Abstract
The synthesis of zeolites using waste as a source of Si and Al is well known, and light coal ash has been studied to minimize the problems of waste management and mitigate environmental effects. The residue used in this work was supplied by Coal Workers Assistance Society (SATC) Criciúma–SC/Brazil, and had 24.09% Al2O3 and 54.25% SiO2 in its chemical composition. Synthesis studies using this residue with the objective of obtaining LTA zeolites were carried out by hydrothermal means, alkaline fusion, and the combination of the two methods, varying parameters such as crystallization time, Na/T ratio, OH/ratio, ultrasound exposure, gel agitation temperature, and the alkaline melting temperature of the residue. The results were characterized by X-ray diffraction (XRD) techniques and scanning electron microscopy (SEM-FEG). It was possible to obtain 70% crystalline zeolite type LTA for the first time at mild conditions with temperatures below 200 °C by alkaline fusion with smaller amounts of NaOH and short times (2 h). Thus, suitable parameters were determined for future scaling.
1. Introduction
Coal ash is generated by the combustion of coal in thermoelectric power plants as a waste product. Given the increasing use of mineral coal, there is a need to minimize the impact it causes. The amount of coal ash produced in the world is approximately 500 million tons per year [1]. According to Sabedot et al. (2015) [1], burning this fuel produces ashes that are classified as slag, bottom ash (heavy), and fly ash (light). Slag and bottom ash are particles of varying dimensions, usually coarse, that accumulate in temporary deposits in companies that burn coal; light ash, or fly ash, is made up of particles smaller than 0.15 nm and is carried away by combustion gases [2]. The largest amount of waste generated from this burning is fly ash (84% of the total coal ash) [1,3]. The “ecoba”, European Coal Combustion Products Association [4] classifies fly ash (CF) as a fine-grained powder composed of spherical glassy particles. These ashes mainly contain silicon and aluminum (SiO2 and Al2O3) and are obtained from electrostatic or mechanical precipitates. Approximately 20% of fly ash that is produced on a worldwide scale in the combustion of mineral coal is reused as raw material for cement industries to produce Portland cement. Other applications are in soil improvement, ceramic industries, and the production of catalysts and zeolites [5]. According to Rocha Junior et al. (2012) [6], the consumption of mineral coal in Brazil should reach 3.5 or 4 million tons per year; however, the cement industry will not have a significant increase to compensate for this growth. Therefore, processes that can transform these ashes into a material with high added value have become necessary to reduce the accumulation of this material in the environment.
The use of fly ash as a precursor to zeolitic materials occurs due to the great similarity in the chemical composition with the volcanic material responsible for the formation of natural zeolites, thus allowing through this residue to synthesize new zeolites as a high-value product and further stimulating its use in this process [5]. Zeolites are crystalline aluminosilicates with wide industrial applications, such as oil refining, the petrochemical industry, and fine chemical production that use zeolite-based catalysts [7]. The actual market for synthetic zeolite materials is approximately US$5.2 billion per year, and it is expected that this value will increase to US$5.9 billion per year by 2023. The most used zeolite types are MFI (ZSM-5), LTA, and FAU (X, Y, and USY). LTA zeolite is the most commercialized in the world both in volume and in value; it is used mainly used as an additive in laundry detergents. The most recent estimate of the size of the zeolite market in the detergent market in 2018 was US$1.4 billion, and that figure is forecast to rise to US$1.8 billion in 2028 [8]. In addition to the use of zeolite LTA for laundry detergents [9], it is also used as a catalyst for the industrial dehydration of ethanol [10], for antimicrobial materials when exchanged with Ag ions [11], and for the separation of gases [12]. LTA zeolites have an Si/Al ratio of 1. It belongs to the cubic system and, when it is completely hydrated and in solid form, has a unit cell parameter equal to 24.60 Å. The chemical formula for the unit cell can be expressed as Na96Al96Si96O384·27H2O [7].
The LTA zeolite was the first synthetic zeolite to be commercialized [8]; these materials have been continuously studied. This can be seen in the number of papers that have been published about this zeolite concerning novel synthesis methods, diversification of applications, and upgrading of waste materials containing aluminum and silicon for raw synthesis materials. The synthesis of zeolite LTA can be performed using fly ash as a source of silicon and aluminum [1,13,14,15,16,17,18,19,20]. These studies show different methods of synthesis. For example, the study by Molina and Polle [19] compared two methods, the traditional hydrothermal and an alkaline fusion of fly ash with NaOH prior to hydrothermal treatment. This study demonstrated that, regardless of the method adopted, the fly ash can be converted into X and A zeolites, which later transform to the more stable P zeolite. Tanaka et al. [1] presented a new method in two steps using microwaves to produce LTA zeolite as a single phase. Iqbal et al. [18] used a fly ash extract of Si-Al-Na species to prepare 4A zeolites by following the synthesis procedure of a two-step method by applying a stepwise change of temperature during the treatment. Kunecki et al. [20] used the fusion method to obtain X and A zeolites in a single phase, varying the synthesis parameters. Although much research has been done regarding this synthesis, fly ash has not yet been used as a raw material on an industrial scale, mainly due to the lack of optimization of the synthesis parameters for adequate scaling.
The present study aims to investigate some new parameters of the synthesis of LTA-type zeolites using light coal ash, aiming for a future industrial application of this synthesis. Thus, this study is expected to make use of a residue generated in the combustion of mineral coal viable to produce products with greater added value and to mitigate the effects on the environment.
2. Materials and Methods
The synthesis of the standard LTA zeolite was carried out following the methodology described by the International Zeolite Association (IZA), that is, 40 g of H2O, 3.585 g of NaOH, 2.5 g of SiO2, and 3.86 g of sodium aluminate were used. Crystallization occurred statically at 100 °C for 4 h. This synthesis was defined as the standard LTA.
The synthesis of LTA-type zeolites using coal fly ash (CF) was carried out using the route proposed by Bieseki et al. [21]. Two methodologies were employed: synthesis through the conventional hydrothermal treatment and the two-stage alkaline fusion treatment followed by the conventional hydrothermal treatment. This procedure is shown in Figure 1.
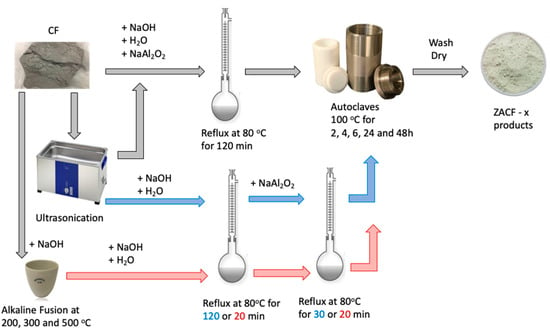
Figure 1.
Schematic procedure for material synthesis employing the coal fly ash (CF) residue.
In the hydrothermal process, the mixture of sodium hydroxide, fly ash, and water can be subjected to a much milder temperature (80–200 °C) [22]. The use of temperature facilitates the dissolution of less stable phases; however, phases such as quartz may remain. The conventional hydrothermal method (open or closed reflux systems) for alkaline fly ash activation has been widely used in previous studies [17,23,24]. The other methodology applied in the study of the synthesis of zeolite LTA using fly ash follows in two stages: alkaline fusion followed by hydrothermal treatment. When performing an alkaline fusion, the quartz present in the residue is dissolved totally or partially, depending on the temperature used [18,25,26]. After the fusion, the conventional hydrothermal treatment is followed in the synthesis process of the LTA zeolite. Many parameters were studied, some of them for the first time: gel stirring time and temperature; residue ultrasonication; reflux at 80 °C for 20 or 120 min; addition of Al; Na/T ratio; OH/T ratio; and crystallization time.
The raw material CF and the synthesized zeolites were characterized by X-ray fluorescence (FRX), X-ray diffraction, and scanning electron microscopy (SEM-FEG). The analyses of the studied samples were performed in a Bruker diffractometer model D2 Phaser® using CuKα radiation (λ = 1.54 Å) with a Ni filter, operating at a current of 10 mA and a voltage of 30 kV in an angle range 2θ = 3–50°. The analyses were performed with a divergent slit of 0.6 mm and a central slit of 1.0 mm, and the collection of the signals was done with a Lynxeye detector. The scan was carried out per step with an increment of 0.02 and an acquisition time of 0.1 s. The crystallinity of the materials was calculated using the 2θ diffraction angles (7.2°; 12.5°; 16.1°; 21.7°; 30°; and 34.2°) [27] with Equation (1), using the Standard LTA synthesized as a reference.
Scanning electron microscopy (SEM) analysis was performed on a Zeiss Auriga 40 device operating at 3 kV. In the study of the samples, a small portion was taken and placed under a sample holder where scans were acquired at different points within the sample with different magnifications.
3. Results and Discussion
3.1. Characterization of Raw Material
The data from the chemical analysis of the light coal ash (CF) residue from thermoelectric power plants in the region of Florianópolis-SC/Brazil are shown in Table 1. It appears that the residue mainly contains Al2O3 (24.09%) and SiO2 (54.25%), making it a suitable material for zeolite synthesis. The Si/Al molar ratio of the residue is approximately 2.2; however, for the synthesis of the LTA zeolite, the ideal is a Si/Al = 1 ratio [21]. Thus, it will be necessary to add Al to complement this relationship.

Table 1.
Composition of the CF residue.
The residue has in its crystalline species such as quartz and mullite (Figure 2a). The mullite present in the ashes is the result of the reactions that occur during the combustion of coal and quartz; it is present in the precursor coal and does not melt in the burning conditions in the thermoelectric plants [15]. The morphology of the waste is spherical (Figure 2b) because the burning of the coal occurs in its pulverized form [28].
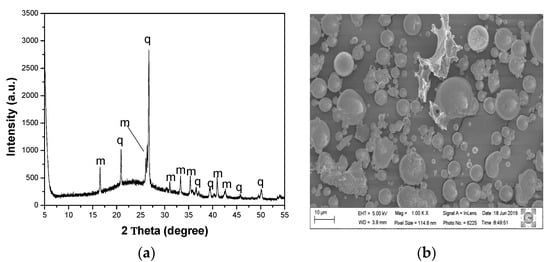
Figure 2.
(a) X-ray diffractogram of the CF residue where m—mullite and q—quartz, and (b) SEM image of the CF residue.
3.2. Syntheses by the Conventional Hydrothermal Method
The study of syntheses using light ash from mineral coal as the primary source of Si and Al was carried out, modifying parameters such as the stirring time, Na/T ratio, OH/T ratio (where T = Si + Al), synthesis time, the temperature of agitation of the synthesis gel, the sonochemical treatment, and the alkaline fusion of the residue. Table 2 shows the general picture of the changes made in the study of the syntheses of the LTA-type zeolite samples using fly ash. The materials are denoted as ZACF (Zeolite A from Coal Ash) plus the number of the experiment.

Table 2.
Nomenclature and parameters studied for the samples.
Comparing the ZACF-1 and ZACF-2 samples with the standard LTA (Figure 3), the variation of the Na/T and OH/T ratios in the synthesis gel did not lead to the formation of the LTA structure (the crystallinities of the samples were below 20%). In Figure 3b, the diffractogram demonstrates the effect of the crystallization time; small peaks indicative of the LTA zeolite appear after 4 h in the ZACF-2 sample. The quartz and mullite that did not dissolve can be observed, and this dissolution is necessary for the formation of the zeolitic structure. At longer times, there is no increase in the dissolution of these phases, but rather sodalite appears, which is a more stable zeolitic phase than LTA. Quartz is a very stable phase, and therefore, remains in the product; for its complete dissolution, an alkaline fusion is necessary [21].
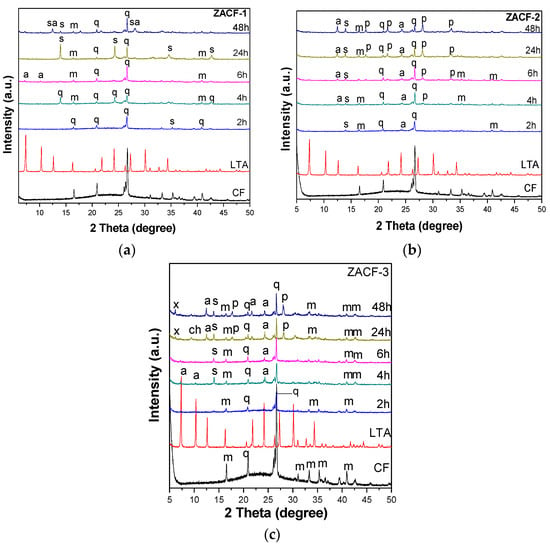
Figure 3.
X-ray diffractogram of (a) ZACF-1, (b) ZACF-2, and (c) ZACF-3 zeolites; where: m—mullite, q—quartz, sa—SAPO, p—zeolite P, s—sodalite, x—zeolite X, a—zeolite LTA.
In the ZACF-3 and ZACF-4 samples, the synthesis gel was refluxed at 80 °C for 120 min under magnetic stirring. In the ZACF-4 sample, the fly ash residue was also subjected to a sonochemical treatment. The X-ray diffractograms for these samples are shown in Figure 3c and Figure 4a. The increase in temperature and the sonochemical treatment favor the formation of the LTA zeolite after 2 h of crystallization. At longer times, more stable phases are observed, such as sodalite (2θ = 13.96), P (2θ = 17.8; 28.02; and 33.38), and Chabasite (2θ = 24.2), leading to a decrease of the crystallinity of the LTA zeolite. The quartz and mullite phases were not fully dissolved. The crystallinity of the product obtained in the ZACF-4 samples was below 20%, and there was no formation of the desired zeolitic phase in the ZACF-3.
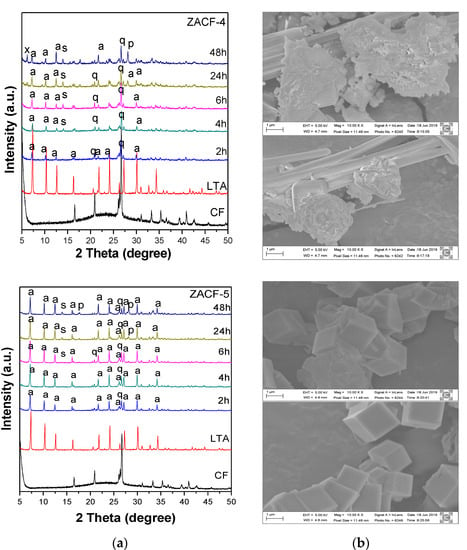
Figure 4.
(a) X-ray diffractogram of zeolites ZACF-4 and ZACF-5, where q—quartz, p—zeolite P, s—sodalite, x—zeolite X, a—zeolite LTA; (b) SEM Images of ZACF-4 and ZSCF-5 obtained at 2 h and 4 h.
In samples ZACF-4 and ZACF-5, both the residues were submitted to sonochemical treatment. However, in the sample ZACF-5, the addition of sodium aluminate was proposed after magnetic stirring for 120 min under reflux in the oil bath at 80 °C and remained for another 30 min under the same conditions of temperature and stirring under the reflux. The result of this modification in the synthesis process resulted in a crystallinity of more than 50% in a synthesis time of 2 h. This finding indicates that the 80 °C treatments at reflux and sonochemical treatment favor the dissolution of the residue when the sodium aluminate is not present. Figure 4 and Table 3 present these results; the presence of more intense peaks is observed, identifying the zeolitic material of the LTA type. There is also a decrease in the peak of the quartz and mullite.

Table 3.
Crystallinity of samples ZACF-4 and ZACF-5.
The scanning electron microscopy analysis shows the morphology of cubic crystals characteristic of LTA-type zeolite in the ZACF-5 sample. In the ZACF-4 image, a stick-like structure is observed, indicating the presence of quartz, while agglomerated crystals of spherical shape possibly indicate the sodalite phase.
In the ZACF-5 and ZACF-6 samples, the aluminate was added after the initial stirring under reflux at 80 °C in an oil bath for 120 min; however, for the ZACF-6 sample, the residue was not ultrasonicated. The sonochemical treatment allowed the formation of more intense peaks that are characteristic of LTA-type zeolite, indicating a greater formation of the material and consequently, crystallinity (Figure 5). The percentage of crystallinity in the ZACF-6 sample did not reach 15%.
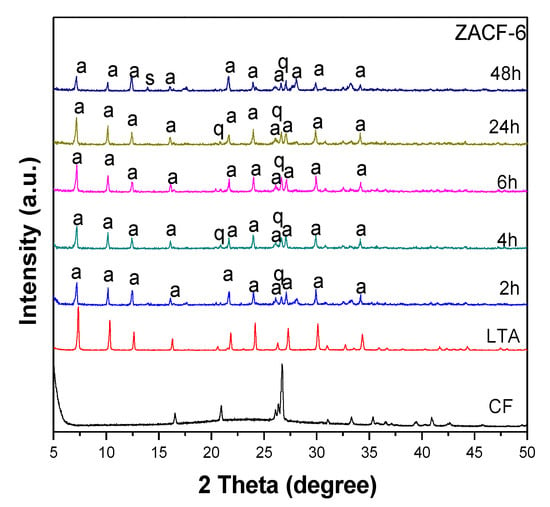
Figure 5.
X-ray diffractograms of ZACV-6 samples, where q—quartz, s—sodalite, a—zeolite LTA.
3.3. Syntheses by the Two-Step Method: Alkaline Fusion Followed by Hydrothermal Treatment
In the alkaline melting process, the mixture of fly ash and sodium hydroxide was melted. After melting, water was added, and followed by conventional hydrothermal treatment. When performing an alkaline fusion, the quartz present in the residue is allowed to be totally or partially dissolved, depending on the temperature used [13]. The fusion with NaOH before the hydrothermal reaction was applied by Shigemoto et al. [29], where they studied different NaOH/fly ash ratios and observed that the ideal amount would be an NaOH/Ash ratio = 1.5 g/g.
In the experiments carried out, no extra NaOH was added; maintaining the amount needed for the composition of the standard gel, the NaOH/Ash value remained at 0.9 g/g. Table 4 shows the description of the parameters used for the zeolite samples synthesized by the two-stage methodology.

Table 4.
Parameters used in the synthesis of LTA-type zeolites using light coal ash in two stages: alkaline fusion followed by hydrothermal treatment.
Figure 6 shows the diffractogram of the residual samples melted with NaOH at different temperatures; thus, this finding verifies that at higher temperatures (500 °C), complete dissolution occurs in the residue, making it amorphous.
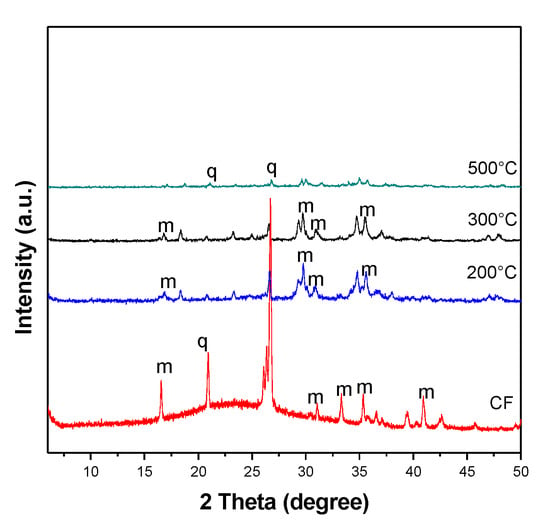
Figure 6.
Comparison of X-ray diffractogram of coal samples fused with NaOH at different temperatures, where q—quartz and m—mullite.
The samples ZACF-200, ZACF-300, and ZACF-500 were all synthesized at the same conditions described in Table 3, varying only the alkaline fusion temperature. It was verified through the X-ray diffractograms (Figure 7) that the fusion alkaline followed by the hydrothermal step led to zeolitic phases. The alkaline fusion treatment provides greater availability of Si and Al, leading to more crystalline zeolitic phases. The formation of zeolite LTA was observed; at longer times and/or higher temperatures, they tend to form more stable structures in the melting stage, such as sodalite (S). The crystallization percentages (Table 5) support this, where crystallinity was observed above 70% in 2 h of synthesis for the ZACF-200 sample. The cubic morphology proves the formation of LTA zeolite (Figure 8); at longer times, the growth of sodalite crystals (spherical agglomerates) was observed.
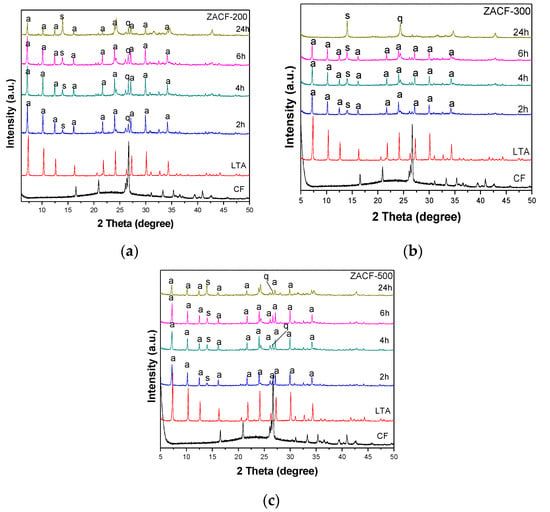
Figure 7.
Diffractograms of samples prepared by alkaline fusion followed at (a) 200 °C, (b) 300 °C, and (c) 500 °C by the hydrothermal treatment, where q—quartz, s—sodalite, a—zeolite LTA.

Table 5.
Crystallinity of samples prepared by alkaline fusion followed by the hydrothermal treatment.
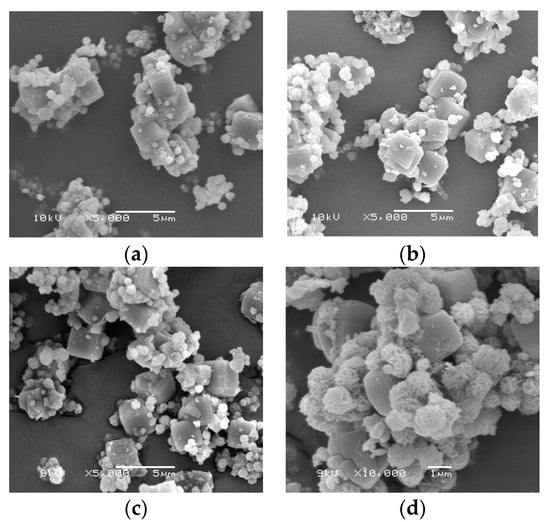
Figure 8.
SEM image analysis of ZACF-200 °C samples obtained after (a) 2 h, (b) 4 h, (c) 6 h, and (d) 24 h of synthesis.
4. Conclusions
The study of different synthesis parameters and methodologies for the formation of LTA-type zeolites from light coal ash showed us that the synthesis of the materials was feasible, as expected since the residue has large amounts of Si and Al. It was observed that time and more drastic conditions led us to obtain more stable zeolitic phases, such as sodalite.
On the other hand, it was possible to prove new parameters of synthesis. The use of treatments at reflux at 80 °C, sonochemical treatment, and alkaline fusion assist in the dissolution of the residue, and thus make Si and Al available for the formation of the zeolitic structure. Once the residue was dissolved and the quartz structures and other minerals present were destroyed, it was of great importance the subsequent addition of sodium aluminate to correct the composition of the synthesis gel.
By controlling the synthesis parameters to optimize the availability of Al and Si from the residue and controlling the stages and the composition of the synthesis gel, it is possible to direct the synthesis of a specific zeolite with a high degree of crystallinity. In this work, we managed to achieve more than 70% crystallinity using mild conditions, temperatures below 200 °C, alkaline fusion with smaller amounts of NaOH, and short times (2 h).
Author Contributions
Conceptualization, S.P.; Data curation, T.J.T.C. and M.I.S.M.; Formal analysis, M.I.S.M.; Funding acquisition, S.P.; Investigation, T.J.T.C.; Methodology, T.J.T.C. and S.P.; Project administration, S.P.; Supervision, M.I.S.M. and S.P.; Validation, T.J.T.C. and M.M.; Writing—original draft, T.J.T.C.; Writing—review & editing, S.P. All authors contributed substantially to the work reported. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development–CNPq., grant number 430485/2018-2.
Acknowledgments
To CNPq for financial support. To PPG and UFRN.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tanaka, H.; Fujji, A.; Fujimoto, S.; Tanaka, Y. Microwave-Assited Two-Step Process for the Synthesis of a Single-Phase Na-A Zeolite from Coal Fly Ash. Adv. Powder Tecnol. 2008, 19, 83–94. [Google Scholar] [CrossRef]
- Sabedot, S.; Sundstron, M.G.; Miltzarek, G.L.; Sampaio, C.H. Tecnologia Mineral para Cinzas da Combustão de Carvão Mineral da Região Carbonífera do Baixo Jacuí-Rs. Tecnol. Metal. Mater. Mineração 2015, 12, 244–250. [Google Scholar] [CrossRef][Green Version]
- Pedrolo, D.R.S.; De Menezes Quines, L.K.; De Souza, G.; Marcilio, N.R. PEDROLO, Débora Regina Strossi e colab. Synthesis of zeolites from Brazilian coal ash and its application in SO2 adsorption. J. Environ. Chem. Eng. 2017, 5, 4788–4794. [Google Scholar] [CrossRef]
- ECOBA. First European conference on ‘Coal Combustion Products-Sustainable materials for the future’. In VGB Power Tech; ECOBA: Hanoi, Vietnam, 2005. [Google Scholar]
- Yao, Z.; Ji, X.; Sarker, P.K.; Tang, J.; Ge, L.; Xia, M.; Xi, Y. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Junior, C.A.F.R.; Santos, S.C.A.; Souza, C.A.G.; Angélica, R.S.; Neves, R.F. Síntese de zeólitas a partir de cinza volante de caldeiras: Caracterização física, química e mineralógica. Cerâmica 2012, 58, 43–52. [Google Scholar] [CrossRef]
- Rigo, R.T.; Pergher, S.B.C.; Petkowicz, D.I.; Dos Santos, J.H.Z. A new procedure for a zeolite synthesis from natural clays. Quimica Nova 2009, 32, 21–25. [Google Scholar] [CrossRef]
- Collins, F.; Rozhkovskaya, A.; Outram, J.G.; Millar, G.J. A critical review of waste resources, synthesis, and applications for Zeolite LTA. Microporous Mesoporous Mater. 2020, 291, 109667. [Google Scholar] [CrossRef]
- Sutili, F.K.; Miotto, N.; Rigoti, E.; Pergher, S.B.C.; Penha, F.G. Application of synthetic zeolites as builder in detergent formulation. Quim. Nova 2009, 32, 879–883. [Google Scholar] [CrossRef]
- Sato, K.; Aoki, K.; Sugimoto, K.; Izumi, K.; Inoue, S.; Saito, J.; Ikeda, S.; Nakane, T. Dehydrating performance of commercial LTA zeolite membranes and application to fuel grade bio-ethanol production by hybrid distillation/vapor permeation process. Microporous Mesoporous Mater. 2008, 115, 184–188. [Google Scholar] [CrossRef]
- De Araújo, L.O.; Anaya, K.; Pergher, S.B.C. Synthesis of Antimicrobial Films Based on Low-Density Polyethylene (LDPE) and Zeolite A Containing Silver. Coatings 2019, 9, 786. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite membranes—Recent developments and progress. Microporous Mesoporous Mater. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Feng, W.; Wan, Z.; Daniels, J.; Li, Z.; Xiao, G.; Yu, J.; Xu, D.; Guo, H.; Zhang, D.; May, E.F.; et al. Synthesis of high quality zeolites from coal fly ash: Mobility of hazardous elements and environmental applications. J. Clean. Prod. 2018, 202, 390–400. [Google Scholar] [CrossRef]
- Querol, X.; Plana, F.; Alastuey, A.; Lopez-Soler, A. Synthesis of Na-zeolites from fly ash. Science 1997, 76, 793–799. [Google Scholar] [CrossRef]
- Jafari, M.; Nouri, A.; Mousavi, S.F.; Mohammadi, T.; Kazemimoghadam, M. Optimization of synthesis conditions for preparation of ceramic (A-type zeolite) membranes in dehydration of ethylene glycol. Ceram. Int. 2013, 39, 6971–6979. [Google Scholar] [CrossRef]
- Behin, J.; Bukhari, S.S.; Dehnavi, V.; Kazemian, H.; Rohani, S. Using Coal Fly Ash and Wastewater for Microwave Synthesis of LTA Zeolite. Chem. Eng. Technol. 2014, 37, 1532–1540. [Google Scholar] [CrossRef]
- Jha, B.; Singh, D.N. Fly Ash Zeolites; Advanced Structured Materials Series; Springer: Berlin/Heidelberg, Germany, 2016; Volume 78. [Google Scholar]
- Iqbal, A.; Sattar, H.; Haider, R.; Munir, S. Synthesis and characterization of pure phase zeolite 4A from coal fly ash. J. Clean. Prod. 2019, 219, 258–267. [Google Scholar] [CrossRef]
- Molina, A.; Polle, C. Acomparative study using two methods to produce zeolites from fly ash. Miner. Eng. 2004, 17, 167–173. [Google Scholar] [CrossRef]
- Kunecki, P.; Panek r Wdowin, M.; Franus, W. Synthesis od Faujasite (FAU) and tschernichite (LTA) type zeolites as a potential direction of the development of lime Class C fly ash. Int. J. Miner. Proc. 2017, 166, 69–78. [Google Scholar] [CrossRef]
- Bieseki, L.; Penha, F.G.; Pergher, S.B.C. Zeolite A synthesis employing a brazilian coal ash as the silicon and aluminum source and its applications in adsorption and pigment formulation. Mater. Res. 2012, 16, 38–43. [Google Scholar] [CrossRef]
- Steenbruggen, G.; Hollman, G. The synthesis of zeolites from fly ash and the properties of the zeolite products. J. Geochem. Explor. 1998, 62, 305–309. [Google Scholar] [CrossRef]
- Izidoro, J.D.E.C. Estudos Sobre a Remoção de Íons Metálicos em Água Usando Zeólitas Sintetizadas a Partir de Cinzas de Carvão. Master’s Thesis, IPEN, São Paulo–SP, Brazil, 2008. [Google Scholar]
- Łach, M.; Grela, A.; Komar, N.; Mikuła, J.; Hebda, M. Calcined Post-Production Waste as Materials Suitable for the Hydrothermal Synthesis of Zeolites. Materials 2019, 12, 2742. [Google Scholar] [CrossRef] [PubMed]
- Ríos, R.; Williams, C.D.; Roberts, C.L. A comparative study of two methods for the synthesis of fly ash-based sodium and potassium type zeolites. Fuel 2009, 88, 1403–1416. [Google Scholar] [CrossRef]
- Tanaka, H.; Eguchi, H.; Fujimoto, S.; Hino, R. Two-step process for synthesis of a single phase Na–A zeolite from coal fly ash by dialysis. Fuel 2006, 85, 1329–1334. [Google Scholar] [CrossRef]
- Bieseki, L. Síntese de Zeólitas e Argilas Ácidas Pilarizadas a Partir de Matérias-Primas Naturais. Master’s Thesis, UFRN, Natal–RN, Brazil, 2012. [Google Scholar]
- Giannetto, P.G. Zeólitas: Características, Propriedades y Aplicaciones Industriales; Editorial Innovacín Tecnológica, Facultad de Ingeniería; UCV: Caracas, Venezuela, 1990. [Google Scholar]
- Shigemoto, N.; Hayashi, H.; Miyaura, K. Selective formation of Na-X zeolite from coal fly ash by fusion with sodium hydroxide prior to hydrothermal reaction. J. Mater. Sci. 1993, 28, 4781–4786. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).