Abstract
The aim of this study was to determine the effects of pre-sowing seed treatment with cold plasma (CP) and an electromagnetic field (EMF) on the agricultural performance of two cultivars of common buckwheat (Fagopyrum esculentum Moench)—‘VB Vokiai’ and ‘VB Nojai’. For this, the effects of CP and EMF on seed germination, plant growth in the field, photosynthetic efficiency, biomass production, seed yield, and the amount of secondary metabolites and minerals in the harvested seeds were estimated. Although the percentage of seedlings that emerged under field conditions decreased by 11–20%, seed treatments strongly improved buckwheat growth and yield. Irrespective of differences in the dynamics of changes in the growth and photosynthetic activity between the two cultivars, the weight of seeds collected per plant for both cultivars was significantly higher (up to 70–97%) compared to the control. The biochemical composition of the harvested seeds (Fe, Zn, quercetin content) was also altered by seed treatments. Thus, pre-sowing treatment of buckwheat seeds with CP and EMF substantially stimulated plant growth in the field, increased biomass production, seed yield and nutritional quality. The results obtained strongly support the idea that plant seed treatment with physical stressors has great potential for use in agriculture.
1. Introduction
Common buckwheat (Fagopyrum esculentum Moench) belongs to the genus Fagopyrum of the knotweed family (Polygonaceae) comprised of numerous species of which F. esculentum is the most widely cultivated [1]. It is an important crop used both for human food and animal feed in Asia, as well as in the USA, Central and Eastern Europe [2,3]. The plant is valued for its high nutritional value: high-quality proteins, essential amino and fatty acids, fiber, vitamins and minerals, such as iron, zinc, and selenium [4,5]. In addition, buckwheat contains high amounts of flavonoids, such as rutin and quercetin, and other phenolic substances exerting antibacterial and antioxidant activity [6]. These substances are important for plant antimicrobial properties and ultraviolet (UV) protection [7,8,9]. The presence of bioactive phytochemicals in buckwheat can lead to positive effects on health due to anti-tumor, anti-oxidant, anti-inflammatory, anti-aging, hepatoprotective, hypoglycemic, anti-allergic, anti-fatigue activities [6,9]. Due to numerous health benefits, gluten-free buckwheat is considered as an important component of functional food [10,11,12]. The anti-glycemic effects of fagomine as well as the photosensitizer potential of fagopyrin are under intensive investigation [6,13].
Although common buckwheat is able to adapt to difficult soil and climatic conditions, the production is greatly affected by a low seed set which results in low and unstable grain yield relative to the major cereals. Difficulties in genetic improvement and breeding through conventional methods are explained by the complex reproductive system of this crop, mainly due to the peculiar dimorphic and sporophytic type of dimorphic self-incompatibility [5,14], or the indeterminate type of growth and embryo abortion [15]. In addition, seed setting in common buckwheat is dependent on the abundance of pollinators or frequency of flower-visiting insects [16,17]. Recently, new reports on genomic interventions in buckwheat have emerged and it is expected that molecular breeding using integrative genomics and breeding could lead to successful genetic improvement of buckwheat [5,18]. However, such molecular technologies are still under development.
Different pre-sowing seed treatment modes have been used in agriculture to increase seed quality and crop productivity. Dormancy breaking agents including mechanical, thermal and chemical scarification, enzymes, dry storage, percussion, low temperatures, radiation and high atmospheric pressures are used to alleviate seed dormancy [19]. Other technique frequently applied to improve seed germination are seed priming or osmopriming by soaking seeds in water, salt or polyethylene glycol solution under controlled conditions for a specified period of time and then dehydrating them [19]. Modern trends in sustainable agriculture involve the use of biostimulants, which are natural or synthetic substances or microorganisms applied with the aim to enhance plant nutrition efficiency, abiotic stress tolerance and crop quality [20,21]. A large variety of commercial biostimulants is currently available on the market that are applied in small quantities compared to fertilizers for improving plant productivity [22]. In addition, a large number of studies have shown that pre-sowing seed treatment with low-temperature plasma (cold plasma, CP) and an electromagnetic field (EMF) can improve germination, early growth and the further development of plants (reviewed in [23,24,25,26,27,28,29]).
We have recently demonstrated that the yield of red clover biomass production may be substantially (up to almost 50%) increased by using seed treatment with CP and EMF [30]. In this study, we aimed to test the hypothesis that the yield of common buckwheat may be also increased using such treatments that are technically easier in comparison to molecular genomics. However, in most of the studies performed (reviewed in [23,24,25,26,27,28,29]) only the effects on germination and early seedling growth were estimated, and only a single study has been reported for common buckwheat [31]. Previously, we have demonstrated that a several fold increase in biomass production of perennial plants may be achieved after one year of plant growth [32]. Our experience showed that the results from laboratory germination tests in vitro may be different from those obtained when seedling emergence in a substrate was estimated [33,34,35]. Field observations for the entire vegetation season are required to test the effects under conditions close to those of agricultural crop cultivation, as well as to estimate the persistence and dynamics of plant response to seed treatment.
The aim of this study was to perform an investigation of the effects of seed treatment with radio-frequency EMF or CP on two cultivars of common buckwheat (‘VB Nojai’ and ‘VB Vokiai’) both in the laboratory conditions as well as during plant cultivation in the field for the entire vegetation season. For this, the effects of treatments on germination in vitro and on seedling emergence under field conditions were estimated. We also evaluated the effects on seedling growth in the field, on the parameters of photosynthetic efficiency and radical scavenging activity in leaves after 4 and 8 weeks, as well as changes in the crop biomass production, seed harvest and nutritional value after 14 weeks of common buckwheat cultivation.
2. Materials and Methods
2.1. Plant Material
The study was conducted using common buckwheat (Fagopyrum esculentum Moench) seeds of two cultivars: ‘VB Vokiai’ and ‘VB Nojai’ obtained from the Vokė branch of Lithuanian Research Centre for Agriculture and Forestry. The seeds were treated with CP and EMF in B.I. Stepanov Institute of Physics, National Academy of Sciences of Belarus.
2.2. Seed Treatment with Cold Plasma (CP) and Electromagnetic Field (EMF)
The equipment and the conditions used for seed treatment have been described earlier [32,36] in more detail. Seeds were treated with radiofrequency (RF) EMF using RF generator frequency 5.28 MHz. The EMF strength components at the center of the induction coil, where the packages with seeds were placed, were following: root-mean-square value of magnetic component H–590 A/m (B ≈ 0.74 mT); the electric component E −12.7 kV/m; amplitude values H* = and E* = were 835 A/m (B ≈ 1 mT) and 17.96 kV/m, respectively. The treatment was performed for 10 and 15 min (these treatments are further abbreviated as EMF10 and EMF15, respectively) at atmospheric pressure, room temperature (20–22 °C) and 35–50% relative humidity.
Seed treatment with CP was performed in a planar geometry plasma reactor. The capacitively coupled RF discharge operated at 5.28 MHz in air at a pressure of 200 Pa. The RF voltage from an RF generator was applied to the upper electrode by a commutator, and the input power density of 0.025 W/cm3 was applied in all experiments. An open sterile Petri dish with evenly dispersed seeds was placed on a grounded electrode before pumping air from the chamber. A pressure of 200 Pa was achieved in the chamber by pumping air from it for approximately 7 min before plasma ignition. Separate experiments were performed to test the effect of vacuum treatment (for 7 min) on seed germination, as an additional control for CP effects. The results showed that exposure to a vacuum (without plasma discharge) does not have an effect on the germination of buckwheat seeds. CP treatments lasted for 5 or 7 min (these treatments are abbreviated as CP5 and CP7, respectively). Control, CP- and EMF-treated seeds were stored at room temperature (19–22 °C) until further investigation. All experiments were performed in at least three replicates as indicated in the legends.
2.3. Seed Germination Tests
Germination tests in vitro were performed 4 days after CP treatment as described earlier [30,32,33,36], using three replicates (50 seeds each). The germination results of each experimental replicate were analyzed using the Richards’ function [37] for the analysis of germinating seed population [38]. The indices of germination kinetics: Vi (%)—the final germination percentage indicating seed viability, Me (hours)—the median germination time (t50%) or t when the percentage of the germinated seeds = Vi/2, indicating the germination halftime of a seed lot or germination rate. Germination is delayed as Me increases. The fitting of the germination results to Richards plots was very good: the measure of the goodness fit Er [38] variated from 1.44 ± 0.36% for the ‘VB Nojai’ EMF15 plots to 4.81 ± 0.19% for the ‘VB Vokiai’ CP5 group.
2.4. Plant Cultivation in Field and Morphometric Analysis
For the field observation seeds were sown in the experimental plots of the Voke Branch of the Lithuanian Research Centre for Agriculture and Forestry, located in Traku Voke (54°63′ N, 25°10′ E). The experimental plots were established on sandy loam on carbonaceous fluvial-glacial gravel eluviated soil (IDp), according to FAO-UNESCO classification Haplic Luvisols (LVh) [39]. Soil agrochemical characteristics: pHKCl 5.2–6.2, humus 2.11–2.18%, mobile P2O5 108–152 mg kg−1, mobile K2O 150–165 mg kg−1. Soil for buckwheat sowing was ploughed in the autumn, two times cultivated and harrowed in the spring. The size of the experimental plots for both cultivars was 1 m2, rows—20 cm, sowing density—100 seeds per 1 m2. There were 4 replicates of each experimental group (400 seeds for each group in total). The randomized complete block design of experimental plots was used in this study. Buckwheat was sown during the third decade of May. No herbicides and fertilizers were applied in the field tests. Weeds were controlled manually. Experimental plots were periodically watered to avoid drought due to the hot season. The percentage of the emerged seedlings in field conditions was determined 6 days after sowing as the ratio of the number of the emerged seedlings and the number of seeds sown in each experimental plot (4 replicates).
Morphometric analysis of seedlings was carried out 4, 8 and 14 weeks after sowing. The total number of plants used for morphometric seedling analysis after 4 weeks was 12–15. Four randomly chosen seedlings from each of 4 plots (as 2 samples with 2 seedlings) were excavated and placed (with roots still in the soil) to plastic containers using a separate container for each group. The pit formed was immediately filled with the soil taking care that the remaining plants are not damaged. The removed plants were transferred for morphometric analysis to the laboratory, where the soil was carefully separated from the roots before the analysis. Plants with accidentally damaged above-ground parts or roots were not used for analysis. Such procedure was possible only 4 weeks after sowing but not on later stages when seedling roots became larger. Therefore, the morphometric analysis of the above-ground part of seedlings was performed 8 and 14 weeks after sowing.
2.5. Measurement of Photosynthetic Indices
Functions of the photosynthetic system were measured in the field 8 weeks after sowing using a portable plant efficiency analyser (PEA, Hansatech Instruments, Ltd., King’s Lynn, Norfolk, UK). The measurements were taken on three healthy top intact leaves of 15 plants from each group. Prior to measurement, leaves were adapted to the dark for 15 min and then chlorophyll fluorescence transients of the dark-adapted leaves were measured. The transients were induced by 1 s illumination with an array of three light-emitting diodes providing a maximum light intensity of 1800 μmol (photon) m−2 s−1 and a homogeneous irradiation over a 4-mm diameter leaf area. The fast fluorescence kinetics was recorded from 10 μs to 1 s. Analysis of the fluorescence transients was performed with WINPEA 32 software and BiolyzerP3 to a spreadsheet [40]. The effectiveness of the second photosystem (FSII) was determined by the maximum quantum efficiency coefficient Fv/Fm ratio (a measure of light use efficiency [41], and two indices that are used to estimate the response of photosynthesis to environmental stress: photosynthesis performance index PIabs and ET0/CSm (electron transport ET0 per excited cross-section, CS) derived from leaf chlorophyll a fluorescence measurement [40,42].
2.6. Measurement of Radical Scavenging Activity and Total Amount of Phenolic Compounds
The extracts of buckwheat seed for measurements of radical scavenging activity and total amount of phenolic compounds were prepared from homogenized seed powder, obtained by milling 3 g of buckwheat with a Tube-Mill grinding machine for 2 min at the 22,000 rpm rotation rate. For each of the three replicates per experimental group 0.3 g of the resulting powder was extracted using 0.4 mL 80% methanol solution in water [43]. To facilitate extraction, it was carried out in an EMMI® ultrasonic bath at 20 kHz frequency for 15 min. The solution obtained was centrifuged at 13,400× g for 10 min, the supernatant was separated from the pelleted raw material, transferred into test tubes, closed tightly and stored at −20 °C until analysis.
The total amount of phenolic compounds in buckwheat seeds was determined using Folin–Ciocalteu (FC) reagent [44]. The reagent was diluted 10 times to obtain a working solution. For analysis, 0.2 mL of buckwheat seed extract was mixed with 1 mL of 10% FC reagent and 0.8 mL of 7.5% sodium carbonate solution and left in the dark for 30 min. The absorption of the samples obtained was measured using a spectrophotometer at 760 nm. The total number of phenolic compounds was determined using the standard calibration curve obtained using gallic acid. The total amount of phenolic compounds was expressed in milligrams of gallic acid equivalents per 100 g of seed weight.
Antioxidant activity of buckwheat seed extracts was determined using the DPPH• (2,2-diphenyl-1-picryhydrazyl hydrate) method [45]. DPPH• solution was prepared dissolving 5.5 mg DPPH• in acetonitrile, 80% methanol and 0.1 M sodium acetate buffer. After mixing 1950 μL DPPH solution with 50 μL of the 10 times diluted buckwheat seed extract, the reaction proceeded for 15 min in complete darkness. A decrease in the DPPH• concentration was registered spectrophotometrically by absorption at 515 nm. The calibration curve was obtained using standard Trolox solution; radical scavenging activity was expressed in equivalents of Trolox in mg/mL 100 g of seed weight.
2.7. Detection of Seed Mineral Content
Mineral (K, Ca, Mg, Na, Fe, Cu and Zn) content in buckwheat seeds (with hull) with was determined with atomic absorption spectrometry measurement on AAnalyst 200 (PerkinElmer, Waltham, MA, USA) using wet digestion process with sulfuric acid of 200 mg of plant material, after milling by ultra-centrifugal mill (Retch 200, mesh size 1 mm). For atomic absorption spectrometry an air-acetylene flame and hollow cathode multi-elements lamps were used.
2.8. Analysis of Flavonoid and Fagomine Amount
A flavonoid assay was carried out according to a previously reported Reversed-phase High-performance liquid chromatography Photo-diode array analysis (RP-HPLC-PD) protocol [46]. An assay of D-fagomine was carried out on an Acquity H-class Ultra-performance liquid chromatography (UPLC) system (Waters, Milford, CT, USA) equipped with Cortecs HILIC 1.6 µm, 2.1 mm × 100 mm (Waters, Milford, CT, USA) column. Linear gradient was mixed using solvent A (200 mM ammonium acetate, pH 4.5), B (acetonitrile) and C (water). Initial (0–0.5 min) gradient composition was 15% A and 85% B, 2 min-15% A, 75% B, 10% C, 3.5 min-15% A, 65% B, 20% C, 5 min-15% A, 55% B, 30% C. The column was termostated at 40 °C. Positive electrospray ionization was applied to monitor fagomine fragments using the following settings of a Xevo TQD triple quadrupole mass spectrometer (Waters, Millford, CT, USA): capillary voltage was set on 1.5 kV, source temperature 150 °C, desolvation temperature 350 °C, desolvation gas flow 650 L/h, cone gas flow 25 L/h. Collision energy and cone voltage was 14 eV and 24 V. MRM transition 148 > 86 was used for quantification.
2.9. Statistical Analysis
Statistical analysis of the results was performed using Statistica 10 software (issued by IBM Lietuva, Vilnius, Lithuania to Vytautas Magnus University). One-way analysis of variance (ANOVA) and Fisher’s least significant difference (LSD) test was used to compare the means of treated groups for field experiments. Results were considered statistically significant at p < 0.05.
3. Results
3.1. Effects on Germination and Seedling Emergence
Dry seeds of common buckwheat seeds were treated with CP for 5 or 7 min (these treatments are abbreviated further as CP5 and CP7, respectively). The treatment of dry seeds with EMF was performed for 10 and 15 min (these treatments are further abbreviated as EMF10 and EMF15, respectively). The early response of two buckwheat cultivars ‘VB Nojai’ and ‘VB Vokiai’ to pre-sowing seed treatment with CP and EMF was estimated by the germination tests in vitro 4 days after seed treatment. Richards plots were used to determine the main indices of germination kinetics presented in Table 1.

Table 1.
Indices of buckwheat germination kinetics in vitro.
Seeds of both cultivars showed high in vitro germination ability: maximal germination percentage was close to 100% in all experimental groups. The median germination time (Me) was also similar and, therefore, there was no difference in germination kinetics between the two cultivars or treatment groups. The only exception was the CP7 treated ‘VB Nojai’ seeds, where Me was reduced by 7% in comparison to the control, indicating a slight stimulating effect on germination rate. The morphometric analysis of germinated seedlings performed 5 days after sowing did not reveal any statistically significant differences in seedling length and weight between cultivars and treatment groups (data not shown).
The percentage of buckwheat seedlings that emerged under field conditions was determined by counting the number of seedlings on the 6th day after sowing in the experimental field plots of Vokė branch, the Lithuanian Research Centre for Agriculture and Forestry. The obtained results are shown in Table 2.

Table 2.
The percentage of the emerged seedlings 6 days after sowing in the field.
The difference in the percentage of the emerged seedlings between the two cultivars in the control was not statistically significant. The number of seedlings in the control group was larger in comparison to certain groups of the treated seeds: the percentage of the emerged seedlings was reduced by EMF15 (11%) and CP5 (13%) in ‘VB Vokiai’; and by EMF10 (15%) and CP5 (20%) treatments in ‘VB Nojai’. Thus, in contrast to CP and EMF effects obtained under in vitro germination conditions (Table 1), negative effects on buckwheat emergence were revealed in field experiments (Table 2).
3.2. Changes Induced in Growth Dynamics in the Field
The morphometric analysis of seedling growth in the field was performed 4, 8 and 14 weeks after sowing; the latter time point coincided with buckwheat harvesting. The obtained results are presented in Figure 1, Figure 2 and Figure 3 respectively. Morphometric parameters of shoot and root growth in the control groups of two buckwheat cultivars were similar 4 weeks after sowing (Figure 1a,b).
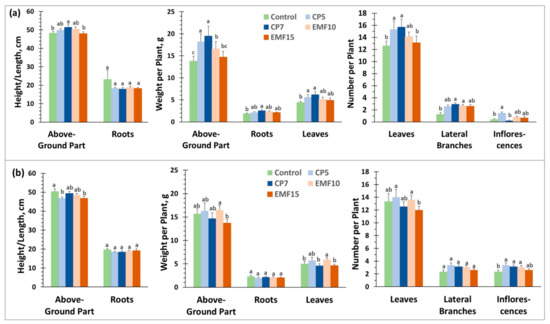
Figure 1.
Morphometric parameters of buckwheat seedlings grown for 4 weeks after sowing in experimental field plots: (a) ‘VB Vokiai’ parameters; (b) ‘VB Nojai’ parameters. The means ± standard errors are presented (n = 12–15). Different lowercase letters indicate significant differences (p < 0.05, Fisher’s LSD test).
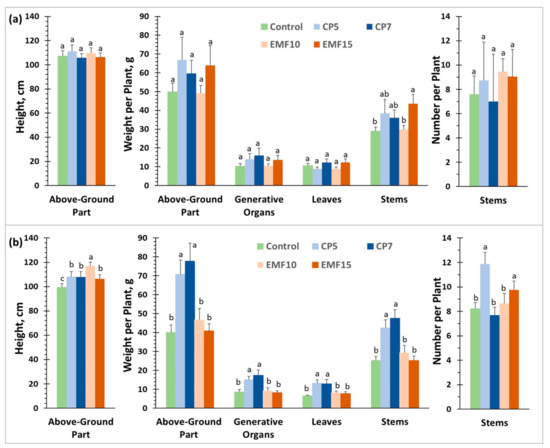
Figure 2.
Morphometric parameters of buckwheat seedlings grown for 8 weeks after sowing in experimental field plots: (a) ‘VB Vokiai’ parameters; (b) ‘VB Nojai’ parameters. The means ± standard errors are presented (n = 12–15). Different lowercase letters indicate significant differences (p < 0.05; Fisher’s LSD test).
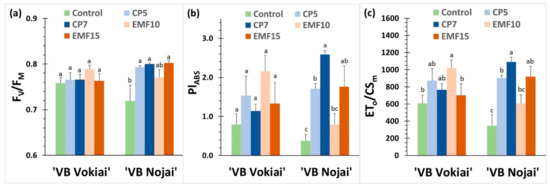
Figure 3.
Photosynthetic efficiency index Fv/Fm (a), photosynthesis performance index PIabs (b) and electron transport per excited leaf cross-section ET0/CSm (c) in buckwheat leaves measured 8 weeks after sowing in experimental field plots: (a) ‘VB Vokiai’; (b) ‘VB Nojai’. The means ± standard errors are presented (n = 15). Different lowercase letters indicate significant differences (p < 0.05, Fisher’s LSD test).
However, the number of lateral branches in ‘VB Nojai’ seedlings was 84% higher, and the number of inflorescences was 5.8 times larger compared to ‘VB Vokiai’, indicating that ‘VB Nojai’ started branching and flowering earlier (significance of differences between the control groups of two cultivars was estimated by Student’s test, p ≤ 0.05). ‘VB Vokiai’ seedlings in the CP7 group differed from other groups by the fastest growth of the above-ground part and root: seedling height was increased by 7%, weight 41%, root weight 36%, and plants developed 2 times more lateral branches, 24% more leaves, which weighed 41% more compared to the control. Certain morphometric indices such as seedling weight, number and weight of the leaves were also better in plants of the CP5 group compared to the controls. The most notable difference was 3.6 times higher number of inflorescences. Seed treatment with EMF15 had no effect on the morphometric parameters of ‘VB Vokiai’ 4 weeks after sowing, but EMF10 increased the seedling weight (by 20%) and the number of lateral branches (2.1-fold). The effects of CP and EMF on the growth of ‘VB Nojai’ cultivar 4 weeks after sowing was significantly different from the effect on ‘VB Vokiai’ (Figure 1a,b): the effect on seedling height was weak but negative (CP5 and EMF15 reduced height by 7%), there was no effect on weight of the above-ground part and root, root length, leaf number and weight; only EMF10 increased the average weight per leaf by 14%. Seed exposure to CP5, CP7 and EMF10 increased the number of inflorescences by (46%, 36% and 32%, respectively) in seedlings of ‘VB Nojai’ cultivar after 4 weeks (Figure 1b).
Only the above-ground part of seedlings was used for morphometric analysis 8 weeks after sowing (Figure 2), because the roots of buckwheat are thin and fragile and it is hard to separate them from the soil without damage. The above-ground parts of both cultivars were significantly larger after 8 weeks in comparison to their size on the 4th week after sowing (Figure 1): the values of morphometric indices of the above-ground part were more than twice as large compared to the indices after 4 weeks. However, the control plants of ‘VB Vokiai’ cultivar grew at the same rate as plants from CP and EMF treated seeds. The only statistically significant difference between the treatment groups and the control was a 51% increase in stem weight in EMF15 group plants (Figure 2a).
Meanwhile, the mean height of the above-ground part of ‘VB Nojai’ cultivar seedlings exceeded the control in all treatment groups after 8 weeks (Figure 2b), e.g., the EMF-10 group showed statistically significant height increase by 18%. CP5 and CP7 treatments strongly enhanced the weight of the above-ground part (by 76 and 94%, respectively), the number of leaves per plant (2 times), and the weight of the regenerative organs (1.8 and 2 times, respectively), in comparison to the control (Figure 2b). Only the CP5 treatment was effective in increasing the number of stems (45%). The effect of EMF on ‘VB Nojai’ morphometric parameters was smaller compared to CP: EMF-10 only increased plant height, and EMF-15 increased stem number by 20%.
3.3. Changes Induced in Photosynthetic Efficiency
To evaluate the effects of seed treatments with CP and EMF on physiological activity, the photosynthetic efficiency was determined in leaves of seedlings of two buckwheat cultivars growing in the experimental field plots 8 weeks after sowing.
Although the mean values of the efficiency of the second photosystem Fv/Fm ratio in leaves of control plants were 5% lower in ‘VB Nojai’ plants compared to’VB Vokiai’, the difference was not statistically significant. ‘VB Vokiai’ seed treatments did not induce appreciable changes in Fv/Fm, in contrast to ‘VB Nojai’, where values of Fv/Fm in all treated groups were significantly (from 7% to 11%) higher in comparison to the control. The only significant change in ‘VB Vokiai’ was a more than 40% increase in the electron transport flux per cross section ET0/CSm in plants of the EMF10 group, although this group did not demonstrate better growth 8 weeks after sowing (Figure 2). The photosynthetic indices of the EMF10 group did not differ from the control in ‘VB Nojai’. However, the values of the performance index PIabs and ET0/CSm values in the leaves of CP5, CP7 and EMF15 groups were substantially higher (4.5, 6.9, 4.7 and 2.6, 3.2, 2.7 times, respectively), while the Fv/Fm index was 10–11% higher in comparison to the control.
Thus, the effects of treatments on the indices of photosynthetic system efficiency were cultivar dependent. Better growth (larger weight parameters) of ‘VB Nojai’ plants 8 weeks after sowing (Figure 2) was related to positive changes in the indices of photosynthetic efficiency (Figure 3), although such a correlation was less obvious for the EMF15 group.
3.4. Changes in the Harvest Yield
The above-ground part of the plants was cut 14 weeks after sowing, dried and used for the analysis of morphometric plant parameters, seed yield per plant and nutritional seed value. The results showed (Figure 4) that CP5, CP7 and EMF15 treatments increased the number of matured seeds per plant of the ‘VB Vokiai’ cultivar by 46%, 41% and 44%, respectively compared to the control; and seed weight per plant in CP5 and EMF15 groups was increased by 70 and 56%, respectively. Seed treatments with CP5 and EMF15 also improved plant biomass production: plant weight increased by 37% and 33% and leaf weight by 80% and 47%, respectively. EMF had no effect on seed number for ‘VB Nojai’ cultivar but EMF15 increased plant and leaf weight by 41 and 71%, respectively, compared to the control. The effect of CP was stronger for ‘VB Nojai’, especially in the case of CP5 treatment which resulted in an 85% increase in the number of matured seeds per plant, a 97% increase in seed and plant weight and as much as 129% increase in leaf weight. CP7 increased the number of seeds by 45%, seed weight by 55%, and plant weight–by 44%. It is important to note that the mean weight of a single seed did not differ between the experimental groups for both ‘VB Vokiai’ and ‘VB Nojai’ (data not shown).
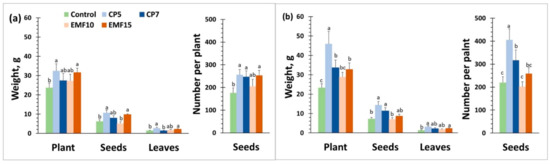
Figure 4.
Morphometric parameters of buckwheat plants and seed yield measured 14 weeks after sowing in experimental field plots. (a) ‘VB Vokiai’ parameters and seed yield; (b) ‘VB Nojai’ parameters and seed yield. The means ± standard errors are presented (n = 21–28). Different lowercase letters indicate significant differences (p < 0.05; Fisher’s LSD test).
3.5. Changes in Antioxidant Activity, Content of Secondary Metabolites and Minerals in Harvested Seeds
Harvested seeds were used to examine the effects of seed treatments on antioxidant activity, content of total phenolic compounds, minerals and secondary metabolites specific for buckwheat. Changes induced in the radical scavenging activity and the total amount of phenolic compounds in seed extracts are presented in Figure 5.
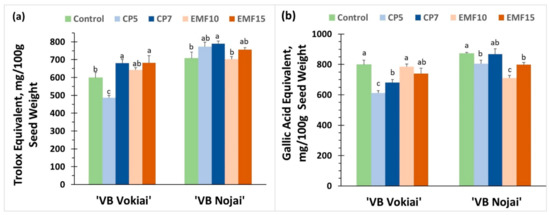
Figure 5.
Radical scavenging activity and amount of total phenolic compounds in the harvested seeds of buckwheat. (a) radical scavenging activity in ‘VB Vokiai’ and ‘VB Nojai’ seeds; (b) content of total phenolic compounds in ‘VB Vokiai’ and ‘VB Nojai’ seeds. The means ± standard errors of three replicates are presented (n = 3). Different lowercase letters indicate significant differences (p < 0.05; Fisher’s LSD test).
Both the radical scavenging activity and the total amount of phenolic compounds in the control seeds of ‘VB Vokiai’ was slightly lower (12% and 9%, respectively) in comparison to the control of ‘VB Nojai’ seeds (Figure 5). The treatment-induced changes in radical scavenging activity were cultivar dependent: for ‘VB Nojai’ the only observed effect was a negligible (2%) increase in radical scavenging activity in the CP7 group, whereas CP5 decreased this activity by 20% in ‘VB Vokiai’ seeds, and CP7 and EMF15 treatments enhanced radical scavenging activity (by 10% and 18%, respectively). CP but not EMF treatments reduced the content of the total phenolic compounds in ‘VB Vokiai’ seeds (CP5 by 24% and CP7 by 15%), in contrast to ‘VB Nojai’ seeds, where the amount of total phenolic compounds was reduced by EMF treatments (EMF15 by 19%, EMF10 by 9%) more so than by the CP treatment (CP5 by 7%). There was no correlation found between changes induced in the radical scavenging activity and the content of the total phenolic compounds in buckwheat seeds.
The analysis of the content of mineral elements has revealed certain cultivar-dependent differences in the mineral content of the control seeds (Figure 6). The content of K and Fe in ‘VB Vokiai’ seeds was (by 8.6% and 28%, respectively) smaller, and the content of Zn by 30% larger compared to ‘VB Nojai’ seeds, while the amounts of calcium, magnesium, sodium, and copper were similar.
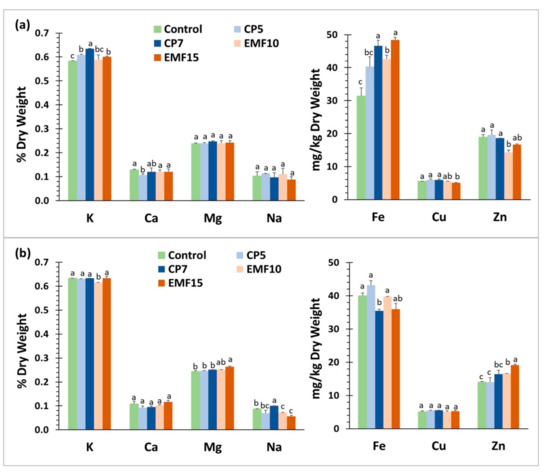
Figure 6.
Mineral content in the harvested seeds of two buckwheat cultivars. (a) ‘VB Vokiai’ seeds; (b) ‘VB Nojai’ seeds. The amount of K, Ca, Mg, Na is presented in % of dry weight, the amount of Fe, Cu and Zn–in μg per g. The means ± standard errors of three replicates are presented (n = 3). Different lowercase letters indicate significant differences (p < 0.05; Fisher’s LSD test).
Treatment-induced changes in seed mineral content were also significantly different for the two buckwheat cultivars. A slight increase (4–9%) in K content was observed in VB ‘Vokiai’ seeds after treatment with CP5, CP7 and EMF15, but the only effect in ‘VB Nojai’ was a 3% decrease of K content in the EMF15 group. The amount of Ca in ‘VB Nojai’ seeds was not affected by stressors, but in ‘VB Vokiai’ seeds CP5 reduced Ca content by 17%. There was no change in the amount of Mg and Na in ‘VB Vokiai’ seeds, while in ‘VB Nojai’ EMF15 increased the content of Mg by 8%; Na was increased by 15% in the CP7 group but significantly decreased by EMF10 and EMF15 (17% and 36%, respectively). The effect of stressors on Fe content in ‘VB Vokiai’ seeds was particularly significant: Fe amount increased by 49%, 36% and 54% for CP7, EMF10 and EMF15, respectively. However, no positive effects on Fe content were observed in ‘VB Nojai’ seeds; on the contrary, Fe content decreased by 11% in the CP7 group. Treatments did not change Cu content in seeds of both cultivars. CP treatments did not have an effect on the amount of Zn in seeds of both cultivars, whereas the effects of EMF treatments were the opposite: EMF10 treatment decreased Zn amount by 24% in ‘VB Vokiai’ seeds, and increased it in ‘VB Nojai’ seeds (by 19% in the EMF10 group and 37% in the EMF15 group).
In summary, the largest effects on buckwheat seed mineral content were observed on the amounts of Fe and Zn, but the effects were opposite for the two cultivars-CP7, EMF10 and EMF15 significantly increased Fe and decreased Zn content in ‘VB Vokiai’ seeds, while in seeds of ‘VB Nojai’ CP7 decreased Fe content and EMF treatments increased Zn content.
The results of HPLC analysis of the amounts of secondary metabolites rutin, quercetin and D-fagomine in buckwheat are presented in Figure 7.
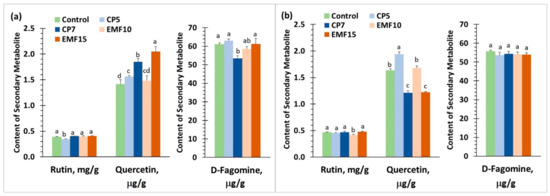
Figure 7.
Secondary metabolite content in harvested seeds of two buckwheat cultivars. (a) ‘VB Vokiai’ seeds; (b) ‘VB Nojai’ seeds. Rutin content is presented in mg/g, quercetin and D-fagomine amount–in μg per g. The means ± standard errors of three replicates are presented (n = 3). Different lowercase letters indicate significant differences (p < 0.05; Fisher’s LSD test).
In the control, ‘VB Vokiai’ seeds contained less rutin and quercetin (by 17% and 13%, respectively) but 10% more D-fagomine compared to ‘VB Nojai’ seeds (Figure 7). The amount of rutin in ‘VB Nojai’ seeds was reduced by 11% in EMF10 group; CP5 reduced rutin amount (by 10%), but EMF15 slightly increased it (by 5%). Changes in quercetin levels were also cultivar-dependent. In ‘VB Nojai’ seeds, quercetin levels were reduced in CP7 and EMF15 groups (by 26% and 25%, respectively), but increased by 19% in the CP5 group. In ‘VB Vokiai’ seeds CP7, EMF10 and EMF15 increased quercetin 31%, 5% and 45%, respectively. The amount of D-fagomine in ‘VB Nojai’ seeds was unaffected by the treatments, while in ‘VB Vokiai’ seeds, CP7 increased it by 12%.
The amount of secondary metabolites per plant was calculated by multiplying the average weight of seeds per plant (Figure 4) by the average amount of secondary metabolite per weight unit (Figure 7). Secondary metabolite production per plant found to be most significantly increased by seed treatment with CP5 in ‘VB Nojai’ seed rutin content per one plant increased 1.9, quercetin 2.3, D-fagomine 1.9 times. EMF15 treatment was the most efficient for ‘VB Vokiai’: rutin content per plant increased 1.6 times, quercetin content 2.3, D-fagomine content 1.6 times (although the total production of seed D-fagomine was the highest in the CP5 group, which increased 1.8 times compared to control seeds). In comparison to rutin and D-fagomine, treatments were more potent in changing the content of quercetin.
4. Discussion
The purpose of this study was to observe the effects of pre-sowing buckwheat seed treatment with CP and EMF on different indices of plant agronomic performance in field conditions during cultivation for the entire vegetation period. It has been reported previously [31] that treatment of buckwheat seeds with gliding arc discharge for 3 min increased the number of germinated seeds by 9%; however, this effect was not statistically significant. In addition, inhibitory effects on buckwheat seed germination have been observed with increasing processing time or using other devices for CP treatments [31], and only the very early growth of sprouts has been tested.
We showed that the effects of seed treatments on buckwheat growth persist for the entire vegetation season, and that the effects on biomass production and seed harvest are considerably larger in comparison to the effects on seed germination. We have previously demonstrated that the effects of seed treatments on germination in vitro are different from those in substrate for Norway spruce [33] and sunflower [34,35], as well as on seedling emergence in the field for industrial hemp [36]. In the case of buckwheat, little to no effect on germination was observed in vitro, while no effect or a moderate negative effect (11–20%) was found on seedling emergence in field conditions. It is possible that some of the seedlings that germinated from treated seeds did not survive 2 weeks after sowing under field conditions. Nevertheless, strong positive effects on plant growth on a longer time scale (14 weeks) were determined (Figure 4). Such findings are similar to the results obtained previously with red clover (Trifolium pratense) grown for 5 months in the field [30], when EMF strongly stimulated plant growth although such treatment did not affect germination. Taken together, these results contradict the widespread opinion that the effects on germination can be regarded as an informative estimate for the response of plants to seed treatments. Moreover, such findings demonstrate the importance of longer observations (at least for the entire vegetation season) under conditions used for plant agricultural cultivation. The results obtained show that the effects of seed treatments on various plant growth traits are different in two common buckwheat cultivars, similar to the results obtained for red clover [30]. The dynamics of the induced changes in plants of the two buckwheat cultivars followed different patterns over time: 4 weeks after sowing, a stronger positive effect on morphometric parameters of ‘VB Vokiai’ cultivar was observed in comparison to that after 8 weeks; meanwhile, the effects on ‘VB Nojai’ seedlings were much smaller or absent after 4 weeks, but positive effects of CP and EMF on growth (plant weight, the number and weight of stems and generative organs) became apparent after 8 weeks (stronger in comparison to the effects on ‘VB Vokiai’). At this time point, seed treatment led to an improvement in the indices of photosynthetic efficiency in leaves of ‘VB Nojai’, but not ‘VB Vokiai’ seedlings. This result indicates that stimulation of plant growth by seed treatments is related to the activation of photosynthetic processes, which also confirms the results obtained in sunflowers [35,36]. Stimulation of the net rate of photosynthesis in seedlings growing from CP treated seeds has been demonstrated directly in wheat [47]. It has been shown that treatment of sunflower seeds with CP and EMF induced substantial changes in leaf proteome, resulting in stimulated expression of proteins involved in photosynthetic processes and their regulation [34].
Irrespective of the different dynamics of changes in the morphometric parameters observed in the two cultivars, the yield of seeds collected from treated seedlings was significantly higher in both cultivars, compared to the control. Both CP and EMF increased seed yield (seed number up to 46%, seed weight up to 70%) per plant of ‘VB Vokiai’ cultivar, but only CP had a positive effect on seed yield (seed number up to 85%, seed weight up to 97%) in ‘VB Nojai’. Biomass production in both cultivars was strongly increased by CP5, CP7 and by EMF15 treatment. It is important to note that strong positive effects on biomass production were obtained in groups with the reduced emergence of buckwheat seedlings under field conditions (Table 2).
The biochemical composition of the harvested buckwheat seeds was altered by seed treatments, and changes induced in antioxidant capacity, content of minerals, and secondary metabolites were cultivar-dependent. Treatment of buckwheat seeds with CP and EMF had a negative effect on the total amount of phenolic compounds in seed extracts, but changes induced in seed radical scavenging activity were stronger in ‘VB Vokiai’ seeds compared to ‘VB Nojai’; moreover, the total phenolic compound content was reduced by CP treatment in ‘VB Vokiai’ seeds, and by EMF treatments in ‘VB Nojai’. The effects on seed mineral content were cultivar-dependent also. The strongest effects were found on seed Fe and Zn content: CP7 reduced Fe content, and EMF increased Zn in ‘VB Nojai’ seeds, while CP7, EMF10 and EMF15 significantly increased Fe and decreased Zn content in ‘VB Vokiai’ seeds. Although the change in the amount of secondary metabolites per unit seed mass was significant only in the case of quercetin, considering the differences in seed yield between experimental groups, it was estimated that seed treatment had a strong effect (from 1.6 to 2.3 times) on secondary metabolite production per buckwheat plant. In this respect, EMF15 treatment was the most effective for ‘VB Vokiai’, whereas CP5 treatment–for ‘VB Nojai’.
The reasons behind the strong observed differences in the response to various modes of seed treatment (the effects on different plant traits) between the two cultivars of the same plant species remain to be understood. Certain genetically determined characteristics of molecular stress signal perception and transduction in seeds, or peculiarities in plant stress response pathways may be responsible for such differences. Seed treatments may induce cultivar-dependent shift in the balance of phytohormonal networks in plant tissues (in particular, stress response phytohormones such as abscisic, salicylic acids, or jasmonate [48]) leading to variety of induced changes at metabolic and physiological level.
The significant increase in plant productivity may be also achieved by using biostimulants, and the observed effects variate in the range between 30% and 75% in dependence on the applied biostimulant type and plant species [49]; in addition, biostimulants can increase the nutritional quality of fruits and vegetables [50]. The effects of various types of biostimulants on the production of common buckwheat have been reported recently [51,52,53,54]. It has been reported that seed soaking for 30 min. in solutions with biostimulants and microorganisms can boost nutrient content [51] and antioxidant activity [52] in buckwheat sprouts. Some plant growth biostimulants applied in the beginning of blooming reduced (by 4–12-fold) embryo abortion and thus contributed to higher seed yield in populations of common buckwheat with a biased flower morph ratio [53]. Silicon biostimulant applied 3 weeks after germination improved numerous morphometric parameters including increased seed number per plant (8%) and seed weight per plant (3 times). However, it is not easy to compare the effects of biostimulants on the productivity of common buckwheat with effects of seed treatments performed in this study due to the essential differences in the protocols used. Further investigations of both methods or their combinations are needed to achieve the reliable results relevant for application in sustainable agriculture.
The persistence of changes induced in plant growth by seed exposure to CP or other stressors implies that response to seed stress is manifested by alterations not only in seed germination and early growth but also in all following stages of plant development. It has been recognized that seed treatment with CP induces numerous adaptive changes observable in the growing plants (see the most recent reviews [23,24,25,26,27,28,29]). Seed treatment with CP results in chemical modifications in seed coat leading to increased surface wettability [55], increased seed electron paramagnetic resonance (EPR) signal [33,56,57], rapid changes in the balance of phytohormones [34,35,58,59] and DNA methylation in dry seeds [60] resulting in changes of protein expression and enzymatic activities underlying the effects on germination. The systematic response of plants and seeds to EMF treatment has been reviewed recently [25]. Although EMF treatment did not affect seed coat structure [32], in dry seeds EMF increased EPR signal [33], induced changes in the balance of phytohormones [58,59], and increased reactive oxygen species (ROS) production in germinating seeds [33]. Seed treatments with CP and EMF are further followed by changes in DNA methylation [61], protein expression and numerous enzymatic activities in tissues of growing seedlings [25,34] including proteins that are important for photosynthetic activity and antioxidant defense. Substantial changes in plant associated microbiome due to sunflower seed treatment with CP have been reported [62]. Numerous findings indicate that secondary metabolism is activated in plants growing from the CP- or EMF-treated seeds [30,36,45,63], and that may lead to increased plant fitness and improved growth due to stimulated communication with beneficial microorganisms [59,64]. It remains to be determined which of these mechanisms are involved in the improvement of agricultural performance of common buckwheat and other plants. It has been suggested that plant adaptations can bring novel solutions for invigoration of agriculture and increasing crop productivity [65]. However, systematic and interdisciplinary knowledge on the detailed sequence of the internal molecular changes induced both in seeds and in plants is needed for the complete understanding of CP and EMF treatment effects and reliable their application in agriculture.
5. Conclusions
The field observations performed for the whole vegetation season revealed that treatment of buckwheat seeds with CP and EMF can significantly improve agronomic plant properties. Although treatments slightly reduced the percentage of the emerged seedlings under field conditions, physiological and developmental processes in growing plants were positively affected. Seed treatments improved growth, increased biomass production, almost doubled seed yield, modulated seed mineral content and significantly increased production of secondary metabolites per plant. The results obtained strongly supported the idea that plant seed treatment with physical stressors has a great potential for use in agriculture. A significant increase in the production of secondary buckwheat metabolites and changes in seed mineral content are important findings both for the pharmaceutical industry and production of healthy food. The presented results imply that field observations are needed to reveal the actual potential of seed treatments with CP and EMF for use in agriculture.
Author Contributions
Conceptualization, V.M. and I.F.; methodology, V.M., I.F., L.I., V.J. and A.Š.; investigation, A.I., Z.N., L.D.-F., R.Ž., I.J., A.M., V.J., D.R., A.Š., V.L. and V.M.; data curation, A.I.; writing—original draft preparation, V.M.; writing—review and editing V.M., I.F., L.I., V.J., A.Š., A.I., Z.N., L.D.-F., R.Ž., I.J., A.M., V.J., D.R. and A.Š.; visualization, Z.N.; funding acquisition, V.M. and V.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, M.; Tang, Y.; Deng, X.; Ruan, C.; Wu, Y. Classification and nomenclature of buckwheat plants. In Buckwheat Germplasm in the World; Zhou, M., Kreft, I., Suvorova, G., Tang, Y., Woo, S.H., Eds.; Academic Press: London, UK, 2018; pp. 9–20. [Google Scholar]
- Kreft, I.; Zhou, M.-L.; Golob, A.; Germ, M.; Likar, M.; Dziedzic, K.; Luthar, Z. Breeding buckwheat for nutritional quality. Breed. Sci. 2020, 70, 67–73. [Google Scholar] [CrossRef]
- Li, H. Buckwheat. In Bioactive Factors and Processing Technology for Cereal Foods; Wang, J., Sun, B., Cao, R., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2019; pp. 137–150. [Google Scholar]
- Kim, S.-L.; Kim, S.-K.; Park, C.-H. Introduction and nutritional evaluation of buckwheat sprouts as a new vegetable. Food Res. Int. 2004, 37, 319–327. [Google Scholar] [CrossRef]
- Joshi, D.C.; Chaudhari, G.V.; Sood, S.; Kant, L.; Pattanayak, A.; Zhang, K.; Fan, Y.; Janovská, D.; Meglič, V.; Zhou, M. Revisiting the versatile buckwheat: Reinvigorating genetic gains through integrated breeding and genomics approach. Planta 2019, 250, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Huda, N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef] [PubMed]
- Kreft, M. Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef]
- Chitarrini, G.; Nobili, C.; Pinzari, F.; Antonini, A.; De Rossi, P.; Del Fiore, A.; Procacci, S.; Tolaini, V.; Scala, V.; Scarpari, M.; et al. Buckwheat achenes antioxidant profile modulates Aspergillus flavus growth and aflatoxin production. Int. J. Food Microbiol. 2014, 189, 1–10. [Google Scholar] [CrossRef]
- Jing, R.; Li, H.; Hu, C.; Jiang, Y.; Qin, L.; Zheng, C. Phytochemical and Pharmacological Profiles of Three Fagopyrum Buckwheats. Int. J. Mol. Sci. 2016, 17, 589. [Google Scholar] [CrossRef]
- Ahmed, A.; Khalid, N.; Ahmad, A.; Abbasi, N.A.; Latif, M.S.Z.; Randhawa, M.A. Phytochemicals and biofunctional properties of buckwheat: A review. J. Agric. Sci. 2014, 152, 349–369. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Zieliński, H. Buckwheat as a functional food and its effects on health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Piskuła, M.; Zieliński, H. Recent advances in development of gluten-free buckwheat products. Trends Food Sci. Technol. 2015, 44, 58–65. [Google Scholar] [CrossRef]
- Benković, E.T.; Kreft, S. Fagopyrins and protofagopyrins: Detection, analysis, and potential phototoxicity in buckwheat. J. Agric. Food Chem. 2015, 63, 5715–5724. [Google Scholar] [CrossRef]
- Mendler-Drienyovszki, N.; Cal, A.J.; Dobránszki, J. Progress and prospects for interspecific hybridization in buckwheat and the genus Fagopyrum. Biotechnol. Adv. 2013, 31, 1768–1775. [Google Scholar] [CrossRef]
- Woo, S.-H.; Roy, S.K.; Kwon, S.J.; Cho, S.-W.; Sarker, K.; Lee, M.-S.; Chung, K.-Y.; Kim, H.-H. Concepts, prospects, and potentiality in buckwheat (Fagopyrum esculentum Moench): A research perspective. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.-H., Chrungoo, N., Wieslander, G., Eds.; Academic Press: Cambridge, UK, 2016; pp. 21–49. [Google Scholar]
- Cawoy, V.; Ledent, J.F.; Kinet, J.M.; Jacquemart, A.L. Floral biology of common buckwheat (Fagopyrum esculentum Moench). Eur. J. Plant Sci. Biotechnol. 2009, 3, 1–9. [Google Scholar]
- Katagiri, C.; Morishita, T.; Suzuki, T.; Mukasa, Y. Growth and yield of self-compatible and hybrid common buckwheat lines pollinated with and without flies. Plant Prod. Sci. 2017, 20, 384–388. [Google Scholar] [CrossRef]
- Matsui, K.; Yasui, Y. Genetic and genomic research for the development of an efficient breeding system in heterostylous self-incompatible common buckwheat (Fagopyrum esculentum). Theor. Appl. Genet. 2020, 133, 1641–1653. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: NewYork, NY, USA, 2014; pp. 79–161. [Google Scholar]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Frederiks, C.; Wesseler, J.H.H. A comparison of the EU and US regulatory frameworks for the active substance registration of microbial biological control agents. Pest Manag. Sci. 2019, 75, 87–103. [Google Scholar] [CrossRef]
- Mafei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant. Sci. 2014, 5, 445. [Google Scholar] [CrossRef]
- Pietruszewski, S.; Martínez, E. Magnetic field as a method of improving the quality of sowing material: A review. Int. Agrophys. 2015, 29, 377–389. [Google Scholar] [CrossRef]
- Kaur, S.; Vian, A.; Chandel, S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Sensitivity of plants to high frequency electromagnetic radiation: Cellular mechanisms and morphological changes. Rev. Environ. Sci. Biotechnol. 2021, 20, 55–74. [Google Scholar] [CrossRef]
- Šerá, B.; Šerý, M. Non-thermal plasma treatment as a new biotechnology in relation to seeds, dry fruits, and grains. Plasma Sci. Technol. 2018, 20, 044012. [Google Scholar] [CrossRef]
- Staric, P.; Vogel-Mikuš, K.; Mozetic, M.; Junkar, I. Effects of nonthermal plasma on morphology, genetics and physiology of seeds: A Review. Plants 2020, 9, 1736. [Google Scholar] [CrossRef]
- Holubová, L.; Kyzek, S.; Durovcová, I.; Fabová, J.; Horváthová, E.; Ševcovicová, A.; Gálová, E. Non-thermal plasma—A new green priming agent for plants? Int. J. Mol. Sci. 2020, 21, 9466. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Mildaziene, V.; Pauzaite, G.; Nauciene, Z.; Zukiene, R.; Malakauskiene, A.; Norkeviciene, E.; Stukonis, V.; Slepetiene, A.; Olsauskaite, V.; Padarauskas, A.; et al. Effect of seed treatment with cold plasma and electromagnetic field on red clover germination, growth and content of major isoflavones. J. Phys. D Appl. Phys. 2020, 53, 26. [Google Scholar] [CrossRef]
- Šerá, B.; Gajdovâ, I.; Černák, M.; Gavril, B.; Hnatiuc, E.; Kováčik, D.; Kříha, V.; Sláma, J.; Šerý, M.; Špatenka, P. How various plasma sources may affect seed germination and growth. In Proceedings of the 2012 13th International Conference on Optimization of Electrical and Electronic Equipment (OPTIM), Brasov, Romania, 24–26 May 2012; pp. 1365–1370.
- Mildaziene, V.; Pauzaite, G.; Malakauskiene, A.; Zukiene, R.; Nauciene, Z.; Filatova, I.; Azharonok, V.; Lyushkevich, V. Response of perennial woody plants to seed treatment by electromagnetic field and low-temperature plasma. Bioelectromagnetics 2016, 37, 536–548. [Google Scholar] [CrossRef]
- Paužaitė, G.; Malakauskienė, A.; Naučienė, Z.; Žūkienė, R.; Filatova, I.; Lyushkevich, V.; Azarko, I.; Mildažienė, V. Changes in Norway spruce germination and growth induced by pre-sowing seed treatment with cold plasma and electromagnetic field: Short-term versus long-term effects. Plasma Process. Polym. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Mildažienė, V.; Aleknavičiūtė, V.; Žūkienė, R.; Paužaitė, G.; Naučienė, Z.; Filatova, I.; Lyushkevich, V.; Haimi, P.; Tamošiūnė, I.; Baniulis, D. Treatment of Common sunflower (Helianthus annus L.) seeds with radio-frequency electromagnetic field and cold plasma induces changes in seed phytohormone balance, seedling development and leaf protein expression. Sci. Rep. 2019, 9, 6437. [Google Scholar] [CrossRef]
- Zukiene, R.; Nauciene, Z.; Januskaitiene, I.; Pauzaite, G.; Mildaziene, V.; Koga, K.; Shiratani, M. DBD plasma treatment induced changes in sunflower seed germination, phytohormone balance, and seedling growth. Appl. Phys. Express 2019, 12, 126003. [Google Scholar] [CrossRef]
- Ivankov, A.; Nauciene, Z.; Zukiene, R.; Degutyte-Fomins, L.; Malakauskiene, A.; Kraujalis, P.; Venskutonis, P.R.; Filatova, I.; Lyushkevich, V.; Mildaziene, V. Changes in growth and production of non-psychotropic cannabinoids induced by pre-sowing treatment of hemp seeds with cold plasma, vacuum and electromagnetic field. Appl. Sci. 2020, 10, 8519. [Google Scholar] [CrossRef]
- Richards, F.J.A. Flexible growth function for empirical use. J. Exp. Bot. 1959, 10, 290–300. [Google Scholar] [CrossRef]
- Hara, Y. Calculation of population parameters using Richards function and application of indices of growth and seed vigor to rice plants. Plant Prod. Sci. 1999, 2, 129–135. [Google Scholar] [CrossRef]
- Buivydaite, V.V. Soil Survey and Available Soil Data in Lithuania 2005, ESB-RR9. pp. 211–223. Available online: https://esdac.jrc.ec.europa.eu/ESDB_Archive/eusoils_docs/esb_rr/n09_soilresources_of_europe/Lithuania.pdf (accessed on 11 May 2021).
- Rasineni, G.K.; Guha, A.; Reddy, A.R. Elevated atmospheric CO2 mitigated photoinhibition in a tropical tree species, Gmelina arborea. J. Photochem. Photobiol. B Biol. 2011, 103, 159–165. [Google Scholar] [CrossRef]
- Harbinson, J. Chlorophyll fluorescence as a tool for describing the operation and regulation of photosynthesis in vivo. In Light Harvesting in Photosynthesis, 1st ed.; Croce, R., van Grondelle, R., van Amerongen, H., van Stokkum, I., Eds.; CRC Press: London, UK, 2018; pp. 539–572. [Google Scholar]
- Strasser, R.J.; Srivatava, A.; Tsimilli-Michael, M. Screening the vitality and photosynthetic activity of plants by fluorescence transient. In Crop Improvement for Food Security, 1st ed.; Behl, R.K., Punia, M.S., Lather, B.P.S., Eds.; SSARM: Hisar, India, 1999; pp. 72–115. [Google Scholar]
- Zielinski, H.; Kozłowska, H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J. Agric. Food Chem. 2000, 48, 2008–2016. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press: London, UK, 1998; Volume 299, pp. 152–178. [Google Scholar]
- Mildažienė, V.; Paužaitė, G.; Naučienė, Z.; Malakauskiene, A.; Žūkienė, R.; Januškaitienė, I.; Jakštas, V.; Ivanauskas, L.; Filatova, I.; Lyushkevich, V. Pre-sowing seed treatment with cold plasma and electromagnetic field increases secondary metabolite content in purple coneflower (Echinacea purpurea) leaves. Plasma Process. Polym. 2018, 14, 1700059. [Google Scholar] [CrossRef]
- Žvikas, V.; Pukelevicienė, V.; Ivanauskas, L.; Pukalskas, A.; Ražukas, A.; Jakštas, V. Variety-based research on the phenolic content in the aerial parts of organically and conventionally grown buckwheat. Food Chem. 2016, 213, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Saberi, M.; Modarres-Sanavy, S.A.M.; Zare, R.; Ghomi, H. Amelioration of photosynthesis and quality of wheat under non-thermal radio frequency plasma treatment. Sci. Rep. 2018, 8, 11655. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress—A Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Quality and safety of fresh fruits and vegetables at harvest. Sci. Hortic. 2018, 239, 78–79. [Google Scholar] [CrossRef]
- Witkowicz, R.; Biel, W.; Chłopicka, J.; Galanty, A.; Gleń-Karolczyk, K.; Skrzypek, E.; Krupa, M. Biostimulants and microorganisms boost the nutritional composition of buckwheat (Fagopyrum esculentum Moench) sprouts. Agronomy 2019, 9, 469. [Google Scholar] [CrossRef]
- Witkowicz, R.; Biel, W.; Skrzypek, E.; Chłopicka, J.; Gleń-Karolczyk, K.; Krupa, M.; Prochownik, E.; Galanty, A. Microorganisms and biostimulants impact on the antioxidant activity of buckwheat (Fagopyrum esculentum Moench) Sprouts. Antioxidants 2020, 9, 584. [Google Scholar] [CrossRef] [PubMed]
- Słomka, A.; Michno, K.; Dubert, F.; Dziurka, M.; Kopeć, P.; Płażek, A. Embryological background of low seed set in distylous common buckwheat (Fagopyrum esculentum Moench) with biased morph ratios, and biostimulant-induced improvement of it. Crop Pasture Sci. 2017, 68, 680. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Park, B.S.; Adnan, M.; Germ, M.; Kreft, I.; Woo, S.H.; Park, C.H. Silicon biostimulant enhances the growth characteristics and fortifies the bioactive compounds in common and Tartary buckwheat plant. J. Crop Sci. Biotechnol. 2021, 24, 51–59. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold Radio frequency Plasma Treatment Modifies Wettability and Germination Speed of Plant Seeds. Sci. Rep. 2012, 12, 741. [Google Scholar] [CrossRef]
- Koga, K.; Attri, P.; Kamataki, K.; Itakagi, N.; Shiratani, M.; Mildažienė, V. Impact of radish sprouts seeds coat color on the electron paramagnetic resonance signals after plasma treatment. Jpn. J. Appl. Phys. 2020, 59, SHHF01. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M.; Mildaziene, M. Impact of seed color and storage time on the radish seed germination and sprout growth in plasma agriculture. Sci. Rep. 2021, 11, 2539. [Google Scholar] [CrossRef] [PubMed]
- Degutytė-Fomins, L.; Paužaitė, G.; Žukienė, R.; Mildažienė, V.; Koga, K.; Shiratani, M. Relationship between cold plasma treatment-induced changes in radish seed germination and phytohormone balance. Jpn. J. Appl. Phys. 2020, 59, SH1001. [Google Scholar] [CrossRef]
- Mildažienė, V.; Ivankov, A.; Paužaitė, G.; Naučienė, Z.; Žūkienė, R.; Degutytė-Fomins, L.; Pukalskas, A.; Venskutonis, P.R.; Filatova, I.; Lyuskevich, V. Seed treatment with cold plasma and electromagnetic field induces changes in red clover root growth dynamics, flavonoid exudation, and activates nodulation. Plasma Proc. Polym. 2020, 18, 2000160. [Google Scholar] [CrossRef]
- Suriyasak, S.; Hatanaka, K.; Tanaka, H.; Okumura, T.; Yamashita, D.; Attri, P.; Koga, K.; Shiratani, M.; Hamaoka, N.; Ishibashi, Y. Alterations of DNA Methylation Caused by Cold Plasma Treatment Restore Delayed Germination of Heat-Stressed Rice (Oryza sativa L.) Seeds. ACS Agric. Sci. Technol. 2021, 1, 5–10. [Google Scholar] [CrossRef]
- Zhang, J.J.; Jo, J.O.; Huynh, D.L.; Mongre, R.K.; Ghosh, M.; Singh, A.K.; Lee, S.B.; Mok, Y.S.; Hyuk, P.; Jeong, D.K. Growth-inducing effects of argon plasma on soybean sprouts via the regulation of demethylation levels of energy metabolism-related genes. Sci. Rep. 2017, 7, 41917. [Google Scholar] [CrossRef] [PubMed]
- Tamošiūnė, I.; Gelvonauskienė, D.; Haimi, P.; Mildažienė, V.; Koga, K.; Shiratani, M.; Baniulis, D. Cold plasma treatment of sunflower seeds modulates plant-associated microbiome and stimulates root and lateral organ growth. Front. Plant Sci. 2020, 11, 568924. [Google Scholar] [CrossRef] [PubMed]
- Iranbakhsh, A.; Oraghi Ardebili, Z.; Molaei, H.; Oraghi Ardebili, N.; Amini, M. Cold plasma up-regulated expressions of WRKY1 transcription factor and genes involved in biosynthesis of cannabinoids in hemp (Cannabis sativa L.). Plasma Chem. Plasma Proc. 2020, 40, 527–537. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Cejas, E.; Zilli, C.; Prevosto, L.; Mancinelli, B.; Santa-Cruz, D.; Yannarelli, G.; Balestrasse, K. Enhancement of soybean nodulation by seed treatment with non–thermal plasmas. Sci. Rep. 2020, 10, 4917. [Google Scholar] [CrossRef] [PubMed]
- Ewel, J.J.; Schreeg, L.A.; Sinclair, T.R. Resources for crop production: Accessing the unavailable. Trends Plant Sci. 2019, 24, 121–129. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).