Abstract
Toxic metals remain a current important threat to aquatic ecosystems, despite regulatory efforts to reduce their release. Several toxic metals already appear in the list of priority substances polluting surface waters, while concerns arise from the increasing use of technology-critical metals such as metallic nanoparticles, rare-earth, and platinum group metals. In aquatic environments, various chemical, biological and physical processes determine the impact of metals on the biota. This review provides insights into responses to toxic metals recently reported in freshwater and marine animals. The specific emphasis is on: (i) common cellular and molecular responses; (ii) stress proteins; (iii) redox homeostasis; (iv) cytoskeleton rearrangement; (v) metabolism reshuffle; (vi) free cellular energy and mitochondrial metabolism; and (vi) immunity. These endpoints are promising, notably in multi-biomarker approaches to identify precise cellular toxicity pathways and anticipate the impact of environmental metal pollution.
1. Introduction
Although considerable efforts for reducing pollutant release have been made, contamination in surface waters is still of concern. In this context, a deeper understanding of contaminant flux, dynamics, and impact on biota in aquatic systems are needed to better predict their response to changing conditions and efficiently anticipate risks for ecosystems. Nearly all water bodies are contaminated to some level with transition metals, such as cadmium (Cd), lead (Pb), and mercury (Hg), due to anthropogenic sources [1,2]. Transition metals may or may not have an essential biological role. For example, copper (Cu) is essential to all animals as it participates in fundamental physiological processes (e.g., mitochondrial respiration) and is a cofactor for many enzymes, e.g., superoxide dismutases (SOD) and cytochrome c oxidases (CCOX) [3]. Because of its high reactivity, Cu concentration is tightly regulated inside cells by a complex homeostasis network [4]. This homeostasis network has been studied in several model species and there is evidence of high conservation throughout evolution [5,6]. However, Cu in excessive concentrations causes oxidative stress due to adverse effects on the above-mentioned necessary cellular processes, such as enzyme activity and the electron transport chain [7,8,9]. Similarly, nonessential metals such as Cd are also highly reactive with cellular components and are toxic to cells at low concentrations. Nonessential metals are generally taken up through essential metal homeostasis networks. In different species, metals will bioaccumulate variably in different organs. Moreover, some metals are more toxic than others in different species. For example, mussels seem more sensitive to Cu than to Cd [10].
Metal concentrations are consequently an important parameter for environmental quality in natural environments. Elevated metal concentrations in aquatic ecosystems are directly related to human activities involving the production of industrial (e.g., pesticide use and agricultural run-off, mine tailings) and domestic wastes (e.g., urbanization, automobile exhausts). In pristine and lightly contaminated systems, the total metal concentration ranges are 100–500 ng Cu/L, 30–200 ng Zn/L, 10–70 ng Cd/L, and 0.05–4.0 ng Hg/L, but total concentrations in oxic surface waters can easily reach 2000 ng Cu/L, 560 ng Zn/L, 350 ng Cd/L and 100 ng Hg/L and higher in severely contaminated sites. However, many toxic metals and metalloids that enter aquatic systems are absorbed into suspended particles or dissolved organic matter prompting their accumulation in surficial sediments and reducing their bioavailability [11,12]. Bioavailability is the proportion of total metals that can be incorporated into biota (bioaccumulation). Knowledge of geochemical parameters in both water and sediment is necessary to understand controls on metal bioavailability and subsequent toxicity in natural waters. Bioavailability studies indicate that aquatic organisms uptake free metal ions (metal hydroxides) from solution quite efficiently and models such as the Biotic Ligand Model were developed to estimate metal bioavailability and subsequent toxicity [13]. Nonetheless, some metals (e.g., Hg) and many biological factors impacting metal bioaccumulation in aquatic organisms in natural waters are not well modeled, limiting the prediction of metal toxicity [13]. However, acute toxic concentrations of dissolved metals resulting in obvious and rapid systemic toxicity (i.e., on survival, growth, and/or reproduction) are uncommon in oxic surface waters. Nevertheless, these metals in environmental concentrations are bioaccumulated and can directly affect aquatic species [14,15].
Ecotoxicology aims to study the effects of toxic substances on the health of ecological systems and their constituent species. In the environment, toxic substances generate adverse effects at all levels of biological organization, from the molecular level to communities and ecosystems. It is hypothesized, for example, in the adverse outcome pathway theory, that cascading chains of causal events occur at different levels of biological organization, i.e., molecular events producing a measurable ecotoxicological effect. In this context, current research priorities in ecotoxicology are to understand mechanisms by which contaminants disturb normal biological performance at a sublethal level relevant in chronic exposure scenarios, and to use this knowledge to develop new early-warning biomarkers.
In recent years, early, specific and robust biomarkers directly linking responses at cellular levels to the whole organism, population, and community-level effects have been envisioned. The development of multi-biomarker strategies that allow the simultaneous monitoring of a wide range of biological responses is seen as useful for improving environmental hazard assessment in widespread non-model sentinel species to generalize the use of these techniques in field applications. Integrating data obtained on different levels of the cellular cascade triggered by a pollutant gives a more complete picture of the health status of an organism. In this context, this paper reviews a set of cellular biomarkers that are seen as promising to assess early cellular toxic effects in aquatic animals, in light of recent researches.
2. A Common Response to Toxic Metal Exposure
Once metal toxicity is established, a similar chain of events linked to oxidative stress sets in motion at the subcellular level: the formation of metal-rich granules and the increased production of free radicals, either strictly by the direct effect of a reactant with the metal or by the indirect response to an increase in metal cellular concentration. The production of reactive oxygen species (ROS) has several cellular effects on aquatic species. For example, Cu generates free radicals through the Fenton and Haber-Weis reaction:
Cu(II) + O2− → Cu(I) + O2
Cu(I) + H2O2 → Cu(II) + ·OH + OH−
2O2− + 2H+ → H2O2 + O2
By contrast, Cd, which is not a Fenton metal, generates them indirectly [16]. For example, in copepods, 100 µg/L of Cu for four days trigger a significant production of ROS, but a similar exposure to Cd does not [17]. However, an overproduction of ROS induces redox homeostasis disturbances and macromolecule damage. For instance, Cu and Cd exposure induced DNA damage and hedgehog cells (i.e., heavily DNA damage cells) in medaka larvae [18], likely due to ROS overproduction as shown in rainbow trout [19,20] and/or to the inhibition of DNA repair mechanisms [21,22,23]. Moreover, an excess of metal ions can cause protein mismetallation resulting in a loss of function of proteins [24], further increasing cellular damage. Metal toxicity (specifically Cd) has recently been reviewed by Zhang et al. [25] and will not be detailed here.
Cellular defense mechanisms against toxic metals include canonical antioxidant systems aimed at capturing and neutralizing ROS and more metal-specific responses like sequestration by metallothioneins or into lysozymes. The latter is related to the cell’s ability to detect the presence of metals. Cellular metal overload sensing mainly relies on MTF-1 (metal response transcription factor 1) as recently reviewed by Park et al. [26]. This TF was first described in mammals and more recently identified in aquatic species such as oysters and tilapia [27,28]. Briefly, activation of MTF-1 triggers its nuclear translocation; the TF can then bind to metal response elements and activate downstream gene expression, for instance, metallothionein encoding genes. In addition, cross-regulation between metal-response pathways and heat stress response (HSR) has been highlighted [26], suggesting a synergistic response to metal stress.
In the context of environmental risk assessment, stress response biomarkers can be categorized as (i) overexpression of stress proteins, i.e., metallothioneins and chaperones, (ii) redox homeostasis alteration, (iii) cytoskeleton rearrangement, i.e., toxic effects and resistance effects prompting differences in the cell structure, (iv) disruption of metabolic homeostasis, (v) free cellular energy as well as mitochondrial metabolism, and (vi) immunity modulation.
3. Overexpression of Stress Proteins
3.1. Metallothioneins
Metallothioneins (MTs) are a highly conserved family of small cysteine-rich proteins implicated in metal binding, such as Zn or Fe, and responses to metal stress (Table 1). These proteins have been described extensively in aquatic species, e.g., marine bivalves [29,30], seawater shrimps Palaemonetes argentines [31], copepods [17], freshwater gammarids and teleostean fish [32].

Table 1.
Summary of studies describing metal impacts on stress proteins, i.e., metallothioneins (MTs) and Heat Shock Proteins (HSPs).
MTs are widely used as an environmental surveillance tool. Their expression is highly correlated with metal concentrations in the medium, although the induction threshold varies according to metals [26,33]. MTs binding capacity can also be modified by different abiotic factors, e.g., temperature [34]. In coastal areas, Mytilus sp. has been identified as a model species that reflects a linear relationship between MT production and toxic metal exposure: mussels transplanted to a metal-rich site during eight months, exhibited an increase in MTs content. In digestive glands, MTs and metal concentrations were strongly correlated and remained observable throughout the study, despite seasonal variations [35]. Metal cross-influences have also been studied and a correlation between Zn, Cu, and Cd uptake was demonstrated in bivalves [36]: Zn-exposed oysters Crassostrea hongkongensis showed higher bioaccumulation of Cu and Cd. Authors hypothesized that Zn induced MT production that also had a high affinity with other metals resulting in increased polymetallic bioaccumulation.
Most aquatic species display a rise in expression of MTs at the transcriptional and proteic levels when exposed from 30 ng/L to 500 mg/L of Cd and Cu [29,32,34]. For example, freshwater mussels Dreissena r. bugensis show a four-fold increase in MTs when exposed to 50 or 100 µg/L of Cd for seven days [37]. Mytilus spp. require higher metal concentrations to induce MTs and therefore seem particularly resistant to this metal toxicity. Mussels also have a second defense line against toxic metal poisoning that is biomineralization [38]. In higher vertebrates, the quickest upregulation of MT appears in gastrointestinal tissue, gills, and other organs known to be involved in detoxification processes [32]. Since the aforementioned review, protein expression variations have been found to be species-specific and seem to be linked to the physiological state of the organism [35]. For example, size and season impact the production of MT in exposed freshwater species [32,35].
3.2. HSP
Heat shock proteins (HSPs) were first discovered in a thermal regulation experiment context. They form a family of proteins involved in folding, refolding, and remodeling during protein synthesis [10,39]. These proteins have been reviewed in fish and bivalves by Basu et al. [40] and Fabbri et al. [41], respectively. With its inducible and constitutive isoforms, HSP70 is the most studied of the HSP family and has been described in the cytosol, mitochondria, and the endoplasmic reticulum [40]. Depending on the species, HSPs show different responses to toxic metal exposure (Table 1). For instance, HSP60 avoids misfolding whereas HSP70 prevents aggregation in stress situations.
Toxic metals have been shown to bind to HSPs, inhibiting their function and causing protein misfolding. As such, the modulation of HSP’s expression reflects the level of damage of tissues in mussels and crustaceans [17,42,43].
In fish, three HSPs have been described in a metal contamination context: HPS50, HSP70, and HSP90. Briefly, HSP50 interacts with the endoplasmic reticulum (ER) and functions in the procollagen helix assembly. HSP90 exhibits cytosolic and nuclear locations with the additional roles of signal transduction and transcriptional activation [40]. The abundance of these HSPs is variable throughout the whole organism. In Sparus auratus for instance, intraperitoneal Cd injection (1.25 mg Cd/kg body mass) triggers tissue-specific responses with HSP90 and HSP70 being more abundant only in liver and kidney, respectively, and show no significant variation in gills [44].
HSPs expression as general stress biomarkers has been extensively studied in invertebrates. In oysters, overexpression of HSP60 and HSP70 mRNA and proteins were documented in concentrations ranging from 100-500 µg/L of Cd [44]. Primary cell cultures isolated from gill or hepatopancreas were submitted to a short Cd exposure (4 h, 5.6 to 22.4 µg/mL). HSP70 and HSP60 exhibited a similar response pattern with an increased abundance in gill cells but not in hepatopancreas cells, while HSP90 level was not modified in both cell types. One part of this study focused on Cd’s impact on cell metabolism and will be described in a forthcoming paragraph. In Mytilus spp., an increased abundance of HSP70 and HSP60 were described in presence of 100 µg Cd/mussel for seven days [45] and 10 µg Cu/L for seven days [43], respectively. In small crustaceans, i.e., Tigriopus japonicus, HSP20 and HSP70 mRNA were overexpressed when exposed to 100 µg/L and 50 µg/L respectively for 96 h to most metals, including Cu and Cd [17]. Moreover, this study highlights a downregulation of the HSP40 gene when exposed to 100 µg/L of Cd and Cu, a potential biomarker for toxic metals. In this species, Cu-induced overproduction of most HSP mRNAs (except the aforementioned HSP40 gene) and Cd-induced HSP20, HSP70, and HSP90 genes and a downregulated HSP10 gene.
4. Redox Homeostasis Disturbance
As mentioned above, metal toxicity induces ROS production. When cell defense capacities are overwhelmed, ROS may trigger oxidative stress. Antioxidant enzymes, such as superoxide dismutases (SOD), catalases (CAT) or antioxidant molecules such as glutathione (GSH), are widely used as molecular markers for metal exposure monitoring, alone or in combination with multi-biomarker approaches (Table 2). Scientists also track macromolecular and cellular damages. In animal cells, the mitochondria are very often analyzed, showing destabilization and function modification due to oxidative stress resulting from toxic metals [16,46]. This destabilization has direct consequences on cellular respiration (see Section 7). At the cellular level, lipid peroxidation and lysosomal activity can be affected by metal toxicity [16,46]. These effects have been reviewed in Mytilus spp. [47].

Table 2.
Summary of studies describing metal impacts on redox homeostasis disturbance.
Effects of Cd on reduced glutathione (GSH), a major non-enzymatic compound of antioxidant defense, are well documented and have been reviewed by Nuran et al. [48]. In Mytilus edulis, a metal-specific response was identified when exposed to Cu at 40 µg/L for six days, with a GSH decrease (−25%) in the digestive gland and gills, but no significant variation when exposed to 40 µg/L of Cd or Zn [49]. A decrease in GSH was also observed in C. virginica gill and hepatopancreas cell cultures in response to Cd exposure (four hours at 22.4 µg/mL; [50]). By contrast, in the copepod T. japonicus, 100 μg/L of Cu during 96 h increased GSH content [17], but 100 μg/L of Cd had no effect. Moreover, in both exposures, increased activities were observed for GST (glutathione S transferases), GR (glutathione reductases), and SOD enzymes. SOD proteins are the first defenses against ROS damages. In Mytilus spp., three isoforms have been characterized in response to Cu (25 μg/L for seven days; [47]). Transcriptomic studies on sea bream cell cultures exposed to toxic metals revealed contrasted results: for example, no SOD gene expression modification was detected by DNA microarray in the fibroblast cell line (SAF1) following a 24 h exposure to Cu or Cd (15.9 mg/L and 1.12 mg/L, respectively) [46]. SOD(Mn) and SOD(Cu) gene transcription levels were not significantly modulated in medaka larvae and embryos following exposure to Cd-spiked sediments [21]. Similar results were reported in isolated leukocytes exposed to higher concentrations of Cd but for shorter times (5.6 mg/L for two hours), while CAT mRNA expression was enhanced [51]. Indeed, CAT protein has been described as a sensitive biomarker to toxic metals [52]. A three-year field study concerning Mytilus spp. issuing from contrasted metal contamination sites seems to confirm CAT protein level robustness [53].
5. Cytoskeleton Rearrangement
The cytoskeleton can be defined as proteins that structure the cell through three main networks: microtubules (tubulin), microfilaments (actin), and intermediary filaments (filamin and lamin). The cytoskeleton is implicated in molecule transport and in signaling via vesicular trafficking. Studies have demonstrated cytoskeleton disruption due to metal exposure (Table 3) [10].

Table 3.
Summary of studies describing metal impacts on cytoskeleton rearrangement.
In hemocytes isolated from Mytilus spp., actin immunostaining revealed a modified cellular distribution of filaments after Cd and Cu exposure, evidencing cytoskeleton disruption [54]. For lower tested concentrations (5.6 mg Cd/L; 3.18 mg Cu/L), the actin cytoskeleton was increased in the perinuclear zone and reduced in the cortical cell areas. At higher tested concentrations (112 mg Cd/L; 12.72 mg Cu/L), microfilaments appeared less developed and cells exhibited a round shape [54]. Whether these effects are direct or indirect is yet to be determined. Indeed, disturbance of the cytoskeleton is mainly documented by proteomic studies that show variations in the abundance of cytoskeletal elements. In fish, for instance, effects of Cu (50 µg/L, three days) on gill proteomes have revealed species characteristic profiles: actin is decreased in common carp and rainbow trout but shows no modification in gibel carp. Furthermore, F-actin capping protein subunit β is more abundant in gills of gibel carp but not in the two other analyzed species [55]. Tissue-specific responses were observed in M. galloprovincialis exposed to Cu (10 µg/L, 15 days): actin abundance was reduced in gills and hepatopancreas, while β-tubulin was upregulated only in the latter [56]. In rock oysters, a metal-specific response was described following a four-day exposure to Cd, Cu, Pb or Zn (5, 50, 100 µg/L): actin, for instance, was downregulated by Cu (5 µg/L) and Zn (100 µg/L), upregulated by Pb (100 µg/L), and not affected by Cd [57].
6. Metabolism Reshuffle
Metal toxicity increases energy demand requiring higher ATP production [58]. A widely observed cellular response consists of switching from an aerobic to an anaerobic metabolism producing more ATP (Table 4). This can be triggered by the aforementioned oxidative stress, caused directly by redox metals or indirectly by non-redox metals. This metabolism switch seems to be supported by increased glucose levels and decreased glycogen reserves in fish treated for 10 days with 1 mg Cd/L [59]. The maximal aerobic and anaerobic capacities of the first steps of glycolysis can be assessed by the integrative glycolytic flux method [60]. We achieved a laboratory study in freshwater fish revealing a disturbance of aerobic metabolism in juvenile Rutilus rutilus exposed to Cu. During this experiment, juvenile fishes were exposed to 0, 10, 50, and 100 µg/L of Cu for seven days. At 50 µg Cu/L, the glycolytic flux measured in white muscle compared to control, revealed a downward trend in anaerobic flux after one day and an upward trend after seven days, while the aerobic flux remained unchanged throughout the whole exposure [61]. These data suggest a potential switch to anaerobic metabolism induced by Cu in juvenile roaches. Concomitantly, an increased expression of the CCOX1 gene was observed, while adenylate energy charge was maintained (AEC > 0.7) after one day (T1) despite a decrease in ATP concentration [62,63]. After seven days, however, AEC decreased with increasing concentrations of Cu (AEC < 0.7), while the increase in expression of CCOX1 was lowered compared to T1. Taken together, these observations support that roaches were first able to cope with Cu exposure maintaining their aerobic metabolism. Eventually, after one week of exposure, organisms seem unable to compensate for this stress and anaerobic metabolism was thereafter mobilized (Figure 1) [61]. Nevertheless, ATP production in bivalves has been described as decreased by metals, with 6-phosphofructokinase (PFK)/pyruvate kinase (PK) ratio decreasing when exposed to Cd (50 µg/L) for 10 days [64,65], a mechanism that is in accordance with the ATP/ADP decrease observed in green mussels exposed seven days to Cd (20 µg/L) and/or Cu (50 µg/L) using metabolomic strategy [66]. However, in some species such as clams, ATP production is maintained during Cu (150 µg/L) exposure, probably due to anaerobic metabolism stimulation, in most tissues except hemolymph [67]. Moreover, the oxidation of NADH during electron transport is affected by Cd [37]. In fish, Cd treatment can inhibit Na+/K+-ATPase activity and expression in the major osmoregulatory organs: gills and kidneys [44]. Cu impacts the early stages of glycolysis and for instance, has been shown to inhibit hexokinase and phosphofructokinase activity in concentrations as low as 10 µg/L in mussels [68]. Interestingly, in mammals, PFK can counteract both Cu and Cd effects on the cytoskeleton [69].

Table 4.
Summary of studies describing toxic metal impacts on metabolism reshuffle.
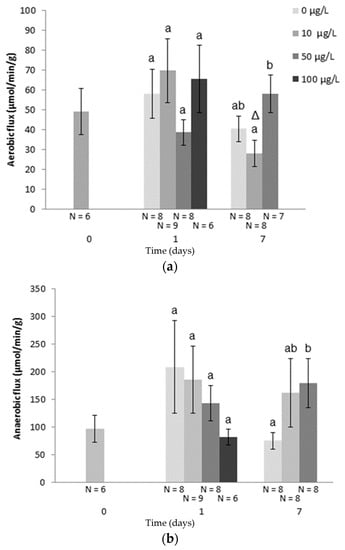
Figure 1.
(a) Aerobic flux (μmol/min/g) and (b) anaerobic flux (μmol/min/g) in white muscle of juvenile roach during Cu exposure. Bars represent S.E. Different letters indicate significant differences for the same time point. Δ indicates significant differences for the same concentration among individual samples at T1 and T7.
7. Impacts of Metals on Free Cellular Energy and Mitochondrial Metabolism
Energy metabolism provides particularly relevant information about stress responses and tolerance (Table 5). The balance between energy demand and energy supply is critical for the survival of organisms facing environmental stressors such as metal toxicants. Metal contamination increases energetic costs of cell maintenance to ensure protection, detoxification as well as repair (see above), and finally survival [70,71]. The scope for growth measurement (SFG) reflects energy allocation to growth and reproduction. SFG in aquatic organisms shows that metal exposure shifts the use of the energy towards the implementation of defense mechanisms (see references in [72,73]). Indeed, in freshwater mussels Dreissena polymorpha exposed to 10 µg Cd/L in controlled conditions, SFG decreased after 90 min [74]. At the cellular level, the mitochondrion is the key organelle for free energy synthesis as respiratory chain and oxidative phosphorylation provide ATP supply. Mitochondrial electron transport system activity is altered by metal exposure as evidenced by the decrease observed in juvenile roach exposed for one day to 100 µg Cu/L [62]. The activity of respiratory chain enzymes varies depending on the complexes they form. For instance, in rainbow trout mitochondria exposed in vitro to Cu (0–1.3 mg/L), complex I and III basal respiration rates were stimulated while complex II and IV basal respiration rates were inhibited [75]. In mollusks such as marine clams, succinate dehydrogenase (complex II) activity is similarly inhibited in mitochondria in vitro exposed to Cu (5 mg/L) [76]. In eastern oysters, sensitivity and response differences to Cd were also observed between electron transport chain complexes [58]. However, in the same species, metals were shown to induce a decrease of the whole mitochondrial respiration rate [77,78]. Free energy availability is consequently depressed, as reflected by the ATP/ADP ratio and the AEC decline in Cd (25 µg/L; three weeks) exposed oysters [77]. Such a decline of ATP concentration is also observed in oyster hemocytes exposed to Cd (0–22.5 mg/L) [79]. Cd effect on mitochondrial bioenergetics depends on the concentration: an increase of proton leak is observed in oysters’ isolated mitochondria exposed to 112.4–562 µg/L of Cd with no effect on ADP-stimulated state 3 respiration rates, while the reverse is observed at 1.12–5.62 mg/L of Cd with the inhibition of state 3 respiration [80]. However, if they are all inhibited in Cd exposed oysters, mitochondrial matrix enzymes, such as citrate synthase or isocitrate-dehydrogenase, are more sensitive to Cd than the membrane enzymes of the respiratory chain complexes [58]. The disturbance of energy metabolism in Cd exposed oysters can differ from their isolated mitochondria, but both support complex phenomenon implying diverse enzymatic impairments [78]. Finally, exposure to a metal such as Cd in oysters, decreases the activation energy of most mitochondrial enzymes, consequently limiting the ability of organisms to face additional environmental stress such as temperature increase [58]. The metabolic effect of metals can also be assessed at the molecular level targeting the same pathways. Indeed, expression of genes involved in cellular energy metabolism, notably in the respiratory chain, may also give relevant information on stress responses. For example, under laboratory conditions, the expression of the CCOX gene increased in several species of freshwater and marine mollusks exposed to Cd [81] and in zebra mussels exposed to different metals [82].

Table 5.
Summary of studies describing toxic metal impacts on free cellular energy and mitochondrial metabolism.
8. Impacts of Metals on Immunity
Immunity is a physiological system involved in the organism’s main homeostatic functions. Its role in anti-pathogen defense (against bacteria, viruses, fungi, etc.) is widely known, but it is also implicated in the tissue remodeling regulation, associated with reproduction, growth, development, and in stress response. Its role in organism integrity maintenance gives it a central position in physiology. Metal toxicity results in immune function modulation in aquatic organisms, involving immune parameter inhibition and/or stimulation of cellular and molecular immunological functions.
Cu increases leucocyte oxidative activity in carp (Cyprinus carpio) exposed from four to eight days to metal at environmental concentrations (100 and 250 mg/L in the Champagne region, France) [83]. The humoral immune factor responses of Cu-treated carp were modulated by the presence of a parasite (Ptychobothrium sp.), as shown by a high increase in lysozyme activity observed in parasitized carp after exposition [84]. Similar responses were observed in roach (Rutilus rutilus) exposed to environmental concentrations of Al [85]. A significant pro-oxidant effect was observed in head kidney leukocytes of roach exposed to 100 μg Al/L for two days. These pro-oxidant effects were higher in fish whose immunity was stimulated with LPS-bacterial endotoxin. These results demonstrate that environmental concentrations of Al induce oxidative and immunotoxic effects in fish and are associated with an immunomodulatory process in relation to inflammatory responses.
Co-exposure of fish to both chemical and biological (parasitic) stress may increase the effects of metals exposure. Identical observations were made in other fish species in laboratory studies [86]. The central question of the environmental reality of these fish responses in their natural environment has rarely been studied to date [87].
9. Conclusions
In recent years, responses to toxic metals in aquatic animals were studied in various experimental conditions which helped to confirm that they trigger common toxicity pathways. In this context, various datasets showed that molecular regulation at the gene, protein, and metabolite level indicated physiological adaptation. Metals induce an early response as evidenced by alterations both at structural and functional levels of different organs, including enzymatic and genetic effects. Thus, they affect redox homeostasis, energy metabolism, and the innate immune system of exposed biota and/or potentially increase susceptibility to multiple types of disease (Figure 2). Moreover, the analysis of these endpoints, notably in multi-biomarker strategies, seems highly promising in identifying molecular signatures that will allow the early and sensitive detection of metal contamination. Nevertheless, further work is needed to efficiently use these molecular and cellular responses to enable risk assessment in the environment at population and community levels. To reach this aim, an effort to better link biomarker responses with whole-organism impact has to be a research priority notably to take into account biological variability and the influence of confounding factors [88]. Biomarkers are valuable tools for the implementation of guidelines for effective environmental management as they offer additional biologically and ecologically relevant information. However, biomarker validation in wild populations is necessary, to normalize background levels and eventually to complement standard tests to increase relevant legislation regarding the protection of aquatic environments [89].
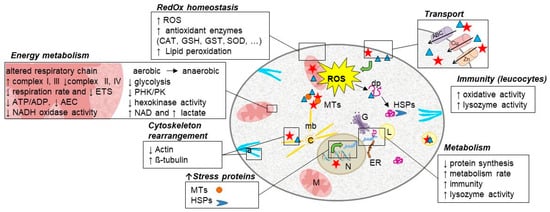
Figure 2.
Exposure to toxic metals in aquatic animals triggers common toxicity pathways (ABC: ABC transporter; a: actin; C: centrosome; dp: denatured protein; L: lysosome; ER: endoplasmic reticulum; G: Golgi; HSPs: heat shock proteins; M: mitochondria; mb: microtubules; MTs: metallothioneins; N: nucleus; ROS: reactive oxygen species).
Author Contributions
Writing—review A.L.S., E.D., S.B., F.B., B.R., I.B.; writing—review and editing C.C.; All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Azimi, S.; Rocher, V. Influence of the water quality improvement on fish population in the Seine River (Paris, France) over the 1990–2013 period. Sci. Total Environ. 2016, 542, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Loizeau, J.; Edder, P.; De Alencastro, L.F.; Corvi, C.; Ramseier Gentile, S. La contamination du léman par les micropolluants—Revue de 40 ans d’études. Arch. Sci. 2013, 66, 117–136. [Google Scholar]
- Castruita, M.; Casero, D.; Karpowicz, S.J.; Kropat, J.; Vieler, A.; Hsieh, S.I.; Yan, W.; Cokus, S.; Loo, J.A.; Benning, C.; et al. Systems Biology Approach in Chlamydomonas Reveals Connections between Copper Nutrition and Multiple Metabolic Steps. Plant Cell 2011, 23, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Colás, N.; Sancenón, V.; Rodriguez-Navarro, S.; Mayo, S.; Thiele, D.J.; Ecker, J.R.; Puig, S.; Peñarrubia, L. The Arabidopsis heavy metal P-type ATPase HMA5 interacts with metallochaperones and functions in copper detoxification of roots. Plant J. 2006, 45, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Burkhead, J.L.; Reynolds, K.A.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef] [PubMed]
- Page, M.D.; Kropat, J.; Hamel, P.P.; Merchant, S. Two Chlamydomonas CTR Copper Transporters with a Novel Cys-Met Motif Are Localized to the Plasma Membrane and Function in Copper Assimilation. Plant Cell 2009, 21, 928–943. [Google Scholar] [CrossRef]
- Monferran, M.V.; Agudo, J.A.S.; Pignata, M.L.; Wunderlin, D.A. Copper-induced response of physiological parameters and antioxidant enzymes in the aquatic macrophyte Potamogeton pusillus. Environ. Pollut. 2009, 157, 2570–2576. [Google Scholar] [CrossRef]
- Razinger, J.; Drinovec, L.; Zrimec, A. Real-time visualization of oxidative stress in a floating macrophyte Lemna minor L. exposed to cadmium, copper, menadione, and AAPH. Environ. Toxicol. 2010, 25, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.; Sharma, G.D.; Panda, S. Responses of antioxidant metabolism and defense mechanism of aquatic macrophyte, Pistia stratiotes L. to zinc treatment under copper stress. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2011, 81, 422–427. [Google Scholar]
- Quintá, H.R.; Galigniana, N.M.; Erlejman, A.G.; Lagadari, M.; Piwien-Pilipuk, G.; Galigniana, M.D. Management of cytoskeleton architecture by molecular chaperones and immunophilins. Cell Signal. 2011, 23, 1907–1920. [Google Scholar] [CrossRef]
- Doig, L.E.; Liber, K. Influence of dissolved organic matter on nickel bioavailability and toxicity to Hyalella azteca in water-only exposures. Aquat. Toxicol. 2006, 76, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Van Cappellen, P.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K.M. Biogeochemical Redox Processes and their Impact on Contaminant Dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Mebane, C.A.; Chowdhury, M.J.; De Schamphelaere, K.; Lofts, S.; Paquin, P.R.; Santore, R.C.; Wood, C.M. Metal Bioavailability Models: Current Status, Lessons Learned, Considerations for Regulatory Use, and the Path Forward. Environ. Toxicol. Chem. 2020, 39, 60–84. [Google Scholar] [CrossRef] [PubMed]
- RajeshKumar, S.; Li, X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol. Rep. 2018, 5, 288–295. [Google Scholar] [CrossRef]
- Mahino, F.; Nazura, U.; Mobarak, H. Heavy metal in aquatic ecosystem emphasizing its effect on tissue bioaccumulation and histopathology: A review. J. Environ. Sci. Technol. 2014, 7, 1–15. [Google Scholar]
- Banfalvi, G. Heavy Metals, Trace Elements and Their Cellular Effects. Cell. Eff. Heavy Met. 2011, 3–28. [Google Scholar] [CrossRef]
- Kim, B.-M.; Rhee, J.-S.; Jeong, C.-B.; Seo, J.S.; Park, G.S.; Lee, Y.-M.; Lee, J.-S. Heavy metals induce oxidative stress and trigger oxidative stress-mediated heat shock protein (hsp) modulation in the intertidal copepod Tigriopus japonicus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 166, 65–74. [Google Scholar] [CrossRef]
- Barjhoux, I.; Baudrimont, M.; Morin, B.; Landi, L.; Gonzalez, P.; Cachot, J. Effects of copper and cadmium spiked-sediments on embryonic development of Japanese medaka (Oryzias latipes). Ecotoxicol. Environ. Saf. 2012, 79, 272–282. [Google Scholar] [CrossRef]
- Risso-de-Faverney, C.; Devaux, A.; Lafaurie, M.; Girard, J.; Bailly-Maitre, P.G.A.B.; Delescluse, C. Cadmium induces apoptosis and genotoxicity in rainbow trout hepatocytes through generation of reactive oxygene species. Aquat. Toxicol. 2001, 53, 65–76. [Google Scholar] [CrossRef]
- Bopp, S.K.; Abicht, H.K.; Knauer, K. Copper-induced oxidative stress in rainbow trout gill cells. Aquat. Toxicol. 2008, 86, 197–204. [Google Scholar] [CrossRef]
- Barjhoux, I.; Gonzalez, P.; Baudrimont, M.; Cachot, J. Molecular and phenotypic responses of Japanese medaka (Oryzias latipes) early life stages to environmental concentrations of cadmium in sediment. Environ. Sci. Pollut. Res. 2016, 23, 17969–17981. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, F.; Baudrimont, M.; Gonzalez, P.; Cuenot, Y.; Bourdineaud, J.; Boudou, A.; Gauthier, L. Genotoxic and stress inductive potential of cadmium in Xenopus laevis larvae. Aquat. Toxicol. 2006, 78, 157–166. [Google Scholar] [CrossRef]
- Giaginis, C.; Gatzidou, E.; Theocharis, S. DNA repair systems as targets of cadmium toxicity. Toxicol. Appl. Pharmacol. 2006, 213, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. The Mismetallation of Enzymes during Oxidative Stress. J. Boil. Chem. 2014, 289, 28121–28128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Reynolds, M. Cadmium exposure in living organisms: A short review. Sci. Total Environ. 2019, 678, 761–767. [Google Scholar] [CrossRef]
- Park, C.; Jeong, J. Synergistic cellular responses to heavy metal exposure: A minireview. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1584–1591. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.P.-L.; Au, C.Y.-M.; Chan, W.W.-L.; Chan, K.M. Characterization and localization of metal-responsive-element-binding transcription factors from tilapia. Aquat. Toxicol. 2010, 99, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Zhang, G.; Li, L.; Li, C.; Wang, T.; Zhang, G. Transcription factor CgMTF-1 regulates CgZnT1 and CgMT expression in Pacific oyster (Crassostrea gigas) under zinc stress. Aquat. Toxicol. 2015, 165, 179–188. [Google Scholar] [CrossRef]
- Ale, A.; Liberatori, G.; Vannuccini, M.L.; Bergami, E.; Ancora, S.; Mariotti, G.; Bianchi, N.; Corsi, I.; DeSimone, M.F.; Cazenave, J.; et al. Exposure to a nanosilver-enabled consumer product results in similar accumulation and toxicity of silver nanoparticles in the marine mussel Mytilus galloprovincialis. Aquat. Toxicol. 2019, 211, 46–56. [Google Scholar] [CrossRef]
- Roesijadi, G. Metallothioneins in metal regulation and toxicity in aquatic animals. Aquat. Toxicol. 1992, 22, 81–113. [Google Scholar] [CrossRef]
- Bertrand, L.; Monferrán, M.V.; Mouneyrac, C.; Amé, M.V. Native crustacean species as a bioindicator of freshwater ecosystem pollution: A multivariate and integrative study of multi-biomarker response in active river monitoring. Chemosphere 2018, 206, 265–277. [Google Scholar] [CrossRef]
- Mijošek, T.; Marijić, V.F.; Dragun, Z.; Ivanković, D.; Krasnići, N.; Erk, M.; Gottstein, S.; Lajtner, J.; Perić, M.S.; Kepčija, R.M. Comparison of electrochemically determined metallothionein concentrations in wild freshwater salmon fish and gammarids and their relation to total and cytosolic metal levels. Ecol. Indic. 2019, 105, 188–198. [Google Scholar] [CrossRef]
- Bourdineaud, J.-P.; Baudrimont, M.; Gonzalez, P.; Moreau, J.-L. Challenging the model for induction of metallothionein gene expression. Biochimie 2006, 88, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Piano, A.; Valbonesi, P.; Fabbri, E. Expression of cytoprotective proteins, heat shock protein 70and metallothioneins, in tissues ofOstrea edulis exposed to heat andheavy metals. Cell Stress Chaperones 2004, 9, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Geffard, A.; Amiard-Triquet, C.; Amiard, J.-C. Do seasonal changes affect metallothionein induction by metals in mussels, Mytilus edulis? Ecotoxicol. Environ. Saf. 2005, 61, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, W.-X. Facilitated Bioaccumulation of Cadmium and Copper in the OysterCrassostrea hongkongensisSolely Exposed to Zinc. Environ. Sci. Technol. 2013, 47, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Hanana, H.; Kleinert, C.; André, C.; Gagné, F. Influence of cadmium on oxidative stress and NADH oscillations in mussel mitochondria. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 216, 60–66. [Google Scholar] [CrossRef]
- Amiard, J.; Amiardtriquet, C.; Barka, S.; Pellerin, J.; Rainbow, P. Metallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 2006, 76, 160–202. [Google Scholar] [CrossRef]
- Niforou, K.; Cheimonidou, C.; Trougakos, I.P. Molecular chaperones and proteostasis regulation during redox imbalance. Redox Boil. 2014, 2, 323–332. [Google Scholar] [CrossRef]
- Basu, N.; Todgham, A.; Ackerman, P.; Bibeau, M.; Nakano, K.; Schulte, P.M.; Iwama, G.K. Heat shock protein genes and their functional significance in fish. Gene 2002, 295, 173–183. [Google Scholar] [CrossRef]
- Fabbri, E.; Valbonesi, P.; Franzellitti, S. HSP expression in bivalves. Inf. Syst. J. 2008, 5, 135–161. [Google Scholar]
- Pestana, J.L.; Novais, S.C.; Norouzitallab, P.; Vandegehuchte, M.B.; Bossier, P.; De Schamphelaere, K. Non-lethal heat shock increases tolerance to metal exposure in brine shrimp. Environ. Res. 2016, 151, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B.; Martin, L.; Howe, S.; Nelson, W.; Hegre, E.; Phelps, D. Tissue-Specific Differences in Accumulation of Stress Proteins in Mytilus edulis Exposed to a Range of Copper Concentrations. Toxicol. Appl. Pharmacol. 1994, 125, 206–213. [Google Scholar] [CrossRef]
- Santos, S.G.G.; Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Varela, J.L.; Mancera, J.; Fontaínhas-Fernandes, A.; Wilson, J.M. Metabolic and osmoregulatory changes and cell proliferation in gilthead sea bream (Sparus aurata) exposed to cadmium. Ecotoxicol. Environ. Saf. 2011, 74, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Tedengren, M.; Olsson, B.; Bradley, B.; Zhou, L. Heavy metal uptake, physiological response and survival of the blue mussel (Mytilus edulis) from marine and brackish waters in relation to the induction of heat-shock protein. Hydrobiologia 1999, 393, 261–269. [Google Scholar] [CrossRef]
- Minghetti, M.; Leaver, M.; Taggart, J.B.; Casadei, E.; Auslander, M.; Tom, M.; George, S.G. Copper induces Cu-ATPase ATP7A mRNA in a fish cell line, SAF1. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 93–99. [Google Scholar] [CrossRef]
- Manduzio, H.; Monsinjon, T.; Rocher, B.; Leboulenger, F.; Galap, C. Characterization of an inducible isoform of the Cu/Zn superoxide dismutase in the blue mussel Mytilus edulis. Aquat. Toxicol. 2003, 64, 73–83. [Google Scholar] [CrossRef]
- Nuran, E.; Hande, G.-O.; Nukhet, A.-B. Toxic Metals and Oxidative Stress Part I: Mechanisms Involved in Me-tal induced Oxidative Damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef]
- Viarengo, A.; Canesi, L.; Pertica, M.; POli, G.; Moore, M.N.; Orunesu, M. Heavy metal effects on lipid peroxidation in the tissues of Mytilus galloprovincialis lam. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 1990, 97, 37–42. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Cherkasov, A.S.; Sokolova, I.M. Effects of cadmium on cellular protein and glutathione synthesis and expression of stress proteins in eastern oysters, Crassostrea virginica Gmelin. J. Exp. Boil. 2008, 211, 577–586. [Google Scholar] [CrossRef]
- Morcillo, P.; Cordero, H.; Meseguer, J.; Esteban, M.A.; Cuesta, A. Toxicological in vitro effects of heavy metals on gilthead seabream (Sparus aurata L.) head-kidney leucocytes. Toxicol. Vitr. 2015, 30, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Regoli, F.; Pellegrini, D.; Winston, G.W.; Gorbi, S.; Giuliani, S.; Virno-Lamberti, C.; Bompadre, S. Application of biomarkers for assessing the biological impact of dredged materials in the Mediterranean: The relationship between antioxidant responses and susceptibility to oxidative stress in the red mullet (Mullus barbatus). Mar. Pollut. Bull. 2002, 44, 912–922. [Google Scholar] [CrossRef]
- Vlachogianni, T.; Dassenakis, M.; Scoullos, M.J.; Valavanidis, A. Integrated use of biomarkers (superoxide dismutase, catalase and lipid peroxidation) in mussels Mytilus galloprovincialis for assessing heavy metals’ pollution in coastal areas from the Saronikos Gulf of Greece. Mar. Pollut. Bull. 2007, 54, 1361–1371. [Google Scholar] [CrossRef]
- Gómez-Mendikute, A.; Cajaraville, M. Comparative effects of cadmium, copper, paraquat and benzo[a]pyrene on the actin cytoskeleton and production of reactive oxygen species (ROS) in mussel haemocytes. Toxicol. Vitr. 2003, 17, 539–546. [Google Scholar] [CrossRef]
- Eyckmans, M.; Benoot, D.; Van Raemdonck, G.A.; Zegels, G.; Van Ostade, X.; Witters, E.; Blust, R.; De Boeck, G. Comparative proteomics of copper exposure and toxicity in rainbow trout, common carp and gibel carp. Comp. Biochem. Physiol. Part D Genom. Proteom. 2012, 7, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Chora, S.; Pereira, C.G.; Cardoso, C.; Bebianno, M.J. Proteomic response of mussels Mytilus galloprovincialis exposed to CuO NPs and Cu2+: An exploratory biomarker discovery. Aquat. Toxicol. 2014, 155, 327–336. [Google Scholar] [CrossRef]
- Thompson, E.; Taylor, D.A.; Nair, S.V.; Birch, G.; Haynes, P.A.; Raftos, D.A. Proteomic discovery of biomarkers of metal contamination in Sydney Rock oysters (Saccostrea glomerata). Aquat. Toxicol. 2012, 109, 202–212. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Habinck, E.; Sokolova, I.M. Differential sensitivity to cadmium of key mitochondrial enzymes in the eastern oyster, Crassostrea virginica Gmelin (Bivalvia: Ostreidae). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 148, 72–79. [Google Scholar] [CrossRef]
- Cicik, B.; Engin, K. The effects of cadmium on levels of glucose in serum and glycogen reserves in the liver and muscle tissues of Cyprinus carpio (L., 1758). Turkish J. Vet. Anim. Sci. 2005, 29, 113–117. [Google Scholar]
- Meléndez-Morales, D.; Lugo, P.D.P.; Meléndez-Hevia, E.; Paz-Lugo, P. Glycolysis activity in flight muscles of birds according to their physiological function. An experimental model in vitro to study aerobic and anaerobic glycolysis activity separately. Mol. Cell. Biochem. 2009, 328, 127–135. [Google Scholar] [CrossRef]
- Maes, V. Le Métabolisme Énergétique Chez un Cyprinidé D’eau Douce, le Gardon Rutilus Rutilus: Vers le Développement de Nouveaux Biomarqueurs en Lien Avec la Contamination Par des Produits Phytosanitaires. Ph.D. Thesis, Université de Reims Champagne-Ardenne, Reims, France, December 2014. [Google Scholar]
- Maes, V.; Betoulle, S.; Jaffal, A.; Dedourge-Geffard, O.; Delahaut, L.; Geffard, A.; Palluel, O.; Sanchez, W.; Paris-Palacios, S.; Vettier, A.; et al. Juvenile roach (Rutilus rutilus) increase their anaerobic metabolism in response to copper exposure in laboratory conditions. Ecotoxicology 2016, 25, 900–913. [Google Scholar] [CrossRef] [PubMed]
- Maes, V.; Vettier, A.; Jaffal, A.; Dedourge-Geffard, O.; Delahaut, L.; Geffard, A.; Betoulle, S.; David, E. Energy metabolism and pesticides: Biochemical and molecular responses to copper in roach Rutilus rutilus. J. Xenobiotics 2013, 3, 7. [Google Scholar] [CrossRef][Green Version]
- Shi, W.; Han, Y.; Guan, X.; Rong, J.; Du, X.; Zha, S.; Tang, Y.; Liu, G. Anthropogenic Noise Aggravates the Toxicity of Cadmium on Some Physiological Characteristics of the Blood Clam Tegillarca granosa. Front. Physiol. 2019, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Viarengo, A.; Pertica, M.; Mancinelli, G.; Capelli, R.; Orunesu, M. Effects of copper on the uptake of amino acids, on protein synthesis and on ATP content in different tissues of Mytilus galloprovincialis Lam. Mar. Environ. Res. 1980, 4, 145–152. [Google Scholar] [CrossRef]
- Wu, H.; Wang, W.-X. NMR-based metabolomic studies on the toxicological effects of cadmium and copper on green mussels Perna viridis. Aquat. Toxicol. 2010, 100, 339–345. [Google Scholar] [CrossRef]
- Giacomin, M.; Jorge, M.B.; Bianchini, A. Effects of copper exposure on the energy metabolism in juveniles of the marine clam Mesodesma mactroides. Aquat. Toxicol. 2014, 152, 30–37. [Google Scholar] [CrossRef]
- Canesi, L.; Ciacci, C.; Piccoli, G.; Stocchi, V.; Viarengo, A.; Gallo, G. In vitro and in vivo effects of heavy metals on mussel digestive gland hexokinase activity: The role of glutathione. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 120, 261–268. [Google Scholar] [CrossRef]
- Liliom, K.; Wágner, G.; Pácz, A.; Cascante, M.; Kovács, J.; Ovádi, J. Organization-dependent effects of toxic bivalent ions. J. Boil. Inorg. Chem. 2000, 267, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Calow, P.; Forbes, V.E. How do physiological responses to stress translate into ecological and evolutionary processes? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 120, 11–16. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Lannig, G. Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: Implications of global climate change. Clim. Res. 2008, 37, 181–201. [Google Scholar] [CrossRef]
- Le Gal, Y.; Lagadic, L.; Lebras, S.; Caquet, T. Charge énergétique en adénylates (CEA) et autres biomarqueurs associés au métabolisme énergétique. In Biomarqueurs en Ecotoxicologie: Aspects Fondamentaux, 1st ed.; Lagadic, L., Caquet, B., Amiard, J.C., Ramade, F., Eds.; Masson: Paris, France, 1997; pp. 241–285. [Google Scholar]
- Mouneyrac, C.; Péry, A. Conséquences des perturbations du métabolisme énergétique. In Biomarqueurs en Écotoxicologie Aquatique, 2nd ed.; Amiard, J.C., Claude, A.-T., Eds.; Lavoisier Tec & Doc: Paris, France, 2017; pp. 270–297. [Google Scholar]
- Louis, F.; Devin, S.; Giambérini, L.; Potet, M.; David, E.; Pain-Devin, S. Energy allocation in two dreissenid species under metal stress. Environ. Pollut. 2019, 245, 889–897. [Google Scholar] [CrossRef]
- Sappal, R.; MacDougald, M.; Fast, M.; Stevens, D.; Kibenge, F.; Siah, A.; Kamunde, C. Alterations in mitochondrial electron transport system activity in response to warm acclimation, hypoxia-reoxygenation and copper in rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 2015, 165, 51–63. [Google Scholar] [CrossRef]
- Jorge, M.B.; Lauer, M.M.; Martins, C.D.M.G.; Bianchini, A. Impaired regulation of divalent cations with acute copper exposure in the marine clam Mesodesma mactroides. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 179, 79–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sokolova, I.M.; Sokolov, E.P.; Ponnappa, K.M. Cadmium exposure affects mitochondrial bioenergetics and gene expression of key mitochondrial proteins in the eastern oyster Crassostrea virginica Gmelin (Bivalvia: Ostreidae). Aquat. Toxicol. 2005, 73, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.; Ivanina, A.; Kurochkin, I. Effects of temperature and cadmium exposure on the mitochondria of oysters (Crassostrea virginica) exposed to hypoxia and subsequent reoxygenation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 163, S8. [Google Scholar] [CrossRef][Green Version]
- Sokolova, I.M.; Evans, S.; Hughes, F.M. Cadmium-induced apoptosis in oyster hemocytes involves disturbance of cellular energy balance but no mitochondrial permeability transition. J. Exp. Boil. 2004, 207, 3369–3380. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, I.M. Cadmium effects on mitochondrial function are enhanced by elevated temperatures in a marine poikilotherm, Crassostrea virginica Gmelin (Bivalvia: Ostreidae). J. Exp. Boil. 2004, 207, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Achard-Joris, M.; Gonzalez, P.; Marie, V.; Baudrimont, M.; Bourdineaud, J.-P. Cytochrome c Oxydase Subunit I Gene is Up-regulated by Cadmium in Freshwater and Marine Bivalves. BioMetals 2006, 19, 237–244. [Google Scholar] [CrossRef]
- Navarro, A.; Faria, M.; Barata, C.; Piña, B. Transcriptional response of stress genes to metal exposure in zebra mussel larvae and adults. Environ. Pollut. 2011, 159, 100–107. [Google Scholar] [CrossRef]
- Dautremepuits, C.; Betoulle, S.; Paris-Palacios, S.; Vernet, G. Immunology-Related Perturbations Induced by Copper and Chitosan in Carp (Cyprinus carpio L.). Arch. Environ. Contam. Toxicol. 2004, 47, 370–378. [Google Scholar] [CrossRef]
- Dautremepuits, C.; Betoulle, S.; Paris-Palacios, S.; Vernet, G. Humoral immune factors modulated by copper and chitosan in healthy or parasitised carp (Cyprinus carpio L.) by Ptychobothrium sp. (Cestoda). Aquat. Toxicol. 2004, 68, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Jolly, S.; Jaffal, A.; Delahaut, L.; Palluel, O.; Porcher, J.-M.; Geffard, A.; Sanchez, W.; Betoulle, S. Effects of aluminium and bacterial lipopolysaccharide on oxidative stress and immune parameters in roach, Rutilus rutilus L. Environ. Sci. Pollut. Res. 2014, 21, 13103–13117. [Google Scholar] [CrossRef] [PubMed]
- Le Guernic, A.; Sanchez, W.; Palluel, O.; Bado-Nilles, A.; Floriani, M.; Turies, C.; Chadili, E.; Della Vedova, C.; Cavalié, I.; Adam-Guillermin, C.; et al. Acclimation capacity of the three-spined stickleback (Gasterosteus aculeatus, L.) to a sudden biological stress following a polymetallic exposure. Ecotoxicology 2016, 25, 1478–1499. [Google Scholar] [CrossRef][Green Version]
- Petitjean, Q. Response variability to multiple stressors exposure in wild gudgeons (Gobio occitaniae). Ph.D. Thesis, Université Toulouse III Paul Sabatier, Toulouse, France, 2019. [Google Scholar]
- Ciliberti, A.; Chaumot, A.; Recoura-Massaquant, R.; Chandesris, A.; François, A.; Coquery, M.; Ferréol, M.; Geffard, O. Caged Gammarus as biomonitors identifying thresholds of toxic metal bioavailability that affect gammarid densities at the French national scale. Water Res. 2017, 118, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, D.; Chaumot, A.; Charnot, A.; Almunia, C.; François, A.; Navarro, L.; Armengaud, J.; Salvador, A.; Geffard, O. Ecotoxico-Proteomics for Aquatic Environmental Monitoring: First in Situ Application of a New Proteomics-Based Multibiomarker Assay Using Caged Amphipods. Environ. Sci. Technol. 2017, 51, 13417–13426. [Google Scholar] [CrossRef]
- McDonagh, B.; Tyther, R.; Sheehan, D. Carbonylation and glutathionylation of proteins in the blue mussel Mytilus edulis detected by proteomic analysis and Western blotting: Actin as a target for oxidative stress. Aquat. Toxicol. 2005, 73, 315–326. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).