Abstract
The use of chloride-based deicing salts, particularly sodium chloride (NaCl) and calcium chloride (CaCl2), is a common practice in cold regions for maintaining road safety during winter. However, the accumulation of salt residues in adjacent soils poses serious environmental threats, including reduced pH, increased electrical conductivity (EC), disrupted soil structure, and plant growth inhibition. This study aimed to evaluate the combined effect of activated carbon (AC) and Pennisetum alopecuroides, a salt-tolerant perennial grass, in alleviating salinity stress under deicer-treated soils. A factorial greenhouse experiment was conducted using three fixed factors: (i) presence or absence of Pennisetum alopecuroides, (ii) deicer type (NaCl or CaCl2), and (iii) activated carbon mixing ratio (0, 1, 2, 5, and 10%). Soil pH, EC, and ion concentrations (Na+, Cl−, Ca2+) were measured, along with six plant growth indicators. The results showed that increasing AC concentrations significantly increased pH and reduced EC and ion accumulation, with the 5% AC treatment being optimal in both deicer systems. Plant physiological responses were improved in AC-amended soils, especially under CaCl2 treatment, indicating less ion toxicity and better root zone conditions. The interaction effects between AC, deicer type, and plant presence were statistically significant (p < 0.05), supporting a synergistic remediation mechanism involving both adsorption and biological uptake. Despite the limitations of short-term controlled conditions, this study offers a promising phytomanagement strategy using natural adsorbents and salt-tolerant plants for sustainable remediation of salt-affected soils in road-adjacent and urban environments.
1. Introduction
In cold-climate regions, the application of chloride-based deicing salts such as sodium chloride (NaCl) and calcium chloride (CaCl2) has become standard practice for ensuring winter road safety. However, the widespread and repeated use of these salts has raised serious environmental concerns due to their long-term impacts on soil quality, vegetation, and surrounding ecosystems [1]. Once applied, these salts are often displaced from the pavement and accumulate in adjacent soils, where they increase electrical conductivity (EC), disrupt soil ionic balance, and lead to osmotic stress and nutrient imbalances in plants [2]. In particular, Na+ and Cl− ions are readily absorbed into the soil profile and plant tissues, causing cellular toxicity, foliar necrosis, and impaired root function, especially in salt-sensitive species [3]. While CaCl2 is generally considered less phytotoxic than NaCl, both salts contribute significantly to the salinization of roadside soils. Research has shown that soil salinity can persist for several months beyond winter application, accumulating with each freeze–thaw cycle and precipitation event [4,5]. Despite these well-documented impacts, mitigation strategies for salt-affected soils—particularly under different deicer types—remain insufficiently developed or applied in real-world roadside management. This raises the need for cost-effective, environmentally sound solutions that can restore soil function and support vegetation growth in salt-impacted environments.
Phytoremediation using salt-tolerant plant species has emerged as a sustainable and low-cost strategy to remediate saline soils and restore productivity in degraded lands [6,7]. Among the promising candidates, Pennisetum alopecuroides (Chinese fountain grass) has shown notable physiological stability and growth performance under saline stress [8,9]. This species achieved up to a 50.92% increase in fresh weight and maintained significant shoot and root development under NaCl concentrations of up to 100 mM, indicating strong salt resilience [10]. Furthermore, its fibrous root system enhances rhizospheric aeration and microbial activity, which contributes to its ecological suitability for phytoremediation. It was also ranked among the most effective species for removing soil-borne contaminants in a comparative study involving six plant species [11]. While Miscanthus sinensis, a related species, has also demonstrated salinity tolerance, P. alopecuroides offers the additional advantages of rapid establishment and lower maintenance requirements in roadside and urban settings. Additionally, compared to more extensively studied grasses such as Miscanthus giganteus and Vetiveria zizanioides, P. alopecuroides offers advantages including low maintenance requirements, rapid establishment, and strong adaptability to urban and roadside environments. These attributes make it a promising candidate for integration with activated carbon in phytoremediation strategies. Simultaneously, activated carbon (AC) has gained attention for its high porosity and ion adsorption capacity, particularly in binding Na+ and Cl− and buffering pH in saline soils [12]. AC not only reduces the bioavailability of toxic ions but also improves soil structure, creating more favorable conditions for root growth and nutrient uptake. However, most existing studies have evaluated either plant-based or carbon-based treatments in isolation. The combined application of phytoremediative grasses and activated carbon, particularly under exposure to different deicing salts such as NaCl and CaCl2, remains largely underexplored. There is a lack of factorial studies that quantify how such combinations affect both soil chemistry and plant physiological responses. Addressing this gap is essential for developing integrated salt mitigation strategies that are ecologically effective and practically deployable in roadside or urban landscapes.
The primary objective of this study was to evaluate the effectiveness of activated carbon in improving soil chemical properties and promoting plant growth under deicer-induced salinity stress. Specifically, we investigated the combined application of Pennisetum alopecuroides and activated carbon across two commonly used deicing salts—NaCl and CaCl2—at varying carbon ratios (0, 1, 2, 5, and 10%). The study aimed to identify optimal treatment combinations that mitigate salt accumulation, reduce ion toxicity, and enhance vegetative performance under stress conditions.
We hypothesized that (i) activated carbon would significantly regulate soil pH and moderate EC, Na+, and Cl− concentrations; (ii) P. alopecuroides would exhibit improved growth performance (height, biomass, chlorophyll content) in activated carbon-amended soils; and (iii) the magnitude of these benefits would differ between NaCl and CaCl2 treatments due to their distinct ionic effects on soil and plant systems. This factorial experiment contributes to the development of integrated mitigation strategies for roadside or urban soils impacted by winter salt application and supports the broader use of natural adsorbents and salt-tolerant vegetation in phytomanagement.
2. Materials and Methods
2.1. Experimental Setup
This experiment was conducted from April to September 2024 in a glass greenhouse at the Konkuk University Global Campus, located in Chungju, Chungcheongbuk-do, Republic of Korea. The greenhouse was maintained under standardized conditions with an average daytime temperature of 24.0 ± 4 °C, relative humidity of 62.7 ± 5%, and natural light conditions averaging 15,000 lux. The experimental design was structured as a three-factor factorial arrangement, with fixed effects assigned to each factor: the presence or absence of Pennisetum alopecuroides planting (2 levels), type of deicing agent (sodium chloride [NaCl] and calcium chloride [CaCl2]), and activated carbon (AC) mixing ratio (0%, 1%, 2%, 5%, and 10%). A total of 20 treatment combinations (2 × 2 × 5) were established, and each treatment was replicated five times, resulting in 100 experimental units (n = 5 per treatment).
The soil used was a commercial horticultural potting mix (Yuanjo Mix, Nongkyung Co., Jincheon-gun, Republic of Korea), consisting primarily of cocopeat (55–60%), peat moss (10–15%), perlite (5–10%), vermiculite (5–10%), and zeolite (5–10%). This artificial substrate provided adequate water retention and drainage. Pre-experimental analysis showed the substrate had a pH of 6.3, electrical conductivity (EC) of 1.05 dS/m, and organic matter content of 1.2%.
The activated carbon used in this study was a fine powder derived from hardwood biomass (Yakuri Pure Chemicals, Kyoto, Japan), with a particle size of ≤ 2 mm. According to manufacturer specifications and literature values, its Brunauer–Emmett–Teller (BET) surface area was approximately 720 m2/g, with an average pore volume of 0.45 cm3/g and mesopore-dominated structure. The point of zero charge (pHpzc) was reported as 6.8, indicating that the surface carries a negative charge under most soil pH conditions [13].
The deicers were analytical-grade sodium chloride (NaCl, 99%) and calcium chloride (CaCl2, 98%) obtained from Daejung Chemicals Co., Ltd. (Siheung-si, Republic of Korea). Each pot was treated with a 0.5% solution of NaCl or CaCl2, calculated based on the dry soil weight. Pots used in the experiment were plastic containers with a diameter of 12 cm and a height of 10 cm. Nonwoven fabric was placed at the bottom to prevent soil loss. Each pot was filled with 100 g of the horticultural substrate. Activated carbon was thoroughly mixed into the soil according to each treatment level.
Uniform Pennisetum alopecuroides seedlings at the three-leaf stage, approximately 5 cm in height, were selected and planted individually in each pot. However, in a portion of the control treatments, no plants were transplanted in order to distinguish soil chemical changes attributable solely to deicer and activated carbon treatment, independent of plant influence. All pots were irrigated three times weekly with 100 mL of purified water (≥99.9% H2O, trace chlorine ≤ 0.2 mg/L). One weekly irrigation was replaced by 100 mL of either 0.5% (w/v) NaCl solution (approximately 85 mM) or 0.5% (w/v) CaCl2 solution (approximately 45 mM). The negligible ionic strength of irrigation water ensured observed changes in soil EC and ion concentrations could be solely attributed to the treatments (activated carbon and deicer application).
2.2. Plant and Soil Analysis
At the end of the experimental period, all plants were harvested, and soil and plant samples were collected and analyzed as follows. Soil samples were taken from the upper 0–10 cm layer, and the aboveground portions of the plants were separated and used for analysis. Soil pH was determined using a 1:5 (w/v) soil-to-distilled water suspension with a calibrated pH meter (Orion Star A215, Thermo Scientific, Waltham, MA, USA). Electrical conductivity (EC) was measured using the same extract and an EC meter (Orion Star A212, Thermo Scientific, USA), with results expressed in decisiemens per meter (dS/m). Elemental analysis was conducted using both an inductively coupled plasma optical emission spectrometer (ICP-OES, Optima 8000, PerkinElmer, Springfield, IL, USA) and an ion chromatography system (930 Compact IC Flex, Metrohm, Herisau, Switzerland). All ion concentrations were expressed on a dry weight basis in mg/kg. Plant growth was evaluated using five indicators. Plant height and root length were measured as net growth (final length minus initial length) using a ruler with 1 mm gradations and recorded in centimeters. Leaf number was counted based on the number of fully expanded leaves at the end of the experiment. Chlorophyll content was measured using a portable SPAD meter (SPAD-502 Plus, Konica Minolta, Osaka, Japan). Measurements were taken from the adaxial surface of the central part of mature leaves, avoiding the midrib, between 11:00 a.m. and 1:00 p.m. in the greenhouse. A total of 120 SPAD readings per treatment were collected and averaged. Fresh weight was measured immediately after harvest using a precision balance (FX-3000i, A&D, Kyoto, Japan), and dry weight was recorded after drying the plant material at 70 °C for 72 h using the same device.
2.3. Statistical Analysis
All collected data were first assessed for normality using the Shapiro–Wilk test and for homogeneity of variance with Levene’s test. Data satisfied both assumptions (p > 0.05), allowing parametric analyses. A two-way analysis of variance (ANOVA) was conducted to evaluate the main and interaction effects of two fixed factors: activated carbon (AC) mixing ratio (0%, 1%, 2%, 5%, and 10%) and the presence or absence of Pennisetum alopecuroides planting, separately for each type of deicer (NaCl and CaCl2). Additionally, separate one-way ANOVAs were conducted within each deicer group to further assess the effects of AC ratios on soil chemical properties and plant growth parameters. When ANOVA results were significant (p < 0.05), Tukey’s honestly significant difference (HSD) test was performed for post hoc comparisons among treatment means. Furthermore, a Multivariate Analysis of Variance (MANOVA) was applied to collectively assess plant growth indicators, including plant height, root length, leaf number, chlorophyll content, fresh weight, and dry weight, using Wilks’ Lambda as the test statistic. Principal component analysis (PCA) was conducted on standardized soil chemical data to comprehensively interpret multivariate treatment effects. All statistical analyses were performed using Python 3.11 (Python Software Foundation, Beaverton, OR, USA), employing the libraries pandas, scipy, statsmodels, scikit-learn, seaborn, and matplotlib. Statistical significance was established at p < 0.05.
3. Results
3.1. Soil Response Under NaCl Treatment
Application of activated carbon (AC) under NaCl deicer conditions led to significant improvements in soil chemical properties. As the AC mixing ratio increased from 0% to 10%, soil pH rose steadily while EC and Na+/Cl− concentrations decreased (Table 1). These patterns were particularly enhanced in planted treatments, where the combined effects of AC and Pennisetum alopecuroides yielded the most substantial remediation.

Table 1.
Summary of soil chemical properties (mean ± SE) under NaCl treatment with combinations of activated carbon ratio and Pennisetum alpecuroides planting.
Two-way ANOVA confirmed that both AC and planting had significant main effects on all measured parameters (pH, EC, Na+, and Cl−), and the interaction between AC and planting was also statistically significant for Na+ and Cl− concentrations (Table 2). These results suggest that physical adsorption via AC and biological uptake by plants act synergistically to mitigate salt-induced stress.

Table 2.
Factorial ANOVA results for the effects of activated carbon treatment and planting on soil ion concentrations (Na+ and Cl−).
3.2. Soil Response Under CaCl2 Treatment
Under CaCl2 stress, soil responses followed a similar pattern to those under NaCl, with AC application leading to increased pH and decreased EC, Ca2+, and Cl− concentrations (Table 3). These changes were more substantial in the 10% AC and planted conditions, again confirming a synergistic effect.

Table 3.
Summary of soil chemical properties (mean ± SE) under CaCl2 treatment with combinations of activated carbon ratio and Pennisetum alpecuroides planting.
Two-way ANOVA revealed significant main effects of AC and planting on all parameters, as well as strong interaction effects for Ca2+ and Cl− (Table 4). While planting alone contributed to ion reduction, the chemical properties of activated carbon appear to play a dominant role in ion regulation under CaCl2 exposure, especially for Ca2+ removal.

Table 4.
Factorial ANOVA results for the effects of activated carbon treatment and planting on soil ion concentrations (Ca2+ and Cl−).
3.3. Principal Component Analysis of Soil Chemical Responses
To comprehensively evaluate the overall patterns of soil chemical responses across all treatment combinations, principal component analysis (PCA) was performed (Figure 1). The analysis included four standardized variables: pH, electrical conductivity (EC), chloride (Cl−), and ion concentration (Na+ for NaCl, or Ca2+ for CaCl2). The dataset was integrated across three treatment factors: deicer type (NaCl or CaCl2), activated carbon (AC) mixing ratio (0, 1, 2, 5, and 10%), and Pennisetum alopecuroides with or without planting. The first principal component (PC1) explained 78.27% of the total variance and was primarily associated with EC, Cl−, and ion concentration, reflecting the degree of salt stress and ionic accumulation. The second principal component (PC2) accounted for 19.53% of the variance and was more strongly influenced by pH, representing the soil’s acid–base buffering response. Together, PC1 and PC2 explained 97.80% of the total variance, enabling highly effective dimensionality reduction and interpretation of multivariate soil responses. The PCA scatter plot clearly visualized the separation between treatment combinations based on deicer type and planting status. NaCl-treated groups clustered in the lower-left region of the PC1–PC2 space, indicating high salinity and ion accumulation, while CaCl2-treated groups appeared in the upper-right quadrant, corresponding to improved pH and reduced salt stress conditions. Notably, the 10% AC + planted treatment was positioned farthest from the origin in both PC1 and PC2 dimensions, demonstrating the strongest deviation from stress-affected controls and the most pronounced recovery effect. In addition, ellipses representing 95% confidence intervals for each treatment group were overlaid to visualize the dispersion within groups and the separation between groups. The spacing and orientation of group centroids and ellipses suggest that the synergistic effect of combining physical treatment (activated carbon) with biological treatment (Pennisetum alopecuroides) played a key role in enhancing soil remediation outcomes. In conclusion, PCA proved to be a powerful tool for summarizing complex soil chemical responses and clearly illustrated that combined treatments were more effective than individual approaches, regardless of deicer type.
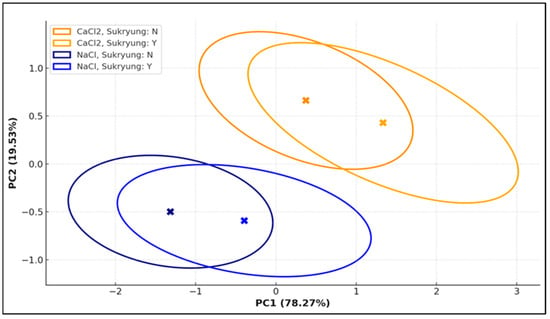
Figure 1.
PCA Ellipse Plot of Soil Chemical Properties across Deicer Treatments and Planting; Colored ellipses represent the 95% confidence regions of each treatment group in the PC1–PC2 space. Blue indicates NaCl-treated groups and orange indicates CaCl2-treated groups. Circle markers represent non-planting treatments, and squares represent planted treatments. X-shaped markers show the group centroids, and greater separation between ellipses suggests stronger treatment differentiation.
3.4. Growth Responses of Pennisetum alopecuroides to Activated Carbon Ratios Under NaCl Deicer Treatment
This section investigates the effects of activated carbon (AC) mixing ratios (0, 1, 2, 5, amd 10%) on the growth of Pennisetum alopecuroides under NaCl stress. Six growth indicators were analyzed, of which plant height, root length, and leaf number represent net growth increments (Δ) during the experiment, while chlorophyll content (SPAD), fresh weight, and dry weight reflect final measured values. One-way ANOVA indicated statistically significant differences (p < 0.05) for all variables. Tukey’s HSD test confirmed that the 5% AC group significantly outperformed the 2% and 10% treatments in most indicators (Figure 2). The 5% AC treatment achieved plant height increase of 32.2 cm, root length of 7.58 cm, 2.4 additional leaves, chlorophyll value of 49.8, fresh weight of 7.46 g, and dry weight of 3.04 g. Notably, a slight decline was observed at 10% AC in some traits (e.g., leaf number, dry weight), indicating a possible inhibitory effect of overapplication. These findings support that moderate AC application (5%) optimally enhances plant growth under salt-stress conditions.
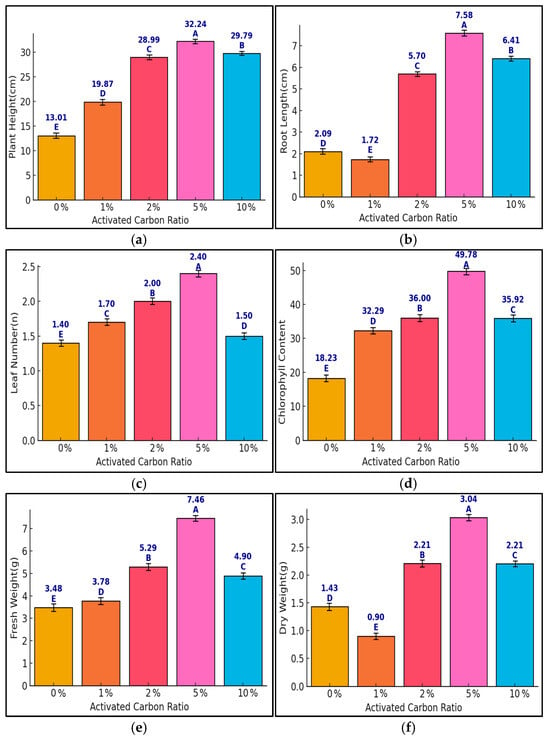
Figure 2.
Growth responses of Pennisetum alopecuroides to different activated carbon (0, 1, 2, 5, and 10%) ratios under NaCl stress across six growth parameters ((a) plant height, (b) root length, (c) leaf number, (d) chlorophyll content, (e) fresh weight, (f) dry weight). Bars show means ± standard error (SE), and capital letters (A–E) represent statistically significant differences based on Tukey’s HSD test (p < 0.05). Sample size: n = 5. Plant height, root length, and leaf number are presented as net growth increments (Δ) from the baseline, while other indicators represent final values.
3.5. Growth Responses of Pennisetum alopecuroides to Activated Carbon Ratios Under CaCl2 Deicer Treatment
This section analyzed the effects of activated carbon (AC) ratios on Pennisetum alopecuroides growth under CaCl2 deicer stress. Six growth parameters were evaluated, with plant height, root length, and leaf number measured as net growth increments (Δ), while chlorophyll content (SPAD), fresh weight, and dry weight were final values. One-way ANOVA showed statistically significant differences (p < 0.05) for all traits. The 5% AC treatment group had the highest values: plant height = 28.04 cm, root length = 13.18 cm, leaf number = 2.70, SPAD = 49.98, fresh weight = 18.34 g, dry weight = 5.24 g. Tukey’s HSD post hoc test revealed that the 10% group showed significantly lower performance than the 5% group in several traits, suggesting potential growth inhibition at high AC concentrations. The statistical groupings (A–E) in Figure 3 clearly illustrate these treatment differences, particularly for growth increment indicators. These findings confirm that 5% AC is optimal for growth improvement under CaCl2 conditions, and higher concentrations may require caution due to possible inhibitory effects.
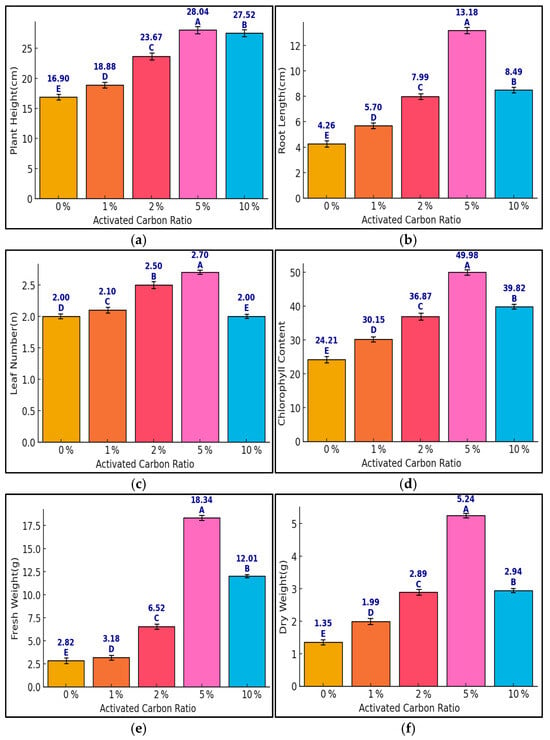
Figure 3.
Growth responses of Pennisetum alopecuroides to different activated carbon (0, 1, 2, 5, and 10%) ra-tios under CaCl2 stress across six growth parameters ((a) plant height, (b) root length, (c) leaf number, (d) chlorophyll content, (e) fresh weight, (f) dry weight). Bars show means ± standard error (SE), and capital letters (A–E) represent statistically significant differences based on Tukey’s HSD test (p < 0.05). Sample size: n = 5. Plant height, root length, and leaf number are presented as net growth increments (Δ) from the baseline, while other indicators represent final values.
4. Discussion
Our findings highlight a synergistic mechanism between activated carbon and salt-tolerant vegetation in remediating salt-affected soils contaminated by common deicing salts.
The addition of activated carbon (AC) significantly improved soil chemical properties under both NaCl and CaCl2 stress conditions. As hypothesized, increasing AC concentrations led to elevated pH and decreased electrical conductivity (EC), suggesting enhanced buffering capacity and reduced ionic strength. These findings are supported by a range of previous studies demonstrating the ion-binding ability of AC in saline or salt-contaminated environments [14,15,16,17,18]. Specifically, EC reduction was closely linked with declining Na+ and Cl− concentrations, consistent with the results of ion-selective adsorption reported in earlier filtration and field trials. Moreover, changes in pH were more prominent in CaCl2 treatments, likely due to the precipitation or exchange behavior of calcium ions [19,20,21]. The beneficial effects of AC in salt-affected soils may also stem from its large specific surface area, pore size distribution, and surface functional groups capable of cation exchange [22].
In salt-affected soils, the removal of Na+ and Ca2+ ions by activated carbon (AC) occurs via physicochemical mechanisms, primarily cation exchange and surface adsorption. The surface of AC is rich in functional groups such as carboxyl (–COOH), hydroxyl (–OH), and phenolic groups. When the pH of the soil solution exceeds the point of zero charge (pHpzc) of the activated carbon, these functional groups acquire negative charges, thereby promoting the binding of cations. Na+ ions are effectively removed through electrostatic exchange [23], while Ca2+ ions form stable bridging complexes with –COOH groups, resulting in stronger adsorption [24]. Furthermore, AC typically exhibits a specific surface area exceeding 700 m2/g [24] and a mesoporous structure, which enhances ion contact and fixation. These properties increase the desorption capacity of salts, stabilize ionic balance within the soil, and ultimately contribute to vegetation recovery and the improvement of soil structure under salt stress conditions.
The combined application of Pennisetum alopecuroides and activated carbon (AC) yielded significant improvements in plant growth under saline stress. In particular, shoot and root length, leaf number, chlorophyll content, and biomass were all enhanced in planted treatments compared to unplanted controls. These improvements were most pronounced at 5% AC, indicating an optimal range for synergy between phytoremediation and chemical adsorption mechanisms. Previous research has demonstrated that Pennisetum alopecuroides possesses moderate salt tolerance and maintains biomass productivity under NaCl concentrations as high as 100 mM [10]. Other studies showed that AC can reduce oxidative stress in plants by lowering salt-induced ion toxicity and enhancing nutrient uptake efficiency [12,23]. The AC-treated soils also maintained better root aeration and enzymatic activity, promoting root health [24,25]. Furthermore, the rhizosphere interaction between Pennisetum alopecuroides and AC likely enhanced microbial and enzymatic functions, aiding stress mitigation [11,26]. In addition, secondary metabolite accumulation in Pennisetum species under saline conditions may further protect photosynthetic machinery [27]. Altogether, these findings confirm that activated carbon enhances phytoremediation efficiency by providing a stable chemical environment, while Pennisetum alopecuroides supports salt mitigation through biological uptake and adaptive physiology.
Comparative studies have shown that grasses such as Miscanthus sinensis, Vetiveria zizanioides, and Phragmites communis possess varying levels of salt tolerance and phytoremediation capacity. For instance, Miscanthus sinensis displayed genotype-dependent variations in biomass and cell wall composition under 100–200 mM NaCl, with certain lines maintaining structural integrity under severe salinity [28]. Vetiver has been shown to outperform several hyperaccumulators in heavy metal remediation, owing to its high biomass and physiological plasticity [29]. Similarly, Phragmites communis exhibited notable ultrastructural adaptations in chloroplast and mitochondrial morphology when exposed to saline-alkaline stress, enabling sustained photosynthetic function [30]. These findings collectively highlight the diversity in stress response strategies among salt-tolerant grasses. Although Pennisetum alopecuroides has been comparatively underexplored, its dual advantages of physiological resilience and ecological suitability position it as a promising candidate for AC-assisted remediation under chloride-based salinity.
Although direct measurements of water retention were not conducted in this study, several studies have reported that activated carbon, due to its high porosity and sponge-like internal structure, can significantly enhance soil moisture retention [31]. Increased moisture availability in the rhizosphere can promote nutrient uptake, enzymatic activity, and photosynthesis—contributing to the observed improvements in biomass and chlorophyll content in AC-treated soils. Future research should quantify this effect under varied soil textures and irrigation regimes. The observed synergy presents a promising eco-compatible strategy for the rehabilitation of salt-affected soils.
The differential impact of NaCl and CaCl2 on soil and plant response highlights the ion-specific behavior associated with chloride-based deicers. While both salts increased soil salinity, the nature of their dissociated ions influenced outcomes distinctly. NaCl treatment resulted in a greater increase in Na+ concentration, EC, and pH depression compared to CaCl2, which tended to raise Ca2+ availability and moderately buffer pH due to cation exchange and carbonate reactions [19,32,33]. The pH-lowering effect of NaCl is attributed to Na-induced soil acidification through exchangeable sodium accumulation and poor flocculation, leading to structural breakdown [34]. In contrast, Ca2+ from CaCl2 improves soil aggregation and reduces SAR (sodium adsorption ratio), which mitigates the loss of soil structure and buffering capacity [35,36]. Plant responses also varied between the two deicers. Under equivalent EC levels, NaCl treatments caused more severe reductions in chlorophyll content and biomass than CaCl2, likely due to Na+ toxicity and disruption of K+ uptake [37,38]. CaCl2 treatments, by increasing Ca2+ availability, supported membrane stabilization and photosynthesis under stress [39,40]. Taken together, these findings demonstrate that deicer-induced stress is not solely a function of salinity level but also of the ionic species involved. This underscores the importance of ion-specific strategies for soil remediation and vegetation selection in salt-impacted environments.
The findings of this study offer valuable insights into practical applications for managing salt-affected soils in temperate and roadside ecosystems. The combined use of activated carbon (AC) and Pennisetum alopecuroides demonstrated effective mitigation of salinity-induced stress, confirming its potential as a low-cost, environmentally friendly solution for soil remediation [41,42,43]. However, some limitations must be acknowledged. Most notably, this was a short-term, greenhouse-based study, which does not capture the seasonal variability or biological complexity of field conditions [44,45]. While the 5% AC rate was optimal under controlled conditions, its performance in large-scale applications may vary due to material cost, uniformity of soil mixing, and water leaching dynamics [46]. Furthermore, the phytoremediation efficiency of Pennisetum alopecuroides could differ under prolonged stress, especially in combination with drought or low organic matter conditions [47,48,49]. To ensure successful field implementation, future studies should focus on long-term monitoring, microbial interactions, and cost–benefit analysis under variable soil and climate conditions [50]. Establishing site-specific protocols and refining AC dosage in coordination with salt-tolerant plant species will enhance both ecological resilience and scalability of this approach.
5. Conclusions
This study confirmed that the combined application of activated carbon (AC) and Pennisetum alopecuroides offers a synergistic approach to mitigate salt-induced stress in soils affected by deicing agents. The dual mechanism—chemical immobilization of Na+ and Ca2+ ions by AC, and biological uptake and resilience provided by salt-tolerant grass—significantly improved both soil quality and plant growth, particularly at 5% AC treatment. This combination not only buffered soil pH and reduced EC, but also promoted biomass accumulation, chlorophyll synthesis, and root health.
From an application perspective, this strategy provides a cost-effective and eco-compatible solution for managing salinity in roadside and urban soils. The findings are especially relevant for temperate climates with seasonal salt applications. The use of moderate AC loading (5%) presents a practical balance between performance and material cost.
Future studies should explore the long-term field performance of AC–plant systems under varying climatic and soil conditions. Additional focus should be placed on measuring water retention, microbial interactions, and conducting life-cycle or cost–benefit analyses to optimize deployment strategies. Integrating this method with other phytoremediation species could further enhance its robustness and scalability in diverse environments.
Author Contributions
Conceptualization, J.-H.P. and J.-H.J.; methodology, J.-H.P. and J.-H.J.; software, J.-H.P.; validation, J.-H.P., J.-H.J. and Y.-H.Y.; formal analysis, J.-H.P. and M.-H.L.; investigation, J.-H.P. and J.-H.J.; resources, J.-H.P. and J.-H.J.; data curation, J.-H.P.; writing—original draft preparation, J.-H.P. and J.-H.J.; writing—review and editing, H.-I.L., Y.-H.Y., J.-H.P. and J.-H.J.; visualization, J.-H.P.; supervision, J.-H.J. and Y.-H.Y.; project administration, J.-H.J.; funding acquisition, J.-H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by Konkuk University in 2025.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Devecchi, M.; Remotti, D. Effect of Salts on Ornamental Ground Covers for Green Urban Areas. Acta Hortic. 2004, 643, 143–150. [Google Scholar] [CrossRef]
- Devitt, D.A.; Wright, L.; Landau, F.; Apodaca, L. Deicing Salts; Assessing Distribution, Ion Accumulation in Plants and the Response of Plants to Different Loading Rates and Salt Mixtures. Environ. Nat. Resour. Res. 2014, 4, 73–88. [Google Scholar] [CrossRef]
- Wrochna, M.; Malecka-Przybysz, M.; Gawrońska, H. Effect of Road De-icing Salts with Anti-Corrosion Agents on Selected Plant Species. Acta Sci. Pol. Hortorum Cultus 2010, 9, 183–195. Available online: https://scispace.com/papers/effect-of-road-de-icing-salts-with-anti-corrosion-agents-on-3rteasqsky (accessed on 18 July 2025).[Green Version]
- Barker, A.; Cox, D.A.; Ebdon, J.S.; Bryson, G.M.; Hamlin, R.L. Deicing Salts, Salt-Tolerant Vegetation and Calcium Sulfate. Massachusetts Highway Department Research Report 2003. Available online: https://scispace.com/papers/deicing-salts-salt-tolerant-vegetation-and-calcium-sulfate-3s7thtdwcs (accessed on 18 July 2025).
- Yamamoto, Y.; Sone, S.; Kimura, K.; Namikawa, Y. Research on Environmental Impact of Spread De-icing Salts. Unpublished Technical Report, Japan. 2010. Available online: https://scispace.com/papers/research-on-environmental-impact-of-spread-de-icing-salts-325vqg9xg9 (accessed on 18 July 2025).
- Nainwal, R.C.; Chaurasiya, P.C.; Kumar, A.; Singh, M.; Singh, D.P.; Tewari, S.K. Phytoremediation: A Sustainable Approach to Combat Soil Salinity. Adv. Environ. Eng. Res. 2024, 4, 1–14. [Google Scholar] [CrossRef]
- Romantschuk, L.; Matviichuk, N.; Mozharivska, I.; Matviichuk, B.; Ustymenko, V.; Tryboi, O. Phytoremediation of Soils by Cultivation of Miscanthus × Giganteus L. and Phalaris arundinacea L. Ecol. Eng. Environ. Prot. 2024, 62, 45–58. [Google Scholar] [CrossRef]
- Praveen, A.; Pandey, V.C. Miscanthus-a Perennial Energy Grass in Phytoremediation. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 67–85. [Google Scholar] [CrossRef]
- Liang, K.; Peng, X.; Liu, F. Physiological Response of Miscanthus Genotypes to Salinity stress under elevated CO2. GCB Bioenergy 2022, 14, 737–749. [Google Scholar] [CrossRef]
- Mane, A.V.; Karadge, B.A.; Samant, J.S. Salt Stress Induced Alteration in Growth Characteristics of a Grass Pennisetum alopecuroides. J. Environ. Biol. 2011, 32, 753–758. Available online: https://pubmed.ncbi.nlm.nih.gov/22471212/ (accessed on 18 July 2025). [PubMed]
- He, B.Y. Research on the Physiological Responses of Six Plants Including Pennisetum alopecuroides to BDE-209 in Soil and Their Phytoremediation Effect. J. Agro-Environ. Sci. 2012, 31, 1745–1751. Available online: https://scispace.com/papers/research-on-the-physiological-responses-of-six-plants-1p0to33jh8 (accessed on 18 July 2025).
- Mamirova, A.; Pidlisnyuk, V.; Amirbekov, A.; Ševců, A.; Nurzhanova, A. Phytoremediation potential of Miscanthus Sinensis And. in Organochlorine Pesticides-Contaminated Soil Amended with Tween 20 and Activated Carbon. Environ. Sci. Pollut. Res. 2020, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hidayu, A.R.; Mohamad, N.F.; Matali, S.; Sharifah, A.S.A.K. Characterization of Activated Carbon Prepared from Oil Palm Empty Fruit Bunch Using BET and FT-IR Techniques. Procedia Eng. 2013, 68, 379–385. [Google Scholar] [CrossRef]
- Lima, S.S.A.; Lima Filho, H.J.B.; de Paiva, S.C.; Messias, A.S. Saline Waters Treatment Using Activated Carbon Filled Filter. Curr. J. Appl. Sci. Technol. 2019, 37, 1–7. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Chen, H.; Liu, F.; Jin, S.; Yin, X.; Zheng, Y.; Liu, B. Effects of Calcium Ion and pH on the Adsorption/Regeneration Process by Activated Carbon Permeable Reactive Barriers. RSC Adv. 2018, 8, 16834–16841. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Koupai, J.A.; Heidarpour, M. The Effect of Modified Zeolite, Activated Carbon and Peat with Cationic Surfactant and Sodium Hydroxide on Removing Anions from Irrigation Saline Waters. Desalin. Water Treat. 2017, 92, 196–204. [Google Scholar] [CrossRef]
- Hassan, S.; Yasin, T. Role of Tailored Surface of Activated Carbon for Adsorption of Ionic Liquids for Environmental Remediation. Int. J. Environ. Sci. Technol. 2015, 12, 2711–2722. [Google Scholar] [CrossRef]
- Mohamed, E.F.; Awad, G.; Andriantsiferana, C.; Delmas, H. Effect of Salinity and PH on the Industrial Effluent Treatment by Activated Carbon: Modeling of the Kinetic Adsorption and Equilibrium Isotherms. Environ. Manag. Sustain. Dev. 2019, 8, 77–94. [Google Scholar] [CrossRef]
- Lou, Y. Effects of Soil Salinity Accumulating and Ion Constitution on pH in the Soil of Protected Field. Agric. Res. Arid Areas 2009, 27, 16–20. Available online: https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=201002220839550068 (accessed on 18 July 2025).
- Bessaim, M.M.; Missoum, H.; Bendani, K.; Laredj, N.; Bekkouche, M.S. Sodic-Saline Soil Remediation by Electrochemical Treatment under Uncontrolled pH Conditions. Arab. J. Geosci. 2020, 13, 199. [Google Scholar] [CrossRef]
- Setia, R.; Rengasamy, P.; Marschner, P. Effect of Exchangeable Cation Concentration on Sorption and Desorption of Dissolved Organic Carbon in Saline Soils. Sci. Total Environ. 2013, 465, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Zhao, Q.; Wan, L.; Li, Y.; Zhou, Q. Carbon Fiber Enhanced Bioelectricity Generation in Soil Microbial Fuel Cells. Biosens. Bioelectron. 2016, 85, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, H.; Song, J.-B.; Chen, W.; Shi, L. Biochar/Vermicompost Promotes Hybrid Pennisetum Plant Growth and Soil Enzyme Activity in Saline Soils. Plant Physiol. Biochem. 2022, 183, 96–110. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Xu, Y.; He, G.; Zhao, X.; Wang, C.; Li, S.; Zhou, G.; Hu, R. Combined Application of Acidic Biochar and Fertilizer Synergistically Enhances Miscanthus Productivity in Coastal Saline-Alkaline Soil. Sci. Total Environ. 2023, 893, 164811. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Chen, X.; Brouwers, H.J.H. Application of Miscanthus to Enhance Plant Growth Adaptability of Bio-Based Vegetal Concrete. Constr. Build. Mater. 2024, 425, 136096. [Google Scholar] [CrossRef]
- Chen, M.; Wang, D.; Chen, T.S.; Kang, A.P.; Liu, Y.; Wang, B. Effects of Phosphorus Nutrition on Growth, Photosynthesis, and Ion Accumulation of Energy Plant Hybrid Pennisetum Seedlings under Salinity. Adv. Mater. Res. 2013, 726–731, 4362–4370. [Google Scholar] [CrossRef]
- Mane, A.V.; Karadge, B.A.; Samant, J.S. Salt Stress Induced Alteration in Photosynthetic Pigments and Polyphenols of Pennisetum Alopecuroides (L.). J. Ecophysiol. Occup. Health 2010, 10, 177–182. [Google Scholar]
- van der Cruijsen, K.; Vanparys, V.; Suárez-González, M.; Verbruggen, N.; De Coninck, B. Salt Stress Alters the Cell Wall Components and Structure in Miscanthus sinensis Stems. Physiol. Plant. 2024, 172, e14430. [Google Scholar] [CrossRef] [PubMed]
- Danh, L.T.; Truong, P.; Mammucari, R.; Tran, T.; Foster, N. Vetiver Grass, Vetiveria zizanioides: A Choice Plant for Phytoremediation of Heavy Metals and Organic Wastes. Int. J. Phytoremediat. 2009, 11, 664–691. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Liu, M.; Cao, B.; Tan, H.; Wang, J.; Li, X. Responses of three different ecotypes of reed (Phragmites communis Trin.) to their natural habitats: Leaf surface micro-morphology, anatomy, chloroplast ultrastructure and physio-chemical characteristics. Plant Physiol. Biochem. 2012, 51, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Doszhanov, Y.; Sabitov, A.; Saurykova, K.; Mansurov, Z.A.; Kurmanbaeva, M.S.; Doszhanov, O.M.; Atamanov, M. Production and Optimization of Activated Carbon from Plant Waste with High Specific Surface Area for Moisture-Saving Applications in Agriculture. Goren. I Plazmohimiâ 2024, 22, 159–167. [Google Scholar] [CrossRef]
- Eom, J.-S.; Pros, K.; Kim, K.-S.; Kim, Y.-G.; Lee, J.-S.; Na, H.-S.; Cho, H.-J.; Shim, J.; Han, G.-H. Differential Reponses of Electrical Conductivity and Chloride Concentration in Soil and Plant Extracts to Chemical and Organic Fertilizations in Salt-Affected Greenhouses. Korean J. Soil. Sci. Fertil. 2022, 55, 239–245. [Google Scholar] [CrossRef]
- Hammde, L.; Sulieman, A. Effect of NaCl Saline Irrigation Water on Soil Salinity. Nat. Appl. Sci. Ser. 2020, 2, 31–52. Available online: https://ejournal.mutah.edu.jo/index.php/NASS/article/view/1827 (accessed on 18 July 2025).
- Fan, Q.F.; Zhang, Y.L.; Chen, Z. Effects of Protected Field Vegetable Cultivation on Soil Salinity Accumulating and pH. J. Soil Water Conserv. 2009, 1, 103–106. Available online: https://scispace.com/papers/effects-of-protected-field-vegetable-cultivation-on-soil-3ac718jhrc (accessed on 18 July 2025).
- El-Agrodi, M.W.M.; Ahmed, G.L.; El-Hamad, M.A. Effect of Different Soil Salinity Levels on Some Soil Properties and Wheat Plant. J. Soil. Sci. Agric. Eng. 2012, 3, 175–188. [Google Scholar] [CrossRef]
- Papadopoulos, I.; Rendig, V.V. Tomato Plant Response to Soil Salinity1. Agron. J. 1983, 75, 696–700. [Google Scholar] [CrossRef]
- Trajkova, F.; Papadantonakis, N.; Savvas, D. Comparative Effects of NaCl and CaCl2 Salinity on Cucumber Grown in a Closed Hydroponic System. HortScience 2006, 41, 437–441. [Google Scholar] [CrossRef]
- Ayad, J.Y. Comparative Effects of CaCl2 and NaCl Salinity on Growth and Ion Partitioning of Atriplex halimus L. Dirasat Shari’a Law Sci. 2010, 37, 82–90. Available online: https://archives.ju.edu.jo/index.php/law/article/view/2112 (accessed on 18 July 2025).
- Bernstein, N. Plants and Salt: Plant Response and Adaptations to Salinity. In Plant Stress Physiology; Academic Press: Cambridge, MA, USA, 2019; pp. 101–112. [Google Scholar] [CrossRef]
- Pätsch, R.; Midolo, G.; Dítě, Z.; Dítě, D.; Wagner, V.; Pavonič, M.; Danihelka, J.; Preislerová, Z.; Ćuk, M.; Stroh, H.G.; et al. Beyond Salinity: Plants Show Divergent Responses to Soil Ion Composition. Glob. Ecol. Biogeogr. 2024, 33, e13821. [Google Scholar] [CrossRef]
- Clay, S.A. Benefits of Phytoremediation When Repairing Salt-Affected Soils. In Salinity and Sodicity: A Global Challenge to Food Security, Environmental Quality and Soil Resilience; ASA, CSSA, SSSA Book Series; American Society of Agronomy, Inc.: Madison, WI, USA, 2024; pp. 93–97. [Google Scholar] [CrossRef]
- Hilber, I.; Bucheli, T.D. Activated Carbon Amendment to Remediate Contaminated Sediments and Soils: A Review. Glob. Nest J. 2010, 12, 305–317. [Google Scholar] [CrossRef]
- Amini, S.; Ghadiri, H.; Chen, C.; Marschner, P. Salt-Affected Soils, Reclamation, Carbon Dynamics, and Biochar: A Review. J. Soils Sediments 2016, 16, 939–953. [Google Scholar] [CrossRef]
- Jesus, J.M.; Danko, A.S.; Fiúza, A.; Borges, M.T. Phytoremediation of Salt-Affected Soils: A Review of Processes, Applicability, and the Impact of Climate Change. Environ. Sci. Pollut. Res. 2015, 22, 6511–6525. [Google Scholar] [CrossRef] [PubMed]
- Imadi, S.R.; Shah, S.W.; Kazi, A.G.; Azooz, M.M.; Ahmad, P. Phytoremediation of Saline Soils for Sustainable Agricultural Productivity. In Plant Metal Interaction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 455–468. [Google Scholar] [CrossRef]
- Clay, D.E.; Pandit, S.; Bhattarai, D. Case Studies on Salt-Affected Soil Remediation. In Salinity and Sodicity: A Global Challenge to Food Security, Environmental Quality and Soil Resilience; ASA, CSSA, SSSA Book Series; American Society of Agronomy, Inc.: Madison, WI, USA, 2024; pp. 129–142. [Google Scholar] [CrossRef]
- Freire, M.B.G.S.; Freire, F.J.; Pessoa, L.G.M.; Souza, E.R.; Gheyi, H.R. Salt-Affected Soils in the Brazilian Semiarid and Phytoremediation as a Reclamation Alternative. In Soil and Water Management; Springer: Cham, Switzerland, 2021; pp. 119–139. [Google Scholar] [CrossRef]
- Datta, A.K.; Setia, R.; Barman, A.; Guo, Y.; Basak, N. Carbon Dynamics in Salt-Affected Soils. In Salinity and Agriculture; Springer: Singapore, 2019; pp. 369–389. [Google Scholar] [CrossRef]
- Basak, B.B.; Smitha, G.R.; Chinchmalatpure, A.R.; Patel, P.K.; Prem, K.B. Aromatic Plants as a Tool for Phytoremediation of Salt Affected Soils. In Phytoremediation Technology; CRC Press: Boca Raton, FL, USA, 2021; pp. 138–153. [Google Scholar] [CrossRef]
- Srivastava, N. Reclamation of Saline and Sodic Soil Through Phytoremediation. In Soil Pollution and Phytoremediation; Springer: Singapore, 2020; pp. 279–306. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).