Abstract
The last two decades have witnessed increasing use of X-ray imaging and, hence, the exposure of humans to potentially harmful ionizing radiation. Computed tomography accounts for the largest portion of medically-related X-ray exposure. Accurate knowledge of ionizing radiation dose from Cone-Beam CT (CBCT) imaging is of great importance to estimate radiation risks and justification of imaging exposures. This work aimed to review the published evidence on CBCT dose estimation by focusing on studies that employ Geant4-based toolkits to estimate radiation dosage. A systematic review based on a scientometrics approach was conducted retrospectively, from January 2021, for a comprehensive overview of the trend, thematic focus, and scientific production in this topic. The search was conducted using WOS, PubMed, and Scopus databases, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. In total, 93 unique papers were found, of which only 34 met the inclusion criteria. We opine that the findings of this study provides a basis to develop accurate simulations of CBCT equipment for optimizing the trade-off between clinical benefit and radiation risk.
1. Introduction
X-ray medical imaging has undergone tremendous technological advances for the diagnosis and treatment of a breadth of examinations and procedures [1]. In particular, due to its rich and robust image information and shorter examination time [2,3,4,5], cone-beam computed tomography (CBCT) has become standard in recent clinical applications as adaptive radiotherapy treatment [6], image-guided radiation therapy (IGRT) [6,7,8,9,10,11,12], maxillofacial applications [13,14,15], cone-beam breast tomography (CBBCT) [16,17,18,19,20,21,22,23], or proton computed tomography for particle therapy [24,25,26,27,28]. CBCT is a new technology using a cone-shaped beam and a detector that rotate 360° around the patient, able to acquire projected data in a single rotation [29].
Nevertheless, these advances have resulted in a rise in radiation dose, being the medical exposure to ionizing radiation from CT examinations a major issue of concern nowadays [30,31,32,33,34,35,36]. Ionization radiation exposure can induce harmful biological effects, depending on the radiosensitivity of the organ, radiation dose, dose distribution, age, gender, or genetic factors [37]. Due to the harmfulness of X-rays for living beings, the ALARA principle, “as low as reasonably achievable”, has become the criterion to apply for medical X-ray imaging [31,38]. This criterion has brought positive effects on protocol standardization [39,40,41,42].
Although CBCT imaging dose is known to be smaller than typical CT scan, it cannot be neglected. Indeed, an overuse of CBCT procedures may increase the risk of the secondary side effects [43]. Lifetime attributable risks (LARs) of cancer incidence for several solid tumors have been classified by patient age and time of exposure [44]. Statistically significant dependence of relative risk (RR) of leukemia and brain cancer incidence following CT scans in childhood was first reported by Pearce et al. [45] showing a linear-no-threshold risk hypothesis [46]. LARs and RR of cancer incidence for various organs in standard- and low-dose CBCT modes from a single scan were analyzed showing that the highest LARs are for incidence of colon and bladder cancers, while the highest RRs are for incidence of stomach and liver cancers for the abdominal CBCT [47]. Therefore, accurate evaluation of the dose delivered from CBCT scans is crucial to provide physicians’ awareness of radiation risks [48].
One of the most common applications of CBCT is image-guided procedures during proton therapy [49]. This technique improves the accuracy of radiation therapy but significantly contributes to an extra radiation dose to cancer patients, thus increasing the risk of second malignant neoplasms [50,51] and other side-effects, as in Reference [52]. These effects are related to different factors as age at irradiation, type of irradiated tissue or volume, treatment technique, or previous radiological procedures [46,53]. Besides, the potential risks of second cancers from scattered proton radiotherapy for childhood cancers is a cause of major concern [54]. Indeed, the use of low-dose mode to avoid unnecessary radiation exposure is recommended in the particular case of pediatric IGRT [47].
In response to the increasing awareness, large-scale efforts have been made to develop new methodologies for improving radiation protection standards [55,56]. Monte Carlo (MC) simulations of the radiation transport and interaction are a powerful method for dose distributions calculations in complex geometries and the presence of extended spectra of multi-radiation sources [57,58,59,60,61,62]. Not only can they accurately predict the distributions of absorbed doses but also other amounts of interest in radiation procedures, as well as the evaluation of the image quality [8]. During the last few years, MC techniques have played a major role in dosimetry; however, until its development, any comprehensive dosimetric study was thought to be impossible and dangerous.
Current applications of the MC method in medical physics cover a wide variety of topics, such as radiation protection, diagnostic radiology, radiation therapy, nuclear medicine, and the design and optimization of imaging protocols. Nevertheless, there is still a wide need to adapt and validate the MC simulation codes in dosimetry studies in order to unveil its potential for testifying more radiation protection measures and also new techniques to reduce the high computational cost required.
Although there is a wide range of MC codes capable of simulating radiation transport [63,64,65,66,67,68,69], not all available codes are capable of precisely handling all radiation-induced aspects, and they require very high computational costs and advanced programming skill. Among all of the above, it is worth highlighting the software Geant4, GEometry And Tracking [68,69], which is a flexible set of tools for simulating the passage of particles through matter by iteratively calculating the trajectories and interactions between particles and atoms. One of the advantages of this toolkit is the capacity of simulating the most physical processes in the areas of high-energy physics, nuclear, space, and medical physics, among others [70,71,72,73]. In addition, due to its great functionality, there are other architectures based on Geant4 with specific applications for radiation therapy and dosimetry applications, such as GATE (Geant4 Application for Emission Tomography) [74,75,76], or GAMOS (Geant4-based Architecture for Medicine Oriented Simulation), which has a set of field-specific utilities for nuclear medicine, radiotherapy, hadrontherapy, and radiation protection [77,78,79,80,81,82,83].
Since CBCT is a quite novel development in clinical practice, data on radiation doses and possible effects of CBCT are still being gathered and analyzed. Therefore, it is worth elucidating knowledge on CBCT applications that estimate delivered dosage using Geant4 simulation toolkit.
Bibliometric analysis in academic medicine is a relatively new suitable method to analyze a large amount of information extracted from scientific databases by using quantitative tools [84,85,86,87,88]. The aim of this systematic review (SR) is to investigate the most impactful and influential studies on CBCT applications using Geant4 architecture to estimate the dose, unraveling the following research questions (RQ):
- RQ1: What are the most significant current publications estimating radiation dose using Geant4 in CBCT applications?
- RQ2: Which are the most common CBCT applications that use Geant4 simulations to estimate the dose and how have they changed over the years?
- RQ3: How advances in Geant4-based CBCT dose estimator may help to evaluate the risk of developing stochastic effects?
- RQ4: What are the current research trends and disciplines for prospective research on this topic?
A holistic understanding of these results may contribute to the development of new theoretical and applied works that can be used to support new research from this field.
The manuscript is organized as follows. Section 2 exposes the search methodology to find all relevant papers by defining a suitable search query in Section 2.1, the inclusion and exclusion criteria in Section 2.2, and the data extraction and analysis process in Section 2.3. Next, Section 3 presents individually the obtained results and the corresponding discussion where the above RQ are answered. Finally, the conclusions are presented in Section 4.
2. Methodology
2.1. Literature Search
A SR of electronic data bases was conducted retrospectively between January 1975 and January 2021. The identified studies were subjected to pre-identified inclusion criteria according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement based on key words and MeSH terms specific to publications indexed in PubMed, Web of Science (WOS), and Scopus databases.
The search strategy was restricted to English-language papers via WOS, Scopus, and Pubmed. To construct a suitable query and increase the sensitivity of the search strategy, the research question included the following combined terms and their synonyms and related topics: “Geant4”, “GATE”, “GAMOS”, “radiation dosage”, “radiation protection”, “dose*”, and “cone-beam computed tomography”.
For the purpose of this search, these terms were defined as:
- Geant4 OR GATE OR GAMOS to include all the toolkit based on these codes.
- Cone beam computed tomography according to medical subject headings (MeSH).
- Cone beam computed tomography as a generic term that refers to the medical imaging technique with a conic/pyramidal X-ray beam of radiation.
- Radiation dosage and radiation protection according to medical subject headings (MeSH).
- Dose OR Dosimetry as a generic term including all types of doses described by the International Commission on Radiological Protection (ICRP) [38].
The final analysis contained primary research articles estimating physician knowledge of radiation dosage of cone-beam computed tomographies based on Geant4. These results were supplemented by a literature hand search. The full query is displayed in Table 1.

Table 1.
Search strings, keywords, and items per database.
2.2. Inclusion and Exclusion Criteria
Research studies based on publications estimating radiation dose using Geant4 in different CBCT applications published in international peer-reviewed journals were included. Reviews, technical notes, conference proceedings, reports, and articles were considered.
The exclusion criteria were:
- not medical use;
- papers that do not focus on CBCT;
- studies that do not focus on humans; and
- papers that do not estimate the dose using Geant4-based toolkits.
2.3. Data Extraction and Analysis
Three examiners performed the eligibility and quality assessment of relevant studies. To construct a query suitable for WOS, PubMed, and Scopus, we extracted the following important information: author, journal, year of publication, citations, country or region, source, keywords, and CBCT applications. We also searched the reference lists of the studies encountered. We examined key elements of their titles, abstracts, and keywords analyzing the obtained results. If this information did not give enough evidence about the eligibility, the full text was screened. Furthermore, the whole text was also screened if one of the authors detected that one of the inclusion criteria was not accomplished. We also individually extracted the simulation toolkit (source Geant4, GATE, GAMOS), as well as the specific application (breast imaging, proton therapy, IGRT, etc.) from each study. In case of the author’s disagreement over the study selection or data extraction, the issue was solved by consensus discussion among the reviewers. The selected articles were ranked based on the total citations received from the three databases.
The screening of the results from the literature search, based on title and abstract reading, revealed that 41 papers were potentially eligible for full-text reading. After examination of the full texts, papers that did not meet the inclusion criteria were excluded. The principal reasons for exclusion were: studies did not focus on humans (1 article); not CBCT applications (1 article); dose was not evaluated by MC simulations (2 articles); dose was not estimated by Geant4 codes (1 article). Each stage of the search and the eligibility screening processes with the number of papers identified, included, and excluded is illustrated in Figure 1.
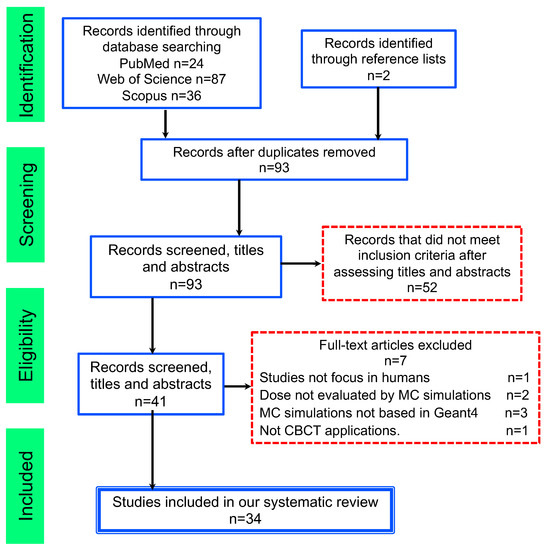
Figure 1.
Flowchart consistent with preferred reporting items for systematic reviews (PRISMA) statement.
To answer the proposed research question, we performed a bibliometric analysis based on Bibliometrix R-package for scientometric factors and biblioshiny [89]. For visualization, the author’s name, corresponding author’s country, the total number of publications, author’s keywords, and keywords plus were generated. For keywords, the co-occurrence of keyword and keyword plus and abstract phrases were evaluated. VOSviewer (version 1.6.16) was also used to perform a cluster analysis and construct a knowledge map of the topics.
3. Results and Discussion
Finally, we found 34 studies that satisfied the inclusion criteria coming from 14 different sources between 2008 and 2021. An overview of this information is shown in Table 2, which displays the main information about the selected papers, and Table 3, which presents the selected publications regarding author, application, and year of publication. Below, the studies from this selection will be discussed individually, addressing the aforementioned research questions.

Table 2.
Main information about selected articles.

Table 3.
Overview of extracted data from papers regarding reference named by first author, applications, and year of publication.
3.1. RQ1: What Are the Most Significant Current Publications Estimating Radiation Dose Using Geant4 in CBCT Applications?
In order to answer this question, special attention is paid to the most influential references and the author’s productivity and relevance. Productivity was measured by the number of publications of each author. Influence was taken into account by the number of total citations (TC) received by the articles included in our selection from documents indexed on the different bibliographic databases. Table 4 presents the top 10 cited articles per author and journal, comparing the TC, TC per year, and the Local citations (LC) and its percentage. LC are citations received by a certain article internally in our selection. The three most influential papers in dosimetry studies by Geant4 simulation were Sechopoulos I. et al. [121], with 63 TC, an average of 5.25 cites per year, 4 LC, and 6.35% of LC; Chen L. et al. [123], with 37 TC and an average of 2.64 cites per year; and Chen L. et al. [122], with 26 TC and an average of 2.0 cites per year. The main topic addressed in his authored and co-authored publications corresponds to Cone-beam breast CT applications.

Table 4.
Top 10 cited articles per author and journal. TC column shows the total citations, TC per year are the total citations per year, and LC are local citations, while LC % are the percentage of local citations.
Figure 2 illustrates the progression of publications available in the selected databases in the period 2008–2020. Despite the fact that our research was conducted retrospectively from 1975, since CBCT is a quite new development in clinical practice, and Geant4 is a recent simulation toolkit, the first publications combining both technologies appear in 2008. There was an upsurge in publications, from just 2 documents published in 2008 to 7 documents in 2017. The main reason for this surge may be attributed to the increase in CPU power and the development of MC simulations integrating new techniques to reduce the high computational cost required as explained in References [90,93,94,96,99,100,104]. The dashed blue line denotes the average TC per year showing two peaks in 2010 and 2016 corresponding to the references [110,121], respectively.
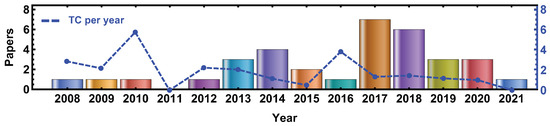
Figure 2.
Annual publication trend of selected documents retrieved from WOS, PubMed, and Scopus in the period 2008–2021. The blue dashed line correspond to the average TC per year.
A further insight into the most relevant publications in terms of author’s productivity, author’s co-citation network, collaboration countries and co-occurrence average citation of the most relevant author’s keywords is described in the Supplementary Material.
3.2. RQ2: Which Are the Most Common CBCT Applications That Use Geant4 Simulations to Estimate the Dose, and How Have They Changed over the Years?
To identify the main research themes, the co-occurrence keyword network map shown in Figure 3 was created by selecting co-occurrence as the target variable [87]. The size of a node is determined by the frequency of its occurrence as a keyword, while the line thickness connection of two terms shows how frequently they co-occurred as keywords. The most frequent keywords, such as “MC simulation”, “CBCT”, “dosimetry”, “computed-tomography”, “radiotherapy”, or “Geant4”, have the biggest sizes. We can distinguish 5 different intertwined nodes sharing the most common terms, but which, in turn, are grouped into more specific topics. The annual tendency show that around 2010 (blue-green colors) the articles were related to “breast imaging”, “mammography”, and “image-quality”, while, around 2015 (green-yellow colors), the main topic was “dosimetry” involving “mc simulations” and “radiotherapy” and “geant4” by 2017. The latest articles (orange-red colors) are related to “validation”, “cancer”, and “radiation dose’.
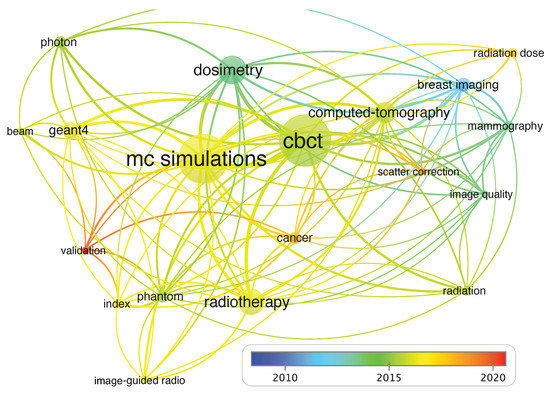
Figure 3.
Co-occurrence network of keywords. The thicker line indicates a strong association between those keywords, while thinner lines depict weak association.
Regarding Table 3, the most common application of Geant4-based toolkit are dedicated to breast imaging, such as CBCCT [90,109,110,118,120,121,122,123]. Tseng et al. found a potential for radiation dose reduction estimating the normalized glandular dose coefficients (DgNCT) that provide air kerma-to-mean glandular dose conversion factors by GATE software, version 8 [90]. GATE was also used in Reference [109] simulating a multi-energy spectrum to radiate same-size built-in calcifications and lump breast motifs under the condition of the same number of particles, while References [110,118,120,121,122,123] investigate the feasibility of Geant4 codes to estimate the dose in CBBCT.
The second most common application corresponds to IGRT [101,116,117]. In the first publication in 2013, S. Bartzch et al. present a novel analytical method that can be employed in a fast treatment planning system for kV photon dose calculations in IGRT [117]. They validate their results by simulations performed with the Geant4 toolkit and found that dose calculation times can be significantly reduced in convolution-based algorithms. In 2018, S. Leotta et al. evaluated organ doses in IGRT due to CBCT and therapeutic MV irradiation in head-neck, thorax, and pelvis districts through GAMOS simulation toolkit [101]. After verification by comparing with experimental quality indexes, they found that scattered radiation in therapy was one to two orders of magnitude larger than that diffused by CBCT. GAMOS toolkit was also used for a deeper understanding of late toxicity risk in external beam radiotherapy patients by simulating dose depositions integrated from different sources over the entire treatment plan in the reference [113].
Besides, using GATE simulation tool, dose deposited during MV-CBCT procedure was simulated and compared to measurements obtained with dedicated detectors in References [104,105], being able to reconstruct imaged volumes and to estimate doses deposited by simple and complex irradiation fields. GATE was also employed by K. Son et al. to investigate the imaging radiation dose delivered to radiosensitive organs from IGRT [116] and the imaging radiation doses resulting from six different acquisition protocols for the adult and pediatric numerical XCAT phantoms [107]. In the first article, they calculated the absorbed doses of head-and-neck, chest, abdomen, and pelvis regions, in both standard CBCT scan mode (125 kVp, 80 mA, and 25 ms) and low-dose scan mode (125 kVp, 40 mA, and 10 ms). They found that the reduction in the radiation dose in the low-dose mode compared to the standard mode was about 20%. In the second article, they provide a platform for developing a new kV-CBCT scanning protocol in conjunction with a low-dose capability. A few years later, the same first author successfully demonstrated the feasibility of incorporating a tube current modulation method in a kV-CBCT system for IGRT to optimized dose reductions without degrading image quality [116]. Thus, the results from these studies would significantly facilitate decisions regarding the administration of extra imaging doses to radiosensitive organs and may reduce the occurrence probability of adverse effects on health derived from radiation exposure.
Proton therapy is a promising high resolution image reconstruction modality applied to CBCT that maintain the improved stopping power estimation of proton CT. Hansen et al. [115] propose a method to overcome the problem of limited angles using a dual modality reconstruction combining the proton data with a CBCT. However, it does not fully achieve the quality of a 360° proton CT scan. In 2018, O. Ardenfors et al. accurately characterized a proton gantry-mounted CBCT system. To examine the influence on organ doses from different acquisition modes and repeated imaging, they used two MC codes: MCNP6 and GATE. They found that organ doses significantly fluctuated depending on the acquisition mode, recommending posterior scans [97,98]. T.E. Marchant also proved that the CBCT imaging doses simulated by GATE depend greatly on the imaging isocenter location, patient sex, and partial rotation angles [108].
A method to estimate dose accuracy in volumetric modulated arc therapy treatment plans by MONACO, an independent Geant4-based dose calculation algorithm, is presented in Reference [119]. J.H. Choi et al. propose new dose point measurement-based method to characterize the dose distributions and the mean dose from a single partial rotation of a CBCT system over a stationary, large, body-shaped phantom validated by a Geant4 simulation [112].
From 2018 to 2020, some publications have been dedicated to developing new software tools for rapidly and accurately estimating scatter in x-ray projection images by solving the deterministic linear Boltzmann transport equation (LBTE) [92,96,100]. These methods use Linear Discontinuous Finite Elements, Multigroup, and Discrete Ordinates methods to maintain the accuracy of a continuous solution. To solve the high computational complexity and achieve the speeds required for clinical utilization, implementation in CUDA allowed to exploit the parallel computing capabilities of GPUs. M. Shi et al. propose a novel strategy to accelerate simulations by also using a Geant4-based GPU code for photon transport for the simulation of an MV-CBCT [93].
3.3. RQ3: How Advances in Geant4-Based CBCT Dose Estimator May Help to Evaluate the Risk of Developing Stochastic Effects?
Estimating potential risks of developing stochastic from a CBCT scanning protocol requires analysis of both the radiation dose to specific organs and the risk of developing health effects as a result of this dose. To this aim, we study the quantities of interest that provided organ doses [124] reported in our bibliographic dataset for the most common applications described in the previous section.
Radiological doses from CBCT are closely related to the field of view, the imaging detector, and voxel sizes used for scanning and can be borderline between low (<1 mGy) and high radiological doses (5–100) mGy [125]. The potential health effects from radiation do not just depend on the absorbed dose but also on how sensitive a certain organ is to radiation [37]. The use of daily standard mode CBCT can result in up to 1.5–2 Gy to some critical organs and an effective dose of 600–800 mSv to the body, which may induce an additional secondary cancer risk of 3–4% [126]. As an example, a daily standard mode CBCT for a prostate of 35 fractions protocol may deliver an additional effective dose of 800 mSv to the patient, which could induce an additional secondary cancer risk of 4%. If only weekly standard mode CBCT were done, the additional risk of secondary cancer would be 0.8%. A chest treatment of 25 to 30 fractions with daily standard mode CBCT can result in an additional effective dose of 600–700 mSv, which could induce an additional secondary cancer risk of 3–3.5% [126].
High-precision radiotherapy is crucial when the surrounding tissues are highly radiosensitive [127]. IGRT can reduce the treatment margin and reduce normal tissue exposure, but it also adds a dose to patients, and its contribution has to be assessed and minimized [126]. The dose delivered, in both standard CBCT scan mode and low-dose scan mode, was studied in different treatment sites as the head-and-neck, chest, abdomen, and pelvis regions [116]. The reduction in the radiation dose in the low-dose mode compared to the standard mode was about 20%. The average absorbed doses in standard mode doses of the organs in the head and neck and chest regions 40.9–82.8 mGy and 43.0–74.8 mGy for the abdomen and pelvis region, while, for low-dose, ranged 16.1–18.9 mGy and 7.9–18.5 mGy, respectively. The variation in organ doses between the different imaging protocols is a consequence of the employed imaging parameters. The average organ doses from 360° scans for the head, thorax, and pelvis protocols were calculated with values between 6–8 mGy, 15–17 mGy, and 24–54 mGy, respectively [98]. Absorbed doses to organs located inside the FOV ranged between 6–54 mGy per 360° scan translating into cumulative doses from repeated daily CBCT imaging of 0.2–1.6 Gy.
Organ doses in IGRT in head-neck, thorax, and pelvis districts were also evaluated due to CBCT and therapeutic MV irradiation [101]. The results showed that the scattered radiation during therapy (MV range) is larger than that diffused by CBCT by one to two orders of magnitude; therefore, it allows to optimize the dose delivery, and it also does not cause excessive dose absorption to the organs at risk. Similarly, the imaging organ doses for the six different CBCT scanning protocols doses were calculated to range from 0.1–24.1 mGy for the adult phantom, and up to 36.8 mGy for the pediatric phantom in Reference [107]. In contrast, the imaging dose to the pediatric phantom in the chest region turns out to be 1.5–2 times higher than that of the adult phantom. For the female organs in the pelvic region, such as the ovary, uterus, and vagina, the imaging doses for the pediatric phantom were calculated to be 1.6–2.24 times higher than those for the adult phantom. These results may shed light on this issue, providing reference data for clinicians to make better decisions on the related clinical procedures, particularly for the pediatric patients. Furthermore, the feasibility of implementing a tube current modulation method to provide optimized dose reductions without degrading image quality has been successfully proven in a kV-CBCT system for IGRT [102].
On the other hand, breast cancer is becoming increasingly common among young women [128]. CBBCT improves breast cancer detection and characterization providing high-quality images with the potential to better visualize overlapping breast tissue compared to mammography or ultrasound [129,130,131]. However, there is a lack of standardized operation criteria in CBBCT [109]. The mean glandular dose using the CBBCT automatic tube current settings can vary from 5.5–17.5 mGy, depending on the breast size and composition [121]. The glandular dose distribution inside the breast can vary by up to 50% of the mean value, while, for a typical mammographic acquisition, the glandular dose distribution varies from 15–400% of the mean. Lower dose values can be achieved if lower tube current settings are selected instead of those automatically suggested by the system, although the image quality may be reduced, as well. Chen et al. proposed that it is possible to selectively image a VOI with a reduced breast dose and scatter effect [122]. Furthermore, Lanconelli et al. [118] found that the more energetic beams provide a more uniform dose distribution than at low energy. Dose values within a PMMA phantom ranged from 0.28–0.46 mGy (at 50 kVp), and 0.44–0.60 mGy (at 80 kVp), while the 50 kVp beam presented an almost double coefficient of variation (ratio between the standard deviation and the mean of the distribution) than that for the 80 kVp beam. A recent study proposed a CBBCT system with an offset detector that estimates the normalized glandular dose coefficients with a potential dose reduction [90].
Let us remark that it is necessary to consider the tradeoffs between image quality and radiation dose. The ALARA principle does not necessarily mean the lowest radiation dose, an accurate diagnosis must be achieved. Conventional methods for improving image quality (scatter and artifacts reduction) based on physical equipment or measurements may lead to a dose increase. S. Vedantham et al. proposed a method that fulfills the necessary conditions for data sufficiency employing a circle-plus-line trajectory instead of a single circular trajectory with less than 0.18% increase in average glandular dose to the breast per projection [120]. In addition, a precomputed scatter library-based software approach to suppress scatter without a dose or scan time increase has been recently proposed by M. Shi et al. [110]. However, a very promising solution based on deep learning-based methods which uses MC simulations results as real data to train a CNN for image restoration and scatter correction has demonstrated high efficiency, accuracy, and reliability [132].
3.4. RQ4: What Are the Current Research Trends and Disciplines for Prospective Research on This Topic?
We conducted an analysis of the trending topic based on the author’s keywords from the dataset. Author’s keywords are generally related to such publication content and, hence, adequate to derive thematic aspects of a certain topic [133,134]. Despite the fact that several authors’ keywords have been shown in the network map of Figure 3, a further insight is given in Figure 4. A hierarchical classification of the topics is shown in this map, representing the log (frequency) of the authors’ keywords over the years. In this case, we have analyzed all the authors’ keywords regardless of their frequency. For instance, in 2017 and 2016, computed tomography was the most discussed topic, followed by simulation in 2017. The dosimetry, photon, cancer, and radiotherapy topics were also quite cited from 2015–2018. In 2020, the term “validation” appears, suggesting that the last tendency point to the development of new methods for dose estimator is dedicated to the validation and effectiveness of new simulations codes suitable for the current technologies.
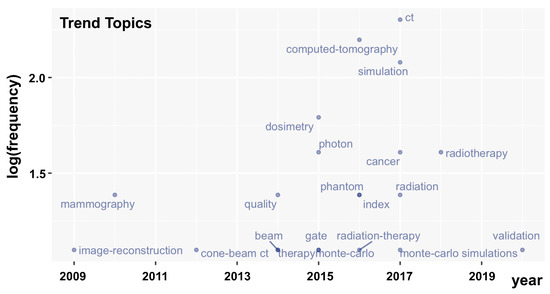
Figure 4.
Trending topics between 2008 to 2021.
Finally, it is worthy of mention that, based on the most recent advances, new promising dose estimator algorithms involves Deep-Learning-based methods [135]. Deep Learning is a subfield of machine learning that employs algorithms inspired by the brain function and structure called artificial neural networks. The work of each neuron is entwined in a distributed manner to collectively learn from the input in order to optimize its final output [136,137]. In particular, convolutional neural network (CNN) architectures have witnessed rapid growth in medical physics [138,139]. Although CNNs were primarily used in the field of image pattern recognition [140], they can be also applied to accurately determine a dose distribution from an approximated simulated input dose [141,142,143,144,145]. In this framework, H. Lee et al. [132] proposed the use of a CNN combined with MC simulations for a CBCT equipment to generate the training data.
4. Conclusions
CBCT has emerged as a key technique for patient positioning and target localization in radiotherapy and other types of procedures; hence, the radiation exposure of human beings has been increased. An accurate estimation of ionizing radiation dose from CBCT procedures is of great importance to quantify the derived risks and for the justification of imaging exposures.
A significant number of publications have emerged in this field with several different approaches investigated due to different devices, medical applications, protocols, dose estimation methods, metrics of comparison, and treatment planning systems being discussed in the different studies. Due to the lack of standardization in dose estimator methods for CBCT applications, large-scale efforts have been made to develop new methodologies for improving radiation protection standards. Simulations based on MC have become one of the most accurate methods to predict the distributions of absorbed doses and other quantities of interest in radiation treatments.
In this systematic review we have adopted a scientometrics approach to investigate the most impactful and influential studies in the field of Geant4-based toolkits dose estimator for CBCT applications. An effective application of bilbiometric tools can help for quantitative research and further insight of the state of the art. We opine that the findings of this study provides a helpful direction for medical physicists to elaborate new theoretical and applied works that can be used to develop accuracy simulations for radiation protection of patients and workers, while optimizing the trade-off between clinical benefit and radiation risk.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11136136/s1, Figure S1: Top Author’s Productivity over time, Figure S2: Author co-citation network, Figure S3: Rate of collaboration between countries based on authors’ affiliations, and top 12 countries of publication based on corresponding author’s country, Figure S4: Co-occurrence average citation of the most relevant author’s keywords and dynamic view over time.
Author Contributions
Conceptualization, A.M.C. and C.U.; methodology, A.M.C. and M.A.-B.; software, A.M.C.; validation, A.M.C., C.U., O.M.-R., and P.A.; formal analysis, A.M.C. and M.A.-B.; investigation, A.M.C.; resources, A.M.C. and O.M.-R.; data curation, A.M.C., M.A.-B., and O.M.-R.; writing original draft preparation, A.M.C.; writing, review and editing, A.M.C., M.A.-B., O.M.-R., C.U., and P.A.; visualization, A.M.C.; supervision, C.U. and P.A.; project administration, A.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been found by UTA-Mayor Project 5794-21.
Institutional Review Board Statement
Ethics approval was not required for this study.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors acknowledge the UTA-Mayor Project 5794-21.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pfeiffer, D.; Pfeiffer, F.; Rummeny, E. Advanced X-ray Imaging Technology. Recent Results Cancer Res. 2020, 216, 3–30. [Google Scholar] [CrossRef]
- Jaffray, D.A.; Siewerdsen, J.H. Cone-beam computed tomography with a flat-panel imager: Initial performance characterization. Med. Phys. 2000, 27, 1311–1323. [Google Scholar] [CrossRef]
- Jaffray, D.A.; Siewerdsen, J.H.; Wong, J.W.; Martinez, A.A. Flat-panel cone-beam computed tomography for image-guided radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 1337–1349. [Google Scholar] [CrossRef]
- Orth, R.C.; Wallace, M.J.; Kuo, M.D. C-arm cone-beam CT: General principles and technical considerations for use in interventional radiology. J. Vasc. Interv. Radiol. JVIR 2008, 19, 814–820. [Google Scholar] [CrossRef]
- Scarfe, W.C.; Farman, A.G.; Sukovic, P. Clinical applications of cone-beam computed tomography in dental practice. J. Can. Dent. Assoc. 2006, 72, 75–80. [Google Scholar]
- Ding, G.X.; Coffey, C.W. Radiation dose from kilovoltage cone beam computed tomography in an image-guided radiotherapy procedure. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Cho, P.S.; Johnson, R.H.; Griffin, T.W. Cone-beam CT for radiotherapy applications. Phys. Med. Biol. 1995, 40, 1863–1883. [Google Scholar] [CrossRef] [PubMed]
- Chetty, I.J.; Curran, B.; Cygler, J.E.; DeMarco, J.J.; Ezzell, G.; Faddegon, B.A.; Kawrakow, I.; Keall, P.J.; Liu, H.; Ma, C.M.; et al. Report of the AAPM Task Group No. 105: Issues associated with clinical implementation of Monte Carlo-based photon and electron external beam treatment planning. Med. Phys. 2007, 34, 4818–4853. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Tsai, S.H.; Cheng, Y.; Chen, J.H.; Chai, J.W.; Chen, C.C.C. Percutaneous Transthoracic Lung Biopsy: Comparison between C-Arm Cone-Beam CT and Conventional CT Guidance. Transl. Oncol. 2015, 8, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.X.; Alaei, P.; Curran, B.; Flynn, R.; Gossman, M.; Mackie, T.R.; Miften, M.; Morin, R.; Xu, X.G.; Zhu, T.C. Image guidance doses delivered during radiotherapy: Quantification, management, and reduction: Report of the AAPM Therapy Physics Committee Task Group 180. Med. Phys. 2018, 45, e84–e99. [Google Scholar] [CrossRef]
- Alaei, P.; Spezi, E. Imaging dose from cone beam computed tomography in radiation therapy. Phys. Med. 2015, 31, 647–658. [Google Scholar] [CrossRef]
- Bryce-Atkinson, A.; de Jong, R.; Bel, A.; Aznar, M.C.; Whitfield, G.; van Herk, M. Evaluation of Ultra-low-dose Paediatric Cone-beam Computed Tomography for Image-guided Radiotherapy. Clin. Oncol. R. Coll. Radiol. 2020, 32, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, D.A.; Rathore, S. Cone-beam CT diagnostic applications: Caries, periodontal bone assessment, and endodontic applications. Dent. Clin. N. Am. 2008, 52, 825–841. [Google Scholar] [CrossRef]
- Venskutonis, T.; Plotino, G.; Juodzbalys, G.; Mickevičienė, L. The importance of cone-beam computed tomography in the management of endodontic problems: A review of the literature. J. Endod. 2014, 40, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Barghan, S.; Tetradis, S.; Mallya, S. Application of cone beam computed tomography for assessment of the temporomandibular joints. Aust. Dent. J. 2012, 57 (Suppl. 1), 109–118. [Google Scholar] [CrossRef]
- O’Connell, A.; Conover, D.L.; Zhang, Y.; Seifert, P.; Logan-Young, W.; Lin, C.F.L.; Sahler, L.; Ning, R. Cone-Beam CT for Breast Imaging: Radiation Dose, Breast Coverage, and Image Quality. Am. J. Roentgenol. 2010, 195, 496–509. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, B.; O’Connor, J.M.; Didier, C.S.; Glick, S.J. Characterization of scatter in cone-beam CT breast imaging: Comparison of experimental measurements and Monte Carlo simulation. Med. Phys. 2009, 36, 857–869. [Google Scholar] [CrossRef]
- Uhlig, J.; Uhlig, A.; Biggemann, L.; Fischer, U.; Lotz, J.; Wienbeck, S. Diagnostic accuracy of cone-beam breast computed tomography: A systematic review and diagnostic meta-analysis. Eur. Radiol. 2019, 29, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, A.M.; Karellas, A.; Vedantham, S.; Kawakyu-O’Connor, D.T. Newer Technologies in Breast Cancer Imaging: Dedicated Cone-Beam Breast Computed Tomography. Semin. Ultrasound CT MR 2018, 39, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ning, R. Cone-beam volume CT breast imaging: Feasibility study. Med. Phys. 2002, 29, 755–770. [Google Scholar] [CrossRef]
- Wienbeck, S.; Lotz, J.; Fischer, U. Review of clinical studies and first clinical experiences with a commercially available cone-beam breast CT in Europe. Clin. Imaging 2017, 42, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, K.K.; Boone, J.M.; Nelson, T.R.; Yang, K.; Kwan, A.L.C.; Miller, D.F. Dedicated breast CT: Initial clinical experience. Radiology 2008, 246, 725–733. [Google Scholar] [CrossRef]
- Thacker, S.C.; Glick, S.J. Normalized glandular dose (DgN) coefficients for flat-panel CT breast imaging. Phys. Med. Biol. 2004, 49, 5433–5444. [Google Scholar] [CrossRef] [PubMed]
- Poludniowski, G.; Allinson, N.M.; Evans, P.M. Proton radiography and tomography with application to proton therapy. Br. J. Radiol. 2015, 88, 20150134. [Google Scholar] [CrossRef] [PubMed]
- Amblard, R.; Floquet, V.; Angellier, G.; Hannoun-Lévi, J.M.; Hérault, J. Proton imaging applications for proton therapy: State of the art. Cancer Radiother. 2015, 19, 136–139. [Google Scholar] [CrossRef]
- Johnson, R.P. Review of medical radiography and tomography with proton beams. Rep. Prog. Physics. Phys. Soc. 2018, 81, 16701. [Google Scholar] [CrossRef]
- Giacometti, V.; Hounsell, A.R.; McGarry, C.K. A review of dose calculation approaches with cone beam CT in photon and proton therapy. Phys. Med. 2020, 76, 243–276. [Google Scholar] [CrossRef]
- Bongrand, A.; Koumeir, C.; Villoing, D.; Guertin, A.; Haddad, F.; Métivier, V.; Poirier, F.; Potiron, V.; Servagent, N.; Supiot, S.; et al. A Monte Carlo Determination of Dose and Range Uncertainties for Preclinical Studies with a Proton Beam. Cancers 2021, 13, 1889. [Google Scholar] [CrossRef]
- Nasseh, I.; Al-Rawi, W. Cone Beam Computed Tomography. Dent. Clin. N. Am. 2018, 62, 361–391. [Google Scholar] [CrossRef]
- Rehani, M.M.; Berry, M. Radiation doses in computed tomography. The increasing doses of radiation need to be controlled. BMJ 2000. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.J.; Kaste, S.C. The ALARA (as low as reasonably achievable) concept in pediatric CT intelligent dose reduction: Multidisciplinary conference organized by the Society of Pediatric Radiology. Pediatr Radiol. 2002, 32, 217–313. [Google Scholar] [CrossRef]
- Tsapaki, V.; Aldrich, J.E.; Sharma, R.; Staniszewska, M.A.; Krisanachinda, A.; Rehani, M.; Hufton, A.; Triantopoulou, C.; Maniatis, P.N.; Papailiou, J.; et al. Dose reduction in CT while maintaining diagnostic confidence: Diagnostic reference levels at routine head, chest, and abdominal CT–IAEA-coordinated research project. Radiology 2006, 240, 828–834. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J. Computed tomography—An increasing source of radiation exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H. Computed Tomography Technology-and Dose-in the 21st Century. Health Phys. 2019, 116, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Schauer, D.A.; Linton, O.W. Ncrp Report No. 160, Ionizing Radiation Exposure of the Population of the United States, Medical Exposure—Are We Doing Less with More, and Is There A Role for Health Physicists? Health Physics 2009, 97, 1–5. [Google Scholar] [CrossRef]
- Muhammad, N.A.; Kayun, Z.; Abu Hassan, H.; Wong, J.H.D.; Ng, K.H.; Karim, M.K.A. Evaluation of Organ Dose and Image Quality Metrics of Pediatric CT Chest-Abdomen-Pelvis (CAP) Examination: An Anthropomorphic Phantom Study. Appl. Sci. 2021, 11, 2047. [Google Scholar] [CrossRef]
- Allan, J.M. Genetic susceptibility to radiogenic cancer in humans. Health Phys. 2008, 95, 677–686. [Google Scholar] [CrossRef]
- Valentin, J. Avoidance of radiation injuries from medical interventional procedures. Ann. ICRP 2000, 30, 7–67. [Google Scholar] [CrossRef]
- Bhargavan-Chatfield, M.; Morin, R.L. The ACR Computed Tomography Dose Index Registry: The 5 million examination update. J. Am. Coll. Radiol. JACR 2013, 10, 980–983. [Google Scholar] [CrossRef]
- Shope, T.B.; Gagne, R.M.; Johnson, G.C. A method for describing the doses delivered by transmission X-ray computed tomography. Med. Phys. 1981, 8, 488–495. [Google Scholar] [CrossRef]
- McCollough, C.H. It is time to retire the computed tomography dose index (CTDI) for CT quality assurance and dose optimization. Against the proposition. Med. Phys. 2006, 33, 1190–1191. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Leng, S.; Yu, L.; Cody, D.D.; Boone, J.M.; McNitt-Gray, M.F. CT dose index and patient dose: They are not the same thing. Radiology 2011. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yoshizumi, T.T.; Toncheva, G.; Yoo, S.; Yin, F.F. Comparison of radiation doses between cone beam CT and multi detector CT: TLD measurements. Radiat. Prot. Dosim. 2008, 132, 339–345. [Google Scholar] [CrossRef]
- Council, N.R. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2; The National Academy of Sciences: Washington, DC, USA, 2006; pp. 1–406. [Google Scholar] [CrossRef]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Sir Craft, A.W.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef]
- Mullenders, L.; Atkinson, M.; Paretzke, H.; Sabatier, L.; Bouffler, S. Assessing cancer risks of low-dose radiation. Nat. Rev. Cancer 2009, 46, 596–604. [Google Scholar] [CrossRef]
- Kim, S.; Yoshizumi, T.T.; Frush, D.P.; Toncheva, G.; Yin, F.F. Radiation dose from cone beam CT in a pediatric phantom: Risk estimation of cancer incidence. Am. J. Roentgenol. 2010, 194, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Jarry, G.; Graham, S.A.; Moseley, D.J.; Jaffray, D.J.; Siewerdsen, J.H.; Verhaegen, F. Characterization of scattered radiation in kV CBCT images using Monte Carlo simulations. Med. Phys. 2006, 33, 4320–4329. [Google Scholar] [CrossRef]
- Landry, G.; Hua, C.H. Current state and future applications of radiological image guidance for particle therapy. Med. Phys. 2018, 45, e1086–e1095. [Google Scholar] [CrossRef]
- Newhauser, W.D.; Durante, M. Assessing the risk of second malignancies after modern radiotherapy. Nat. Rev. Cancer 2011, 11, 438–448. [Google Scholar] [CrossRef]
- Jeong, H.; Rah, J.E.; Hwang, U.J.; Yoo, S.H.; Min, B.J.; Lee, S.Y.; Yoon, M.; Shin, D.H.; Park, S.Y.; Lee, S.B.; et al. Estimation of the secondary cancer risk induced by diagnostic imaging radiation during proton therapy. J. Radiol. Prot. 2011, 31, 477–487. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, H.; Chen, Z.; Knisely, J.P.S.; Nath, R.; Feng, Z.; Bao, S.; Deng, J. Concomitant Imaging Dose and Cancer Risk in Image Guided Thoracic Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.G. Photons—Radiobiological issues related to the risk of second malignancies. Phys. Med. 2017, 42, 213–220. [Google Scholar] [CrossRef]
- Trott, K.R. Special radiobiological features of second cancer risk after particle radiotherapy. Phys. Med. 2017, 42, 221–227. [Google Scholar] [CrossRef] [PubMed]
- ICRP. 2000 Annual Report of the International Commission on Radiological Protection; ICRP: Ottawa, ON, Canada, 2000; pp. 1–30. [Google Scholar]
- Kase, K.R. Radiation protection principles of NCRP. Health Phys. 2004, 87, 251–257. [Google Scholar] [CrossRef]
- Rogers, D.W. The role of Monte Carlo simulation of electron transport in radiation dosimetry. Int. J. Radiat. Appl. Instrum. 1991, 42, 965–974. [Google Scholar] [CrossRef]
- Ma, C.M.; Jiang, S.B. Monte Carlo modelling of electron beams from medical accelerators. Phys. Med. Biol. 1999, 44, R157–R189. [Google Scholar] [CrossRef] [PubMed]
- Andreo, P. Monte Carlo simulations in radiotherapy dosimetry. Radiat. Oncol. 2018, 13, 121. [Google Scholar] [CrossRef]
- Lewis, R.D.; Ryde, S.J.; Seaby, A.W.; Hancock, D.A.; Evans, C.J. Use of Monte Carlo computation in benchmarking radiotherapy treatment planning system algorithms. Phys. Med. Biol. 2000, 45, 1755–1764. [Google Scholar] [CrossRef]
- Chow, J.C.L. Depth Dose Enhancement on Flattening-Filter-Free Photon Beam: A Monte Carlo Study in Nanoparticle-Enhanced Radiotherapy. Appl. Sci. 2020, 10, 7052. [Google Scholar] [CrossRef]
- Nazemi, E.; Six, N.; Iuso, D.; De Samber, B.; Sijbers, J.; De Beenhouwer, J. Monte-Carlo-Based Estimation of the X-ray Energy Spectrum for CT Artifact Reduction. Appl. Sci. 2021, 11, 3145. [Google Scholar] [CrossRef]
- Verhaegen, F.; Seuntjens, J. Monte Carlo modelling of external radiotherapy photon beams. Phys. Med. Biol. 2003, 48, R107–R164. [Google Scholar] [CrossRef]
- Kawrakow, I.; Mainegra Hing, E.; Tessier, F.; Walters, B.R.B. The EGSnrc Code System: Monte Carlo Simulation of Electron and Photon Transport. 2021. Available online: https://nrc-cnrc.github.io/EGSnrc/doc/pirs701-egsnrc.pdf (accessed on 15 April 2021).
- Briesmeister, J.F. MCNP: A General Monte Carlo N-Particle Transport Code; Los Alamos National Laboratory: Santa Fe, NM, USA, 2000.
- Salvat, F.; Fernandez-Varea, J.M.; Acosta, E.; Sempau, J. Penelope—A Code System for Monte Carlo Simulation of Electron and Photon Transport; Nuclear Energy Agency: Paris, France, 2001. [Google Scholar]
- Tapiovaara, M.; Lakkisto, M.; Servomaa, A. PCXMC A PC-Based Monte Carlo Program for Calculating Patient Doses in Medical X-ray Examinations; Technical Report; IAEA: Vienna, Austria, 1997. [Google Scholar]
- Agostinelli, S.; Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. GEANT4—A simulation toolkit. Nucl. Instrum. Methods Phys. Res. 2003, 506, 250–303. [Google Scholar] [CrossRef]
- Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Arce Dubois, P.; Asai, M.; Barrand, G.; Capra, R.; Chauvie, S.; Chytracek, R.; et al. Geant4 developments and applications. IEEE Trans. Nucl. Sci. 2006, 53, 270–278. [Google Scholar] [CrossRef]
- Jahnke, L.; Fleckenstein, J.; Wenz, F.; Hesser, J. GMC: A GPU implementation of a Monte Carlo dose calculation based on Geant4. Phys. Med. Biol. 2012, 57, 1217–1229. [Google Scholar] [CrossRef]
- Bert, J.; Perez-Ponce, H.; El Bitar, Z.; Jan, S.; Boursier, Y.; Vintache, D.; Bonissent, A.; Morel, C.; Brasse, D.; Visvikis, D. Geant4-based Monte Carlo simulations on GPU for medical applications. Phys. Med. Biol. 2013, 58, 5593–5611. [Google Scholar] [CrossRef]
- Sarrut, D.; Bardiès, M.; Boussion, N.; Freud, N.; Jan, S.; Létang, J.M.; Loudos, G.; Maigne, L.; Marcatili, S.; Mauxion, T.; et al. A review of the use and potential of the GATE Monte Carlo simulation code for radiation therapy and dosimetry applications. Med. Phys. 2014, 41, 64301. [Google Scholar] [CrossRef] [PubMed]
- Boone, J.M.; Shah, N.; Nelson, T.R. A comprehensive analysis of coefficients for pendant-geometry cone-beam breast computed tomography. Med. Phys. 2004, 31, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Santin, G.; Strul, D.; Staelens, S.; Assié, K.; Autret, D.; Avner, S.; Barbier, R.; Bardiès, M.; Bloomfield, P.M.; et al. GATE: A simulation toolkit for PET and SPECT. Phys. Med. Biol. 2004, 49, 4543–4561. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Benoit, D.; Becheva, E.; Carlier, T.; Cassol, F.; Descourt, P.; Frisson, T.; Grevillot, L.; Guigues, L.; Maigne, L.; et al. GATE V6: A major enhancement of the GATE simulation platform enabling modelling of CT and radiotherapy. Phys. Med. Biol. 2011, 56, 881–901. [Google Scholar] [CrossRef]
- Papadimitroulas, P. Dosimetry applications in GATE Monte Carlo toolkit. Phys. Med. 2017, 41, 136–140. [Google Scholar] [CrossRef]
- Townsend, D. In Proceedings of the 2011 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC 2011), Valencia, Spain, 23–29 October 2011.
- Arce, P.; Ignacio, J.; Harkness, L.; Pérez-astudillo, D.; Cañadas, M.; Rato, P.; Prado, M.D.; Abreu, Y.; Lorenzo, G.D.; Kolstein, M.; et al. Nuclear Instruments and Methods in Physics Research A G AMOS: A framework to do G EANT 4 simulations in different physics fi elds with an user-friendly interface. Nucl. Inst. Methods Phys. Res. 2014, 735, 304–313. [Google Scholar] [CrossRef]
- Arce, P.; Rato, P.; Canadas, M.; Lagares, J.I. GAMOS: A Geant4-based easy and flexible framework for nuclear medicine applications. In Proceedings of the 2008 IEEE Nuclear Science Symposium Conference Record, Dresden, Germany, 19–25 October 2008; pp. 3162–3168. [Google Scholar] [CrossRef]
- Karaoglu, A.; Arce, P.; Obradors, D.; Lagares, J.I.; Unak, P. Calculation by GAMOS/Geant4 simulation of cellular energy distributions from alpha and lithium-7 particles created by BNCT. Appl. Radiat. Isot. 2018, 132, 206–211. [Google Scholar] [CrossRef]
- Auditore, L.; Amato, E.; Italiano, A.; Arce, P.; Campennì, A.; Baldari, S. Internal dosimetry for TARE therapies by means of GAMOS Monte Carlo simulations. Phys. Med. 2019, 64, 245–251. [Google Scholar] [CrossRef]
- Bongrand, A.; Busato, E.; Force, P.; Martin, F.; Montarou, G. Use of short-lived positron emitters for in-beam and real-time β(+) range monitoring in proton therapy. Phys. Med. 2020, 69, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Arce, P.; Lagares, J.I.; Azcona, D. A utility to read automatically DICOM format data for GAMOS/Geant4 simulation. Phys. Med. 2016, 32, 256. [Google Scholar] [CrossRef]
- Antoniadi, A.M.; Du, Y.; Guendouz, Y.; Wei, L.; Mazo, C.; Becker, B.A.; Mooney, C. Current Challenges and Future Opportunities for XAI in Machine Learning-Based Clinical Decision Support Systems: A Systematic Review. Appl. Sci. 2021, 11, 5088. [Google Scholar] [CrossRef]
- Longo, U.G.; De Salvatore, S.; Candela, V.; Zollo, G.; Calabrese, G.; Fioravanti, S.; Giannone, L.; Marchetti, A.; De Marinis, M.G.; Denaro, V. Augmented Reality, Virtual Reality and Artificial Intelligence in Orthopedic Surgery: A Systematic Review. Appl. Sci. 2021, 11, 3253. [Google Scholar] [CrossRef]
- Lucchese, A.; Bonini, C.; Noviello, M.; Lupo Stanghellini, M.T.; Greco, R.; Peccatori, J.; Biella, A.; Tassi, E.; Beretta, V.; Ciceri, F.; et al. The Effect of Removable Orthodontic Appliances on Oral Microbiota: A Systematic Review. Appl. Sci. 2021, 11, 2881. [Google Scholar] [CrossRef]
- Chen, C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef]
- White, H.D.; McCain, K.W. Visualizing a discipline: An author co-citation analysis of information science, 1972–1995. J. Am. Soc. Inf. Sci. 1998, 49, 327–355. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-tool for comprehensive science mapping analysis. J. Inf. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Tseng, H.W.; Karellas, A.; Vedantham, S. Radiation dosimetry of a clinical prototype dedicated cone-beam breast CT system with offset detector. Med. Phys. 2021, 48, 1079–1088. [Google Scholar] [CrossRef]
- Cho, S.; Lim, S.; Kim, C.; Wi, S.; Kwon, T.; Youn, W.S.; Lee, S.H.; Kang, B.S.; Cho, S. Enhancement of soft-tissue contrast in cone-beam CT using an anti-scatter grid with a sparse sampling approach. Phys. Med. 2020, 70, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Principi, S.; Wang, A.; Maslowski, A.; Wareing, T.; Jordan, P.; Schmidt, T.G. Deterministic linear Boltzmann transport equation solver for patient-specific CT dose estimation: Comparison against a Monte Carlo benchmark for five realistic scanner configurations and patient models. Med. Phys. 2020, 47, 6470–6483. [Google Scholar] [CrossRef]
- Shi, M.; Myronakis, M.; Jacobson, M.; Ferguson, D.; Williams, C.; Lehmann, M.; Baturin, P.; Huber, P.; Fueglistaller, R.; Lozano, I.V.; et al. GPU-accelerated Monte Carlo simulation of MV-CBCT. Phys. Med. Biol. 2020, 65, 235042. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, S.; Cha, J.G.; Baek, T.; Yang, K.M. Postmortem Computed Tomography and Computed Tomography Angiography: Cardiothoracic Imaging Applications in Forensic Medicine. J. Thorac. Imaging 2019, 34, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Gao, H.; Zhang, L.; Xing, Y.; Zheng, J. Dental and maxillofacial cone beam computed tomography absorbed dose distribution calculation by GEANT4. In Proceedings of the SPIE Medical Imaging, San Diego, CA, USA, 16–21 February 2019; Volume 10948. [Google Scholar] [CrossRef]
- Wang, A.; Maslowski, A.; Wareing, T.; Star-Lack, J.; Schmidt, T.G. A fast, linear Boltzmann transport equationsolver for computed tomography dose calculation (Acuros CTD). Med. Phys. 2019, 46, 925–933. [Google Scholar] [CrossRef]
- Ardenfors, O.; Henry, T.; Gudowska, I.; Poludniowski, G.; Dasu, A. Comparison of Beam Characteristicsand Organ Doses for a Proton Gantry-Mounted CBCT System Modelled with MCNP6 and GATE. Med. Phys. 2018, 45, E430. [Google Scholar]
- Ardenfors, O.; Henry, T.; Gudowska, I.; Poludniowski, G.; Dasu, A. Organ doses from a proton gantry-mounted cone-beam computed tomography system characterized with MCNP6 and GATE. Phys. Med. 2018, 53, 56–61. [Google Scholar] [CrossRef]
- Gholami, S.; Longo, F.; Nedaie, H.A.; Berti, A.; Mousavi, M.; Meigooni, A.S. Application of Geant4 Monte Carlo simulation in dose calculations for small radiosurgical fields. Med. Dosim. 2018, 43, 214–223. [Google Scholar] [CrossRef]
- Maslowski, A.; Wang, A.; Sun, M.; Wareing, T.; Davis, I.; Star-Lack, J. Acuros CTS: A fast, linear Boltzmann transport equation solver for computed tomography scatter—Part I: Core algorithms and validation. Med. Phys. 2018, 45, 1899–1913. [Google Scholar] [CrossRef] [PubMed]
- Leotta, S.; Amato, E.; Settineri, N.; Basile, E.; Italiano, A.; Auditore, L.; Santacaterina, A.; Pergolizzi, S. Patient Dose in Image Guided Radiotherapy: Monte Carlo Study of the CBCT Dose Contribution. Atti Accad. Peloritana Dei-Pericolanti-Cl. Sci. Fis. Mat. Nat. 2018, 96. [Google Scholar] [CrossRef]
- Son, K.; Chang, J.; Lee, H.; Kim, C.; Lee, T.; Cho, S.; Park, S.; Kim, J.S. Optimal dose reduction algorithm using an attenuation-based tube current modulation method for cone-beam CT imaging. PLoS ONE 2018, 13, e0192933. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Penfold, S.N. Europium-155 as a source for dual energy cone beam computed tomography in adaptive proton therapy: A simulation study. Med. Phys. 2017, 44, 5143–5152. [Google Scholar] [CrossRef]
- Benhalouche, S.; Bert, J.; Boussion, N.; Autret, A.; Pradier, O.; Visvikis, D. GATE Monte-Carlo Simulation of an MV-CBCT Flat Panel for Synergistic Imaging and Dosimetric Applications in Radiotherapy. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 39, 4529–4544. [Google Scholar] [CrossRef]
- Myronakis, M.; Star-Lack, J.; Baturin, P.; Rottmann, J.; Morf, D.; Wang, A.; Hu, Y.H.; Shedlock, D.; Berbeco, R.I. A novel multilayer MV imager computational model for component optimization. Med. Phys. 2017, 44, 4213–4222. [Google Scholar] [CrossRef] [PubMed]
- Sakata, D.; Haga, A.; Kida, S.; Imae, T.; Takenaka, S.; Nakagawa, K. Effective atomic number estimation using kV-MV dual-energy source in LINAC. Phys. Med. 2017, 39, 9–15. [Google Scholar] [CrossRef]
- Son, K.; Kim, J.S.; Lee, H.; Cho, S. Imaging dose of human organs from KV-CBCT in image-guided radiation therapy. Radiat. Prot. Dosim. 2017, 175, 194–200. [Google Scholar] [CrossRef]
- Marchant, T.E.; Joshi, K.D. Comprehensive Monte Carlo study of patient doses from cone-beam CT imaging in radiotherapy. J. Radiol. Prot. 2017, 37, 13–30. [Google Scholar] [CrossRef]
- Xu, M.; Cheng, X.; Cheng, X.; Lan, X.; Chen, S.; Ji, J. Areas of breast tissue covered in cone beam breast CT imaging. Exp. Ther. Med. 2017, 13, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Vedantham, S.; Karellas, A.; Zhu, L. Library based X-ray scatter correction for dedicated cone beam breast CT. Med. Phys. 2016, 43, 4529–4544. [Google Scholar] [CrossRef] [PubMed]
- Baldacci, F.; Mittone, A.; Bravin, A.; Coan, P.; Delaire, F.; Ferrero, C.; Gasilov, S.; Létang, J.M.; Sarrut, D.; Smekens, F.; et al. A track length estimator method for dose calculations in low-energy X-ray irradiations: Implementation, properties and performance. Z. Fur Med. Phys. 2015, 25, 36–47. [Google Scholar] [CrossRef]
- Choi, J.H.; Constantin, D.; Ganguly, A.; Girard, E.; Morin, R.L.; Dixon, R.L.; Fahrig, R. Practical dose point-based methods to characterize dose distribution in a stationary elliptical body phantom for a cone-beam C-arm CT system. Med. Phys. 2015, 42, 4920–4932. [Google Scholar] [CrossRef] [PubMed]
- Brochu, F.M.; Burnet, N.G.; Jena, R.; Plaistow, R.; Parker, M.A.; Thomas, S.J. Geant4 simulation of the Elekta XVI kV CBCT unit for accurate description of potential late toxicity effects of image-guided radiotherapy. Phys. Med. Biol. 2014, 59, 7601–7608. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, D.; Yang, J.; Liu, B. A study of the short- to long-phantom dose ratios for CT scanning without table translation. Med. Phys. 2014, 41, 091912. [Google Scholar] [CrossRef]
- Hansen, D.C.; Petersen, J.B.B.; Bassler, N.; Sørensen, T.S. Improved proton computed tomography by dual modality image reconstruction. Med. Phys. 2014, 41, 031904. [Google Scholar] [CrossRef]
- Son, K.; Cho, S.; Kim, J.S.; Han, Y.; Ju, S.G.; Choi, D.H. Evaluation of radiation dose to organs during kilovoltage cone-beam computed tomography using Monte Carlo simulation. J. Appl. Clin. Med. Phys. 2014, 15, 295–302. [Google Scholar] [CrossRef]
- Bartzsch, S.; Oelfke, U. A new concept of pencil beam dose calculation for 40–200 keV photons using analytical dose kernels. Med. Phys. 2013, 40, 111714. [Google Scholar] [CrossRef]
- Lanconelli, N.; Mettivier, G.; Lo Meo, S.; Russo, P. Investigation of the dose distribution for a cone beam CT system dedicated to breast imaging. Phys. Med. 2013, 29, 379–387. [Google Scholar] [CrossRef][Green Version]
- Fleckenstein, J.; Jahnke, L.; Lohr, F.; Wenz, F.; Hesser, J. Development of a Geant4 based Monte Carlo Algorithm to evaluate the MONACO VMAT treatment accuracy. Z. Fur Med. Phys. 2013, 23, 33–45. [Google Scholar] [CrossRef]
- Vedantham, S.; Shi, L.; Karellas, A.; Noo, F. Dedicated breast CT: Radiation dose for circle-plus-line trajectory. Med. Phys. 2012, 39, 1530–1541. [Google Scholar] [CrossRef]
- Sechopoulos, I.; Feng, S.; D’Orsi, C. Dosimetric characterization of a dedicated breast computed tomography clinical prototype. Med. Phys. 2010, 37, 4110–4120. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shen, Y.; Lai, C.J.; Han, T.; Zhong, Y.; Ge, S.; Liu, X.; Wang, T.; Yang, W.T.; Whitman, G.J.; et al. Dual resolution cone beam breast CT: A feasibility study. Med. Phys. 2009, 36, 4007–4014. [Google Scholar] [CrossRef]
- Chen, L.; Shaw, C.C.; Altunbas, M.C.; Lai, C.J.; Liu, X.; Han, T.; Wang, T.; Yang, W.T.; Whitman, G.J. Feasibility of volume-of-interest (VOI) scanning technique in cone beam breast CT—A preliminary study. Med. Phys. 2008, 35, 3482–3490. [Google Scholar] [CrossRef]
- ICRP International Commission on Radiological Protection. The 2007 Recommendations of the International Commission on Radiological Protection; ICRP International Commission on Radiological Protection: Ottawa, ON, Canada, 2017; pp. 1–337. [Google Scholar]
- Nations, U.; Committee, S.; Radiation, A. UNSCEAR 2012 Report. Report to the General Assembly; UNSCEAR: New York, NY, USA, 2012. [Google Scholar]
- Kan, M.W.K.; Leung, L.H.T.; Wong, W.; Lam, N. Radiation dose from cone beam computed tomography for image-guided radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 272–279. [Google Scholar] [CrossRef]
- Verellen, D.; De Ridder, M.; Linthout, N.; Tournel, K.; Soete, G.; Storme, G. Innovations in image-guided radiotherapy. Nat. Rev. Cancer 2007, 7, 949–960. [Google Scholar] [CrossRef]
- DeSantis, C.; Ma, J.; Bryan, L.; Jemal, A. Breast cancer statistics, 2013. CA Cancer J. Clin. 2014, 64, 52–62. [Google Scholar] [CrossRef]
- Wienbeck, S.; Uhlig, J.; Luftner-Nagel, S.; Zapf, A.; Surov, A.; von Fintel, E.; Stahnke, V.; Lotz, J.; Fischer, U. The role of cone-beam breast-CT for breast cancer detection relative to breast density. Eur. Radiol. 2017, 27, 5185–5195. [Google Scholar] [CrossRef]
- O’Connell, A.M.; Kawakyu-O’Connor, D. Dedicated Cone-beam Breast Computed Tomography and Diagnostic Mammography: Comparison of Radiation Dose, Patient Comfort, And Qualitative Review of Imaging Findings in BI-RADS 4 and 5 Lesions. J. Clin. Imaging Sci. 2012, 2, 7. [Google Scholar] [CrossRef]
- He, N.; Wu, Y.P.; Kong, Y.; Lv, N.; Huang, Z.M.; Li, S.; Wang, Y.; Geng, Z.J.; Wu, P.H.; Wei, W.D. The utility of breast cone-beam computed tomography, ultrasound, and digital mammography for detecting malignant breast tumors: A prospective study with 212 patients. Eur. J. Radiol. 2016, 85, 392–403. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J. A Deep Learning-Based Scatter Correction of Simulated X-ray Images. Electronics 2019, 8, 944. [Google Scholar] [CrossRef]
- Song, Y.; Chen, X.; Hao, T.; Liu, Z.; Lan, Z. Exploring two decades of research on classroom dialogue by using bibliometric analysis. Comput. Educ. 2019, 137, 12–31. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Tseng, H.H.; Luo, Y.; Ten Haken, R.K.; El Naqa, I. The Role of Machine Learning in Knowledge-Based Response-Adapted Radiotherapy. Front. Oncol. 2018, 8, 266. [Google Scholar] [CrossRef]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef]
- Xu, L.; Ren, J.S.J.; Liu, C.; Jia, J. Deep Convolutional Neural Network for Image Deconvolution. In Advances in Neural Information Processing Systems; Ghahramani, Z., Welling, M., Cortes, C., Lawrence, N., Weinberger, K.Q., Eds.; Curran Associates, Inc.: Red Hook, NY, USA, 2014; Volume 27. [Google Scholar]
- Avanzo, M.; Trianni, A.; Botta, F.; Talamonti, C.; Stasi, M.; Iori, M. Artificial Intelligence and the Medical Physicist: Welcome to the Machine. Appl. Sci. 2021, 11, 1691. [Google Scholar] [CrossRef]
- Chartrand, G.; Cheng, P.M.; Vorontsov, E.; Drozdzal, M.; Turcotte, S.; Pal, C.J.; Kadoury, S.; Tang, A. Deep Learning: A Primer for Radiologists. Radiographics 2017, 37, 2113–2131. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. arXiv 2015, arXiv:1505.04597v1. [Google Scholar]
- Cagni, E.; Botti, A.; Micera, R.; Galeandro, M.; Sghedoni, R.; Orlandi, M.; Iotti, C.; Cozzi, L.; Iori, M. Knowledge-based treatment planning: An inter-technique and inter-system feasibility study for prostate cancer. Phys. Med. Eur. Med. Phys. 2017, 36, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Götz, T.I.; Lang, E.W.; Schmidkonz, C.; Kuwert, T.; Ludwig, B. Dose voxel kernel prediction with neural networks for radiation dose estimation. Z. Med. Phys. 2021, 31, 23–36. [Google Scholar] [CrossRef]
- Götz, T.I.; Schmidkonz, C.; Chen, S.; Al-Baddai, S.; Kuwert, T.; Lang, E.W. A deep learning approach to radiation dose estimation. Phys. Med. Biol. 2020, 65, 35007. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.; Doken, S.; Zhuang, T.; Wingerter, D.; Gidwani, M.; Mistry, N.; Ladic, L.; Kamen, A.; Abazeed, M.E. An image-based deep learning framework for individualizing radiotherapy dose. Lancet Digit. Health 2019, 1, e136–e147. [Google Scholar] [CrossRef]
- Nguyen, D.; Long, T.; Jia, X.; Lu, W.; Gu, X.; Iqbal, Z.; Jiang, S. A feasibility study for predicting optimal radiation therapy dose distributions of prostate cancer patients from patient anatomy using deep learning. Sci. Rep. 2019, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).