Abstract
(1) Background: Mercury is a threat for the aquatic environment. Nonetheless, the entrance of Hg into food webs is not fully understood. Macrophytes are both central for Hg entry in food webs and are seen as good candidates for biomonitoring and bioremediation; (2) Methods: We review the knowledge gained on the uptake and effects of inorganic Hg (IHg) and methyl-Hg (MMHg) in the macrophyte Elodea nuttallii found in temperate freshwaters; (3) Results: E. nuttallii bioaccumulates IHg and MMHg, but IHg shows a higher affinity to cell walls. At the individual level, IHg reduced chlorophyll, while MMHg increased anthocyanin. Transcriptomics and metabolomics in shoots revealed that MMHg regulated a higher number of genes than IHg. Proteomics and metabolomics in cytosol revealed that IHg had more effect than MMHg; (4) Conclusions: MMHg and IHg show different cellular toxicity pathways. MMHg’s main impact appears on the non-soluble compartment, while IHg’s main impact happens on the soluble compartment. This is congruent with the higher affinity of IHg with dissolved OM (DOM) or cell walls. E. nuttallii is promising for biomonitoring, as its uptake and molecular responses reflect exposure to IHg and MMHg. More generally, multi-omics approaches identify cellular toxicity pathways and the early impact of sublethal pollution.
1. Introduction
Anthropogenic activities cause environmental contamination [1,2], affecting biota and human populations via contaminated food [3,4]. In this context, mercury (Hg) is a past as well as a current priority for legislators, because of its widespread occurrence and high toxicity. Freshwater Hg contamination results from atmospheric Hg depositions as rainfall or dry settling, polluted soils runoffs, effluents and emissions of industrial and gold-mining activity, as well as reemission from historical contaminations [5,6]. Anthropogenic activities release mainly (i) the highly volatile Hg0 that is transported in the atmosphere and (ii) inorganic Hg (HgII, IHg). IHg is the most abundant form in the freshwater environment. In surface waters, divalent HgII can be bio-converted to methylated Hg (CH3Hg+, MMHg), generally representing > 1% of total Hg (THg = MMHg + IHg) [7,8]. In rivers and lakes, measured concentrations typically range between 0.003–30·10−9 M THg [9]. In natural waters, IHg and MMHg are both mainly bound to thiol groups of dissolved organic matter (DOM) [5,9,10]. Water chemistry (e.g., chloride concentration and pH) governs the abundance of the minor Hg fraction not bound with DOM among the different complexes and dissolved chemical species [11]. The data revealed that IHg and MMHg show different affinities to ions and DOM [5,9,10]. Nonetheless, in natural waters the analysis and the modeling of Hg speciation remain challenging. Consequently, uptake in biota is difficult to link to classical water chemistry analysis. However, data repeatedly show IHg and MMHg bioaccumulation in aquatic biota. In addition, MMHg is biomagnified along the trophic chain, reaching very high and toxic body burdens in fish and threatening humans’ health through fish consumption, including when low concentrations of IHg and MMHg are found in primary producers [3,7].
Primary producers are central for Hg uptake and transfer to higher trophic levels [7,11,12]. In numerous littoral and shallow freshwater ecosystems (e.g., lakes, marshes, ponds and rivers), macrophytes are dominant in terms of primary production, biomass and have major ecological roles as a source of food for animals as well as in nutrient cycling [13,14]. Understanding macrophytes’ interactions with Hg and the factors controlling IHg and MMHg bioavailability to them in more detail will enhance our capacity to anticipate Hg impact and fate in aquatic ecosystems. Moreover, macrophytes could be used for biomonitoring and risk assessment of ecosystems and for ecotoxicology. However, current standardized ecotoxicology tests fail to measure the impact of Hg on primary producers in chronic exposure that involve realistic environmental concentrations [12]. In ecotoxicology, a current research priority is identifying the mechanisms of the disturbance of contaminants at a sublethal level to normal biological performance and to use this knowledge to develop new early-warning biomarkers relevant in the context of chronic exposure. High-throughput omics have revolutionized the understanding of molecular responses to sublethal stresses [15]. Early, specific and robust biomarkers directly linking responses at the level of the molecule and the cell to the effects at the level of the whole organism, population and community are envisioned in line with the adverse outcome pathway theory [10,16,17,18].
In recent years, we identified the macrophyte Elodea nuttallii as an interesting model to study Hg bioaccumulation and develop innovative biomarkers of exposure [9,19,20,21]. E. nuttallii is a rooted macrophyte that, as an aggressive invader in temperate climates, is often found in contaminated environments in all temperate regions of the world. Here, the paper aims to review the recent knowledge gained on Hg bioaccumulation and effects in this species. In more detail, we focused on Hg bioaccumulation and responses to Hg exposure in E. nuttallii at different levels of biological organization, including various omics studies. Indeed, this fundamental knowledge is essential to develop innovative biomarkers for the future environmental risk assessment of Hg in situ.
2. Bioaccumulation of Hg in Elodea nuttallii
2.1. Uptake
Primary producers, at the base of trophic webs, represent an important entry for Hg into food chains. It is thus essential to understand their Hg bioaccumulation to anticipate transfer and fate to higher trophic levels. As well as this, the bioaccumulation of IHg and MMHg is expected to determine responses to Hg exposure in primary producers. Even when Hg concentrations were very low in freshwaters, high bioaccumulation has been observed in macrophytes [7,21,22]. Macrophytes collected in the field show concentrations ranging between 1–20·10−2 µg·g−1 THg dry weight (dw) [12], but show much higher concentrations in contaminated sites. Indeed, in Valdeazogues River (Spain), roots of Typha domingensis reached 6 µg·g−1dw THg [23]. Overall, for macrophytes in the field, bioaccumulated Hg normalized by measured Hg in the water (L·kg−1) presents log10 values between 4 and 6 [7,21]. This points to an elevated bioaccumulation capacity and evidenced the importance of Hg bioaccumulation in aquatic plants for its further transfer in the food web. As such, bioaccumulation in primary producers is identified as the major bioconcentration step for Hg in the environment [11,12]. Nevertheless, among primary producers, macrophytes receive surprisingly little attention in research programs dedicated to Hg transfer and fate, despite their key ecological role in the shallow aquatic environment.
In 2009, we conducted a study to examine the biogeochemistry of Hg and its biomagnification in the Olt River (Romania). We studied the Babeni Reservoir that is affected by Hg containing effluents from a chlor-alkali plant. We identified phytoplankton and macrophytes at the base of food webs, characterized by N and C isotopic ratio, as well as MMHg concentrations [7]. Both showed a high accumulation and resulted in a similar biomagnification of MMHg in fish, e.g., up to 10 µg·g−1dw and 95% of THg [7]. The MMHg bioaccumulation step from water to primary producers was shown to be the largest increase in MMHg concentrations in this system, reaching 105-fold. We then performed a detailed study on Hg bioaccumulation in several macrophytes sampled in the Babeni Reservoir. Amongst the macrophytes, the submerged and rooted macrophyte E.nuttallii showed among the higher IHg and MMHg bioaccumulation. More in detail, E. nuttallii reached close to 2 µg·g−1dw THg concentrations [21], similar to another submerged macrophyte Potamogeton pectinatus, and three times higher than THg measured in plankton (0.7 µg·g−1dw THg) [7]. We subsequently studied Hg bioaccumulation in this plant in the field and in the laboratory in detail [9,19,21,24].
First we performed a detailed measurement of Hg bioaccumulation and uptake in E. nuttallii in the laboratory [21]. We investigated (i) accumulation of IHg and MMHg in E. nuttallii in Lake Geneva water (1 mg·L−1 DOC) spiked with increasing IHg and MMHg concentrations (3.5–3500 pM), (ii) to determine the source of Hg found in shoots and roots, as well as (iii) Hg fate in this representative aquatic plant. Tolerance to both forms of Hg was high, as evidenced by the lack of visible symptoms on leaves after 7 days of exposure for all concentrations. The accumulation of Hg was high in the microcosm with an enrichment factor (EF) of 103 for IHg and 104 for MMHg [21]. While EF decreased with increasing concentration of exposure for IHg, the EF increased for MMHg. IHg at 0.35 nM resulted in a higher bioaccumulation in leaves than stems (1.4 vs. 0.4 mgTHg·kg−1 dw), while 0.1 nM MMHg resulted in 0.6 and 0.3 mgTHg·kg−1 dw in stems and leaves, respectively. An uptake in the absence of DOM gave highly similar EF for IHg and MMHg, resulting in similar intracellular THg concentration in E. nuttallii, pointing to a role of DOM in the observed difference between the fate of IHg and MMHg observed in biota in the field [25]. Death, cold and Cu+ co-exposure highly reduced accumulation in shoots [21]. Metabolism and copper transporters govern Hg uptake at the cellular level.
A two-compartment exposure setting in the laboratory further revealed the predominant IHg basipetal transport, whereas MMHg acropetal transport was evidenced [21]. It has to be noted that in planta no measurable methylation or demethylation was evidenced. Data were confirmed with exposure to Babeni sediments for 2 months in controlled conditions that resulted in a 102.7% ± 6.4% MMHg to THg proportion in shoots of E. nuttalli, evidencing the remobilization of MMHg from sediments to shoots [24]. In 2014, we conducted another field campaign to study Hg dispersion in the Babeni Reservoir and three reservoirs located immediately downstream in the Olt River (Romania), to further test E. nuttallii potential for Hg biomonitoring [26]. A significant correlation of bioaccumulated THg, and intracellular THg bioaccumulation was found in E. nuttalii vs. THg concentration in water filtered at 45 µm (Pearson’s coefficients 0.80; 0.88), and MMHg concentration in sediments (Pearson’s coefficients 0.82; 0.89), confirming previous observations in controlled conditions [26]. In addition, because analysis of MMHg proportion in water in the Babeni Reservoir showed an increase along the part of the reservoir favorable for macrophytes development, we measured the impact of E. nuttallii rhizosphere on bacterial communities in Hg-contaminated sediments. A 2-month-long experiment revealed an increased MMHg/THg proportion in pore water and a significant change in the structure of bacterial communities in rhizospheric sediments when compared to bulk sediments [24]. E. nuttallii roots microenvironment appeared to be appropriate for Hg methylation.
To summarize, in E. nuttallii shoots, IHg mainly bioaccumulates from the water column, while Hg methylation seems to be favored in its rhizosphere and MMHg is remobilized from the sediments to its shoots. As such, THg in E. nuttallii reflected local MMHg production in sediments. The occurrence of E. nuttallii in sediments contaminated by Hg should therefore be given attention, as it could participate in MMHg transfer to food webs.
2.2. Subcellular Distribution
Because toxicity is hypothesized to originate from the intracellular uptake, we further investigated the subcellular fate of Hg in E. nuttallii. In plants, vacuoles and cell walls have a recognized role for Hg sequestration and are expected to protect the cellular components [27,28]. In shoots of E. nuttallii exposed for 24 h to 3.5·10−10 M IHg and 1·10−10 M MMHg in the presence of 1 mg·L−1 DOC, close to 68% and 73% of THg was internalized, likely in the vacuole, and around 32% and 26% was found in cell walls, respectively [19,21]. However, in these experimental conditions 99.9% of Hg was expected to be bound to DOM. On the contrary, in E. nuttallii exposed to 1·10−11 M IHg and MMHg without DOM, 33% of Hg was intracellular for the IHg treatment, while close to 100% of Hg was intracellular for the MMHg treatment [25] (Figure 1). Data revealed earlier that IHg and MMHg affinities to DOM and ions are contrasted [5,9,10]. In the same line, the distinct affinities of IHg and MMHg to cell walls could explain the higher EF of MMHg than IHg in primary producers. Besides, intracellular Hg is expected to be highly available to predators, and could thus result in the higher transfer and biomagnification of MMHg in natural waters [9].
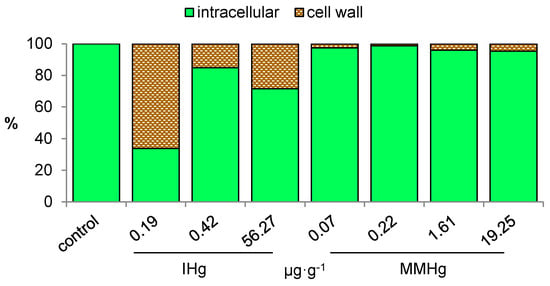
Figure 1.
Distribution (%) of THg at the subcellular level in E. nuttallii exposed 2 h to IHg or MMHg in water without DOM [25].
3. Responses to Hg Exposure at Different Levels of Biological Organization in Elodea nuttallii
3.1. Physiological Responses
Among the effects of Hg toxicity at the individual level, growth is a widely used endpoint [22]. For example, the growth of cells in the presence of IHg or MMHg in Thalassiosira weissflogii was compared and showed a reduction of 50% at 5 × 10−10 M IHg and MMHg [29]. In addition, scientists observed different toxicity pathways at the cell level: growth rate was reduced by MMHg, while strong cell damage, but no effect on division rate, resulted from IHg exposure [29]. Exposure for 7d to 3.5 × 10−10 M IHg reduced growth of roots and increased cell wall lignification in E. nuttallii, whereas exposure for 7d to 1 × 10−10 M MMHg had no effect on those endpoints [19] (Table 1). Under similar conditions, an inhibition of root growth was observed for 1 nM MMHg [21]. When uptaken, Hg compounds bind to DNA, proteins and various enzymes that are primordial for cell normal physiology. Hg also increases the production of reactive oxygen species (ROS) that might result in oxidative stress, and is evidenced by the alteration in the activity of enzymes involved in ROS regulation, such as catalases, class III peroxidases (POD), lipoxygenases, or superoxide dismutases (SOD) [30,31]. SOD, POD and catalase activities were induced by 5 µM IHg 7d in Lemna minor [22] and excessive ROS generation was evidenced in Chlamydomonas reinhardtii exposed to 1 × 10−7 M IHg or 1 × 10−9 M MMHg [32] or for 96 h to 2 × 10−6 M IHg [33], and resulting in lipid peroxidation after 24 h exposure to 5 × 10−6 M IHg [34]. In E. nuttallii, after 24 h exposure to 4 × 10−10 M and 4 × 10−5 M IHg, SOD and POD activities showed a 1.3× and 1.3× increase and a 2.1× and 4.6× decrease, respectively [35] (Table 1). In addition, numerous studies in plants and algae evidenced the impact of Hg (mostly 10−6 M IHg) on photosynthesis, notably the chlorophyll breakdown and the reduction in photosynthesis efficiency [11,22,31,36,37]. Hg toxicity results from its binding to various groups in proteins, e.g., sulfhydryl (-SH), phosphate and active groups of ADP or ATP, as well as essential major cations’ substitution [36,38]. The impact of IHg in all kinds of phytoplankton and macrophyte species on the photosynthesis and the generation of an oxidative stress were widely reported. However, MMHg toxicity in aquatic primary producers has been globally less studied than IHg. The data reveal similar targets for MMHg and IHg (e.g., oxidative stress, photosynthesis), but when compared in strictly identical experimental conditions, MMHg seems to impact distinct toxicity pathways than IHg [25,39]}. Nevertheless, physiological responses are rarely significant at environmental concentrations [21]. Indeed, many of the studies described above were obtained in controlled laboratory experiments at concentrations 103 to 106 higher than Hg concentrations generally found in natural waters. Recently, a study in C. reinhardtii exposed for 2 h to 10−8 and 10−11 M MMHg showed an increase in photosynthesis efficiency. This paradoxical effect observed for lower range concentration is called hormesis and results from the overcompensation of a moderate stress [37,40]. A distinct impact of IHg and MMHg was also reported on photosynthesis in T. weissflogii, a marine diatom. Exposure for 72 h to 2–11·10−7 M IHg blocked the electron chain of photosynthesis and increased chlorophyll fluorescence lifetime. In identical experimental conditions, exposure to up to 2.8·10−6 M MMHg had no effect [29]. Similarly, chlorophyll content was reduced by IHg, while a significant antioxidant response was induced by MMHg in E. nuttallii (Table 1) [25]. As such, in E. nuttallii, a direct impact of IHg on the integrity of chloroplast membranes was hypothesized, while MMHg would impact the metabolism of cytoplasmic organelles. A more recent study in E. nuttallii confirmed that 0.1 nM MMHg for 24 h did not impact chlorophyll content (Table 1) [19]. To summarize, IHg toxic action appears to target the photosynthetic components with, for example, the chelation of IHg with chloroplast proteins and the Mg ion replacement in the heme of chlorophyll [41]. MMHg toxicity would rather target the cellular components, increasing the production of ROS.

Table 1.
Responses to Hg revealed by metabolomic (M), proteomic (P) or transcriptomic (T) in E. nuttallii.
Investigating mechanisms at a low level of biological organization increases the understanding of IHg and MMHg toxicity at the molecular level. Short exposure lengths reveal early toxicity responses that are thought to be more specific to each stress than a longer exposure [40]. As such, omics appear to be particularly promising for new ecotoxicology endpoints, because their sensitivity makes them suitable for environmental realism, including low concentrations and short (transient) exposures [42].
3.2. Gene Level Responses
In recent years, ecotoxicological studies using omics have increased because of the availability of new sequencing methods that can be applied to non-model species of interest (e.g., RNAseq). Transcriptomic compares gene expression in chosen conditions vs. a control condition (reference). Until our studies in E. nuttallii (Table 1), transcriptomic had not been applied to the analyses effects of Hg in aquatic plants.
In E. nuttallii, we first performed the analysis of effects of 0.04, 4 and 40·10−7 M IHg for 24 h through differential gene analysis by illumina RNAseq in E. nuttallii [20]. Treatments of up-regulated genes coding for protein of stress such as chaperones, a regulation of genes found in energy metabolism pathways (e.g., sugar catabolism), attributed to an inhibition of energy reserves production by photosynthesis [20]. Concomitantly, we measured the down-regulation of genes related to homeostasis, such as metal transporters, for reducing and controlling Hg accumulation [20]. Indeed, the down-regulation of the gene EnCOPT1, with increasing concentrations of IHg, was observed. The data supported the uptake of Hg through high affinity Cu transporters [20]. Transcriptomic analysis also pointed towards IHg toxicity to trigger an anti-oxidative response and alter protein structure. In E. nuttallii, IHg (24 h 4·10−7 M) also up-regulated genes involved in N-metabolism and the biosynthesis of lipids [35]. We also compared transcriptomes upon 2 h exposure to increasing IHg and MMHg concentrations (10−11 to 10−8 M) [25]. The significantly regulated contigs are involved in similar gene ontology (GO) categories, including amino acid, energy and secondary metabolisms, as well as development, and transport (Figure 2a). The main difference was that MMHg regulated up to 20 times more contigs at similar intracellular concentration, evidencing that MMHg had a higher molecular impact than IHg (Figure 2b). A recent study in the Olt River (Romania), on in situ caged E. nuttallii downstream a chlor-alkali plant, revealed a strong gene regulation after 2 h exposure, in line with the expected impact at much higher Hg concentrations (Figure 2), despite 1.2·10−11 M Hg concentration in water and a DOC of 3 mg·L−1 resulting in nonsignificant uptake [10]. It has to be noted that, among GO terms of significantly regulated contigs, a predominant part in all treatments show an unknown function, indicating some level of uncertainty in the analysis of responses, as well as a high potential for discoveries in the biology of Hg stress.
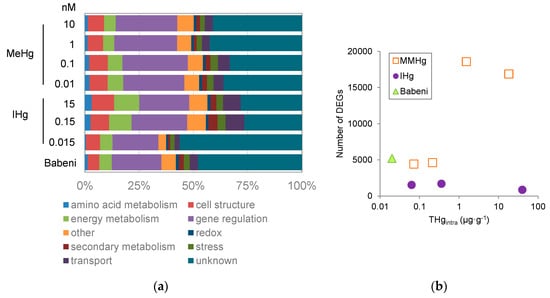
Figure 2.
(a) proportion of regulated genes among gene ontology terms (Mapman terminology) and (b) number of deregulated genes (DEGs) as a function of THg bioaccumulated in the intracellularly in E. nuttallii exposed for 2 h with IHg, MMHg in the laboratory or in the Babeni Reservoir (Romania) [10,25].
The use of gene expression has shown promising results for the characterization of the precise early cellular toxicity pathways of Hg as well as for the definition of new biomarker of exposure. One strong interest of omics is its high sensitivity, allowing the measurement of significant responses at environmental concentrations. In E. nuttallii, the analysis of the expression of selected genes directly on RNA could be realized, with the nCounter method developed by Nanostring [20,25]. Statistical analysis (hierarchical clustering) of treatments using global gene expression signatures discriminated pM from nM concentrations of IHg or other stress exposure for concentrations as low as 1·10−9 M THg [20,25]. Transcriptomics could thus be used to derive more sensitive biomarkers for ecotoxicological testing than higher organization level endpoints.
3.3. Responses at the Proteome and Metabolome Level
As mentioned above, transcriptomic targets gene expression level, but it is its protein that carries the function of a gene in the cell. Eventually, proteins have numerous cell functions involved in signaling and tolerance to abiotic stressors such as the metabolism of amino acids and sugars, the energy production, primary and secondary metabolites that have a key role for remodeling, and cell physiological repair in plants [44]. Proteomics inform not only on protein expression level but also on the occurrence of regulation at post-transcription level. However, the availability and completeness of a database with identified proteins profiles still limits its use [42]. Early proteomic study with 2D-DIGE in E. nuttallii identified 30 proteins involved in energy (sugar metabolism, photosynthesis) and cell structure to be dysregulated by 24 h exposure to 4·10−10 M IHg [19] (Table 1). Those observations were in line with previous transcriptomic data, as similar functions were observed in regulated genes in E. nuttallii exposed to IHg. On the other hand, 1·10−10 M MMHg did not result in significant proteomic response, which is opposite to the transcriptomic observation of a higher number of genes regulated by MMHg than IHg exposure [25]. This discrepancy might come from a lower uptake (0.19 µg·g−1) of MMHg in those conditions than for IHg (0.67 µg·g−1). Nonetheless, a few years later, the effects of the same concentrations (4·10−10 M IHg, 1·10−10 M MMHg 24 h resulting in 0.98 and 0.81 µg·g−1 THg respectively) were analyzed by GC-MS- and LC-MS-targeting metabolites in a way that is complementary to other molecular approaches to confirm the Hg cellular toxicity pathway in E. nuttallii (Table 1) [43]. In whole shoots, MMHg resulted in the significant regulation of 15 metabolites, while no metabolite was significantly regulated by IHg treatment. In the cytosol, IHg and MMHg exposure regulated three metabolites and four metabolites respectively [43]. Pathway analysis by the Kyoto Encyclopedia of Genes and Genomes (KEGG) of deregulated metabolites revealed that MMHg exposure resulted in biochemical changes in aminoacyl-tRNA biosynthesis, glycine, serine and threonine metabolism, nitrogen metabolism, arginine and proline metabolism, cyanoamino acid metabolism, while IHg treatment caused no significant variations at the pathway level. The data supported an impact of MMHg on N homeostasis, while IHg had a low impact. In sum, metabolomics highlighted that sub-lethal concentrations of IHg and MMHg resulted in significant changes in the nutrition pathways and induced a protective response. Moreover, significant differences in the responses supported different molecular toxicity pathways of MMHg and IHg. Indeed, MMHg affects components of the non-soluble compartment of cells, while IHg affects components of the soluble compartment of cells. This point might explain the absence of the effect of MMHg, measured by proteomics using 2D-DIGE, which is known to better identify soluble proteins. Another discrepancy was the observation of no effect on sugars measured by metabolomics, contrary to the previously reported significant regulation of genes and proteins involved in the metabolism of sugars. As such, we concluded that gene regulation happened to keep the level of those metabolites stable [20,25,35,37]. Nonetheless this apparent disconnection can be explained in part by technical issues, as well as by the complexity of the metabolic network structure. Although the linear relationship of transcripts with protein levels and their biological functions is sometimes observed, in general there is a major discrepancy that results from the circular nature and branching of metabolic pathways, notably when regulatory loops are operating [45]. In addition, the transformation of a metabolite depends more on the kinetic specificities of enzymes than on the concentration of enzymes or metabolites. Similar sequences can encode for enzymes catalyzing different reactions, and enzymatic activity can vary according to cellular and subcellular compartmentation [45,46]. Posttranslational modification, resulting in the modification of the activity and affinities of an enzyme, is more predominant for the regulation of enzymes than a change in the abundance of a protein through transcription, translation or degradation [46]. As such, a non-linearity of responses is observed in most cases. This must be expected and accounted for when analyzing multi-omic data aiming to integrate transcript, protein and metabolite abundance together with biological responses. The analysis of the kinetics of molecular responses is a major research priority for better understanding of metabolic networks in future research. However, in E. nuttallii metabolomics was very useful to complement transcriptome and proteome differential analysis, revealing the potential of multi-omics to reveal cellular toxicity pathways in more detail and, as such, the early impact of environmental pollution.
4. Conclusions
Bioaccumulation and responses to IHg and MMHg in E. nuttallii were studied in various experimental conditions and helped to confirm that, globally, MMHg has a higher bioavailability than IHg and that they trigger different toxicity pathways. As such, omics helped to reach a better mechanistic understanding of Hg impact in E. nuttallii. The various datasets showed that molecular regulation at the gene, protein and metabolite level was measured at much lower Hg concentrations than bioaccumulation or other physiological endpoints. Moreover, the analysis of gene signature appeared highly promising to identify molecular biomarkers that will allow the early and sensitive detection of Hg contamination. Nevertheless, more studies concerning primary producers are needed. Notably, highly variable experimental settings make comparisons between species difficult, and consequently limit extrapolation to the ecosystem level. In primary producers, further work is needed to efficiently link responses at the molecular level to risk assessment in the environment at the population and at community level. In addition, the validation of biomarkers based on omic analysis is requested in wild populations, to normalize background levels and eventually complement standard tests [16].
Funding
This research received no external funding.
Acknowledgments
Author thanks Rebecca Beauvais-Fluck, Andrea G. Bravo, Perrine Dranguet, Alexander Grosse-Honebrink, Floriane Larras, Beatriz Lobo, Nicole Regier and Debora Tanaami for their help in performing the experiments published previously elsewhere and discussed here.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Azimi, S.; Rocher, V. Influence of the water quality improvement on fish population in the Seine River (Paris, France) over the 1990-2013 period. Sci. Total Environ. 2016, 542, 955–964. [Google Scholar] [CrossRef]
- Loizeau, J.-L.; Edder, P.; De Alencastro, L.F.; Corvi, C.; Ramseier Gentile, S. La contamination du Léman par les micropolluants—Revue de 40 ans d’études. Arch. Sci. 2013, 66, 117–136. Available online: http://archive-ouverte.unige.ch/unige:35814 (accessed on 10 February 2020).
- Bravo, A.G.; Loizeau, J.L.; Bouchet, S.; Richard, A.; Rubin, J.F.; Ungureanu, V.G.; Amouroux, D.; Dominik, J. Mercury human exposure through fish consumption in a reservoir contaminated by a chlor-alkali plant: Babeni reservoir (Romania). Environ. Sci. Pollut. Res. 2010, 17, 1422–1432. [Google Scholar] [CrossRef]
- Niane, B.; Guedron, S.; Moritz, R.; Cosio, C.; Ngom, P.; Deverajan, N.; Pfeifer, H.; Pote, J. Human exposure to mercury in artisanal small-scale gold mining areas of Kedougou region, Senegal, as a function of occupational activity and fish consumption. Environ. Sci. Pollut. Res. 2014, 1–11. [Google Scholar] [CrossRef]
- Dranguet, P.; Flück, R.; Regier, N.; Cosio, C.; Le Faucheur, S.; Slaveykova, V.I. Towards mechanistic understanding of mercury availability and toxicity to aquatic primary producers. CHIMIA 2014, 68, 799–805. [Google Scholar] [CrossRef]
- UNEP. Global Mercury Assessment 2018: Sources, Emissions, Releases and Environmental Transport; United Nations Environment Programme (UNEP) Chemicals: Geneva, Switzerland, 2018. [Google Scholar]
- Bravo, A.G.; Cosio, C.; Amouroux, D.; Zopfi, J.; Cheualley, P.A.; Spangenberg, J.E.; Ungureanu, V.G.; Dominik, J. Extremely elevated methyl mercury levels in water, sediment and organisms in a Romanian reservoir affected by release of mercury from a chlor-alkali plant. Water Res. 2014, 49, 391–405. [Google Scholar] [CrossRef]
- Bravo, A.G.; Cosio, C. Biotic formation of methylmercury: A bio-physico-chemical conundrum. Limnol. Ocean. 2019. [Google Scholar] [CrossRef]
- Beauvais-Flück, R.; Slaveykova, V.; Cosio, C. Molecular effects of inorganic and methyl mercury in aquatic primary producers: Comparing impact to a macrophyte and a green microalga in controlled conditions. Geosciences 2018, 8, 393. [Google Scholar] [CrossRef]
- Dranguet, P.; Cosio, C.; Le Faucheur, S.; Beauvais-Fluck, R.; Freiburghaus, A.; Worms, I.A.M.; Petit, B.; Civic, N.; Docquier, M.; Slaveykova, V.I. Transcriptomic approach for assessment of the impact on microalga and macrophyte of in-situ exposure in river sites contaminated by chlor-alkali plant effluents. Water Res. 2017, 121, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Le Faucheur, S.; Campbell, P.G.C.; Fortin, C.; Slaveykova, V.I. Interactions between mercury and phytoplankton: Speciation, bioavailability, and internal handling. Environ. Toxicol. Chem. 2014, 33, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Cosio, C.; Fluck, R.; Regier, N.; Slaveykova, V.I. Effects of macrophytes on the fate of mercury in aquatic systems. Environ. Toxicol. Chem. 2014, 24. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Noges, T.; Luup, H.; Feldmann, T. Primary production of aquatic macrophytes and their epiphytes in two shallow lakes (Peipsi and Vrtsjarv) in Estonia. Aquat. Ecol. 2010, 44, 83–92. [Google Scholar] [CrossRef]
- Brinke, A.; Buchinger, S. Toxicogenomics in Environmental Science. Adv. Biochem. Eng. Biotechnol. 2017, 157, 159–186. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, D.; Chaumot, A.; Charnot, A.; Almunia, C.; Francois, A.; Navarro, L.; Armengaud, J.; Salvador, A.; Geffard, O. Ecotoxico-proteomics for aquatic environmental monitoring: First in situ application of a new proteomics-based multibiomarker assay using caged amphipods. Environ. Sci. Technol. 2017, 51, 13417–13426. [Google Scholar] [CrossRef]
- Trapp, J.; Almunia, C.; Gaillard, J.-C.; Pible, O.; Chaumot, A.; Geffard, O.; Armengaud, J. Proteogenomic insights into the core-proteome of female reproductive tissues from crustacean amphipods. J. Proteom. 2016, 135, 51–61. [Google Scholar] [CrossRef]
- Prud’homme, S.M.; Renault, D.; David, J.-P.; Reynaud, S. Multiscale approach to deciphering the molecular mechanisms involved in the direct and intergenerational effect of ibuprofen on Mosquito Aedes aegypti. Environ. Sci. Technol. 2018, 52, 7937–7950. [Google Scholar] [CrossRef]
- Larras, F.; Regier, N.; Planchon, S.; Pote, J.; Renaut, J.; Cosio, C. Physiological and proteomic changes suggest an important role of cell walls in the high tolerance to metals of Elodea nuttallii. J. Hazard. Mater. 2013, 2, 575–583. [Google Scholar] [CrossRef]
- Regier, N.; Baerlocher, L.; Munsterkotter, M.; Farinelli, L.; Cosio, C. Analysis of the Elodea nuttallii transcriptome in response to mercury and cadmium pollution: Development of sensitive tools for rapid ecotoxicological testing. Environ. Sci. Technol. 2013, 47, 8825–8834. [Google Scholar] [CrossRef]
- Regier, N.; Larras, F.; Bravo, A.G.; Ungureanu, V.-G.; Amouroux, D.; Cosio, C. Mercury bioaccumulation in the aquatic plant Elodea nuttallii in the field and in microcosm: Accumulation in shoots from the water might involve copper transporters. Chemosphere 2013, 90, 595–602. [Google Scholar] [CrossRef]
- Yang, J.; Li, G.; Bishopp, A.; Heenatigala, P.P.M.; Hu, S.; Chen, Y.; Wu, Z.; Kumar, S.; Duan, P.; Yao, L.; et al. A comparison of growth on mercuric chloride for three Lemnaceae species reveals differences in growth dynamics that effect their suitability for use in either monitoring or remediating ecosystems contaminated with mercury. Front. Chem. 2018, 6, 2018. [Google Scholar] [CrossRef] [PubMed]
- Lominchar, M.A.; Sierra, M.J.; Jimenez-Moreno, M.; Guirado, M.; Martin-Doimeadios, R.C.R.; Millan, R. Mercury species accumulation and distribution in Typha domingensis under real field conditions (Almaden, Spain). Environ. Sci. Pollut. Res. Int. 2019, 26, 3138–3144. [Google Scholar] [CrossRef] [PubMed]
- Regier, N.; Frey, B.; Converse, B.; Roden, E.; Grosse-Honebrick, A.; Bravo, A.G.; Cosio, C. Elodea nuttallii roots effect on bacterial communities and MMHg proportion in a Hg polluted sediment. PLoS ONE 2012, 7, e45565. [Google Scholar] [CrossRef] [PubMed]
- Beauvais-Fluck, R.; Slaveykova, V.I.; Skyllberg, U.; Cosio, C. Molecular effects, speciation and competition of inorganic and methyl mercury in the aquatic plant. Elodea nuttallii. Environ Sci Technol. 2018, 52, 8876–8884. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.G.; Loizeau, J.-L.; Dranguet, P.; Makri, S.; Björn, E.; Ungureanu, V.G.; Slaveykova, V.I.; Cosio, C. Persistent Hg contamination and occurrence of Hg methylating gene (hgcA) downstream a chlor-alkali plant in the Olt River (Romania). Environ. Sci. Pollut. Res. 2016, 23, 10529–10541. [Google Scholar] [CrossRef]
- Castro, R.; Pereira, S.; Lima, A.; Corticeiro, S.; Valega, M.; Pereira, E.; Duarte, A.; Figueira, E. Accumulation, distribution and cellular partitioning of mercury in several halophytes of a contaminated salt marsh. Chemosphere 2009, 76, 1348–1355. [Google Scholar] [CrossRef]
- Carrasco-Gil, S.; Alvarez-Fernandez, A.; Sobrino-Plata, J.; Millan, R.; Carpena-Ruiz, R.O.; Leduc, D.L.; Andrews, J.C.; Abadia, J.; Hernandez, L.E. Complexation of Hg with phytochelatins is important for plant Hg tolerance. Plant Cell Environ. 2011, 34, 778–791. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, Y.; Qu, J.Y.; Wang, W.-X. Mercury effects on Thalassiosira weissflogii: Applications of two-photon excitation chlorophyll fluorescence lifetime imaging and flow cytometry. Aquat. Toxicol. 2012, 110–111, 133–140. [Google Scholar] [CrossRef]
- Ferrat, L.; Romeo, M.; Gnassia-Barelli, M.; Pergent-Martini, C. Effects of mercury on antioxidant mechanisms in the marine phanerogam Posidonia oceanica. Dis. Aquat. Org. 2002, 50, 157–160. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Chi, W.-C.; Huang, T.-L.; Lin, C.-Y.; Quynh Nguyeh, T.T.; Hsiung, Y.-C.; Chia, L.-C.; Huang, H.-J. Mercury-induced biochemical and proteomic changes in rice roots. Plant Physiol. Biochem. 2012, 55, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Siebman, C.; Velev, O.D.; Slaveykova, V.I. Two-dimensional algal collection and assembly by combining AC-dielectrophoresis with fluorescence detection for contaminant-induced oxidative stress sensing. Biosensors 2015, 5, 319–336. [Google Scholar] [CrossRef]
- Elbaz, A.; Wei, Y.Y.; Meng, Q.A.; Zheng, Q.; Yang, Z.M. Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicology 2010, 19, 1285–1293. [Google Scholar] [CrossRef]
- Cheloni, G.; Slaveykova, V.I. Optimization of the C11-BODIPY581/591 Dye for the Determination of Lipid Oxidation in Chlamydomonas reinhardtii by Flow Cytometry. Cytom. Part A 2013, 83, 952–961. [Google Scholar] [CrossRef]
- Regier, N.; Beauvais-Fluck, R.; Slaveykova, V.I.; Cosio, C. Elodea nuttallii exposure to mercury exposure under enhanced ultraviolet radiation: Effects on bioaccumulation, transcriptome, pigment content and oxidative stress. Aquat. Toxicol. 2016, 180, 218–226. [Google Scholar] [CrossRef]
- Patra, M.; Sharma, A. Mercury toxicity in plants. Bot. Rev. 2000, 66, 379–422. [Google Scholar] [CrossRef]
- Beauvais-Fluck, R.; Slaveykova, V.I.; Cosio, C. Effects of two-hour exposure to environmental and high concentrations of methylmercury on the transcriptome of the macrophyte Elodea nuttallii. Aquat. Toxicol. 2018, 194, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Bhowmik, N.; Bandopadhyay, B.; Sharma, A. Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ. Exp. Bot. 2004, 52, 199–223. [Google Scholar] [CrossRef]
- Beauvais-Fluck, R.; Slaveykova, V.I.; Cosio, C. Cellular toxicity pathways of inorganic and methyl mercury in the green microalga Chlamydomonas reinhardtii. Sci. Rep. 2017, 7, 017–08515. [Google Scholar] [CrossRef] [PubMed]
- Beauvais-Fluck, R.; Slaveykova, V.I.; Cosio, C. Transcriptomic and physiological responses of the green microalga Chlamydomonas reinhardtii during short-term exposure to subnanomolar methylmercury concentrations. Environ. Sci. Technol. 2016, 50, 7126–7134. [Google Scholar] [CrossRef]
- Küpper, H.; Küpper, F.; Spiller, M. Environmental relevance of heavy metal-substituted chlorophylls using the example of water plants. J. Exp. Bot. 1996, 47, 259–266. [Google Scholar] [CrossRef]
- Snape, J.R.; Maund, S.J.; Pickford, D.B.; Hutchinson, T.H. Ecotoxicogenomics: The challenge of integrating genomics into aquatic and terrestrial ecotoxicology. Aquat. Toxicol. 2004, 67, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Cosio, C.; Renault, D. Effects of cadmium, inorganic mercury and methyl-mercury on the physiology and metabolomic profiles of shoots of the macrophyte Elodea nuttallii. Environ. Pollut. 2019, 5, 113557. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J. Exp. Biol. 2005, 208, 2819. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Stitt, M. On the discordance of metabolomics with proteomics and transcriptomics: Coping with increasing complexity in logic, chemistry, and network interactions scientific correspondence. Plant Physiol. 2012, 158, 1139–1145. [Google Scholar] [CrossRef]
- Cosio, C.; Dunand, C. Specific functions of individual class III peroxidase genes. J. Exp. Bot. 2009, 60, 391–408. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).