Recent Progress in Hybrid Solar Cells Based on Solution-Processed Organic and Semiconductor Nanocrystal: Perspectives on Device Design
Abstract
1. Introduction
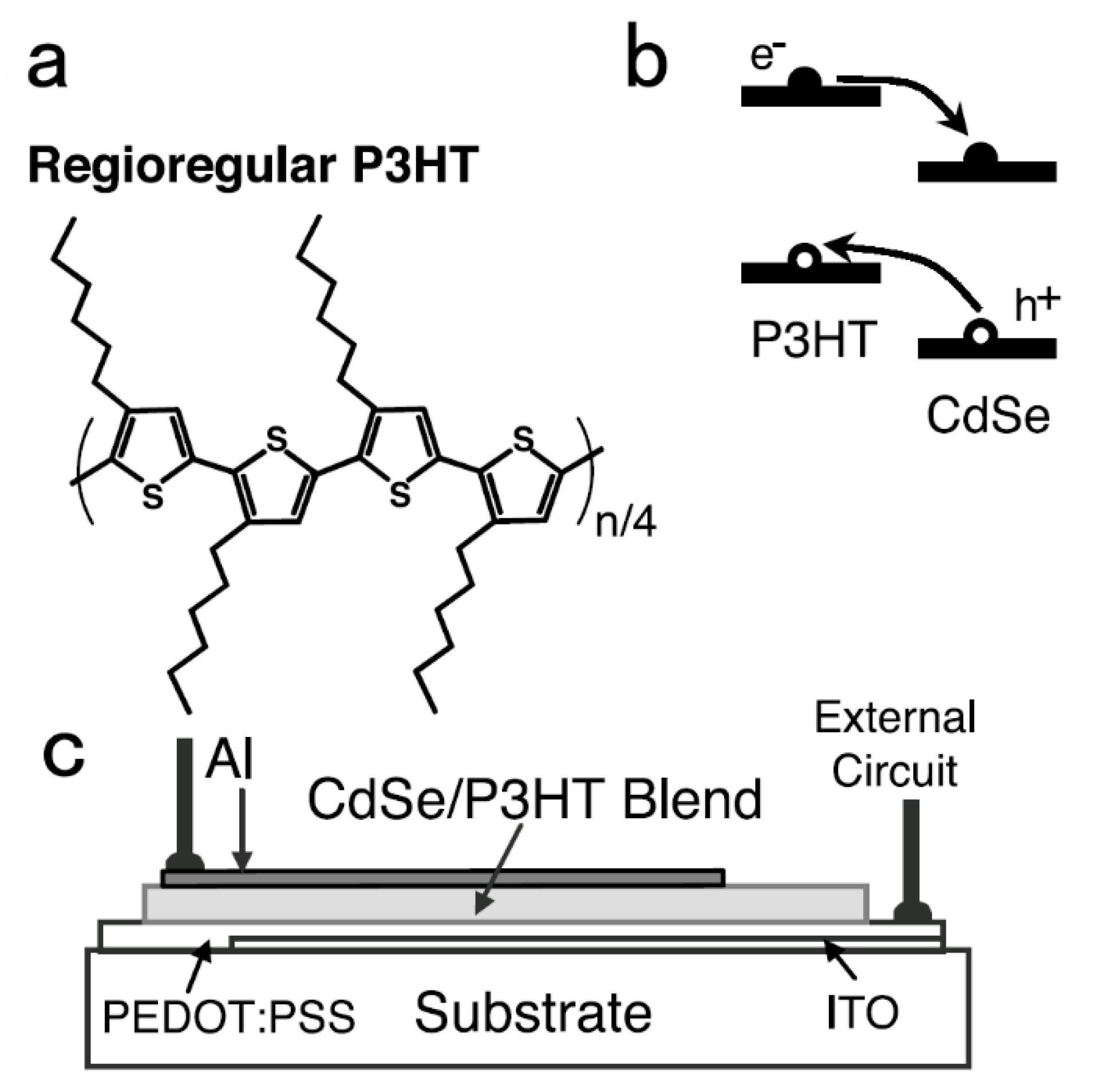
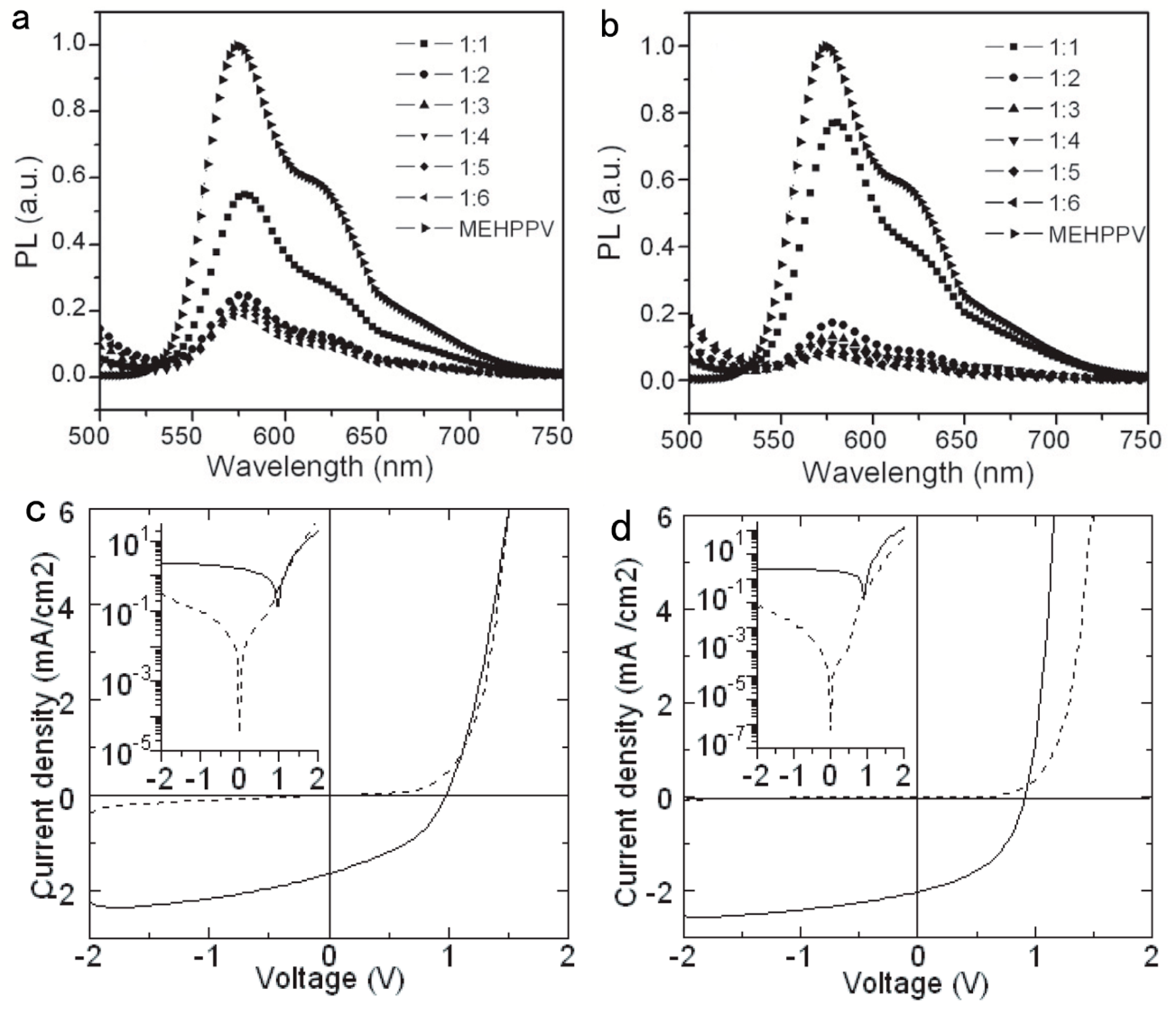
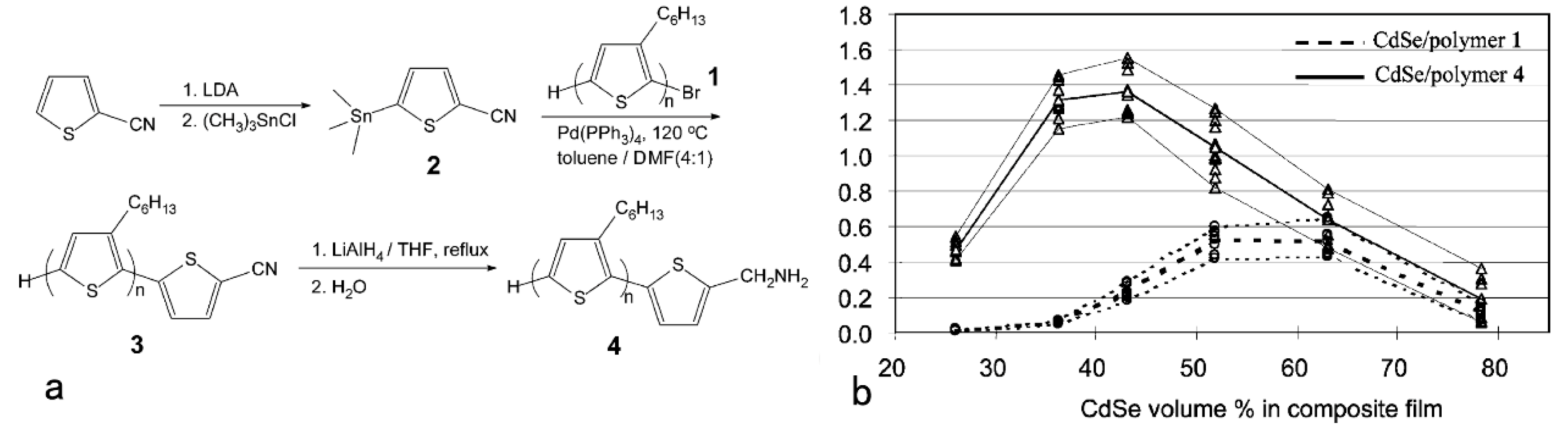
2. Hybrid BHJ Solar Cells with a Large Bandgap Semiconductor NC as an Acceptor
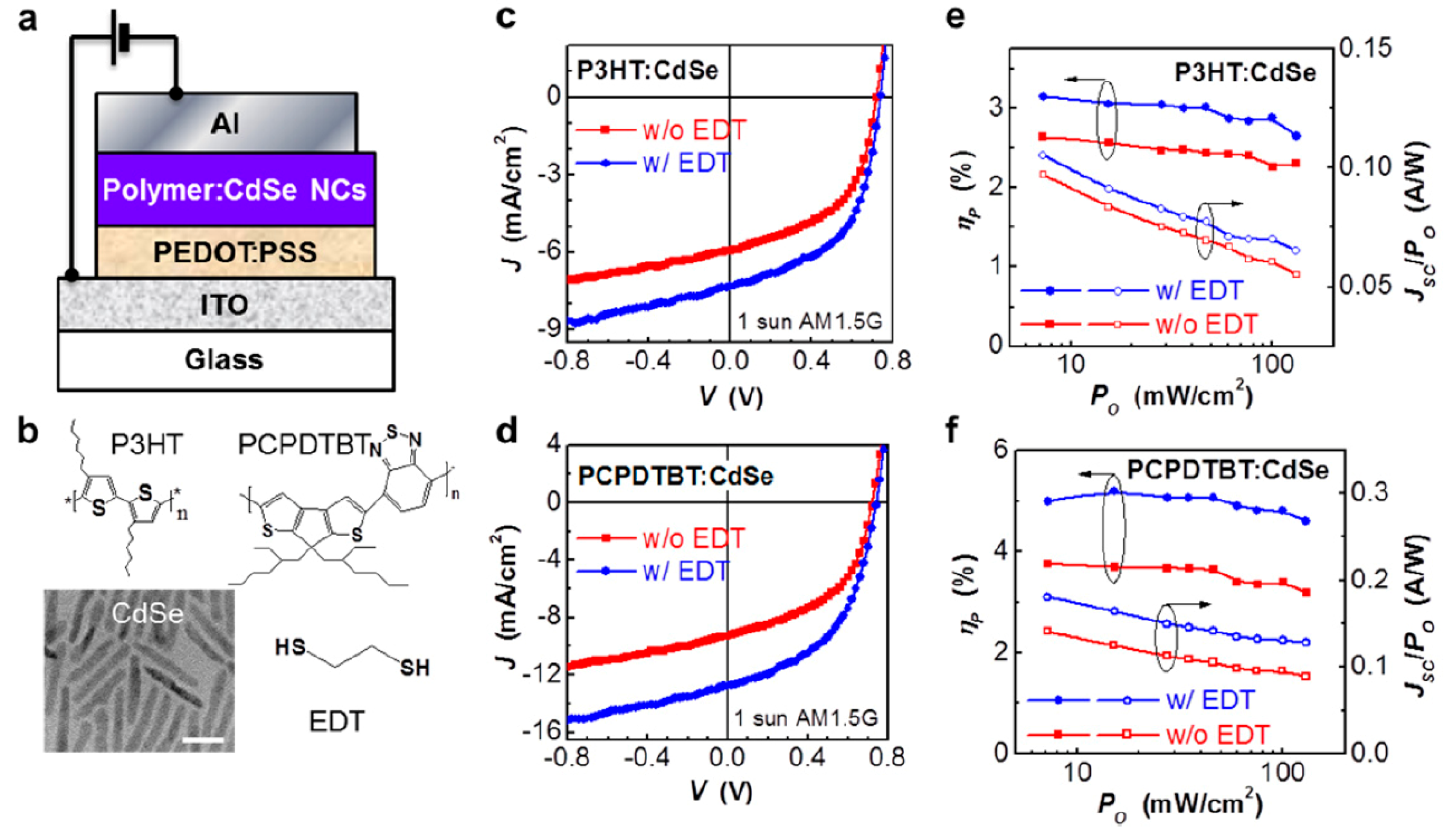
3. Hybrid Bulk Heterojunction Solar Cells Using Low Bandgap Nanocrystals
4. HSC with More Active Layers
5. HSCs Using Organic Materials as the HTL
6. HSCs with Tandem Structure
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kiani, A.; Sutherland, B.R.; Kim, Y.; Ouellette, O.; Levina, L.; Walters, G.; Dinh, C.T.; Liu, M.; Voznyy, O.; Lan, X.; et al. Single-step colloidal quantum dot films for infrared solar harvesting. Appl. Phys. Lett. 2016, 109, 183105. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, Z.; Zhao, Y.; Du, X.; Liu, F.; Jin, G.; Dong, F.; Zhang, H.; Yang, B. Aqueous-Processed inorganic thin-film solar cells based on CdSexTe1–x nanocrystals: The impact of composition on photovoltaic performance. ACS Appl. Mater. Interfaces 2015, 7, 2322–2323. [Google Scholar] [CrossRef] [PubMed]
- Beek, W.J.; Wienk, M.M.; Janssen, R.A. Efficient hybrid solar cells from zinc oxide nanoparticles and a conjugated polymer. Adv. Mater. 2004, 16, 1009–1013. [Google Scholar] [CrossRef]
- Arici, E.; Sariciftci, N.S.; Meissner, D. Hybrid solar cells based on nanoparticles of CuInS2 in organic matrices. Adv. Funct. Mater. 2003, 13, 165–171. [Google Scholar] [CrossRef]
- Arici, E.; Hoppe, H.; Schäffler, F.; Meissner, D.; Malik, M.A.; Sariciftci, N.S. Morphology effects in nanocrystalline CuInSe 2-conjugated polymer hybrid systems. Appl. Phys. A 2004, 79, 59–64. [Google Scholar] [CrossRef]
- Chaure, S. MEH-PPV/CdS Hybrid Nanowire Polymer Solar Cell Array. J. Electron. Mater. 2019, 48, 1074–1078. [Google Scholar] [CrossRef]
- Dayal, S.; Kopidakis, N.; Olson, D.C.; Ginley, D.S.; Rumbles, G. Photovoltaic devices with a low band gap polymer and CdSe nanostructures exceeding 3% efficiency. Nano Lett. 2010, 10, 239–242. [Google Scholar] [CrossRef]
- Jin, G.; Wei, H.T.; Na, T.Y.; Sun, H.Z.; Zhang, H.; Yang, B. High-efficiency aqueous-processed hybrid solar cells with an enormous Herschel infrared contribution. ACS Appl. Mater. Interfaces 2014, 6, 8606–8612. [Google Scholar] [CrossRef]
- Beek, W.J.; Wienk, M.M.; Kemerink, M.; Yang, X.; Janssen, R.A. Hybrid zinc oxide conjugated polymer bulk heterojunction solar cells. J. Phys. Chem. B 2005, 109, 9505–9516. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.C.; Lo, H.H.; Liao, H.C.; Chen, S.; Lin, Y.Y.; Yen, W.C.; Zeng, T.W.; Chen, Y.F.; Chen, C.W.; Su, W.F. Using scanning probe microscopy to study the effect of molecular weight of poly (3-hexylthiophene) on the performance of poly (3-hexylthiophene): TiO2 nanorod photovoltaic devices. Sol. Energy Mater. Sol. Cells 2009, 93, 869–873. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, S.; Zeng, X.; Liu, J.; Zhang, C.; Shi, M.; Tan, F.; Wang, Z. The synthesis of MDMO-PPV capped PbS nanorods and their application in solar cells. Curr. Appl. Phys. 2009, 9, 1175–1179. [Google Scholar] [CrossRef]
- Jung, J. Preparation of anisotropic CdSe-P3HT core-shell nanorods using directly synthesized Br-functionalized CdSe nanorods. Surf. Coat. Technol. 2019, 362, 84–89. [Google Scholar] [CrossRef]
- Tulsiram, N.; Kerr, C.; Chen, J.I. Photoinduced charge transfer in poly (3-hexylthiophene)/TiO2 hybrid inverse opals: Photonic vs. interfacial effects. J. Phys. Chem. C 2017, 121, 26987–26996. [Google Scholar] [CrossRef]
- Greaney, M.J.; Brutchey, R.L. Ligand engineering in hybrid polymer: Nanocrystal solar cells. Mater. Today 2015, 18, 31–38. [Google Scholar] [CrossRef]
- Tsang, S.W.; Fu, H.; Wang, R.; Lu, J.; Yu, K.; Tao, Y. Highly efficient cross-linked PbS nanocrystal/C 60 hybrid heterojunction photovoltaic cells. Appl. Phys. Lett. 2009, 95, 183505. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, W.; Liang, Q.; Zhu, J.; Cong, Z.; Lin, F.; Yi, S.; Luo, G.; Yang, T.; Liu, S.; et al. High-performance fullerene-free polymer solar cells featuring efficient photocurrent generation from dual pathways and low nonradiative recombination loss. ACS Energy Lett. 2018, 4, 8–16. [Google Scholar] [CrossRef]
- Yang, T.; Cai, W.; Qin, D.; Wang, E.; Lan, L.; Gong, X.; Cao, Y. Solution-processed zinc oxide thin film as a buffer layer for polymer solar cells with an inverted device structure. J. Phys. Chem. C 2010, 114, 6849–6853. [Google Scholar] [CrossRef]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid nanorod-polymer solar cells. Science 2002, 295, 2425–2427. [Google Scholar] [CrossRef]
- Couderc, E.; Greaney, M.J.; Brutchey, R.L.; Bradforth, S.E. Direct spectroscopic evidence of ultrafast electron transfer from a low band gap polymer to CdSe quantum dots in hybrid photovoltaic thin films. J. Am. Chem. Soc. 2013, 135, 18418–18426. [Google Scholar] [CrossRef]
- Sun, B.; Marx, E.; Greenham, N.C. Photovoltaic devices using blends of branched CdSe nanoparticles and conjugated polymers. Nano Lett. 2003, 3, 961–963. [Google Scholar] [CrossRef]
- Sun, B.; Snaith, H.J.; Dhoot, A.S.; Westenhoff, S.; Greenham, N.C. Vertically segregated hybrid blends for photovoltaic devices with improved efficiency. J. Appl. Phys. 2005, 97, 014914. [Google Scholar] [CrossRef]
- Han, L.; Qin, D.; Jiang, X.; Liu, Y.; Wang, L.; Chen, J.; Cao, Y. Synthesis of high -quality zinc-blende CdSe nanocrystals and their application in hybrid solar cells. Nanotechnology 2006, 17, 4736. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Jiang, X.; Qin, D.; Cao, Y. Enhancement of photovoltaic characteristics using a suitable solvent in hybrid polymer/multiarmed CdS nanorods solar cells. J. Phys. Chem. C 2007, 111, 9538–9542. [Google Scholar] [CrossRef]
- Peng, Z.A.; Peng, X. Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor. J. Am. Chem. Soc. 2001, 123, 183–184. [Google Scholar] [CrossRef]
- Peng, Z.A.; Peng, X. Nearly monodisperse and shape-controlled CdSe nanocrystals via alternative routes: Nucleation and growth. J. Am. Chem. Soc. 2002, 124, 3343–3353. [Google Scholar] [CrossRef]
- Peng, X.; Manna, L.; Yang, W.; Wickham, J.; Scher, E.; Kadavanich, A.; Alivisatos, A.P. Shape control of CdSe nanocrystals. Nature 2000, 404, 59–61. [Google Scholar] [CrossRef]
- Huynh, W.U.; Peng, X.; Alivisatos, A.P. CdSe nanocrystal rods/poly (3-hexylthiophene) composite photovoltaic devices. Adv. Mater. 1999, 11, 923–927. [Google Scholar] [CrossRef]
- Kramer, I.J.; Sargent, E.H. The architecture of colloidal quantum dot solar cells: Materials to devices. Chem. Rev. 2014, 114, 863–882. [Google Scholar] [CrossRef]
- Liu, J.; Tanaka, T.; Sivula, K.; Alivisatos, A.P.; Fréchet, J.M. Employing end-functional polythiophene to control the morphology of nanocrystal− polymer composites in hybrid solar cells. J. Am. Chem. Soc. 2004, 126, 6550–6551. [Google Scholar] [CrossRef]
- Lan, X.; Voznyy, O.; García de Arquer, F.P.; Liu, M.; Xu, J.; Proppe, A.H.; Walters, G.; Fan, F.; Tan, H.; Liu, M.; et al. 10.6% certified colloidal quantum dot solar cells via solvent-polarity-engineered halide passivation. Nano Lett. 2016, 16, 4630–4634. [Google Scholar] [CrossRef]
- Chuang, C.H.M.; Brown, P.R.; Bulović, V.; Bawendi, M.G. Improved performance and stability in quantum dot solar cells through band alignment engineering. Nat. Mater. 2014, 13, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Kemp, K.W.; Hoogland, S.; Jeong, K.S.; Liu, H.; Levina, L.; Furukawa, M.; Wang, X.; Debnath, R.; Chou, K.W.; et al. Colloidal-quantum-dot photovoltaics using atomic-ligand passivation. Nat. Mater. 2011, 10, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.; Voznyy, O.; Pan, J.; Hoogland, S.; Adinolfi, V.; Xu, J.; Li, M.; Kirmani, A.R.; Sun, J.P.; Kemp, K.W.; et al. Air-stable n-type colloidal quantum dot solids. Nat. Mater. 2014, 13, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Chang, L.Y.; Lim, S.K.; Zhao, J.; Smith, M.; Zhao, N.; Bulovic, V.; Bawendi, M.; Gradecak, S. Inorganic–organic hybrid solar cell: Bridging quantum dots to conjugated polymer nanowires. Nano Lett. 2011, 11, 3998–4002. [Google Scholar] [CrossRef]
- Cui, C.; He, Z.; Wu, Y.; Cheng, X.; Wu, H.; Li, Y.; Wong, W.Y.; Cao, Y. High-performance polymer solar cells based on a 2D-conjugated polymer with an alkylthio side-chain. Energy Environ. Sci. 2016, 9, 885–891. [Google Scholar] [CrossRef]
- Fu, W.; Wang, L.; Ling, J.; Li, H.; Shi, M.; Xue, J.; Chen, H. Highly efficient hybrid solar cells with tunable dipole at the donor–acceptor interface. Nanoscale 2014, 6, 10545–10550. [Google Scholar] [CrossRef]
- Zhou, R.; Stalder, R.; Xie, D.; Cao, W.; Zheng, Y.; Yang, Y.; Plaisant, M.; Holloway, P.H.; Schanze, K.S.; Xue, J.; et al. Enhancing the efficiency of solution-processed polymer: Colloidal nanocrystal hybrid photovoltaic cells using ethanedithiol treatment. ACS Nano 2013, 7, 4846–4854. [Google Scholar] [CrossRef]
- Kerr, C.S.; Kryukovskiy, A.; Chen, J.I. Effects of Surface Passivation on Trap States, Band Bending, and Photoinduced Charge Transfer in P3HT/TiO2 Hybrid Inverse Opals. J. Phys. Chem. C 2018, 122, 17301–17308. [Google Scholar] [CrossRef]
- Liao, H.C.; Lee, C.H.; Ho, Y.C.; Jao, M.H.; Tsai, C.M.; Chuang, C.M.; Shyue, J.J.; Chen, Y.F.; Su, W.F. Diketopyrrolopyrrole-based oligomer modified TiO2 nanorods for air-stable and all solution processed poly (3-hexylthiophene): TiO2 bulk heterojunction inverted solar cell. J. Mater. Chem. 2012, 22, 10589–10596. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Recent Developments of Solar Cells from PbS Colloidal Quantum Dots. Appl. Sci. 2020, 10, 1743. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Wu, H.; Yan, L.; Wang, Z.; Zhao, J.; Yu, W.W.; Rogach, A.L. PbSe quantum dot films with enhanced electron mobility employed in hybrid polymer/nanocrystal solar cells. RSC Adv. 2016, 6, 17029–17035. [Google Scholar] [CrossRef]
- Song, T.; Cheng, H.; Fu, C.; He, B.; Li, W.; Xu, J.; Tang, Y.; Yang, S.; Zou, B. Influence of the active layer nanomorphology on device performance for ternary PbS x Se1− x quantum dots based solution-processed infrared photodetector. Nanotechnology 2016, 27, 165202. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Luther, J.M.; Zheng, H.; Wu, Y.; Alivisatos, A.P. Photovoltaic devices employing ternary PbSxSe1-x nanocrystals. Nano Lett. 2009, 9, 1699–1703. [Google Scholar] [CrossRef]
- Xu, J.; Voznyy, O.; Liu, M.; Kirmani, A.R.; Walters, G.; Munir, R.; Abdelsamie, M.; Proppe, A.H.; Sarkar, A.; Wei, M.; et al. 2D matrix engineering for homogeneous quantum dot coupling in photovoltaic solids. Nat. Nanotechnol. 2018, 13, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Midgett, A.G.; Luther, J.M.; Stewart, J.T.; Smith, D.K.; Padilha, L.A.; Klimov, V.I.; Nozik, A.J.; Beard, M.C. Size and composition dependent multiple exciton generation efficiency in PbS, PbSe, and PbSxSe1–x alloyed quantum dots. Nano Lett. 2013, 13, 3078–3085. [Google Scholar] [CrossRef] [PubMed]
- Noone, K.M.; Strein, E.; Anderson, N.C.; Wu, P.T.; Jenekhe, S.A.; Ginger, D.S. Broadband absorbing bulk heterojunction photovoltaics using low-bandgap solution-processed quantum dots. Nano Lett. 2010, 10, 2635–2639. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Cho, M.J.; Lee, D.; Cartwright, A.N.; Prasad, P.N. Efficient heterojunction photovoltaic cell utilizing nanocomposites of lead sulfide nanocrystals and a low-bandgap polymer. Adv. Mater. 2011, 23, 3984–3988. [Google Scholar] [CrossRef]
- Carey, G.H.; Abdelhady, A.L.; Ning, Z.; Thon, S.M.; Bakr, O.M.; Sargent, E.H. Colloidal quantum dot solar cells. Chem. Rev. 2015, 115, 12732–12763. [Google Scholar] [CrossRef]
- Piliego, C.; Manca, M.; Kroon, R.; Yarema, M.; Szendrei, K.; Andersson, M.R.; Heiss, W.; Loi, M.A. Charge separation dynamics in a narrow band gap polymer–PbS nanocrystal blend for efficient hybrid solar cells. J. Mater. Chem. 2012, 22, 24411–24416. [Google Scholar] [CrossRef]
- Lu, H.; Joy, J.; Gaspar, R.L.; Bradforth, S.E.; Brutchey, R.L. Iodide-passivated colloidal PbS nanocrystals leading to highly efficient polymer: Nanocrystal hybrid solar cells. Chem. Mater. 2016, 28, 1897–1906. [Google Scholar] [CrossRef]
- Su, Y.W.; Lin, W.H.; Hsu, Y.J.; Wei, K.H. Conjugated polymer/nanocrystal nanocomposites for renewable energy applications in photovoltaics and photocatalysis. Small 2014, 10, 4427–4442. [Google Scholar] [CrossRef] [PubMed]
- Gur, I.; Fromer, N.A.; Chen, C.P.; Kanaras, A.G.; Alivisatos, A.P. Hybrid solar cells with prescribed nanoscale morphologies based on hyperbranched semiconductor nanocrystals. Nano Lett. 2007, 7, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Van Bavel, S.S.; Bärenklau, M.; de With, G.; Hoppe, H.; Loos, J. P3HT/PCBM bulk heterojunction solar cells: Impact of blend composition and 3D morphology on device performance. Adv. Funct. Mater. 2010, 20, 1458–1463. [Google Scholar] [CrossRef]
- Campoy-Quiles, M.; Ferenczi, T.; Agostinelli, T.; Etchegoin, P.G.; Kim, Y.; Anthopoulos, T.D.; Stavrinou, P.N.; Bradley, D.D.C.; Nelson, J. Morphology evolution via self-organization and lateral and vertical diffusion in polymer: Fullerene solar cell blends. Nat. Mater. 2008, 7, 158–164. [Google Scholar] [CrossRef]
- Xue, J.; Rand, B.P.; Uchida, S.; Forrest, S.R. A hybrid planar–mixed molecular heterojunction photovoltaic cell. Adv. Mater. 2005, 17, 66–71. [Google Scholar] [CrossRef]
- Wang, Z.; Yokoyama, D.; Wang, X.F.; Hong, Z.; Yang, Y.; Kido, J. Highly efficient organic p–i–n photovoltaic cells based on tetraphenyldibenzoperiflanthene and fullerene C 70. Energy Environ. Sci. 2013, 6, 249–255. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Z.; Du, X.; Zeng, Q.; Ji, T.; Cheng, Z.; Jin, G.; Yang, B. High efficiency aqueous-processed MEH-PPV/CdTe hybrid solar cells with a PCE of 4.20%. J. Mater. Chem. A 2016, 4, 1105–1111. [Google Scholar] [CrossRef]
- Yao, S.; Chen, Z.; Li, F.; Xu, B.; Song, J.; Yan, L.; Jin, G.; Wen, S.; Wang, C.; Tian, W.; et al. High-efficiency aqueous-solution-processed hybrid solar cells based on P3HT Dots and CdTe nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 7146–7152. [Google Scholar] [CrossRef]
- Jin, G.; Chen, N.; Zeng, Q.; Liu, F.; Yuan, W.; Xiang, S.; Feng, T.; Du, X.; Ji, T.; Wang, L.; et al. Aqueous-Processed Polymer/Nanocrystal Hybrid Solar Cells with Double-Side Bulk Heterojunction. Adv. Energy Mater. 2018, 8, 1701966. [Google Scholar] [CrossRef]
- Chen, N.N.; Jin, G.; Wang, L.J.; Sun, H.N.; Zeng, Q.S.; Yang, B.; Sun, H.Z. Highly efficient aqueous-processed hybrid solar cells: Control depletion region and improve carrier extraction. Adv. Energy Mater. 2019, 9, 1803849. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Yuan, J.; Wei, H.; Huang, X.; Han, L.; Wang, W.; Wang, H.; Ma, W. High-efficiency hybrid solar cells based on polymer/PbSxSe1-x nanocrystals benefiting from vertical phase segregation. Adv. Mater. 2013, 25, 5772–5778. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Gallagher, A.; Liu, Z.; Sun, Y.; Ma, W. High-efficiency polymer–PbS hybrid solar cells via molecular engineering. J. Mater. Chem. A 2015, 3, 2572–2579. [Google Scholar] [CrossRef]
- Baek, S.W.; Jun, S.; Kim, B.; Proppe, A.H.; Ouellette, O.; Voznyy, O.; Kim, C.; Kim, J.; Walters, G.; Jeong, S.; et al. Efficient hybrid colloidal quantum dot/organic solar cells mediated by near-infrared sensitizing small molecules. Nat. Energy 2019, 4, 969–976. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.K. Physics and chemistry of CdTe/CdS thin film heterojunction photovoltaic devices: Fundamental and critical aspects. Energy Environ. Sci. 2014, 7, 45–102. [Google Scholar] [CrossRef]
- Lin, H.; Xia, W.; Wu, H.N.; Tang, C.W. CdS/CdTe solar cells with MoOx as back contact buffers. Appl. Phys. Lett. 2010, 97, 123504. [Google Scholar] [CrossRef]
- Shen, K.; Yang, R.; Wang, D.; Jeng, M.; Chaudhary, S.; Ho, K.; Wang, D. Stable CdTe solar cell with V2O5 as a back contact buffer layer. Sol. Energy Mater. Sol. Cells 2016, 144, 500–508. [Google Scholar] [CrossRef]
- Xiao, D.; Li, X.; Wang, D.; Li, Q.; Shen, K.; Wang, D. CdTe thin film solar cell with NiO as a back contact buffer layer. Sol. Energy Mater. Sol. Cells 2017, 169, 61–67. [Google Scholar] [CrossRef]
- Paudel, N.R.; Xiao, C.; Yan, Y. CdS/CdTe thin-film solar cells with Cu-free transition metal oxide/Au back contacts. Prog. Photovolt. Res. Appl. 2015, 23, 437–442. [Google Scholar] [CrossRef]
- Paudel, N.R.; Yan, Y. Application of copper thiocyanate for high open-circuit voltages of CdTe solar cells. Prog. Photovolt. Res. Appl. 2016, 24, 94–101. [Google Scholar] [CrossRef]
- Paudel, N.R.; Yan, Y. CdTe thin-film solar cells with cobalt-phthalocyanine back contacts. Appl. Phys. Lett. 2014, 104, 143507. [Google Scholar] [CrossRef]
- Wang, W.; Paudel, N.R.; Yan, Y.; Duarte, F.; Mount, M. PEDOT: PSS as back contact for CdTe solar cells and the effect of PEDOT: PSS conductivity on device performance. J. Mater. Sci. Mater. Electron. 2016, 27, 1057–1061. [Google Scholar] [CrossRef]
- Du, X.; Chen, Z.; Liu, F.; Zeng, Q.; Jin, G.; Li, F.; Yao, D.; Yang, B. Improvement in open-circuit voltage of thin film solar cells from aqueous nanocrystals by interface engineering. ACS Appl. Mater. Interfaces 2016, 8, 900–907. [Google Scholar] [CrossRef]
- Guo, X.; Tan, Q.; Liu, S.; Qin, D.; Mo, Y.; Hou, L.; Liu, A.; Wu, H.; Ma, Y. High-efficiency solution-processed CdTe nanocrystal solar cells incorporating a novel crosslinkable conjugated polymer as the hole transport layer. Nano Energy 2018, 46, 150–157. [Google Scholar] [CrossRef]
- Paudel, N.R.; Poplawsky, J.D.; Moore, K.L.; Yan, Y. Current enhancement of CdTe-based solar cells. IEEE J. Photovolt. 2015, 5, 1492–1496. [Google Scholar] [CrossRef]
- Rong, Z.; Guo, X.; Lian, S.; Liu, S.; Qin, D.; Mo, Y.; Xu, W.; Wu, H.; Zhao, H.; Hou, L. Interface engineering for both cathode and anode enables low-cost highly efficient solution-processed CdTe nanocrystal solar cells. Adv. Funct. Mater. 2019, 29, 1904018. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Yip, H.L.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Gilot, J.; Wienk, M.M.; Janssen, R.A. Optimizing polymer tandem solar cells. Adv. Mater. 2010, 22, E67–E71. [Google Scholar] [CrossRef]
- Dou, L.; You, J.; Yang, J.; Chen, C.C.; He, Y.; Murase, S.; Moriarty, T.; Emery, K.; Li, G.; Yang, Y. Tandem polymer solar cells featuring a spectrally matched low-bandgap polymer. Nat. Photonics 2012, 6, 180–185. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, S.; Zhang, M.; Zhao, K.; Ye, L.; Chen, Y.; Yang, B.; Hou, J. Highly efficient tandem polymer solar cells with a photovoltaic response in the visible light range. Adv. Mater. 2015, 27, 1189–1194. [Google Scholar] [CrossRef]
- Speirs, M.J.; Groeneveld, B.G.H.M.; Protesescu, L.; Piliego, C.; Kovalenko, M.V.; Loi, M.A. Hybrid inorganic–organic tandem solar cells for broad absorption of the solar spectrum. Phys. Chem. Chem. Phys. 2014, 16, 7672–7676. [Google Scholar] [CrossRef]
- Kim, T.; Gao, Y.; Hu, H.; Yan, B.; Ning, Z.; Jagadamma, L.K.; Zhao, K.; Kirmani, A.R.; Eid, J.; Sargent, E.H.; et al. Hybrid tandem solar cells with depleted-heterojunction quantum dot and polymer bulk heterojunction subcells. Nano Energy 2015, 17, 196–205. [Google Scholar] [CrossRef]
- Aqoma, H.; Azmi, R.; Oh, S.H.; Jang, S.Y. Solution-processed colloidal quantum dot/organic hybrid tandem photovoltaic devices with 8.3% efficiency. Nano Energy 2017, 31, 403–409. [Google Scholar] [CrossRef]
- Tong, J.; Yang, X.; Xu, Y.; Li, W.; Tang, J.; Song, H.; Zhou, Y. Efficient top-illuminated organic-quantum dots hybrid tandem solar cells with complementary absorption. ACS Photonics 2017, 4, 1172–1177. [Google Scholar] [CrossRef]
- Kim, T.; Palmiano, E.; Liang, R.Z.; Hu, H.; Murali, B.; Kirmani, A.R.; Firdaus, Y.; Gao, Y.; Sheikh, A.; Mohammed, O.F.; et al. Hybrid tandem quantum dot/organic photovoltaic cells with complementary near infrared absorption. Appl. Phys. Lett. 2017, 110, 223903. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Firdaus, Y.; Kirmani, A.R.; Liang, R.Z.; Hu, H.; Liu, M.; Labban, A.E.; Hoogland, S.; Beaujuge, P.M.; Amassian, A.; et al. Hybrid tandem quantum dot/organic solar cells with enhanced photocurrent and efficiency via ink and interlayer engineering. ACS Energy Lett. 2018, 3, 1307–1314. [Google Scholar] [CrossRef]
- Aqoma, H.; Imran, I.F.; Mubarok, M.A.; Hadmojo, W.T.; Do, Y.R.; Jang, S.Y. Efficient hybrid tandem solar cells based on optical reinforcement of colloidal quantum dots with organic bulk heterojunctions. Adv. Energy Mater. 2020, 10, 1903294. [Google Scholar] [CrossRef]
- Green, M.A.; Dunlop, E.D.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Ho-Baillie, A.W. Solar cell efficiency tables (Version 55). Prog. Photovolt. Res. Appl. 2019, 28. [Google Scholar] [CrossRef]
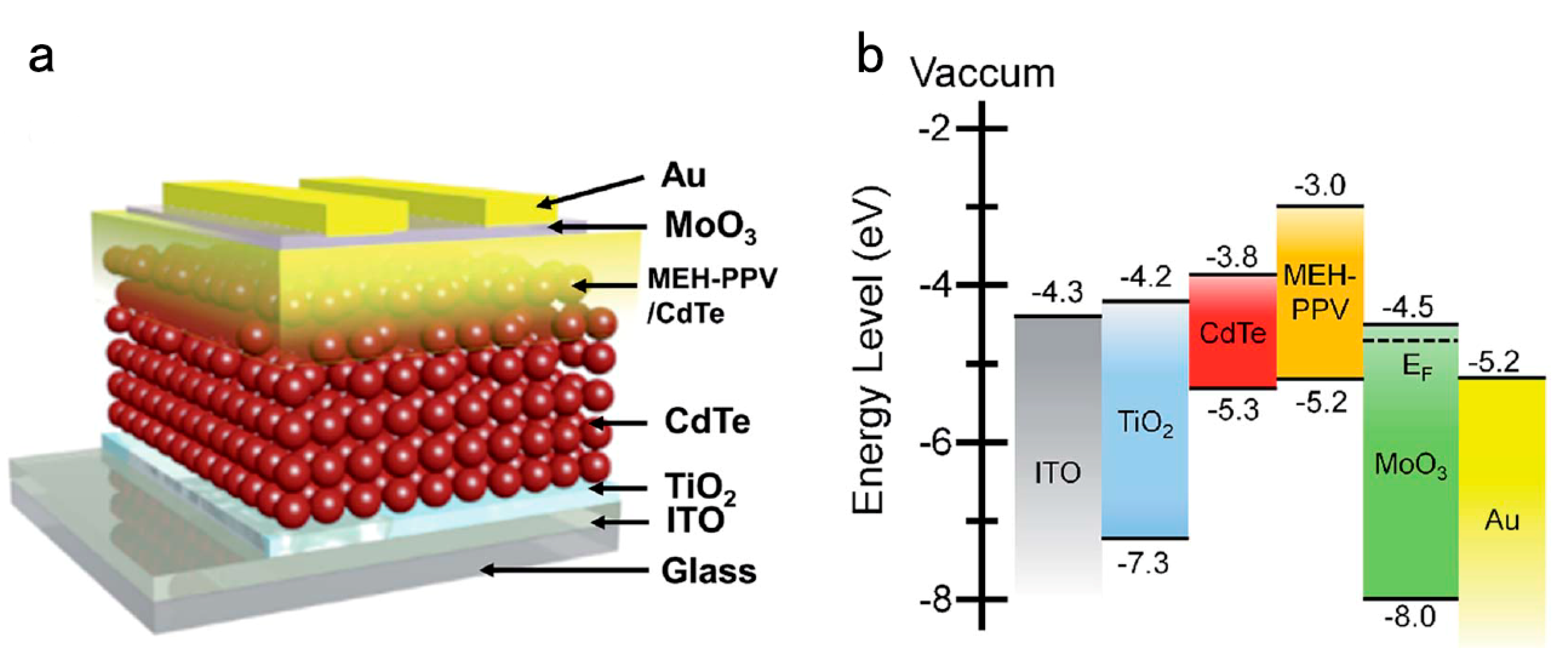

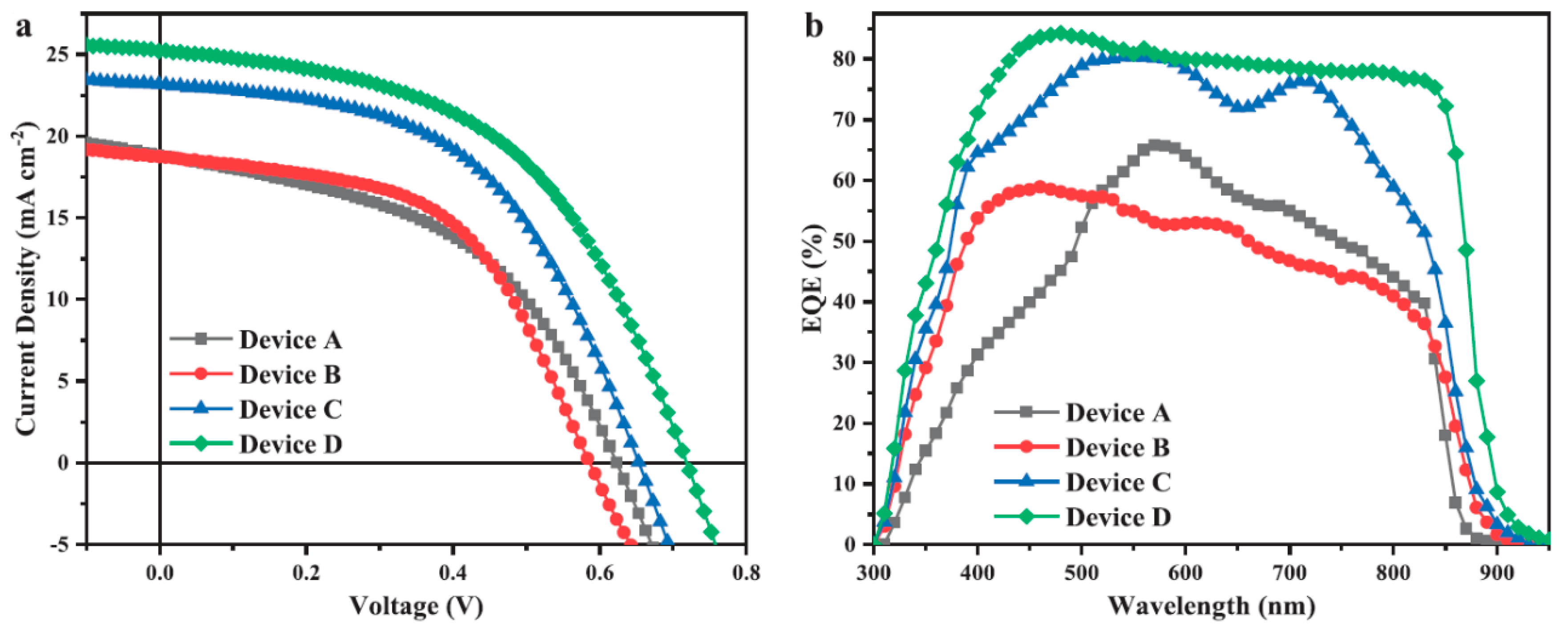
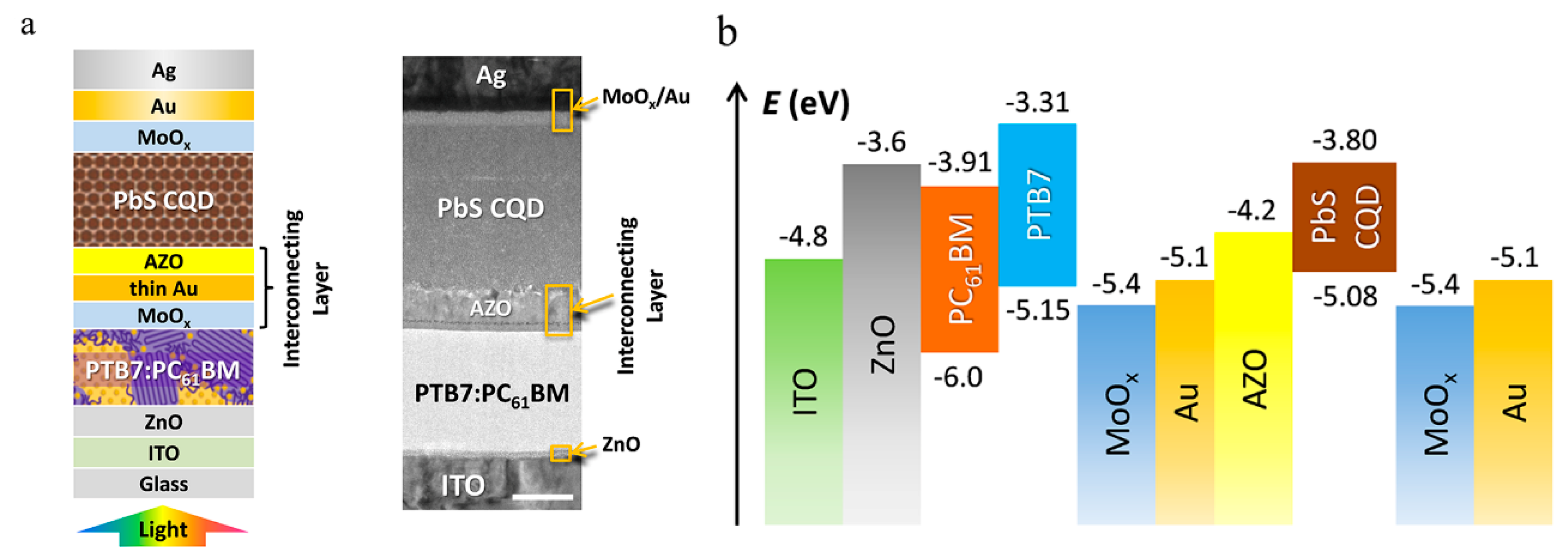
| Type | Device Architecture | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | Published Year | Ref. |
|---|---|---|---|---|---|---|---|
| A | ITO/P3HT: CdSe NC/Al | 0.70 | 5.70 | 40.0 | 1.7 | 2002 | [18] |
| ITO/PEDOT: PSS/MEH-PPV:CdSe NC/Al | 0.90 | 2.03 | 47.0 | 0.85 | 2006 | [22] | |
| ITO/PEDOT:PSS/P3HT:CdS/BCP/Mg:Ag | 1.10 | 10.90 | 35.0 | 4.1 | 2011 | [34] | |
| ITO/ PEDOT:PSS/PCPDTBT:CdSe/PFN/Al | 0.69 | 10.17 | 57.0 | 3.99 | 2014 | [36] | |
| ITO/PEDOT:PSS/P3HT:CdSe NC/Al | 0.73 | 7.40 | 54.0 | 2.9 | 2013 | [37] | |
| ITO/PEDOT:PSS/PCPDTBT:CdSe NC/Al | 0.74 | 12.80 | 50.0 | 4.7 | 2013 | [37] | |
| ITO/PEDOT:PSS/PDTPBT:PbS/TiO2/LiF/Al | 0.57 | 13.06 | 51.0 | 3.78 | 2011 | [47] | |
| ITO/PEDOT:PSS/Si-PCPDTBT:PbS/ZnO/Al | 0.48 | 18.20 | 55.0 | 4.78 | 2016 | [50] | |
| B | ITO/TiO2/CdTe/MEH-PPV:CdTe/MoO3/Au | 0.60 | 13.56 | 51.7 | 4.20 | 2016 | [57] |
| ITO/TiO2/CdTe/P3HT:CdTe/MoO3/Au | 0.54 | 16.59 | 47.2 | 4.32 | 2015 | [58] | |
| ITO/TiO2/CdTe:TiO2/CdTe/PPV:CdTe/MoO3/Au | 0.615 | 18.90 | 51.7 | 6.01 | 2018 | [59] | |
| ITO/ZnO/CdTe:ZnO/CdTe/PPV:CdTe/MoO3/Au | 0.62 | 19.50 | 53.9 | 6.51 | 2019 | [60] | |
| ITO/ZnO/PbS/PBDTTT-E-T:IEICO/MoO3/Ag | 0.66 | 29.60 | 67.0 | 13.1 | 2019 | [63] | |
| C | Glass/SnO2:F/SnO2/CdS/CdTe/PEDOT:PSS/Au | 0.71 | 21.42 | 60.0 | 9.1 | 2016 | [71] |
| ITO/TiO2/CdTe NC/spiro-OMeTAD/Au | 0.71 | 18.78 | 49.2 | 6.56 | 2016 | [72] | |
| ITO/ZnO/CdSe/CdTe/Si-TPA/Au | 0.66 | 23.38 | 54.1 | 8.34 | 2018 | [73] | |
| ITO/ZnO/CdS/CdSe/CdTe/P-TPA/Au | 0.72 | 25.31 | 50.5 | 9.20 | 2019 | [75] | |
| D | ITO/ZnO/PFN-Br/PBDB-T:F-M/M-PEDOT/ZnO/PTB7-Th:O6T-4F:PC71BM/MoO3/Ag | 1.64 | 14.35 | 73.7 | 17.36 | 2018 | [76] |
| ITO/PbS/WO3/Al/P3HT:PCBM/Al | 0.89 | 3.90 | 53.0 | 1.8 | 2014 | [80] | |
| FTO/TiO2/PbS NC/MoOx/ZnO/PFN/Polymer -:fullerene/MoOx/Ag | 1.30 | 5.76 | 68.1 | 5.25 | 2015 | [81] | |
| ITO/ZnO/PbS/MoO3/Au/ZnO/PTB7-Th/PC71BM /MoO3/Ag | 1.27 | 10.36 | 63.0 | 8.27 | 2017 | [82] | |
| ITO/ZnO/PTB7:PCBM/MoOx/Au/AZO/PbS/ -MoOx/Au/Ag | 1.31 | 12.50 | 56.7 | 9.4 | 2018 | [85] | |
| ITO/ZnO/PbS/EDTPbS/Au/ZnO/PTBTTh:IEICO -4F/MoO3/Ag | 1.36 | 13.63 | 69.0 | 12.82 | 2020 | [86] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Li, X.; Jiang, Y.; Yang, R.; Fu, M.; Li, W.; Pan, Y.; Qin, D.; Xu, W.; Hou, L. Recent Progress in Hybrid Solar Cells Based on Solution-Processed Organic and Semiconductor Nanocrystal: Perspectives on Device Design. Appl. Sci. 2020, 10, 4285. https://doi.org/10.3390/app10124285
Xie S, Li X, Jiang Y, Yang R, Fu M, Li W, Pan Y, Qin D, Xu W, Hou L. Recent Progress in Hybrid Solar Cells Based on Solution-Processed Organic and Semiconductor Nanocrystal: Perspectives on Device Design. Applied Sciences. 2020; 10(12):4285. https://doi.org/10.3390/app10124285
Chicago/Turabian StyleXie, Sihang, Xueqi Li, Yasi Jiang, Rourou Yang, Muyi Fu, Wanwan Li, Yiyang Pan, Donghuan Qin, Wei Xu, and Lintao Hou. 2020. "Recent Progress in Hybrid Solar Cells Based on Solution-Processed Organic and Semiconductor Nanocrystal: Perspectives on Device Design" Applied Sciences 10, no. 12: 4285. https://doi.org/10.3390/app10124285
APA StyleXie, S., Li, X., Jiang, Y., Yang, R., Fu, M., Li, W., Pan, Y., Qin, D., Xu, W., & Hou, L. (2020). Recent Progress in Hybrid Solar Cells Based on Solution-Processed Organic and Semiconductor Nanocrystal: Perspectives on Device Design. Applied Sciences, 10(12), 4285. https://doi.org/10.3390/app10124285






