Abstract
The Gulf of California is the most productive fishing region in Mexico; its ecosystems contain a vast diversity of species with exploitation potential, some of them potentially vulnerable to climate change. This research was conducted to analyze, through habitat suitability models, the possible alterations in the distribution of the three shrimp species of the most importance for commercial fishing in the region: Litopenaeus stylirostris, Litopenaeus vannamei, and Farfantepenaeus californiensis. Habitat suitability models were built using the MaxEnt software, primary productivity data, temperature, salinity, bathymetry, substratum, coastal type, and geo-referenced occurrence records of the three species. Of the data, 70% was used on training, while the remaining 30% was used for validation. To make estimates of climate change impact on this fishery, projections on distribution of the three species from environmental forecasts generated by the intergovernmental panel on climate change until 2100 were made. The used model, that is in full development and expansion, could be considered as an applicable tool to other problems and showed efficiency rates above 90%. The species will maintain most of their historical distribution, but L. stylirostris and L. vannamei will have a new distribution area within the zones of the Magdalena-Almejas Bay and the Gulf of Ulloa, with an increase of 80% and 148% respectively; all species will have loss areas in the proportion of 16%, 2%, and 11%, respectively, along the southern Gulf of California.
1. Introduction
In the Mexican Pacific, eight shrimp species are utilized, from which three constitute more than the 90% of the total catch: brown shrimp (Farfantepenaeus californiensis), blue shrimp (Litopenaeus stylirostris), and white shrimp (Litopenaeus vannamei). Both the industrial fleet and the small scale one or riverside participate in the fishing [1,2]. The capture of these species is within the top ten by tons produced, but in first place by the economic benefits at the national level [3]. Their distribution is associated with estuarine systems, coastal lagoons, and the coastline of tropical and subtropical zones, which are zones characterized by great environmental, biologic, and ecologic variability [4].
The main three shrimp species with high commercial value are captured largely from the Gulf of California (GC), where each shrimp has its preferred environmental conditions. The blue shrimp is caught with more frequency in the areas near the estuaries, and in salinities higher than normal [5]. The brown shrimp is located over harder substrates and clearer waters [5]. The white shrimp is found regularly on zones influenced by river deltas, estuaries, or coast lagoons; it does not tolerate high salinities neither cold temperatures, and temperatures over 33 °C and below 5 °C are lethal in the long term; the optimal intervals are between 24 to 30 °C [6]. Nowadays, the states in the northwest of the country contribute most of the capture (75%), which is composed mainly of brown shrimp (F. californiensis) (approximately 70–80% of the total catch) and blue shrimp (L. stylirostris) [6,7,8]; the white shrimp (L. vannamei) are captured to to a lesser extent, as well as a variety of crystal shrimp species (F. brevirostris) and rock shrimp (Sicyonia spp.).
The shrimp capture in Mexico has important fluctuations that are mainly related to the environmental variability, creating evidence suggesting that these are species greatly affected by environmental changes which impact their abundance, feeding, and mainly their distribution. Besides this, the high fishing effort may cause high variability of captures [9,10,11] and an effect on biomass production [12,13,14]. According to official statistics in the Gulf of California, during the 2000–2018 period, the reported catches by shrimp fisheries were upwards of 30,000 tons yearly. During 2017–2018, the catch was 51,611 tons, with a value of $290 million USD.
The ENSO Niño-Niña phenomenon has a profound influence on shrimp’s biomass, causing a reproduction gap that has repercussions in recruitment periods in lagoons, estuaries, and later to high seas [4]. The blue shrimps are the species that shows a higher correlation with environmental variability, since in Niña events the capture increases significantly, and the opposite happens in Niño events; in white and brown shrimps it is possible to observe tendencies which indicate some environmental influence on captures; the former seems to be favored by Niña events and the latter by Niño events [6].
Nowadays, records exist on environmental variability behavior which accumulate data series of many years, but there are few published studies about what will happen as a consequence of climate change in the future. One of these studies is the one from the Intergovernmental Panel on Climate Change (IPCC), established in 1988; the IPCC publishes periodical assessment reports to understand the climate change induced by human activities, and which mention that, in the last 30 years, the atmospheric temperature increased by between 0.3 °C and 0.6 °C and that increments between 1.4 and 5.5 °C are expected for the last part of the century. These variations within the sea environment affect the ocean’s climate regulation, since they have a heat capacity (heat absorption) around 1000 times higher than that of the atmosphere, suggesting that each atmospheric increase of 1 °C will cause an increase of 0.5 °C in the ocean [15]. In this way, the future scenarios on climate change imply a global increase in the ocean’s temperature, a decrease in total primary productivity, and an increase of salinity [16,17,18].
The fishing sector is strongly affected by extreme climate conditions [19]. In Mexico, the shrimp is a high priority fishing resource due to its high demand and value on the market, providing a considerable source of currency and job generation for an important proportion of the fishers’ population [20]. Climate change could have severe repercussions on Mexico, since it will not only be temperature increases, but the frequency of extreme hydrometeorological events will increase too, having severe impacts on some species and causing their extinction if temperature variations surpass their adaptive capability [21]. Therefore, information is needed regarding spatial and temporal variability of this type of fishery to provide a view on the changes that shrimp populations can expect [22], and to provide useful scenarios to integrate strategies for climate change adaptation and thereby reduce potential future impacts in the fishing industry and fishers.
The habitat suitability model (HSM) portrays information about relationships between species and environmental variables; therefore, it must be considered as a useful and improvable approach, and as an applicable tool to other problems such as the forecast of potential environmental impacts on species distribution [23,24,25]. These models are in full development and expansion, with new methods that allow evaluations on environmental variations impacts, which could ease the development of strategies that help to elaborate scientific answers on how the species react to the changes that global warming causes [26]. Many algorithms exist for HSM creation, but the Maximum entropy (MaxEnt) model is the most used because it implements one of the algorithms of precise mathematical formulation and only requires presence data and environmental variables [27].
Considering what was discussed previously, this work had the purpose of using HSM to analyze the historical distribution of brown shrimp (F. californiensis), blue shrimp (L. stylirostris), and white shrimp (L. vannamei) to project the models in function of the possible variations on oceanographic conditions caused by climate change, using the scenarios 2.6, 4.5, and 8.5 of the Representative Concentration Pathways (RCP) proposed by the Intergovernmental Panel on Climate Change (IPCC). This effort generated unpublished information about the potential effects on the distribution of the main species of the fishery in the face of climate change, and identified the variables with the greatest contribution to the modelling process.
2. Materials and Methods
2.1. Study Area
The Gulf of California (Figure 1) has hydrological characteristics that vary from the large and the narrow sea [28]. The northern region is characterized by high superficial salinities and temperatures that oscillate from 10 °C to 30 °C in winter to summer; in the central region, superficial temperature shows a strong difference between winter and summer, reaching values of 16 °C and 31 °C, respectively, and the southern region, which is in open communication with the tropical Pacific Ocean through the mouth, has a complex hydrographic structure due to the confluence of many water masses [29]. On the other hand, the mixture by tides and the upwelling processes generate a high primary productivity [30], which contributes a great volume of resources of fishing importance to the region’s coastal communities.
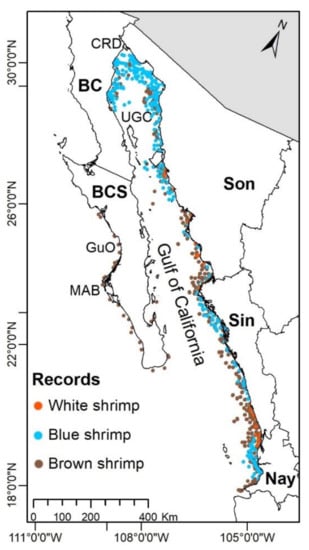
Figure 1.
Study Area and shrimps’ occurrence records.
2.2. Habitat Suitability Models
To develop the HSM, two essential elements were required: data on species presence and an environmental database which serves to detect the potential habitable sites on the GC.
2.2.1. Occurrence Records of Shrimps
The occurrence data for each shrimp species were obtained from free access sources: the Global Biodiversity Information Facility (GBIF; www.gbif.org; accessed on 28 February 2019), the Ocean Biogeographic Information System (OBIS; https://obis.org/; accessed on 13 September 2020), and INaturalist (https://www.inaturalist.org/; accessed on 13 September 2020), but the majority were obtained by digitizing maps from scientific publications related to shrimp fishing (Table 1). The digitization was made in QGIS 3.8 software; in order to decrease the spatial autocorrelation, a filter to eliminate duplicate data on a buffer of 9.2 km was applied to the occurrence records at the same spatial resolution of environmental layers [31,32]. Additionally, the elimination of records with mistakes pertaining to coordinates, land records, or that were outside the species’ natural habitat were done. In total, 595 records for the blue shrimp, 650 for the brown shrimp, and 388 for the white shrimp were obtained (Figure 1).

Table 1.
Environmental and occurrence records used to create the species distribution model of shrimps.
2.2.2. Environmental Database
The environmental database was built from data of: Primary productivity (PP, mg C m−2 d−1), sea surface temperature (SST, °C), bathymetry (m), sea surface salinity (SSS), and substratum and coastal type.
The primary productivity data (PP), obtained from Oregon State University, were based on data obtained by the sensor MODIS Aqua, which consisted of satellite images complemented with the carbon-based productivity model [33]. SST data was obtained from the sensor MODIS Aqua. Both variables’ data were obtained from 2003–2019 on a resolution of 9.2 km. The SSS was obtained from a HYCOM (HYbrid Coordinate Ocean Model) [34] model from 2003–2019 and were interpolated using an inverse distance weighted (IDW) scheme on the same resolution and spatial coverage as the SST and the PP. These data were averaged to generate climatology for each season, which were used to develop models of the shrimp distribution (Table 1).
To prevent the high correlation between environmental layers, we used Pearson’s correlation analysis in R software [35] and excluded the environmental layers with correlation indices higher than 0.9 because high correlation between environmental layers may alter model predictions [36,37].
The bathymetry data was downloaded from the General Bathymetric Chart of the Oceans (GEBCO) on a resolution of 30 arc seconds and was re-sampled on the same spatial resolution as the rest of the variables. For the creation of the coastal type, a raster of Normalized Difference Vegetation Index (NDVI), downloaded from the Global Land Cover Facility (GLCF: http://www.landcover.org/; accessed on 13 September 2020), was implemented to differentiate between mangrove ecosystems and other types of non-submerged vegetation [38,39]. The substratum type data were obtained by digitizing the sea sedimentology published by INAPESCA (1994) [40].
2.2.3. Current Shrimps’ Distribution Model
The habitat suitability model (HSM) was made individually for each species using MaxEnt 3.4.1 [27]; 30 replicates of each model were made, and for each one of them 30% of the randomly selected records were used and the remaining 70% were used as model training. With this finished, the average of the 30 remaining replicates was considered as a result of the three shrimps’ habitat suitability modelling. The output on logistics format and the area under the curve (AUC) value from the receiver operating characteristics (ROC) chart were used to evaluate the model efficiency.
2.3. Climate Change Scenarios
The climate change scenarios were obtained from the Coupled Model Intercomparison Project Phase 5 (CMIP5) generated by the Max Planck Institute (MPI). These scenarios propose weather tendencies derived from the concentration of greenhouse gases on the atmosphere; the scenarios used were the RCP, 2.6, 4.5, and 8.5, which indicated 2.6 W/m2 for scenario 2.6, 4.5 W/m2, for scenario RCP 4.5, and 8.5 W/m2 in the case of scenario RCP 8.5 [41].
The scenarios consisted of the variables SST (K), SSS (PSU), and PP (mol m−2s−1) for the period 2006–2100; this data was selected due to its spatial resolution (0.25°), which included data along the Gulf of California, unlike other models [17]. The MPI projection units became the same historical variables (SST in °C and PP in mg C m−2 d-1), but the scenarios presented a bias in regard to local conditions, which was corrected using the quantile method to escalate towards real environmental data [42]. Taking as reference the historical variables of SST, SSS, and PP (2006–2018), the quantiles were calculated using the R software [35]. Once the biases in RCP scenarios were corrected, Pearson‘s correlation between the historical variables (2006–2018) and the RCP models for the same period were made; a high correlation between historical variables and RCP scenarios indicated bias reduction.
Once the HSM was made, the tenth percentile threshold provided by MaxEnt was used to generate binary maps (presence/absence; Melo-Merino et al., 2020) [43] and to make estimations on the current coverage in km2 of these species’ distribution. Later, projections were made for every decade (current to 2100) according to each RCP scenario to detect the change in the shrimps’ habitat caused by the changes in environmental variables according to the RCP scenarios.
3. Results
According to Pearson’s correlation analysis, the selected environmental variables for making the habitat suitability models were temperature and primary productivity during winter and summer. In the case of salinity, all seasons showed a high correlation between them, so we selected the autumn salinity because it had the lowest correlation in relation to the rest of the environmental variables (R ≤ 0.68; Table A1).
On the other hand, Pearson’s correlation analysis between historical variables and RCP scenarios found that the SST had a correlation higher than 0.87, the salinity had a correlation higher than 0.91 and, finally, the primary productivity had the lowest correlation of 0.67.
The MaxEnt results indicated that the areas under the curve (AUC) of the blue, white, and brown shrimp species’ potential distribution were 0.973, 0.988, and 0.961, respectively. The values of the tenth percentile threshold were 0.387 for the blue shrimp, 0.372 for the white shrimp, and 0.266 for the brown shrimp.
3.1. Environmental Contribution on Shrimps’ Habitat Suitability Models
The bathymetry, maximum temperature, and minimum salinity were the variables which most influenced species distribution with more than 94% contribution; the remaining variables (substratum, maximum primary productivity, minimum, minimum temperature and coastal type) had less than 6% contribution (Table 2).

Table 2.
Contribution of each selected variable to create the habitat model for each shrimp species using MaxEnt.
According to the response curve generated by MaxEnt (Figure A1, Figure A2 and Figure A3), the blue shrimp preferred depths less than 100 m, temperatures between 13 to 32 °C, primary production of 250 to 380 mg C m−2 d−1, a salinity of 35.5, coasts with little unsubmerged vegetation, and sandy soils with gravel and silt (Figure A1).
The white shrimp preferred depths less than 100 m, temperatures between 25 to 32 °C, primary production of 400 to 600 mg C m−2 d−1, a minimum salinity of 34.5, and coasts with vegetation and soils of sand and silt (Figure A2). The brown shrimp preferred depths less than 100 m, temperatures between 18 to 32 °C, primary production of 100 to 3500 mg C m−2 d−1, a minimum salinity of 34–34.5, and coasts with plentiful vegetation and soils of sand, silt, and gravel (Figure A3).
3.2. Current Shrimps’ Distribution
According to the habitability models, the blue shrimp (Figure 2a) had a continuous distribution from Nayarit to the upper Gulf of California, which equals to an extension of 59,778 km2 approximately. On the other hand, the white shrimp had its distribution in the coasts of Nayarit, Sinaloa, and south of Sonora, and the distribution area was 24,543 km2 with limits around the Guaymas bay (Figure 2b). Finally, the brown shrimp had a distribution in all the states found within the Gulf of California but the species had its major concentration in Sinaloa; the distribution area was 106,110 km2 and included zones outside the GC, such as Magdalena Bay in the Pacific Ocean (Figure 2c).
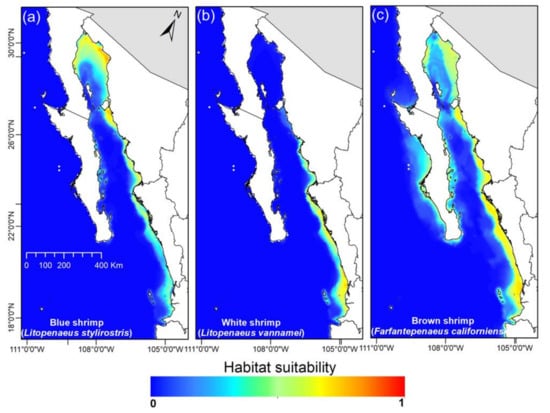
Figure 2.
Current habitats suitability model for each shrimp species in the Gulf of California.
3.3. Climate Change Effects on Shrimps’ Distribution
The estimated areas from the climate change projections (Figure 3 and Figure 4, Figure A4 and Figure A5) show the amount of available area tendency for the three shrimp species; these show that the blue and white shrimp will have an increase of area above their historical distribution in the whole time set, while the brown shrimp will have fluctuations, both increasing and decreasing in regard to its historical distribution.
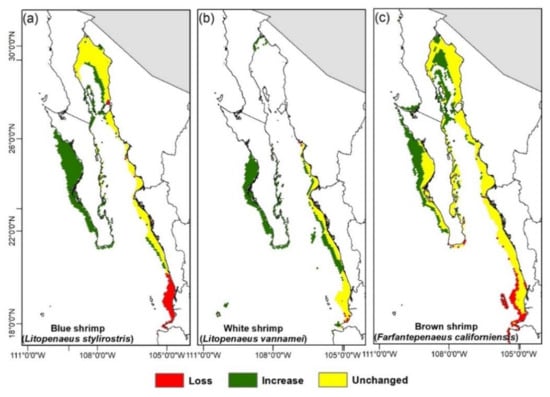
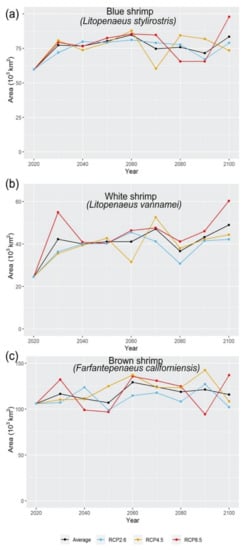
Figure 4.
Changes in habitat suitability areas for each shrimps’ species based on climate change scenarios (current to 2100).
The geographical changes projected towards 2100 (Figure 3 and Figure 4, Figure A4 and Figure A5) show that the blue shrimp will have a 16% loss zone including the coasts of Nayarit and south of Sinaloa, while the coasts of BCS are where the higher area gain will appear with an increase of 80%. For the white shrimp, there will be a small spatial loss in Nayarit and the gain areas will be distributed in the upper Gulf of California and BCS, including the zones of the Gulf of Ulloa (GuO) and Magdalena-Almejas Bay (MAB) with a 148% area increase. The brown shrimp will have an increase of 40% in the west coast of BCS and the greater part of the upper Gulf of California; the loss zones will be only 11%, distributed in Nayarit and the southern part of Sinaloa.
4. Discussion
4.1. Uncertainties and Assumptions
The shrimps’ distribution has limits related to the environmental conditions they live in, highlighting the variable values of temperature, salinity, and primary productivity in the depths wherein adult-size shrimps are found. Considering that records of these values are not available due to the high cost implicit in generating this data, they are uncommon in governmental agencies statistics, and occasionally published as research products; these type of studies are complex due to the lack of reports, or because the existent ones correspond to specific periods and not to a constant data series.
Additionally, it has to be considered that in the case GC upwellings occur, induced by the wind along the coast, there will be impacts on food availability, temperature, salinity, and turbidity [20,44]; these conditions are a reflection of what lies at sea bottom, which rises to the surface carrying with it these conditions that are present in historical variables data. In accordance with all of the above, we considered that the use of historical variables at a surface level is not ideal, but in the absence of information, it offered an approach to the environmental conditions that are found in the sea bottom.
4.2. Model Accuracy and Current Distribution
The MaxEnt algorithm is considered as one of the best due to its performance and precision [27,45], since it provides AUC values based on the assessment locations used in each model execution [46]. In this study, the average values of AUC calculated from 30 model repetitions were used to measure the model performance, since generally the AUC is the measure accepted as the best to evaluate the model performance [47]; values between 0.8–0.9 indicated a very good model quality [48].
The habitat suitability models (Figure 2) are a congruent reflection of the abundancy and species capture zones, since the digitized points correspond to abundancy maps and fishing boats; therefore, it is assumed that in the upper Gulf of California only blue and brown shrimp are found, and in the zone exist two activities of special attention: the resources exploitation and the ecosystem conservation [49]. The space occupied by fishing in the UGC is large and it includes approximately 75% of the aquatic surface; the core zone and the vaquita refuge area are zones not highly exploited, and they must function as recruitment areas and for population recovery of sea species affected by fishing [50,51].
In the coast of Sonora, Guaymas is one of the most important zones in terms of riparian shrimp fishing due to its habitats that include shallow waters, sandy beaches, rocky areas, islands, coastal dunes, mangrove vegetation, and seaweed beds; likewise, it is a place for reproduction and the breeding of brown, blue, and white shrimp [52], which is congruent with the habitability models because these species have a preference for these types of oceanographic conditions. In the state of Sinaloa, the habitability models indicated that the three shrimps are distributed in most of the state, because the coast has a series of estuaries, bays, coastal lagoons, and wetlands, which generate protected environments from wave activity and predators that are perfect for reproduction development [53].
Finally, in the state of Baja California Sur (BCS), the lagoon complex of Magdalena-Almejas Bay is the main zone for brown shrimp extraction, which is the most conspicuous species in the captures, significantly partaking in seasons both with high and low total production [5]. The lagoon complex is divided into three zones: the northwest zone, the Magdalena Bay zone, and the Almejas Bay zone. Each of them is irregular in shape and is composed of a large number of estuaries, small lagoons, and by waterways with an average depth of 3.6 m [5].
4.3. Environmental Drivers of Shrimps’ Distribution
Among the environmental variables used for the HSM, the bathymetry showed most influence on predictions of potential distribution zones (Table 2), which is consistent with other articles reporting that the bathymetry is extremely important in shrimp distribution [3,22]; additionally, Basher and Costello (2016) reported contribution percentages of 61% for bathymetry, it being the most important variable in the distribution of the Nematocarcinus lanceopes shrimp in Antarctica, by using the ManEnt algorithm with AUC results of 0.958 [54]. On the other hand, Dambach et al., (2012) also reported that the bathymetry variable was the one which presented the major contribution in their model for the Nematocarcinus lanceopes and Notocrangon antarcticus species, with contributions of 42.8% and 66.6% respectively, and with AUC values of 0.960 and 0.980 respectively, and highlighted its importance for their survival [55]. The temperature and salinity are also very important variables on shrimp distribution due to their direct effect on oxygen consumption, metabolism, growth, and molting [56].
Lluch-Belda (1982) showed that the blue shrimp dominates in waters protected by high salinities or over the sea average (35), being able to sporadically decrease due to continental freshwater discharges [57]. This habitat is typical of sea waters linked to desert and semi-desert climates in the Gulf of California, such as the states of Baja California (BC), Sonora, and northern Sinaloa, but it was observed that its distribution decreases once entering the south of Sinaloa, where the white shrimp dominates.
The white shrimp dominates in waters protected by a permanent or seasonal low salinity that responds to the rain cycle, from southern Sinaloa to Guatemala [58]; the zones with 20 °C temperatures make them inactive and lowers their food consumption, unlike the shrimps from the 35 °C zones, which show hyperactivity and a higher food consumption [59]. Additionally, the white shrimp tolerates low salinities, these commonly found in protected waters, becoming the dominant species in inland waters from southern Sinaloa to Tehuantepec [2].
For the brown shrimp, the zones with 35°C temperatures are deadly; in contrast, in zones below 19 °C it is demonstrated that the shrimp grows without apparent problems [60], also considering that it presents an optimal growth and survivability to salinities of 35 [61,62]. Our habitat suitability models (Figure 2) were congruent with the distribution of each shrimp found in the bibliography.
In the Mexican Pacific it has been demonstrated that the shrimps present different times and intensities in their reproduction. It has been demonstrated that latitudinal gradients exist where there is a presence of mature females all year in tropical zones and the reproduction is restricted only to the warm summer months in temperate zones for the same species [63,64,65].
4.4. Range Shift in Response to Climate Change
According to our analysis, the three shrimp species will continue to be distributed from Sinaloa to Guaymas, Sonora within the GC, including the coast of the municipality of La Paz and Comondú in BCS in the Pacific Ocean coast. In addition, two or more species will continue to be distributed in areas where there are currently more permits for vessels dedicated to shrimp fishing and are also the sites where the highest catches are reported according to official fishery statistics (Figure 5); therefore, the negative impacts of climate change on this fishery within the GC could be lower.
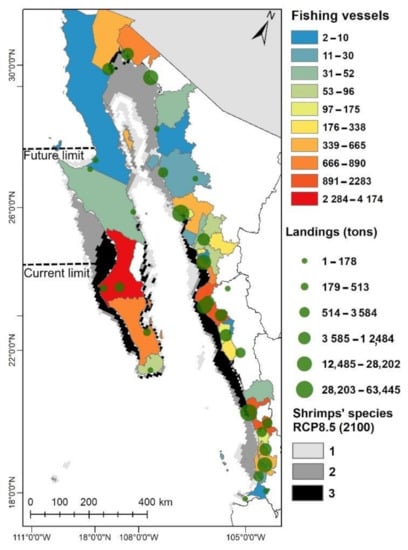
Figure 5.
Historical landings (2000–2018) of shrimp fishery and the spatial distribution of fishing vessels for the fishery in northwestern Mexico. Gray colors represent shrimp species richness in 2100 according to RCP 8.5.
The distribution maps projected to 2100 (Figure 3 and Figure 5, Figure A4 and Figure A5) showed the potential zones for the shrimps’ new distribution and clearly identified that the MAB lagoon complex, the GoU, and zones adjacent to the west coast of BCS could have the most adequate environmental conditions for the blue and white shrimp establishment; records on their distribution or capture do not exist in the zone. Nowadays, the brown shrimp dominates the shrimp fishery in MAB and the GoU, which is fundamental for the economy of the two fishing communities [66]. On the other hand, the blue shrimp’s landings reported in MAB represents the 20% of the total shrimp fishery; however, in the future this species could increase its distribution and abundance with the tropicalization of this transitional area by ocean warming due climate change [17], and also by the presence of soft bottom, lagoons, and mangroves ecosystems [65]. In the case of the white shrimp, although this species increases its habitat area in MAB and GoU, its distribution is limited to the Gulf of California wherein the connectivity is limited to inside the GC or southern areas by the current patterns. However, the white shrimp could be an important species for aquaculture in MAB [66]. Aquaculture activity for the white shrimp even could be extended across the GoU in the future (Figure 3b).
The establishment of blue (Figure 3a and Figure 4a) and white shrimp (Figure 3b and Figure 4b) will economically benefit not only the local fishers, but also fishers from Sinaloa and Sonora who move to the communities at the start of seasons [63]. If their basic needs are not covered, the fishers will have an incentive to keep exploiting the resource, which means that they could exceed the established quota limits, leading to possible consequences of less brown shrimp abundance for the next seasons or very small-size catches. The future establishment of the blue and white shrimps (from 25° N to 28° N, Figure 5) would bring variety to shrimp fishing and potentially higher income generation to the MAB and GoU communities.
On the other hand, the maps also indicate the establishment of the white shrimp in the upper Gulf of California (Figure 3b), specifically in the Colorado River Delta (CRD); this is a surprising fact because, nowadays, records of this shrimp’s presence in past years do not exist. A reason for the white shrimp to have this distribution towards the year 2100 is due to the low salinity values that will be in the zone. Because no other variable presents any change in the CRD zone, these salinity values match with the white shrimp’s preferences.
The upper Gulf of California presents a problem due to the spatial dispersion and social disparity in the riparian fishing [67,68,69] and, nowadays, an important percentage of Puerto Peñasco and San Felipe fishers consider that the reserve establishment has had a positive impact on shrimp fishery; more than the 40% pointed out that they obtained an increase in the capture [65], which may indicate that, over time, a white shrimp fishery able to compete with the blue and white shrimp fisheries can be established, since the CRD oceanographic conditions are essential for the white shrimp’s development and reproduction. These results could be used to complement efforts on spatial planning in this region, such as the model proposed by Morzaria-Luna et al. (2020) [69].
The maps projected to 2100 also showed the loss zones, which included most of the coasts of Nayarit, where the fishing activity is very important for the state. The white shrimp is the main species in the captures, representing 98.6% of the catch, while the blue shrimp represents 1.1% and the brown shrimp 0.3% (Figure 3) [6]. The white shrimp will have little area loss, which indicates that there will not be economic losses for the fishing communities of Nayarit. For the blue and brown shrimp, the area loss would not suppose fishing changes, since these tend to be caught in minimal quantities and are not usually abundant in the local market.
5. Conclusions
The models developed in this study achieved high levels of significance, so the results will be useful for future studies and for the development of adaptation/management plans for this fishery.
According to the model projections, the three shrimp species will have a displacement tendency towards the coasts of BCS, mainly the areas within the GoU and the MAB lagoon complex, zones wherein the blue and white shrimp are not currently present, which will be a great benefit for the fishing communities and also prevent the overexploitation of brown shrimp. On the other hand, blue and brown shrimp area losses in the coasts of Nayarit do not suppose an economic risk for the affected communities, since these species are present in minor quantities and are not the main extraction species.
Spatial changes found by the effect of climate change may be considered by institutions working on adaptation plans to this phenomenon, and especially can serve to strengthen management plans for this important fishery with considerable economic and social impacts in Mexico.
Author Contributions
A.C.-D.: Conceptualization, analysis, writing—original draft. D.P.-R.: Methodology, databases, writing—reviewing. M.Á.O.-R.: supervision, reviewing and editing. E.A.M.-M.: writing—reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Funding from CONACYT through Problemas Nacionales grant No. 6428.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was funded by Mexico’s CONACYT through a postgraduate scholarship to D.P.-R. (grant number 445870). A.C.-D. and M.Á.O.-R. holds funding from CONACYT (Problemas Nacionales, 6428).
Conflicts of Interest
The authors report no declarations of interest.
Appendix A

Table A1.
Pearson’s correlation between environmental layers.
Table A1.
Pearson’s correlation between environmental layers.
| Layer | NPP Winter | NPP Spring | NPP Summer | NPP Autumn | SST Winter | SST Spring | SST Summer | SST Autumn | SSS Winter | SSS Spring | SSS Summer | SSS Autumn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NPP winter | 1.00 | |||||||||||
| NPP spring | 0.76 | 1.00 | ||||||||||
| NPP summer | 0.67 | 0.94 | 1.00 | |||||||||
| NPP autumn | 0.91 | 0.78 | 0.79 | 1.00 | ||||||||
| SST winter | 0.33 | 0.03 | −0.07 | 0.17 | 1.00 | |||||||
| SST spring | 0.44 | 0.18 | 0.06 | 0.28 | 0.95 | 1.00 | ||||||
| SST summer | 0.55 | 0.39 | 0.25 | 0.38 | 0.85 | 0.95 | 1.00 | |||||
| SST autumn | 0.45 | 0.20 | 0.07 | 0.27 | 0.97 | 0.97 | 0.95 | 1.00 | ||||
| SSS winter | 0.27 | 0.33 | 0.23 | 0.17 | 0.48 | 0.61 | 0.76 | 0.65 | 1.00 | |||
| SSS spring | 0.38 | 0.35 | 0.24 | 0.25 | 0.65 | 0.78 | 0.88 | 0.79 | 0.96 | 1.00 | ||
| SSS summer | 0.27 | 0.31 | 0.21 | 0.14 | 0.55 | 0.67 | 0.79 | 0.70 | 0.97 | 0.97 | 1.00 | |
| SSS autumn | 0.20 | 0.31 | 0.22 | 0.09 | 0.39 | 0.52 | 0.68 | 0.56 | 0.97 | 0.91 | 0.97 | 1.00 |
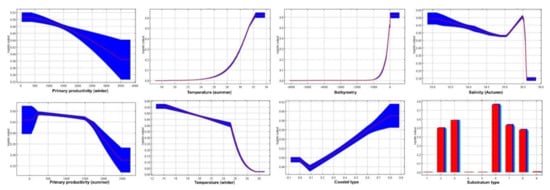
Figure A1.
Response of blue shrimp (L. stylirostris) for each environmental variable.
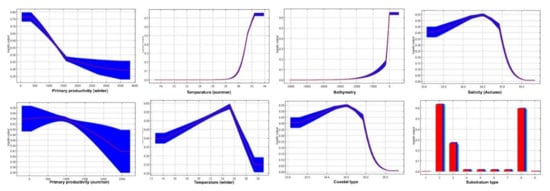
Figure A2.
Response of white shrimp (L. vannamei) for each environmental variable.
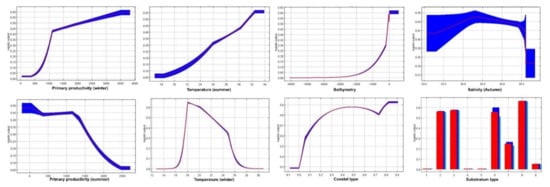
Figure A3.
Response of brown shrimp (F. californiensis) for each environmental variable.
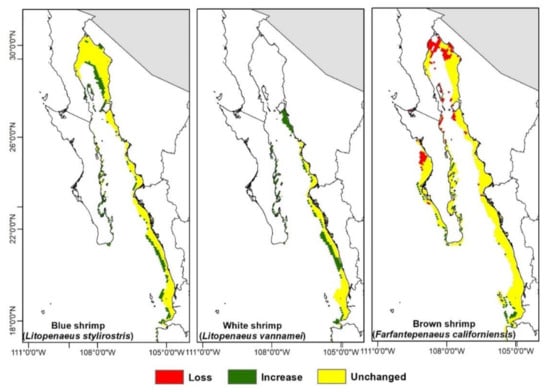
Figure A4.
Habitat suitability models for each shrimps species for the 2090–2100 period according to the RCP2.6 scenario.
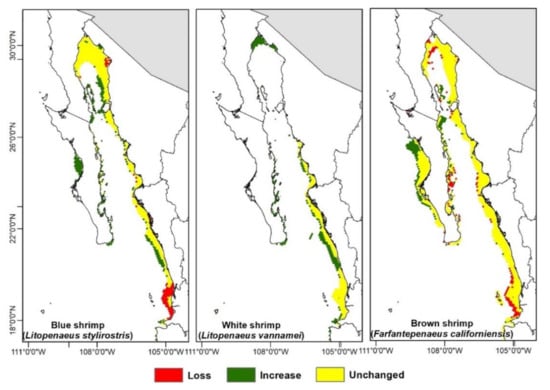
Figure A5.
Habitat suitability models for each shrimps species for the 2090–2100 period according to the RCP4.5 scenario.
References
- Instituto Nacional de Pesca. Evaluación Biológica de las Poblaciones de Camarón Durante la Veda 2014 en el Litoral del Pacífico Mexicano; INAPESCA: Mazatlán, Mexico, 2014; pp. 1–96.
- Aranceta-Garza, F.; Arreguín-Sánchez, F.; Seijo, J.C.; Ponce-Díaz, G.; Lluch-Cota, D.; del Monte-Luna, P. Determination of catchability-at-age for the Mexican Pacific shrimp fishery in the southern Gulf of California. Reg. Stud. Mar. Sci. 2020, 33, 100967. [Google Scholar] [CrossRef]
- Instituto Nacional de Pesca. La Pesquería de Camarón del Pacifico; INAPESCA: La Paz, Mexico, 2012; pp. 1–50.
- López-Martínez, J.; Hernández-Vásquez, S.; Herrera-Valdivia, E.; Rodríguez-Romero, J.; Chávez, E.A. Influencia Ambiental en la Pesquería de Camarón. In Variabilidad Ambiental y Pesquería en Mexico; Comision Nacional de Acuacultura y Pesca: Zacatepec, Morelos, Mexico, 2008; pp. 111–123. [Google Scholar]
- García-Borbón, J.A.; Balart, E.F.; Gallo-Ramírez, J.J.; Loreto-Campos, P.A. La Pesquería de Camarón. In Estudio del Potencial Pesquero y Acuícola de Baja California Sur; Casas-Valdéz, M., Ponce-Diaz, G., Eds.; INAPESCA: La Paz, Mexico, 1996; Volume 1, pp. 187–206. [Google Scholar]
- Instituto Nacional de Pesca. Evaluación y Manejo de la Pesquería de Camarón del Pacifico Mexicano; INAPESCA: La Paz, Mexico, 2016; pp. 1–42.
- López-Martínez, J.; Morales-Bojorques, E.; Paredes-Mallon, F.; Lluch-Belda, D.; Cervantes, C. La Pesquería de Camarón de Altamar en Sonora; Centros de Actividad Biológica del Pacífico Mexicano: La Paz, Mexico, 2001; pp. 301–312. [Google Scholar]
- Rodríguez de la Cruz, C.; Chávez, E. La pesquería de camarón en altamar. Pacífico de Mexico. Memorias 1994, 35, 11. [Google Scholar]
- García, S.; Le Reste, L. Ciclos Vitales, Dinámica, Explotación y Ordenación de las Poblaciones de Camarones Peneidos Costeros; FAO Doc. Téc. Pesca; Food and Agricultural Organization of United Nations: Rome, Italy, 1987; 180p. [Google Scholar]
- Mendo, J.; Tam, J. Multiple environmental states affecting penaeid shrimp production in Peru. Naga ICLARM Q. 1993, 16, 44–47. [Google Scholar]
- Sheridan, P. Forecasting the fishery for pink shrimp, Penaeus duorarum, on the Tortugas Grounds, Florida. Fish. Bull. 1996, 94, 743–755. [Google Scholar]
- Criales, M.M.; Lee, T.N. Larval distribution and transport of penaeoid shrimps during the presence of the Tortugas Gyre in May–June 1991. Oceanogr. Lit. Rev. 1996, 6, 471–482. [Google Scholar]
- García, S. Tropical Penaeid Prawns. In Fish Population Dynamics, 2nd ed.; Gulland, J.A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1988; pp. 219–249. [Google Scholar]
- Hannah, R.W. Influence of environmental variation and spawning stock levels on recruitment of ocean shrimp (Pandalus jordani). Can. J. Fish. Aquat. Sci. 1993, 50, 612–622. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Dubash, N.K. Climate Change 2014: Synthesis Report—Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef]
- Petatán-Ramírez, D.; Ojeda-Ruiz, M.Á.; Sánchez-Velasco, L.; Rivas, D.; Reyes-Bonilla, H.; Cruz-Piñón, G.; Salvadeo, C. Potential changes in the distribution of suitable habitat for Pacific sardine (Sardinops sagax) under climate change scenarios. Deep Sea Res. Part. II Top. Stud. Oceanogr. 2019, 169, 104632. [Google Scholar] [CrossRef]
- Rahel, F.J.; Olden, J.D. Assessing the effects of climate change on aquatic invasive species. Conserv. Biol. 2008, 22, 521–533. [Google Scholar] [CrossRef]
- Magaña-Rueda, V.O.; García, C.G. Vulnerabilidad y adaptación regional ante el cambio climático y sus impactos ambientales, sociales y económicos. Gaceta Ecológica 2002, 65, 7–23. [Google Scholar]
- López-Martínez, J.; Herrera-Valdivia, E.; Hernández-Saavedra, N.; Serviere-Zaragoza, E.; Rodríguez-Romero, J.; Rábago-Quiroz, C.H.; Pedrín-Aviles, S. Efectos de la Pesca de Arrastre del Camarón en el Golfo de California: Síntesis de las Investigaciones Desarrolladas por el Centro de Investigaciones Biológicas del Noroeste SC. In Efectos de la Pesca de Arrastre en el Golfo de California; López-Martínez, J., Morales-Bojórquez, E., Eds.; Cibnor: La Paz, Mexico, 2012; pp. 15–26. [Google Scholar]
- Sosa-Rodríguez, F.S. Política del cambio climático en Mexico: Avances, obstáculos y retos. Revista Internacional de Estadística y Geografía 2015, 6, 4–23. [Google Scholar]
- Morales-Bojórquez, E.; Madrid-Vera, J.; Díaz-Uribe, J.G.; Aguirre-Villaseñor, H.; Liedo-Galindo, A.; Chávez-Herrera, D.; Hernández-López, A. Distribución y Abundancia de Camarón Café (Fanfantepenaeus californiensis) en el Norte de Sinaloa, Mexico. In Efectos de la Pesca de Arrastre en el Golfo de California; López-Martínez, J., Morales-Bojórquez, E., Eds.; Cibnor: La Paz, Mexico, 2012; pp. 385–398. [Google Scholar]
- Mateo, R.G.; Felicísimo, Á.M.; Muñoz, J. Modelos de distribución de especies y su potencialidad como recurso educativo interdisciplinar. Reduca 2012, 5, 137–153. [Google Scholar]
- Cuspilici, A.; Monforte, P.; Ragusa, M.A. Study of Saharan dust influence on PM10 measures in Sicily from 2013 to 2015. Ecol. Indic. 2017, 76, 297–303. [Google Scholar] [CrossRef]
- López-Martínez, J.; Rábago-Quiroz, C.H.; Herrera-Valdivia, E.; Morales-Azpeitia, R.; Padilla-Serrato, J.G. Distribution and abundance of Sicyonia shrimp in the Gulf of California and western coast of the Baja California Peninsula, Mexico. Reg. Stud. Mar. Sci. 2020, 38, 101381. [Google Scholar] [CrossRef]
- Wilson, K.A.; Westphal, M.I.; Possingham, H.P.; Elith, J. Sensitivity of conservation planning to different approaches to using predicted species distribution data. Biol. Conserv. 2005, 122, 99–112. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Calmus, T.; Burquez, A.; Martínez-Yrizar, A. El Golfo de California: Un océano joven, región megadiversa, vínculo entre tectónica y ecología. Ciencia UANL 2017, 85, 59–64. [Google Scholar]
- Soto-Mardones, L.; Marinone, S.; Parés-Sierra, A. Variabilidad espaciotemporal de la temperatura superficial del mar en el Golfo de California. Ciencias Marinas 1999, 25, 1–30. [Google Scholar] [CrossRef]
- Álvarez-Borrego, S.; Lara-Lara, J.R. The Physical Environment and Productivity of the Gulf of California. In The Gulf and Peninsular Province of the Californias; Dauphin, J.P., Simoneit, B., Eds.; American Association of Petroleum Geologists: Tulsa, OK, USA, 1991; pp. 555–567. [Google Scholar]
- Veloz, S.D. Spatially autocorrelated sampling falsely inflates measures of accuracy for presence-only niche models. J. Biogeogr. 2009, 36, 2290–2299. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Westberry, T.; Behrenfeld, M.J.; Siegel, D.A.; Boss, E. Carbon-based primary productivity modeling with vertically resolved photoacclimation. Global Biogeochem. Cycles 2008, 22. [Google Scholar] [CrossRef]
- Chassignet, E.P.; Hurlburt, H.E.; Smedstad, O.M.; Halliwell, G.R.; Hogan, P.J.; Wallcraft, A.J.; Bleck, R. The HYCOM (hybrid coordinate ocean model) data assimilative system. J. Mar. Syst. 2007, 65, 60–83. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing. 2021. Available online: https://www.r-project.org (accessed on 6 February 2021).
- Gilani, H.; Goheer, M.A.; Ahmad, H.; Hussain, K. Under predicted climate change: Distribution and ecological niche modelling of six native tree species in Gilgit-Baltistan, Pakistan. Ecol. Indic. 2020, 111, 106049. [Google Scholar] [CrossRef]
- Heikkinen, R.K.; Luoto, M.; Araújo, M.B.; Virkkala, R.; Thuiller, W.; Sykes, M.T. Methods and uncertainties in bioclimatic envelope modelling under climate change. Prog. Phys. Geogr. 2006, 30, 751–777. [Google Scholar] [CrossRef]
- Euán-Avila, J.I.; Cuevas-Jiménez, A. Regionalizing Coastal Zones with Geospatial Tools for Integrated Coastal Zone Management. In Coastal and Marine Geospatial Technologies; Green, D.R., Ed.; Springer Press: Dordrecht, The Netherlands, 2010; pp. 139–151. [Google Scholar] [CrossRef]
- Petatán-Ramírez, D.; Hernández, L.; Becerril-García, E.E.; Berúmen-Solórzano, P.; Auliz-Ortiz, D.; Reyes-Bonilla, H. Potential distribution of the tiger shrimp Penaeus monodon (Decapoda: Penaeidae), an invasive species in the Atlantic Ocean. Rev. Biol. Trop. 2020, 68, 156–166. [Google Scholar] [CrossRef]
- Instituto Nacional de Pesca. Atlas Pesquero de Mexico; INAPESCA: Ciudad de Mexico, Mexico, 1994.
- Intergovernmental Panel on Climate Change (IPCC). Resumen para Responsables de Políticas. In Cambio Climático 2013: Bases Físicas; Contribución del Cambio Climático; IPCC: Geneva, Switzerland, 2013. [Google Scholar]
- Gudmundsson, L.; Bremnes, J.B.; Haugen, J.E.; Engen-Skaugen, T. Downscaling RCM precipitation to the station scale using statistical transformations—A comparison of methods. Hydrol. Earth Syst. Sci. 2012, 16, 3383–3390. [Google Scholar] [CrossRef]
- Melo-Merino, S.M.; Reyes-Bonilla, H.; Lira-Noriega, A. Ecological niche models and species distribution models in marine environments: A literature review and spatial analysis of evidence. Ecol. Model. 2020, 415, 108837. [Google Scholar] [CrossRef]
- Mann, K.H.; Lazier, J.R. Dynamics of Marine Ecosystems: Biological-Physical Interactions in the Oceans; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Peterson, A.T. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Basher, Z.; Bowden, D.A.; Costello, M.J. Diversity and distribution of deep-sea shrimps in the Ross Sea region of Antarctica. PLoS ONE 2014, 9, e103195. [Google Scholar] [CrossRef]
- Peterson, A.; Papeş, M.; Eaton, M. Transferability and model evaluation in ecological niche modeling: A comparison of GARP and Maxent. Ecography 2007, 30, 550–560. [Google Scholar] [CrossRef]
- Elith, J. Quantitative Methods for Modeling Species Habitat: Comparative Performance and an Application to Australian plants. In Quantitative Methods for Conservation Biology; Ferson, S., Burgman, M., Eds.; Springer: New York, NY, USA, 2000; pp. 39–58. [Google Scholar] [CrossRef]
- García-Juárez, A.R.; Rodríguez-Domínguez, G.; Lluch-Cota, D.B. La cuota de captura de camarón azul (Litopenaeus stylirostris) como instrumento de gestión en el Alto Golfo de California. Ciencias Marinas 2009, 35, 297–306. [Google Scholar] [CrossRef]
- Gell, F.R.; Roberts, C.M. Benefits beyond boundaries: The fishery effects of marine reserves. Trends Ecol. Evol. 2003, 18, 448–455. [Google Scholar] [CrossRef]
- Hilborn, R.; Stokes, K.; Maguire, J.J.; Smith, T.; Botsford, L.W.; Mangel, M.; Cochrane, K.L. When can marine reserves improve fisheries management? Ocean. Coast. Manag. 2004, 47, 197–205. [Google Scholar] [CrossRef]
- Balmori-Ramírez, A.; Cervantes-Higuera, H. La Fauna Acompañante de la Pesca Ribereña de Camarón en Sonora 2019. Available online: https://fisheryprogress.org/sites/default/files/documents_actions/Gillnet%20bycatch%20evaluation%202018-19.pdf (accessed on 6 February 2021).
- Berlanga-Robles, C.; Ruiz-Luna, A. Los Sistemas Acuáticos Costeros de Sinaloa. In Atlas de los Ecosistemas de Sinaloa; Cifuentes-Lemus, J.L., Gaxiola-López, J., Eds.; El Colegio de Sinaloa: Culiacán, Mexico, 2003; pp. 197–214. [Google Scholar]
- Basher, Z.; Costello, M.J. The past, present and future distribution of a deep-sea shrimp in the Southern Ocean. PeerJ 2016, 4, e1713. [Google Scholar] [CrossRef]
- Dambach, J.; Thatje, S.; Roedder, D.; Basher, Z.; Raupach, M.J. Effects of Late-Cenozoic glaciation on habitat availability in Antarctic benthic shrimps (Crustacea: Decapoda: Caridea). PLoS ONE 2012, 7, e46283. [Google Scholar] [CrossRef][Green Version]
- Vargas-Albores, F.; Hinojosa-Baltazar, P.; Portillo-Clark, G.; Magallon-Barajas, F. Influence of temperature and salinity on the yellowleg shrimp, Penaeus californieinsis Holmes, 1900, prophenoloxidase system. Aquac. Res. 1998, 29, 549–553. [Google Scholar] [CrossRef]
- Lluch, B.D. La Pesquería del Camarón del Pacífico (Diagnosis Monográfica de los Conocimientos Existentes); Centro de Investigaciones Bilologicas del Noroeste; S.C. Centro Interdisciplinario de Ciencias Marinas: La Paz, Mexico; Consejo Nacional deCiencia y Tecnologia: Cocoyoc, Mexico, 1982. [Google Scholar]
- Hernández-Covarrubias, V.; Muñoz-Rubí, H.A.; Madrid-Vera, J.; Chávez-Herrera, D. Fecundidad del camarón blanco Litopenaeus vannamei de la plataforma continental de Sinaloa, Mexico. Ciencia Pesquera 2012, 20, 17–21. [Google Scholar]
- Ponce-Palafox, J.; Martinez-Palacios, C.A.; Ross, L.G. The effects of salinity and temperature on the growth and survival rates of juvenile white shrimp, Penaeus vannamei, Boone, 1931. Aquaculture 1997, 157, 107–115. [Google Scholar] [CrossRef]
- Magallón, B.F.; Portillo, G.; Campos, R.; Porchas, M.A.; Naranjo, J.; Muhlia, A. Penaeus californiensis as a cold tolerant species in Baja California Sur, Mexico. In Book of Abstracts: World Aquaculture ‘94 New Orleans LA, USA, 14–18 January 1994; World Aquaculture Society: Sorrento, LA, USA, 1994; p. 84. [Google Scholar]
- Villarreal, H.; Hernandez-Llamas, A.; Hewitt, R. Effect of salinity on growth, survival and oxygen consumption of juvenile brown shrimp, Farfantepenaeus californiensis (Holmes 1900). Aquac. Res. 2003, 34, 187–193. [Google Scholar] [CrossRef]
- Romero-Sedano, J.C.; Aragón-Noriega, E.A.; Manzano-Sarabia, M.M.; Salinas-Zavala, C.A.; García-Juárez, A.R. Periodo reproductivo del camarón café Farfantepenaeus californiensis (Holmes, 1900) en la laguna costera de Agiabampo, Sonora/Sinaloa, Mexico. Ciencias Marinas 2004, 30, 465–475. [Google Scholar] [CrossRef]
- Aragón-Noriega, E.A.; Alcántara-Razo, E. Influence of sea surface temperature on reproductive period and size at maturity of brown shrimp (Farfantepenaeus californiensis) in the Gulf of California. Mar. Biol. 2005, 146, 373–379. [Google Scholar] [CrossRef]
- Aragón-Noriega, E.A. Coupling the reproductive period of blue shrimp Litopenaeus stylirostris Stimpson, 1874 (Decapoda: Penaeidae) and sea surface temperature in the Gulf of California. Rev. Biol. Mar. Oceanogr. 2007, 42, 167–175. [Google Scholar] [CrossRef]
- Aragón-Noriega, E.; Pérez-Arvizu, E.; Valenzuela-Quiñonez, W. Latitudinal variation in reproduction timing of whiteleg shrimp Litopenaeus vannamei (Decapoda, Penaeidae) of the Mexican Pacific coast. Crustaceana 2012, 85, 287–300. [Google Scholar] [CrossRef]
- García-Martínez, S.; Chávez-Ortiz, E.A. La Pesquería de Camarón en Puerto San Carlos, Bahía Magdalena: Una Perspectiva Socioeconómica. In Estudios Ecológicos en Bahía Magdalena; Funes-Rodríguez, R., Gómez-Gutiérrez, J., Palomares-García, R.S., Eds.; Centro de Investigaciones Biológicas del Noroeste: La Paz, Mexico, 2007; pp. 277–287. [Google Scholar]
- Doode, S.; Wong, P. El Golfo de California: Surgimiento de nuevos actores sociales, ambientalismo y región. Estudios Sociales 2001, 11, 25–56. [Google Scholar]
- Rodriguez Quiroz, G.; Bracamontes-Sierra, A. Pertinencia de las ANP como política de conservación y mejoramiento de la calidad de vida: Análisis de percepción en la Reserva de la Biosfera del Alto Golfo de California y Delta del Río Colorado. Estudios Sociales 2008, 16, 141–176. [Google Scholar]
- Morzaria-Luna HNTurk-Boyer, P.; Hernández, J.M.D.; Polanco-Mizquez, E.; Downton-Hoffmann, C.; Cruz-Piñón, G.; Munguia-Vega, A. Fisheries management tools to support coastal and marine spatial planning: A case study from the Northern Gulf of California, Mexico. MethodsX 2020, 7, 101108. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).