Radiotherapy of Conjunctival Melanoma: Role and Challenges of Brachytherapy, Photon-Beam and Protontherapy
Abstract
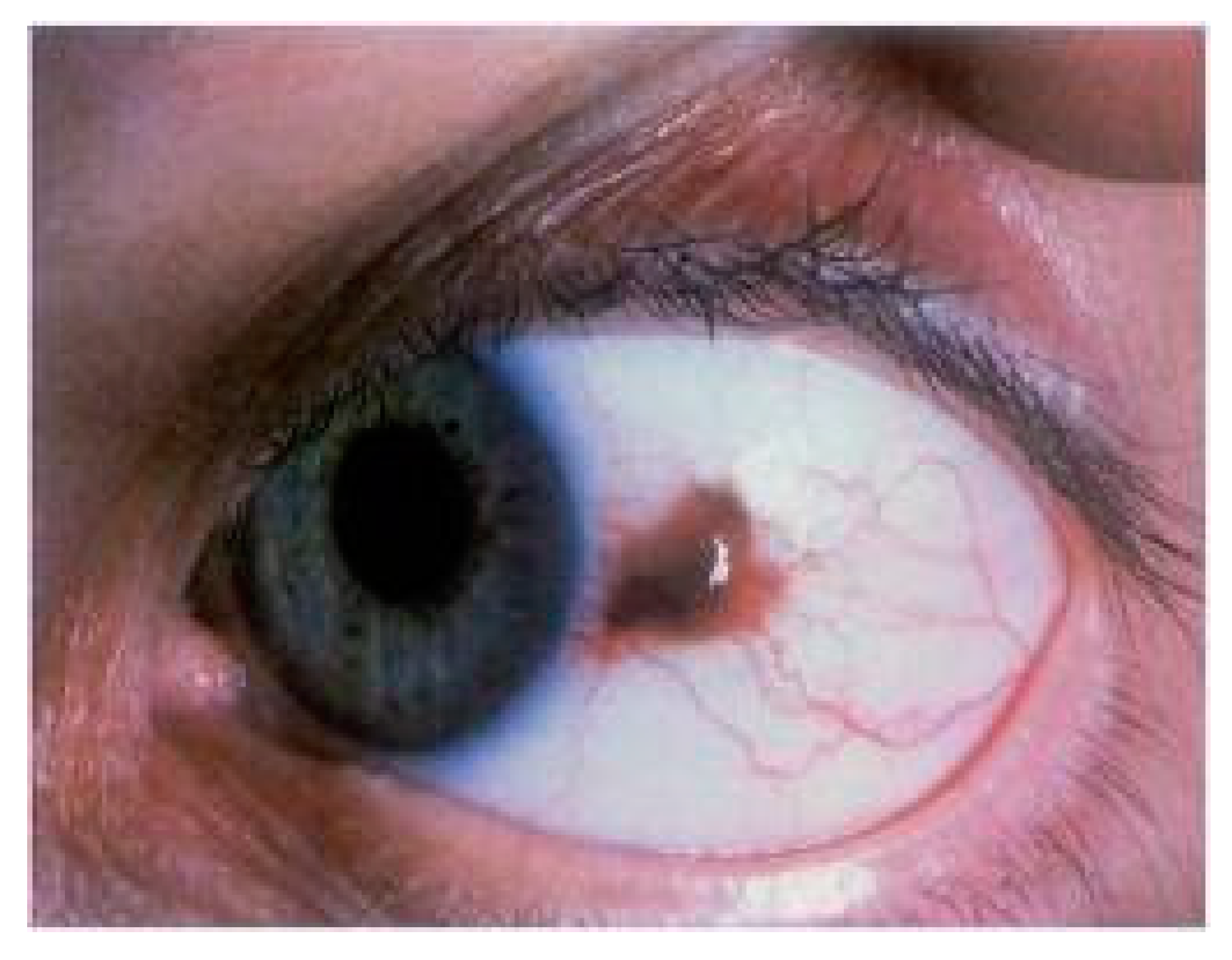
1. Introduction and General Overview
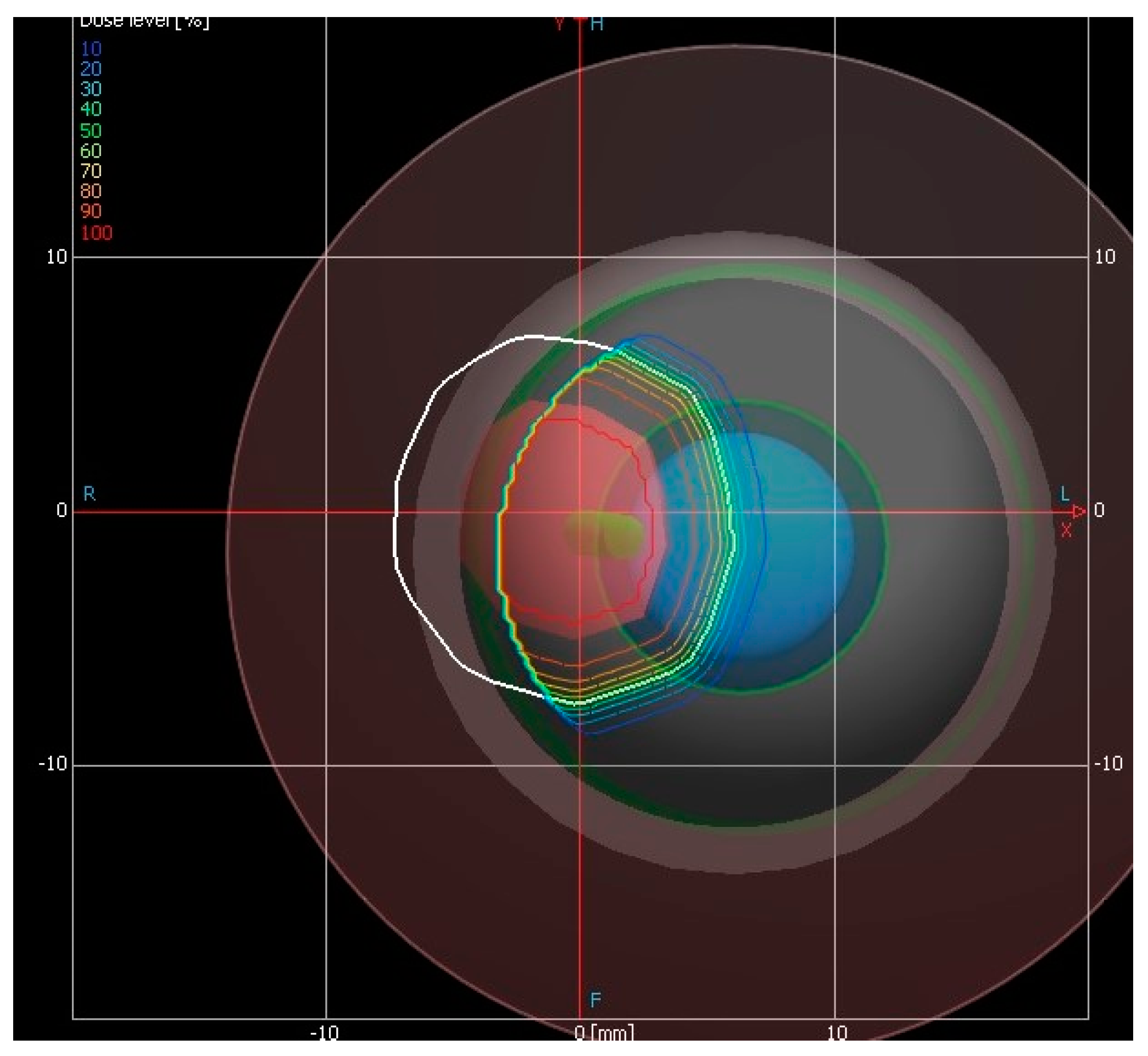
2. Brachytherapy
3. External Photon-Beam Radiotherapy
4. Proton-Beam Irradiation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, G.-P.; Hu, D.-N.; McCormick, S.; Finger, P.T. Conjunctival melanoma: Is it increasing in the United States? Am. J. Ophthalmol. 2003, 135, 800–806. [Google Scholar] [CrossRef]
- Hu, D.-N.; Yu, G.; McCormick, S.A.; Finger, P.T. Population-Based Incidence of Conjunctival Melanoma in Various Races and Ethnic Groups and Comparison With Other Melanomas. Am. J. Ophthalmol. 2008, 145, 418–423.e1. [Google Scholar] [CrossRef] [PubMed]
- Tuomaala, S.; Eskelin, S.; Tarkkanen, A.; Kivelä, T.T. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3399–3408. [Google Scholar]
- Jakobiec, F.A.; Folberg, R.; Iwamoto, T. Clinicopathologic characteristics of premalignant and malignant melanocytic lesions of the conjunctiva. Ophthalmology 1989, 96, 147–166. [Google Scholar] [CrossRef]
- Shields, C.L.; Shields, J.A. Ocular melanoma: Relatively rare but requiring respect. Clin. Dermatol. 2009, 27, 122–133. [Google Scholar] [CrossRef] [PubMed]
- AJCC-UICC Ophthalmic Society Task Force. Malignant Melanoma of the Conjunctiva. In AJCC Cancer Staging Manual; Edge, S.E., Byrd, D.R., Carducci, M.A., Compton, C.A., Eds.; Springer: New York, NY, USA, 2009; pp. 539–546. [Google Scholar]
- Griewank, K.G.; Westekemper, H.; Murali, R.; Mach, M.; Schilling, B.; Wiesner, T.; Schimming, T.; Livingstone, E.; Sucker, A.; Grabellus, F.; et al. Conjunctival Melanomas Harbor BRAF and NRAS Mutations and Copy Number Changes Similar to Cutaneous and Mucosal Melanomas. Clin. Cancer Res. 2013, 19, 3143–3152. [Google Scholar] [CrossRef]
- Kurli, M.; Chin, K.; Finger, P.T. Whole-body 18 FDG PET/CT imaging for lymph node and metastatic staging of conjunctival melanoma. Br. J. Ophthalmol. 2008, 92, 479–482. [Google Scholar] [CrossRef]
- Shields, J.A.; Shields, C.L.; De Potter, P. Surgical management of conjunctival melanoma. The 1994 Lynn B. McMahan Lecturwe. Arch Ophthalmol. 1997, 115, 808–815. [Google Scholar] [CrossRef]
- Damato, B.; E Coupland, S. An audit of conjunctival melanoma treatment in Liverpool. Eye 2009, 23, 801–809. [Google Scholar] [CrossRef]
- Kim, J.W. Topical treatment options for conjunctival neoplasms. Clin. Ophthalmol. 2008, 2, 503–515. [Google Scholar] [CrossRef]
- Missotten, G.S.; Keijser, S.; De Keizer, R.J.W.; De Wolff-Rouendaal, D. Conjunctival Melanoma in The Netherlands: A Nationwide Study. Investig. Opthalmol. Vis. Sci. 2005, 46, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Cohen, V.M.L.; Papastefanou, V.P.; Liu, S.; Stoker, I.; Hungerford, J.L. The Use of Strontium-90 Beta Radiotherapy as Adjuvant Treatment for Conjunctival Melanoma. J. Oncol. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, D.; Karan, K. Plaque brachytherapy for posterior uveal melanoma in 2018: Improved techniques and expanded indications. Curr. Opin. Ophthalmol. 2018, 29, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Stöckela, E.; Eichmanna, M.; Flühsb, D.; Sommer, H.; Biewald, E.; Bornfeld, N.; Spaan, B.; Sauerwein, W. Dose Distributions and Treatment Margins in Ocular Brachytherapy with 106Ru Eye Plaques. Ocul. Oncol. Pathol. 2018, 4, 122–128. [Google Scholar] [CrossRef]
- Ebrahimi-Khankook, A.; Vejdani-Noghreiyan, A. Dosimetric comparison between realistic ocular model and other models for COMS plaque brachytherapy with 103Pd, 131Cs, and 125I radioisotopes. Radiat. Environ. Biophys. 2018, 57, 265–275. [Google Scholar] [CrossRef]
- Lee, Y.C.; Kim, Y.; Huynh, J.W.-Y.; Hamilton, R.J. Failure modes and effects analysis for ocular brachytherapy. Brachytherapy 2017, 16, 1265–1279. [Google Scholar] [CrossRef]
- Brouwer, N.J.; Marinkovic, M.; Peters, F.P.; Hulshof, M.C.C.M.; Pieters, B.R.; De Keizer, R.J.W.; Horeweg, N.; Laman, M.S.; Bleeker, J.C.; Van Duinen, S.G.; et al. Management of conjunctival melanoma with local excision and adjuvant brachytherapy. Eye 2020, 1–9. [Google Scholar] [CrossRef]
- Fallico, M.; Reibaldi, M.; Avitabile, T.; Longo, A.; Bonfiglio, V.; Chronopoulos, A.; Caltabiano, R.; Spatola, C.; Russo, A. Intravitreal aflibercept for the treatment of radiation-induced macular edema after ruthenium 106 plaque radiotherapy for choroidal melanoma. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1547–1554. [Google Scholar] [CrossRef]
- Russo, A.; Reibaldi, M.; Avitabile, T.; Uva, M.G.; Franco, L.M.; Gagliano, C.; Bonfiglio, V.; Spatola, C.; Privitera, G.; Longo, A. Dexamethasone intravitreal implant vs ranibizumab in the treatment of macular edema secondary to brachytherapy for choroidal melanoma. Retina 2018, 38, 788–794. [Google Scholar] [CrossRef]
- Messineo, D.; Barile, G.; Morrone, S.; La Torre, G.; Turchetti, P.; Accetta, L.; Battagliola, E.T.; Agostinelli, E.; Pacella, F. Meta-analysis on the utility of radiotherapy for the treatment of Ocular Melanoma. Clin. Ter. 2020, 170, e89–e98. [Google Scholar]
- Levy, R.P.; Schulte, R.W.M. Stereotactic radiosurgery withcharged-particle beams: Technique and clinical experience. Transl. Cancer Res. 2012, 1, 159–172. [Google Scholar] [CrossRef]
- Graue, G.F.; Tena, L.B.; Finger, P.T. Electron Beam Radiation for Conjunctival Squamous Carcinoma. Ophthalmic Plast. Reconstr. Surg. 2011, 27, 277–281. [Google Scholar] [CrossRef]
- Finger, P.T. Radiation Therapy for Orbital Tumors: Concepts, Current Use, and Ophthalmic Radiation Side Effects. Surv. Ophthalmol. 2009, 54, 545–568. [Google Scholar] [CrossRef] [PubMed]
- Doyen, J.; Falk, A.T.; Floquet, V.; Hérault, J.; Hannoun-Lévi, J. Proton beams in cancer treatments: Clinical outcomes and dosimetric comparisons with photon therapy. Cancer Treat. Rev. 2016, 43, 104–112. [Google Scholar] [CrossRef]
- Wuestemeyer, H.; Sauerwein, W.; Meller, D.; Chauvel, P.; Schueler, A.; Steuhl, K.-P.; Bornfeld, N.; Anastassiou, G. Proton radiotherapy as an alternative to exenteration in the management of extended conjunctival melanoma. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.L.; Hérault, J.; Stang, A.; Griewank, K.G.; Meller, D.; Thariat, J.; Steuhl, K.-P.; Westekemper, H.; Sauerwein, W. Proton radiotherapy in advanced malignant melanoma of the conjunctiva. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1309–1318. [Google Scholar] [CrossRef]
- Thariat, J.; Salleron, J.; Maschi, C.; Fevrier, E.; Lassalle, S.; Gastaud, L.; Baillif, S.; Claren, A.; Baumard, F.; Herault, J.; et al. Oncologic and visual outcomes after postoperative proton therapy of localized conjunctival melanomas. Radiat. Oncol. 2019, 14, 239. [Google Scholar] [CrossRef]
- Cirrone, G.A.P.; Cuttone, G.; Raffaele, L.; Salamone, V.; Avitabile, T.; Privitera, G.; Spatola, C.; Amico, A.G.; Larosa, G.; Leanza, R.; et al. Clinical and research activities at the CATANA facility of INFN-LNS: From the conventional hadrontherapy to the laser-driven approach. Front. Oncol. 2017, 7, 223. [Google Scholar] [CrossRef]
- Cuttone, G.; Cirrone, G.A.P.; Di Franco, G.; La Monaca, V.; Nigro, S.L.; Ott, J.; Pittera, S.; Privitera, G.; Raffaele, L.; Reibaldi, A.; et al. CATANA protontherapy facility: The state of art of clinical and dosimetric experience. Eur. Phys. J. Plus 2011, 126, 65–67. [Google Scholar] [CrossRef]
- Spatola, C.; Privitera, G. Clinical aspects and potential clinical applications of laser accelerated proton beams. In Proceedings of the 2nd Elimed Workshop and Panel, Catania, Italy, 18–19 October 2012; AIP Publishing: Melville, NY, USA, 2013; Volume 1546, pp. 108–111. [Google Scholar]
- Tocco, A.; Privitera, G.; Raffaele, L.; Salamone, V.; Scoglio, C.; Milazzotto, R.; Marletta, D.; Cuttone, G.; Cirrone, G.A.P.; Russo, A.; et al. Porocarcinoma of the eyelid treated with proton beam radiotherapy: Case report and literature review. Acta Medica Mediterr. 2018, 34, 709. [Google Scholar]
- Milazzotto, R.; Liardo, R.L.E.; Privitera, G.; Raffaele, L.; Salamone, V.; Arena, F.; Pergolizzi, S.; Cuttone, G.; Cirrone, G.A.P.; Russo, A.; et al. Proton beam radiotherapy of locally advanced or recurrent conjunctival squamous cell carcinoma: Experience of the CATANA Centre. J. Radiother. Pract. 2020, 1–8. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spatola, C.; Liardo, R.L.E.; Milazzotto, R.; Raffaele, L.; Salamone, V.; Basile, A.; Foti, P.V.; Palmucci, S.; Cirrone, G.A.P.; Cuttone, G.; et al. Radiotherapy of Conjunctival Melanoma: Role and Challenges of Brachytherapy, Photon-Beam and Protontherapy. Appl. Sci. 2020, 10, 9071. https://doi.org/10.3390/app10249071
Spatola C, Liardo RLE, Milazzotto R, Raffaele L, Salamone V, Basile A, Foti PV, Palmucci S, Cirrone GAP, Cuttone G, et al. Radiotherapy of Conjunctival Melanoma: Role and Challenges of Brachytherapy, Photon-Beam and Protontherapy. Applied Sciences. 2020; 10(24):9071. https://doi.org/10.3390/app10249071
Chicago/Turabian StyleSpatola, Corrado, Rocco Luca Emanuele Liardo, Roberto Milazzotto, Luigi Raffaele, Vincenzo Salamone, Antonio Basile, Pietro Valerio Foti, Stefano Palmucci, Giuseppe Antonio Pablo Cirrone, Giacomo Cuttone, and et al. 2020. "Radiotherapy of Conjunctival Melanoma: Role and Challenges of Brachytherapy, Photon-Beam and Protontherapy" Applied Sciences 10, no. 24: 9071. https://doi.org/10.3390/app10249071
APA StyleSpatola, C., Liardo, R. L. E., Milazzotto, R., Raffaele, L., Salamone, V., Basile, A., Foti, P. V., Palmucci, S., Cirrone, G. A. P., Cuttone, G., Russo, A., Avitabile, T., Reibaldi, M., Longo, A., Broggi, G., Bonfiglio, V., Caltabiano, R., Pergolizzi, S., & Arena, F. (2020). Radiotherapy of Conjunctival Melanoma: Role and Challenges of Brachytherapy, Photon-Beam and Protontherapy. Applied Sciences, 10(24), 9071. https://doi.org/10.3390/app10249071








