Abstract
The coal structure deformation attributed to actions of tectonic stresses can change characteristics of nanopore structure of coals, affecting their CO2 adsorption. Three tectonically deformed coals and one undeformed coal were chosen as the research objects. The isotherm adsorption experiments of four coal specimens were carried out at the temperature of 35 °C and the pressure of 0 to 7 MPa. Nanopore structures were characterized using the liquid nitrogen adsorption method. The results show that there exist maximum values of excess and absolute adsorption capacity, which increase with increasing coal deformation degree. As the degree of coal deformation increases, the pore volume and specific surface area present an obvious increasing trend in the case of micropores, exhibiting an increase at first (cataclastic coal and ganulitic coal) and then stabilization (crumple coal), in the case of mesopores, and showing a gradual decrease in the case of macropores. The mesopores are the key factor of CO2 adsorption of tectonically deformed coals, followed by the micropores and the limited effect of macropores at the strong coal deformation stage.
1. Introduction
In China, the exploration, development, and use of coalbed methane has been paid more and more attention to in recent years. Although coalbed methane resources are very rich in China, the output of coalbed methane shows a steady downward trend [1]. A great amount of coalbed methane is stored in the coal seam damaged by action of tectonic stress. The effect of tectonic stress causes the damage and transformation of coal body structure, and thereby induces the tectonically deformed coals [2,3,4]. However, “two high and two low” characteristics of tectonically deformed coals, i.e., high gas content, high gas desorption rate, low permeability, and low coal strength, cause the dangerous areas of gas outburst in the tectonically deformed coal development areas, and thereby restrict the efficient exploitation of coalbed methane [5,6]. Since the coal has larger adsorption capacity of CO2 compared to CH4, the replacement effect by injecting CO2 into the coal seam can enhance the recovery rate of coalbed methane (CO2-ECBM) and reduce greenhouse gas emissions effectively [7]. Therefore, CO2 adsorption characteristics of tectonically deformed coal is one of key problems during the coalbed methane exploitation by CO2 injection.
The gas in coal mainly at adsorption state occurs on the inner surface of coal matrix particles. Therefore, the pore structure is an important factor affecting the gas adsorption characteristics of coal. Many previous studies have indicated the relationship between pore structure and gas adsorption of coal [8,9]. Meanwhile, they have proposed that the coal adsorption is affected by coal pore volume, pore area, pore size, pore shape, and pore complexity, and revealed that developmental characteristics of micropores or nanopores are main factors affecting coal adsorption capacity to a large extent. For example, Castello et al. [10] found that the adsorption capacity of coal was proportional to the micropore volume. Chen et al. [11] believed that the specific surface area of nanopores determined the adsorption capacity of coal. Jian et al. [12] studied the relationships between the pore structure and adsorption characteristics of low rank coal, and found that developmental characteristics of microporous pore volume determined change laws of gas adsorption characteristics.
At present, there have been some studies on tectonically deformed coal adsorption, and some basic laws have been instituted. It has been found that the adsorption capacity of tectonically deformed coal is significantly greater than that of primary coal at the same coal rank, which is attributed to significant changes in pore volume and the specific surface area of micropores, as well as the developmental degree and connectivity of pore fractures of coal suffering from tectonic stress [13,14]. However, there are still many problems to be investigated related to the CO2 adsorption characteristics of tectonically deformed coal. For example, what are basic laws of CO2 adsorption characteristics of tectonically deformed coal? How do vary the CO2 adsorption capacity with increasing coal deformation degree? What are the evolutionary characteristics of nanopores of coal with coal deformation degrees, and their effects on the CO2 adsorption?
Therefore, tectonically deformed coals with different deformation degrees and deformation types are chosen as the research object. Isothermal adsorption experiments and pore structure tests and analysis were carried out to reveal evolution laws of CO2 adsorption and nanopores with coal deformation degree. Finally, the control mechanisms of nanopore structure evolution on CO2 adsorption of tectonically deformed coals were investigated.
2. Specimens and Experimental Methods
2.1. Coal Specimens and Their Characteristics
2.1.1. Coal Specimen Collection
In the present study, the sampling site is located at the Zhuxianzhuang coal mine in the Suxian mining area of Huaibei coalfield (Figure 1). The main coal bearing strata are the Permian Shihezi and Shanxi formations. The Shanxi formation, with a thickness of 96 m to 143 m and 120 m on average, is widely distributed throughout the whole area. As one of the main coal bearing sections in the area, the Shanxi formation presents well-developed number 10 and 11 coal seams. The stable number 10 coal seam has a cumulative thickness varying from 2.0 m to 3.5 m, and is the main minable coal seam in the Suxian mining area. The regional control structure of the Suxian mining area is composed of the Sudong syncline in the overlying system, and the Sunan and Nanping synclines in the underlying system of the Xishipo thrust fault. The Zhuxianzhuang coal mine is located in the overlying system of the Xisipo thrust fault, with very large compression and shear stress and intense tectonic deformation. Therefore, complete types of tectonically deformed coals have developed there.
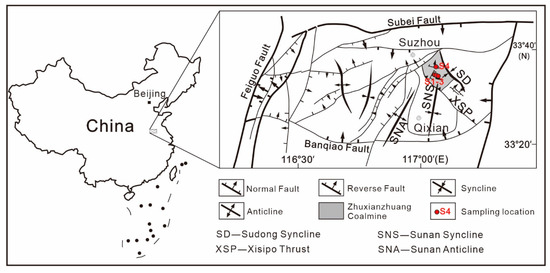
Figure 1.
Regional structure of the study area and sampling locations.
The underground sampling is located near the newly exposed structure of the number 10 coal seam. Tectonically deformed coal samples of different deformation degrees are collected from different distances to the fault plane (Figure 1). The methods of collection and preservation of coal specimens are based on the “Sampling of coal seams” (GB/T 482-2008) and “Sampling of coal petrology” (GB/T 19222-2003). During sampling, the original structure of the coal body should be maintained as fully as possible. Three tectonically deformed coal specimens and one undeformed coal specimen are chosen as the research objects.
2.1.2. Coal Specimen Properties
The basic properties of the coal specimens are shown in Table 1. Vitrinite reflectance and macerals were carried on a microspectrophotometer (AXIO Imager M1m, Carl Zeiss, Germany). According to proximate analysis of coal (GB/T 212-2008), the proximate analyses of coal specimens were carried out by employing the Self Employed Industrial Instrument and Analyzer (SDTGA8000a, Hunan Sundy Technology Co., Ltd., China). The ultimate analyses were carried out by an element analyzer (PerkinElmer 2400 II). Table 1 shows that the maximum vitrinite reflectance varies from 0.9% to 1.17%, and the volatile content (wt.%) changes from 30.27% to 34.06%. Therefore, the collected specimens are highly volatile bituminous coal A.

Table 1.
Properties of coal specimens.
Macro- and microstructure deformation characteristics of four coal specimens were observed, including coal body structure, macro- and microfractures and folds, and etc. Then the three types of technically deformed coal specimens were recognized, i.e., undeformed coal, cataclastic coal, and crumpl coal; meanwhile, their deformation degree and deformation series were determined (Table 2 and Figure 2).

Table 2.
Macro and micro deformation characteristics of coal specimens.
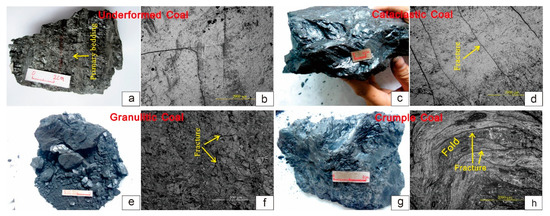
Figure 2.
Macro and micro photos of coal specimens. (a) Undeformed coal (macroscope); (b) undeformed coal (microscope); (c) cataclastic coal (macroscope); (d) cataclastic coal (microscope); (e) granulitic coal (macroscope); (f) granulitic coal (microscope); (g) crumple coal (macroscope); (h) crumple coal (microscope).
2.2. Experimental
2.2.1. Adsorption Experiments
The coal specimens were ground, and the particle size was in the range of 180–250 µm (60–80 mesh). About 100 g for each coal specimen were weighed to put into a thermostat with supersaturated K2SO4 solution for 48 h, in order to make coal specimens containing equilibrium moisture. Then, the isotherm adsorption experiments on the coal specimens were carried out by an ISO-300 isotherm adsorption instrument with the temperature of 35 °C and pressure varying from 0 to 7 MPa. The experimental principle is shown in Figure 3.
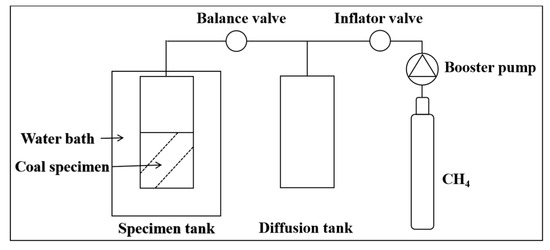
Figure 3.
Schematic diagram of isothermal adsorption experiment apparatus.
The detailed experiment procedure is as follows:
- (1)
- Put the coal specimens in the specimen tank and check the air tightness.
- (2)
- The free space volume of specimen tank and reference tank was measured.
- (3)
- Open the water bath heating system and set the experimental temperature to 35 °C.
- (4)
- Open the booster pump and inflation valve and close the balance valve to charge CH4 into the reference tank, and after the pressure is stable, open the balance valve and close the inflation valve. Then, the isothermal adsorption experiment begins.
- (5)
- After adsorption equilibrium, record the experimental equilibrium pressure and adsorption data, and repeat step (4) to obtain the adsorption data of other pressure points. Seven pressure points were set in this experiment, and the pressure balance time was 72 h.
- (6)
- After the completion of the adsorption experiment, open the balance valve and vent valve, and adjust the exhaust speed to make the maximum experimental pressure drop to standard atmospheric pressure within 2 h.
- (7)
- After the experiment, close the booster pump, cylinder valve, and water bath heating system, and take out the coal specimens.
According to the experimental method of high-pressure isothermal adsorption to coal (capacity method) (GB/T 19560-2008), and based on the dynamic change of gas pressure before and after adsorption, the gas adsorption increment is calculated using the material balance equation, and thereby the gas adsorption amount in the coal under different pressures can be obtained. The detailed calculation process is as follows.
Attributed to the conservation of mass during gas adsorption, the difference between the sum of the gas amount in the reference tank after the ith inflation, the gas amount in the specimen tank, and the total gas amount in both tanks after adsorption balance is regarded as the adsorption increment of the adsorption balance pressure point. The ith adsorption increment is given by
Since the gas pressure in the reference tank is equal to that in the specimen tank at the adsorption equilibrium state, i.e., Pi2 = Pi3, the equation is written as
The adsorption amount after the ith adsorption equilibrium is described as
Therefore, the adsorption capacity can be calculated by
where Δηiex is the adsorption increment after the ith adsorption equilibrium (mol); Vi is the adsorption capacity after the ith adsorption equilibrium (cm3·g−1); m is the mass of coal specimen (g); T is the experimental temperature (K); R is universal constant (R = 8.314); Vs is the free space volume of the experimental tank (cm3); Vr is the free space volume of the reference tank (cm3); Pi−1 is the gas pressure in the experimental tank (MPa); Pi1 is the gas pressure in the reference tank before the ith adsorption (MPa); Pi2 is the gas pressure in the experimental tank after the ith adsorption equilibrium (MPa); Pi3 is the gas pressure in the reference tank after the ith adsorption equilibrium (MPa); and Zi−1, Zi1, Zi2, and Zi3 are compression factors corresponding to the pressures of Pi−1, Pi1, Pi2, and Pi3, respectively.
2.2.2. Liquid Nitrogen Adsorption
The liquid nitrogen adsorption method reflects the pore volume, pore specific surface area, and pore size distribution of a solid through the adsorption law of nitrogen on the solid surface. The specimens with particle sizes in the range of 60–80 mesh were dried for 4 h at 80 °C. Liquid nitrogen absorption tests were carried out by the surface area and porosimetry system (ASAP-2020, Micromeritics Instrument Corp., Norcross, GA, USA) at 77.3 K. We can obtain the isotherm adsorption curve of adsorption amount versus relative pressure. The specific surface area and volume of nanopores (from 2 to 200 nm) of coal specimens were analyzed by the Barrett-Joyner-Halenda (BJH) method.
3. Results and Discussion
3.1. Adsorption Characteristics’ Evolution during Coal Structure Deformation
3.1.1. Excess Adsorption Curve
The experimental measurement of the adsorption capacity is known as Gibbs excess adsorption capacity. Figure 4 shows that isotherms of four coal specimens are not monotonous at the pressure of 0–7 MPa. The excess adsorption capacity of CO2 presents an increase at first, and then a decrease with increasing pressure, and shows the single peak curve, which coincides with type I of Gibbs adsorption isotherms proposed by Donohue and Aranovich [15]. An increase of coal deformation degree causes the overall increase in the excess adsorption capacity. According to granulitic coal and crumple coal with strongly structural deformation, the adsorption isotherms present crossing points; in the low pressure zone of 0–3.5 MPa, the adsorption capacity of granulitic coal is greater than that of crumple coal; as the pressure exceeds 3.5 MPa, the adsorption capacity of crumple coal is greater than that of granulitic coal. The maximum values of excess adsorption capacity of undeformed coal, cataclastic coal, granulitic coal, and crumple coal were 9.48 m3·g−1, 12.04 m3·g−1, 15.83 m3·g−1, and 16.74 m3·g−1, respectively. The maximum excess adsorption capacity increased with increasing degrees of coal deformation.
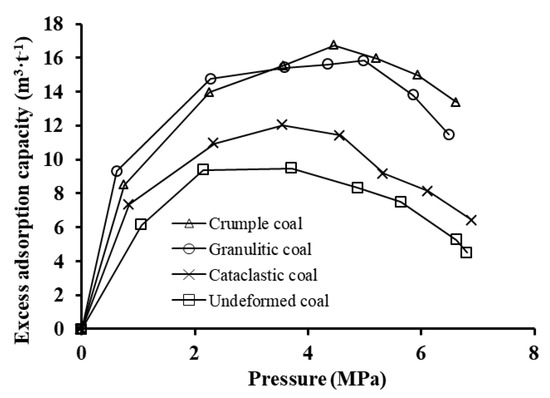
Figure 4.
Isotherms for excess adsorption of CO2 on coal.
The maximum excess adsorption capacity is present at pressure ranging from 4 MPa to 5 MPa, and with increasing coal deformation degree the pressure corresponding to maximum excess adsorption amount shifts to the high pressure. As shown in Figure 4, the maximum adsorption amount is located at the pressure of 4 MPa in cases of undeformed coal and cataclastic coal, and at the pressure larger than 5 MPa in cases of strongly deformed granulitic coal and crumple coal. More researches will be carried out in the future to validate the results attributed to the limited number of specimens in the present study.
3.1.2. Absolute Adsorption Curve
As mentioned above, isotherms of four coal specimens exhibited an increase at first and then a decrease with increasing pressure, and showed a single peak curve rather than monotonous characteristics. That indicates that with increasing pressure, CO2 gradually approaches a critical state and exhibits supercritical fluid quality, especially with pressure larger than 5 MPa. Therefore, the adsorption capacity measured by isothermal adsorption experiment is the excess adsorption capacity, also known as apparent adsorption capacity; the corresponding absolute adsorption capacity is the real adsorption capacity. According to the relationship between the excess adsorption capacity and the real adsorption capacity, as described in Equation (5) [16,17], the difference between them is mainly attributed to the densities of the adsorbed phase and bulk gas phase.
where Vab and Vex denotes absolute and excess adsorption capacity, respectively (m3·t−1); and ρad and ρg denote the density of the adsorption phase and gas phase, respectively (kg m−3). When supercritical CO2 is in the adsorption phase, it is often regarded as the gas with ultimate compression, which his attributed to its non-liquefying property. The volume occupied by a CO2 molecule is its intrinsic volume, and reduces the free space of the molecule, which is consistent with the meaning of volume correction term b in a van der Waals gas state equation. Therefore, the volume correction term b value given by Equation (6) [18] is employed to calculated the density of CO2 adsorption phase, i.e., the ultimate compression density of CO2 equal to 1028 kg·cm−3. The gas phase density of CO2 is chosen according to values determined by U.S. National Institute of standards and Technology (NIST):
where ρad denotes the density of adsorption phase (kg·m−3) and M represents the gas molar mass (g·mol−1). Therefore, according to Equations (5) and (6), the real adsorption capacity can be obtained using the apparent adsorption capacity under the same temperature and pressure conditions (Figure 5).
Vab = Vex/(1 − ρg/ρad)
ρad = 1/b × M
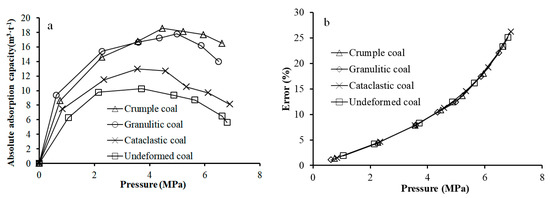
Figure 5.
Absolute adsorption capacity of coal (a), and its error with excess adsorption capacity (b).
Figure 5a shows that the absolute adsorption capacity of tectonically deformed coals presents similar evolution laws with excess adsorption capacity. An increase of pressure at first causes an increase, and then a decrease of the isotherm curve, which indicates type I isotherm characteristics. An increase of coal deformation degree induces a gradual increase in the absolute adsorption capacity. The maximum adsorption value shifts to the high pressure point. In order to compare and analyze the relationship between absolute adsorption capacity and excess adsorption capacity with pressure, the error between excess adsorption capacity and absolute adsorption capacity at each pressure point is calculated, as shown in Equation (7):
Error = (Vex − Vab)/Vex
The error analysis indicates that an increase of pressure causes the rapid increase in the error between excess adsorption and absolute adsorption shown in Figure 5b. Meanwhile, error curves of four specimens almost coincide, which indicates that the error has no relationship with the degree of coal deformation.
It should be noted that although the adsorption phase is taken into account in the absolute adsorption correction, the downward trend of the adsorption curve has not been eliminated. According to this downward trend, if the pressure continues to increase, the adsorption capacity may be negative, as indicated by Krooss et al. [19] and Hall et al. [20]. During CO2 adsorption, an increase of pressure causes the large amount of CO2 adsorbed on the surface of the coal matrix as the adsorption phase and the coal matrix to expand, which results in the change of free space volume of specimen tank, and finally causes the deviation of theoretical calculation results of Gibbs excess adsorption.
3.2. Nanopore Structural Evolutions during Coal Structure Deformation
Figure 6 shows that the relationship between the pore volume/pore-specific surface area of coal and the pore diameter presents a three-stage change, with boundaries of pore diameters of 10 nm and 50 nm, respectively. Therefore, nanopores of tectonically deformed coals are divided into the micropore (2–10 nm), mesopore (10–50 nm), and macropore (50–200 nm). The nanopore structures of tectonically deformed coals are obviously affected by the tectonic deformation of coal. An increase of degree of coal deformation, i.e., in the order of undeformed coal, cataclastic coal, granulitic coal, and crumple coal, causes obvious increases in the total pore volume and total specific surface area of tested pore size range (2–200 nm); the total pore volume increases from 4.371 × 10−3 cm3·g−1 to 14.746 × 10−3 cm3·g−1, and the total specific surface area increases from 0.517 m2/g to 7.211 m2·g−1 (Figure 7a,e).
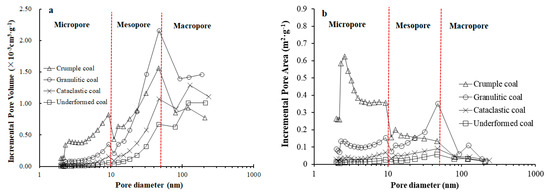
Figure 6.
Relationships between incremental pore volume (a), incremental pore specific surface area, (b) and average pore diameter.
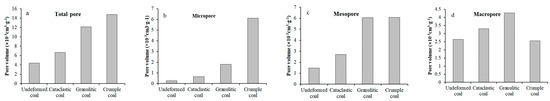
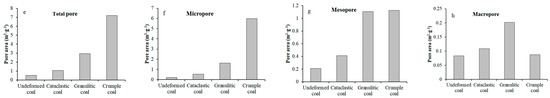
Figure 7.
Various types of pore volume (a–d) and specific surface area (e–h): (a) Pore volume of total pore; (b) Pore volume of micropore; (c) Pore volume of mesropore; (d) Pore volume of macropore; (e) Pore area of total pore; (f) Pore area of micropore; (g) Pore area of mesopore; (h) Pore area of macro pore.
It is clearly seen that an increase of degree of coal deformation causes the relatively consistent change trends of various types of pore volume and specific surface area. Specifically, as the degree of coal deformation increases, the pore volume and specific surface area present an obvious increasing trend in the case of micropores (Figure 7b,f), an increase at first (cataclastic coal and granulitic coal) and then stabilization (crumple coal) in the case of mesopores (Figure 7c,g), and a gradual decrease in the case of macropores (Figure 7d,h).
3.3. Relationships between CO2 Adsorption Capacity and Nanopore Structure
It is shown in Figure 8 that the effects of pore volume and specific surface area on the absolute adsorption capacity of tectonically deformed coals is relatively consistent at the pressure stage of 0–7 MPa. In the order of undeformed coal, cataclastic coal, and granulitic coal, the absolute adsorption capacity of coal specimens presents obviously linear positive correlation with the pore volume and specific surface area of macropores (black lines in Figure 8a,e); as it changes from granulitic coal to crumple coal, the pore volume and specific surface area of macropores decreases obviously, and the absolute adsorption capacity increases slightly (red lines in Figure 8a,e). The absolute adsorption capacity of tectonically deformed coals is obviously controlled by mesopores; the volume and specific surface area of mesopores both present good linear positive correlation with the absolute adsorption capacity (Figure 8b,f).
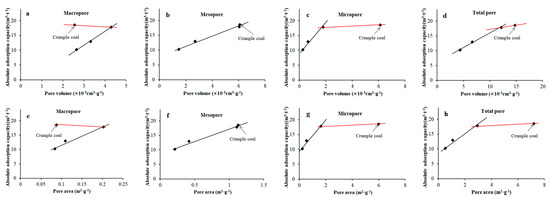
Figure 8.
Relationships between absolute adsorption capacity and pore volume (a–d) and specific surface area of coals (e–h): (a) Absolute adsorption capacity versus pore volume of macropore; (b) Absolute adsorption capacity versus pore volume of mesopore; (c) Absolute adsorption capacity versus pore volume of micropore; (d) Absolute adsorption capacity versus pore volume of total pore; (e) Absolute adsorption capacity versus pore area of macropore; (f) Absolute adsorption capacity versus pore area of mesopore; (g) Absolute adsorption capacity versus pore area of micropore; (h) Absolute adsorption capacity versus pore area of total pore.
Increases in micropores mainly contribute to increases in the total pore volume and specific surface area. Therefore, the relationships between total pore volume and specific surface area of tectonically deformed coals and absolute adsorption capacity are consistent with those of micropores. In the order of undeformed coal, cataclastic coal, and granulitic coal, the absolute adsorption capacity of the coal specimens presents obviously linear positive relationships with pore volume, specific surface area of micropores, total pore volume, and specific surface area of the coals (black lines in Figure 8c,d,g,h). As it changes from granulitic to crumple coal, there are obvious increases in the pore volume and specific surface area of micropores and total pores; however, the absolute adsorption capacity increases more gently (red lines in Figure 8c,d,g,h).
Therefore, during coal deformation, tectonic stress causes damage of the coal matrix block from the undeformed coal to cataclastic coal and granulitic coal. The volume and specific surface area of all types of pores exhibit obvious increases, especially for macropores and mesopores, which causes the increase in the CO2 adsorption capacity of the coal. As the tectonic deformation increases gradually (crumple coal), the coal structure is more broken; the tectonic stress has affected the smaller nanopores with newly formed, large numbers of micropores, as well as micropores formed due to damages of existing macropores and mesopores [21]. Therefore, in the case of crumple coal, an increase of tectonic deformation causes obvious decreases in the pore volume and specific surface area of macropores, slight increases in the pore volume and specific surface area of mesopores, and sharp increases in pore volume and specific surface area of micropores. However, the absolute adsorption capacity of crumple coal presents a slight increase, which coincides with the change trend of mesopores, and does not increase or decrease obviously with changes of macropores and micropores. The above analyses indicate that the mesopores are the key factor of CO2 adsorption of tectonically deformed coals at the pressure stage of 0–7 MPa, followed by the micropores and the limited effect of macropores at the strong coal deformation stage. Those also reveal that curves of excess adsorption and absolute adsorption of granulitic coal and crumple coal present intersection points. As indicated in Figure 9, when the pressure is smaller than 3.5 MPa, CO2 is mainly adsorbed in larger pores (23–200 nm) of coals, and the pore volume and specific surface area of larger pores (23–200 nm) of granulitic coal are larger than those of crumple coal, which indicates the larger adsorption capacity of granulitic coal as compared to crumple coal. As the pressure increases (3.5–7 MPa stage), CO2 is adsorbed in smaller pores of coals (2–23 nm); at this stage, the pore volume and specific surface area of crumple coal are larger than those of crumple coal, which indicates a larger adsorption capacity of crumple coal.
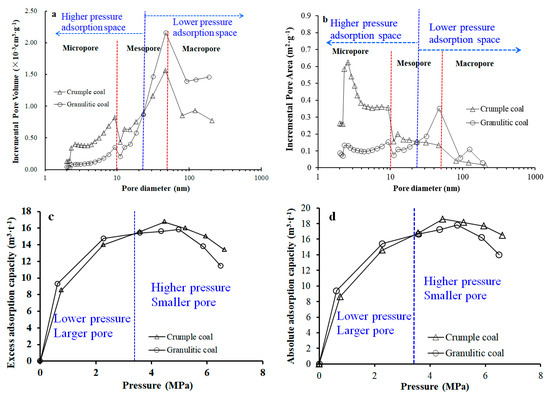
Figure 9.
Adsorption space distributions at higher and lower pressure stages: (a) Division of higher and lower pressure adsorption space of pore volume distribution curve; (b) Division of higher and lower pressure adsorption space of pore area distribution curve; (c) Division of higher pressure and lower pressure stage of isothermal excess adsorption curve; (d) Division of higher pressure and lower pressure stage of isothermal absolute adsorption curve.
4. Conclusions
This study presents evolutions of CO2 adsorption and nanopore structure characteristics with coal structure deformation, and reveals the effects of nanopore structure on CO2 adsorption in tectonically deformed coals.
Isotherms of excess and absolute adsorption of four coal specimens exhibit an increase at first, and then a decrease with increasing pressure. There exist maximum values of excess and absolute adsorption capacity increasing with increasing coal deformation degree. Maximum values of excess adsorption capacity of undeformed coal, cataclastic coal, granulitic coal, and crumple coal are 9.48 m3·g−1, 12.04 m3·g−1, 15.83 m3·g−1, and 16.74 m3·g−1, respectively. The maximum excess adsorption capacity is present at a pressure ranging from 4 MPa to 5 MPa, and with increasing coal deformation degree the pressure corresponding to the maximum excess adsorption amount shifts to the higher pressure. The error analysis indicates that an increase of pressure causes the rapid increase in the error between excess adsorption and absolute adsorption.
In addition, as the degree of coal deformation increases, the pore volume and specific surface area present an obviously increasing trend in the case of micropores, exhibiting an increase at first (cataclastic coal and granulitic coal) and then stabilization (crumple coal), in the case of mesopores, and showing the gradual decrease in the case of macropores. The effects of pore volume and specific surface area on the absolute adsorption capacity of tectonically deformed coals is relatively consistent at the pressure stage of 0–7 MPa. The mesopores are the key factor of CO2 adsorption of tectonically deformed coals at the pressure stage of 0–7 MPa, followed by the micropores and the limited effect of macropores at the strong coal deformation stage. The volume and specific surface area of mesopores both present good linear positive correlation with the absolute adsorption capacity. Differences in mesopore development result in the intersection of curves of excess adsorption and absolute adsorption in granulitic coal and crumple coal.
Author Contributions
Specimen preparation, macro- and microstructure analysis, Z.L.; isotherm adsorption experiment and liquid nitrogen adsorption, L.W. All authors discussed the results and contributed to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, grant numbers 41702169 and 41572138, and China Postdoctoral Foundation, grant number 2019M662013.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hou, S.Y. Development status of China coalbed methane industry in recent years. China Coalbed Methane 2018, 15, 42–45. [Google Scholar]
- Gou, D.Y. Research on Structural Physics of Coal and Gas Outburst. Ph.D. Thesis, China University of Mining and Technology, Beijing, China, 1996. [Google Scholar]
- Zhang, H.R. Discussion on the origns of structural coal in Xiahuayuan coal mine. Min. Saf. Environ. Prot. 1999, 5, 31–34. [Google Scholar]
- Ju, Y.W.; Jiang, B.; Hou, Q.L.; Wang, G.L. The new structure-genetic classification system in tectonically deformed coals and its geological significance. J. China Coal Soc. 2004, 29, 513–517. [Google Scholar]
- Jiang, B.; Qin, Y.; Ju, Y.W.; Wang, J.L.; Li, M. The coupling mechanism of the evolution of chemical structure with the characteristics of gas of tectonic coals. Earth Sci. Front. 2009, 16, 262–271. [Google Scholar]
- Zhang, Y.G. Evolution of Deformed Coal and Process of Coal Mechanochemistry. Ph.D. Thesis, Taiyuan University of Technology, Taiyuan, China, 2006. [Google Scholar]
- Xing, W.L. Study on Characteristics of Adsorption/Desorption and Diffusion for CO2, CH4, N2, and Their Mixtures in Coal. Ph.D. Thesis, Dalian University of Technology, Dalian, China, 2016. [Google Scholar]
- Chen, R.; Qin, Y.; Zhang, P.F.; Wang, Y.Y. Changes in pore structure of coal caused by CS2 treatment and its methane adsorption response. Geofluids 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Zhao, J.C.; Qin, Y.; Shen, J.; Zhou, B.Y.; Li, C.; Li, G. Effects of pore structures of different maceral compositions on methane adsorption and diffusion in anthracite. Appl. Sci. 2019, 9, 5130. [Google Scholar] [CrossRef]
- Castello, D.L. Advances in the study of methane storage in a porous carbon aceous materials. Fuel 2002, 81, 1777–1803. [Google Scholar] [CrossRef]
- Chen, X.J.; Liu, J.; Wang, L.; Qi, L.L. Influence of pore size distribution of different metamorphic grade of coal on adsorption constant. J. China Coal Soc. 2013, 39, 294–300. [Google Scholar]
- Jian, K.; Fu, X.H.; Ding, Y.M.; Wang, H.D.; Li, T. Characteristics of pores and methane adsorption of low-rank coal in China. J. Nat. Gas Sci. Eng. 2015, 27, 207–218. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, Y.B.; Gao, S.S.; Hong, P.F.; Zhang, M.J. Differences in pore structures and absorptivity between tectonically deformed and undeformed coals. Geol. J. China Univ. 2012, 18, 528–532. [Google Scholar] [CrossRef]
- Li, Y.K. Research on the Methane Adsorption/Desorption/Diffusion Characteristics of Tectonic Coal. Master’s Thesis, Henan Polytechnic University, Jiaozuo, China, 2015. [Google Scholar]
- Donohue, M.D.; Aranovich, G.L. A new classification of isotherms for Gibbs adsorption of gases on solids. Fluid Phase Equil. 1999, 158, 557–563. [Google Scholar] [CrossRef]
- Moffat, D.H.; Weale, K.E. Sorption by coal of methane at high pressures. Fuel 1955, 34, 449–462. [Google Scholar]
- Kim, H.J.; Shi, Y.; He, J.W.; Lee, H.H.; Lee, C.H. Adsorption characteristic of CO2 and CH4 on dry and wet coal from subcritical to supercritical conditions. Chem. Eng. J. 2011, 171, 45–53. [Google Scholar] [CrossRef]
- Cui, Y.J. Adosrption of CH4, N2, CO2, Single and Multicomponent Gas on Coal; Xian Branch, China Coal Researeh Institute: Xian, China, 2003. [Google Scholar]
- Kroos, B.M.; Bergen, F.V.; Gensterblum, Y.; Siemons, N.; Pagnier, H.J.M.; David, P. High-pressure methane and carbon dioxide adsorption on dry and moisture-equilibrated pennsylvanian coals. Int. J. Coal Geol. 2002, 51, 69–92. [Google Scholar] [CrossRef]
- Hall, F.E.; Chunhe, Z.; Gasem, K.A.M.; Robinson, R.L.; Yee, D. Adsorption of pure methane, nitrogen, and carbon dioxide and their binary mixtures on wet Fruitland coal. In Proceedings of the SPE Eastern Regional Conference and Exhibition, Charleston, WV, USA, 8–10 November 1994; pp. 329–344. [Google Scholar]
- Pan, J.N.; Zhu, H.T.; Hou, Q.L.; Wang, H.C.; Wang, S. Macromolecular and pore structures of Chinese tectonically deformed coal studied by atomic force microscopy. Fuel 2015, 139, 94–101. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).