Featured Application
The recycling of the lavender solid by-products remaining after the extraction of essential oil could represent an important goal in this industrial field. The innovative and eco-compatible pulsed ultrasound-assisted extraction (PUAE) demonstrated to be a promising strategy to valorize this waste by obtaining, using only food-grade extraction solvents (a mixture of ethanol and water), a new potential product rich in polyphenolic compounds to use in different fields (agronomic, food, cosmetic, etc.).
Abstract
FINNOVER is an EU Interreg-Alcotra project that aims to bring new perspectives to floriculture enterprises by recovering useful bioproducts from the waste produced during processing of several aromatic species. In this study, a new operation strategy to recover lavender (Lavandula angustifolia Mill.) solid by-products remaining after the extraction of the essential oil was developed. Pulsed ultrasound-assisted extraction was employed as a sustainable and eco-compatible technology to extract, in a very short time (10 min), this agricultural waste using a food-grade solvent (a mixture of ethanol/water). All the extracts obtained from both flower and leaf waste and flower-only residues, exhibit a promising total phenolic content (38–40 mg gallic acid/g of dry waste), radical scavenging activity (107–110 mg Trolox/g of dry waste) and total flavonoid content (0.11–0.13 mg quercetin/g of dry waste). Moreover, the chromatographic analysis of these extracts has shown that this overlooked agriculture waste can represent a valuable source of multifunctional compounds. Particularly, they exhibit a content of polyphenols and flavonoids up to 200 times higher than the corresponding leachate, and they are a valuable source of gentisic acid (1.4–13 mg/g dry waste) representing a new low-cost ingredient usable in different fields (i.e., cosmetic).
1. Introduction
In this research, the solid by-products remaining after the distillation of lavender, which are still little studied in scientific literature, are taken into account as a potential and still underutilized source of bioactive compounds to be valorized. These solid wastes, sometimes called “distilled straws of lavender” are lignocellulosic-rich materials, which have been proposed as an interesting source to be converted into biofuel [1]. However, this biomass is still rich in bioactive compounds, mainly antioxidants, whose extraction could represent a useful first step towards its utilization as an economic resource still to be exploited. This recycling possibility has been evaluated inside a European research project called Finnover.
Finnover (for “Innovative strategies for the development of cross border green supply chains”), is a cross-border Italy/France EU Interreg Alcotra project started in 2017 and finished in 2021 [2]. The aims of this research project were the innovation and the sustainable implementation of several agro-industrial processing chains in view of the green circular economy and the valorization of the biodiversity of the Alcotra territory. In particular, the management of waste deriving from agricultural and food processing is one of the main topics of the project, offering new perspectives to floriculture enterprises by recovering useful bioproducts from the waste produced during the distillation of essential oils from lavender and other aromatic species.
The Lavendula genus, which belongs to the Lamiaceae family and includes numerous varieties and hybrids, is one of the best-known essential oil crops in the world [3]. Lavandula angustifolia Miller (syn. L. vera or L. officinalis) is an evergreen and perennial flowering shrub and it represents, among aromatic plants, the most common cultivated species in the Mediterranean coasts [4].
Lavender has been widely used as herbal medicine for centuries in the traditional medicine. Particularly, lavender essential oil (LEO) is commonly used in pharmaceutical products, phytomedicine preparations, as well as in foods and cosmetics [5]. One of the main uses of LEO is in inhalation aromatherapy, due to its numerous sedative [6], anti-inflammatory [7], antidepressant [8], antiseptic [9], analgesic [10] and antispasmodic [11] healing properties. In the food industry, LEO is mainly employed as a natural antibacterial to preserve food products and extend their shelf life, and as a flavoring agent in several foodstuffs (i.e., beverages, sweets, candies and chewing gums) [12,13].
As concerns the extraction process, steam distillation has traditionally been used to recover the essential oil from lavender flowers. This extraction procedure is industrially the most frequently used method because its simplicity, low cost, and safety (no environmental impact) [14]. LEO is produced from raw plant materials (flowers, buds, stems and leaves) by steam distillation, but yields are low with only 2–10% volume/dry weight [1] with a large amount of by-products: liquid and solid secondary products.
The liquid secondary products of the distillation process of essential oils are the distillation wastewaters namely hydrolats (or hydrosols or aromatic waters) and leachates. During steam distillation, volatile plant constituents are vaporized and then condensed on cooling to produce an immiscible mixture of an oil phase and an aqueous phase, named hydrolat. The hydrolat contains both traces of the essential oil (generally less than 1 g/L) and several water-soluble components with low molecular weight [15]. Leachates are aqueous mixtures too, containing water-soluble compounds with high molecular weight, which are extracted from the plant matrix by heating but do not undergo the distillation process (Figure 1).
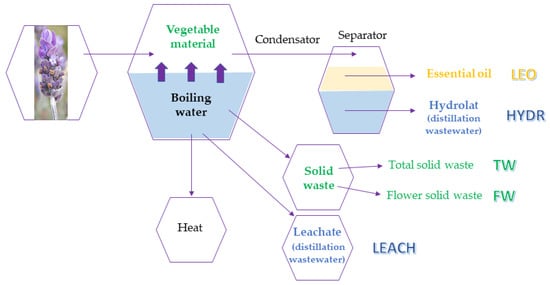
Figure 1.
Lavender steam distillation scheme.
Furthermore, after obtaining the essential oil (LEO) there remains a solid waste represented by the residues of the flowers and leaves, which does not yet have any real industrial value. Sometimes this distilled biomass, called “distilled straws”, is partly recovered to be used as animal fodder, as a bedding substrate for domestic animals, manure after composting or as a base for the growth of mushrooms [1].
Nowadays, the eco-friendly extraction of bio-active compounds from agro-industrial wastes is of great interest to answer the serious economic and environmental problem of significant waste amounts generated by the processing industries [16]. Ultrasound is an innovative eco-compatible food processing technique endowed with an important role in the promotion of the sustainable food industry [17].
This technique has recently emerged among green technologies due to their advantages shown in several processing steps such as degassing, filtration, preservation (i.e., inactivation of microorganism and enzymes), and extraction of natural products [18]. According to the six principles of green extraction [19], ultrasound-assisted extraction (UAE) allows one to extract, in very short time, with high reproducibility, and with increased extraction efficiency (extraction yield) compared to the conventional extraction methods, several classes of bioactive compounds from different plants and foodstuffs [20,21,22]. In pulsed ultrasound-assisted extraction (PUAE), ultrasounds are applied in pulsed mode, i.e., the ultrasound processor is switched on and off intermittently during the entire extraction process. This extraction modality, compared to the continuous sonication, leads to less heat generation allowing a better control of the temperature rise. For this reason, it is the most suitable method for the extraction of thermolabile biomolecules (e.g., polyphenols) [23]. Moreover, as recently reported by Saratale et al. [24], ultrasounds are a very useful method for the treatment of biomass.
In this research, a strategy to recycle and valorize the solid waste remaining after LEO distillation, both the total waste (TW, distilled floral spikes) and the only-flower waste (FW, flower material detached from the distilled spikes), has been proposed. Particularly, PUAE was employed for the first time, according to our knowledge, to extract polyphenols and flavonoids from this natural matrix. The obtained extracts, using a food-grade solvent (a mixture of ethanol and water) named respectively total waste extract (TWE) and flower waste extract (FWE), were spectrophotometrically and chromatographically analyzed and compared to the corresponding liquid aqueous by-products (hydrolat and leachate) of the distillation process.
The goal of this study has been the development and the characterization of a new natural extract starting from an agro-industrial waste according to eco-sustainable and circular economy criteria. Figure 2 shows the global scheme of the present work.
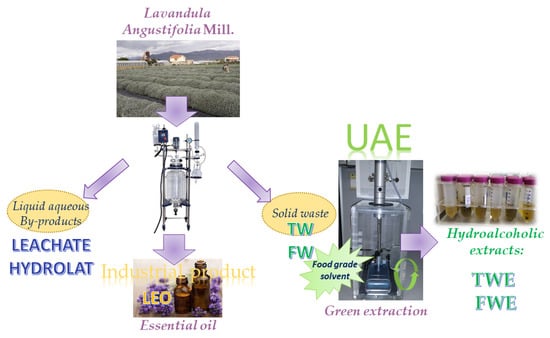
Figure 2.
The global scheme of this research.
2. Materials and Methods
2.1. Raw Materials
Lavender of Lavandula angustifolia cultivar Rosa was collected in August 2017 from plants growing in the outdoor facilities of Franco Stalla at Albenga, Savona Province (Italy) and was immediately authenticated by an agronomist at Istituto Regionale per la Floricoltura (IRF). The conventional steam distillation technique was immediately applied for the extraction of the LEO. The total solid waste (TW) composed from flower and leaf residues remaining after the distillation of essential oil, was supplied to the food chemistry laboratory of the University of Genoa Department of Pharmacy for their further extraction. Moreover, the only-flower residues (FW) were manually separated from TW to make a comparison. The distillation wastewaters namely hydrolat (HYDR) and leachate (LEACH), which represent the liquid aqueous by-products of the LEO distillation, were also provided for further investigation as reported in Figure 2. Leachate concentration was calculated considering an average production of 1400 L of leachate from 35,000 kg of fresh plant material and a 77% moisture content for lavender spikes, resulting in a dry weight/volume ratio of 5.75 g/mL.
2.2. Chemicals
For the extraction step, ethanol 96% of analytical grade was used and it was purchased from VWR Chemical (Radnor, PA, USA), while high purity water (18 MΩ) was produced by a Milli-Q system (Millipore, Bedford, MA, USA). HPLC gradient grade acetonitrile and water were supplied by VWR. Folin−Ciocalteu reagent, sodium carbonate (Na2CO3), gallic acid, DPPH (1,1-diphenyl-2-picrylhydrazyl), Trolox (6-hydroxy-2,5,7,8-tetramethylchromane-2 carboxylic acid), ascorbic acid, aluminium chloride (AlCl3), quercetin, and sodium acetate (CH3COONa) were supplied by Sigma Aldrich (Steinheim, Germany).
2.3. Equipment
A traditional Binder FED53 heating oven (Goettingen, Germany) and a Retsch Grindomix 200 M (Haan, Germany) were used for preliminary drying and grinding of the samples, respectively. A MA40 infrared moisture analyzer (Sartorius, Goettingen, Germany) was used to detect the residual moisture content of the solid wastes before their drying. UAE was performed directly by a Hielscher UP200St sonicator (Teltow, Germany). An UV-Vis Agilent 8453 spectrophotometer (Waldbronn, Germany) was used for the determinations of total phenolic content (TPC) and radical scavenging activity (RSA). Total flavonoids were determined using a T60 spectrophotometer (PG Instruments Ltd., Leichestershire, UK). HPLC analyses were performed using a Perkin-Elmer (Waltham, MA, USA) Series 200 LC pump coupled with a 785A UV/VIS detector.
2.4. Ultrasonic-Assisted Extraction of the Solid By-Products
The solid wastes (TW and FW) were dried at 40 °C for 48 h until the residual water amounts, evaluated by a moister analyzer, were lower than 11%. After drying, wastes were finely ground by a Grindomix (company, city, state abbrev if USA, country; process conditions: 20 s at 5000 rpm) and then sieved through a 150 µm sieve. 1 g of ground solid waste (TW and FW, respectively) were immersed in 40 mL of ethanol:water mixture (70:30) in a polypropylene 50 mL centrifuge tube with 30 mm of internal diameter. Then, this mixture was submitted to ultrasounds applied in pulsed modality, using an ultrasonic titanium probe (7 mm diameter), at a 26 kHz frequency and an effective power of 200 W, and keeping temperature under control during all the extraction time (<60 °C). Process conditions of UAE were set up based on published results and several preliminary trials were made to adapt them [25,26]. The experimental details are reported in Table 1.

Table 1.
UAE experimental condition details.
The obtained extracts, named TWE and FWE, were centrifuged at 3500 rpm for 10 min (RCF or G-force equal to 2016) and then they were filtered through a Büchner funnel, using Whatman no. 1 paper. The final extracts were stored frozen at −20 °C until their analysis.
2.5. Analytical Procedures
2.5.1. Determination of the Radical Scavenging Activity (RSA)
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity was determined according to the in vitro assay described by Brand-Williams et al. [27]. 0.250 mL of the extracts were transferred into a 10 mL volumetric flask and were mixed with a daily prepared DPPH solution (10−4 M in methanol). After 30 min incubation in the dark and at room temperature, the residual absorbance was read at 515 nm against a blank (solution without radical). The initial DPPH concentration was measured by control samples (without extracts) obtained by diluting 0.250 mL of methanol with the DPPH solution in a 10 mL volumetric flask. The absorbance measurements were transformed in antioxidant activity using Trolox and ascorbic acid as reference standards. A multi-point calibration curves in Trolox and ascorbic acid (R2 = 0.9976 and R2 = 0.9975, respectively) were obtained. The RSA was expressed as Trolox equivalent antioxidant capacity (TEAC, mg TE/gof dry waste) and ascorbic acid equivalent antioxidant capacity (AEAC, mg AAE/g of dry waste), respectively. Two replicated determinations were performed for each extract and the averaged results were reported.
2.5.2. Determination of Total Phenolic Content (TPC)
The total phenolic content (TPC) of the extracts was determined by the Folin-Ciocalteu method previously reported by Singleton et al. [28]. Briefly, 200 µL of suitably diluted extracts or standard, 1 mL of Folin–Ciocalteu reagent (previously diluted 1:10 with deionized water) and 800 µL of aqueous sodium carbonate 7.5% w/v were mixed in a test tube and vortexed. The mixture was equilibrated in the dark at room temperature for 30 min and then the absorbance was read spectrophotometrically at 760 nm using quartz cuvettes with 1 cm of path length. TPC was calculated using gallic acid as reference standard for the calibration curve (R2 = 0.9887) and results were expressed as milligrams of gallic acid in one gram of dry waste (mg GA/gdry waste). Determinations were replicated for each sample and the averaged results were reported.
2.5.3. Determination of Total Flavonoid Content (TFC)
The total flavonoid content (TFC) was estimated following the aluminium chloride colorimetric method [29]. Aliquots of 0.5 mL of lavender extracts or diluted standards (quercetin) were mixed with 0.1 mL of AlCl3, 0.1 mL of CH3COONa and 2.8 mL of deionized water. Absorbance of the samples was measured at 415 nm wavelength after 40 min of incubation in the dark at room temperature. Diluted samples of commercial quercetin in a range between 5 and 150 µg/mL were used as standards for the calibration curve (R2 = 0.9987). Flavonoid concentrations were expressed as mg/g of quercetin equivalents (QE).
2.5.4. HPLC Analysis
Reversed phase (RP) chromatography of the studied extracts was performed by using a Kinetex EVO C18 column 25 cm × 4.6 mm, 4.7 µm particle size (Phenomenex Inc., Torrance, CA, USA). The solvent gradient method used 0.1% H3PO4 in water (A) and acetonitrile (B). Gradient (% in A): 0–30 min from 10% to 58% B, 1 min from 58% to 90% B, 5 min isocratic 90% B, 1 min from 90% to 10% B, and 25 min isocratic 10% B [30]. Injection volume was 50 µL; solvent flow rate was set at 1 mL/min. Detection was performed at 280 and 350 nm wavelength to discriminate between phenols and flavonoids. Peak identification and quantification, when possible, was made using external standards. When identification was no possible, peak concentration was calculated as gallic acid equivalents (mg/g) or as quercetin equivalents (mg/g) for unknown phenols and flavonoids, respectively.
2.6. Statistical Analysis
Data were analysed using the R environment [31]. Differences in RSA, TPC, and TFC among the studied extracts (TWE, FWE, LEACH) were analysed via analysis of variance using the aov function. Multiple comparisons between extracts were carried out using Tukey’s Honest Significance tests with the TukeyHSD function. The same approach was used to analyse differences among lavender extracts in the relative composition of single phenolic compounds and flavonoids as observed through HPLC. Results throughout the text were expressed as means ±95% confidence intervals.
3. Results and Discussion
3.1. Overall Differences between Solid Waste Hydroalcoholic Extracts (TWE and FEW) Obtained by PUAE and Distillation Wastewaters (LEACH)
As reported in Table 2, the analyzed extracts significantly differed in their antioxidant activities (ANOVA F2,3 = 365.4, p < 0.001 for TEAC; F2,3 = 374.9, p < 0.001 for AEAC), and in their phenolic and flavonoid contents (ANOVA F2,3 = 1100, p < 0.001, and F2,3 = 177.1, p < 0.001, respectively). Solid wastes (TWE and FWE) showed very similar RSA (expressed as TEAC and AEAC), TPC (expressed as GAE) and TFC values (expressed as QE). In turn, when compared with solid wastes, distillation wastewaters showed a significantly lower antioxidant activity (more than 30 times lower, both for TEAC and AEAC) and phenolic and flavonoid concentration (ca. 52 times and 300 times, respectively).

Table 2.
Radical scavenging activity (RSA), total polyphenol content (TPC) and total flavonoid content (TFC) of the solid waste extracts obtained by PUAE: the total waste extract (TWE) and the only-flower waste extract (FWE). The chemical characterization of the corresponding distillation wastewaters (Leachate: LEACH) was reported too.
Hydrolat (HYDR), generated during the extraction process of essential oils from aromatic plants, is made up of condensation water containing water-soluble, oxygenated, and volatile components in which very small amounts of essential oils remain dispersed [17]. Śmigielski et al. [32] describing the composition of the hydrolat of L. angustifolia reported the following major chemical components: linalool (26.5%), borneol (9.0%), cis-linalool oxide (6.6%), and trans-linalool oxide (5.2%). These hydrosols showed antibacterial in vitro activity [33]. However, no study has been published on the antioxidant activity of lavender hydrolats. As reported in Table 2, HYDR was very poor in phenolic compounds (0.70 + 0.00 mg of gallic acid in 100 mL) while both the antiradical activity (RSA) and the flavonoid content (TFC) were not detectable.
Recently, the application of UAE to different plant matrices has produced very interesting results. Particularly, this extraction method was proposed as an innovative alternative to conventional processes to extract antioxidants, such as phenolic compounds, from several natural matrices [22,23,24,25,26]. The main advantages of UAE are the mass transfer intensification, the vegetal cell disruption, and a higher penetration of the extraction solvent to the plant tissues. Indeed, the high-frequency ultrasonic waves through the cavitation process break the vegetal cells releasing the cell contents into the extraction medium [20]. Several articles have been published on the application of UAE to extract phenolic compounds from plants such as myrtle (Myrtus communis L.) [34], pomegranate (Punica granatum L.) by-products [22,25,26], maqui (Aristotelia chilensis) [35], black chokeberry (Aronia melanocarpa L.) [36], sloes (Prunus spinosa L.) [37] and buds (i.e., Ribes nigrum, Castanea sativa) [23,24]. All of these studies described ultrasound as an efficient and a more suitable method for the extraction of these degradable bioactive compounds, also in comparison to other green extractive technologies (e.g., microwave).
Moreover, several comparative studies on the extraction of phenolic compounds from the flowers and buds of different species were carried out using ultrasounds, traditional maceration, and extraction assisted by heat (i.e., decoction). These studies confirmed that UAE is a promising rapid and efficient green technology, which allows to significantly improve the extraction of phenolic compounds compared to other conventional methods [38,39].
In 2006, Proestos and Komaitis [40] demonstrated the superiority of UAE in extracting phenolics from aromatic plants (such as Rosmarinus officinalis, Vitex agnus–castus, Origanum dictamnus, O. majorana, Salvia officinalis and Teucrium polium) over conventional extraction techniques. The content of phenolic compounds extracted in an ultrasonic bath at the optimized conditions of 1 h at 60 °C using methanol as extraction solvent, are between 11 and 23 mg of gallic acid per g of dry sample depending on the species. In this work, using an eco-compatible food-grade solvent (a mixture of EtOH and water) and a direct UAE through a probe, which is much more powerful because the ultrasonic intensity is delivered on a small surface (only the tip of the probe) compared to the ultrasonic bath [41], about double extractive yields were obtained (38–40 mg GA/g of dry waste). As regards RSA, Chrysargyris et al. [42] reported the antioxidant activity of several medicinal and aromatic plants (i.e., Artemisia abrotanum L., Pelargonium roseum L., Laurus nobilis L., Rosmarinus officinalis L., Mentha spicata L., Lavandula angustifolia L., Aloysia triphylla L.) in the range between 6.1–47.7 mg of trolox/g of fresh weight. Particularly, the methanolic extract of L. angustifolia (stem and leaves) showed RSA equal to 16.06 mg of Trolox/g of fresh weight. In this work, both the extracts obtained from lavender waste (TWE and FW) showed a higher antioxidant activity (107–110 mg Trolox/g dry waste).
In the present study, we observed relatively low contents of flavonoid compounds per g dry weight as compared to TPC (Table 2). TFC values were within the concentration range (between µg/g and mg/g) of those observed by previous authors in distilled straws [1,43]. Besides the expected variability in flavonoid concentrations between lavender cultivars from different geographic locations, differences in flavonoid quantification may also result from variability in distillation procedures (water and/or steam distillation, distillation time, temperature, etc.) affecting the quality of the remaining plant material. In addition, the use of a AlCl3 chelation method for the quantification of total flavonoids (this study) may result in underestimation of flavonoid concentrations because glycoside sugar moieties can prevent chelation with AlCl3 and the associated bathochromic shifts, rendering undetectable these molecules [44]. Since glycoside flavonoids are frequent in distilled straws [45], flavonoid quantification using colorimetric (through AlCl3 chelation) or chromatographic (quantification of HPLC peaks) may result in significant discrepancies. In the present study, reported TFC values agreed with flavonoid concentrations estimated from the corresponding HPLC peak areas (data not shown).
3.2. Differences between Solid Waste Hydroalcoholic Extracts (TWE and FEW) and Distillation Wastewaters (LEACH) in the Relative Composition of Single Phenolic and Flavonoid Compounds
The analysis of the HPLC profiles for phenolic and flavonoid compounds revealed clear differences in the number and relative abundance of single compounds depending on the type of extract considered (Figure 3A–C). Overall number of chromatographed compounds ranged between 29 and 30 (Table 3).
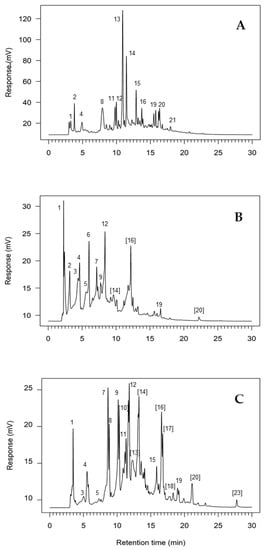
Figure 3.
Chromatographic profiles of distillation wastewaters and hydroalcoholic extracts of distilled straws obtained after essential oil distillation from flowers of Lavandula angustifolia cultivar Rosa. (A): distillation wastewater (LEACH); (B): PUAE of dry distillation straws (TWE) in 70:30 ethanol:water; (C): PUAE of dry petals from distillation straws (FWE) in 70:30 ethanol:water. Chromatograms were obtained at 280 nm wavelength after RP-HPLC. Note that retention times may vary for the same compounds depending on the specific laboratory conditions during the analysis. Numbers within square brackets indicate unidentified compounds. Peak numbers correspond to those reported in Table 3. The studied compounds differed in molecular weight and structure and not all of them showed maximum absorbance at 280 nm wavelength; therefore, observed peak areas and/or peak heights do not necessarily match calculated concentrations.

Table 3.
Observed differences in the relative composition of 32 phenolic compounds present in the distillation wastewaters (LEACH) and the corresponding distilled straws of inflorescences (TWE and FWE) from Lavandula angustifolia var. Rosa. In Table S1 the same data quantified as μg/g DW are reported.
At the qualitative level, distillation wastewaters were characterized by the absence of p-coumaric acid and two non-polar unidentified flavonoids, likely aglycones. In turn, TWE samples were apparently lacking syringic acid, rutin and an unidentified polar flavonoid. These were the three most abundant compounds in LEACH samples, suggesting that steam distillation was a relatively efficient extraction method for some abundant compounds in the selected lavender cultivar. Syringic acid, rutin and the abovementioned flavonoid were present instead in the FEW samples, likely due to a concentration effect resulting from discarding the stem and the few leaves usually present in floral spikes used for essential oil distillation. The presence of flavonoid glycosides is very variable within the subgenus Lavandula, resulting in flavonoid profiles that may differ between groups of species [46]. There is little information of flavonoid variation at the cultivar level, although our results suggest that PUAE of distillation by-products may also contribute to lavender characterization at this level. The most abundant compound in TWE samples was the phenol gentisic acid (2,5-dihydroxybenzoic acid), accounting for more than 40% of the polyphenolic fraction obtained by PUAE (Table 3).
This is indicative of the fact that polar phenolic compounds were more abundant in TWEs than in FWEs, likely due to the relatively higher concentrations of phenolic compounds (about four times higher in the case of gentisic acid) in leaves and/or stem tissues. Other phenolic compounds, such as caffeic, chlorogenic and p-coumaric acids were also present in the studied extracts, although their relative abundance significantly depended on the type of extract (Table 3). In general, as in the case of gentisic acid, the proportion of these compounds was higher in TWE than in the other two extracts. Regarding the identity of the chromatographed phenolics, the occurrence of caffeic and p-coumaric acids was already reported in lavender distilled straws [1] as typical phenolic constituents of this kind of distillation by-product. Besides simple phenols, Torras-Claveria et al. [45] showed that lavender distilled straws are characterized by the presence of numerous glycosylated forms, which renders unlikely their identification using common external standards, as we tried to do in this study. In addition, distilled straws are a source of new polyphenolic compounds [47], which likely differ in composition and abundance between lavender species and cultivars.
The overall amount of flavonoids was much higher in PUAE samples than in distillation wastewaters (Table 2), and the relative proportion of these compounds significantly varied between extracts (Table 3). Distillation wastewaters (LEACH) and PUAE samples (TWE and FWE) showed clearly different chromatographic profiles (Figure 3A–C), mostly due to differences in the relative amounts of flavonoids. As expected, hydroalcoholic extracts resulted in more concentrated flavonoid samples, likely due to the affinity of many of these compounds for solvents less polar than water [48]. Further processing of the distillation straws by collecting and extracting only flower tissues (FWE) resulted in a different chromatographic profile showing an even proportion of some of the compounds, mostly flavonoids, obtained in the TWE (Figure 3B,C). Among the identified flavonoids, the aglycones luteolin and apigenin were already reported in flower distilled straws from L. angustifolia [1]. The presence of these two compounds agreed with the observed accumulation of flavone 7-O-glycosides derived from luteolin and apigenin, as a typical biochemical trait of L. angustifolia [49]. The ability of PUAE to extract a wide range of phenolic and flavonoid compounds that were just partially released from the plant material during the essential oil distillation process confirmed that distilled straws constitute a rich source of secondary metabolites. These compounds can be used, e.g., as flavour or as antioxidants [50], or even as a potential synergist of insecticides [51].
Since phenolic compounds and flavonoids show different biological activities depending on their structure [52], observed differences in the relative abundances of these compounds in the studied extracts likely resulted in overall differences in extract properties. By comparing the proportional abundance of the most abundant compound in each type of extract, it was possible to clearly characterize these hypothesized differences. As mentioned before, TWE extracts were distinguished by the abundance of gentisic acid (43.84% of the total concentration), while the most abundant compounds in LEACH and FWE samples were rutin (21.33%) and syringic acid (11.09%), respectively. Maximum percentage abundances are equivalent to the Berger-Parker index of dominance [53]. Therefore, an increase of the reciprocal form of this index, in this case 2.28 for TWE, 4.69 for LEACH and 9.02 for FWE indicated an increase in chemical diversity associated with a reduction in compound dominance [54]. These results reinforced the view that the two extracts from distilled straws (TW and FW) were significantly different between them (Figure 3B,C), pointing to the potential importance of post-processing of distillation by-products.
3.3. Future Research Directions
Lavandula angustifolia is mainly grown for the purpose of obtaining essential oils by hydrodistillation and the oil yield is estimated to be 40 kg per hectare [55]. Lavender flowers have up to 3% essential oil, so the amount of residual vegetable material remaining after processing is copious. Even if some process wastes of essential oil, as hydrolat, have already found a reuse possibility, the solid straws remaining after the process still represent a natural source to be further exploited. The high-power ultrasound corresponding to frequencies of 20 or 25 kHz seem to offer a good and they could be applied using two types of devices, ultrasonic bath, or probe-type ultrasound equipment. The probe system is more powerful due to an ultrasonic intensity delivered through a smaller surface, when comparing to the ultrasonic bath [18]. Even though the quantity of product that can be treated by an ultrasonic probe is restricted to rather small volumes, nevertheless many lavender essential oil producers are small enterprises which could exploit this extraction system on pilot scale after reoptimising the process variables to adapt them on larger scale. Both the PUAE extracts obtained are rich in gentisic acid (2,5-dihydroxybenzoic acid) whose amounts are comparable with the richest natural sources of this phenolic acid already reported in literature [56,57]. There are several industrial uses of this compound and many published studies on its biological activities that make the natural extracts rich in gentisic acid very interesting to be exploited in numerous food, cosmetic and pharmaceutical applications.
4. Conclusions
The solid by-products remaining after the traditional distillation of lavender essential oil constitute an interesting source of antioxidant compounds such as polyphenols and flavonoids, whose recovery could be performed as a first step in their valorization before using the remaining lignocellulosic biomass for other purposes (e.g., biofuel production). The “environmentally friendly” PUAE demonstrated to be a promising strategy to valorize these wastes obtaining, using only food-grade solvent (a mixture of ethanol and water), new potential ingredients rich in polyphenolic compounds that can be used in the cosmetic and pharmaceutical industries. Gentisic acid (2,5-dihydroxybenzoic acid) which is particularly abundant in both the solid waste extracts (TWE, FEW) is described as an analgesic, anti-inflammatory, antirheumatic, antiarthritic, cytostatic agent, and a fibroblast growth factor (FGF) inhibitor and it seems able to inhibit low-density lipoprotein oxidation in human plasma [58]. Moreover, extracts rich in gentisic acid could be exploited as cosmetic ingredient for skin-lightening applications since this phenolic compound is largely used in cosmetics just as lightening and whitening skin ingredient [58,59]. Likewise, the relative abundance of quercetin derivatives might find application in skin care formulates for their anti-inflammatory properties [60]. As recently reported in the literature [61], the size of the market for natural phenolic acids will grow exponentially over the next five years. Also, as far as gentisic acid is concerned, plants are considered its best source, and lavender flowers (Lavandula officinalis L.) have been reported as one of the richest sources. However, to our knowledge, the highest amount reported in the literature (8.6 mg/g DW) was obtained from the flowers by maceration with methanol [62]. In this research the change in the extraction method, using PUAE with a food grade solvent, allows to reach a similar or even higher quantity of gentisic acid (TWE: 10–13 mg/g DW; FWE: 1.4–4 mg/g DW; Table S1) in the corresponding extract in an ecological way. This approach could represent an opportunity for lavender essential oil producers who could increase their production revenues by valorising solid by-products.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11125495/s1, Table S1: Peak quantification.
Author Contributions
Conceptualization, F.T, F.M., M.B., L.M., P.Z., P.C. and R.B.; methodology, F.T. and F.M.; software, F.M.; formal analysis, F.T., G.T. and F.M.; investigation, F.M. and F.T.; writing—original draft preparation F.T. and F.M.; writing—review and editing, R.B. and M.B.; funding acquisition, M.B. and R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an European Union project called FINNOVER (n◦ 1198), http://www.interreg-finnover.com/ (Accessed on 11 June 2021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the present manuscript or in the supplementary materials.
Acknowledgments
The authors are grateful to “Stalla Franco” company for providing lavender samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lesage-Meessen, L.; Bou, M.; Ginies, C.; Chevret, D.; Navarro, D.; Drula, E.; Bonnin, E.; del Río, J.C.; Odinot, E.; Bisotto, A.; et al. Lavender- and lavandin-distilled straws: An untapped feedstock with great potential for the production of high-added value compounds and fungal enzymes Biotechnol. Biofuels 2018, 11, 217. [Google Scholar] [CrossRef] [PubMed]
- Finnover Interreg Alcotra Project 2017–2020. Available online: http://www.interreg-finnover.com/ (accessed on 29 March 2021).
- Kıvrak, Ş. Essential oil composition and antioxidant activities of eight cultivars of Lavender and Lavandin from western Anatolia. Ind. Crop. Prod. 2018, 117, 88–96. [Google Scholar] [CrossRef]
- Mohammadpourhodki, R.; Sadeghnezhad, H.; Ebrahimi, H.; Basirinezhad, M.H.; Maleki, M.; Bossola, M. The Effect of Aromatherapy Massage with Lavender and Citrus Aurantium Essential Oil on Quality of Life of Patients on Chronic Hemodialysis: A Parallel Randomized Clinical Trial Study. J. Pain Symptom Manag. 2021, 61, 456–463. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K.B. Composition, biological properties and therapeutic effects of lavender (Lavandula Angustifolia L). A review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Tomi, K.; Kitao, M.; Murakami, H.; Matsumura, Y.; Hayashi, T. Classification of lavender essential oils: Sedative effects of Lavandulaoils. J. Essent. Oil Res. 2018, 30, 56–68. [Google Scholar] [CrossRef]
- Da Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; Melo, D.A.D.S.; Donadio, M.; Nunes, F.B.; De Azambuja, M.S.; Santana, J.C.; Moraes, C.M.; et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. Anais Acad. Bras. Ciências 2015, 87, 1397–1408. [Google Scholar] [CrossRef]
- Al-Sayari, A.; Ghazwani, M.; Alhamhoom, Y.; Almaghaslah, D.; Louis, J.V.; Gurusamy, N. The antidepressant-like effect of almond oil: An additive effect with lavender oil. Biomed. Res. 2018, 29, 3402–3407. [Google Scholar] [CrossRef]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Seol, G.H. Lavandula Angustifolia Mill. Oil and Its Active Constituent Linalyl Acetate Alleviate Pain and Urinary Residual Sense after Colorectal Cancer Surgery: A Randomised Controlled Trial. Evid.-Based Complement. Altern. Med. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Bikmoradi, A.; Seifi, Z.; Poorolajal, J.; Araghchian, M.; Safiaryan, R.; Oshvandi, K. Effect of inhalation aromatherapy with lavender essential oil on stress and vital signs in patients undergoing coronary artery bypass surgery: A single-blinded randomized clinical trial. Complement. Ther. Med. 2015, 23, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Decorti, D.; Kikic, I. Flavour compounds of Lavandula Angustifolia L. to use in food manufacturing: Comparison of three different extraction methods. Food Chem. 2009, 112, 1072–1078. [Google Scholar] [CrossRef]
- Deng, X.; Chen, J.; Chen, W. Hydrogel particles as a controlled release delivery system for lavender essential oil using pH triggers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125134. [Google Scholar] [CrossRef]
- Perović, A.; Stanković, M.Z.; Veljković, V.B.; Kostić, M.D.; Stamenković, O.S. A further study of the kinetics and optimization of the essential oil hydrodistillation from lavender flowers. Chin. J. Chem. Eng. 2021, 29, 126–130. [Google Scholar] [CrossRef]
- D’Amato, S.; Serio, A.; López, C.C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control. 2018, 86, 126–137. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kotsanopoulos, K.V.; Savva, A.G. Use of ultrasounds in the food industry–Methods and effects on quality, safety, and organoleptic characteristics of foods: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 109–128. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochemistry 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Turrini, F.; Zunin, P.; Catena, S.; Villa, C.; Alfei, S.; Boggia, R. Traditional or hydro-diffusion and gravity microwave coupled with ultrasound as green technologies for the valorization of pomegranate external peels. Food Bioprod. Process. 2019, 117, 30–37. [Google Scholar] [CrossRef]
- Turrini, F.; Donno, D.; Beccaro, G.L.; Zunin, P.; Pittaluga, A.; Boggia, R. Pulsed Ultrasound-Assisted Extraction as an Alternative Method to Conventional Maceration for the Extraction of the Polyphenolic Fraction of Ribes nigrum Buds: A New Category of Food Supplements Proposed by The FINNOVER Project. Foods 2019, 8, 466. [Google Scholar] [CrossRef]
- Turrini, F.; Donno, D.; Boggia, R.; Beccaro, G.L.; Zunin, P.; Leardi, R.; Pittaluga, A.M. An innovative green extraction and re-use strategy to valorize food supplement by-products: Castanea sativa bud preparations as case study. Food Res. Int. 2019, 115, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Turrini, F.; Boggia, R.; Donno, D.; Parodi, B.; Beccaro, G.L.; Baldassari, S.; Signorello, M.G.; Catena, S.; Alfei, S.; Zunin, P. From pomegranate marcs to a potential bioactive ingredient: A recycling proposal for pomegranate-squeezed marcs. Eur. Food Res. Technol. 2020, 246, 273–285. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Varjani, S.; Cho, S.-K.; Ghodake, G.S.; Kadam, A.; Mulla, I.S.; Bharagava, R.N.; Kim, D.-S.; Shin, H.S. Development of ultrasound aided chemical pretreatment methods to enrich saccharification of wheat waste biomass for polyhydroxybutyrate production and its characterization. Ind. Crop. Prod. 2020, 150, 112425. [Google Scholar] [CrossRef]
- Turrini, F.; Boggia, R.; Leardi, R.; Borriello, M.; Zunin, P. Optimization of the Ultrasonic-Assisted Extraction of Phenolic Compounds from Oryza Sativa L. ‘Violet Nori’ and Determination of the Antioxidant Properties of its Caryopses and Leaves. Molecules 2018, 23, 844. [Google Scholar] [CrossRef] [PubMed]
- Boggia, R.; Turrini, F.; Villa, C.; LaCapra, C.; Zunin, P.; Parodi, B. Green Extraction from Pomegranate Marcs for the Production of Functional Foods and Cosmetics. Pharmaceuticals 2016, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Cangelosi, B.; Clematis, F.; Monroy, F.; Roversi, P.F.; Troiano, R.; Curir, P.; Lanzotti, V. Filiferol, a chalconoid analogue from Washingtonia filifera possibly involved in the defence against the Red Palm Weevil Rhynchophorus ferrugineus Olivier. Phytochemistry 2015, 115, 216–221. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 10 May 2021).
- Śmigielski, K.B.; Prusinowska, R.; Krosowiak, K.; Sikora, M. Comparison of qualitative and quantitative chemical composition of hydrolate and essential oils of lavender (Lavandula Angustifolia). J. Essent. Oil Res. 2013, 25, 291–299. [Google Scholar] [CrossRef]
- Moon, T.; Wilkinson, J.; Cavanagh, H. Antibacterial activity of essential oils, hydrosols and plant extracts from Australian grown Lavandula spp. Int. J. Aromather. 2006, 16, 9–14. [Google Scholar] [CrossRef]
- De Peredo, A.V.G.; Vázquez-Espinosa, M.; Espada-Bellido, E.; González, M.F.; Amores-Arrocha, A.; Palma, M.; Barbero, G.F.; Jiménez-Cantizano, A. Alternative Ultrasound-Assisted Method for the Extraction of the Bioactive Compounds Present in Myrtle (Myrtus Communis L.). Molecules 2019, 24, 882. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Espinosa, M.; De Peredo, A.V.G.; González, M.F.; Carrera, C.; Palma, M.; Barbero, G.F.; Espada-Bellido, E. Assessment of Ultrasound Assisted Extraction as an Alternative Method for the Extraction of Anthocyanins and Total Phenolic Compounds from Maqui Berries (Aristotelia Chilensis (Mol.) Stuntz). Agronomy 2019, 9, 148. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-De-Peredo, A.V.; Espada-Bellido, E.; González, M.F.; Toledo-Domínguez, J.J.; Carrera, C.; Palma, M.; Barbero, G.F. Ultrasound-Assisted Extraction of Two Types of Antioxidant Compounds (TPC and TA) from Black Chokeberry (Aronia Melanocarpa L.): Optimization of the Individual and Simultaneous Extraction Methods. Agronomy 2019, 9, 456. [Google Scholar] [CrossRef]
- González-De-Peredo, A.V.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Álvarez, J.Á.; Barbero, G.F.; Ayuso, J. Optimization of Analytical Ultrasound-Assisted Methods for the Extraction of Total Phenolic Compounds and Anthocyanins from Sloes (Prunus spinosa L.). Agronomy 2020, 10, 966. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chang, W.C.; Chen, P.S.; Chang, T.C.; Chang, S.T. Ultrasound-assisted extraction of phenolic antioxidants from Acacia confusa flowers and buds. J. Sep. Sci. 2011, 34, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Turrini, F.; Donno, D.; Beccaro, G.; Pittaluga, A.; Grilli, M.; Zunin, P.; Boggia, R. Bud-Derivatives, a Novel Source of Polyphenols and How Different Extraction Processes Affect Their Composition. Foods 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C.; Komaitis, M. Ultrasonically assisted extraction of phenolic compounds from aromatic plants: Comparison with conventional extraction technics. J. Food Qual. 2006, 29, 567–582. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Mikallou, M.; Petropoulos, S.; Tzortzakis, N. Profiling of Essential Oils Components and Polyphenols for Their Antioxidant Activity of Medicinal and Aromatic Plants Grown in Different Environmental Conditions. Agronomy 2020, 10, 727. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Dzięcioł, M. Comparison of phenolic acids and flavonoids contents in various cultivars and parts of common lavender (Lavandula Angustifolia) derived from Poland. Nat. Prod. Res. 2017, 31, 2575–2580. [Google Scholar] [CrossRef]
- Mammen, D.; Daniel, M. A critical evaluation on the reliability of two aluminum chloride chelation methods for quantification of flavonoids. Food Chem. 2012, 135, 1365–1368. [Google Scholar] [CrossRef]
- Torras-Claveria, L.; Jauregui, O.; Bastida, J.; Codina, C.; Viladomat, F. Antioxidant Activity and Phenolic Composition of Lavandin (Lavandula x Intermedia Emeric ex Loiseleur) Waste. J. Agric. Food Chem. 2007, 55, 8436–8443. [Google Scholar] [CrossRef]
- Upson, T. Phytochemistry flavonoids. In The Genus Lavandula; Upson, T., Andrews, S., Eds.; Timber Press: Portland, OR, USA, 2004; p. 442. [Google Scholar]
- Yadikar, N.; Bobakulov, K.; Li, G.; Aisa, H.A. Seven new phenolic compounds from Lavandula Angustifolia. Phytochem. Lett. 2018, 23, 149–154. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: Berlin, Germany, 1970; p. 354. [Google Scholar]
- Upson, T.M. Systematics of the Genus Lavandula L. (Lamiaceae). Ph.D. Thesis, University of Reading, Reading, UK, 1999. [Google Scholar]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.-C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin: A review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Beruto, M.; Mela, L.; Boggia, R.; Cangelosi, B.; Turrini, F.; Curir, P.; Monroy, F. Novel bioproducts from lavander to be tested against Myzus Persicae Sulzer (Homoptera: Aphididae). In In Vitro Cellular & Developmental Biology–Plant; Springer: New York, NY, USA, 2018; Volume 54, p. 5117. [Google Scholar]
- Harborne, J.B.; Baxter, H.; Moss, G.P. Phytochemical Dictionary A Handbook of Bioactive Compounds from Plants, 2nd ed.; Taylor & Francis: London, UK, 1999; p. 976. [Google Scholar]
- McCarthy, B.C.; Magurran, A.E. Measuring Biological Diversity. J. Torrey Bot. Soc. 2004, 131, 277. [Google Scholar] [CrossRef]
- Morris, E.K.; Caruso, T.; Buscot, F.; Fischer, M.; Hancock, C.; Maier, T.S.; Meiners, T.; Müller, C.; Obermaier, E.; Prati, D.; et al. Choosing and using diversity indices: Insights for ecological applications from the German Biodiversity Exploratories. Ecol. Evol. 2014, 4, 3514–3524. [Google Scholar] [CrossRef]
- Lis-Balchin, M. Lavender: The Genus Lavandula; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Abedi, F.; Razavi, B.M.; Hosseinzadeh, H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects. Phytother. Res. 2019, 34, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Juurlink, B.H.; Azouz, H.J.; Aldalati, A.M.; AlTinawi, B.M.; Ganguly, P. Hydroxybenzoic acid isomers and the cardiovascular system. Nutr. J. 2014, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Khadem, S.; Marles, R. Monocyclic Phenolic Acids; Hydroxy- and Polyhydroxybenzoic Acids: Occurrence and Recent Bioactivity Studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, J. The Use of Botanical Extracts as Topical Skin-Lightening Agents for the Improvement of Skin Pigmentation Disorders. J. Investig. Dermatol. Symp. Proc. 2008, 13, 20–24. [Google Scholar] [CrossRef]
- Bonina, F. Flavonoids as potential protective agents against photo-oxidative skin damage. Int. J. Pharm. 1996, 145, 87–94. [Google Scholar] [CrossRef]
- Valanciene, E.; Jonuskiene, I.; Syrpas, M.; Augustiniene, E.; Matulis, P.; Simonavicius, A.; Malys, N. Advances and Prospects of Phenolic Acids Production, Biorefinery and Analysis. Biomolecules 2020, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Zgórka, G.; Głowniak, K. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biomed. Anal. 2001, 26, 79–87. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).