Abstract
Oral mucocele is a benign cystic exophytic lesion affecting the minor salivary gland and is especially present in pediatric patients (3% under 14 years). It is characterized by an extravasation or retention of fluid or mucus in the submucosal tissue of the minor salivary glands. Several surgical techniques have been proposed over the years, including the excision of the mucocele by using the injection of a hydrocolloid impression material in the light of the cyst to prevent the collapse of the cystic wall and solidify the lesion, resulting in a better cleavage plan. The combined clinical approach between the combination of Shira’s technique and the surgical excision of the cystic lesion results in a conservative surgical removal of the lesion. Here, we reported the removal of a labial mucocele in a 14-year-old male patient, using the injection of a hydrocolloid impression material. At a 12 months follow up, the patient showed complete healing of the surgical site, showing a pinkish lip lining mucosa without scarring or recurrence of the primary lesion. The combined therapeutic approach between Shira’s technique and surgical excision allows a safe and predictable excision of the labial mucocele, minimizing the risk of recurrence.
1. Introduction
Mucocele is a benign, exophytic and asymptomatic cystic lesion, found in the oral cavity, in the appendix, in the gallbladder, in the paranasal sinuses or in the lacrimal sac [1,2]. Clinically, the lesion is characterized by the accumulation of liquid or mucoid material, which generates a translucent nodular lesion, circumscribed with rounded edges, bluish in color and of variable size. The consistency is typically soft and fluctuating in response to palpation and exhibits a slow growth. In the oral cavity, the most affected structures are the lower lip, in 77% of cases, followed by the floor of the mouth and the tongue (antero-ventral portion) at 15%, while the rarer mucoceles of the cheek and the palate occur around 9% of the time [3,4,5,6].
The lesion has no gender predilection and affects patients of all ages, in the order of 2.5 lesions per 1000 individuals, but has the most affected subjects in children and young adults; these data are more relevant if it is calculated in comparisons of all lesions, with a percentage of 17% [7,8].
The size of oral mucoceles varies from 1 mm to several centimeters in diameter. Mucoceles are generally asymptomatic, although in some patients they may cause discomfort by interfering with speech, chewing or swallowing [1]. Some oral mucoceles evolve and disappear spontaneously after a short time, while others become chronic and require surgical removal.
The etiological mechanisms of development of these lesions are the extravasation of mucus, generally considered as of traumatic origin, and the retention of mucus, resulting from the obstruction of the duct of a minor or accessory gland [1].
Mucoceles are histologically classified into the extravasation variant and the retention variant [1,9,10].
The extravasation histotype accounts for 92% of cases and evolves following a trauma to a duct gland, located in the lower labial mucosa and in the buccal mucosa. The trauma tears the duct, resulting in the saliva leaking into the connective tissue. Symptoms of chronic inflammation occur, resulting in the formation of a granulation tissue capsule and the presence of foamy mucus-filled macrophages in the cystic fluid. It is found in individuals under the age of 30 and is mainly located in the lower lip (80%) [10,11].
Oral retention mucocele is caused by the occlusion or subocclusion of the excretory duct of a minor salivary gland, by a sialolith, a mucin plug formed by a very thick salivary secretion or an anomaly in the shape or thickness of the duct, which determines the non-excretion of the glandular secretion. The resulting increase in intraluminal pressure determines the dilation of the canal upstream of the obstruction. Therefore, a cystic formation is generated whose wall is made up of the “stretched” ductal epithelium following the abnormal dilation of the duct itself. The retention mucocele, on the other hand, appears as a cavity delimited by fibrous tissue and covered by epithelium with variable characteristics of cuboid cells, cylindrical or flattened, and mono, pseudo or multilayered. The inflammatory infiltrate is scarce [12].
The literature describes various therapeutic procedures for the removal of the mucocele lesion: surgical excision with scalpel, ablation with carbon dioxide (CO2) and LASER ablation, intralesional injection of corticosteroids, marsupialization and cryosurgery [1,13]. The main problem when removing the mucocele is the high frequency of recurrence of the primary lesion following the permanence in situ of epithelial fragments of the capsule that circumscribe the lesion [14].
An easy-to-use technique that allows the total removal of the lesion, including its wall, is the combination of filling the lesion lumen with a hydrocolloid impression material (dental alginate), followed by surgical excision [1].
The technique reported by us was described for the first time in 1962 by Shira [15], highlighting its practical advantages. The injection of a solid hydrocolloid impression material into the lumen prevents its collapse, and therefore it is easier to remove the cyst wall in its entirety, preserving the integrity of the surrounding tissues. In case of relapse, it is necessary to remove the adjacent salivary gland. Below, we report the treatment of a pediatric lower lip mucocele using Shira’s technique and surgical excision of the lesion.
2. Case History
A 14-year-old male patient came to the dental clinic of the University of L’Aquila, complaining about the presence of a swelling on the mucosal surface of the inferior lip. While completing the anamnestic interview, the patient stated that the swelling started 25 days ago and that it slowly increased in volume. The intraoral inspection revealed a swelling measuring 10 mm, with elastic consistency, red and blue colored, not fluctuant and painless (Figure 1). Based on the age and the position, a mucous extravasation mucocele was the presumed clinical diagnosis. The parents of the patient gave their consent to the publication of this case.
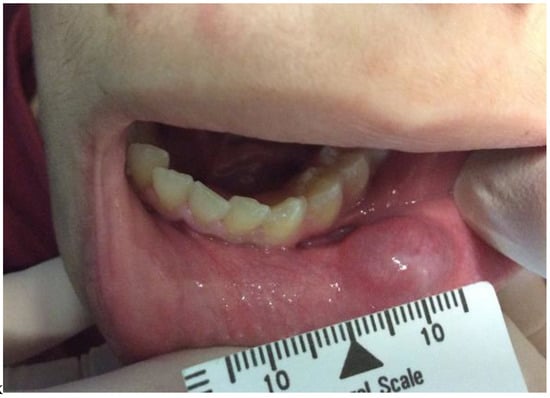
Figure 1.
Male patient of 14 years showed swelling of the lower lip mucosa, of elastic consistency, red/blue color, not fluctuating and painless. The lesion measured 10 mm.
Surgical Procedure
The patient underwent antibiotic prophylaxis by administering 2 g amoxicillin to be taken 1 h before surgery. The aforementioned therapy was continued by taking one tablet every 12 h for the next 3 days. The patient was provided with postoperative instructions which included pre-running antibiotic prophylaxis as indicated, use of routine pain relievers as needed and intake of a liquid diet for 3 days. In addition, the patient was recommended to use chlorhexidine spray 0.20% for 4 days, cleansing with 10% hydrogen peroxide using a sterile hydrophilic gauze to be passed over the sutures [16].
Local anesthesia, OPTOCaIN® (20 mg/m with adrenalin 1:80,000. Molteni Dental—Italy), was applied. The anesthesia was administered at the level of the mental nerve, on the bottom of the fornix and on the mental foramen contralateral to the lesion, avoiding tension in the incision area.
Through use of an intradermic syringe, the cystic fluid was aspired. Intralesional fluid was suctioned by single-use sterile syringe (5 mL). Subsequently, we kept the needle in the light of the lesion and injected, through a second sterile syringe, the hydrocolloid material from imprint in fluid consistency.
Therefore, in the same hole, the alginate material was inserted to fill the cavity (Figure 2).
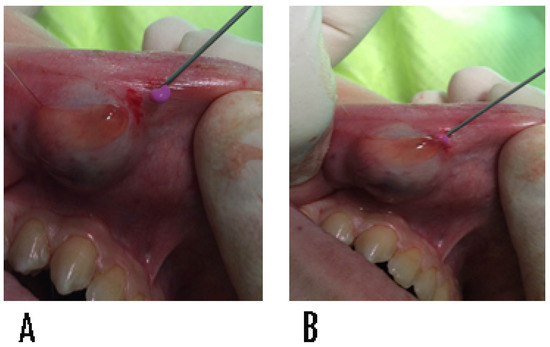
Figure 2.
(A,B) Injection of hydrocolloid impression material (alginate) by syringe into the lumen of the mucocele. The technique prevents the collapse of the wall of the lesion, safeguarding the health of the surrounding tissue.
The hydrocolloid material used in this case report was Hydrogum 5® extra fast (Zhermack dental, Italy), with 65 s of processing time, mixing included. The material remained in the oral cavity for 45 s for a total of 110 s. Our working group operated in a sterile operating field. Regarding the hydrocolloid material, we used alginate taken from a new package and cold sterilized the instrument for the preparation of the same by gluteraldehyde 2%. The volume of hydrocolloid impression material was equal to the amount of liquid aspirated from the lesion. Once the material became solid, the formation was enucleated (Figure 3).
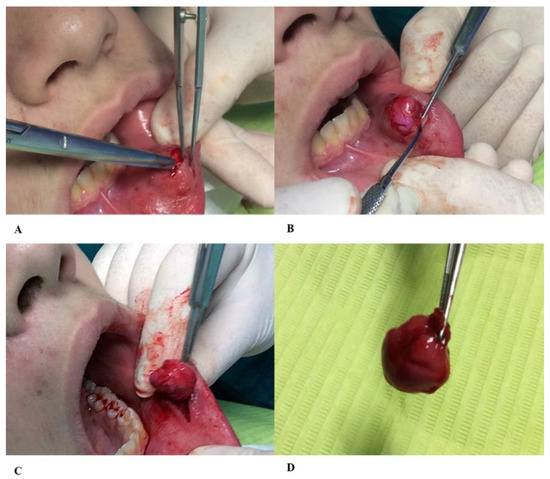
Figure 3.
(A) After insertion into the lumen of the hydrocolloid material injury impression, we proceeded with the dissection of the flap through scissors with rounded ends and (B) the removal of the cyst wall, (C) preserving the healthy surrounding tissue. (D) The complete removal of the cyst wall is the key to preventing recurrence of mucocele.
The site was then sutured by VICRYLTM—Ethicon (Ethicon code: V304H, caliber: 4/0, color: purple, shape: cylindrical, needle length: 17.4 mm, gauge: 21, needle: RB-1) and the sutures were removed 7 days later. At the 12 months follow up no recurrence appeared (Figure 4).
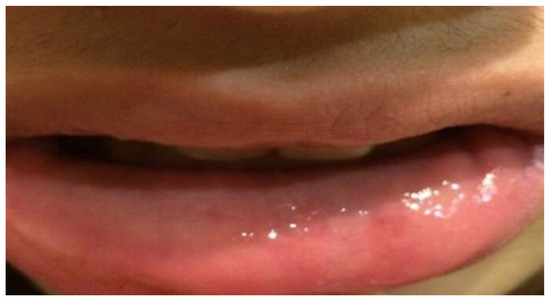
Figure 4.
Twelve months follow up. The patient showed the labial mucosa of conformation and normal color, without any scars.
The histological examination, performed at the San Salvatore dell’Aquila Hospital, showed a fibro-collagenous cystic wall, foamy microphages, scanty skeletal muscle fibers and benign salivary gland tissue showing mixed inflammation, confirming the diagnosis of extravasation mucocele (Figure 5).
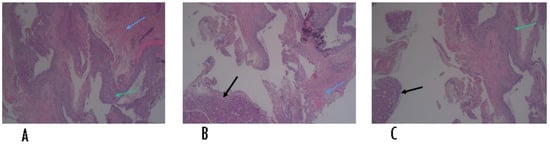
Figure 5.
Histological slides of the cyst specimen. Black arrows (B,C) indicate benign salivary gland tissue, green arrows (A,C) indicate foamy microphages, blue arrows (A,B) indicate skeletal muscle fibers.
The patient was seen after one week for a follow up and the sutures were removed. He did not report any complication other than mild discomfort on the day of the surgery. The site of excision appeared to be healing and was non-tender to palpation on day seven.
3. Discussion
Mucocele lesions usually occur without particular symptoms and are usually diagnosed during routine clinical checks or if they create discomfort for the patient. Buccal mucosal mucoceles can be bothersome and painful due to their susceptibility to trauma, so surgical removal is almost mandatory. Considering the clinical aspect of the mucocele with other lesions of the oral cavity, such as gingival cysts, palatal cysts, thyroglossal cysts, congenital epulis, vascular hamartoma, lymphangioma and oropharyngeal teratoma, [2,17] it is essential to always perform a biopsy examination of the removed lesion to confirm the diagnostic hypothesis.
Although the traditional excision technique is considered a simple surgical procedure [18], over the years the development of new technologies has aimed at minimizing tissue and psychological trauma to the patient [19]. In the literature, the authors have demonstrated the efficacy and simplicity of the micro-marsupialization procedure for the treatment of oral ranulae and mucoceles [20].
Authors in the literature argue that a valid therapeutic approach that allows the removal of the lesion is LASER ablation [21]. The effect of CO2 LASER on soft tissues is poorly penetrating and limited to the soft tissue surface alone, but both post-operative bleeding and paresthetic problems of the lower lip have been documented [22].
An effective and non-invasive technique is the intralesional injection of corticosteroids (bethamethasone), recently reported as an efficient, repeatable and economical procedure [23]. Its non-surgical nature makes it widely acceptable for patients. However, the protocol includes multiple injections, and therefore patient compliance is critical.
The technique reported by us was described for the first time in 1962 by Shira [15], highlighting its practical advantages. The injection of a solid hydrocolloid impression material into the lumen prevents its collapse, and therefore it is easier to remove the cyst wall entirely, preserving the integrity of the surrounding tissues. Complete removal of the cystic wall is essential as the presence of epithelial residues in situ determines the appearance of future relapses of the primary lesion. Shira’s technique is relatively minimally invasive and fulfills the objective described above. The use of hydrocolloid impression material, due to its consistency and its work-hardening time, can present side effects during the procedure such as the possibility of extension of the material in the deep tissue planes with the risk of losing the material to inside the tissues. This complication can lead to the development of a secondary foreign body inflammatory reaction [24]. However, the compatibility of the hydrocolloid material with moist oral soft tissues [25] makes it the material of choice for this type of procedure.
The use of irreversible hydrocolloid material to delineate the mucocele boundaries, beyond the simplicity and the cost advantages, facilitates the cleavage plan while keeping the anatomical references of the lesion intact, and might allow a complete removal and minimize the chances of recurrence.
This type of combined procedure has been shown to be effective in pediatric patients, especially those suffering from anxiety and fear of the dentist.
The mucocele is a pathology recognized as being principally caused by the mechanical trauma deriving from biting minor salivary glands in the inferior lips.
Even though the nature of the lesion is benign, an accurate diagnosis should always include a histology analysis to confirm its nature, since sometimes the clinical appearance of mucocele can be similar to the mucoepidermoid tumor or the primitive rhabdomyosarcoma of the inferior lip.
The clinical case presented in our case report shows the labial mucosa in health, pink in color, with the absence of scarring 12 months after surgery. The absence of relapses at the follow up visit confirmed the success of this technique in the removal of labial and buccal mucoceles. Further clinical findings and systematic reviews are needed to confirm the safe use of this surgical procedure.
4. Conclusions
The complete surgical excision of the mucocele as therapy is fundamental and highly recommended to avoid the recurrence of the lesion.
In our experience, the therapeutic decision of sculpting with respect to LASER is conditioned by the size of the lesion and its tissue depth. The use of irreversible hydrocolloid material to delineate the boundaries of the mucocele, due to its easy management, allows us to focus the anatomical references of the lesion to ensure complete removal and minimize the chances of recurrence.
Author Contributions
Investigation, G.B., C.F. and M.S.; Resources, G.F. and R.G.; Data curation, G.B., C.F. and M.S.; Writing—original draft preparation, G.B., C.F. and M.S.; Writing—review and editing, G.F., R.G., G.F.F., P.V.V. and C.D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Internal Review Board of the University of L’Aquila, n. 55/2018.19.
Informed Consent Statement
Informed consent was obtained from the subject involved in the study.
Data Availability Statement
Data will be available upon reasonable request to the corresponding author.
Acknowledgments
The authors are grateful to Sara Bernardi for data, critical discussions and advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baurmash, H.D. Mucoceles and ranulas. J. Oral. Maxillofac. Surg. 2003, 61, 369–378. [Google Scholar] [CrossRef]
- Ozturk, K.; Yaman, H.; Arbag, H.; Koroglu, D.; Toy, H. Submandibular gland mucocele: Report of two cases. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2005, 100, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Re Cecconi, D.; Achilli, A.; Tarozzi, M.; Lodi, G. Mucoceles of the oral cavity: A large case series (1994–2008) and a literature review. Med. Oral. Pathol. Oral. Cir. Bucal. 2010, 15, e551–e556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- More, C.B.; Bhavsar, K.; Varma, S.; Tailor, M. Oral mucocele: A clinical and histopathological study. J. Oral. Maxillofac. Pathol. 2014, 18 (Suppl. 1), S72–S77. [Google Scholar] [CrossRef] [Green Version]
- Graillon, N.; Mage, C.; Le Roux, M.K.; Scemama, U.; Chossegros, C.; Foletti, J.M. Mucoceles of the anterior ventral surface of the tongue and the glands of Blandin-Nuhn: 5 cases. J. Stomatol. Oral. Maxillofac. Surg. 2019, 120, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Ata-Ali, J.; Carrillo, C.; Bonet, C.; Balaguer, J. Oral mucocele: Review of literature. J. Clin. Exp. Dent. 2010, 2, e18–e21. [Google Scholar] [CrossRef]
- Hong, C.; Dean, D.R.; Hull, K.; Hu, S.J.; Sim, Y.F.; Nadeau, C.; Gonçalves, S.; Lodi, G.; Hodgson, T.A. World Workshop on Oral Medicine VII: Relative frequency of oral mucosal lesions in children, a scoping review. Oral. Dis. 2019, 25 (Suppl. 1), 193–203. [Google Scholar] [CrossRef]
- Jensen, J.L. Superficial mucoceles of the oral mucosa. Am. J. Dermatopathol. 1990, 12, 88–92. [Google Scholar] [CrossRef]
- Harrison, J.D. Salivary mucoceles. Oral. Surg. Oral. Med. Oral. Pathol. 1975, 39, 268–278. [Google Scholar] [CrossRef]
- Bhaskar, S.N.; Bolden, T.E.; Weinmann, J.P. Pathogenesis of mucoceles. J. Dent. Res. 1956, 35, 863–874. [Google Scholar] [CrossRef]
- Jinbu, Y.; Kusama, M.; Itoh, H.; Matsumoto, K.; Wang, J.; Noguchi, T. Mucocele of the glands of Blandin-Nuhn: Clinical and histopathologic analysis of 26 cases. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2003, 95, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Sfasciotti, M.; Perfetti, G.; Annibali, S.; Pippi, R. Il mucocele delle ghiandole salivari minori. Parte I: Eziopatogenesi e istopatologia. Dental. Cadmos 1991, 11, 66–78. [Google Scholar]
- Sagari, S.K.; Vamsi, K.C.; Shah, D.; Singh, V.; Patil, G.B.; Saawarn, S. Micromarsupialization: A minimally invasive technique for mucocele in children and adolescents. J. Indian Soc. Pedod. Prev. Dent. 2012, 30, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Byun, J.S.; Choi, J.K.; Jung, J.K. Identification of predictive variables for the recurrence of oral mucocele. Med. Oral. Pathol. Oral. Cir. Bucal 2019, 24, e231–e235. [Google Scholar] [CrossRef]
- Anastassov, G.E.; Haiavy, J.; Solodnik, P.; Lee, H.; Lumerman, H. Submandibular gland mucocele: Diagnosis and management. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2000, 89, 159–163. [Google Scholar] [CrossRef]
- Johnson-Jahangir, H.; Agrawal, N. Perioperative Antibiotic Use in Cutaneous Surgery. Dermatol Clin. 2019, 37, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Dinoi, M.T.; Marchetti, E.; Garagiola, U.; Caruso, S.; Mummolo, S.; Marzo, G. Orthodontic treatment of an unerupted mandibular canine tooth in a patient with mixed dentition: A case report. J. Med. Case Rep. 2016, 10, 170. [Google Scholar] [CrossRef] [Green Version]
- Paglia, L.; Gallusi, S.; de Giorgio, S.; Cianetti, S.; Lupatelli, E.; Lombardo, G.; Montedori, A.; Eusebi, P.; Gatto, R.; Caruso, S. Reliability and validity of the Italian versions of the Children’s Fear Survey Schedule—Dental Subscale and the Modified Child Dental Anxiety Scale. Eur. J. Paediatr. Dent. 2017, 18, 305–312. [Google Scholar]
- Delbem, A.; Cunha, R.; Vieira, A.; Ribeiro, L. Treatment of mucus retention phenomena in children by the micro-marsupialization technique: Case reports. Pediatr. Dent. 2000, 22, 1558. [Google Scholar]
- Bernardi, S.; Mummolo, S.; Zeka, K.; Pajewski, L.; Continenza, M.A.; Marzo, G. Use and Evaluation of a Cooling Aid in Laser-Assisted Dental Surgery: An Innovative Study. Photomed. Laser. Surg. 2016, 34, 258–262. [Google Scholar] [CrossRef]
- Sinha, R.; Soumyabrata, S.; Khaitan, T.; Kabiraj, A.; Maji, A. Nonsurgical Management of Oral Mucocele by Intralesional Corticosteroid Therapy. Int. J. Dent. 2016, 2896748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, I.Y.; Chen, C.M.; Kao, Y.H.; Worthington, P. Treatment of mucocele of the lower lip with carbon dioxide laser. J. Oral. Maxillofac. Surg. 2007, 65, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Shira, R.B. Simplified technic for the management of mucoceles and ranulas. J. Oral. Surg (Chic) 1962, 20, 374–379. [Google Scholar]
- De Santana Santos, T.; Martins, V.B.; Frota, R.; Karam, F.K. Excision of ranula using injection of hydrocolloid dental impression material. J. Craniofac. Surg. 2013, 24, 1859–1860. [Google Scholar] [CrossRef]
- Bernardi, S.; Marzo, G.; Continenza, M.A. Dorzalna površina jezika i halitoza: Morfološki aspekti Dorsal Lingual Surface and Halitosis: A Morphological Point of View. Acta Stomatol. Croat. 2016, 50, 151–157. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).