Abstract
The organic fraction of municipal solid waste (OFMSW) is recognized as a suitable substrate for the anaerobic digestion (AD) process and is currently considered a mature technology. A promising strategy to enhance biogas yield and productivity is the co-digestion of OFMSW with other organic biomass, such as green waste (GW), a mixture of leaves, grass, and woody materials originated from private yards and public greenspace management. The main limitation to the use of GW for biogas production is the high percentage of the lignocellulosic fraction, which makes necessary a pretreatment of delignification to dissolve the recalcitrant structure. In this study, a new strategy of sustainable bio-delignification using the white-rot fungi Bjerkandera adusta (BA) in comparison with other chemical pretreatments were investigated. Untreated and treated GW were, respectively, submitted to anaerobic co-digestion with OFMSW. AD processes were carried out in a lab-scale plant for 30 days in thermophilic conditions (55 °C). Biogas cumulative production was increased by about 100% in the case of treated GW compared with that of just OFMSW, from 145 to 289 Nm3 CH4/ton SV, and productivity almost doubled from 145 to 283 Nm3/ton FM * day. The measured average methane content values in the cumulative biogas were 55% from OFMSW and 54% from GW. Moreover, over 95% of the biogas was produced in 20 days, showing the potential opportunity to reduce the AD time.
1. Introduction
Management of municipal solid waste (MSW) has become a major global concern due to the increase of urbanization and consumption standards [1]. In Europe, the generation of municipal solid waste has been growing annually, reaching 502 kg per capita in 2019, of which today less than 60% is fully recycled, incinerated, or composted [2]. As a result of public health and environmental protection issues, efforts have been made towards increasing this percentage by improving recovery strategies and/or recycling useful materials according to the Waste Directive 2008/98/EC [3]. MSW typically comprises about 50–55% organic materials, principally derived from food scraps and food waste (the organic fraction of MSW, or OFMSW), and green waste (GW), originated from private yards and public greenspace (i.e., roadside edges, public lawns) management [4]. OFMSW disposal is typically associated with composting or landfilling [5], which is strongly discouraged, because if inappropriately performed, they can contaminate water sources and soil with leachate and air with greenhouse gas emissions [6]. Composting is facing a lack of public interest mainly due to the low value of the product (i.e., compost) and limited waste volume reduction [7]. Moreover, the majority of OFMSW is generally rich in nutrient substances, such as proteins, minerals, and sugars, which can be recovered in the production of green energy before composting, saving more resources and improving the circularity of biological nutrients.
Anaerobic digestion (AD) is an established biological process suitable for stabilizing organic solid wastes and is coupled with the recovery of energy and nutrients. It provides a sustainable option for energy recovery, thereby contributing to a circular economy [8]. According to several recent studies [9,10,11], biogas/biomethane will play a key role in helping Europe’s transition to a clean energy system, achieving the goal of sustainability with a special focus on the economic and environmental dimensions, and enabling a carbon-neutral (or even carbon-negative) Europe with zero pollution by 2050.
As a consequence of the Paris Climate Agreement, which requires global decarbonization, the development of green energy can contribute significantly to tackling climate change [12]. The transformation of organic waste such as OFMSW and GW through the process of AD would encourage EU member states to unlock the national potential of their renewable gases, which would generate ample benefits: adequate waste management, resource-efficient agriculture, and displacement of fossil energy, triggering significant emission savings [13]. A European-wide target can help cities and municipalities to establish a local and circular bio-economy; organic residues, such as OFMSW and GW, can be separated and fermented in anaerobic digestion plants, producing biogas and biomethane that can then be used to fuel, for instance, urban garbage trucks, thereby reducing noise pollution and improving air quality by replacing diesel-powered trucks [14].
AD under controlled conditions is a proficient technique for OFMSW treatment, and it is currently a mature technology, mostly found in Europe [15]. It presents a double advantage as it recovers energy from waste-producing biogas and simultaneously treats the residues, reducing their disposal in landfills [16]. AD refers to a series of biological processes in which a microbial consortium synergistically decomposes organic matters to biogas (a mixture of CH4 at 45–70% and CO2 at 24–40%, on average), leaving a digestate (composed by the not-promptly fermentable fraction of organic materials). The process occurs in four sequential stages: hydrolysis, acidogenesis, acetogenesis, and methanogenesis [17]. Biogas is a promising renewable source of bioenergy that can be used for different applications including vehicular fuel, heating, and electricity production [18].
OFMSW is highly biodegradable and has a high-energy-yield potential of up to 200 m3 of biogas per metric ton of treated OFMSW [19]. Moreover, the use of digestate, in place of mineral fertilizers, provides a further environmental benefit due to reduced CO2 emissions estimated at 30–40 kg per metric ton of biowaste [20]. However, OFMSW has certain characteristics that may limit its efficacy as such a resource due to issues such as high solids content, large particle size, slowly biodegradable components (lignin-rich, woody wastes), and the waste’s heterogeneous nature, which makes process control challenging. These difficulties are usually overcome with biomass pretreatments and by co-digestion with different combinations of municipal, industrial, agricultural, and farming waste materials [21]. The main advantage of the co-digestion process is the improvement of biogas production (25 to 400% over the mono-digestion of the same substrate) and methane yield, as well as the economic advantages from the sharing of apparatuses and costs [22].
GW is a complex structure of cellulose, hemicellulose, and lignin, which makes it resistant to hydrolysis by microbial enzymes during AD. Consequently, biogas generation and digestion time of GW are negatively influenced, resulting in an inefficient process [23]. This explains why only a limited number of experiments have been reported for mono-digestion of GW and none for co-digestion with OFMSW [24,25,26], even though biogas production from GW and lignocellulosic materials in general have a remarkable potential in terms of both environmental and social sustainability [27].
The large gap between the actual and potential biogas production from such feedstocks can in fact be ascribed to the their recalcitrant structure [28]. This makes pretreatments before AD a desired step to overcome the obstacle and make the biomass accessible to microorganisms [29]. Many pretreatments, generally classified into mechanical, physical, chemical, and combined, are available for cellulosic biomass as the basis for biological biorefineries [30]. In recent years, many studies have evaluated the feasibility of these methods for accelerating the AD process and improving biogas production from lignocellulosic biomass [31].
Several attempts have been reported for delignification of GW, and herbaceous waste in general, in order to obtain fermentable sugars, fine chemicals, or renewable biofuels using mechanical, chemical, or physical pretreatments [32]. Most of the processes involve harsh conditions, require the use of chemicals, and produce waste and toxic byproducts, making such processes unsuitable from economic and environmental points of view [33].
A promising and low-cost alternative is biological pretreatment, or bio-delignification, which involves the direct action of microbial metabolism on lignocellulosic substrates and can be used to increase biogas production [34].
In nature, B. adusta plays an ecologically important role in the global carbon cycle by decomposing wood and leaf litter [35], whereas its biotechnological advantages have gained interest in the last decades; it is used as a dye-decolorizing fungus and is able to degrade aromatic xenobiotics in textile wastewater [36] and transform halogenated toxic compounds in pesticides [37]. Previous experiments in lignin degradation by B. adusta have been reported for pulp and paper mill wastewater [38] and for wheat straw for bioethanol production [39,40]. The aim of this study was to evaluate the application of B. adusta to bio-delignification pretreatment on GW to enhance biogas production yield from anaerobic co-digestion of OFMSW and GW and to realize a sustainable alternative for the valorization of waste biomasses, whose potential from a circular economy perspective is often underestimated. The bio-delignification process was also compared with chemical treatments of GW, based on alkali, sulfuric acid, and chlorine, in terms of yield and overall sustainability. To our best knowledge, this study represents the first attempt to use B. adusta for bio-delignification of GW destined for biogas production in co-digestion with OFMSW.
Bio-Delignification with White-Rot Fungi
Bio-delignification is an attractive approach because of its cost-effectiveness, low-energy requirement, low environment impact, and low formation of toxic materials such as furfural, hydroxymethylfurfural, etc., which could negatively affect anaerobic digestion [41].
However, long pretreatment times (from 13 up to 50 days) to obtain high yields of delignification have been reported, limiting the use of these processes in commercial applications [42]. In addition, there could be competition for carbohydrates between pretreatment and downstream biogas production, because certain levels of carbohydrates are required by microbes during biological pretreatment. On the other hand, the accessibility of cellulose is increased after pretreatment, which can improve biogas production [43]. Therefore, one major objective of biological pretreatment is to minimize the loss of carbohydrates and maximize the lignin removal for AD feedstocks with high digestibility [44]. Until now, bio-delignification has followed two main approaches: treatments with fungal lignolytic enzymes as free mixtures or immobilized [31,32] and submerged or solid-state fermentations with fungal cells [45]. The microbial treatment includes fungi, such as white-rot fungi, brown-rot fungi, and soft-rot fungi, and bacteria. Both brown-rot and soft-rot fungi principally degrade the plant polysaccharides with minimal lignin degradation, while white-rot fungi are capable of complete mineralization of both the lignin and the polysaccharide components [46].
Limited research related to the improvement of biogas yield by using white-rot fungi has been done, even though data regarding the bio-delignification and hydrolysis yields are available that are vital parameters for biogas production [47]. Previous experiments of fungal pretreatments of woody and herbaceous feedstocks have been performed using a variety of white-rot fungal strains, and several studies have been carried out on different biomass, such as Polyporus brumalis on wheat straw [48], Trametes versicolor on corn silage [49], Ceriporiopsis subvermispora on yard trimmings [50], Pleurotus eryngii on corn stover [51], and Trichoderma reesei on rice straw [52], reporting sugar yields between 25 and 70% with 20–52% lignin degradation. In fact, white-rot fungi are the most abundant degraders of wood in nature. Under optimal conditions, the rates at which they mineralize lignin is exploitable for industrial applications [53]. Their strategy is to decompose the lignin in wood so that they can gain access to the digestible polysaccharides (cellulose and hemicellulose) embedded in the lignin matrix. Given the chemical recalcitrance of lignin, it is evident that white-rot fungi must employ unusual mechanisms to degrade it, based on the generation of lignin-free radicals that, due to their chemical instability, subsequently undergo a variety of spontaneous cleavage reactions [54]. The white-rot fungi have been found to produce extracellular peroxidases and laccases, which usually appear in response to nutrient depletion [55]. The best characterized white-rot fungus is Phanerochaete chrysosporium, since the discovery of the lignin peroxidase from this fungus by Tien and Kirk [56] and Glenn et al. [57]. Recently, some information has become available in the literature on Bjerkandera adusta, which is now considered a promising candidate for further studies on bio-delignification, because it can produce a large amount of lignin peroxidases, similar to P. chrysosporium, but also manganese peroxidase, versatile peroxidase, and laccase, all highly effective at decomposing lignocellulose substrates [58,59].
2. Materials and Methods
2.1. Bio-Delignification Process
Bjerkandera adusta DSM 23426(BA) was purchased from the DSMZ (Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures GmbH) company and belongs to the collection of microorganisms of the Life Sciences and Biotechnology Department of the University of Ferrara. The inoculum was prepared by inoculating 100.0 mL of milk whey at a pH adjusted to 5.5 with HCl 1 M and incubation at 24 ± 2 °C on a rotary shaker (60 rpm) for 5 days. The obtained inoculum (100 mL) was used for inoculation of a 250 mL Erlenmeyer flask containing glucose (1 g/L) and the fresh biomass (25 g) diluted in 1 L of distilled water. The treatment was carried out at the above-mentioned conditions of pH, temperature, and agitation for 7 days.
2.2. Chemical Delignification
For delignification based on active chlorine, the approach followed by Tamburini et al. [60] for wheat straw was carried out. A diluted brine solution containing 5 g/L of NaCl was treated in an electrochemical cell for about 15 min, using 12 V power generation and adjusting pH to 6 with HCl 37%. As a result, an active chlorine solution at a concentration of about 1500 ppm was obtained. Then, 250 mL of this solution was put in contact with 5 g of fresh biomass (GW) for 60 min under slight agitation (60 rpm) and then was washed with 5 L of pure water to completely remove chlorine and chloride residues.
Treatment with alkali was carried out based on the protocol suggested by Asghar et al. [61]. First, 2 g of GW was soaked in 2.5% NaOH for 60 min at room temperature and then autoclaved at 121 °C for 90 min. Biomass was washed with pure water up to a neutral pH.
In the dilute acid delignification, H2SO4 3% (w/v) was added to 2 g GW samples, heated in an oil bath at 160 °C for 45 min [62], and washed with pure water up to a neutral pH.
At the end of the treatments, all samples were dried at 105 °C overnight and stored at room temperature until used.
2.3. Substrate Collection and Inoculum
For this study, OFMSW and GW were used as substrates. The OFMSW was supplied by a local multiutility company. It was composed of a great variety of raw and cooked food waste, as obtained from a door-to-door collection system from residences. The GW was collected from the university campus. It was mainly constituted of leaves, grass, and wooden sticks.
After being collected, the OMSFW and GW samples were ground in a domestic food processor to reduce the particle size to 0.5–1 cm. When not immediately used, they were frozen at a temperature of −20 °C for later use.
The inoculum was obtained from a local biogas plant (SESA Spa, Padova, Italy) that treats triticale and corn under mesophilic conditions.
2.4. Anaerobic Digestion (AD) Process
The experiments were carried out in a lab-scale anaerobic digester plant, equipped with four glass reactors with a working volume of 2 L and a plastic twin-hose screw cap GL45. Biogas was measured through the water displacement method, the volume of water displaced in the container was equal to that of the volume of the gas. One end of the gassing gadget was connected to the biogas plant and the other end was connected to the inverted measuring cylinder, which contained water. Biogas production was measured daily equal to the mL of water displaced. The biogas was allowed to collect in the inverted measuring cylinder by displacing water. A similar method was used by Zhang et al. [63], Huang et al. [64], and Pavi et al. [16]. The reactors were kept under a thermostatic water bath with a temperature fixed at 55 °C. Based on data reported by Liu et al. [25], a feed/inoculum ratio of 1.6 was maintained in all reactors with an initial loading of 10.6 g VS/L. Biogas production was reported as normal cubic meters (Nm3), that is the amount of gas in 1 cubic meter in normal conditions (20 °C and 1 Atm). Data on biogas production were normalized based on the equation:
where:
- V0 = normalized volume of biogas (Nm3)
- V = volume of biogas measured by the amount of water displaced (m3)
- pL = external pressure (Atm)
- pw = vapor pressure of water at room temperature (Atm)
- T0 = standard temperature (293 °K = 20 °C)
- P0 = standard pressure (1 Atm)
- T = room temperature (°K)
The following combinations of biomass were tested:
- Control (only inoculum)
- OFMSW
- Untreated GW
- OFMSW + GW
- OFMSW + GW pretreated
In the case of mixed matrices, the ratio of 1:1 in terms of volatile solids (VS) contribution was maintained. Immediately after the biomass addition, the headspace of the reactors was saturated with nitrogen to ensure anaerobic conditions.
Each reactor was manually shaken for 1 min twice a day and biogas production data were collected daily for 30 days. OFMSW and GW pre- and post-bio-delignification were characterized for total solids (TS), volatile solids (VS), lignin content, and reducing sugars content. All experiments were carried out in triplicate.
Biochemical methane potential (BMP) values were expressed per VS of the original waste, i.e., the cumulative methane production obtained at the end of the biodegradability tests (after subtraction of the value obtained in the blank assays) was divided by the equivalent VS content of the amount of waste that was pretreated and transferred to the biodegradability tests. To quantify methane content in biogas, the biogas flux passed through a CO2 capturing trap consisting of a 3 M NaOH solution with 0.4% thymolphtalein as the pH indicator.
2.5. Analytical Methods
The lignin determination was carried out using the Klason—TAPPI Standard T 222 method [65]. The sample (0.5 g) was treated with H2SO4 72% (10 mL) at 20 °C for 2 h followed by dilution to 3% H2SO4 and refluxing for 4 h. The lignin was filtered in a tared crucible, washed, dried, and weighed. The isolated lignin corresponded (by weight) closely to the original amount of lignin in the sample. Total solids (TS), volatile solids (VS), and ash content were determined following the standard procedures [66].
3. Results and Discussion
3.1. Bio-Delignification and Chemical Delignification Processes
The raw characterization of the fresh biomasses used in this study for biogas production are reported in Table 1. Typically, OFMSW is a highly heterogeneous material with high moisture content that makes it highly biodegradable due to the large fraction of food scraps and fresh food waste. On average, the TS content is about 20%, in a range between 9% and 30% [67], depending on region, waste collection, and management by authorities.

Table 1.
Chemical compositions and characteristics of fresh biomasses (OFMSW and untreated GW) (OFMSW = organic fraction of municipal solid waste; GW = green waste).
The heterogeneity of GW is caused by various factors, including the place where it is collected, the composition, and seasonal variations. It is very difficult to refer to average values because TS an vary from 5–8% in the case of a prevalence of flowers, grass, and leaves to 35–40% with woody materials [68].
In order to evaluate the performance of bio-delignification in comparison with chemical treatments, GW was submitted to four different treatments with different characteristics, as summarized in Table 2.

Table 2.
Characteristics of delignification and bio-delignification treatments carried out on GW samples (BA = Bjerkandera adusta).
Bio-delignification showed a yield of about 40% after 7 days of treatment, which is a very long time in comparison with the time for chemical delignification, but it is worth noting the total absence of toxic chemicals and the working temperature of room value. The yield obtained is comparable to values reported in the literature for bio-delignification, in the range 20–42% [69,70]. As expected, treatment based on sodium hydroxide had the highest yield, due to the strong effect of caustic degradation on lignocellulosic materials [23], provoking relevant damage on cellulose fibrils [60]. Chemical treatments with sulfuric acid and sodium hydroxide need some distilled water for biomass washing until reaching a neutral pH (pH = 7 ± 0.1), and 5 liters of distilled water is required for chlorine treatments. This is different from bio-delignification, which does not alter the pH of the solution. For residual rinsing water from chlorine treatments, an evaluation of the amount of reducing (fermentable) sugars lost in water was carried out, showing a value of 926.7 ± 55.6 mg/L.
As a whole, pretreatments on lignocellulosic matters based on microbial technology are perceived to be promising and low-impact methods for improving process efficiency, but their effective application at a plant-scale has been a subject of controversy due to the many conflicting literature reports about how they can be conveniently used to enhance the AD process. For example, a recent techno-economic analysis carried out by Vasco-Correa et al. [71] underlined that bio-delignification is not economically feasible when it is realized as a pretreatment for bioethanol production, because in that case, bio-delignification had to be preceded by a sterilization step followed by enzymatic hydrolysis to obtain sugars to be fermented to ethanol. In the case of bio-delignification used as a former step of biogas production, hydrolysis is carried out directly during the AD and sterilization is not needed. Moreover, there are no cost to feedstock, as it is already a collected waste, and there is no need to wash it with pure water, because of the absence of toxic residues or toxic byproducts. Overall, it seems a promising technique of pretreatment in order to increase the quantity of fermentable sugars to biogas.
The addition of glucose in the culture medium during bio-delignification has probably favored the production of laccase and peroxidase as lignolytic enzymes and promoted the growth of B. adusta on the easily available glucose rather than inducing cellulolytic activity, which would have reduced the amount of fermentable sugars in the subsequent AD process [72]. In fact, as reported by Quiroz-Castañeda et al. [73], B. adusta exhibits its maximum cellulolytic activity on the 6th day of culture, when lignocellulosic material is the sole carbon source. In the conditions maintained in our experiments, we can exclude a significant cellulose concentration depletion due to the growing metabolism of B. adusta in the 7 days of pretreatment. For the subsequent 30 days, the anaerobic conditions for biogas production, not favorable to fungal growth, provoked fungal death, and so there is no cellulose depletion, making DA available to the organic material of cells debris.
Compared to the untreated GW, delignification treatments modified the TS and VS content of GW, as shown in Table 3.

Table 3.
Variation of the TS and VS content of GW with different delignification treatments.
A consistent loss of both TS and VS occurred with sodium hydroxide treatment and at a lesser extent in the case of the other chemical treatments, whereas the bio-delignification did not significantly alter the characteristics of GW. Moreover, without water flushing for biomass washing, all the soluble matter was available for AD.
3.2. Anaerobic Digestion (AD) of OFMSW and Bio-Delignificated GW
In order to assess the effectiveness of bio-delignification in improving biodegradability and enhancing biogas generation, batch AD assays of untreated and bio-delignified GW co-digested with OFMSW were carried out. Figure 1 compares the cumulative biogas production as a function of time (30 days) that occurred during AD of OFMSW, untreated GW, OFMSW + untreated GW, and OFMSW + GW treated with BA.
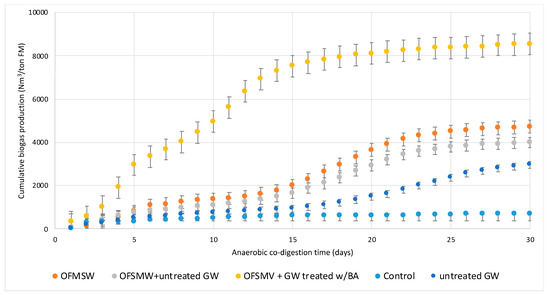
Figure 1.
Cumulative biogas production during anaerobic co-digestion of OFMSW and GW, treated with BA and untreated, compared with AD of OFMSW and untreated GW. Control corresponds to the sole inoculum AD without the addition of biomass. Data are expressed as Nm3 of biogas per ton of FM (OFMSW = organic fraction of municipal solid waste; GW = green waste; BA = Bjerkandera adusta; AD = anaerobic digestion; FM = fresh matter).
As seen, pretreatment of GW with BA was certainly advantageous in increasing biogas yield during co-digestion. After 30 days, the cumulative biogas production increased by more than 80% compared to the cumulative biogas production from the sole OFMSW, from 4720 to 8550 Nm3/ton SV, respectivey. This occurrence validates the fact that pretreatment with BA promotes lignin degradation and consequently promotes hydrolysis of the cellulose and hemicellulose present in GW and their biodigestibility to biogas. Compared with the co-digestion of OFMSW and untreated GW, it is evident how the presence of ligneous residues mixed with OFMSW has a slightly negative effect on the final cumulative yield, with a reduction of about 15% (4000 Nm3/ton FM), probably due to spatial interferences induced by untreated GW fragments that make it difficult for hydrolytic bacteria to reach the promptly fermentable sugars in OFMSW. Untreated GW alone obtained a cumulative production lower than 3000 Nm3/ton FM. These results suggest that bio-delignification is suitable as a pretreatments for AD, including accessibility of particles to microbial activity.
As observed in Figure 2, about 90% of the overall biogas production from co-digestion of OFMSW and treated GW was obtained in the first 15 days, arriving at over 95% in 20 days. The two peaks, at days 5 and 12, were probably due to the different types of organic matters available for the hydrolytic microbial consortium. OFSMW shows a daily production, characterized by two peaks as well, the first due to promptly fermentable sugars in food waste and the second at days 7–20 by AD of slowly fermentable or complex organic matter. OFMSW + untreated GW had the same trend as the sole OFMSW, with smooth peaks of production.
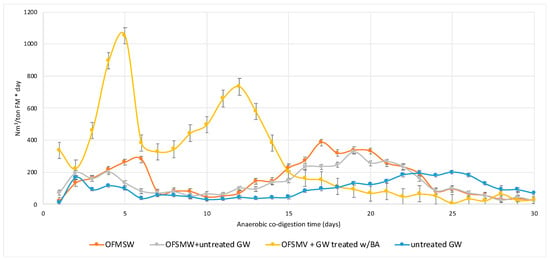
Figure 2.
Daily production of biogas from the co-digestion of OFMSW and GW, treated with BA and untreated, compared with AD of the sole OFMSW and untreated GW. Since biogas production was collected discontinuously, the conjunction lines have the sole function of helping with the comprehension of daily trends and do not correspond to continuous measurements. OFMSW = organic fraction of municipal solid waste; GW = green waste; BA = Bjerkandera adusta; AD = anaerobic digestion.
Reduction in AD time along with an increased biogas production is another advantageous outcome of BA pretreatment of GW in co-digestion with OFMSW. A duration as short as possible is a pivotal parameter in the AD process setup, to increase plant productivity, use the smallest reactor volume, and reduce the cost of operation [74]. Moreover, the opportunity to increase substrate biodegradability also permits for the enhancement of the overall efficiency of the AD plant [75]. Table 4 shows the increase of productivity during co-digestion of OFMSW and treated GW based on 30 days of process compared with those of the other two cases. Moreover, considering the opportunity to reduce the hydraulic retention time to 20 days, productivity increased to 500 Nm3/ton FM*day. This could be of relevance in the case of application on a higher scale in terms of economic issues of the plant’s management. Reduction in digestion time along with increased biogas production are yet more advantageous outcomes of bio-delignification of GW co-digested with OFMSW. Attaining the shortest digestion time is a key variable to using the smallest reactor volume and minimizing the cost of operation. Reduction in digestion time means increased substrate biodegradability and, therefore, it is an indicator of enhanced efficiency during anaerobic digestion. BMP values are also reported. The measured average methane content values in cumulative biogas were 55% from OFMSW and 54% from GW, in accordance with the values reported in the literature [76].

Table 4.
Parameters of the AD and anaerobic co-digestion processes considered in this study (OFMSW = organic fraction of municipal solid waste; GW = green waste; BA = Bjerkandera adusta; FM = fresh matter; BMP = biochemical methane potential; VS = volatile solids).
It is worthwhile noting the difficulty of comparing these values with others found in the literature for BMP and those related to other GW pretreatments and co-digestion with OFMSW because of the great heterogeneity of the materials utilized. By comparing our results with those reported in the literature regarding different fungal pretreatments of agricultural residues and their effects on biogas yield improvements, we can see that B. adusta could ensure a promising combination between biogas yield increase and time of treatment. Table 5 is a comparative evaluation describing the combination of biological treatment and biogas production with references to the published literature.

Table 5.
Different fungal strains used in delignification of agricultural residues and effects of pretreatments on biogas production (AD = anaerobic digestion; BMP = biochemical methane potential).
Despite B. adusta pretreatments of GW being eco-friendly, cost-effective, and seemingly not affected by sugar losses [41,47,65,71], in comparison with other chemical treatments, it is a time consuming process, which must have a detrimental effect on the overall process costs. This application deserves a specific technoeconomic analysis that accounts for capital costs, energy consumption, requirements of chemicals, and waste management; fungal pretreatment imposes extra costs, which often forms a chief hindrance for industrial scaling up of lignocellulosic biomass.
It is worth noting that with respect to the other fungal pretreatments (Table 5), B. adusta could ensure the best performance in terms of both time of treatment and biogas yield improvement. The activities and generation of ligninolytic enzymes secreted by white-rot fungi are influenced by several characteristics such as the availability of substrate, moisture content, incubation time, temperature, O2 concentration, pH, and nutrients supplementation. The optimization of the process is crucial for efficient pretreatment for high fungi activity and production of ligninolytic enzymes, and subsequently higher lignin digestion, for reducing process costs and for managing a scaling up at the industrial level.
4. Conclusions
This study investigated the effect of bio-delignification with the white-rot fungi Bjiekandera adusta on anaerobic biodegradability of GW, which is rich in lignocellulosic matter, co-digested with OFMSW. A bio-delignification yield of 42% was obtained after treating GW for 7 days with B. adusta at room temperature and with mild agitation. Although the yield of delignification was significantly lower than what obtained was with the sodium hydroxide treatment (78% of delignification), co-digestion of OFMSW and GW treated with B. adusta showed an increase of biogas yield in 30 days, reaching 289 Nm3 CH4/ton VS, leading to a 98% increase in biogas yield compared to that of untreated biomass. Considering the opportunity to diminish the hydraulic retention time by about 10 days, productivity increased to 500 Nm3/ton FM*day, emphasizing the effect of bio-delignification on lignocellulosic structure and the increasing matrix accessibility to microbial attack. The increase in biogas production is attributed to lignin degradation induced by the fungi strain, releasing cellulose and hemicellulose that is then available for subsequent microbial attachment.
To conclude, bio-delignification with B. adusta was shown to be very efficient for improving the biodegradability of lignocellulosic biomass. It is inexpensive, environmental-friendly, and has great room for further improvements in terms of optimization of pretreatment conditions (duration, etc.), which should certainly improve these results. In future research, pretreatments with B. adusta can give access to new feedstocks for biogas plants, improving the circular economy paradigm.
Author Contributions
Conceptualization, E.T. and S.C.; methodology, data curation, and investigation F.Z.; B.S., and D.S.; writing—original draft preparation, E.T.; writing—review and editing, all authors; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tyagi, V.K.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Garcia, L.I.R.; Ng, W.J. Anaerobic Co-Digestion of Organic Fraction of Municipal Solid Waste (OFMSW): Progress and Challenges. Renew. Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar] [CrossRef]
- Municipal Waste Statistics—Statistics Explained. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Municipal_waste_statistics (accessed on 13 March 2021).
- Inghels, D.; Dullaert, W.; Aghezzaf, E.-H.; Heijungs, R. Towards Optimal Trade-Offs between Material and Energy Recovery for Green Waste. Waste Manag. 2019, 93, 100–111. [Google Scholar] [CrossRef]
- Malinauskaite, J.; Jouhara, H.; Czajczyńska, D.; Stanchev, P.; Katsou, E.; Rostkowski, P.; Thorne, R.J.; Colón, J.; Ponsá, S.; Al-Mansour, F.; et al. Municipal Solid Waste Management and Waste-to-Energy in the Context of a Circular Economy and Energy Recycling in Europe. Energy 2017, 141, 2013–2044. [Google Scholar] [CrossRef]
- Logan, M.; Visvanathan, C. Management Strategies for Anaerobic Digestate of Organic Fraction of Municipal Solid Waste: Current Status and Future Prospects. Waste Manag. Res. 2019, 37, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveen, B.P.; Sumalatha, J.; Malik, R.K. A Study on Contamination of Ground and Surface Water Bodies by Leachate Leakage from a Landfill in Bangalore, India. Int. J. Geo-Eng. 2018, 9, 27. [Google Scholar] [CrossRef]
- Bekchanov, M.; Mirzabaev, A. Circular Economy of Composting in Sri Lanka: Opportunities and Challenges for Reducing Waste Related Pollution and Improving Soil Health. J. Clean. Prod. 2018, 202, 1107–1119. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.; et al. Resource Recovery and Circular Economy from Organic Solid Waste Using Aerobic and Anaerobic Digestion Technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef]
- Cucchiella, F.; D’Adamo, I.; Gastaldi, M. An Economic Analysis of Biogas-Biomethane Chain from Animal Residues in Italy. J. Clean. Prod. 2019, 230, 888–897. [Google Scholar] [CrossRef]
- Turcios, A.E.; Cayenne, A.; Uellendahl, H.; Papenbrock, J. Halophyte Plants and Their Residues as Feedstock for Biogas Production—Chances and Challenges. Appl. Sci. 2021, 11, 2746. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Sebastia-Saez, D.; Pastor-Pérez, L.; Reina, T.R. Analysis of the Potential for Biogas Upgrading to Syngas via Catalytic Reforming in the United Kingdom. Renew. Sustain. Energy Rev. 2021, 144, 110939. [Google Scholar] [CrossRef]
- Ardolino, F.; Arena, U. Biowaste-to-Biomethane: An LCA Study on Biogas and Syngas Roads. Waste Manag. 2019, 87, 441–453. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Circular Economy Action Plan—For a Cleaner and More Competitive Europe; European Union: Brussels, Belgium, 2020; Available online: https://ec.europa.eu/environment/pdf/circular-economy/new_circular_economy_action_plan.pdf (accessed on 1 June 2021).
- Battista, F.; Frison, N.; Pavan, P.; Cavinato, C.; Gottardo, M.; Fatone, F.; Eusebi, A.L.; Majone, M.; Zeppilli, M.; Valentino, F.; et al. Food Wastes and Sewage Sludge as Feedstock for an Urban Biorefinery Producing Biofuels and Added-Value Bioproducts. J. Chem. Technol. Biotechnol. 2020, 95, 328–338. [Google Scholar] [CrossRef]
- Shahriari, H.; Warith, M.; Hamoda, M.; Kennedy, K.J. Anaerobic Digestion of Organic Fraction of Municipal Solid Waste Combining Two Pretreatment Modalities, High Temperature Microwave and Hydrogen Peroxide. Waste Manag. 2012, 32, 41–52. [Google Scholar] [CrossRef]
- Pavi, S.; Kramer, L.E.; Gomes, L.P.; Miranda, L.A.S. Biogas Production from Co-Digestion of Organic Fraction of Municipal Solid Waste and Fruit and Vegetable Waste. Bioresour. Technol. 2017, 228, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Kougias, P.G.; Angelidaki, I. Biogas and Its Opportunities—A Review. Front. Environ. Sci. Eng. 2018, 12, 14. [Google Scholar] [CrossRef]
- Sahota, S.; Shah, G.; Ghosh, P.; Kapoor, R.; Sengupta, S.; Singh, P.; Vijay, V.; Sahay, A.; Vijay, V.K.; Thakur, I.S. Review of Trends in Biogas Upgradation Technologies and Future Perspectives. Bioresour. Technol. Rep. 2018, 1, 79–88. [Google Scholar] [CrossRef]
- Bolzonella, D.; Pavan, P.; Mace, S.; Cecchi, F. Dry Anaerobic Digestion of Differently Sorted Organic Municipal Solid Waste: A Full-Scale Experience. Water Sci. Technol. 2006, 53, 23–32. [Google Scholar] [CrossRef]
- Giuliano, A.; Catizzone, E.; Freda, C.; Cornacchia, G. Valorization of OFMSW Digestate-Derived Syngas toward Methanol, Hydrogen, or Electricity: Process Simulation and Carbon Footprint Calculation. Processes 2020, 8, 526. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic Co-Digestion Process for Biogas Production: Progress, Challenges and Perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Rajendran, K.; Drielak, E.; Sudarshan Varma, V.; Muthusamy, S.; Kumar, G. Updates on the Pretreatment of Lignocellulosic Feedstocks for Bioenergy Production—A Review. Biomass Conv. Bioref. 2018, 8, 471–483. [Google Scholar] [CrossRef]
- Dasgupta, A.; Chandel, M.K. Enhancement of Biogas Production from Organic Fraction of Municipal Solid Waste Using Hydrothermal Pretreatment. Bioresour. Technol. Rep. 2019, 7, 100281. [Google Scholar] [CrossRef]
- Ionescu, G.; Rada, E.C.; Ragazzi, M.; Mărculescu, C.; Badea, A.; Apostol, T. Integrated Municipal Solid Waste Scenario Model Using Advanced Pretreatment and Waste to Energy Processes. Energy Convers. Manag. 2013, 76, 1083–1092. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, R.; El-Mashad, H.M.; Dong, R. Effect of Feed to Inoculum Ratios on Biogas Yields of Food and Green Wastes. Bioresour. Technol. 2009, 100, 5103–5108. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, W.; Sheng, K.; Sanati, M. Comparison of High-Solids to Liquid Anaerobic Co-Digestion of Food Waste and Green Waste. Bioresour. Technol. 2014, 154, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Ho, M.C.; Ong, V.Z.; Wu, T.Y. Potential Use of Alkaline Hydrogen Peroxide in Lignocellulosic Biomass Pretreatment and Valorization—A Review. Renew. Sustain. Energy Rev. 2019, 112, 75–86. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Zamani, A.; Amiri, H.; Horváth, I.S. Enhanced Solid-State Biogas Production from Lignocellulosic Biomass by Organosolv Pretreatment. BioMed Res. Int. 2014, 2014, e350414. [Google Scholar] [CrossRef]
- Abraham, A.; Mathew, A.K.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.H.; Sang, B.-I. Pretreatment Strategies for Enhanced Biogas Production from Lignocellulosic Biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef]
- Kumar, G.; Dharmaraja, J.; Arvindnarayan, S.; Shoban, S.; Bakonyi, P.; Saratale, G.D.; Nemestóthy, N.; Bélafi–Bakó, K.; Yoon, J.; Kim, S. A Comprehensive Review on Thermochemical, Biological, Biochemical and Hybrid Conversion Methods of Bio-Derived Lignocellulosic Molecules into Renewable Fuels. Fuel 2019, 251, 352–367. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Tocco, D.; Carucci, C.; Monduzzi, M.; Salis, A.; Sanjust, E. Recent Developments in the Delignification and Exploitation of Grass Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2021, 9, 2412–2432. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Review of Second Generation Bioethanol Production from Residual Biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef]
- Mutschlechner, M.; Illmer, P.; Wagner, A.O. Biological Pre-Treatment: Enhancing Biogas Production Using the Highly Cellulolytic Fungus Trichoderma Viride. Waste Manag. 2015, 43, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A Review on Lignin Structure, Pretreatments, Fermentation Reactions and Biorefinery Potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging Technologies for the Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valor. 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Wagner, A.O.; Lackner, N.; Mutschlechner, M.; Prem, E.M.; Markt, R.; Illmer, P. Biological Pretreatment Strategies for Second-Generation Lignocellulosic Resources to Enhance Biogas Production. Energies 2018, 11, 1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rencoret, J.; Pereira, A.; del Río, J.C.; Martínez, Á.T.; Gutiérrez, A. Delignification and Saccharification Enhancement of Sugarcane Byproducts by a Laccase-Based Pretreatment. ACS Sustain. Chem. Eng. 2017, 5, 7145–7154. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Zhao, L.; Ding, Z.; Ma, H.; Terry, N. Fungal Laccase Production from Lignocellulosic Agricultural Wastes by Solid-State Fermentation: A Review. Microorganisms 2019, 7, 665. [Google Scholar] [CrossRef] [Green Version]
- Kainthola, J.; Podder, A.; Fechner, M.; Goel, R. An Overview of Fungal Pretreatment Processes for Anaerobic Digestion: Applications, Bottlenecks and Future Needs. Bioresour. Technol. 2021, 321, 124397. [Google Scholar] [CrossRef]
- Rouches, E.; Zhou, S.; Sergent, M.; Raouche, S.; Carrere, H. Influence of White-Rot Fungus Polyporus Brumalis BRFM 985 Culture Conditions on the Pretreatment Efficiency for Anaerobic Digestion of Wheat Straw. Biomass Bioenergy 2018, 110, 75–79. [Google Scholar] [CrossRef]
- Tišma, M.; Planinić, M.; Bucić-Kojić, A.; Panjičko, M.; Zupančič, G.D.; Zelić, B. Corn Silage Fungal-Based Solid-State Pretreatment for Enhanced Biogas Production in Anaerobic Co-Digestion with Cow Manure. Bioresour. Technol. 2018, 253, 220–226. [Google Scholar] [CrossRef]
- Zhao, J.; Ge, X.; Vasco-Correa, J.; Li, Y. Fungal Pretreatment of Unsterilized Yard Trimmings for Enhanced Methane Production by Solid-State Anaerobic Digestion. Bioresour. Technol. 2014, 158, 248–252. [Google Scholar] [CrossRef]
- Wyman, V.; Henríquez, J.; Palma, C.; Carvajal, A. Lignocellulosic Waste Valorisation Strategy through Enzyme and Biogas Production. Bioresour. Technol. 2018, 247, 402–411. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Poulsen, T.G.; Sheng, K. Fungal Pretreatment of Rice Straw with Pleurotus Ostreatus and Trichoderma Reesei to Enhance Methane Production under Solid-State Anaerobic Digestion. Appl. Energy 2016, 180, 661–671. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of Lignocellulosic Biomass for Enhanced Biogas Production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Christian, V.; Shrivastava, R.; Shukla, D.; Modi, H.A.; Vyas, B.R.M. Degradation of Xenobiotic Compounds by Lignin-Degrading White-Rot Fungi: Enzymology and Mechanisms Involved. IJEB 2005, 43, 301–312. [Google Scholar]
- Kaal, E.E.J.; Field, J.A.; Joyce, T.W. Increasing Ligninolytic Enzyme Activities in Several White-Rot Basidiomycetes by Nitrogen-Sufficient Media. Bioresour. Technol. 1995, 53, 133–139. [Google Scholar] [CrossRef]
- Tien, M.; Kirk, T.K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete Chrysosporium Burds. Science 1983, 221, 661–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glenn, J.K.; Morgan, M.A.; Mayfield, M.B.; Kuwahara, M.; Gold, M.H. An Extracellular H2O2-Requiring Enzyme Preparation Involved in Lignin Biodegradation by the White Rot Basidiomycete Phanerochaete Chrysosporium. Biochem. Biophys. Res. Commun. 1983, 114, 1077–1083. [Google Scholar] [CrossRef]
- Kocher, G.S.; Kaur, P.; Taggar, M.S. An Overview of Pretreatment Processes with Special Reference to Biological Pretreatment for Rice Straw Delignification. Curr. Biochem. Eng. 2017, 4, 151–163. [Google Scholar] [CrossRef]
- Chandel, A.K.; Gonçalves, B.C.M.; Strap, J.L.; da Silva, S.S. Biodelignification of Lignocellulose Substrates: An Intrinsic and Sustainable Pretreatment Strategy for Clean Energy Production. Crit. Rev. Biotechnol. 2015, 35, 281–293. [Google Scholar] [CrossRef]
- Kang, B.R.; Kim, S.B.; Song, H.A.; Lee, T.K. Accelerating the Biodegradation of High-Density Polyethylene (HDPE) Using Bjerkandera Adusta TBB-03 and Lignocellulose Substrates. Microorganisms 2019, 7, 304. [Google Scholar] [CrossRef] [Green Version]
- Bouacem, K.; Rekik, H.; Jaouadi, N.Z.; Zenati, B.; Kourdali, S.; El Hattab, M.; Badis, A.; Annane, R.; Bejar, S.; Hacene, H.; et al. Purification and Characterization of Two Novel Peroxidases from the Dye-Decolorizing Fungus Bjerkandera Adusta Strain CX-9. Int. J. Biol. Macromol. 2018, 106, 636–646. [Google Scholar] [CrossRef]
- Davila-Vazquez, G.; Tinoco, R.; Pickard, M.A.; Vazquez-Duhalt, R. Transformation of Halogenated Pesticides by Versatile Peroxidase from Bjerkandera Adusta. Enzym. Microb. Technol. 2005, 36, 223–231. [Google Scholar] [CrossRef]
- Costa, S.; Dedola, D.G.; Pellizzari, S.; Blo, R.; Rugiero, I.; Pedrini, P.; Tamburini, E. Lignin Biodegradation in Pulp-and-Paper Mill Wastewater by Selected White Rot Fungi. Water 2017, 9, 935. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.-Q.; Zhao, R.-Y.; Chen, Z.-C. Review of the Pretreatment and Bioconversion of Lignocellulosic Biomass from Wheat Straw Materials. Renew. Sustain. Energy Rev. 2018, 91, 483–489. [Google Scholar] [CrossRef]
- Dinis, M.J.; Bezerra, R.M.F.; Nunes, F.; Dias, A.A.; Guedes, C.V.; Ferreira, L.M.M.; Cone, J.W.; Marques, G.S.M.; Barros, A.R.N.; Rodrigues, M.A.M. Modification of Wheat Straw Lignin by Solid State Fermentation with White-Rot Fungi. Bioresour. Technol. 2009, 100, 4829–4835. [Google Scholar] [CrossRef] [Green Version]
- Tamburini, E.; Bernardi, T.; Castaldelli, G.; Tumiatti, G.; Ferro, S. Green Electrochemical Approach for Delignification of Wheat Straw in Second-Generation Bioethanol Production. Energy Environ. Sci. 2011, 4, 551–557. [Google Scholar] [CrossRef]
- Asghar, U.; Irfan, M.; Iram, M.; Huma, Z.; Nelofer, R.; Nadeem, M.; Syed, Q. Effect of Alkaline Pretreatment on Delignification of Wheat Straw. Nat. Prod. Res. 2015, 29, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Knierim, B.; Manisseri, C.; Arora, R.; Scheller, H.V.; Auer, M.; Vogel, K.P.; Simmons, B.A.; Singh, S. Comparison of Dilute Acid and Ionic Liquid Pretreatment of Switchgrass: Biomass Recalcitrance, Delignification and Enzymatic Saccharification. Bioresour. Technol. 2010, 101, 4900–4906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Hu, J.; Lee, D.-J. Biogas from Anaerobic Digestion Processes: Research Updates. Renew. Energy 2016, 98, 108–119. [Google Scholar] [CrossRef]
- Huang, X.; Yun, S.; Zhu, J.; Du, T.; Zhang, C.; Li, X. Mesophilic Anaerobic Co-Digestion of Aloe Peel Waste with Dairy Manure in the Batch Digester: Focusing on Mixing Ratios and Digestate Stability. Bioresour. Technol. 2016, 218, 62–68. [Google Scholar] [CrossRef]
- TAPPI T 222—Acid-Insoluble Lignin in Wood and Pulp|Engineering360. Available online: https://standards.globalspec.com/std/10402544/TAPPI%20T%20222 (accessed on 15 March 2021).
- Mauer, L.J.; Bradley, R.L. Moisture and Total Solids Analysis. In Food Analysis; Nielsen, S.S., Ed.; Food Science Text Series; Springer: Cham, Switzerland, 2017; pp. 257–286. ISBN 9783319457765. [Google Scholar]
- Cabbai, V.; Ballico, M.; Aneggi, E.; Goi, D. BMP Tests of Source Selected OFMSW to Evaluate Anaerobic Codigestion with Sewage Sludge. Waste Manag. 2013, 33, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Langsdorf, A.; Volkmar, M.; Holtmann, D.; Ulber, R. Material Utilization of Green Waste: A Review on Potential Valorization Methods. Bioresour. Bioprocess. 2021, 8, 19. [Google Scholar] [CrossRef]
- Sun, F.; Li, J.; Yuan, Y.; Yan, Z.; Liu, X. Effect of Biological Pretreatment with Trametes Hirsuta Yj9 on Enzymatic Hydrolysis of Corn Stover. Int. Biodeterior. Biodegrad. 2011, 65, 931–938. [Google Scholar] [CrossRef]
- Vasco-Correa, J.; Ge, X.; Li, Y. Fungal Pretreatment of Non-Sterile Miscanthus for Enhanced Enzymatic Hydrolysis. Bioresour. Technol. 2016, 203, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Vasco-Correa, J.; Shah, A. Techno-Economic Bottlenecks of the Fungal Pretreatment of Lignocellulosic Biomass. Fermentation 2019, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Heinfling, A.; Martínez, M.J.; Martínez, A.T.; Bergbauer, M.; Szewzyk, U. Purification and Characterization of Peroxidases from the Dye-Decolorizing Fungus Bjerkandera Adusta. FEMS Microbiol. Lett. 1998, 165, 43–50. [Google Scholar] [CrossRef]
- Quiroz-Castañeda, R.E.; Balcázar-López, E.; Dantán-González, E.; Martinez, A.; Folch-Mallol, J.; Martínez Anaya, C. Characterization of Cellulolytic Activities of Bjerkandera Adusta and Pycnoporus Sanguineus on Solid Wheat Straw Medium. Electron. J. Biotechnol. 2009, 12, 5–6. [Google Scholar]
- Angelidaki, I.; Boe, K.; Ellegaard, L. Effect of Operating Conditions and Reactor Configuration on Efficiency of Full-Scale Biogas Plants. Water Sci. Technol. 2005, 52, 189–194. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. A Review of the Chemistry of Anaerobic Digestion: Methods of Accelerating and Optimizing Process Efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef] [Green Version]
- Seruga, P.; Krzywonos, M.; Seruga, A.; Niedźwiecki, Ł.; Pawlak-Kruczek, H.; Urbanowska, A. Anaerobic Digestion Performance: Separate Collected vs. Mechanical Segregated Organic Fractions of Municipal Solid Waste as Feedstock. Energies 2020, 13, 3768. [Google Scholar] [CrossRef]
- Müller, H.W.; Trösch, W. Screening of White-Rot Fungi for Biological Pretreatment of Wheat Straw for Biogas Production. Appl. Microbiol. Biotechnol. 1986, 24, 180–185. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V.; Goel, R. Fungal Pretreatment and Associated Kinetics of Rice Straw Hydrolysis to Accelerate Methane Yield from Anaerobic Digestion. Bioresour. Technol. 2019, 286, 121368. [Google Scholar] [CrossRef]
- Albornoz, S.; Wyman, V.; Palma, C.; Carvajal, A. Understanding of the Contribution of the Fungal Treatment Conditions in a Wheat Straw Biorefinery That Produces Enzymes and Biogas. Biochem. Eng. J. 2018, 140, 140–147. [Google Scholar] [CrossRef]
- Liu, S.; Wu, S.; Pang, C.; Li, W.; Dong, R. Microbial Pretreatment of Corn Stovers by Solid-State Cultivation of Phanerochaete Chrysosporium for Biogas Production. Appl. Biochem. Biotechnol. 2014, 172, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).