Abstract
With the increase in global energy requirements, the utilization of fossil fuels has also increased, which has caused global warming. In this study, a process integration framework based on an energy mix system is proposed to simultaneously produce two cleaner fuels (methanol and H2). Aspen Plus is used to develop process models followed by their techno-economic assessment. Case 1 is considered the base case process, where the coal–biomass gasification process is used to produce the synthesis gas, which is further converted into H2 and methanol. Conversely, the case 2 design represents the novel process configuration framework, where the coal–biomass gasification technology in case 1 is sequentially integrated with the methane reforming technology to minimize the energy penalties while increasing the net fuel production. To perform the technical analysis, the fuel production rates, carbon conversion efficiencies and specific energy requirements are compared for both models. It is analyzed from the results that the case 2 design offers higher methanol and H2 production rates with lower energy requirements. Additionally, the specific energy requirement for case 2 is 29% lower compared to the case 1 design, leading to an increase in the process efficiency of case 2 by 3.5%.
1. Introduction
Global warming and greenhouse gas emissions have become one of the biggest debated issues around the globe. With recent industrialization and deforestation, the carbon footprint around the world has increased to dangerous levels during the last few decades. The intergovernmental panel on climate change (IPCC) clearly indicates this in its reports about global warming and the depletion of energy resource [1,2]. A reduction in greenhouse gas emissions can be achieved by either minimizing fuel consumption, by improving the process performance, by reducing the greenhouse gas emissions or by using renewables forms of energy. The current global capacity of renewables is not enough to meet the world energy and supply demand. Therefore, the dependence on conventional fuel resources will remain dominant in the future too. Energy in any form is very valuable around the world to meet societal and industrial needs, and a lot of research has been dedicated to improving the process efficiency or to finding alternative energy resources. Hydrocarbon-based fossil fuels have remained the major source of energy supply during the last few decades, owing to their high efficiency and availability. However, the utilization of fossil fuel based-processes also increased the global CO2 emissions to the highest level ever.
Gasoline, diesel and natural gas are the main fuels that are used in the automotive, chemical, fertilizer and process industries, the use of which steadily increased at a rate of nearly 2–2.5% annually, paralleling the global economic growth, which will be further increased up to 50% by the year 2030 [3]. Compared to conventional fuels used for power generation, methanol and hydrogen are considered not only cleaner fuel sources but also raw materials for many process industries [4]. Oxygenates such as methanol and ethanol are also bended with petroleum fuels to improve various combustion properties such as octane number [5] and emission characteristics such as sooting [6] propensity. Large-scale methanol and hydrogen production processes are based on natural gas feedstocks using intermediate reforming technologies for synthesis gas production. As hydrogen is a key component of methanol production, the increase in methanol production also increases the need for hydrogen production. Yao et al. [7]) also mentioned that the methanol demand has increased two times during the last 5–7 years, which also increases the methanol production requirement by 1.7 times. Methanol can be produced from conventional fuels that can be converted into synthesis gas, mainly composed of CO and H2. For instance, commercially, natural-gas-to-methanol (NGTM) processes are used where the natural gas is first reformed to generate synthesis gases with higher hydrogen-to-carbon (HCR) ratios followed by methanol synthesis. Coal-to-methanol (CTM) production processes have also received a lot of attention due to the lower cost of coal compared to natural gas and the larger reserves around the world. The CTM production process is slightly more complex compared to the natural-gas-to-methanol processes as it contains more unit processes. Khalafalla et al. [8] demonstrated pathways to produce methanol from coal by developing alternative designs and compared the results in terms of carbon conversion, specific energy consumption and methanol production cost.
Reaching a fully sustainable clean energy source for power, electricity and cleaner fuel production is a long ways off, and a lot of research is being focused on finding alternative pathways to increase the efficiency of already existing systems. Castellani et al. [9,10] studied the carbon-neutral process of producing methane, methanol and ammonia using a solar-based renewable hydrogen supply process. Poly-generation processes are under the spotlight for being one of the most promising solutions for this problem. This is because poly-generation processes are flexible in combining multiple energy sources that can enable the sustainable supply of energy demand while meeting environmental regulations in terms of reduced greenhouse gas (GHG) emissions. For a sustainable energy system, available resources should be utilized at the maximum possible efficiency followed by a reduction in GHG emissions. Ng et al. [11] and Jana et al. [12] provided reviews on poly-generation processes using multiple feedstocks with multiple outputs. The poly-generation process can be utilized for co-currently producing multiple chemicals and products including electricity, fuel, chemicals, etc.
In this study, poly-generation process models are discussed, in which coal, natural gas and biomass feedstocks are used for the production of two useful and cleaner fuels (H2 and methanol). Some of the state-of-the-art methanol and H2 production processes involve syngas production from fossil fuels, which is treated in the cleaning and water–gas shift units before the final production and purification of methanol and H2. For instance, the natural-gas-to-methanol (NGTM) process has been extensively used in various industries for the production of both H2 and methanol. The hydrogen-to-carbon (HCR) ratio of 2–2.2 is usually required for methanol production, which can be achieved by steam methane reforming (SMR) processes, which generates a syngas of the HCR ratio of approximately 3:1. Coal to methanol (CTM) has also emerged as an attractive technology to produce H2 due to higher coal reserves globally, but the syngas produced from coal gasification has a drawback of offering a lower amount of HCR in the synthesis gas. This issue is resolved by using additional WGS (water–gas shift) units to enhance H2 production by transforming the CO over the nickel-based catalyst using high-pressure steam. The research studies also showed that retrofitting already developed processes does not need major process modifications to enhance the process sustainability by reducing both the fuel consumption and GHG emissions. Wang et al. [13] and Huang et al. [14] demonstrated the pathways for producing methanol and electricity in the poly-generation process integration framework and employed WGS reactors to achieve the desired HCR in the synthesis gas. Ahmed et al. [15,16] developed the process models based on coal and natural gas feedstocks for generating hydrogen and methanol in a co-production manner. Recently, Rehfeldt et al. [17] discussed the 2030 goal for global energy resources and mentioned the use of biomasses up to 10% with the primary energy resources. Similarly, Bazzanella and Ausfelder [18] developed the process model on using biomasses as a feedstock to explore fuel switch technologies. AlNouss et al. [19] used various biomass feedstocks in the research and highlighted that the dependencies on the conventional fuels can be reduced by energy mix and fuel switch systems. Recently, Hamid et al. [20] and Ahmed et al. [21] also demonstrated a process integration framework using a combination of both gasification and reforming technologies to enhance the power and H2 production capacities. The goal of this work is to investigate the development of standalone models for coal and biomass gasification processes followed by their integration with natural gas reforming units to enhance overall syngas production potentials. Furthermore, the heat exchanger network is developed to utilize the heat from the hot and cold streams to minimize the external energy requirements. This process integration resulted in the utilization of three different feedstocks, which reduced the reliance on a single fuel and improves the H2 and methanol production rates. Finally, a techno-economic analysis is performed for all designs to analyze the project feasibility of the processes.
2. Development of Simulation Model
In this work, Aspen Plus V11 was used as the process simulator to develop the models for the simultaneous methanol and H2 production. The Peng Robinson with Boston Mathias (PR–BM) equation of state was employed as the thermodynamic package for the calculation of physical properties. As the composition of fossil fuels is not consistent globally, the coal and biomass used in this study were modelled as unconventional components in Aspen Plus. Proximate, ultimate and sulfanal analyses were taken from the experimental results to specify the composition and heating values of the feedstock. Table 1 represents the modelling units of all processes developed in Aspen Plus along with their operation conditions.

Table 1.
Design parameter and modelling approach.
2.1. Case 1: Coal and Biomass-Based Model for the Simultaneous Methanol and Hydrogen Production (CBMH Process)
Case 1 is considered the base case model for producing methanol and hydrogen from coal and biomass feedstocks. as represented in Figure 1. The process starts with coal and biomass blending at a weight ratio of 90:10%. The blend feed for the gasifier, which is a mixture of coal and biomass, enters the entrained flow gasifier at 56 bar pressure. A controlled amount of high-purity oxygen (95 vol. %) also enters at the top of the gasifier, which partially oxidizes the feed stream to produce raw syngas. The syngas is then passed through a series of radiant and convective heat exchangers to reduce the temperature to 20 °C, which is further reduced to −35 °C in the AGR unit to remove H2S and CO2. The syngas is then pre-heated to 200 °C and fed to the methanol synthesis unit to produce methanol. As the conversion of syngas to methanol is not complete, the syngas is fed to the WGS unit, where it reacts with steam to convert the CO in the syngas to H2. The mixture of H2 and CO2 is then fed into the CO2 removal unit to capture CO2, and pure H2 is recovered and sent to the storage section.
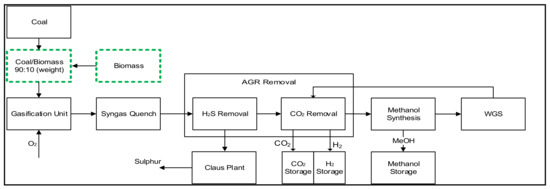
Figure 1.
Process model for converting coal and biomass to methanol and hydrogen.
2.2. Case 2: Co-Production of Methanol and Hydrogen from Gasification and Reformation Models Using Three Fuels (Coal, Biomass and Natural Gas)—CBNMH Process
Case 2 represents the new model and represents the modifications made in case 1. Figure 2 shows the proposed model where the coal + biomass gasification process is integrated with the natural gas reforming process in parallel integration for the simultaneous production of methanol and H2 products. Similar to case 1, the raw syngas obtained from the gasification unit is treated in the AGR unit, where H2S is removed and sent to the Claus plant. However, the location of the CO2 removal unit in case 2 has been changed contrary to case 1. The syngas coming from the reformer is mixed with the H2S-free syngas from the gasification section before being fed to the CO2 removal unit. The heat exchanger network is designed in a way to utilize the heat from the gasification reactor into the reforming reactor. The process configuration in the case 2 design produces a syngas (at syngas mixer) with higher heating value and improves the overall process performance. The CO2 in the syngas is removed in the CO2 removal unit, and the rest of the syngas containing H2 and CO is fed to the methanol synthesis unit, where most of the syngas is transformed into methanol. The un-reacted syngas is directed towards the WGS unit to convert all of the un-reacted CO in the syngas into H2. The mixture of H2 and CO2 is further treated in the AGR unit to remove the CO2 and to recover pure H2.
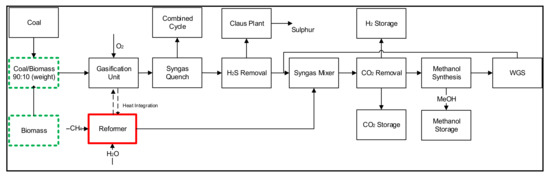
Figure 2.
Process integration framework between reforming and the gasification unit for the production of methanol and hydrogen (CBNMH process).
3. Results and Discussion
This article presents two process models to simultaneously produce methanol and H2 followed by a technical and economic analysis. In case 1, the blend of coal and biomass is partially oxidized in the entrained flow gasification unit using high-purity oxygen to produce syngas at 56 bar pressure and 1370 °C. As the main feedstock is coal in this study, which shares 90% of the total mass flow rate in the gasification unit, the validation of the coal gasification model is conducted with the report published by the Department of Energy [22], as represented in Figure 3. After developing the coal gasification model, biomass is added up to 10% compared to the mass flow rate of the coal. The composition of coal feedstock in combination with the biomass does not vary much; however, the lower cost of the biomass may affect the overall process economics. After the treatment of acid gases in the AGR unit, methanol and hydrogen are produced as already discussed in Figure 1 and Figure 2.
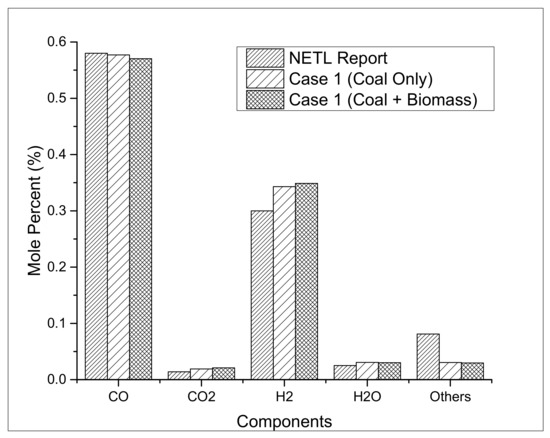
Figure 3.
Validation of process models.
3.1. Equations Used for Comparative Analysis
3.1.1. Process Efficiency
The overall process efficiency for the poly-generation process is an important process performance indicator, which represents the fuel and electricity production per unit of energy consumption, as shown in Equation (1).
3.1.2. CO2-Specific Emission
CO2-specific emissions represent the un-captured CO2 from the process, as represented in Equation (2). In this study, methanol and H2 are produced in the process. Therefore, CO2-specific emissions give an indication of un-captured CO2 during the production of the aforementioned fuels.
3.1.3. Carbon Conversion Efficiency
Carbon conversion efficiency is an important environmental metric that reflects the amount of carbon conversion from the feedstocks to the produced fuel, as represented in Equation (3). In this study, methanol is produced in the process. Therefore, carbon conversion efficiency highlights the carbon flow rates as a part of the methanol per unit feedstock flow rates containing carbon, where CMeOH and Cfeedstock represents the carbon in the methanol and feedstocks, respectively.
3.2. Process Performance Analysis
3.2.1. Methanol and Hydrogen Production Rates
Two case studies are developed in this study for the dual production of methanol and H2. In this study, the fuel production represents the simultaneous production of H2 and methanol. The comparative analysis is performed between two cases to determine the fuel (H2 and methanol) production rates, overall process performance, CO2 emissions and carbon conversion efficiencies. The methanol production rate is calculated as 29.97 kg/s and 37.91 kg/s from the case 1 and case 2 designs, respectively. On the other hand, the hydrogen generation capacity is estimated as 4.22 kg/s and 3.87 kg/s for case 1 and case 2, respectively. The comparative analysis for both cases in terms of methanol and hydrogen flow rates are also represented in Figure 4, where, the case 2 design takes a lead in the methanol production rate between the two cases.
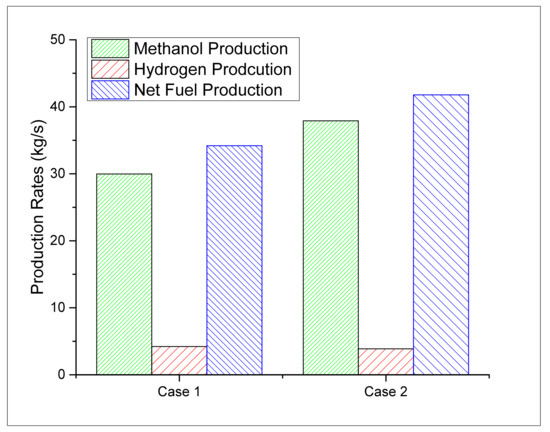
Figure 4.
Hydrogen and methanol production rates.
However, case 1 takes a lead in terms of H2 production rate compared to the case 2 design. The comparative analysis between the two cases showed that the case 2 design offers 22.2% higher overall fuel production rates compared to the case 1 design. The results showed that case 2 offers higher production of the fuel (methanol and H2) and, therefore, requires higher heating and cooling duties compared to case 1. The energy required in the syngas processing is also higher in case 2 due to larger volumes of the syngas. Table 2 provides a summary for both cases in terms of fuel production rates and other process performance indicators for comparative analysis. CO2-specific emission is used for estimating the environmentally friendly nature of the process. The processes showing higher carbon emissions may increase the overall carbon footprint and is therefore less preferred. The comparative analysis between the two cases for the CO2-specific emissions reveals that case 2 has 38% fewer emissions than the case 1 design.

Table 2.
Comparison of case 1 and case 2 in terms of process performance.
3.2.2. Specific Energy Consumption and Process Efficiency
Specific energy consumption and process efficiency indicators were used in this study to evaluate the reliability and sustainability of the process. It can be seen from the results that case 1 and case 2 produce 8.9 kg and 11.5 kg of fuel (including both H2 and methanol) per unit (GJ) energy consumed. By incorporating the heating values of the feedstocks and the fuel produced, the process efficiencies are calculated as 47.1% and 50.6% for case 1 and case 2, respectively. The comparison between the process efficiencies of two cases reveals that case 2 offers 3.5% higher process efficiency and 29% higher fuel production capacity than the base case (case 1) design (Figure 5).
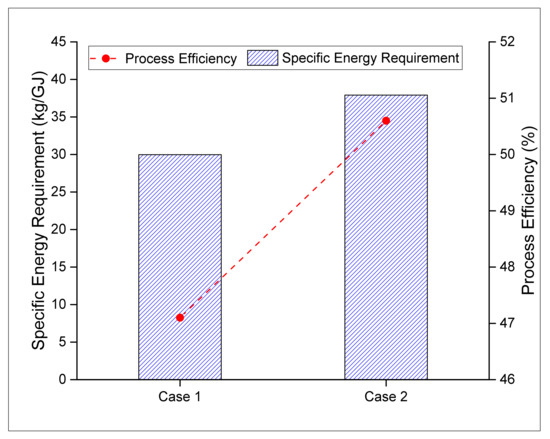
Figure 5.
Process efficiency and energy requirements.
3.2.3. Carbon Conversion and Emissions
The carbon conversion from feedstock to fuel (methanol in this study) and net CO2 emissions are some of the performance indicators that are used to analyse the conversion of carbon from feedstock to product. The comparative analysis between two cases on carbon efficiency (ηc) in case 2 takes a lead of almost 4% over the case 1 design, where the actual ηc of case 1 and case 2 is calculated as 29% and 33%, respectively. Moreover, the CO2-specific emissions for case 1 and case 2 during the production of methanol and hydrogen on a mass basis are estimated to be 2.12 and 1.62, respectively. Figure 6 represents the comparison between CO2-specific emissions and the carbon conversion efficiency of both cases.
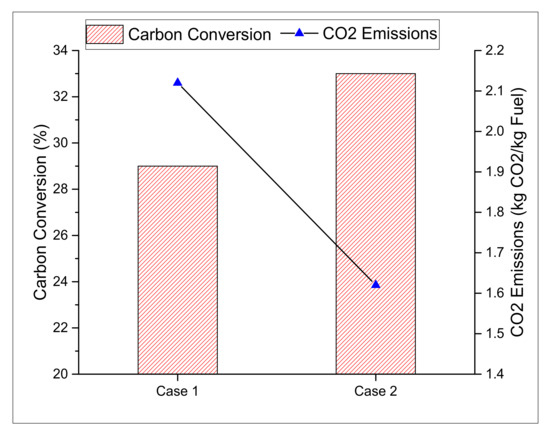
Figure 6.
Comparison of carbon conversion and emissions.
3.3. Economic Analysis
To evaluate the overall sustainability of the process, the capital (CAPEX) and Operational cost (OPEX) have been estimated for both cases. All of the equipment has been sized in Aspen Plus to find the flow rate and size of the equipment. The CAPEX of all equipment is calculated using the capacity, cost of equipment and cost index information, as represented in Equation (4), where the value of x is considered 0.6.
The results show that the CAPEX for case 1 and case 2 is estimated as 2337 M€ and 2578 M€, respectively. Due to the installation of an additional reforming unit, case 2 incurs a higher CAPEX. Moreover, the flow rates of syngas are also higher in case 2, which increases the sizes of all equipment, which also affects the overall CAPEX. Similarly, the OPEX of both cases is calculated on the basis of several assumptions including the feedstock price, catalyst cost and other utility costs. OPEX/yr represents the annual cost of running the plant and is estimated as 270 M€ and 308 M€ for case 1 and case 2, respectively. As natural gas has been used in case 2 as a third fuel, the OPEX/yr for case 2 tends to be higher, as shown in Figure 7. For a reliable analysis, the combination of CAPEX/MT and OEPX/MT during the lifetime of the plant is estimated in terms of fuel production (€/MT) for both cases. The comparative analysis showed that the CAPEX/MT and OPEX/MT required for fuel production are 403.4 M€ and 373.9 M€ for case 1 and case 2, respectively.

Figure 7.
Comparison of capital and operational cost.
While evaluating the process economics, it has been seen that the production cost and selling price of fuels are highly influenced by the CAPEX and OPEX. Table 3 highlights the detailed results of the economic analysis for both cases. Keeping the discount rate at 10%, the selling price of fuel is calculated as 443.74 and 411.30 €/MT of fuel. The cost breakdown reveals that the methanol selling price is significantly lower for the case 2 design. On the other hand, the H2 selling price is lower for the case 1 design. While comparing the results obtained from economic analysis, case 2 reflected a better process feasibility and offers lower fuel production prices.

Table 3.
Economic analysis results for case 1 and case 2.
4. Conclusions
This study involves the development of two process models for the simultaneous production of methanol and H2. Case 1 used the coal and biomass-based gasification process for the production of two clean fuels, namely methanol and hydrogen. On the other hand, the case 2 design integrates both the gasification and reforming techniques while using three different feedstocks, namely coal, biomass and natural gas, for the co-production of fuels, i.e., H2 and methanol. By conducting the technical and economic analysis, it has been found that case 2 outperforms the case 1 design in terms of process efficiencies and economics. The methanol and hydrogen production rates from case 1 and case 2 are calculated as 29.97 kg/s and 4.22 kg/s, and 37.91 kg/s and 3.87 kg/s, respectively. The comparative analysis between the two cases reveals that case 2 offers 3.5% higher process efficiency. Moreover, the analysis on the process economics showed that the fuel production/selling prices in case 2 are 27.8% less compared to the case 1 design. It has been analysed from the results that the proposed integration of reforming technology with the gasification process has a potential to improve the overall process performance. Moreover, retrofitting existing processes is also possible to incorporate the proposed design along with an efficient heat exchanger network that can improve the overall process economics of existing processes.
Author Contributions
Conceptualization, U.A.; methodology, U.A. and U.Z.; Software, U.A.; Formal analysis, H.A.; Investigation, N.A. (Nauman Ahmad); Writing—original draft preparation, U.A.; writing—review and editing, U.Z., A.G.A.J., N.A. (Nauman Ahmad), S.A.O., N.A. (Nabeel Ahmad) and H.A.; visualization, A.G.A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deanship of Research Oversight and Coordination (DROC) at King Fahd University of Petroleum & Minerals (KFUPM), grant number SB201019 and The APC was funded by DROC.
Acknowledgments
The authors acknowledge the support provided by the Deanship of Research Oversight and Coordination (DROC) at King Fahd University of Petroleum & Minerals (KFUPM) for funding this work through Project No. SB201019.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| AGR | Acid Gas Removal |
| CAPEX | Capital Expenditure |
| CBMH | Coal and Biomass to Methanol and Hydrogen |
| CBNMH | Coal Biomass and Natural Gas to Methanol and Hydrogen |
| CTM | Coal to Methanol |
| GHG | Global Greenhouse Gas |
| HCR | Hydrogen-to-Carbon Ratio |
| IPCC | Intergovernmental Panel on Climate Change |
| NGTM | Natural Gas to Methanol |
| OPEX | Operational Expenditure |
| SMR | Steam Methane Reforming |
| WGS | Water–Gas Shift |
References
- Jameel, A.G.A.; van Oudenhoven, V.; Emwas, A.-H.; Sarathy, S.M. Predicting Octane Number Using Nuclear Magnetic Resonance Spectroscopy and Artificial Neural Networks. Energy Fuels 2018, 32, 6309–6329. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, U. Techno-economic analysis of dual methanol and hydrogen production using energy mix systems with CO2 capture. Energy Convers. Manag. 2021, 228, 113663. [Google Scholar] [CrossRef]
- Ahmed, U. Techno-economic feasibility of methanol synthesis using dual fuel system in a parallel process design configuration with control on green house gas emissions. Int. J. Hydrogen Energy 2020, 45, 6278–6290. [Google Scholar] [CrossRef]
- Ahmed, U.; Zahid, U.; Lee, Y. Process simulation and integration of IGCC systems for H2/syngas/electricity generation with control on CO2 emissions. Int. J. Hydrogen Energy 2019, 44, 7137–7148. [Google Scholar] [CrossRef]
- AlNouss, A.; McKay, G.; Al-Ansari, T. A techno-economic-environmental study evaluating the potential of oxygen-steam biomass gasification for the generation of value-added products. Energy Convers. Manag. 2019, 196, 664–676. [Google Scholar] [CrossRef]
- Bazzanella, A.M.; Ausfelder, F. Low Carbon Energy and Feedstock for the European Chemicalindustry (Technical Study). In Dechema, Ges. für Chem. Tech. Biotechnol. eV; Frankfurt am Main, Germany, 2017; Available online: https://dechema.de/en/Low_carbon_chemical_industry-path-123212,124930.html (accessed on 29 June 2021).
- Blumberg, T.; Tsatsaronis, G.; Morosuk, T. On the economics of methanol production from natural gas. Fuel 2019, 256. [Google Scholar] [CrossRef]
- Castellani, B.; Gambelli, A.M.; Morini, E.; Nastasi, B.; Presciutti, A.; Filipponi, M.; Nicolini, A.; Rossi, F. Experimental Investigation on CO2 Methanation Process for Solar Energy Storage Compared to CO2-Based Methanol Synthesis. Energies 2017, 10, 855. [Google Scholar] [CrossRef]
- Castellani, B.; Rinaldi, S.; Morini, E.; Nastasi, B.; Rossi, F. Flue gas treatment by power-to-gas integration for methane and ammonia synthesis—Energy and environmental analysis. Energy Convers. Manag. 2018, 171, 626–634. [Google Scholar] [CrossRef]
- Edenhofer, O. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: New York, NY, USA, 2015; Volume 3. [Google Scholar]
- Zoelle, A.; Keairns, D.; Turner, M.J.; Woods, M.; Kuehn, N.; Shah, V.; Chou, V.; Pinkerton, L.L.; Fout, T. Cost and Performance Baseline for Fossil Energy Plants Volume 1b: Bituminous Coal (IGCC) to Electricity Revision 2b—Year Dollar Update; United States Departmeant of Energy: Washington, DC, USA, 2015.
- Hamid, U.; Rauf, A.; Ahmed, U.; Shah, S.A.S.; Ahmad, N. Techno-economic assessment of process integration models for boosting hydrogen production potential from coal and natural gas feedstocks. Fuel 2020, 266, 117111. [Google Scholar] [CrossRef]
- Huang, H.; Yang, S.; Cui, P. Design concept for coal-based polygeneration processes of chemicals and power with the lowest energy consumption for CO2 capture. Energy Convers. Manag. 2018, 157, 186–194. [Google Scholar] [CrossRef]
- Jameel, A.; Gani, A. Predicting Sooting Propensity of Oxygenated Fuels Using Artificial Neural Networks. Processes 2021, 9, 1070. [Google Scholar] [CrossRef]
- Jana, K.; Ray, A.; Majoumerd, M.M.; Assadi, M.; De, S. Polygeneration as a future sustainable energy solution—A comprehensive review. Appl. Energy 2017, 202, 88–111. [Google Scholar] [CrossRef]
- Khalafalla, S.S.; Zahid, U.; Jameel, A.G.A.; Ahmed, U.; Alenazey, F.S.; Lee, C.-J. Conceptual Design Development of Coal-To-Methanol Process with Carbon Capture and Utilization. Energies 2020, 13, 6421. [Google Scholar] [CrossRef]
- Moavenzadeh, J.; Torres-Montoya, M.; Gange, T. Repowering Transport: Project White Paper; World Economic Forum: Geneva, Switzerland, 2011. [Google Scholar]
- Ng, K.S.; Zhang, N.; Sadhukhan, J. Techno-economic analysis of polygeneration systems with carbon capture and storage and CO2 reuse. Chem. Eng. J. 2013, 219, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Pachauri, R.K.; Meyer, L. Climate Change 2014 Synthesis Report-Summary for Policymakers; Intergovernmetnal Panel on Climate Change (IPCC): Geneva, Switzerland, 2014. [Google Scholar]
- Rehfeldt, M.; Worrell, E.; Eichhammer, W.; Fleiter, T. A review of the emission reduction potential of fuel switch towards biomass and electricity in European basic materials industry until 2030. Renew. Sustain. Energy Rev. 2020, 120, 109672. [Google Scholar] [CrossRef]
- Wang, X.; Demirel, Y. Feasibility of power and methanol production by an entrained-flow coal gasification system. Energy Fuels 2018, 32, 7595–7610. [Google Scholar] [CrossRef]
- Yao, C.; Pan, W.; Yao, A. Methanol fumigation in compression-ignition engines: A critical review of recent academic and technological developments. Fuel 2017, 209, 713–732. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).