from infected wheat heads and rice stubble in China. Pest Manag. Sci. 2014, 70, 1228–1236. [Google Scholar] [CrossRef]
- Spolti, P.; Del Ponte, E.M.; Dong, Y.; Cummings, J.; Bergstrom, G.C. Triazole Sensitivity in a Contemporary Population of Fusarium graminearum from New York Wheat and Competitiveness of a Tebuconazole-Resistant Isolate. Plant Dis. 2014, 98, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Paul, P.A.; Lipps, P.E.; Hershman, D.E.; McMullen, M.P.; Draper, M.A.; Madden, L. Efficacy of Triazole-Based Fungicides for Fusarium Head Blight and Deoxynivalenol Control in Wheat: A Multivariate Meta-Analysis. Phytopathology 2008, 98, 999–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A.J. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Stoev, S.D. Food Safety and Increasing Hazard of Mycotoxin Occurrence in Foods and Feeds. Crit. Rev. Food Sci. Nutr. 2013, 53, 887–901. [Google Scholar] [CrossRef]
- Anderson, J.A.; Stack, R.W.; Liu, S.; Waldron, B.L.; Fjeld, A.D.; Coyne, C.; Moreno-Sevilla, B.; Fetch, J.M.; Song, Q.J.; Cregan, P.B.; et al. DNA markers for Fusarium head blight resistance QTLs in two wheat populations. Theor. Appl. Genet. 2001, 102, 1164–1168. [Google Scholar] [CrossRef]
- Bai, G.; Su, Z.; Cai, J. Wheat resistance to Fusarium head blight. Can. J. Plant Pathol. 2018, 40, 336–346. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Ban, T.; A Anderson, J. QTL mapping and marker-assisted selection forFusariumhead blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Christensen, J.J.; Stakman, E.C.; Immer, F.R. Susceptibility of wheat varieties and hybrids to Fusarial Head Blight in Minnesota. Minn. Agric. Exp. Stn Bull. 1929, 59, 1–29. [Google Scholar]
- Gilbert, J.; Tekauz, A. Review: Recent developments in research on fusarium head blight of wheat in Canada. Can. J. Plant Pathol. 2000, 22, 1–8. [Google Scholar] [CrossRef]
- Wulff, B.B.H.; Jones, J.D.G. Breeding a fungal gene into wheat. Science 2020, 368, 822–823. [Google Scholar] [CrossRef]
- Atanasoff, D. Fusarium blight (scab) of wheat and other cereals. J. Agric. Res. 1920, 20, 1–32. [Google Scholar]
- Haile, J.K.; N’Diaye, A.; Walkowiak, S.; Nilsen, K.T.; Clarke, J.M.; Kutcher, H.R.; Steiner, B.; Buerstmayr, H.; Pozniak, C.J. Fusarium Head Blight in Durum Wheat: Recent Status, Breeding Directions, and Future Research Prospects. Phytopathology 2019, 109, 1664–1675. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Xie, Q.; Li, G.; Jia, H.; Zhou, J.; Kong, Z.; Li, N.; Yuan, Y. Germplasms, genetics and genomics for better control of disastrous wheat Fusarium head blight. Theor. Appl. Genet. 2020, 133, 1541–1568. [Google Scholar] [CrossRef]
- Mesterhazy, A. Types and components of resistance to Fusarium head blight of wheat. Plant Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Pirgozliev, S.R.; Edwards, S.; Hare, M.C.; Jenkinson, P. Strategies for the Control of Fusarium Head Blight in Cereals. Eur. J. Plant Pathol. 2003, 109, 731–742. [Google Scholar] [CrossRef]
- Bai, G.; Kolb, F.L.; Shaner, G.; Domier, L.L. Amplified Fragment Length Polymorphism Markers Linked to a Major Quantitative Trait Locus Controlling Scab Resistance in Wheat. Phytopathology 1999, 89, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Cuthbert, P.A.; Somers, D.J.; Thomas, J.; Cloutier, S.; Brulé-Babel, A. Fine mapping Fhb1, a major gene controlling fusarium head blight resistance in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2006, 112, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.W.; Christensen, J.J. Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology 1963, 53, 831–838. [Google Scholar]
- Anderson, J.A. Marker-assisted selection for Fusarium head blight resistance in wheat. Int. J. Food Microbiol. 2007, 119, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Collard, B.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2007, 363, 557–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnappa, G.; Savadi, S.; Tyagi, B.S.; Singh, S.K.; Mamrutha, H.M.; Kumar, S.; Mishra, C.N.; Khan, H.; Gangadhara, K.; Uday, G.; et al. Integrated genomic selection for rapid improvement of crops. Genomics 2021, 113, 1070–1086. [Google Scholar] [CrossRef]
- Rai, N.; Bellundagi, A.; Kumar, P.K.C.; Thimmappa, R.K.; Rani, S.; Sinha, N.; Krishna, H.; Jain, N.; Singh, G.P.; Singh, P.K.; et al. Marker-assisted backcross breeding for improvement of drought tolerance in bread wheat (Triticum aestivum L. em Thell). Plant Breed. 2018, 137, 514–526. [Google Scholar] [CrossRef]
- Yang, Z.; Gilbert, J.; Somers, D.; Fedak, G.; Procunier, J.; McKenzie, I. Marker Assisted Selection of Fusarium Head Blight Resistance Genes in Two Doubled Haploid Populations of Wheat. Mol. Breed. 2003, 12, 309–317. [Google Scholar] [CrossRef]
- Anderson, J.A.; Chao, S.; Liu, S. Molecular Breeding Using a Major QTL for Fusarium Head Blight Resistance in Wheat. Crop Sci. 2007, 47, S-112–S-119. [Google Scholar] [CrossRef]
- Pumphrey, M.; Bernardo, R.; Anderson, J.A. Validating the Fhb1 QTL for Fusarium Head Blight Resistance in Near-Isogenic Wheat Lines Developed from Breeding Populations. Crop Sci. 2007, 47, 200–206. [Google Scholar] [CrossRef]
- Anderson, J.A.; Wiersma, J.J.; Linkert, G.L.; Kolmer, J.A.; Jin, Y.; Dill-Macky, R.; Wiersma, J.V.; Hareland, G.A. Registration of ‘Sabin’ Wheat. J. Plant Regist. 2012, 6, 174–179. [Google Scholar] [CrossRef]
- Löffler, M.; Schön, C.-C.; Miedaner, T. Revealing the genetic architecture of FHB resistance in hexaploid wheat (Triticum aestivum L.) by QTL meta-analysis. Mol. Breed. 2009, 23, 473–488. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Pumphrey, M.; Stack, R.W.; Gill, B.S.; Anderson, J.A. Complex microcolinearity among wheat, rice, and barley revealed by fine mapping of the genomic region harboring a major QTL for resistance to Fusarium head blight in wheat. Funct. Integr. Genom. 2005, 6, 83–89. [Google Scholar] [CrossRef]
- Cuthbert, P.A.; Somers, D.J.; Brulé-Babel, A. Mapping of Fhb2 on chromosome 6BS: A gene controlling Fusarium head blight field resistance in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 114, 429–437. [Google Scholar] [CrossRef]
- Xue, S.; Li, G.; Jia, H.; Xu, F.; Lin, F.; Tang, M.; Wang, Y.; An, X.; Xu, H.; Zhang, L.; et al. Fine mapping Fhb4, a major QTL conditioning resistance to Fusarium infection in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2010, 121, 147–156. [Google Scholar] [CrossRef]
- Jia, H.; Zhou, J.; Xue, S.; Li, G.; Yan, H.; Ran, C.; Zhang, Y.; Shi, J.; Jia, L.; Wang, X.; et al. A journey to understand wheat Fusarium head blight resistance in the Chinese wheat landrace Wangshuibai. Crop J. 2018, 6, 48–59. [Google Scholar] [CrossRef]
- Xue, S.; Xu, F.; Tang, M.; Zhou, Y.; Li, G.; An, X.; Lin, F.; Xu, H.; Jia, H.; Zhang, L.; et al. Precise mapping Fhb5, a major QTL conditioning resistance to Fusarium infection in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2011, 123, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, R.; Yuan, Y.; Anderson, J.; Pumphrey, M.; Zhang, Z.; Chen, J. Evaluation of the Potential for Genomic Selection to Improve Spring Wheat Resistance to Fusarium Head Blight in the Pacific Northwest. Front. Plant Sci. 2018, 9, 911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arruda, M.P.; Brown, P.J.; Lipka, A.E.; Krill, A.M.; Thurber, C.; Kolb, F.L. Genomic Selection for Predicting Fusarium Head Blight Resistance in a Wheat Breeding Program. Plant Genome 2015, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arruda, M.P.; Lipka, A.E.; Brown, P.J.; Krill, A.M.; Thurber, C.; Brown-Guedira, G.; Dong, Y.; Foresman, B.J.; Kolb, F.L. Comparing genomic selection and marker-assisted selection for Fusarium head blight resistance in wheat (Triticum aestivum L.). Mol. Breed. 2016, 36, 84. [Google Scholar] [CrossRef]
- Gill, B.S.; Appels, R.; Botha, A.-M.; Buell, C.R.; Bennetzen, J.L.; Chalhoub, B.; Chumley, F.; Dvořák, J.; Iwanaga, M.; Keller, B.; et al. A Workshop Report on Wheat Genome Sequencing: International Genome Research on Wheat Consortium. Genetics 2004, 168, 1087–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prat, N.; Guilbert, C.; Prah, U.; Wachter, E.; Steiner, B.; Langin, T.; Robert, O.; Buerstmayr, H. QTL mapping of Fusarium head blight resistance in three related durum wheat populations. Theor. Appl. Genet. 2017, 130, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Szabo-Hever, A.; Zhang, Q.; Friesen, T.L.; Zhong, S.; Elias, E.M.; Cai, X.; Jin, Y.; Faris, J.D.; Chao, S.; Xu, S.S. Genetic Diversity and Resistance to Fusarium Head Blight in Synthetic Hexaploid Wheat Derived From Aegilops tauschii and Diverse Triticum turgidum Subspecies. Front. Plant Sci. 2018, 9, 1829. [Google Scholar] [CrossRef] [PubMed]
- Miedaner, T.; Wilde, F.; Steiner, B.; Buerstmayr, H.; Korzun, V.; Ebmeyer, E. Stacking quantitative trait loci (QTL) for Fusarium head blight resistance from non-adapted sources in an European elite spring wheat background and assessing their effects on deoxynivalenol (DON) content and disease severity. Theor. Appl. Genet. 2005, 112, 562–569. [Google Scholar] [CrossRef]
- Zhao, M.; Leng, Y.; Chao, S.; Xu, S.S.; Zhong, S. Molecular mapping of QTL for Fusarium head blight resistance introgressed into durum wheat. Theor. Appl. Genet. 2018, 131, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Buerstmayr, M.; Matiasch, L.; Mascher, F.; Vida, G.; Ittu, M.; Robert, O.; Holdgate, S.; Flath, K.; Neumayer, A.; Buerstmayr, H. Mapping of quantitative adult plant field resistance to leaf rust and stripe rust in two European winter wheat populations reveals co-location of three QTL conferring resistance to both rust pathogens. Theor. Appl. Genet. 2014, 127, 2011–2028. [Google Scholar] [CrossRef] [Green Version]
- Giancaspro, A.; Giove, S.L.; Zito, D.; Blanco, A.; Gadaleta, A. Mapping QTLs for Fusarium Head Blight Resistance in an Interspecific Wheat Population. Front. Plant Sci. 2016, 7, 1381. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Lemmens, M.; Hartl, L.; Doldi, L.; Steiner, B.; Stierschneider, M.; Ruckenbauer, P. Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat. I. Resistance to fungal spread (Type II resistance). Theor. Appl. Genet. 2002, 104, 84–91. [Google Scholar] [CrossRef]
- Mesterhazy, A. Breeding wheat for Fusarium head blight resistance in Europe. In Fusarium Head Blight of Wheat and Barley; Leonard, K.J., Bushnell, W.R., Eds.; APS Press: St. Paul, MN, USA, 2003; pp. 211–240. [Google Scholar]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glössl, J.; Luschnig, C.; Adam, G. Detoxification of the Fusarium Mycotoxin Deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef] [Green Version]
- Shude, S.P.; Yobo, K.S.; Mbili, N.C. Progress in the management of Fusarium head blight of wheat: An overview. S. Afr. J. Sci. 2020, 116, 1–7. [Google Scholar] [CrossRef]
- Wei, Z.; Jousset, A. Plant breeding goes microbial. Trends Plant Sci. 2020, 22, 555–558. [Google Scholar] [CrossRef]
- Gnanamanickam, S.S.; Vasudevan, P.; Reddy, M.S.; Defago, G.; Kloepper, J. Principles of biological control. In Biological Control of Crop Diseases; Springer: New York, NY, USA, 2002; pp. 1–9. [Google Scholar]
- Pal, K.K.; Gardener, B.M. Biological Control of Plant Pathogens. Plant Health Instr. 2006. [Google Scholar] [CrossRef] [Green Version]
- Vujanovic, V. Fusarium and Fusarium Mycotoxin Biocontrol. PCT: WO2011/022809Al. 2011. Available online: https://patentimages.storage.googleapis.com/b5/54/bf/2c20fbe0ba1f48/WO2011022809A1.pdf (accessed on 3 May 2021).
- Roberti, R.; Veronesi, A.; Cesari, A.; Cascone, A.; Di Berardino, I.; Bertini, L.; Caruso, C. Induction of PR proteins and resistance by the biocontrol agent Clonostachys rosea in wheat plants infected with Fusarium culmorum. Plant Sci. 2008, 175, 339–347. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.K.; Horwitz, B.A.; Herrera-Estrella, A.; Schmoll, M.; Kenerley, C.M. Trichoderma Research in the Genome Era. Annu. Rev. Phytopathol. 2013, 51, 105–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Vujanovic, V. Biodegradation and biodetoxification of Fusarium mycotoxins by Sphaerodes mycoparasitica. AMB Express 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vujanovic, V. Tremellomycetes Yeasts in Kernel Ecological Niche: Early Indicators of Enhanced Competitiveness of Endophytic and Mycoparasitic Symbionts against Wheat Pathobiota. Plants 2021, 10, 905. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.R.; Weston, G.E.; Turner, J.A.; Jennings, P.; Nicholson, P. Differential Control of Head Blight Pathogens of Wheat by Fungicides and Consequences for Mycotoxin Contamination of Grain. Eur. J. Plant Pathol. 2001, 107, 421–431. [Google Scholar] [CrossRef]
- He, J.W.; Bondy, G.S.; Zhou, T.; Caldwell, D.; Boland, G.J.; Scott, P.M. Toxicology of 3-epi-deoxynivalenol, a deoxynivalenol-transformation product by Devosia mutans 17-2-E-8. Food Chem. Toxicol. 2015, 84, 250–259. [Google Scholar] [CrossRef]
- Ikunaga, Y.; Sato, I.; Grond, S.; Numaziri, N.; Yoshida, S.; Yamaya, H.; Hiradate, S.; Hasegawa, M.; Toshima, H.; Koitabashi, M.; et al. Nocardioides sp. strain WSN05-2, isolated from a wheat field, degrades deoxynivalenol, producing the novel intermediate 3-epi-deoxynivalenol. Appl. Microbiol. Biotechnol. 2011, 89, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Kollarczik, B.; Gareis, M.; Hanelt, M. In vitro transformation of theFusarium mycotoxins deoxynivalenol and zearalenone by the normal gut microflora of pigs. Nat. Toxins 1994, 2, 105–110. [Google Scholar] [CrossRef]
- Tan, H.; Hu, Y.; He, J.; Wu, L.; Liao, F.; Luo, B.; He, Y.; Zuo, Z.; Ren, Z.; Zhong, Z.; et al. Zearalenone degradation by two Pseudomonas strains from soil. Mycotoxin Res. 2014, 30, 191–196. [Google Scholar] [CrossRef]
- Yu, Y.; Qiu, L.; Wu, H.; Tang, Y.; Yu, Y.; Li, X.; Liu, D. Degradation of zearalenone by the extracellular extracts of Acinetobacter sp. SM04 liquid cultures. Biodegradation 2011, 22, 613–622. [Google Scholar] [CrossRef]
- Vujanovic, V.; Goh, Y.K. Sphaerodes mycoparasitica sp. nov., a new biotrophic mycoparasite on Fusarium avenaceum, F. graminearum and F. oxysporum. Mycol. Res. 2009, 113, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Busko, M.; Chełkowski, J.; Popiel, D.; Perkowski, J. Solid substrate bioassay to evaluate impact of Trichoderma on trichothecene mycotoxin production byFusarium species. J. Sci. Food Agric. 2008, 88, 536–541. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Popiel, D.; Kwaśny, H.; Chełkowski, J.; Stępień, Ł.; Laskowska, M. Impact of selected antagonistic fungi on Fusarium species—Toxigenic cereal pathogens. Acta Mycol. 2013, 43, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Matarese, F.; Sarrocco, S.; Gruber, S.; Seidl-Seiboth, V.; Vannacci, G. Biocontrol of Fusarium head blight: Interactions between Trichoderma and mycotoxigenic Fusarium. Microbiology 2012, 158, 98–106. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Picollo, E.; Scopel, C.; Causin, R. Trichoderma harzianum T22 induces in maize systemic resistance against Fusarium Verticillioides. J. Plant Pathol. 2014, 96, 133–142. [Google Scholar] [CrossRef]
- Harman, G.E. Myths and Dogmas of Biocontrol Changes in Perceptions Derived from Research on Trichoderma harzinum T-22. Plant Dis. 2000, 84, 377–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, G.E.; Petzoldt, R.; Comis, A.; Chen, J. Interactions Between Trichoderma harzianum Strain T22 and Maize Inbred Line Mo17 and Effects of These Interactions on Diseases Caused by Pythium ultimum and Colletotrichum graminicola. Phytopathology 2004, 94, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Bigirimana, J.; Meyer, G.D.; Poppe, J.; Elad, Y.; Höfte, M. Induction of systemic resistance on bean (Phaseolus vulgaris) by Trichoderma harzianum. Meded. Fac. Landbouwkd. Toegep. Biol. Wet. Univ. Gent. 1997, 62, 1001–1007. [Google Scholar]
- Cardoza, R.E.; Malmierca, M.G.; Hermosa, M.R.; Alexander, N.J.; McCormick, S.P.; Proctor, R.H.; Tijerino, A.M.; Rumbero, A.; Monte, E.; Gutiérrez, S. Identification of Loci and Functional Characterization of Trichothecene Biosynthesis Genes in Filamentous Fungi of the Genus Trichoderma. Appl. Environ. Microbiol. 2011, 77, 4867–4877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degenkolb, T.; Dieckmann, R.; Nielsen, K.F.; Gräfenhan, T.; Theis, C.; Zafari, D.; Chaverri, P.; Ismaiel, A.; Brückner, H.; Von Döhren, H.; et al. The Trichoderma brevicompactum clade: A separate lineage with new species, new peptaibiotics, and mycotoxins. Mycol. Prog. 2008, 7, 177–219. [Google Scholar] [CrossRef] [Green Version]
- Malmierca, M.G.; Cardoza, R.E.; Alexander, N.J.; McCormick, S.P.; Hermosa, R.; Monte, E.; Gutiérrez, S. Involvement of Trichoderma Trichothecenes in the Biocontrol Activity and Induction of Plant Defense-Related Genes. Appl. Environ. Microbiol. 2012, 78, 4856–4868. [Google Scholar] [CrossRef] [Green Version]
- Takahashi-Ando, N.; Ohsato, S.; Shibata, T.; Hamamoto, H.; Yamaguchi, I.; Kimura, M. Metabolism of Zearalenone by Genetically Modified Organisms Expressing the Detoxification Gene from Clonostachys rosea. Appl. Environ. Microbiol. 2004, 70, 3239–3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utermark, J.; Karlovsky, P. Role of Zearalenone Lactonase in Protection of Gliocladium roseum from Fungitoxic Effects of the Mycotoxin Zearalenone. Appl. Environ. Microbiol. 2007, 73, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Xue, A.G. Biological Control of Pathogens Causing Root Rot Complex in Field Pea Using Clonostachys rosea Strain ACM941. Phytopathology 2003, 93, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Hue, A.G.; Voldeng, H.; Savard, M.; Fedak, G.; Tian, X.; Hsiang, T. Biological control of fusarium head blight of wheat withClonostachys roseastrain ACM941. Can. J. Plant Pathol. 2009, 31, 169–179. [Google Scholar] [CrossRef]
- Xue, A.G.; Chen, Y.; Voldeng, H.D.; Fedak, G.; Savard, M.E.; Längle, T.; Zhang, J.; Harman, G.E. Concentration and cultivar effects on efficacy of CLO-1 biofungicide in controlling Fusarium head blight of wheat. Biol. Control 2014, 73, 2–7. [Google Scholar] [CrossRef]
- Vujanovic, V.; Goh, Y.K. Sphaerodes mycoparasitica biotrophic mycoparasite of 3-acetydleoxynivalenol and 15-acetyldeoxynivalenol-producing toxigenic Fusarium graminearum chemotypes. FEMS Microbiol. Lett. 2011, 316, 136–143. [Google Scholar] [CrossRef]
- Vujanovic, V.; Goh, Y.K. qPCR quantification of Sphaerodes mycoparasitica biotrophic mycoparasite interaction with Fusarium graminearum: In vitro and in planta assays. Arch. Microbiol. 2012, 194, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Vujanovic, V.; Kim, S.H. Adaptability of mitosporic stage inSphaerodes mycoparasiticatowards its mycoparasitic-polyphagous lifestyle. Mycologia 2017, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. 2019, 94, 1443–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goh, Y.K.; Vujanovic, V. Sphaerodes quadrangularisbiotrophic mycoparasitism on Fusarium avenaceum. Mycologia 2010, 102, 757–762. [Google Scholar] [CrossRef]
- Chenthamara, K.; Druzhinina, I.S. 12 Ecological Genomics of Mycotrophic Fungi. In Environmental and Microbial Relationships. The Mycota A Comprehensive treatise on Fungi as Experimental Systems for Basic and Applied Research, 3rd ed.; Esser, K., Druzhinina, I.S., Kubicek, C.P., Eds.; Springer: Cham, Switzerland, 2016; Volume IV, pp. 215–246. Available online: https://link.springer.com/chapter/10.1007/978-3-319-29532-9_12 (accessed on 17 April 2021).
- Vujanovic, V.; Daida, M.A.; Daida, P. qPCR assessment of aurofusarin gene expression in mycotoxigenic Fusarium species challenged with mycoparasitic and chemical control agents. Biol. Control 2017, 109, 51–57. [Google Scholar] [CrossRef]
- Hoover, J. Strategies for managing fusarium head blight in western Canada: A review of literature. In Proceedings of the 7th Canadian Workshop on Fusarium Head Blight, Winnipeg, MB, Canada, 27–30 November 2011; Available online: http://www.cwfhb.org/programs/7_CWFHB_2011_Winnipeg.pdf (accessed on 15 April 2021).
- King, C. Fighting Fungus with Fungus. Top Crop Manager. 2010, pp. 32–33. Available online: https://www.mydigitalpublication.com/publication/?m=1031&i=47114&p=32&ver=html5 (accessed on 11 May 2021).
- King, C. A Beneficial Parasite: A Fungus Native to Saskatchewan Helps Control Both Fusarium and its Toxins. Top Crop Manager. 2020. Available online: https://www.topcropmanager.com/a-beneficial-parasite/ (accessed on 11 May 2021).
- Arnason, R. Discovery of Biological Control Benefits Resistant Varieties. The Western Producers Crops. 2011. Available online: https://www.producer.com/crops/discovery-of-biological-control-benefits-resistant-varieties/ (accessed on 10 May 2021).
- Lysøe, E.; Seong, K.-Y.; Kistler, H. The Transcriptome of Fusarium graminearum During the Infection of Wheat. Mol. Plant Microbe Interact. 2011, 24, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Udendhran, R.; Balamurugan, M. Towards secure deep learning architecture for smart farming-based applications. Complex Intell. Syst. 2021, 7, 659–666. [Google Scholar] [CrossRef]
- Bauer, A.; Bostrom, A.G.; Ball, J.; Applegate, C.; Cheng, T.; Laycock, S.; Rojas, S.M.; Kirwan, J.; Zhou, J. Combining computer vision and deep learning to enable ultra-scale aerial phenotyping and precision agriculture: A case study of lettuce production. Hortic. Res. 2019, 6, 70. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powell, A.J.; Vujanovic, V. Evolution of Fusarium Head Blight Management in Wheat: Scientific Perspectives on Biological Control Agents and Crop Genotypes Protocooperation. Appl. Sci. 2021, 11, 8960. https://doi.org/10.3390/app11198960
Powell AJ, Vujanovic V. Evolution of Fusarium Head Blight Management in Wheat: Scientific Perspectives on Biological Control Agents and Crop Genotypes Protocooperation. Applied Sciences. 2021; 11(19):8960. https://doi.org/10.3390/app11198960
Chicago/Turabian StylePowell, Antonia J., and Vladimir Vujanovic. 2021. "Evolution of Fusarium Head Blight Management in Wheat: Scientific Perspectives on Biological Control Agents and Crop Genotypes Protocooperation" Applied Sciences 11, no. 19: 8960. https://doi.org/10.3390/app11198960
APA StylePowell, A. J., & Vujanovic, V. (2021). Evolution of Fusarium Head Blight Management in Wheat: Scientific Perspectives on Biological Control Agents and Crop Genotypes Protocooperation. Applied Sciences, 11(19), 8960. https://doi.org/10.3390/app11198960




