Abstract
Three combined investigations were conducted to examine the sources of PM2.5 in agricultural areas. The first was the measurement of PM2.5 and gaseous compounds in the greenhouse, which is a relatively closed system, while the second was the analysis of pesticide components used in agricultural areas. Finally, the physical and chemical properties of PM2.5 were analyzed in an orchard area and compared with the results of the greenhouse and agricultural chemical analyses. As a result, this research was able to confirm the source of emission and characteristics of PM2.5 originating from the agricultural area. Volatile organic compounds (VOCs) in agricultural areas are emitted by agricultural chemicals, and the discharged agricultural chemicals are first absorbed into the soil, and then released into the air by evaporation. Finally, the secondary products of PM2.5 in agricultural areas were estimated to have positive relationships with the VOCs from agricultural chemicals, and NH3 from fertilizers. The photochemical reactions of VOCs and NH3 were responsible for the impact on secondary products.
1. Introduction
Air pollution caused by industrial development is becoming a major threat to human health at a global level. As of 2016, more than 91% of the regions around the world did not meet the standard of air criteria provided by the World Health Organization (WHO) [1]. In particular, fine particulate matter (PM2.5) is one of the air pollutants which is rapidly increasing in China and other developing countries, where urbanization and industrialization are progressing at great speed [2,3,4,5]. Among several air pollutants, PM2.5 is an important environmental factor, because it has a negative impact on the environment, such as causing a reduction in visibility distance and the global heat budget, and the disturbance of the ecosystem, as well as posing risks to the respiratory system and increasing cardiovascular diseases in humans [6,7,8,9].
PM2.5 in the air can be emitted by various kinds of human activities: agricultural activity, such as soil cultivation, is one of the main human activities that causes PM2.5 emissions in rural areas [10,11]. According to previous studies, PM2.5 originating from agricultural activities can be categorized into mechanical factors during harvest and grain processing, the floating of ground particles by wind, and secondary generation of precursors from the use of chemicals, such as fertilizers and pesticides, used for the growth of crops [11,12,13]. When evaluated at the country level, the total amount of PM2.5 emitted by agricultural activities is higher than those that originate in urban and industrial areas. For example, in Europe, a survey investigating the sources of PM2.5 revealed that PM2.5 emitted by agricultural activities assumes the highest proportion, compared to other human activities [9,14]. Therefore, in order to reduce the amount of PM2.5, it is necessary to confirm the amount of emissions and the mechanism of PM2.5 generation in rural areas.
There has been a wide range of investigations into the amount of PM2.5 emissions from agricultural activities in European countries, the USA, and Canada. These countries use bottom-up tracking methodology based on emission coefficients and activity levels of agricultural activities to estimate the amount of PM2.5 emissions from the agricultural activities [13]. Therefore, emission coefficients and activity coefficients with high accuracy are required to calculate the amount of PM2.5 emissions from agricultural source. However, the emission coefficient derived from agricultural activities has multiple factors of uncertainty, even when the same kind of crop is cultivated, because of regional soil characteristics, different conditions in water quality, and differences in crop cultivation systems [13]. Therefore, to estimate the amount of PM2.5 originating from agricultural activities using emission coefficients, it is necessary to calculate amounts at a regional level by considering variables from conditions such as soil characteristics, cultivation system, and climate. However, it is almost impossible to calculate the emission coefficient for each region, because it requires a huge amount of effort. Therefore, some researchers insist that the measurement of the amount of PM2.5 emitted by agricultural activities should be based on the concentration of PM2.5 by on-site measurement, rather than the use of emission coefficients [15,16,17,18]. Agricultural activities emit various kinds of precursors, which in addition to the PM2.5, could originate secondary aerosols. It was reported that 92.2% of NH3 originated in China is emitted by agricultural activities, and agricultural activities also originate benzene, toluene, ethylbenzene, and xylene (BTEX) [19,20]. Therefore, to reduce the emission of PM2.5 by agricultural activities, it is necessary to measure not only local PM2.5, but also gaseous precursors, and to confirm the secondary formation mechanism of PM.
This research investigated the origination of PM2.5 emitted by agricultural activities. For this purpose, this study was conducted in three steps. The first step was to measure the PM2.5 and PM2.5 precursors originating from agricultural activities in the greenhouse; and the second step was to analyze the VOCs in the agricultural chemicals used in the research area. The final step was to analyze the physicochemical properties of PM2.5 originating in the agricultural environment, and gaseous compounds in the research area. As a result, this research was able to confirm the secondary products of PM2.5 originating from the agricultural source.
2. Materials and Methods
2.1. Sampling Location of Greenhouse
To examine the originations of PM2.5 in agricultural areas, three different experiments were performed. The first was to measure PM2.5 and precursor gases in the greenhouse, which is a relatively closed system, compared to the outdoor area. PM2.5 (5014i, Thermo Scientific, Waltham, MA, USA), NO, NO2 (42iQ, Thermo Scientific, Waltham, MA, USA), and NH3 (Los Gatos Research (LGR), ABB Inc., San Jose, CA, USA) were studied for 28 days in greenhouses (about 8 m × 160 m) in the Wanju area (Figure 1) from 9 October to 7 November 2020. The separated sampling site (2.5 m × 2.5 m) was about 3 m away from the greenhouse. The samples were collected using a half inch diameter Teflon tube.
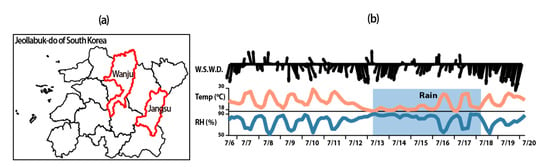
Figure 1.
(a) Sampling site at Jangsu-gun, Jeollanamdo. (b) Meteorological conditions during the sampling periods.
2.2. Sampling Location of Agricultural Areas
To examine the originations of PM2.5 in agricultural areas, PM2.5 and gaseous pollutants were measured for two weeks from 6 July to 20 July 2020 at an apple orchard located in Jang-Su, Jeollabukdo, Republic of Korea (Figure 1). The collected PM2.5 samples were analyzed for organic carbon (OC), elemental carbon (EC) with the National Institute of Occupational Safety and Health (NIOSH5040) protocol, equivalent black carbon (eBC) from multi-angle absorption photometer (MAAP) (5012, Thermo Scientific, Waltham, MA, USA), water soluble organic carbon (WSOC) from total organic carbon (TOC) (Sievers M9, General Electoric, Boston, MA, USA) analyzer, water-soluble ions from ion chromatography (IC) (Metrohm 930, Herisau, Switzerland), and trace elements from Energy Dispersive X-ray Fluorescence Spectrometry, ED–XRF (ARL QUANT’X EDXRF Spectrometer, Thermo Scientific, Waltham, MA, USA), to investigate the characteristics of PM2.5 in agricultural areas. During the measurement period, gaseous compounds, such as NH3, NO and NO2, were analyzed in real time; and additionally, VOCs were collected using solid adsorption. All instruments were operated in an air monitoring trailer with meteorological monitoring during the sampling periods.
2.3. Volatile Organic Compounds (VOCs) Analysis
The sequence VOCs sampler was implemented at a flow rate of 50 mL/min to minimize the loss of low VOCs, and automatically collected using an automatic switch that functions every 3 h (Figure 2). VOCs (Custom (Q-QC27-1), Kemidas (M-VAVOC 503M2), Gyeonggi-do, Korea) were used as standard material for analysis. The internal standard material was chlorobenzene-d5 manufactured by Kemidas. solid adsorption tube (C2-CAXX-5149) (Markes International, Ltd., CF31 3RT, UK) was utilized for sample collection and calibration curve. VOCs were analyzed using Thermal Desorption (Unity2, Markes International, Ltd., CF31 3RT, UK) gas chromatography (GC) (7890A, Agilent, Santa Clara, CA, USA) mass spectrometry (MS) (5975C, Agilent, Santa Clara, CA, USA), and the analysis method was US EPA TO-17 (solid adsorption method). There were 33 compounds for analysis that included benzene, toluene, ethylbenzene, and xylene both for agricultural chemical analyses and orchard ambient (Figure 3). The 1 μL of a diluted solution by mixing two standard VOCs in the solid adsorption tube was collected using nitrogen gas at a flow rate of 50 mL/min. In addition, 1 μL of chlorobenzene-d5 at the concentration of 50 mg/L was injected into the adsorption tube and all the samples at a flow rate of 50 mL/min, using nitrogen gas to finalize the calibration curve. After that, the VOCs collected in the solid adsorption tube were desorbed by thermal desorption, inducing low-temperature concentration and resorption at −20 °C, and then thermally desorbed at 320 °C for 15 min, and transferred to the GC. After GC injection, samples were separated by a column of 60 m length, 0.25 mm inner diameter, and 0.25 μm thickness, and were finally quantified by quadruple mass spectrometry.
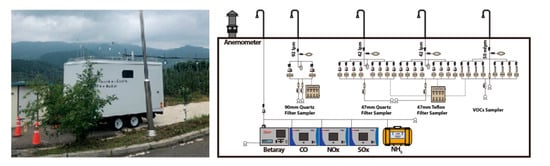
Figure 2.
Picture of air monitoring trailer and schematic of the air monitoring instruments and samplers in the air monitoring trailer.
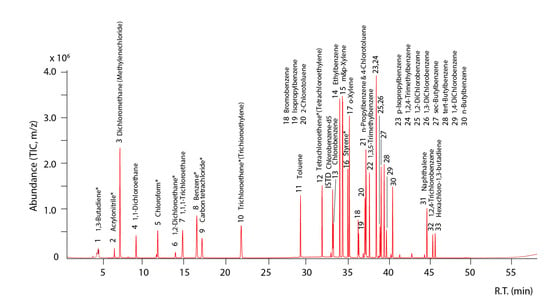
Figure 3.
GC-MS chromatogram of the volatile organic compounds (VOCs) released by thermal desorption.
3. Results
3.1. Observation of Gaseous Components and Analysis of Agricultural Chemicals in Greenhouse
To examine the originations of PM2.5 in agricultural areas, three different experiments were performed. The first was to measure PM2.5 and precursor gases in the greenhouse, which is a relatively closed system, compared to the outdoor area. The second was to analyze the components of pesticides, herbicides, and vapor deposition agents used in the agricultural source. Finally, by measuring the characteristics and precursors of PM2.5 in an outdoor area, the source of PM2.5 in agricultural areas could be defined. For this purpose, PM2.5, NO, NO2 and NH3 were studied for 28 days in greenhouses in the Wanju area (Figure 1), and four different kinds of pesticides, germicides, herbicides, and vapor deposition agents were used to analyze the characteristics of PM2.5 at orchards of the Jangsu area.
Figure 4 shows the results of PM2.5, NO, NO2 and NH3 measured at the Wanju greenhouses. The average concentration of PM2.5 observed during the study period was 12.98 µg/m3, the average concentration of NO was 8.11 ppb, the average concentration of NO2 was 15.11 ppb, and that of NH3 was 101.34 ppb. In addition, the maximum concentration observed during the measurement period of PM2.5 was 29.96 µg/m3, and those of NO, NO2, and NH3 were 55.06, 183.62 and 309.27 ppb, respectively. According to the monthly atmospheric report of Jeonbuk province in October 2020 from the urban atmospheric measurement network data of the monthly atmospheric report, the average concentration of PM2.5 was 17 µg/m3, and the maximum concentration over 24 h was 32 µg/m3, while the average concentration of NO2 was 9 ppb, and the maximum concentration during 24 h was 12 ppb. Comparing the monthly atmospheric report to the measurements of this study, the concentration of PM2.5 was similar, while NO2 was 1.7 times higher than the local concentration on average, and more than 20 times higher at maximum. The concentration of ammonia is not shown in any publication of the same province. When comparing the 6.9 ppb of NH3 concentration in Seoul suggested in the previous study, NH3 in the research area was 14.7 times higher than the average concentration, and 44.8 times higher than the maximum concentration [21]. That means that the above result shows that the concentration of PM2.5 in the greenhouse is similar to or lower than the concentration of the local area, but the precursor gases, such as NO2 and NH3, are higher than the local concentration.
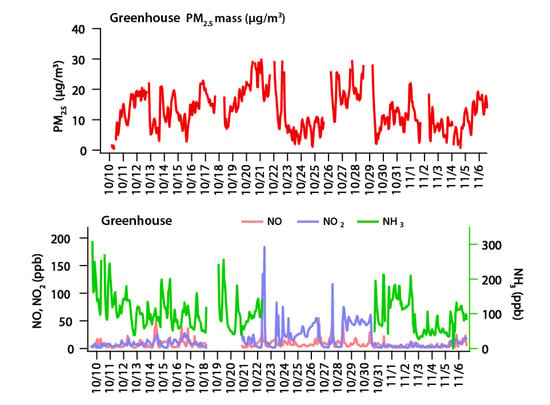
Figure 4.
Concentrations of PM2.5, NH3, NO and NO2 measured in a green house.
Figure 5 shows the concentrations of PM2.5, NO, NO2 and NH3 measured in the greenhouse over time. PM2.5 had concentration range of 11.03–15.01 µg/m3 with standard deviation of 1.23 µg/m3, which means there was no significant change in concentration over time. On the other hand, NO was in the range 3.76–16.9 ppb, with standard deviation of 3.24 ppb; NO2 was in the range 9.93–26.77 ppb, with standard deviation of 5.22 ppb; and NH3 was in the range 78.77–141.54 ppb, with standard deviation of 15.01 ppb, which means that the variation was higher than the variation of PM2.5. When looking at the concentrations of NO and NO2 over time, the concentrations of NO and NO2, which were relatively stable at night and dawn, increased after 7 a.m., and NO showed the maximum concentration at 9 a.m., and then decreased. On the other hand, the maximum concentration of NO2 was observed between 10 and 11 a.m., which was about 1 to 2 h later than the time when the maximum concentration of NO was observed. This is interpreted as NO changing into NO2 by typical photochemical reaction. The change of NH3 concentration overtime was relatively constant at dawn and nighttime, like NO and NO2; and after 8 a.m., the NH3 concentration started to rise, and showed the maximum concentration at 11 a.m. After that, it gradually decreased until 5 p.m., and then remained relatively constant. It is difficult to understand the characteristics of the primary emission source, because fertilizer, crop and soil characteristics, crop management, and meteorological conditions have multiple impacts on the emission of NH3 in the agricultural environment [15,16,17,18]. NH3 in the greenhouse can be evaporated by temperature rise based on the time when the NH3 concentration increases and reaches the maximum during the daytime. As a result of measuring the level of PM2.5 and precursor gases for 28 days in the greenhouse, which is a relatively closed system, agricultural activity did not significantly affect the increase in PM2.5. However, an increase in precursors, such as NO, NO2 and NH3, was observed from agricultural activities; and it was found that each material increase was based on temperature and photoreaction.
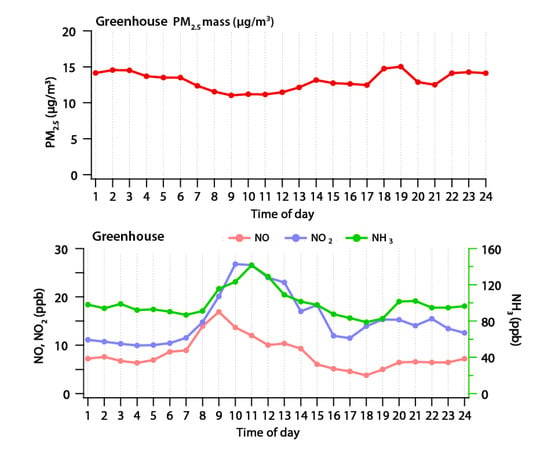
Figure 5.
Time-series concentrations of PM2.5, NH3, NO and NO2.
VOCs are air pollutants with a boiling point of 50 to 260 °C at room temperature (RT) and atmospheric pressure. Among VOCs, benzene, toluene, ethylbenzene, and xylene (BTEX) are all designated by the EPA as hazardous air pollutants (HAPs) [22,23]. In terms of PM2.5 formation, oxygenated volatile organic compounds (OVOCs) generate particulates by themselves in the atmospheric environment through the process of condensation and nucleation, and are also involved in the photochemical reaction of ozone (O3) and nitrogen oxides, which has an impact on the generation of secondary organic aerosols (SOA) and secondary nitric aerosols [24,25,26].
In agricultural areas, VOCs are emitted in various forms. VOCs are formed as products of resistance to environmental factors that put stress on plant growth [27,28]. This means that they are caused by beneficial ecological functions that can promote plant health, such as repelling predators and parasites that affect plant growth, or attracting beneficial animals [29,30,31]. It is also suggested that in agricultural environments, VOCs are emitted when a pesticide is sprinkled. According to a previous study, it was reported that VOCs of 0.6–5000 ppb were contained in the wastewater of pesticide plants, which raised concerns about the increase in VOCs in agricultural soil [32]. Another previous study reported the increase in benzene, toluene, ethylbenzene, xylene, and styrene (BTEXS), which are major components of pesticides in the soil by agricultural activities [33]. Based on these results, it was suggested that VOCs-based organic solvents contained in pesticides are directly discharged into the air and soil by agricultural activities. Therefore, in this study, VOCs content was investigated by analyzing pesticides used in agricultural areas.
To investigate the origination of PM2.5 in agricultural areas, the VOCs contained in four kinds of agricultural chemicals were analyzed. We selected pesticides, fungicides, herbicides, and vapor deposition agents that are available in the market and are mainly used in apple orchards. The reason for selecting the agricultural chemical used in the apple orchard is that the main crop in the orchard area investigated in this study is apple. Table 1 shows the concentrations of VOCs contained in agricultural chemicals. The agricultural chemicals that were analyzed contained VOCs in the range of 13 to 710 mg/L, and the VOCs level was high in this order: vapor deposition agent > germicide > herbicide > pesticides at 710.71, 86.43, 33.63, 13.92 mg/L, respectively. Looking at the representative VOCs contained in the agricultural chemicals that were investigated, the % contents in pesticides, fungicides, and herbicides were high in this order: benzene > ethylbenzene > m&p-Xylene > toluene > styrene > o-Xylene at 33.58, 16.37, 15.33, 12.03, 8.36, 4.76%, respectively, and all other aerosols were less than 3%. Even though benzene was not analyzed in the pesticide sample, the overall average of Benzene is the most abundant among the major VOCs. This means that the main VOCs of pesticides, fungicides, and herbicides, except for vapor deposition agents, were BTEXS, which is consistent with BTEXS soil contamination, which is increasing due to agricultural activities in rural areas in China, as suggested in a previous study [33]. Meanwhile, the vapor deposition agent showed the highest ratio of naphthalene (58.90%) and acrylonitrile (36.63%) among the total VOCs. However, the concentration of BTEXS in the vapor deposition agent was higher than that of the other VOCs, and naphthalene and acrylonitrile are considered to be characteristics of the agricultural chemicals measured in this study.

Table 1.
Concentration of volatile organic compounds in the agricultural chemicals used in orchards.
3.2. OC Characteristics of PM2.5 in Orchard Areas
Table 2 shows the results of PM2.5 and gaseous compounds in real-time at the orchard area observed during the study period. The rePM2.5 presented in the table is the result of summing the concentrations of OC, EC, water-soluble ions, and heavy metals among the analysis compounds. rePM2.5, OC, water-soluble ion, heavy metal, and EC were 47.91, 43.20, 5.02, and 3.87%, respectively. In this study, H, N, S, O, and other water-soluble ions other than the three components, which constitute organic substances other than carbon, were not analyzed. However, since the contents of H, N, S, and O, excluding the carbon component, are generally calculated using the OM/OC ratio based on the molecular weight of each component constituting the OC, the OC presented in this study can represent the amount of organic aerosols included in PM2.5 [34,35,36,37]. Additionally, according to previous studies, the SO42−, NO3− and NH4+ (SNA) analyzed in this study takes the largest portion of the PM2.5 ionic components, and these components are known to be important factors in SOA formation [38]. Therefore, based on the concentration of OC and the three water-soluble ions presented in this study, it is judged that there will be no major issue in interpreting the cause of PM2.5 in agricultural areas. In conclusion, it can be confirmed that OC and ion components have a great impact on PM2.5 in agricultural areas.

Table 2.
Analysis result of PM2.5 chemical concentration in orchard area.
OC can be briefly divided into water-insoluble OC (WIOC) and water-soluble organic carbon (WSOC). WIOC mainly originates through the combustion of fossil fuels, and is known to be emitted from primary origins [39,40]. On the other hand, WSOC can be emitted directly as primary particles from biomass combustion (BB) sources, or can originate by SOA formation [24,41,42]. Therefore, in this study, the proportion of WSOC among OCs was confirmed, and the result showed that WSOC took 95.09% of OCs. In other words, it can be confirmed that the PM2.5 in the study area originated through the combustion of biomass or the reaction of SOA formation, rather than originating from the combustion of fossil fuels.
WSOC can be described in two forms. One is WSOC (biomass-burning, WSOCbb) by biomass combustion, while the other is WSOC (non-biomass-burning, WSOCnbb) by secondary organic compounds. In this study, WSOCnbb was calculated by referring to the calculation method from previous studies to determine the source of WSOC [43]. Briefly, WSOCnbb is determined by differences between total WSOC and biomass burning WSOC calculated from the ratio of levoglucosan to OC. As a result, WSOCnbb accounted for 90.71 and 95.40% of OC and WSOC, respectively. Therefore, it was suggested that the WSOC in the study area originated in secondary organic compounds, rather than in the primary biomass burning, from the results of levoglucosan analyzed in this study. Levoglucosan is a substance that can be produced only by the decomposition of cellulose and hemicellulose when the combustion temperature is higher than 300 °C [44,45]. Therefore, levoglucosan is used as one of the indicators of PM2.5 from biocombustion, and the ratio of levoglucosan to OC (L/OC) is mainly used to track biomass burning combustion using water-soluble model [46,47,48]. Since the L/OC ratio has lots of variables, such as the type of biomass to be burned, and the combustion conditions, an absolute value in standard does not exist [49]. However, it was suggested that the maximum contribution of BB to OC was about 33%, and the minimum contribution was 4% [48]. Based on this, the L/OC of this study was 3.43%, confirming that the contribution of BB to OC was almost zero. In conclusion, OC, which takes a large portion of the PM2.5 composition in the study area, did not originate from the combustion of biomass burning.
As discussed, previous studies maintained that the secondary organic carbon (SOC) can be represented by WSOC without the effect of biomass combustion [50]. In other words, it was determined that most of the OC in PM2.5 in the study area was formed by SOC. Additionally, in terms of secondary products, WSOC involves the conversion of gaseous compounds into particles through the oxidation of VOCs [39,45,50,51]. Therefore, in this study, VOCs were measured for 31 compounds in the study area during the study period (Table 3). Table 2 shows that the total of the average concentration of VOCs (ΣVOCs) in the study area was 10.2351 ppb. The average concentration of Toluene, m&p-Xylene, o-Xylene, ethylbenzene, benzene, and styrene presented 5.62, 1.11, 1.02, 0.68, 0.65 and 0.20 ppb, respectively. In addition, among the 31 compounds analyzed, BTEXS accounted for 90.73% of ΣVOCs, accounting for most of the VOCs in the study area. It is known that BTEX is mainly emitted through the combustion of fossil fuels, such as car exhausts, industrial activities, such as petrochemicals, and the combustion of heating fuel for houses [52,53,54]. However, the research area was an orchard area where apples are harvested, and there are almost no residential or industrial complexes nearby, as it is more than 300 m away from the main road, without much traffic. In addition, the study result of OC also showed that the main causes of WSOC, WIOC, WSOCbb, and WSOCnbb were not clearly confirmed. On the other hand, BTEXS, which showed a high proportion of VOCs observed in the study area, was consistent with the major VOCs components of pesticides, germicides, and herbicides analyzed in this study. Therefore, it is considered that the VOCs observed in the study area are derived from the agricultural chemicals used in the agricultural area.

Table 3.
Analysis result of VOCs concentration in the orchard area.
VOCs in the air are known to be important precursor gases of SOA [55]. According to previous studies, semi-volatile or non-volatile aerosols are originated by photochemical oxidation reaction (mainly OH radical reaction), and then react with nitrogen oxide in the presence of solar radiation to generate SOA [56,57]. Therefore, VOCs in the air can generate secondary PM2.5 based on photochemical reactions. Therefore, in this study, to check the effect of VOCs on PM2.5 generation in the study area, the data were categorized into rainy days, and sunny days without rain, during the study period. This was performed to confirm the relationship between the OC originating from the photochemical reaction, and the concentration of VOCs in the air. As a result, Table 2 shows that the OC in PM2.5 was (4.66 and 2.01) µg/m3 on a sunny and rainy day, respectively, which showed decrease of about 56.89% in rainy days compared to sunny days. Table 3 confirms that ΣVOCs were (11.29 and 7.30) ppb on sunny and rainy days, respectively, which is a 35.35% reduction. Although there is a large difference between the reduction rate of VOCs and the reduction rate of OC, the correlation between the daily VOCs concentration and the daily OC concentration is 0.8 or higher, confirming that VOCs have a significant impact on the OC concentration. Judging from the reduced concentration of OC and VOCs on rainy days compared to sunny days with active photochemical reactions, it was found that OCs in the study area originated from photochemical reactions of VOCs.
The production yield for VOCs was calculated with the assumption that WSOCnbb in the study area originates by secondary products by VOCs, and it was estimated by using the yield production of each component suggested in a previous study with the assumption that under normal atmospheric conditions, the measured BTEXS fully reacts [58,59,60,61,62,63,64]. As a result, particles that originated from BTEXS were found to be 2.35 and 4.01 µg/m3 when the minimum and maximum coefficients of yield production were applied, respectively. When the number of particles calculated from the daily BTEXS was compared with WSOCnbb, the determination coefficient r2 was 0.4125, which was relatively low. This result is based on the yield production used in the above calculation being the coefficient derived from the chamber experiment, hence many variables from the actual atmospheric state were not able to be applied. On the other hand, the calculated amount of BTEXS particle originated was 2.35–4.01 µg/m3, and the measured WSOCnbb average was 3.59 µg/m3, which is a result with significance. It is difficult to accurately calculate the formation of VOCs particles based on the results analyzed so far, but based on the calculated results only, it is estimated that the impact of BTEXS on WSOCnbb in the study area is relatively high.
In order to confirm the emission characteristics of VOCs that originate in agricultural areas, Figure 6 shows the ratio of m,p-xylene/ethylbenzene (X/E) and toluene/benzene (T/B) that was confirmed. The X/E ratio is used as data that can confirm the photochemical time change after the generation of VOCs. In general, m,p-xylene and ethylbenzene originate by the same source of emission, though the theory that the decomposition rate by OH radicals in the air varies is applied, because the rate of oxidation is different [65]. That means that the KOH of m-xylene and p-xylene is 23.6 and 14.3, and the lifetimes in the air are 11.8 and 19.4 h, respectively. However, the KOH of ethylbenzene is 6.05, and the lifetime in the air is about 1.6 days [65]. Therefore, when m,p-xylene and ethylbenzene are emitted from the same source, the ratio of X/E decreases as the photochemical reaction time goes by. Additionally, based on this, the higher the ratio of X/E, the higher the influence of local occurrences, and the lower the ratio of X/E, the larger the radius of influence from external inflows and VOCs from the source [66]. The average X/E measured during the study period was 1.86. The range of X/E for major cities confirmed by previous studies is relatively constant, ranging (2.8 to 4.6) in the absence of strong emission sources in particular [65]. In addition, the X/E ratio of the suburbs of Colorado, USA, confirmed in the previous study, was 2.0, while the urban background concentration of Italy was 1.55, and these areas were found to be affected by the urban area [67,68]. Additionally, the X/E ratio of the four agricultural chemicals analyzed in this study for pesticides, germicides, herbicides, and vapor deposition agents was 14.68, 0.78, 1.26 and 1.20, respectively, with an average of 4.48. Considering that the study period was summer, and the cultivated crop was the apple, it was assumed that the use of pesticides was high. Therefore, it can be concluded that after VOCs are emitted from the emission source, the ratio of X/E measured in the study area, which is 1.86, shows relatively long-term photochemical time change.
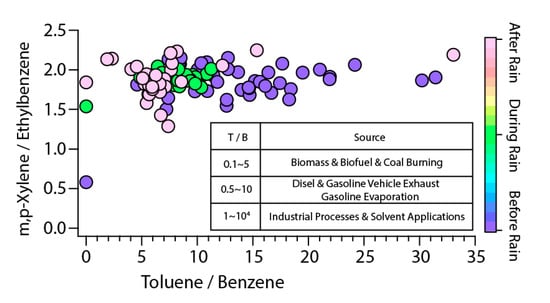
Figure 6.
VOCs cross-plots for the ratio of toluene vs. benzene and m,p-xylene vs. ethylbenzene.
The T/B of this study ranged from 1.89 to 33.05, with an average of 10.05, and 101 out of 106 observed T/B showed 4.0 or higher. T/B is used as data for estimating emission sources. Generally, biomass combustion has T/B of 1 or less, 0.1–5 for coal combustion, 0.5–10 for combustion by vehicles such as gasoline and diesel, and 1 or more for emissions from industrial processes and solvents, which in previous studies showed up to 10,000 [69]. The mean value of T/B in this study is 10.05, and as Figure 6 shows, a ratio of 10 or higher represents more than 40% of the entire observed values. In addition, the research area is the center of the orchard, and there is no industrial area within a radius of 1 km of the observation point. In other words, considering the observed value of T/B in the study area and the surrounding environment, the source of VOCs in the study area is assumed to be solvents, and that seems to be due to the agricultural chemicals used in the orchard area.
In this study, it was suggested that the agricultural chemicals used in agricultural activities are the source of VOCs in agricultural areas. In addition, it was confirmed that the measured VOCs had a photochemical time change for a long time after emission. Therefore, to confirm the process of VOCs emission into the air after agricultural chemicals are applied, the correlation between toluene/ethylbenzene (T/E) was investigated. According to previous studies, toluene and ethylbenzene originate from the same emission source, and the lifetime in the air is close to (1.9 and 1.6) days, respectively, which are quite similar to each other [65]. On the other hand, the boiling points of toluene and ethylbenzene differ by 110 and 136 °C, respectively. Therefore, in the previous study, the emission type was estimated based on the correlation of T/E. When toluene and ethylbenzene were emitted by combustion, such as automobiles and heating, a high correlation of 0.9 or higher was observed. On the other hand, when toluene and ethylbenzene were emitted by evaporation other than combustion, a low correlation of 0.5 or less was observed [65,70]. In addition, the study has shown that volatile aerosols, such as BTEX contained in soil, are volatilized into the air by the action of microorganisms, temperature, and photoreaction. In particular, as moisture evaporates after rain, the amount of evaporation of VOCs contained in the soil increases accordingly [33,71,72,73]. Based on these previous studies, the results of this study were confirmed. As a result of examining whether toluene and ethylbenzene, which are estimated to be the sources of VOCs emission in agricultural areas, are contained in agricultural chemicals, all four agricultural chemicals analyzed in this study were mixed with toluene and ethylbenzene. Therefore, it was possible to confirm the emission form based on the correlation of T/E in previous studies. The correlation of T/E was 0.79 throughout the entire study period, showing a relatively high correlation. However, it is difficult to confirm the emission form of VOCs, because this result includes chemical reactions, such as the oxidation state of VOCs released into the air. Therefore, in this study, the correlation of T/E was confirmed based on the evaporation time of surface moisture after rain during the study period. As a result, the correlation of T/E was found to be 0.49. This means that the study concluded that VOCs contained in the soil were released in the form of evaporation, rather than VOCs released by combustion.
In conclusion, when T/B indicating the emission source by solvent, X/E indicating long photochemical time change, and T/E indicating evaporative emission form are all investigated, VOCs in agricultural areas are emitted by agricultural chemicals, and the discharged agricultural chemicals are first absorbed into the soil, and then released into the air by evaporation. Additionally, when comparing sunny days and rainy days, the reduction of VOCs was 35.35%, of which 31.24% was confirmed to be BTEXS, a major component of agricultural chemicals. These results indicate that SOC is greatly affected by agricultural chemicals among OCs that consist of PM2.5 in agricultural areas.
3.3. Characteristics of Water-Soluble Ions in PM2.5 at Orchard Areas
The 43.20% of rePM2.5 in the study area were identified as water-soluble ionic components, and the concentration for each compound was 0.73, 1.07, and 1.78 ug/m3 for NH4+, NO3− and SO42−, respectively. The ratio of NOx to SOx was examined to estimate the emission source of ionic aerosols affecting PM2.5 generation in the study area. In general, the higher the NO3−/SO42− mass ratio, the greater the effect of mobile pollutants. Previous studies reported the ratio of NOx and SOx originating during gasoline and diesel combustion to be 13:1 and 8:1, respectively [73]. Therefore, the mass ratio of NO3− and SO42− can represent the contribution of fixed and mobile pollutants affecting the generation of PM2.5. The NO3−/SO42− mass ratio measured in this study was 0.6, suggesting that the source of PM2.5 and water-soluble ions in the study area had a greater effect from the fixed source, than the effect from the mobile source. This is consistent with the estimated result of the WSOC emission source described above, and it can be suggested that the combustion of fossil fuels by vehicles has relatively little effect on PM2.5 in the study area.
To confirm the formation process of PM2.5 by water-soluble ions, the concentration of gaseous aerosols, the molar ratio of NH4+ and SO42−, and the molar ratio of NO3− and SO42− among the water-soluble ions of PM2.5 SUM were measured. NH3 in the atmosphere, which is a dominant factor in the SOA formation reaction, neutralizes sulfuric acid (H2SO4) with alkaline gas to generate ammonium sulfate ((NH4)2SO4) or ammonium bisulfate ((NH4)HSO4), and then the remaining NH3 in the air reacts with nitric acid (HNO3) to form ammonium nitrate (NH4NO3) [74,75]. Ammonium sulfate, ammonium bisulfate, and ammonium nitrate originated are all SOA, and their production is dominated by NH3 in the air. Based on this, the effect of NH3 in the atmosphere can be confirmed by using the molar ratio of NO3−, SO42−, and NH4+ contained in PM2.5. According to previous studies, if the molar ratio of NH4+/SO42− in PM2.5 is 1.5 or more, it can be defined as ammonia-rich, in which the NH3 concentration in the air is sufficient for SOA formation; and when this molar ratio is 1.5 or less, it is reported that the contribution of SO42− to the PM2.5 SOA is high [76,77]. That is, as the molar ratio of NO3−/SO42− increases, the contribution of NO3− to SOA increases. In this study, it was confirmed that the molar ratio of NH4+/SO42− was 2.23, and the molar ratio of NO3−/SO42− was 0.95. Therefore, it was determined that the study area was an ammonia-rich state, where the concentration of NH3 in the air was high enough to form SOA, and when NO3− and SO42− were compared, it could be confirmed that SOA had a greater effect on SO42− than on NO3−.
To examine the process of SOA formation in the study area in detail, the data were divided into sunny days and rainy days, to collect the measurement result. During the study period, the molar ratio of NO3−/SO42− was in the range (0.1–4.0), and the period when the molar ratio of NO3−/SO42− was 1.5 or more was mostly sunny days. Additionally, looking at the correlation of NH4+, NO3−, SO42− to rePM2.5, the coefficient of determination r2 was (0.9009, 0.6865 and 0.7746) on a sunny day, respectively, so rePM2.5 during sunny days showed the greatest impact from NH4+. On the other hand, the coefficient of determination r2 from rainy day was (0.1442, 0.4173 and 0.3737) for NH4+, NO3−, and SO42−, respectively, so rePM2.5 during rainy day showed the greatest impact from NO3−. The sum of water-soluble ions, such as NH4+, NO3− and SO42−, is (4.76 and 0.26) µg/m3 on sunny and rainy days, respectively, which is estimated to be the difference from the SOA formation rate on sunny and rainy days. The difference between sunny and rainy days is also observed in the concentration of gas-phase in the air. During the study period, the average of gaseous NH3 measured during sunny and rainy days was (20.22 and 7.73) ppb, respectively, which showed the average gaseous NH3 is higher on sunny than on rainy days. Summarizing the above results, the SOA formation in the study area is made by the photochemical reaction of SNA, and the NH3 concentration in the air, expressed in the ammonia-rich state, is greatly involved in the formation of SNA. NH3 in the air is estimated to have originated by agricultural activities, as suggested in the observation of greenhouses in the previous section of this report. Additionally, the concentration of NH3 observed over time in the greenhouse increases in response to temperature and light. Therefore, the NH3 concentration in the air did not increase on rainy days, and not much SOA formation by the photochemical reaction of SNA occurred. These results clearly confirm the molar ratios of NH4+, NO3− and SO42−, and the concentration and diurnal pattern of NH3 in the air, as presented in Figure 7. The figure shows that when the concentration of NH3 in the air increases, the molar ratio of NO3−/SO42− also increases, and it is confirmed that this reaction occurs when there is no rain.
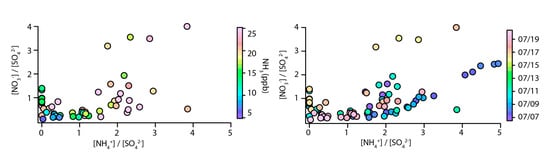
Figure 7.
Ions cross-plots for the molar ratio of NH4+/SO42− vs. NO3−/SO42−.
4. Conclusions
Three combined investigations were conducted to examine the sources of PM2.5 in agricultural areas. The first was the measurement of PM2.5 and gaseous compounds in the greenhouse. The second was the analysis of agricultural chemical components used in agricultural areas. Finally, the chemical properties of PM2.5 were analyzed in the orchard area, and compared with the results of the greenhouse and the agricultural chemical components’ results. As a result, NH3 and NOx, which are precursor gases in the greenhouse, were found to be higher than the local concentration. The four agricultural chemical components analyzed in this study were mainly identified as BTEXS, which showed similar results to the results of previous studies that analyzed the soil in agricultural areas. As a result of analyzing the chemical properties of PM2.5 in the orchard area, it was confirmed that about 48% of rePM2.5 in the orchard area was OC, and 43% was water-soluble ions. Most of the OCs consisted of secondary products; and for the water-soluble ions, the formation of SOA by SNA was confirmed to be the main cause. Based on the characteristics of OC and water-soluble ions, the source of PM2.5 in agricultural areas was estimated to be the main cause of VOCs from agricultural chemicals, NH3 from fertilizers, and it was found that the photochemical reaction has a huge impact on the secondary products.
Author Contributions
M.S. contributed to this work via experiment measurements, data analysis and manuscript preparation. M.K. (Minwook Kim), S.-H.O., C.P., M.K. (Moonsu Kim), M.K. (Minsung Kim), H.L. and S.C. contributed research sample analyses. M.-S.B. contributed to experiment planning, data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Rural Development Administration, National Institute of Agricultural Sciences (Grant Number PJ01490002). We acknowledge the support of the National Research Foundation of Korea (NRF) (2020M3G1A1115000).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shou, Y.; Huang, Y.; Zhu, X.; Liu, C.; Hu, Y.; Wang, H. A review of the possible associations between ambient PM2.5 exposures and the development of Alzheimer’s disease. Ecotoxicol. Environ. Saf. 2019, 174, 344–352. [Google Scholar] [CrossRef]
- Nemery, B.; Hoet, P.H.M.; Nemmar, A. The Meuse Valley fog of 1930: An air pollution disaster. Lancet 2001, 357, 704–708. [Google Scholar] [CrossRef]
- Davis, D. A Look Back at the London Smog of 1952 and the Half Century Since. Environ. Health Perspect. 2002, 110, A734–A735. [Google Scholar] [CrossRef] [PubMed]
- Parrish, D.D.; Singh, H.B.; Molina, L.; Madronich, S. Air quality progress in North American megacities: A review. Atmos. Environ. 2011, 45, 7015–7025. [Google Scholar] [CrossRef]
- Ji, X.; Yao, Y.; Long, X. What causes PM2.5 pollution? Cross-economy empirical analysis from socioeconomic perspective. Energy Policy 2018, 119, 458–472. [Google Scholar] [CrossRef]
- Hazarika, N.; Srivastava, A. Estimation of risk factor of elements and PAHs in size-differentiated particles in the National Capital Region of India. Air Qual. Atmos. Health 2017, 10, 469–482. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, L.; Lee, M.; Liu, P.; Di, Q.; Zanobetti, A.; Schwartz, J.D. Long-term Exposure to PM2.5 and Mortality among Older Adults in the Southeastern US. Epidemiology 2017, 28, 207–214. [Google Scholar] [CrossRef]
- Kelly, V.R.; Lovett, G.M.; Weathers, K.C.; Likens, G.E. Trends in atmospheric concentration and deposition compared to regional and local pollutant emissions at a rural site in southeastern New York, USA. Atmos. Environ. 2002, 36, 1569–1575. [Google Scholar] [CrossRef]
- Bogman, P.; Cornelis, W.; Rollé, H.; Gabriels, D. Prediction of TSP and PM10 emissions from agricultural operations in Flanders, Belgium. In Proceedings of the 14th International Conference “Transport and Air Pollution”, Graz, Austria, 1–3 June 2005. [Google Scholar]
- Cassel, T.; Trzepla-Nabaglo, K.; Flocchini, R. PM10 emission factors for harvest and tillage of row crops. In Proceedings of the 12th International Emission Inventory Conference, San Diego, CA, USA, 29 April–1 May 2003. [Google Scholar]
- Pattey, E.; Qiu, G. Trends in primary particulate matter emissions from Canadian agriculture. J. Air Waste Manag. Assoc. 2012, 62, 737–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aneja, V.P.; Schlesinger, W.H.; Erisman, J.W. Effects of Agriculture upon the Air Quality and Climate: Research, Policy, and Regulations. Environ. Sci. Technol. 2009, 43, 4234–4240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Tong, D.Q.; Zhang, S.; Zhang, X.; Zhao, H. Local PM10 and PM2.5 emission inventories from agricultural tillage and harvest in northeastern China. J. Environ. Sci. 2017, 57, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Walker, J.T.; Jones, M.R.; Bash, J.O.; Myles, L.; Meyers, T.; Schwede, D.; Robarge, W. Processes of ammonia air–surface exchange in a fertilized Zea mays canopy. Biogeosciences 2013, 10, 981–998. [Google Scholar] [CrossRef] [Green Version]
- Nelson, A.J.; Koloutsou-Vakakis, S.; Rood, M.J.; Myles, L.; Lehmann, C.; Bernacchi, C.; Lin, J. Season-long ammonia flux measurements above fertilized corn in central Illinois, USA, using relaxed eddy accumulation. Agric. For. Meteorol. 2017, 239, 202–212. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Cobena, A.; Lassaletta, L.; Estellés, F.; Del Prado, A.; Guardia, G.; Abalos, D.; Billen, G. Yield-scaled mitigation of ammonia emission from N fertilization: The Spanish case. Environ. Res. Lett. 2014, 9, 125005. [Google Scholar] [CrossRef]
- Recio, J.; Montoya, M.; Ginés, C.; Sanz-Cobena, A.; Vallejo, A.; Alvarez, J.M. Joint mitigation of NH3 and N2O emissions by using two synthetic inhibitors in an irrigated cropping soil. Geoderma 2020, 373, 114423. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Q.; Kurokawa, J.-I.; Woo, J.-H.; He, K.; Lu, Z.; Carmichael, G.R. MIX: A mosaic Asian anthropogenic emission inventory under the international collaboration framework of the MICS-Asia and HTAP. Atmos. Chem. Phys. 2017, 17, 935–963. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Chen, W.; Xiu, A.; Zhao, H.; Zhang, X.; Zhang, S.; Tong, D.Q. A comprehensive inventory of agricultural atmospheric particulate matters (PM10 and PM2.5) and gaseous pollutants (VOCs, SO2, NH3, CO, NOx and HC) emissions in China. Ecol. Indic. 2019, 107, 105609. [Google Scholar] [CrossRef]
- Shon, Z.-H.; Ghosh, S.; Kim, K.-H.; Song, S.-K.; Jung, K.; Kim, N.-J. Analysis of water-soluble ions and their precursor gases over diurnal cycle. Atmos. Res. 2013, 132, 309–321. [Google Scholar] [CrossRef]
- Tagiyeva, N.; Sheikh, A. Domestic exposure to volatile organic compounds in relation to asthma and allergy in children and adults. Expert Rev. Clin. Immunol. 2014, 10, 1611–1639. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Weber, R.J.; Sullivan, A.P.; Peltier, R.E.; Russell, A.; Yan, B.; Zheng, M.; Edgerton, E. A study of secondary organic aerosol formation in the anthropogenic-influenced southeastern United States. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, X.; Nie, W.; Chi, X.; Xu, Z.; Zheng, L.; Ding, A. Influence of synoptic condition and holiday effects on VOCs and ozone production in the Yangtze River Delta region, China. Atmos. Environ. 2017, 168, 112–124. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef] [PubMed]
- Gouinguené, S.P.; Turlings, T.C.J. The Effects of Abiotic Factors on Induced Volatile Emissions in Corn Plants. Plant Physiol. 2002, 129, 1296. [Google Scholar] [CrossRef] [Green Version]
- Dicke, M.; van Loon, J.J.A.; Soler, R. Chemical complexity of volatiles from plants induced by multiple attack. Nat. Chem. Biol. 2009, 5, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T. Plant volatiles. Curr. Biol. 2010, 20, R392–R397. [Google Scholar] [CrossRef] [Green Version]
- Bruce, T.J.A.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects—Finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef]
- Girón-Calva, P.S.; Li, T.; Blande, J.D. Plant-plant interactions affect the susceptibility of plants to oviposition by pests but are disrupted by ozone pollution. Agric. Ecosyst. Environ. 2016, 233, 352–360. [Google Scholar] [CrossRef]
- Yang, K.; Wang, C.; Xue, S.; Li, W.; Liu, J.; Li, L. The identification, health risks and olfactory effects assessment of VOCs released from the wastewater storage tank in a pesticide plant. Ecotoxicol. Environ. Saf. 2019, 184, 109665. [Google Scholar] [CrossRef]
- Xu, C.; Lin, X.; Yin, S.; Liu, K.; Liu, W. Spatio-vertical characterization of the BTEXS group of VOCs in Chinese agricultural soils. Sci. Total Environ. 2019, 694, 133631. [Google Scholar] [CrossRef] [Green Version]
- Kamruzzaman, M.; Takahama, S.; Dillner, A.M. Quantification of amine functional groups and their influence on OM/OC in the IMPROVE network. Atmos. Environ. 2018, 172, 124–132. [Google Scholar] [CrossRef]
- Sandrini, S.; Fuzzi, S.; Piazzalunga, A.; Prati, P.; Bonasoni, P.; Cavalli, F.; Gilardoni, S. Spatial and seasonal variability of carbonaceous aerosol across Italy. Atmos. Environ. 2014, 99, 587–598. [Google Scholar] [CrossRef]
- Gentner, D.R.; Isaacman, G.; Worton, D.R.; Chan, A.W.H.; Dallmann, T.R.; Davis, L.; Goldstein, A.H. Elucidating secondary organic aerosol from diesel and gasoline vehicles through detailed characterization of organic carbon emissions. Proc. Natl. Acad. Sci. USA 2012, 109, 18318. [Google Scholar] [CrossRef] [Green Version]
- Chow, J.C.; Watson, J.G.; Lowenthal, D.H.; Chen, L.-W.A.; Motallebi, N. Black and Organic Carbon Emission Inventories: Review and Application to California. J. Air Waste Manag. Assoc. 2010, 60, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.L.; Canagaratna, M.R.; Donahue, N.M.; Prevot, A.S.H.; Zhang, Q.; Kroll, J.H.; Worsnop, D.R. Evolution of Organic Aerosols in the Atmosphere. Science 2009, 326, 1525. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Kondo, Y.; Takegawa, N.; Komazaki, Y.; Fukuda, M.; Kawamura, K.; Weber, R.J. Time-resolved measurements of water-soluble organic carbon in Tokyo. J. Geophys. Res. Atmos. 2006, 111. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-L.; Li, J.; Zhang, G.; Zotter, P.; Huang, R.-J.; Tang, J.-H.; Szidat, S. Radiocarbon-Based Source Apportionment of Carbonaceous Aerosols at a Regional Background Site on Hainan Island, South China. Environ. Sci. Technol. 2014, 48, 2651–2659. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Sullivan, A.P.; Weber, R.J.; Ingall, E.D. Characterization of Water-Soluble Organic Carbon in Urban Atmospheric Aerosols Using Solid-State 13C NMR Spectroscopy. Environ. Sci. Technol. 2006, 40, 666–672. [Google Scholar] [CrossRef]
- Bozzetti, C.; El Haddad, I.; Salameh, D.; Daellenbach, K.R.; Fermo, P.; Gonzalez, R.; Prévôt, A.S.H. Organic aerosol source apportionment by offline-AMS over a full year in Marseille. Atmos. Chem. Phys. 2017, 17, 8247–8268. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Li, M.; Zhang, P.; Gong, S.; Zhong, M.; Wu, M.; Zheng, M.; Chen, C.; Wang, H.; Lou, S. Investigation of the sources and seasonal variations of secondary organic aerosols in PM2.5 in Shanghai with organic tracers. Atmos. Environ. 2013, 79, 614–622. [Google Scholar] [CrossRef]
- Achad, M.; Caumo, S.; de Castro Vasconcellos, P.; Bajano, H.; Gómez, D.; Smichowski, P. Chemical markers of biomass burning: Determination of levoglucosan, and potassium in size-classified atmospheric aerosols collected in Buenos Aires, Argentina by different analytical techniques. Microchem. J. 2018, 139, 181–187. [Google Scholar] [CrossRef]
- Simoneit, B.R.T. A review of biomarker compounds as source indicators and tracers for air pollution. Environ. Sci. Pollut. Res. 1999, 6, 159–169. [Google Scholar] [CrossRef]
- Mkoma, S.L.; Kawamura, K.; Fu, P.Q. Contributions of biomass/biofuel burning to organic aerosols and particulate matter in Tanzania, East Africa, based on analyses of ionic species, organic and elemental carbon, levoglucosan and mannosan. Atmos. Chem. Phys. 2013, 13, 10325–10338. [Google Scholar] [CrossRef] [Green Version]
- Fu, P.; Kawamura, K.; Chen, J.; Miyazaki, Y. Secondary Production of Organic Aerosols from Biogenic VOCs over Mt. Fuji, Japan. Environ. Sci. Technol. 2014, 48, 8491–8497. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.F.; Engling, G.; Sai Hang Ho, S.; Huang, R.; Lai, S.; Cao, J.; Lee, S.C. Seasonal variations of anhydrosugars in PM2.5 in the Pearl River Delta Region, China. Tellus B Chem. Phys. Meteorol. 2014, 66, 22577. [Google Scholar] [CrossRef]
- Bhattarai, H.; Saikawa, E.; Wan, X.; Zhu, H.; Ram, K.; Gao, S.; Cong, Z. Levoglucosan as a tracer of biomass burning: Recent progress and perspectives. Atmos. Res. 2019, 220, 20–33. [Google Scholar] [CrossRef]
- Kondo, Y.; Miyazaki, Y.; Takegawa, N.; Miyakawa, T.; Weber, R.J.; Jimenez, J.L.; Worsnop, D.R. Oxygenated and water-soluble organic aerosols in Tokyo. J. Geophys. Res. Atmos. 2007, 112. [Google Scholar] [CrossRef]
- Hecobian, A.; Zhang, X.; Zheng, M.; Frank, N.; Edgerton, E.S.; Weber, R.J. Water-Soluble Organic Aerosol material and the light-absorption characteristics of aqueous extracts measured over the Southeastern United States. Atmos. Chem. Phys. 2010, 10, 5965–5977. [Google Scholar] [CrossRef] [Green Version]
- Sairat, T.; Homwuttiwong, S.; Homwutthiwong, K.; Ongwandee, M. Investigation of gasoline distributions within petrol stations: Spatial and seasonal concentrations, sources, mitigation measures, and occupationally exposed symptoms. Environ. Sci. Pollut. Res. 2015, 22, 13870–13880. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Grosselin, B.; Daële, V.; Mellouki, A.; Mu, Y. Seasonal and diurnal variations of BTEX compounds in the semi-urban environment of Orleans, France. Sci. Total Environ. 2017, 574, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Marć, M.; Bielawska, M.; Simeonov, V.; Namieśnik, J.; Zabiegała, B. The effect of anthropogenic activity on BTEX, NO2, SO2, and CO concentrations in urban air of the spa city of Sopot and medium-industrialized city of Tczew located in North Poland. Environ. Res. 2016, 147, 513–524. [Google Scholar] [CrossRef]
- Hui, L.; Liu, X.; Tan, Q.; Feng, M.; An, J.; Qu, Y.; Cheng, N. VOC characteristics, sources and contributions to SOA formation during haze events in Wuhan, Central China. Sci. Total Environ. 2019, 650, 2624–2639. [Google Scholar] [CrossRef] [PubMed]
- Aumont, B.; Valorso, R.; Mouchel-Vallon, C.; Camredon, M.; Lee-Taylor, J.; Madronich, S. Modeling SOA formation from the oxidation of intermediate volatility n-alkanes. Atmos. Chem. Phys. 2012, 12, 7577–7589. [Google Scholar] [CrossRef] [Green Version]
- Sheng, J.; Zhao, D.; Ding, D.; Li, X.; Huang, M.; Gao, Y.; Zhang, Q. Characterizing the level, photochemical reactivity, emission, and source contribution of the volatile organic compounds based on PTR-TOF-MS during winter haze period in Beijing, China. Atmos. Res. 2018, 212, 54–63. [Google Scholar] [CrossRef]
- Al-Naiema, I.M.; Offenberg, J.H.; Madler, C.J.; Lewandowski, M.; Kettler, J.; Fang, T.; Stone, E.A. Secondary organic aerosols from aromatic hydrocarbons and their contribution to fine particulate matter in Atlanta, Georgia. Atmos. Environ. 2020, 223, 117227. [Google Scholar] [CrossRef]
- Díaz-de-Mera, Y.; Aranda, A.; Martínez, E.; Rodríguez, A.A.; Rodríguez, D.; Rodríguez, A. Formation of secondary aerosols from the ozonolysis of styrene: Effect of SO2 and H2O. Atmos. Environ. 2017, 171, 25–31. [Google Scholar] [CrossRef]
- Jia, L.; Xu, Y. Different roles of water in secondary organic aerosol formation from toluene and isoprene. Atmos. Chem. Phys. 2018, 18, 8137–8154. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.J.; Kim, J.C.; Lee, S.J.; Kim, Y.P. Evaluation of the optimum volatile organic compounds control strategy considering the formation of ozone and secondary organic aerosol in Seoul, Korea. Environ. Sci. Pollut. Res. 2013, 20, 1468–1481. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, H.; Jia, L.; Ge, S. Effect of particle water on ozone and secondary organic aerosol formation from benzene-NO2-NaCl irradiations. Atmos. Environ. 2016, 140, 386–394. [Google Scholar] [CrossRef]
- Yao, L.; Yang, L.; Chen, J.; Wang, X.; Xue, L.; Li, W.; Wang, W. Characteristics of carbonaceous aerosols: Impact of biomass burning and secondary formation in summertime in a rural area of the North China Plain. Sci. Total Environ. 2016, 557, 520–530. [Google Scholar] [CrossRef]
- Zhang, F.; Shang, X.; Chen, H.; Xie, G.; Fu, Y.; Wu, D.; Chen, J. Significant impact of coal combustion on VOCs emissions in winter in a North China rural site. Sci. Total Environ. 2020, 720, 137617. [Google Scholar] [CrossRef]
- Monod, A.; Sive, B.C.; Avino, P.; Chen, T.; Blake, D.R.; Sherwood Rowland, F. Monoaromatic compounds in ambient air of various cities: A focus on correlations between the xylenes and ethylbenzene. Atmos. Environ. 2001, 35, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Nelson, P.F.; Quigley, S.M. The m,p-xylenes:ethylbenzene ratio. A technique for estimating hydrocarbon age in ambient atmospheres. Atmos. Environ. 1983, 17, 659–662. [Google Scholar] [CrossRef]
- Goldan, P.D.; Trainer, M.; Kuster, W.C.; Parrish, D.D.; Carpenter, J.; Roberts, J.M.; Fehsenfeld, F.C. Measurements of hydrocarbons, oxygenated hydrocarbons, carbon monoxide, and nitrogen oxides in an urban basin in Colorado: Implications for emission inventories. J. Geophys. Res. Atmos. 1995, 100, 22771–22783. [Google Scholar] [CrossRef]
- Brocco, D.; Fratarcangeli, R.; Lepore, L.; Petricca, M.; Ventrone, I. Determination of aromatic hydrocarbons in urban air of Rome. Atmos. Environ. 1997, 31, 557–566. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Wang, X.; Lü, S.; Huang, Z.; Huang, X.; Zhang, Q. Spatiotemporal patterns and source implications of aromatic hydrocarbons at six rural sites across China’s developed coastal regions. J. Geophys. Res. Atmos. 2016, 121, 6669–6687. [Google Scholar] [CrossRef]
- Sigsby, J.E.; Tejada, S.; Ray, W.; Lang, J.M.; Duncan, J.W. Volatile organic compound emissions from 46 in-use passenger cars. Environ. Sci. Technol. 1987, 21, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Sagehashi, M.; Terada, A.; Zhou, S.; Li, F.; Hosomi, M. Adequacy of a Simple Diffusion Model to Predict Benzene Behavior in Soil. Soil Sci. Soc. Am. J. 2011, 75, 2147–2157. [Google Scholar] [CrossRef]
- Rossabi, S.; Choudoir, M.; Helmig, D.; Hueber, J.; Fierer, N. Volatile Organic Compound Emissions From Soil Following Wetting Events. J. Geophys. Res. Biogeosci. 2018, 123, 1988–2001. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, G.; Zhang, X.; Huang, K.; Xu, C.; Tang, A.; An, Z. The ion chemistry, seasonal cycle, and sources of PM2.5 and TSP aerosol in Shanghai. Atmos. Environ. 2006, 40, 2935–2952. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, L.; Evans, G.J.; Yao, X. Variability of atmospheric ammonia related to potential emission sources in downtown Toronto, Canada. Atmos. Environ. 2014, 99, 365–373. [Google Scholar] [CrossRef]
- Pathak, R.K.; Yao, X.; Chan, C.K. Sampling Artifacts of Acidity and Ionic Species in PM2.5. Environ. Sci. Technol. 2004, 38, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Wu, W.S.; Wang, T. Summertime PM2.5 ionic species in four major cities of China: Nitrate formation in an ammonia-deficient atmosphere. Atmos. Chem. Phys. 2009, 9, 1711–1722. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Liu, X.; Liu, L.; Dore, A.J.; Tang, A.; Lu, L.; Zhang, F. Impact of emission controls on air quality in Beijing during APEC 2014: Implications from water-soluble ions and carbonaceous aerosol in PM2.5 and their precursors. Atmos. Environ. 2019, 210, 241–252. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).