Abstract
Background: Smoking tobacco significantly affects the biology of periodontal tissues and contributes to the increased risk of peri-implant diseases. The aim of the study was to investigate whether smoking cigarettes affects the primary and secondary stability of maxillary dental implants, inserted into fresh sockets immediately after extraction. Methods: The study was conducted on 164 patients between the ages of 27–71 years old. 67 individuals smoked more than 20 cigarettes daily and 97 were non-smokers. 190 immediate implants were inserted in the maxilla. Immediate implantations were performed with simultaneous augmentation of the socket with xenogenic bone grafting material. In the posterior region, implants were inserted into the palatal alveolus. The stability of the implants was measured using Insertion Torque Value (ITV) and two types of devices: Periotest (PT) and Osstell (ISQ). Marginal bone loss was evaluated on cone beam computed tomography scans. Results: In an aesthetic area, the PT values at 6 months post-implantation were higher for smokers than non-smokers (p < 0.05), respectively. The ISQ values were significantly lower in smokers compared to non-smokers at 6 months post-implantation (p = 0.0226), respectively. In the posterior region PT values were higher in smokers both on the day of implantation (p = 0.0179), 6 months after surgery (p = 0.0003) as well as 24 months after surgery (p < 0.0001), as compared to non-smokers, respectively. Smokers revealed lower ISQ values than non-smokers (p = 0.0047) on the day of implantation, as well as 6 months after implantation (p = 0.0002), respectively. There were no significant differences in marginal bone loss after 18 months of loading between smokers and non-smokers in the aesthetic, as well as posterior regions (p > 0.05). ITV measurements were lower in smokers than non-smokers in the aesthetic (16.3 vs. 17.5 Ncm) and posterior area (16.8 vs. 17.9 Ncm). Conclusions: This study indicate that smoking cigarettes has a negative effect on the stability of immediate implants in the maxilla. Primary stability of immediate implants may be lower in the posterior area of the maxilla in smokers when compared to non-smokers, which may eliminate smokers from immediate implants in this region. Secondary stability of immediate implants may be lower in both the aesthetic and posterior areas in smokers compared to non-smokers, which may encourage the postponement of final crowns delivery at 6 months post op and the extension of the occlusaly temporary crowns use in some smoker cases.
1. Introduction
Smoking tobacco is considered a risk factor in implantoprosthetic treatment, as the failure rate of implants among smokers is almost 2-fold higher than in non-smokers [1]. Recent meta-analysis shows that the effect of smoking tobacco is dose-dependent, and the implant failure risk is 4-fold higher in patients who smoke more than 20 cigarettes a day [2]. The multidirectional impact of cigarettes on wound healing in the head and neck region, its influence on the process of osseointegration, and on the composition of the oral microbiome, all affect the outcome of implant therapy [3,4,5]. The stability of implants is a key factor in the process of osseointegration. Primary implant stability, acquired through mechanical forces between the surface of the implant and the bone, is dependent mostly on the implant design and dimensions, bone architecture and the insertion protocol. Secondary implant stability is gained after the osseointegration of the implant and is constituted by a biological connection between the bone tissue and the implant surface [6]. Implant stability is a prerequisite for proper osseointegration, as excessive micromobility of the implant may disrupt the formation of bone in contact with the titanium screw, and could lead to fibrous encapsulation of the implant [7].
Literature on dental implant stability among smokers is limited. Most previous studies report that smoking tobacco does not have a significant impact on implant stability after full osseointegration [8,9,10,11,12]. A recent study revealed lower stability among smokers [13], while another indicated that primary stability may be higher in smokers versus non-smokers [14]. Some researchers found that the process of osseointegration may be altered by tobacco use and that the process of acquiring secondary stability may be slower in smokers [11,12]. Existing evidence related to implant stability among smokers is scarce. Heterogenous results appear to be contradictory.
All of the above-mentioned studies evaluated the implants inserted in a conventional way, in the healed alveolar bone crest. Nowadays, there are many new techniques for placing implants in simultaneously regenerated bone, zygomatic implants or implants placed directly into fresh sockets after tooth extraction [15,16,17]. To date there is no research evaluating the stability of immediate implants inserted into fresh sockets, following extraction, in smokers. Moreover, most of the existing studies were performed according to a one-step protocol employing an open model of implant healing. The comparison between posterior and anterior area of implant insertion is lacking [8,9,10,11,13,14]. The current research is focused on immediate implants inserted in the aesthetic, as well as posterior area of the maxilla, where all of the implants underwent a closed healing process.
The aim of this study was to objectively compare the primary and secondary stability (6 and 24 months after implant insertion) of immediate implants in the anterior and posterior area of maxilla in smoking and non-smoking patients by means of resonance frequency analysis (RFA) and damping capacity (PTV).
2. Materials and Methods
2.1. Study Design
The prospective case series study was conducted in the Department of Oral Surgery, Medical University of Warsaw, Poland. The study included 164 patients. Study participants were recruited between the years 2012 and 2015 (during which their surgeries were performed) and the observation period was two years. Patients were informed about the purpose of the study and informed consent was obtained. The study was approved by the Bioethical Committee of the Medical University of Warsaw, permission number: KB/278/2012.
Criteria for inclusion in the study were as follows: generally healthy patient with no chronic diseases, not taking any chronic medication, lack of focal infection in other regions of the oral cavity, proper oral hygiene, patients who lost their teeth due to caries, endodontic problems, periodontitis, tooth fracture, and resorption. The exclusion criteria were as follows: moderate smokers (less than 20 pack years), bruxism, occlusal parafunctions (e.g., clenching, grinding, tapping teeth, biting the mucosa of lips and cheeks, biting nails and chewing gum), defects of the alveolar socket walls except for the vestibular bone plate. In the case of the presence of chronic periapical inflammation, patients qualified for the delayed protocol of implantation and were thus not included in the study.
2.2. Implants Features
SPI implants (Alpha-Bio Tec., Petah Tikwa, Israel) was used. SPI is an active spiral implant, recommended for D3 and D4 bone, with a high thread pitch of 2.4 mm. SPI has self-drilling, self-tapping and self-condensing properties. It has a hexagonal connection 2.5 mm, a patented NanoTec surface, high BIC (bone implant contact) and platform switching. The implants used are characterized by a slightly tapered body and tapering core, that is more pronounced that the body. The double thread design with 2.4 mm step is used with the variable threads design: coronal–thicker square threads, middle–thinner square threads, and apical–V threads. Thread depth increase in the apical direction. Alveoli were simultaneously augmented with bovine xenogenic bone substitute material (granule diameter of 0.5–1 mm; Alpha-Bio’s Natural Bone Graft, Alpha-Bio Tec., Petah Tikva, Israel).
2.3. Surgical Procedures
Cone beam computed tomography (CBCT) scans were routinely performed before surgery to evaluate the future position of the implants and confirm the integrity of the alveolar bony walls. Parameters such as implant diameter and length, angle of insertion, torque, depth of alveolar socket, drilling depth, and level of implant embedment in the bone were recorded. The density of the bone was not assessed.
The surgical procedures were performed by P.W. After obtaining optimal oral hygiene, implants were placed with antibiotic coverage of oral Augmentin (875 mg of amoxicillin with 125 mg of clavulanic acid), given every 12 h for 7 days, starting from the day before surgery. Infiltration anaesthesia was administered using cartridges containing 40 mg articaine hydrochloride and 0.01 mg epinephrine tartrate, in a 1 mL solution.
In the aesthetic region, minimally traumatic extractions of the teeth were performed with use of luxators and forceps. Immediate implantation with SPI implants was performed and implants were inserted according to the manufacturer’s instructions. Alveoli were simultaneously augmented with bovine xenogenic bone substitute material, with a granule diameter of 0.5–1 mm (Alpha-Bio’s Natural Bone Graft, Alpha-Bio Tec., Petah Tikva, Israel). Augmentation in both aesthetic and posterior regions was performed only within the boundaries of the alveolus. Missing teeth in the anterior area were restored temporarily with adhesive Maryland bridges.
In the posterior region, extractions with immediate implantation were performed according to the method described by Wychowański et al. [18]. Shortly, after removal of the crown and root separation, atraumatic extractions were performed with luxators and forceps. Post-extraction sockets were thoroughly curetted. Buccal, as well as palatal alveoli, were filled with bovine xenogenic material (granule diameter of 0.5–1 mm, Alpha-Bio’s Natural Bone Graft). Afterwards, the implant bed in the palatal alveolus was prepared manually with the use of tools of increasing diameter, in order to allow the biomaterial to condense and to enlarge the implant bed. SPI implants (Alpha-Bio Tec., Petah Tikva, Israel) were inserted according to the manufacturer’s instructions. Control CBCT scans were taken, and the wound was protected using surgical cement (Septo-Pack, Septodont, Paris, France). All of the implants underwent closed healing for a period of 6 months [18].
In the aesthetic area, 129 implants with a length of 11.5, 13 or 16 mm and a platform diameter of 3.3, 3.75 or 4.2 mm were inserted. In the posterior region, 61 implants with a length of 10 or 11.5 mm and a diameter of 3.75 or 4.2 were inserted. Each patient received either 1 or 2 implants. Bone Class III and IV by Lekholm and Zarb classification was found in all implant sites [19]. Characteristics of study participants were presented in detail in Table 1.

Table 1.
Characteristics of Study Participants.
2.4. Post-Operative Care
Patients were administered 500 mg ibuprofen, 30 min after the procedure and again later on, if pain was present. Patients were instructed about the negative effects of smoking on implant stability and wound healing and were instructed to avoid smoking for 2 days post-surgery. Patients were advised to rinse their mouth with a solution containing 0.12% chlorhexidine, twice a day for 2 weeks. Professional prophylactic procedures involving removal of bacterial biofilm and dental calculus were performed twice a year.
All implants superstructure was single unit cemented crowns. We performed ceramic crowns on a zirconium foundation. We used Maxcem Elite self-etch and self adhesive resin cement (Kerr Italia) to cement the crowns on the zirconium abutment.
2.5. Stability Measurement
Stability of dental implants was measured using two devices: the Periotest Classic (Medizintechnik Gulden, Modautal, Germany) and the Osstell Mentor (Osstell, Göteborg, Sweden). Stability measurements were performed on the day of implantation, after 6 months (at the day of prosthetic loading) and again after 24 months post-implantation. Stability measurements using the Osstell apparatus 24 months after implantation were not performed due to the presence of cemented prosthetic superstructures.
The Osstell Mentor device uses resonance frequency analysis (RFA) to estimate the stability of the implants. The device magnetically induces vibration of the implant and measures its frequency of movement. The result is expressed with ISQ scale (ISQ—Implant Stability Quotient) values: from 0 to 100—increasing values of ISQ are correlated with increased stability of the implant [20,21]. According to the producer manual and research data there are four main ranges of measured ISQ values: less than 60 (consider conservative approach due to low implant stability), 60–65 (two–stage conventional loading recommended), 65–70 (one–stage early loading possible witch caution), and more than 70 (one stage immediate loading recommended) [22,23].
Measurements were performed three times in the bucco-lingual and antero-posterior plane, and results were averaged to minimize measurement error. The final ISQ result is the average of values obtained from both planes.
The Periotest Classic device is designed to identify the damping capacity and the stiffness of the implant by measuring the contact time between the tip of the monitoring rod and the surface being tested during percussion. The results are expressed using PTV (Periotest Value) units ranging from −8 to +50, where a lower PTV is correlated with higher stability of the implant [24]. Measurements were performed by applying the tip of the device perpendicularly to the long axis of the healing abutment or the prosthetic crown, at its most apical point. Measurements were repeated three times in the bucco-lingual plane and the results were averaged. At the day of implantation Insertion Torque Value (ITV) was measured using the OsseoCare Pro drilling unit Bien Air Dental.
2.6. Marginal Bone Loss Measurement
Marginal bone loss was assessed with the use of CBCT to visualize alveolar bone in a repeatable manner, both on the mesial and distal sides of the implants, as well as on the palatal and vestibular aspects [25,26]. Measurements were performed on the day of prosthetic loading (6 months after implantation) and 18 months after prosthetic restoration, using a Kodak 3000C 3D CBCT apparatus (Kodak Dental Systems, Atlanta, GA, USA), with Dental Imaging Software version 6.12.32 (Kodak Dental Systems, Atlanta, GA, USA). The measurements were made from the implant shoulder level to the nearest bone level. Scans were evaluated in two planes: bucco-palatal (one measurement on the buccal side, one on the palatal side of the implant) and mesio-distal (one measurement on the mesial side and one on the distal side of the implant). The recorded result was the highest of the four measurements. The investigator performing the measurements was blinded with regards to which group the patients were assigned to. The order of analysed material was randomized to compensate potential bias in the learning curve during measurement taking. This was the case for both marginal bone loss measurements and implants stability measurements.
2.7. Statistical Analysis
The sample size was estimated using G*Power software, version 3.1.9.4 for a one-tailed t-test at α = 0.05 with 80% power, assuming d (effect size) = 0.85, for two study groups Effect size 0.85 was reached assuming minimal delta = 0.51 and maximal SD = 0.60. The SD was obtained based on our previous studies. The recommended sample size for one group was 18 patients. All data were expressed as the mean and standard deviation. Data were first examined for normality using the Kolmogorov–Smirnov test (data not shown); Unpaired t-test or Mann-Whitney test (some data was not normally distributed) were used to compare the differences between groups (smokers and non-smokers). If possible, the significance test was 2- tailed and conducted at a 0.05 level of significance. The Pearson test was used for correlation analyses between measured variables. There was no missing data.
3. Results
3.1. Characteristic of Patients
The study was conducted on 164 patients: 74 men and 90 women, aged between 27 and 71 years (mean age of 49 years). The group of patients included 67 patients that smoked more than 20 cigarettes a day, for at least 10 years (more than 10 pack years–heavy-smokers) and 97 people that were non-smokers (had never smoked). The survival rate of the implants after 2 years of observation was 100%.
Since smoking tobacco is one of the risk factors for the development of periodontitis, the reasons for the extraction of the teeth were recorded, and the study groups remained uniform in this regard. The percentage of teeth extracted due to periodontal disease in smokers and non-smokers in the posterior area were 8% and 16%, respectively, whereas teeth extracted due to periodontal disease in the aesthetic area was 9% and 9.5%, respectively. The studied population was limited to a group of patients that fulfilled the eligibility criteria and were admitted between the years 2012 and 2015. Overall, 674 patients were assessed for eligibility, 251 patients were excluded due to general chronic diseases, 3 patients were routinely taking sedative medication, 114 had an active focal infection in the oral cavity, 117 suffered from bruxism/occlusal parafunctions and 23 could not maintain proper oral hygiene. Overall, 508 patients were excluded from the study and the inclusion criteria were met by 166 subjects. The follow-up period for all the participants was 2 years. No patient was lost to follow-up, 2 patients quit smoking in the follow-up period, and were excluded from the study. Reasons for tooth loss were displayed in Table 2.

Table 2.
Reasons for tooth loss.
3.2. Aesthetic Area
Osstell measurements were not significantly different between groups on the day of implantation. Mean Osstell measurements were significantly lower (lower stability) in smokers than in non-smokers 6 months after implantation (p = 0.0226). Periotest measurements in the aesthetic area on the day of implantation and 24 months post-implantation did not reveal any statistically significant differences in implant stability between smokers and non-smokers (p > 0.05). Mean Periotest values 6 months post-implantation were higher among smokers (lower stability) when compared to non-smokers and reached 0.34 (±0.12) and 0.0 (±0.09, p = 0.02), respectively. There were no significant differences in the marginal bone level around implants in the aesthetic area between smokers and non-smokers, 18 months after loading (p = 0.94). Moreover, the vestibular bone plate thickness remained the same between the two groups. The results are presented in Figure 1, and in Table 3. ITV measurements in the posterior area were lower in smokers than non-smokers, but do not revealed statistically significant differences as showed in Table 1. Statistical analysis showed a positive correlation between ITV and periotest (PT), and ITV and ISQ in all study groups, however the above-mentioned correlation was not statistically significant.
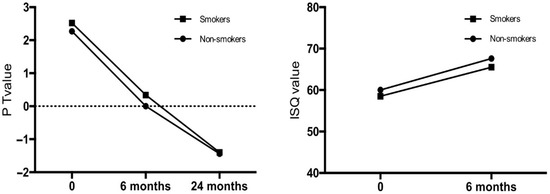
Figure 1.
Implant stability in the aesthetic area of smokers and non-smokers, following immediate implantations in maxilla.

Table 3.
Implant stability and bone atrophy in the aesthetic area of smokers and non-smokers, following immediate implantations in maxilla (ISQ—mean Implant Stability Quotient; PT—periotest measurements).
3.3. Posterior Area
Mean Osstell measurements in the posterior area were lower in smokers than non-smokers, both on the day of implantation (p = 0.0047), as well as 6 months post-implantation (p = 0.0002). Mean Periotest values in the posterior area were higher (lower stability) on the day of implantation (p = 0.0179), 6 (p = 0.0003) and 24 months (p < 0.0001) after implantation in smokers, when compared to non-smokers. Marginal bone loss in the posterior region, 18 months after implantation, was not significantly different between groups (p = 0.49). The results are presented in Figure 2, and in Table 4. ITV measurements in the posterior area of maxilla were lower in smokers than non-smokers, but do not revealed statistically significant differences as shown in Table 1. Statistical analysis showed a positive correlation between ITV and PT, and ITV and ISQ in all study groups, however the above-mentioned correlation was not statistically significant.
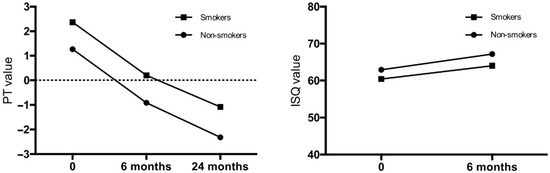
Figure 2.
Implant stability in the posterior area of smokers and non-smokers, following immediate implantations in maxilla.

Table 4.
Implant stability and bone atrophy in the posterior area of smokers and non-smokers, following immediate implantations in maxilla (ISQ—mean Implant Stability Quotient; PT—periotest measurements).
3.4. Other Analyses
The Pearson test was used for correlation analyses between measured variables (Periotest value, ISQ value, and bone atrophy) and other variables (age, implant diameter, implant length, implant angulation, and torque during insertion, depth of alveolar socket, drilling depth, and level of implant embedment in the bone).
Among the groups studied, the only variables that correlated with PT values or ISQ were implant length, implant diameter and torque. These correlations are consistent with previously published data available in the literature [24]. The dynamics of the implant stability increase over time was analyzed for potential differences between smokers and non-smokers. No significant differences were found between groups in the aesthetic and posterior regions (p = 0.124 and p = 0.188, respectively).
4. Discussion
The results of the current study indicate that smoking tobacco has a negative impact on implant stability following immediate implantation in the aesthetic, as well as posterior regions of the maxilla. The marginal bone loss around implants was not significantly different between groups 18 months after prosthetic loading.
Dental implant stability is the key factor enabling proper healing, and, therefore, the success of the implantoprosthetic treatment and depends on the bone quality, implant properties, as well as surgical technique [27,28]. The thread pitch, in implants we used, is 2.4 mm what seems to be a lot, but it is compensated by the double thread design which results in maximum 1.2 mm distance between single threads. The implant primary stability is also enriched due to variable thread design wider in the coronal part and deeper in apical direction. In results this may provide high primary implant stability, even in soft bone. Periotest measurements in the aesthetic area showed no significant differences in implant stability on the day of implantation, and 24 months after surgery between smokers and non-smokers. Although at the time of prosthetic loading (6 months after surgery), smokers displayed lower implant stability than non-smokers. In the posterior area of the maxilla implant stability was lower in smokers on the day of implantation, as well as 6 months after. Sanchez-Perez et al. found no significant differences in PT values in general when comparing smokers to non-smokers, however they revealed that higher Periotest values (lower stability) were correlated with increased risk of implant failure. Moreover, the success rate of the treatment was significantly lower in smokers when compared to non-smokers (84.2% and 98.6%, respectively). Increased tobacco consumption resulted in more pronounced differences, as the failure rate after 5 years rose to 30.76% among patients who smoked more than 20 cigarettes a day [10]. Mesa et al. similarly found no differences in Periotest values between smokers and non-smokers [9].
After 2 years of follow-up no implant failure was observed in both groups of patients. The differences observed in the above-mentioned studies, which described implantations performed in a healed alveolar crest, may be attributed to the different type of surgical protocol, since our patients underwent immediate implantations involving intra-alveolar augmentation with a xenogenic bone substitute.
It has been build up evidence that excessive mobility of implants, exceeding the threshold of about 150 microns, inhibits the osseointegration process and may lead to the formation of a connective tissue layer between the implant and the surrounding bone [29]. Moreover, available clinical stability measurement methods, such as resonance frequency analysis used in Osstell device, have been described as suitable to predict the risk of implant failure, based on secondary stability measurements [30,31]. It is of a great importance that RFA and Insertion Torque Value represent two different features of primary stability. The RFA indicating the resistance to bending load, the ITV indicating the resistance to shear forces [21]. The measurement of ITV or RFA may be significantly overstated in the case of a thick cortical bone, that provides primary stabilization of the implant only in its narrow section in contact with the cortical bone, while the implant may not achieve stabilization in the part emerged in the cancellous bone. In these cases, dynamic measurement methods such as VTW (Variable Torque Work) or IE (Insertion Energy) are essential to establish the actual force, the implant is stabilized in the bone. In our study all the cases were immediate implantation to the fresh alveolar sockets, that mean the cortical bone was missing. The bone was III or even IV class by Lekholm and Zarb classification [19]. This is why RFA seems to be reliable method of primary implant stability assesment and correlated with VTW and IE in these bone conditions [32]. Our results show the mean ISQ value at the time of surgery in the posterior area 60.4 ± 0.4 for smokers and 62.9 ± 0.6 for non-smokers and at the time of loading 64.0 ± 0.5 and 67.2 ± 0.6, respectively. It could be noticed that at the time of surgery in posterior region of maxilla the majority of smokers cases are on the verge of eligibility for immediate implantation, while non-smokers exceed these requirements and can be easily recommended for two-stage conventional loading implantation. After six months the loading of smokers’ implants should be performed in caution (may be with the use of temporary not fully loaded crown for a few months), while non-smokers implants may be loaded with permanent crown. Our results show the mean ISQ Value at the time of surgery in the aesthetic area 58.5 ± 0.4 for smokers and 60.0 ± 5.1 for non-smokers and at the time of loading 65.52 ± 5.05 and 67.61 ± 5.11, respectively. It could be noticed that at the time of surgery in aesthetic region of maxilla the majority of smoker cases are below of eligibility for immediate implantation, while non-smokers, in most cases, meet these requirements. After six months the loading of smokers’ implants should be always performed in caution (with the use of temporary not fully loaded crown for a few months), while non-smokers seems to be ready for permanent loading. Results from previous studies involving implant stability, measured by resonance frequency analysis, are heterogenous. Most of the authors conclude that tobacco consumption is not associated with differences in implant stability [8,11,12]. A recent study by Badenes et al. reported that primary implant stability was not affected by smoking, while secondary stability may be lower among smokers. Moreover, during the healing period, implant stability among smokers decreased by 0.91 ISQ points, while in non-smokers it was increased by 2.69 ISQ points, during osseointegration [13]. On the other hand, Sayardoust et al. showed that stability of implants in smokers was significantly higher than in non-smokers on the day of implantation, and 1 day, 7 days, and 14 days after surgery. However, at 28 days post-implantation the difference between these two groups of patients was not significant [14]. Diverse research protocols and surgical procedures hinder direct comparison of results obtained. Available results regarding the effects of smoking on implant stability are significantly variable which indicates the need for further exploration of the osseointegration process and clarification of the factors contributing to the discrepancies observed.
A recent meta-analysis by Alfadda et al. indicates that smoking cigarettes increases the marginal bone loss around implants by 0.11 mm per year on average [1]. Other meta-analyses confirm increased marginal bone loss around implants in smokers [33,34]. In the current study the marginal bone loss after 18 months was lower than most of the reported results included in the above-mentioned meta-analyses. This may be attributed to different surgical techniques. In our study, the marginal atrophy observed in the alveolar bone was not significantly different between smokers and non-smokers, both in the aesthetic as well as posterior regions of the maxilla, 18 months after prosthetic loading.
The differences in implant stability between smokers and non-smokers in the current study were similar at all time points. Statistical analysis of the implant stability growth dynamics based on the ISQ value increase did not reveal significant differences between the two groups (aesthetic area p = 0.124, posterior area p = 0.188). It can be concluded that smoking did not noticeably affect the osseointegration process itself, during the 6-month post-implantation period.
A study by Sun et al., comparing the process of osseointegration between heavy smokers and non-smokers showed that the healing process after implantation may be slower in smokers. The results indicated initially prolonged period of acquiring secondary stability of the implants in the first weeks after implantation. However, at 12 weeks post-surgery, the differences between smokers and non-smokers were not significant, which is consistent with our observations [11]. Sayardoust on the other hand showed that the stability of implants in smokers was significantly higher than in non-smokers on the day of implantation, and 1 day, 7 days, and 14 days after surgery. However, 28 days post-surgery the difference between these two groups of patients was not significant [14]. Smoking has a negative effect on bone metabolism, it impedes normal function and proliferation of the alveolar bone marrow mesenchymal stem cells. A study by Zhao et al. revealed that these changes were also correlated with disturbances in osseointegration and lower stability of the implants among smokers from the 3rd to the 6th week after surgery [12]. Due to the different surgical techniques (closed healing) used in our study, the implants were unavailable for examination due to the ongoing healing process during the first few weeks after surgery. Upon measuring the implant stability 6 months post-implantation, we found no significant differences in implant stability growth dynamics, which is in line with the abovementioned research.
One of the limitations of the current study involves the use of indirect methods of stability assessment. ISQ index, despite a strong correlation to the magnitude of implant micro-mobility, is not in complete agreement with it [35]. Measurement of the absolute micro-mobility value may have provided more precise information regarding the quality of the bone-implant interface and might have given a broader image of the osseointegration process in smokers. To date there are no devices available that could be used in a clinical setting to assess actual micro-mobility.
Implant stability is affected by a multitude of factors of biological, mechanical, and technical nature. In our study, parameters including age, implant diameter, implant length, angulation of implant in mesio-distal and bucco-palatal dimensions, torque and marginal bone loss were recorded. In the aesthetic area, the depth of the alveolar socket, drilling depth, and level of implant embedment in the bone were additionally assessed. Each of the parameters was separately evaluated for correlation to results of stability measurements to control for potential confounding of the research outcome.
The study by Baltayan et al. proved that implant stability measurement by means of resonance frequency analysis is suitable for predicting the risk of implantoprosthetic treatment—the survival rate was correlated with ISQ measurements. Additionally, all of the failures that were recorded occurred when ISQ was less than 67 at the time of implant loading [36]. In our study, the mean implant stability in smokers on the day of implant loading was below that level in both the posterior and anterior regions. Although a survival rate of 100% was achieved over a 2-year observation period, the significantly lower implant stability observed in smokers may be viewed as a factor affecting the long-term prognosis of the implants in that group of patients, thus procedures with a higher failure risk, such as immediate or early implant loading should be used with caution, especially in the presence of other risk factors [36,37]. Implant stability measurements performed before implant loading may help avoid this condition, where the threshold implant stability level, necessary for a proper osseointegration process, would be exceeded. Many of implants were inserted in angulation forced by alveolar native bone presence. This is why in lots of cases the technological hole for screwing on superstructure projected onto the labial wall of the crown. It was unacceptable especially in aesthetic region. This is why cemented superstructures were administered to all patients to avoid variation in results. The proposed procedure made it impossible to measure ISQ 24 months after implantation. We were able only to use Periotest in this time point of the study. Some newest research considers PT value as a very predictable implant stability measurement option even correlated with marginal bone loss in a time. Routine stability measurements may contribute to predictable long-term success of implantoprosthetic treatment [38].
5. Conclusions
The differences in primary and secondary stability between smokers and non-smoker are not huge in numbers but may decide about the disqualification for the procedure or postponement of final crown delivery at 6 months post op and the extension of the occlusaly temporary crowns use in smokers’ cases. Primary stability of immediate implants may be lower in the posterior area of the maxilla in smokers when compared to non-smokers. Secondary stability of immediate implants may be lower in both the aesthetic and posterior areas of the maxilla in smokers compared to non-smokers. Further studies on a larger group of participants and deeper insights into the field of periodontal analysis (e.g., pocked depth, bleeding on probing) and bone biology (e.g., bone density analysis, influence of bone augmentation on implants healing and stability) during immediate implantation in smokers are still needed.
Author Contributions
Conceptualization, P.W. and A.S.; methodology, P.W., A.S., P.K., P.A. and J.W.; software, P.W. and P.K.; validation, P.W. and P.K.; formal analysis, P.W. and A.S.; investigation, P.W. and A.S.; resources, P.W., P.K. and A.S.; data curation, P.W., P.K. and A.S.; writing—original draft preparation, P.W., P.K., B.A.J.-F. and A.S.; writing—review and editing, P.W., P.K., B.A.J.-F., E.I.-G., J.W., P.A. and A.S.; visualization, A.S., P.K. and P.W.; supervision, A.S. and B.A.J.-F.; project administration, P.W. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Bioethical Committee of the Medical University of Warsaw, permission number: KB/278/2012.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alfadda, S.A. Current Evidence on Dental Implants Outcomes in Smokers and Nonsmokers: A Systematic Review and Meta-Analysis. J. Oral Implantol. 2018, 44, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Naseri, R.; Yaghini, J.; Feizi, A. Levels of smoking and dental implants failure: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47, 518–528. [Google Scholar] [CrossRef]
- Bezerra Ferreira, J.D.; Rodrigues, J.A.; Piattelli, A.; Iezzi, G.; Gehrke, S.A.; Shibli, J.A. The effect of cigarette smoking on early osseointegration of dental implants: A prospective controlled study. Clin. Oral Implants Res. 2016, 27, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Lassig, A.A.D.; Bechtold, J.E.; Lindgren, B.R.; Pisansky, A.; Itabiyi, A.; Yueh, B.; Joseph, A.M. Tobacco exposure and wound healing in head and neck surgical wounds. Laryngoscope 2018, 128, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, S.P.; Fontes, M.; Ribeiro, F.V.; Corrêa, M.G.; Nishii, D.; Cirano, F.R.; Casati, M.Z.; Casarin, R.C.V. Smoking habit modulates peri-implant microbiome: A case-control study. J. Periodontal. Res. 2018, 53, 983–991. [Google Scholar] [CrossRef]
- Monje, A.; Ravidà, A.; Wang, H.L.; Helms, J.A.; Brunski, J.B. Relationship Between Primary/Mechanical and Secondary/Biological Implant Stability. Int. J. Oral Maxillofac. Implants 2019, 34, 7–23. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Reasons for failures of oral implants. J. Oral Rehabil. 2014, 41, 443–476. [Google Scholar] [CrossRef]
- Marcello-Machado, R.M.; Faot, F.; Schuster, A.J.; Bielemann, A.M.; Nascimento, G.G.; Del Bel Cury, A.A. Mapping of inflammatory biomarkers in the peri-implant crevicular fluid before and after the occlusal loading of narrow diameter implants. Clin. Oral Investig. 2020, 24, 1311–1320. [Google Scholar] [CrossRef]
- Mesa, F.; Muñoz, R.; Noguerol, B.; de Dios Luna, J.; Galindo, P.; O’Valle, F. Multivariate study of factors influencing primary dental implant stability. Clin. Oral Implants Res. 2008, 19, 196–200. [Google Scholar] [CrossRef]
- Sanchez-Perez, A.; Villaescusa, M.; Caffesse, R. Tobacco as a Risk Factor for Survival of Dental Implants. J. Periodontol. 2007, 78, 351–359. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, J.; Jianghao, C.; Hong, T. Effect of Heavy Smoking on Dental Implants Placed in Male Patients Posterior Mandibles: A Prospective Clinical Study. J. Oral Implantol. 2016, 42, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhu, B.; Duan, Y.; Wang, X.; Li, D. The Effect of Smoking Behavior on Alveolar Bone Marrow Mesenchymal Stem Cells of Clinical Implant Patient. Biomed. Res. Int. 2018, 2018, 7672695. [Google Scholar] [CrossRef] [PubMed]
- Badenes, J.; Pallarés, A. Influence of smoking upon dental implant osseointegration: A radiofrequency analysis of 194 implants. J. Oral Implantol. 2020. [Google Scholar] [CrossRef]
- Sayardoust, S.; Omar, O.; Thomsen, P. Gene expression in peri-implant crevicular fluid of smokers and nonsmokers. 1. The early phase of osseointegration. Clin. Implant Dent. Relat. Res. 2017, 19, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Wychowanski, P.; Starzynska, A.; Woliński, J.; Kosieradzki, M.; Fiedor, P. New Surgical Technique Using Xenograft as a Microinvasive Method to Avoid Extensive Bone Reconstruction in Patients with Compromised General Health: Promising Surgical Methodology and First Clinical Results. Transplant. Proc. 2020, 52, 2244–2247. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrowicz, P.; Kusa-Podkańska, M.; Grabowska, K.; Kotuła, L.; Szkatuła-Łupina, A.; Wysokińska-Miszczuk, J. Extra-Sinus Zygomatic Implants to Avoid Chronic Sinusitis and Prosthetic Arch Malposition: 12 Years of Experience. J. Oral Implantol. 2019, 45, 73–78. [Google Scholar] [CrossRef]
- Wychowanski, P.; Woliński, J.; Morawiec, T.; Kownacki, P.; Starzynska, A.; Kosieradzki, M.; Fiedor, P. Preliminary Clinical Data and the Comparison of the Safety and Efficacy of Autogenous Bone Grafts Versus Xenograft Implantations in Vertical Bone Deficiencies Before Dental Implant Installation. Transplant. Proc. 2020, 52, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Wychowański, P.; Woliński, J.; Kacprzak, M.; Tomkiewicz, W.; Bartłomiej, I.; Szubińska-Lelonkiewicz, D.; Wojtowicz, A.; Nevis, M. Immediate Palatal Molar Implants: A Simple, Safe, Minimally Invasive Technique. Int. J. Periodontics Restor. Dent. 2017, 37, e297–e301. [Google Scholar]
- Lekholm, U.; Zarb, G.A. Patient selection and preparation. In Tissueintegrated Prostheses: Osseointegration in Clinical Dentistry; Brånemark, P.-I., Zarb, G.A., Albrektsson, T., Eds.; Quintessence: Chicago, IL, USA, 1985; pp. 199–209. [Google Scholar]
- Herrero-Climent, M.; Santos-García, R.; Jaramillo-Santos, R.; Romero-Ruiz, M.M.; Fernández-Palacin, A.; Lázaro-Calvo, P.; Bullón, P.; Ríos-Santos, J.V. Assessment of Osstell ISQ’s reliability for implant stability measurement: A cross-sectional clinical study. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e877–e882. [Google Scholar] [CrossRef]
- Sennerby, L.; Meredith, N. Implant stability measurements using resonance frequency analysis: Biological and biomechanical aspects and clinical implications. Periodontology 2000 2008, 47, 51–66. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Hart, C.N.; Halbritter, S.A.; Morton, D.; Buser, D. Early Loading of Nonsubmerged Titanium Implants with a Chemically Modified Sand-Blasted and Acid-Etched Surface: 6-Month Results of a Prospective Case Series Study in the Posterior Mandible Focusing on Peri-Implant Crestal Bone Changes and Implant Stability Quotient (ISQ) Values. Clin. Implant Dent. Relat. Res. 2009, 11, 338–347. [Google Scholar] [PubMed]
- Kokovic, V.; Jung, R.; Feloutzis, A.; Todorovic, V.; Jurisic, M.; Hämmerle, C. Immediate vs. early loading of SLA implants in the posterior mandible: 5-year results of randomized controlled clinical trial. Clin. Oral Implants Res. 2014, 25, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Lang, N.P.; Rangert, B. Validity and clinical significance of biomechanical testing of implant/bone interface. Clin. Oral Implants Res. 2006, 17 (Suppl. S2), 2–7. [Google Scholar] [CrossRef]
- Bohner, L.O.L.; Mukai, E.; Oderich, E.; Porporatti, A.L.; Pacheco-Pereira, C.; Tortamano, P.; De Luca Canto, G. Comparative analysis of imaging techniques for diagnostic accuracy of peri-implant bone defects: A meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Naveau, A.; Shinmyouzu, K.; Moore, C.; Avivi-Arber, L.; Jokerst, J.; Koka, S. Etiology and Measurement of Peri-Implant Crestal Bone Loss (CBL). J. Clin. Med. 2019, 8, 166. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Herrera-Briones, F.J.; Bassam, T.; Vallecillo-Capilla, M.F.; Reyes-Botella, C. Factors Affecting Dental Implant Stability Measured Using the Ostell Mentor Device: A Systematic Review. Implant Dent. 2015, 24, 565–577. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.A. Current interpretations of the osseointegrated response: Clinical significance. Int. J. Prosthodont. 1993, 6, 95–105. [Google Scholar]
- Szmukler-Moncler, S.; Salama, H.; Reingewirtz, Y.; Dubruille, J.H. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J. Biomed. Mater. Res. 1998, 43, 192–203. [Google Scholar] [CrossRef]
- Baltayan, S.; Pi-Anfruns, J.; Aghaloo, T.; Moy, P.K. The Predictive Value of Resonance Frequency Analysis Measurements in the Surgical Placement and Loading of Endosseous Implants. J. Oral Maxillofac. Surg. 2016, 74, 1145–1152. [Google Scholar] [CrossRef]
- Rodrigo, D.; Aracil, L.; Martin, C.; Sanz, M. Diagnosis of implant stability and its impact on implant survival: A prospective case series study. Clin. Oral Implants Res. 2010, 21, 255–261. [Google Scholar] [CrossRef]
- Degidi, M.; Daprile, G.; Piattelli, A.; Iezzi, G. Development of a New Implant Primary Stability Parameter: Insertion Torque Revisited. Clin. Implant Dent. Relat. Res. 2013, 15, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Clementini, M.; Rossetti, P.H.; Penarrocha, D.; Micarelli, C.; Bonachela, W.C.; Canullo, L. Systemic risk factors for peri-implant bone loss: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2014, 43, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Moraschini, V.; Barboza, E.S. Effect of autologous platelet concentrates for alveolar socket preservation: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Pagliani, L.; Sennerby, L.; Petersson, A.; Verrocchi, D.; Volpe, S.; Andersson, P. The relationship between resonance frequency analysis (RFA) and lateral displacement of dental implants: An in vitro study. J. Oral Rehabil. 2013, 40, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cai, M.; Yang, J.; Aldhohrah, T.; Wang, Y. Immediate versus early or conventional loading dental implants with fixed prostheses: A systematic review and meta-analysis of randomized controlled clinical trials. J. Prosthet. Dent. 2019, 122, 516–536. [Google Scholar] [CrossRef]
- Do, T.A.; Le, H.S.; Shen, Y.W.; Huang, H.L.; Fuh, L.J. Risk Factors related to Late Failure of Dental Implant-A Systematic Review of Recent Studies. Int. J. Environ. Res. Public Health 2020, 17, 3931. [Google Scholar] [CrossRef]
- Khalaila, W.; Nasser, M.; Ormianer, Z. Evaluation of the relationship between Periotest values, marginal bone loss, and stability of single dental implants: A 3-year prospective study. J. Prosthet. Dent. 2020, 124, 183–188. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).