Abstract
Background: Most studies on air pollution (AP) exposure have focused on adverse health effects of particulate matter (PM). Less well-studied are the actions of volatile organic compounds (VOCs) not retained in PM collections. These studies quantified chemical and biological properties of both PM2.5 and VOCs. Methods: Samples were collected near the Port of Los Angeles (Long Beach, LB), railroads (Commerce, CM), and a pollution-trapping topography-site (San Bernardino, SB). Quantitative assays were conducted: (1) chemical—prooxidant and electrophile content, (2) biological—tumor necrosis factor-α (TNF-α) and heme oxygenase-1 (HO-1) expression (3), VOC modulation of PM effects and (4), activation of the antioxidant response element (ARE) using murine RAW 264.7 macrophages. Results: SB site samples were the most potent in the chemical and biological assays, followed by a CM railroad site. Only PM2.5 exhibited significant proinflammatory responses. VOCs were more potent than PM2.5 in generating anti-inflammatory responses; further, VOC pretreatment reduced PM-associated TNF-α expression. VOCs significantly increased ARE activation compared to their corresponding PM2.5 which remained at background levels. Conclusion: Ambient VOCs are major contributors to adaptive responses that can modulate PM effects, in vitro, and, as such, need to be included in comprehensive assessments of AP.
1. Introduction
Epidemiological and clinical studies have firmly established associations between ambient air pollution (AP) levels and adverse health effects (see for examples, [1,2,3,4]). To advance our understanding of the relationships between AP exposure and disease processes, chemical and biological studies are needed to identify and characterize underlying molecular and cellular mechanisms. An essential element of those studies is the use of quantitative methodologies that allow for the comparison of results across other AP studies conducted in different local environments and geographical regions.
Our prior studies have been focused on characterizing reactive chemicals in AP that interact with cellular targets to elicit responses such as inflammation. In ambient air, these chemicals are differentially distributed between particle and vapor phases as obtained in AP sample collections. The particulate matter phase (typically PM2.5) contains mostly prooxidants such as redox active metals together with higher molecular weight quinones and humic-like substances (HULIS) [5,6] together with inorganic electrophiles such as arsenic and zinc. The vapor phase contains mostly volatile organic compounds (VOCs) that include quinones [7,8,9] and electrophilic carbonyls [10]. At the functional level, prooxidants catalyze the reduction of oxygen to reactive oxygen species and electrophiles react with nucleophilic functions, such as thiolate and amino groups, to form covalent bonds (see Graphical Abstract).
Surprisingly, in recent years, research on biological assessments of AP has largely focused only on the consequences of PM exposure without addressing the potential biological contributions of corresponding VOCs. To address this deficiency, the present studies were designed to generate quantitative data on both PM2.5 and VOCs of ambient AP samples for evaluating their distinct chemical reactivities and biological effect profiles.
In AP biological studies, the consequences of chemical exposure upon cellular components involves induction of oxidative stress and/or protein modifications that at low levels activate cytoprotective mechanisms but at higher levels initiate proinflammatory responses [11]. To understand the relationship between levels of reactive chemicals and this continuum of cellular responses, in this study we determined in PM2.5 and VOC fractions of ambient AP samples the prooxidant content based on their ability to transfer electrons from dithiothreitol to oxygen [12] and electrophile content, based on their ability to inactivate glyceraldehyde 3-phosphate dehydrogenase (GAPDH) [13]. The same fractions were then subjected to cellular assays designed to determine relative potencies of their biological responses.
The AP samples for this study were collected at three regions that neighbor railyards (Long Beach (LB), Commerce (CM), and San Bernardino (SB)) in the Los Angeles Basin with the objective of assessing regional differences in the chemical and biological actions of AP [14]. The chemical study results show distinct distributions of reactive chemicals: prooxidants in the PM2.5 and the electrophiles in the VOCs. The biological study results show that the samples with high reactive chemical content were the most potent in eliciting biological responses: PM2.5 promoting an inflammatory response and VOCs promoting an anti-inflammatory response.
Additionally, a second set of AP samples were collected at CM at sites 0.03–1 mile from its railyards to assess their chemical and biological actions as a function of proximity to the emission source. Those CM local samples exhibited profiles similar to the three regions’ data, i.e., high chemical reactivity was associated with potent biological effects; furthermore, distance-dependent chemical and biological reactivities were also observed.
The pro- and anti-inflammatory biological responses to the PM2.5 and VOC samples raised the question of whether additive or antagonistic interactions occur following ambient AP exposures (i.e., PM2.5 + VOCs). We could not address this issue directly insofar as the concentrations of PM2.5 and VOC extracts precluded simultaneous exposure studies, however, we did conduct a two phase exposure in which RAW 264.7 macrophages were pre-exposed to VOCs, followed 24 h later by exposure to PM2.5. The results show that pre-exposure to VOCs reduces the magnitude of an inflammatory response to subsequent PM2.5. Based on prior studies showing that compounds contained in VOCs can activate the Nrf2-ARE pathway [15], the current samples were similarly tested. VOCs, but not PM2.5, activated this pathway, suggesting a cellular mechanism for their anti-inflammatory actions. Collectively, our in vitro studies show that VOCs contain reactive chemicals capable of inducing significant anti-inflammatory effects and, as such, need to be included with PM analyses to enable comprehensive studies of ambient AP.
2. Materials and Methods
2.1. Materials
Rabbit muscle glyceraldehyde-3-phosphate dehydrogenase (GAPDH), nicotinamide adenine dinucleotide (NAD+), ethylenediaminetetraacetic acid (EDTA), glyceraldehyde-3-phosphate (GAP), dithiothreitol (DTT), 5,5′-dithiobisbis-(2-dinitrobenzoic acid (DTNB), and diethylenetriaminepentaacetic acid (DTPA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other reagents were of the highest grade available and purchased from Fisher Scientific (Pittsburgh, PA, USA). Murine macrophage cell line RAW 264.7 cells were purchased from American Type Cell Culture (Manassas, VA, USA). Dulbecco’s modified Eagle medium (DMEM) and penicillin/streptomycin were purchased from Life Technologies (Carlsbad, CA, USA). Fetal bovine serum was purchased from Gemini Bio-Products (Sacramento, CA, USA). The ELISA kits for TNF- α were purchased from BD Biosciences Pharmingen (San Diego, CA, USA) and the kits for HO-1 were purchased from Enzo Life Sciences (Farmington, NY, USA). Lipofectamine LTX transfection reagent was obtained from Life Technologies (Carlsbad, CA, USA).
2.2. Sample Collection and Extraction
2.2.1. PM2.5
Medium-volume samplers (Tish, Cleves, OH) were located in the selected locations; PM2.5 and vapor samples were collected continuously for 48 h as one time collections. The LB and SB samples were collected in early summer (June). The CM samples were collected continuously over a 5 d period in late spring (May). Teflon-coated glass fiber filters (Pall Corp, East Hills, NY) were used for PM2.5 collection and XAD-4 resin beds (Acros, Thermo Fisher Scientific) for the vapor-phase. Sampling details and matrix cleaning procedures have been previously published (16). Estimates of the volume equivalent for each vapor sample analyzed were based on the air volume collected divided by the fraction of the total extract used in each analysis. Aqueous suspensions of PM2.5 samples were prepared by sonicating filter punches in cell culture water for 20 min and the corresponding volume of air (m3/cm2) calculated to normalize the results. As the mass of the PM2.5 on the filter was not measured, the volume equivalent of air was used to describe the final concentration of the particles in the aqueous suspension, which was 2.2–6.0 m3/mL.
2.2.2. VOCs
XAD-4 resin beds containing the trapped vapor phase organic components corresponding to each particle sample were extracted by sonication (30 min) with dichloromethane. The suspension was filtered through a 0.45 µm nylon filter (Millipore, Billerica, Massachusetts), the volume reduced, and solvent evaporated into a known volume of dimethyl sulfoxide (DMSO), so the concentration for analysis could be expressed as m3 per mL of DMSO. The final concentration of the organic extract was approximately 300 m3/mL of DMSO. Blank XAD-4 resin extracts were prepared as described previously and used as controls [16]. Highly polar compounds such as those with multiple hydroxyl moieties that are associated with the vapor phase would not be extracted with dichloromethane.
2.3. Chemical Assays
2.3.1. DTT Assay
This assay measured the prooxidant content of the sample from its ability to transfer electrons from DTT to oxygen [12,13,14,15,16,17]. In the procedure, aliquots of vapor-phase and PM2.5 water suspensions were incubated with DTT (Sigma Chemical Co., MO, USA) for times varying from 10 to 30 min. The reaction was quenched at specific times and after addition of DTNB to the complex with the remaining DTT, the absorbance at 412 nm was measured. Rates were calculated averaging duplicate runs, and were blank-corrected. The units were DTT nmol consumed per min per m3, with 95% confidence intervals derived from regression analysis of rates.
2.3.2. GAPDH Assay
Electrophilic reactivity was measured from the sample’s ability to inhibit or inactivate the thiolate enzyme GAPDH, through covalent bonding [13]. In brief, a mixture of 1 unit of rabbit GAPDH was incubated with aliquots of the organic extracts of vapors or water suspensions of particles under argon gas at 25 °C for 120 min. The reaction was then quenched by adding an equal volume of cold DTT solution; GAPDH activity, based on the rate of nicotinamide adenine dinucleotide (NADH) formation, was measured by its absorption at 340 nm. The ability to inactivate the enzyme was expressed in equivalents of N-ethylmaleimide (NEM), the standard electrophile, which was included in each assay as a control. Samples were run in triplicate and values reported as averages ± SEM. The assay provided a measure for electrophile content that was based on structures capable of interacting with the catalytic center of the enzyme and provided a quantitative estimate of the electrophilic content which could be used in comparison studies. The units used were the equivalents of NEM per m3.
2.4. Cell Culture and Treatment
2.4.1. Cell Viability
Cell viability was determined by the 3-(4,5-dimethylthiazol-2yl)-2,5-triphenyl tetrazolium bromide (MTT) tetrazolium salt colorimetric assay [18]. A 5 mg/mL MTT solution was prepared in phosphate buffered saline and sterilized by filtration through a Steriflip. RAW264.7 cells were exposed to particle and vapor samples in 96-well plates for 16 h then treated with 10 µL of 5 mg/mL MTT for 2 h at 37 °C. The medium was removed and 100 µL DMSO was added to dissolve the formazan. Absorbance was measured at 540 nm on a Biotek Synergy 2 Multi-mode Microplate Reader. To determine suitable sample concentration ranges for these experiments, air sample toxicity was assessed by RAW 264.7 macrophages exposure to concentrations of 1 m3/mL for 16 h. The loss of cells was normalized to that caused by a blank filter extract for the PM2.5 and a blank XAD resin extract for the vapors. The PM2.5 loss was 113.6% ± 3.9% (SEM for N = 8) and that for XAD was 94.7% ± 3.0% of their respective controls. Based on these results, all subsequent cell experiments used concentrations of 1 m3/mL or less.
2.4.2. Cell Exposure
Murine RAW 264.7 cells were cultured in DMEM, supplemented by 1% penicillin-streptomycin and 10% FBS as described by Li et al. [11] with slight modifications. Cells were exposed to three concentrations (0.1 to 2.0 m3 air equivalent/mL) of PM2.5 or VOC extracts in duplicate for 16 h, after which the medium and cells were separated and the cells subjected to lysis to obtain a cell extract. The cell extracts were used in the ELISA assays for HO-1 and the medium used to assay TNF-α. The results were analyzed by linear regression procedures (Graph Pad Prism, (San Diego CA, USA) to determine (a) concentration dependency of the response, and (b), if the slope was significant, its value, which is a measure of the potency of the sample.
2.5. Two Phase Study VOC Pretreatment/PM Exposure
In phase 1, cells were exposed to the VOC at a single concentration in triplicate (1 m3/mL) together with the relevant controls for 24 h. In phase 2, the medium was removed and replaced with fresh medium containing the challenge agent, or PM2.5, also at 1 m3/mL for 16 h. Cells and media were then processed for HO-1 and TNF-α analyses
2.5.1. ELISA Assays
The ELISA assays were performed following instructions provided by the manufacturers (HO-1; Enzo Life Sciences; TNF-α, BD Pharmingen). The results reported are the differences between the control and the experimental conditions. Values for HO-1 (ng/mg cell protein) and TNF-α (pg/mg cell protein) were normalized to cell protein.
2.5.2. ARE/EpRE Activation
DNA transfections were performed with Lipofectamine LTX transfection reagent (Life Technologies, Carlsbad, CA) following the manufacturer’s instructions performing cell culture in 12-well plates. ARE-luciferase cDNA (1 μg/well) and pRL-TK cDNA (0.1 μg/well) or transfection reagent (2 μL/well) were mixed with serum-free media. Before addition to the cells, the DNA solution and transfection reagent solution were mixed together and incubated for 20 min at room temperature to allow the formation of complexes. The complexes were mixed with the culture media and incubated for 24 h to allow transfection. After transfection, the cells were exposed to the samples as described above and luciferase activity measured in cellular extracts according to the manufacturer’s instructions (Dual-Luciferase reporter assay system; Promega, WI, USA) with a multi-mode microplate reader (Synergy 2, Bio-Tek, Winooski, VT, USA).
2.6. Collection Sites
The ambient air samples for this study were collected in three communities with railyards in the Los Angeles Basin: Commerce (CM), Long Beach (LB) and San Bernardino (SB) (Figure 1). CM, with two railyards, is located in the midtown area of Los Angeles. LB is southwest of CM and neighbors the Pacific Ocean and the Port of Los Angeles, which is the largest container port in the United States as measured by container volume and cargo value. Its emission sources would include both local railyards and the Port. The SB site is located in the eastern end of the Basin, approximately 80 miles from the coast and represents a receptor site that receives air parcels that have been subjected to modifications by photochemical and chemical reactions as they move from east to west across the Basin [19], together with emissions from a SB railyard. We previously observed that as the air mass moves east, a trend toward higher levels of 9,10-phenanthroquinone was found in the air samples, consistent with its formation from phenanthrene by atmospheric processes [7].
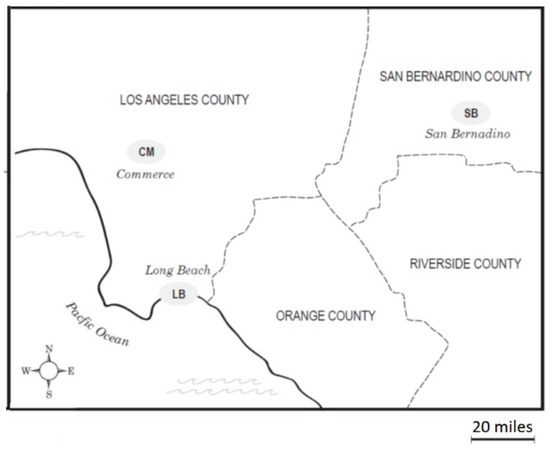
Figure 1.
Map of the Los Angeles Basin with locations of the air collection sites: Long Beach (LB), Commerce (CM) and San Bernardino (SB). The Los Angeles Basin contains a coastal area bordered by the San Gabriel Mountains located in northern Los Angeles County and western San Bernardino County.
The second set of collections focused on CM at different distances nearby its railyards to assess ambient air properties as a function of distance from emission sources (Figure 2).
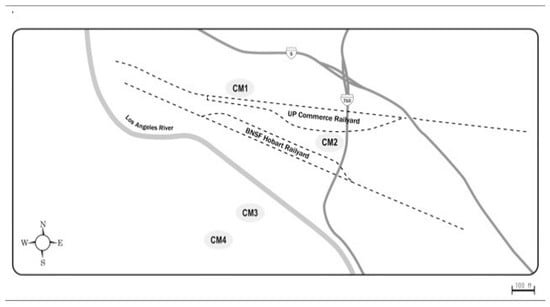
Figure 2.
Map of the Commerce railyards with locations of air collection sites.
The sites, CM1 and CM2, were closest to the CM-Union Pacific railyard and downwind from the Burlington Northern and Santa Fe (BNSF) Hobart yard. Sites CM3 and CM4 were both upwind of the BNSF yard, but CM3 was closer to the yards and to a diesel truck processing center. CM4 was considered as a background site, minimally directly impacted by the railyard activities. It should be pointed out that as the samples were collected continuously 24-h/d for 5-d, changes in air movement during that period could have minimized differences due to changes from prevailing onshore to infrequent offshore winds. The distances from the railyards for the CM collections are summarized in Table 1.

Table 1.
Location of collection sites at Commerce.
2.7. Biological Reactivities
Two markers were used to assess cellular responses by the RAW 264.7 macrophage cell line: TNF-α as a proinflammatory response [20,21] and HO-1 as an anti-inflammatory response [22]. Cellular responses were determined at three concentrations (0.1, 0.5 and 1.0 m3/mL) to ensure that the responses compared were linear over the concentrations used. The results were analyzed by linear regression to obtain slopes of the concentration vs. response curves. The results provide an assessment of the concentration-dependency of the response with the slope providing a quantitative assessment of potency in stimulating expression of the target markers, TNF-α and HO-1 (see Supplementary Materials).
2.8. Computational Procedures
All computations were performed with GraphPad Prism 8.12 (San Diego, CA, USA). Linear regression procedures were used to generate the best-fit values, the standard error of the slope and its 95% confidence intervals together with an assessment of the significance of the slope provided by its p value. These procedures generated Pearson correlation coefficients and their respective p values.
3. Results
3.1. Chemical and Biological Reactivities for Basin Sites
The PM and VOC samples from SB were the most reactive compared to those collected at CM and LB. For all three regions, the prooxidant content was mostly associated with the PM2.5 fraction (Figure 3).
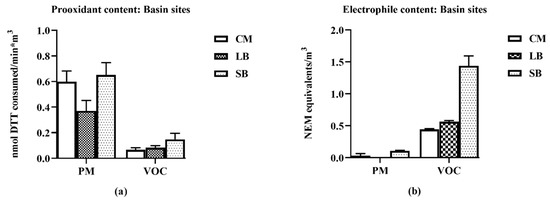
Figure 3.
Chemical properties of particulate matter (PM) and volatile organic compound (VOC) air samples collected at CM, LB and SB. For each region’s PM and VOC samples, nmol dithiothreitol (DTT) consumed (prooxidant content (a)) and N-ethylmaleimide (NEM) equivalents derived from a glyceraldehyde 3-phosphate dehydrogenase (GAPDH)-inhibition assay (electrophile content (b)) were measured. The VOCs represent the dichloromethane extracts of XAD resin traps of the vapor phases. Error bars indicate 95% CI (n = 3) for DTT results; SEM (n = 3) for the NEM results (values of the CIs are detailed in supplemental data—Table S1).
In contrast, electrophile content was mostly associated with the VOC fractions; electrophile content of the PM2.5 was close to background levels. SB VOC electrophile content was approximately three-fold higher than those for CM, LB. All PM2.5 samples increased TNF-α expression, with SB being 40-fold more potent than CM and LB (Figure 4). Increases in TNF-α expression were not detected in the VOC samples, i.e., they did not exhibit a concentration-dependent response (data not shown).
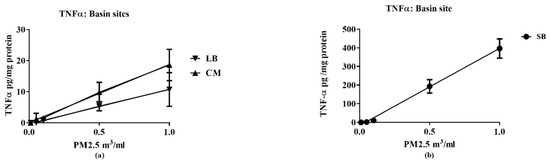
Figure 4.
Relative potencies of air samples from CM, LB (a) and SB (b) to increase TNF-α in RAW 264.7 macrophages. TNF-α expression in the media was measured following exposure to the indicated PM 2.5 concentrations for 16 h. The solid lines represent the best line fit following linear regression analysis of the results (n = 3 for each data point) with potencies indicated by the slopes (values of the regression analysis are detailed in supplemental data—Table S4).
All VOC samples increased HO-1 expression, with SB being three-fold more potent than CM, LB, (Figure 5). The SB PM also increased HO-1 expression but the response was markedly lower than its VOC; concentration-dependent increases in HO-1 expression for CM PM2.5 and LB PM2.5 were not detected.
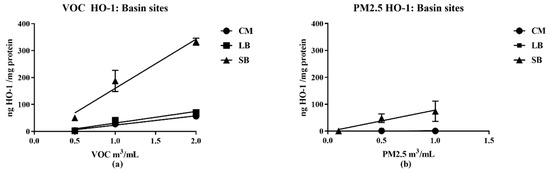
Figure 5.
Relative potencies of VOC and PM 2.5 air samples from CM, LB (a) and SB (b) to increase HO-1 expression in RAW 264.7 macrophages. Cell extracts were measured following exposure to the indicated concentrations of either VOC or its corresponding PM for 16 h. The lines represent the best line fit following linear regression analysis of the results (n = 3 for each data point) with potencies indicated by the slopes (values of the regression analysis are detailed in supplemental data—Table S5).
3.2. Chemical and Biological Reactivities: CM Railroad Sites
The collection sites were located at different distances (0.03 to 1.3 mi from the railyards (Table 1) identified as CM1 to CM4 in increasing distance. The PM2.5 prooxidant and VOC electrophile content decreased as a function of distance from the railroad emission source (Figure 2) with the exception of CM2 which was adjacent to two railyards.
Prooxidant content was mostly associated with PM2.5 samples while electrophile content was mostly associated with the VOC samples. VOC prooxidant and PM2.5 electrophile contents remained close to background levels for all samples (Figure 6).
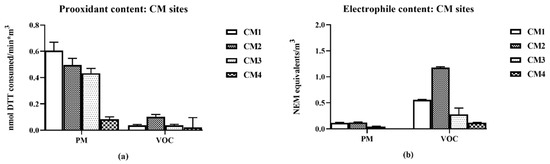
Figure 6.
Chemical properties of PM and VOC air samples collected near to the CM railroad area (CM1—closest, CM4 most distant). For each PM and VOC local sample, the nmol DTT consumed (prooxidant content (a)) and NEM equivalents (electrophile content (b)) were measured. Error bars indicate 95% CI for DTT (n = 3) results and SEM (n = 3) for the GAPDH results; analysis methods as described in Figure 4, (values of the CIs are detailed in supplemental data—Tables S2 and S3).
The biological reactivities paralleled the profiles of the regional samples. Further, PM2.5 TNF-α and VOC HO-1 expression showed a ‘distance’ pattern, with CM2 being the most potent and CM4 (most distant) being least potent; PM2.5 HO-1 expression remained close to background levels for all samples (Figure 7).

Figure 7.
TNF-α expression (a) and HO-1 expression (b,c) in RAW 264.7 macrophages using air samples collected nearby CM railroads (CM1—closest, CM4 most distant). Macrophages were incubated with three concentrations of VOC or PM for 16 h. The lines represent the best line fit following linear regression analysis of the results (n = 3 for each data point) with potencies indicated by the slopes (values of the regression are detailed in supplemental data—Tables S4 and S5).
3.3. SB VOC Pretreatment Effects on SB PM2.5 2.5 Expression of TNF-α and HO-1 in RAW 264.7 Macrophages
The anti-inflammatory properties of the VOC suggested that VOC pretreatment may affect the attenuation of the pro-inflammatory effects of a subsequent PM2.5 challenge. We selected the most potent samples—SB VOC for the pretreatment and SB PM2.5 for the challenge. A sequential exposure experiment was performed in which cells were first exposed to the SB VOC for 24 h. The media was then replaced with fresh media containing control filter extracts or suspensions of the SB PM2.5 and the exposure continued for 16 h. Macrophages and media were analyzed for HO-1 and TNF-α content, respectively (Figure 8).
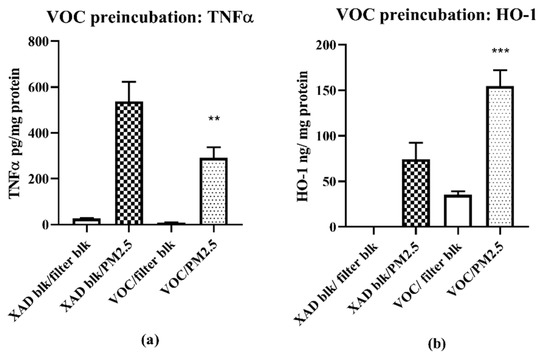
Figure 8.
SB VOC pretreatment effects on SB PM 2.5 expression of TNF-α (a) and HO-1 (b) in RAW 264.7 macrophages. Cells were preincubated with VOC (1 m3/mL) for 24 h followed by incubation with PM 2.5 (1 m3/mL) for 16 h; TNF-α—XAD Blk/PM 2.5 vs. VOC/PM 2.5, n = 4; p** < 0.005; HO-1—XAD Blk/PM 2.5 vs. VOC/PM 2.5, n = 4; *** p < 0.001.
The reason for the two-stage exposure was the limitation in the volume of sample solutions that could be added to the plate cells while maintaining the incubation conditions (see Methods for details). The results show a 54% decrease in the TNF-α response to the PM2.5 challenge following pretreatment to SB VOCs at 1 m3/mL, n = 4; p**** < 0.005, together with a significant 200% increase in HO-1 expression (Blank XAD/SB vs. SB VOC/SB 2.5; (n = 4; p < 0.001).
3.4. Activation of the antioxidant Response Element (ARE) in RAW 264.7 Macrophages by Air Samples from CM, LB and SB
In prior studies, we measured an HO-1 response in VOC samples collected at Riverside CA (a sample site nearby SB). Those samples also showed an electrophilic effect on the ARE [23]. Accordingly, we evaluated in this study the three regional samples for their potential to activate the ARE. The results show that all VOC samples significantly increased ARE activation 1.5- to three-fold compared to their corresponding PM2.5 samples which remained at background levels. The SB VOC sample was approximately two-fold more potent than the LB and CM samples (Figure 9).
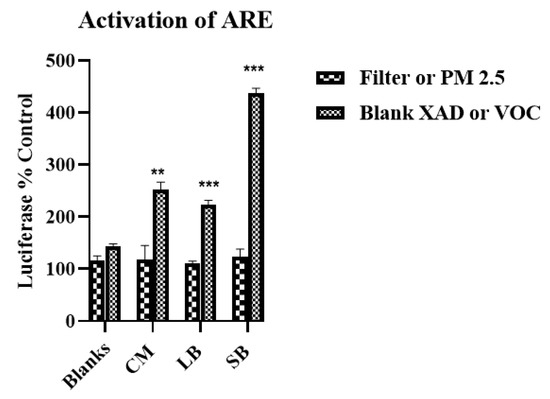
Figure 9.
Activation of the antioxidant response element (ARE) in RAW 264.7 macrophages by air samples from CM, LB and SB. Cells transfected with ARE-luciferase cDNA were exposed to either PM2.5 and VOC from CM, LB, SB at 1 m3/mL for 16 h and ARE activation assessed; (see Methods for ARE procedures). Each value is the mean ± SD, n = 4/sample site; site values normalized to corresponding blanks; CM VOC vs. CM PM2.5, ** p < 0.005; LB VOC vs. LB PM 2.5, SB VOC vs. SB PM2.5, *** p < 0.00001.
4. Discussion
In our prior AP studies on PM2.5 and VOCs, we characterized chemical species that can participate in two reactions associated with acute toxicity, (1) prooxidants that catalyze the formation of hydrogen peroxide and hydroxyl radical, and (2) electrophiles that form covalent bonds with nucleophilic groups such as amino and thiolate found in proteins. Furthermore, we showed that VOC electrophiles associated with AP can activate cellular signaling pathways relevant to adverse health effects [23]. However, VOC biological assessment has received minimal attention in conjunction with PM research despite its apparent contributions to AP toxicology, with the exception of adductomics studies by Rappaport and his associates (e.g., [24]).
For this study, analytical methods we previously developed and validated [14] were used to characterize chemical and biological properties of SB, CM and LB AP samples. The samples were obtained from (1) an aqueous suspension of the particulates, PM2.5, and (2) an organic extract of the VOCs trapped by polystyrene resins placed downstream of the PM2.5 filters which have been shown to contain the majority of the total electrophiles present in ambient air [14]. Based on our previous analyses of seasonal effects on AP properties [14,16], the samples for this study collected in late spring/early summer were likely have relatively higher prooxidant and VOC electrophile content compared to winter collections. Nonetheless, despite such quantitative differences, predominantly PM—TNF-α—and VOC electrophile responses were consistently observed for both seasonal collections. The quantitative nature of the regional data allowed for multiple comparisons between different parameters of AP chemical reactivities in terms of pro- and anti-inflammatory properties, and their distribution between PM 2.5 and VOC phases. Prooxidant reactivity and proinflammatory responses (TNF-α increases) were predominantly associated with PM samples while electrophile reactivity and anti-inflammatory responses (HO-1 increases and ARE activation) were predominantly associated with VOCs. Future studies, both in vitro and in vivo, are needed to determine whether the interactions of these responses are synergistic or antagonistic following concurrent exposure of PM and VOCs. Presently, evidence for a potential interaction is suggested from the results of the two-phase studies we conducted with RAW 264.7 macrophages in which the anti-inflammatory effects of the VOC suppressed the pro-inflammatory effects of the PM2.5.
The following analysis illustrates how the data we generated can be used to explain differences in the response profiles of the two most reactive and potent samples, SB and CM2.
Of particular note, the SB and CM2 samples have comparable chemical reactivities in the DTT assay (0 65, 0.50; Table 2), but the SB pro- and anti-inflammatory potencies as derived from the biological assays were significantly greater (slope values from linear regression analysis; Table 2B). Namely, the SB TNF-α and HO-1 responses were 14 and four times more potent, respectively, than those generated by CM2. We interpret these differences in terms of the chemical content in the samples being collected at different regions of the LA Basin. The CM2 sample consists mostly of railyard traffic emissions, whereas the SB sample has been subjected to atmospheric chemical reactions on the air mass as it moves approximately 80 miles across the Basin. These reactions convert, among other compounds, polynuclear aromatic hydrocarbons to their corresponding quinones as exemplified by 9,10-phenanthroquinone generation (PQ) [7] a potent biological prooxidant associated with PM [25]. Relatively higher levels of such organic prooxidants in the SB PM2.5 sample are suggested by the DTT assay data (Table 2B) that show a larger fraction of the SB PM activity not inhibited by metal chelation (i.e., DTPA-insensitive), in support of this phenomenon of atmospheric formation of organic prooxidants. Analogously, the generation of organic electrophiles such as 1,4-benzoquinone (BQ) likely contribute to the more potent SB anti-inflammatory HO-1 response in the VOC fraction (Table 3). Based on these considerations, we suggest that the higher pro- and anti-inflammatory actions of the SB sample compared to that of CM2 are due to its higher content of organic reactants generated by photochemical processes.

Table 2.
Prooxidant content of SB and CM2 samples.

Table 3.
Biological properties of SB and CM2 samples.
A second consideration in determining relative biological potencies of PM and VOCs is their cellular access upon exposure. PM/metal ions’ entry into cells is regulated by endocytosis and transport processes [6,26], whereas quinones such as PQ and BQ are neutral and non-polar compounds that can readily penetrate membranes to promote oxidative stress-related reactions [27]. These biological differences underscore the complex chemical composition of PM, which limits the interpretive value of a general chemical assay such as the DTT assay and necessitate inclusion of parallel biological assays for more definitive evaluation of PM reactivities.
Direct extrapolation of these results to potential toxicological effects in humans, i.e., PM-induced oxidative stress and VOC-induced covalent adducts, remains to be established. In one of the very few direct comparisons of ‘whole’ diesel exhaust (DE) and VOC components, Campen et al. [28] compared the cardiovascular effects of these two samples in mice and observed major effects consistent with myocardial ischemia, attributable to VOC components. These VOC effects were also seen in vitro using isolated blood vessels. Although some of the chemical components were determined, they did not, however, assay for redox-active species or electrophiles. A second relevant study examined the role of electrophiles that included 1,2-naphthoquinone, a component of VOCs [25] in the actions of DE on mouse lungs [29]. In an intact lung model and in multiple cultured human lung cell models, they showed activation of transient receptor potential ankyrin-1, a pro-inflammatory mediator, on airway C-fibers by a variety of qualitatively and quantitatively different DE particles. These studies demonstrated the actions of VOCs underlying the toxicity of emissions and emphasized the need for further studies that model human exposure conditions.
5. Conclusions
We have shown that quantitative assessments of chemical and biological properties of ambient PM2.5 and VOCs can be used effectively to characterize, compare and contrast AP across different geographical regions, to account for effects of atmospheric modifications on air mass, and to evaluate exposure proximity to an emission source. Of major significance is the consistent characterization of VOCs in terms of their electrophilic chemical reactivity and biological modulation of HO-1 expression and ARE activation. Collectively, these results have human health implications. VOCs, being non-polar volatile species, will readily enter the lungs and penetrate membranes to access all body compartments and thus can potentially contribute to multiple organ effects. Furthermore, electrophilic properties of VOCs can affect the long-term inactivation of protein function resulting from their covalent binding with tissue nucleophiles. Consequently, the effects of low level VOC exposure in AP can accumulate over time and contribute to chronic adverse health effects.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/10/9/3245/s1, Table S1 Basin prooxidant and electrophile content of PM2.5 and VOC; Table S2 CM1 -CM2 prooxidant and electrophile content of PM2.5 and VOC; Table S3 CM3–CM4 prooxidant and electrophile content of PM2.5 and VOC; Table S4. RAW cell TNF-α response to PM2.5; Table S5 RAW cell HO-1 responses to PM2.5 and VOC.
Author Contributions
Conceptualization, A.K.C., J.R.F., W.P.M.; methodology, Y.S., D.A.S., E.D.S., A.E.-F., A.L.N.G., E.M.S.; software, A.K.C.; validation, A.K.C., W.P.M., D.A.S.; writing—original draft preparation, A.K.C., Y.S., W.P.M.; writing—review and editing A.K.C., W.P.M., Y.S.; supervision, A.K.C.; project administration, A.K.C., J.R.F.; funding acquisition, J.R.F., A.K.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the SOUTH COAST AIR QUALITY MANAGEMENT (AQMD) under awards 12865 and 20090799. YS was supported by a grant, “Institutional Program for Young Researcher Overseas Visits” from the Japan Society for the Promotion of Science. EMS was supported by NIH Research Grant # D43TW000623 funded by the Fogarty International Center.
Conflicts of Interest
The authors declare no conflict of interest. The opinions, findings, conclusions, and recommendations are those of the authors and do not necessarily represent the views of AQMD. AQMD, its officers, employees, contractors, and subcontractors make no warranty, expressed or implied, and assume no legal liability for the information in this report. AQMD has not approved or disapproved this report, nor has AQMD passed upon the accuracy or adequacy of the information contained herein.
References
- Berhane, K.; Chang, C.C.; McConnell, R.; Gauderman, W.J.; Avol, E. Association of Changes in Air Quality With Bronchitic Symptoms in Children in California, 1993–2012. JAMA 2016, 315, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Cassee, F.R.; Heroux, M.E.; Gerlofs-Nijland, M.E.; Kelly, F.J. Particulate matter beyond mass: Recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal. Toxicol. 2013, 25, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Delfino, R.J.; Wu, J.; Tjoa, T.; Gullesserian, S.K.; Nickerson, B.; Gillen, D.L. Asthma morbidity and ambient air pollution: Effect modification by residential traffic-related air pollution. Epidemiology 2014, 25, 48–57. [Google Scholar] [CrossRef] [PubMed]
- McConnell, R.; Wu, W.; Berhane, K.; Liu, F.; Verma, G. Inflammatory Cytokine Response to Ambient Particles Varies due to Field Collection. Am. J. Respir. Cell Mol. Biol. 2013, 48, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Soukup, J.M.; Madden, M.C. The toxicology of air pollution predicts its epidemiology. Inhal. Toxicol. 2018, 30, 327–334. [Google Scholar] [CrossRef]
- Ghio, A.J.; Madden, M.C. Human lung injury following exposure to humic substances and humic-like substances. Environ. Geochem. Health 2018, 40, 571–581. [Google Scholar] [CrossRef]
- Eiguren-Fernandez, A.; Miguel, A.; Lu, R.; Purvis, K.; Grant, B. Atmospheric formation of 9,10-phenanthroquinone in the Los Angeles air basin. Atmos. Environ. 2008, 42, 2312–2319. [Google Scholar] [CrossRef]
- Cho, A.; Di Stefano, E.; Ying, Y.; Rodriguez, C.E.; Schmitz, D. Determination of Four Quinones in Diesel Exhaust Particles, SRM 1649a and Atmospheric PM2.5. Aerosol Sci. Technol. 2004, 38, 68–81. [Google Scholar] [CrossRef]
- Jakober, C.A.; Riddle, S.G.; Robert, M.A.; Destaillats, H.; Charles, M.J. Quinone emissions from gasoline and diesel motor vehicles. Environ. Sci. Technol. 2007, 41, 4548–4554. [Google Scholar] [CrossRef]
- Jakober, C.A.; Robert, M.A.; Riddle, S.G.; Destaillats, H.; Charles, M.J. Carbonyl emissions from gasoline and diesel motor vehicles. Environ. Sci. Technol. 2008, 42, 4697–4703. [Google Scholar] [CrossRef]
- Li, N.; Kim, S.; Wang, M.; Froines, J.; Sioutas, C.; Nel, A. Use of a stratified oxidative stress model to study the biological effects of ambient concentrated and diesel exhaust particulate matter. Inhal. Toxicol. 2002, 14, 459–486. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.K.; Sioutas, C.; Miguel, A.H.; Kumagai, Y.; Schmitz, D.A. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ. Res. 2005, 99, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Shinyashiki, M.; Rodriguez, C.E.; Di Stefano, E.W.; Sioutas, C.; Delfino, R.J. On the interaction between glyceraldehyde-3-phosphate dehydrogenase and airborne particles: Evidence for electrophilic species. Atmos. Environ. 2008, 42, 517–529. [Google Scholar] [CrossRef]
- Eiguren-Fernandez, A.; Di Stefano, E.; Schmitz, D.A.; Guarieiro, A.L.N.; Salinas, E.M. Chemical reactivities of ambient air samples in three Southern California communities. J. Air. Waste Manag. Assoc. 2015, 65, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, Y.; Nakajima, S.; Eiguren-Fernandez, A.; Stefano, E.D.; Schmitz, D.A. Ambient vapor samples activate the Nrf2-ARE pathway in human bronchial epithelial BEAS-2B cells. Environ. Toxicol. 2013, 25, 1222–1230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eiguren-Fernandez, A.; Miguel, A.H.; Froines, J.R.; Thurairatnam, S.; Avol, E.L. Seasonal and Spatial Variation of Polycyclic Aromatic Hydrocarbons in Vapor-Phase and PM2.5 in Southern California Urban and Rural Communities. Aerosol Sci. Technol. 2004, 38, 447–455. [Google Scholar] [CrossRef]
- Kumagai, Y.; Koide, S.; Taguchi, K.; Endo, A.; Nakai, Y. Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chem. Res. Toxicol. 2002, 15, 483–489. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Ito, T.; Bekki, K.; Fujitani, Y.; Hirano, S. The toxicological analysis of secondary organic aerosol in human lung epithelial cells and macrophages. Environ. Sci. Pollut. Res. Int. 2016, 26, 22747–22755. [Google Scholar] [CrossRef]
- Sama, P.; Long, T.C.; Hester, S.; Tajuba, J.; Parker, J. The cellular and genomic response of an immortalized microglia cell line (BV2) to concentrated ambient particulate matter. Inhal. Toxicol. 2007, 19, 1079–1087. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, S.R.; Lee, Y.C. Impact of oxidative stress on lung diseases. Respirology 2009, 14, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, R. Carbon monoxide: Endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 2005, 57, 585–630. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, N.; Nishiyama, A.; Eiguren-Fernandez, A.; Hinds, W.; Kumagai, Y. Biochemical and cellular effects of electrophiles present in ambient air samples. Atmos. Environ. 2010, 44, 1483–1489. [Google Scholar] [CrossRef]
- Grigoryan, H.; Edmands, W.; Lu, S.S.; Yano, Y.; Regazzoni, L. Adductomics Pipeline for Untargeted Analysis of Modifications to Cys34 of Human Serum Albumin. Anal. Chem. 2016, 88, 10504–10512. [Google Scholar] [CrossRef] [PubMed]
- Eiguren-Fernandez, A.; Miguel, A.; Di Stefano, E.; Schmitz, D.; Cho, A. Atmospheric Distribution of Gas- and Particle-Phase Quinones in Southern California. Aerosol Sci. Technol. 2008, 42, 854–861. [Google Scholar] [CrossRef]
- Su, R.; Jin, X.; Li, H.; Huang, L.; Li, Z. The mechanisms of PM2.5 and its main components penetrate into HUVEC cells and effects on cell organelles. Chemosphere 2020, 241, 125127. [Google Scholar] [CrossRef]
- Taguchi, K.; Fujii, S.; Yamano, S.; Cho, A.K.; Kamisuki, S. An approach to evaluate two-electron reduction of 9,10-phenanthraquinone and redox activity of the hydroquinone associated with oxidative stress. Free Radic. Biol. Med. 2007, 43, 789–799. [Google Scholar] [CrossRef]
- Campen, M.J.; Babu, N.S.; Helms, G.A.; Pett, S.; Wernly, J. Nonparticulate components of diesel exhaust promote constriction in coronary arteries from ApoE-/- mice. Toxicol. Sci. 2005, 88, 95–102. [Google Scholar] [CrossRef]
- Deering-Rice, C.E.; Memon, T.; Lu, Z.; Romero, E.G.; Cox, J. Differential Activation of TRPA1 by Diesel Exhaust Particles: Relationships between Chemical Composition, Potency, and Lung Toxicity. Chem. Res. Toxicol. 2019, 32, 1040–1050. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).