Abstract
Heterogeneous ice nucleation is a key process for ice cloud formation, snowfall, and freezing of water bodies. Ice nucleating particle (INP) cloud feedbacks are one of the largest sources of uncertainties in Earth’s Energy Budget. Although INPs are essential in the development of mixed-phased and glaciated clouds, their composition, sources, and cloud feedbacks remain poorly constrained. Previous studies have shown mixed results on the potential of light-absorbing particles (LAP), such as black carbon (BC) and high latitude dust (HLD), serving as INPs. However, many of these studies use laboratory or model-generated particles that may not represent the complex morphology and behaviors of ambient light-absorbing particles sufficiently. Here, we use in situ surface snow samples, collected during Spring 2018 in Svínafellsjökull, Iceland. The samples were analyzed by an immersion freezing mechanism for their ice nucleation activity (INA). Portions of the filtered samples were concentrated by lyophilization to observe the potential enhancement of INA. We investigated environmental samples of deposited aerosols to better understand the role activity of HLD and BC in ice nucleating activity in mixed-phase clouds in Iceland. We found concentrations of 16 ± 27 ng g−1 and 33 ± 66 × 106 ng g−1 for BC and HLD, respectively. However, we found that isolated methanol-soluble organic aerosols have a more prominent role than BC and HLD in Iceland. We conclude that BC and HLD are insignificant INP but that they can inhibit INA from other INP.
1. Introduction
Clouds play an integral role in Earth’s climate system by regulating the radiation budget and water exchange between the surface and the atmosphere. The representation of clouds in global climate models directly influences the model estimates of energy budget. In fact, the spread of climate sensitivity among most models is rooted in the differences between models regarding cloud feedbacks on the energy budget [,,,,]. Therefore, cloud feedbacks, especially those regarding aerosol interactions, remain the largest uncertainty in modeling the Earth’s changing energy budget []. Aerosols c serve as ice nucleating particles, which seed precipitation, meanwhile altering cloud microphysical properties and nucleation []. In this study, we aim to examine the role of light-absorbing particles (LAP) on the ice nucleation activity in Iceland.
Tropospheric clouds can be categorized as warm clouds, mixed-phase clouds, and ice clouds. While warm clouds are composed of liquid water droplets, mixed-phase, and ice clouds consist partly or entirely of ice crystals. The presence of liquid water droplets or solid ice crystals in clouds determines their behavior and properties []. For instance, ice clouds reflect a higher amount of shortwave solar radiation back into space in comparison with liquid clouds []. In high latitudes, clouds’ microphysical properties contribute to the Arctic Amplification by enhancing downward long-wave radiation and warming the surface [,]. The Arctic is experiencing the fastest warming through climate change due to amplification and climate feedbacks—such as the sea ice albedo feedback, water vapor, and cloud feedback, aerosol-cloud feedback, and lapse-rate feedback [,,]. Despite the critical role of high-latitude climates in global circulation, global climate models often fail to accurately represent these regions, resulting in poor simulations of contemporary Arctic climate.
It is estimated that around 50% of clouds in the Arctic are in the mixed-phase state [,,,,,,]. In mixed-phase clouds, containing both ice crystals and supercooled water droplets, ice crystals will grow rapidly at the expense of water droplets feeding the crystals water vapor. Once the ice crystals reach a critical size, precipitation may occur. This process is called the Wegener–Bergeron–Findeisen (WBF) process and it is known to dominate the precipitation globally over continental regions and midlatitude oceans [,]. The higher fall speed of large ice crystals can initiate precipitation faster than liquid droplets. The formation of ice crystals can happen through two mechanisms called homogeneous and heterogeneous ice nucleation []. At temperatures below −35 °C, small single droplets would instantaneously freeze in a process called homogeneous freezing []. This freezing mechanism happens when water molecules are arranged in an ice-like pattern and are over a critical cluster size [,]. However, freezing can be triggered at higher sub-zero temperatures with the introduction of a so-called ice nucleating particle (INP) acting as a catalyst, in a process called heterogeneous freezing which is responsible for primary ice formation in mixed-phase clouds at temperatures above −35 °C [,].
Ice-nucleating particles (INPs) are a subset of atmospheric aerosol particles capable of catalyzing the formation of ice at temperatures above the homogeneous freezing limit of pure water (around −38 °C) [,,]. Ice nucleation activity (INA) refers to the measurable efficiency of these particles to induce freezing under given environmental conditions, expressed in terms such as onset temperature or active site density. INPs only make up a tiny fraction of aerosol particles’ mass, yet have key physicochemical properties that reduce the critical energy barrier for spontaneous nucleation []. Therefore, understanding their role in high-latitude clouds is important. INPs originate from a variety of natural and anthropogenic sources, including mineral dust, biogenic aerosol, volcanic ash, and carbonaceous aerosols.
Mineral dust can be classified into high-latitude dust (HLD) and low-latitude dust (LLD). HLD is typically rich in feldspars and other mineral phases that can act as efficient INPs, while low-latitude dust (LLD) often has a different mineralogical composition and can be influenced by longer atmospheric transport times, which may reduce in INA [,,,,]. Low latitude dust (LLD), emitted from arid and semi-arid regions (Middle East, North Africa, and Asia), has been a focus of many Arctic studies [,,,,]. Although high latitude dust (HLD) contributes around 27% to the dust burden in the Arctic and sub-Arctic region [], which includes Iceland [,,,], Canada [], Alaska [], Greenland [,,] it is understudied, similar in other polar regions such as Antarctica []. HLD has different physical, chemical, and optical properties than the LLD, commonly referred to as mineral dust []. In contrast to LLD which is found throughout the troposphere, most of the emitted HLD is restricted at the lower altitudes in the Arctic because of the stratified atmosphere in the cold environment [,]. Iceland is a primary source of HLD, and Icelandic HLD could therefore also be an important contributor to INPs in the Arctic region. However few studies have evaluated HLD ice nucleation activity [,,].
Biogenic aerosols, such as bacteria, fungal spores, and pollen, can be efficient INPs due to the presence of ice-active macromolecules on their surfaces []. Volcanic ash can also act as a potent INP source, with activity linked to its glassy and crystalline mineral content []. In addition to these sources, carbonaceous particles, such as black carbon and other carbonaceous light absorbing impurities, can affect both directly and indirectly the Earth’s radiation balance through snow/ice albedo impacts or by altering cloud microphysical properties, respectively [,,,,,]. These microphysical processes dictate the cloud albedo and cloud lifetime. Clouds’ response to carbonaceous aerosols remains a high uncertainty in global climate models, as it can lead to cloud development, cloud coverage decrease, glaciation, and changes in emissivity, droplet size, and deactivation processes, among others [,,,,,,,,,,,,,]. However, the ice nucleation ability of carbonaceous light-absorbing impurities is not yet fully understood partially due to the scarcity of INP measurements in high latitudes.
Ice nucleation is dependent on the presence of nucleation sites, the freezing pathway, and environmental conditions, particularly temperature and the available water vapor content. The two main ice nucleation pathways in real-world systems are immersion freezing (typical of low-altitude mixed-phase clouds—freezing occurs within a droplet of supercooled water condensed around or on a particle) and deposition nucleation (typical of high-altitude and low-temperature clouds, where ice nucleates at a particle surface without prior condensation) []. In this work, we examined the immersion freezing properties of light-absorbing particles (LAP)—high-latitude dust and black carbon—and organic aerosol in environmental snow samples. While the ice nucleating activity (INA) of mineral dust had been demonstrated, but little is known about the role of HLD and BC [,]. The specific objectives of this study were to (1) expand in situ measurements of LAP in Iceland and (2) analyze the LAP and methanol-soluble organic aerosol ice nucleation activity (i.e., trigger ice nucleation at temperatures higher than −35°).
2. Materials and Methods
2.1. Study Area
The Vatnajökull Ice Cap has one of the most negative mass balance values recorded in the 21st century, with −1.34 ± 0.12 m water equivalent (w.e). a−1 (2002–2010). At the southernmost part of the Ice Cap is Öræfajökull an ice-filled stratovolcano and the highest peak in Iceland at 2110 m []. The 1362 eruption of Öræfajökull, the largest in Europe after Mt. Vesuvius, triggered massive glacial outbursts leading to the formation of volcanic sand deposits or black deserts that surround the southwest area of Vatnajökull Ice. Valley glaciers extending from the volcanic flanks have carved overdeepened valleys as is the case of the outlet glacier Svínafellsjökull (Figure 1). This glacier has retreated 800 m since the Little Ice Age (LIA) maximum (~1890), a common baseline to assess long-term glacial changes. This retreat is smaller than its neighboring glacier Skaftafellsjökull which has retreated 2700 m since LIA maximum []. However, both Skaftafellsjökull and Svínafellsjökull present similar mass balances −0.40 ± 0.96 and −0.32 ± 0.96 m w.e a−1 from 1890 to 2010 []. Svínafellsjökull mass loss is therefore governed through thinning rather than retreat, due to its over deepened bed with a maximum depth of 320 m below sea level [,].
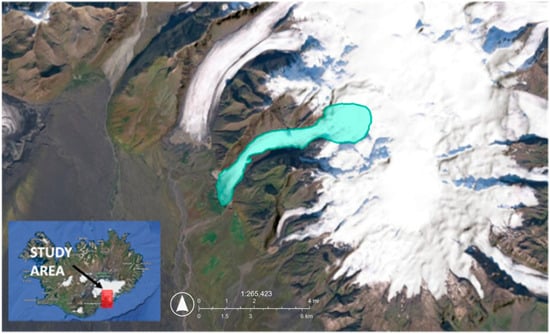
Figure 1.
Overview of Svínafellsjökull, our study area, in Iceland as part of the Vatnajökull Ice Cap. Source: Esri, Vantor, Earthstar Geographics, the GIS User Community, Samsyn Ehf, TomTom, Garmin, FAO, NOAA, & USGS. (2025). Map of the study area. The turquoise overlay represents the Svínafellsjökull glacier region, where surface snow samples were collected. North is indicated by the arrow. Coordinates are shown to orient the site’s location relative to the broader Vatnajökull region.
2.2. Snow Sample Collection
Surface snow samples were taken at three different locations (namely Site A, Site B, and Site C) in Svínafellsjökull (Table 1), in March 2018. Samples have been collected in triplets within an area of 20 m for each sample site (Table 1). All snow samples were stored frozen at −20 °C immediately after collection and remained frozen until INA analysis, which occurred within 9 months from sampling date. No intermediate thawing occurred. Generally, the 9 samples were kept frozen whenever possible until analysis. However, in some instances, partial thawing occurred. Previous studies have shown that freeze–thaw cycles can reduce BC concentrations, but primarily when samples are warmed to room temperature [,]. Our samples, however, remained cold during transport. Consistent with our conditions, Nagorski et al. (2019) found no changes in BC loading when samples underwent partial thawing but remained below room temperature []. Thus, this thawing is not expected to have an impact on the analysis. Polypropylene sample vials were used to directly collect the triplicate samples. Each sample consists of a 3 cm deep snow layer from an area of approximately 1.8 m2. The 1.8 m2 surface area was delineated in the field using a measuring tape to mark the corners directly on the snow surface, ensuring consistent sample size across sites. After putting the snow in the vial, the vial was tapped to help compact the snow in order to collect sufficient samples. To minimize sample contamination, Polypropylene gloves were worn at all times during the sampling, and care was taken to ensure that fibers from clothing did not come in contact with the sample.

Table 1.
Sampling site coordinates and weather conditions on Svínafellsjökul, 2 March 2018. All weather data was obtained from Skaftafell, the closest weather station, except for precipitation *, which was retrieved from Kvísker as it was the only station with precipitation data available. Unless otherwise noted, all data represent daily averages.
Sites A, B, and C were selected along the same glacier to ensure representative sampling across a shared cryospheric system while minimizing environmental variability. Each site was located approximately 100 m apart to capture localized differences without compromising comparability. The area was chosen for being accessible for fieldwork yet outside of common tourist routes, reducing the risk of anthropogenic disturbance.
All weather conditions were taken from Skaftafell, the closest weather station, except for precipitation, which was obtained from Kvísker as it was the only station with precipitation data available.
2.3. Light-Absorbing Particles Analysis
We measured black carbon concentrations using an extended range Single Particle Soot Photometer, SP2 (Droplet Measurement Technologies, Boulder, CO, USA) coupled with a CETAC Marin 5 nebulizer []. Combined with the CETAC Marin-5 nebulizer, the SP2 laser-induced incandescence allows estimating refractory BC (rBC hereby BC) particles between 80–1000 nm in snow-melted liquid samples [,,,,]. Most samples went through cycles of partial melting and refreezing during transport. However, sample 1 from Site C (1C), had intact snow in the bottle, which was removed and measured separately to examine the effect of the melting cycle in the final result. Upon melting in the lab, all samples were sonicated for 10 min and agitated with a magnetic stir bar during sample measurement. Samples were pumped at 143 mL min−1 to a CETAQ Marin-5 nebulizer (operating temperatures of 110 °C and 5 °C for the heating and cooling element, respectively), coupled to an extended range SP2. Reported BC concentrations are blank corrected using deionized water and account for mechanical particle losses in the nebulizer based on an external calibration using Aquadag standards [].
2.4. Dust Measurements
After the BC analysis was completed, the dry or insoluble microparticle mass (hereafter referred to as dry mass) was measured using a gravimetric filter procedure []. Even though quartz filters are not ideal for dust measurements because of pore size uncertainty, they were selected as the filter medium to allow the determination of other carbonaceous materials in future work. Melted snow samples were filtered under a class 100HEPA (High-Efficiency Particulate Air) clean bench using quartz filters (Whatman QM-A, 2.2 μm nominal pore size New York, NY, USA), previously baked at 650 °C for 8 h, to allow for the determination of carbonaceous material. After filtration, these were dried in a Class 100 laminar flow bench. After relative humidity and temperature equilibration (72 h) in an air-conditioned room, gravimetric measurements of dry mass measurements were taken using a microbalance (Sartorius M5P with a range of up to 1 g reading to ±0.5 μg). Each filter was weighed three times to obtain analytical replicates, and the mean value was used in subsequent calculations. While we did not perform particle size distribution analysis, the use of 2.2 μm filters implies that particles retained represent the coarse LAP fraction.
This non-selective procedure, described above, results in the quantification of total dry mass, including all impurities deposited in the snow including carbonaceous particles, organic matter, and dust. To establish if our dry mass measurements are representative of the dust content, we use iron (Fe) as a proxy (r = 0.99) to constrain dust since ferric oxides tend to influence mineral dust radiative forcing []. Fe concentrations were determined through energy-dispersive X-ray fluorescence (ED-XRF) analysis, which is a non-destructive way to analyze filter samples.
We found that dry mass is significantly correlated with Fe concentrations (n = 9, p < 0.001, Figure 2), which suggests that dust dominates the mass obtained by gravimetry []. Given this significant correlation between Fe and dry mass, and the predominance of LAP dust in previous studies of Greenland [,,], we conclude that our gravimetric measurements reflect dust concentrations.
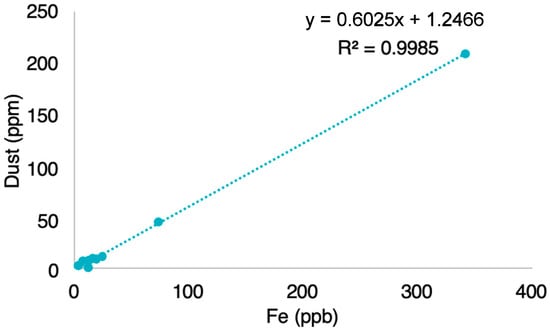
Figure 2.
Average Fe (µg L−1) vs. average dry mass (g L−1) for each of the three sites (n = 9). The correlation coefficient between Fe and Dry Mass was 0.99 (n = 9, p < 0.001). Average dry mass are based on analytical triplicates of each of the 9 filters. The dotted line indicates the linear regression fit (R2 = 0.9985).
2.5. Measurement Setup and Calculations for Ice Nucleation Activity
To counter sedimentation, melted samples were sonicated before pipetting 1 mL aliquot of each into sterile Eppendorf tubes. Ice nucleation activity was determined through cryo-microscopy, using the Vienna Optical Droplet Crystallization Analyzer (VODCA) [,,,]. VODCA is a well-established instrumental method for immersion freezing measurements. Its performance has been benchmarked against other immersion freezing measurement systems, including continuous flow diffusion chambers and cold plate methods, in laboratory and field intercomparisons. It allows for highly sensitive detection of immersion freezing events in deposited droplets, with detection limits as low as a few INPs per liter of air. Intercomparison studies have shown good agreement in onset temperatures and INP concentrations across a range of atmospheric aerosol types and concentrations, confirming the instrument’s robustness and reproducibility [].
The VODCA set-up consists of an airtight cell with a thermoelectric cooler mounted on a copper block that enabled precise cooling rates between 0.1 and 10 °C/min down to −40 °C. A glass window on the cover of the cell allows observation through the 40× magnification lens (Olympus, Shinjuku, Japan) of a light microscope (Olympus BX51M, Tokyo, Japan) to allow monitoring. The VODCA setup has an attached camera (Hengtech MDC320, 2.3 M pixel, Hengtech, Viernheim, Germany) to capture video and pictures along the ice nucleation process. Figure 3 showcases the sampler setup with both a photo of the instrument and a schematic of the different parts of the airtight cell.
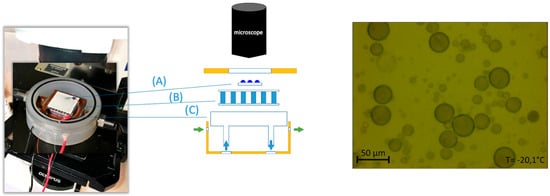
Figure 3.
Vienna Optical Droplet Crystallization Analyzer Sampler Setup. To the left, we observe a photo of the airtight cryo cell placed under the light microscope. In the center is a schematic of the cryocell components: (A) Sample carrier, (B) Peltier element, (C) Cooper cooling block. The right panel shows an example of a microscope view of frozen droplets on the right. Adapted from [].
The aqueous samples were emulsified with an oil phase (90 wt% paraffin and 10 wt% lanolin water-free grade) as proven viable from previous work [,]. The emulsion was directly created on a thin glass slide mixing 2 mL of the sample and 2 mL of the oil phase with a pipette tip until the mixture turned opaque due to Mie scattering [,,]. After placing emulsified samples on the sample carrier, the microscope cell was flushed with dry nitrogen between measurements. Experiments of snow samples were done with a cooling rate of approximately 10 °C min−1. Due to the change in light-scattering properties, we could easily identify frozen droplets as they turn opaque when crystallizing. The diameter of the droplets used for analysis ranged from 15–41 µm (1.8–23 pL). For each sample, three separate slides were prepared. Each slide was measured at three different positions for each samples, allowing the stage to warm to room temperature between measurements to ensure independent cooling cycles. Upon freezing, frozen and unfrozen droplets are counted in each image enabling the determination of the fraction of frozen droplets for every recorded temperature step. All measured droplets were combined in a single freezing curve for analysis. The resulting mean freezing temperature (MFT) is calculated as:
where is the recorded temperature and fice is the frozen fraction and is given by
and ni is the number of droplets freezing at this temperature and ntotal is all the droplets observed in the sample (including those freezing homogeneously). Here, we also present the median ice nucleation temperature T50 values ( at which the fice = 0.5) and the onset ice nucleation temperature, TO, ( at which the fice = 0.01). T50 is commonly used to describe the highest level of INA and TO the initiation of ice nucleation activity. Although T50 is used in common ice nucleation parameterizations, the onset temperature (TO) can still be important, as the first freezing events could prevent the occurrence of additional freezing events in their immediate atmospheric vicinity due to the Wegener–Bergeron–Findeisen (WBF) process or the ice superannuation negative feedback [,,,]. The ice formation initiation at warmer temperatures and lower ice supersaturations in a rising air parcel might deplete water vapor by crystal growth inhibiting homogeneous nucleation and affecting radiative properties, lifetime, and humidity of clouds [,,,,].
2.6. Sample Treatments
Given that arctic snow environmental samples have lower concentration than usual laboratory standards used in these experiments, we tested three sample treatments approaches to pre-concentrate and isolate components of the deposited LAP in order to enhance detectability or INP activity. These sample treatments included: (1) untreated melted snow samples, (2) methanol extractions from filtered particles, and (3) freeze-dried melted samples. For untreated applications, samples were directly analyzed as discussed in Section 3.1 above. Freeze-drying and methanol extraction sample treatment procedures are explained in the sections below and then analyzed for INA.
2.6.1. Freeze Drying of Snow Samples
Lyophilization, or freeze-drying, is a dehydration technique that preserves sensitive chemical and biological properties by freezing the sample and removing water through sublimation under reduced pressure. To understand if freeze-drying could enhance ice nucleation activity on environmental samples, we selected snow samples that displayed activity close to homogenous ice nucleation. A 5 mL aliquot of a sample was transferred to 50 mL centrifuge tubes with pierced lids. The freeze-drying was done using a Christ Alpha 1-4 LD plus (Burladingen, Germany) during a period of 24 h at 1 mbar and −55 °C. The solid residue was then reconstituted with 200 µL of ultrapure water (18.2 MΩcm−1). The samples were then analyzed for ice nucleation activity with the VODCA procedure described above.
2.6.2. Extraction
A 7 mm filter punch of each quartz filter sample was extracted ultrasonically in 10 mL of HPLC grade MeOH for 60 min [,,,]. The extracts were then filtered with a 0.20 μm PTFE syringe filter (VWR, sterile) to remove insoluble material. We used a rotary evaporator at 40 °C and 112 mmHg to remove methanol and replace it with ultrapure water. This procedure was performed to isolate the methanol-soluble fraction of the organic aerosol. We did not determine percent recovery values, as LAPs were quantified prior to extraction and the purpose here was specifically to obtain the methanol-soluble fraction. The resulting methanol-soluble organic aerosol extracts were analyzed as described in Section 3.1.
3. Results and Discussion
Light-absorbing particles presented deposited BC concentrations ranging between 1.65 and 98.72 ng g−1 for an average of 28 ± 25 ng g−1. Our observed range captures the magnitude of concentrations in previous studies, which have found BC mass of 87 ng g−1 during March Spring []. On the other hand, high-latitude dust concentrations ranged between 0.86 and 208.67 ppm (Figure 4a), resulting in an average deposited dust concentration of 0.13 ± 0.25 g m−2. These concentrations are below previous field studies in other areas of the ice cap and modeling estimates for the Svínafellsjökull that find an average of 2 g m−2 and 5 g m−2, respectively [,,]. Some of this discrepancy in dust concentration can be due to the seasonal and temporal difference in measurements, as INPs have a strong seasonality with lower concentrations during winter and spring, due to aerosol transport cycle [,,,,,].
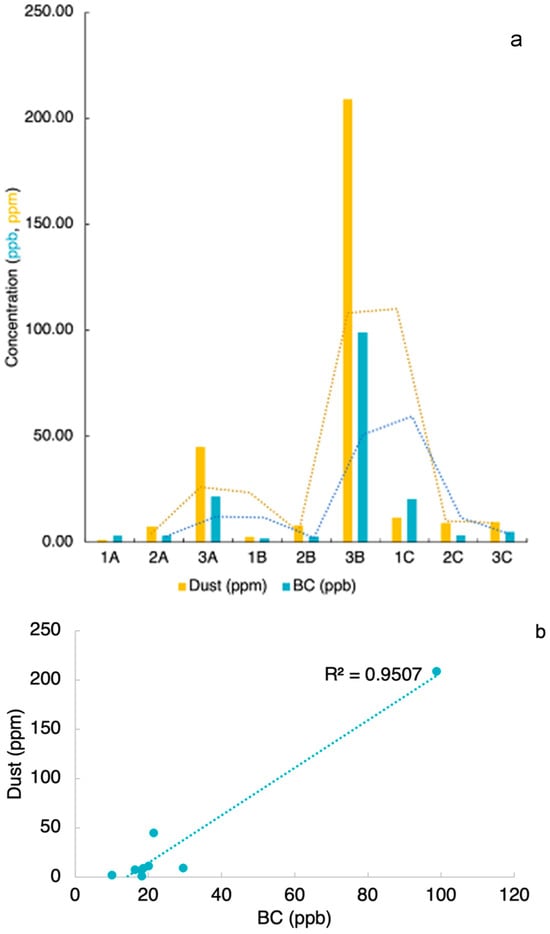
Figure 4.
Light-absorbing particle concentration on Iceland Surface Snow. (a) Concentrations of dust (ppm) are shown in yellow and BC (ppb) are shown in blue, stipple lines show the moving average trendline (b) shows the relationship between dust and BC concentrations (n = 9, p < 0.001).
The highest LAP concentrations are markedly found in sample 3B, while other high concentrations can be observed in samples 3A and 1C. The surface dust deposition distribution showed a similar pattern to black carbon deposition. We analyzed the correlation coefficient of the sample concentration and found that BC is positively correlated with HLD (r = 0.95) (Figure 4b). The highest BC concentration corresponds to a visibly darker snow surface at the sampling site, suggesting localized deposition of light-absorbing particles rather than a measurement artifact. Although the total number of samples analyzed in this study is limited (n = 9), this dataset is particularly valuable given the lack of field observations and the challenges and logistical constraints of collecting and preserving samples in remote Arctic field settings. These measurements provide important insights into LAP composition and sources despite the small sample size.
3.1. Ice Nucleation Activity
3.1.1. Untreated and Freeze-Dried Samples
We first compare unfiltered (melted snow samples prior to gravimetric analysis) and freeze-dried samples to assess the influence of concentration and preservation on INA (Figure 5, Figure 6 and Figure 7). T50 values remain largely consistent across sites before and after freeze-drying, while modest differences emerge in TO (Figure 5). Likewise, mean freezing temperatures (MFT) are also broadly similar (Figure 6) across unfiltered and freeze-dried samples. These results indicate that the freeze-drying process did not significantly alter the average bulk freezing behavior of the overall sample ensemble. However, one notable exception is sample 1C, which shows a significant decrease in both MFT and TO after freeze-drying (Figure 5 and Figure 6). Frozen fraction curves for sites A and B overlap, whereas the freeze-dried curve for Site C is slightly displaced to lower temperatures, consistent with reduced apparent INA after freeze drying (Figure 7). These observations imply that concentration effects alone do not explain change in behavior; freeze-drying likely removes or deactivates labile INP fraction at Site C.
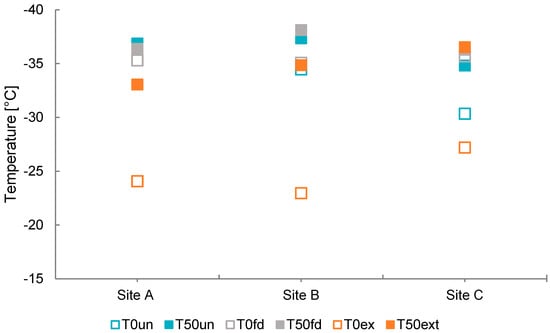
Figure 5.
T50 and TO values for each sample site. Cryo-microscopy results with T50 and TO temperatures of unfiltered, freeze-dried, and extracted samples. TO temperatures are shown with hollow symbols and T50 temperatures with solid squares. Extraction samples are marked with orange squares, freeze-dried with green squares, and unfiltered with blue squares.
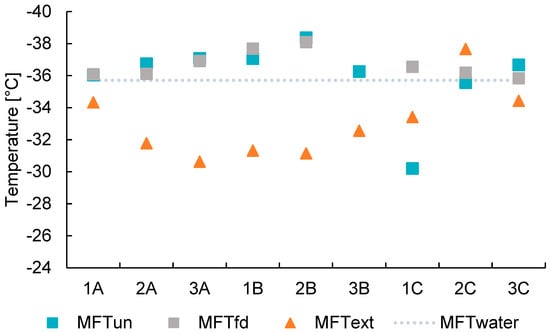
Figure 6.
Mean freezing temperature (MFT) of the different snow samples. Sample labels are shown on the x-axis without. Unfiltered (un) samples are marked with blue squares, freeze-dried samples (fd) with gray squares, and organic aerosol filter extracts (ext) with orange triangles. The dashed line is the MFT of ultrapure ware at −35.71 °C.
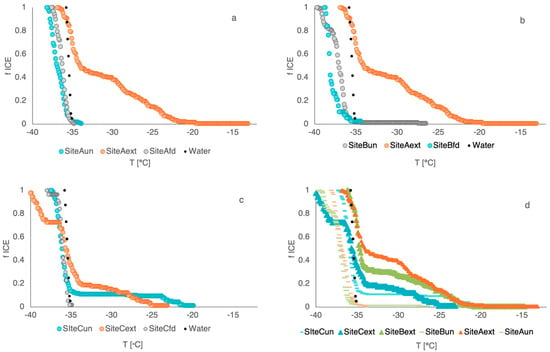
Figure 7.
Frozen fraction (FF) curves as a function of temperature. (a) Site A, (b) Site B, (c) Site C, and (d) all unfiltered and extracted results. The colors used for unfiltered is blue, extracted is orange, freeze-dried is gray and ultrapure water is black for site-specific graphs. Unfiltered samples represent BC and dust INP, while extracted samples represent methanol-soluble compounds. The freeze-dried samples can technically be viewed as concentrated unfiltered samples.
Previous studies have shown that BC alone exhibits relatively low ice-nucleating efficiency compared to other particle types, with activity several orders of magnitude lower than that of mineral dust or biological INPs. In the case of HLD, weak and conflicting ice-nucleating activity may result from its amorphous mineral structure, low silica content, and the relative presence of metal-rich volcanic glass [,,]. Icelandic HLD is characterized for its volcanogenic origin, displays specific mineralogical and chemical characteristics that influence its INA. In our freeze-dried and unfiltered samples (Figure 7), which contain both BC and HLD, the combined contribution shows shallow frozen fraction curves and appears insufficient to produce notable ice nucleation activity, consistent with these earlier findings. While recent studies documented how HLD can strongly enhance INP concentration and exhibit comparable values to low latitude dust (LLD) and feldspar at around −17 °C, ice nucleation sites are governed by factors such as surface topography, chemical composition, and pore structure [,,,,,,,,,,,]. The differences between our results and those of Sánchez-Marroquín et al. may also reflect seasonal and methodological contrasts. While our campaign occurred in the early March campaign, when late-winter conditions remain in Iceland, their April sampling occurred later in the melt season. This timing influences snowpack structure, surface melt, and boundary layer dynamics, all of which can affect LAP concentrations and ice-nucleating activity. In addition, their measurements were obtained in situ from aircraft, whereas our study analyzed surface snow samples, introducing differences in particle sources, atmospheric processing, and deposition patterns. Arctic INPs have been observed to exhibit freezing onset temperature ranging from −9 to −30 °C, depending on season and regional variability [,]. These compositional and structural factors are likely responsible for the comparatively low INA observed in our untreated and freeze-dried samples. Given that these samples (untreated and freeze-dried) represent BC and HLD composition, we conclude that neither of these LAP contributes significantly to INA as this activity cannot be attributed to heterogeneous ice nucleation.
3.1.2. Extracted Samples
Most samples of methanol-soluble organic aerosol (i.e., extracted samples) consistently show MFT values higher than the homogeneous ice nucleation temperature (~−35.71 °C), confirming the presence of effective INPs (Figure 6). The freezing curves (Figure 7) clearly reveal the increase in INA above the water blank (Milli-Q® water, Darmstadt, Germany). Overall, the samples display lesser steep freezing curves but earlier onset temperatures, indicating the presence of a small number but highly effective INPs. We see that for extracted samples, INP activity ranges from −23 to −34 °C (Figure 5). Independent of concentrations, these effective INPs trigger ice nucleation at temperatures up to −23 °C. For instance, the onset temperature for Site A increased from −36.5 °C to −24.4 °C after extraction. We observe the largest difference for Site B, which coincidentally has the largest concentration of LAP. The methanol-soluble organic aerosol extracted from all samples showcases a much shallower slope after T50 and a larger frozen fraction than its unfiltered samples. The results suggest that the extracted fraction (which is the sum of particles either soluble in MeOH or <2 µm in size) is the driving factor for ice nucleation in these samples. These results challenge conventional understanding in two key ways. First, 0.22 µm filtration is generally expected to lead to lesser INA according to classic INP theories [] and laboratory studies [,]. Second, the organic carbon, which we would expect in the extracted fraction, have exhibited significantly reduced or even suppressed INA in previous laboratory studies [,,]. Nevertheless, our results indicate that, in these environmental samples, the extracted fraction may contain a distinct set of potent ice-active compounds, warranting further chemical characterization.
In this study, we observed the difference between the onset ice nucleation temperature and the median ice nucleation temperature to better capture dynamics within the samples. We find that the difference between T50 and TO increases exponentially with LAP concentration for unfiltered samples with an asymptote reaching approximately −10 °C with HLD and BC concentrations higher than 2 × 108 ng g−1 and 90 ng g−1 (Figure 8). In contrast, the extracted samples do not show any relationship with BC and dust concentrations. For unfiltered samples, ΔT50–TO appears to increase with LAP concentration, suggesting a possible concentration-dependent inhibition of ice nucleation. Since this apparent exponential inhibition trend hinges on a high-concentration sample, it should be further studied. However, the sample fits the expectation from a visual examination from where it was collected.
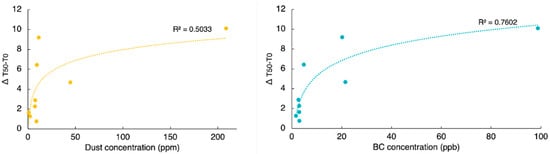
Figure 8.
Delta T50 and TO for unfiltered samples as a function of LAP concentration. Yellow symbols are used for HLD concentrations and blue for BC. As expected, extracted samples did not show any relationship with Dust or BC concentrations therefore it is not shown.
Methanol-soluble organic aerosol samples have a larger difference between T50 and TO (Δ T50–TO). To understand if BC and HLD were influencing the steep onset ice nucleation temperatures on extracted samples we plotted Δ (T50–TO) vs. LAP concentration, but no relationship was found. Moreover, the extracted samples show a pronounced difference between TO and T50 of ice nucleation. In general, the difference between T50 and TO is bigger for the extracted samples with an average of 10.07 °C, compared to 2.97 °C and 1.57 °C for unfiltered and freeze dried, respectively (Figure 5). This suggests a different class of INPs, methanol-soluble organic aerosol, is responsible for the INA likely as a result of a change in the active sites []. Given that this activity is only shown after extraction and no strong shift of T50 is observed in other sample treatments, we infer that the extracted materials have reduced ice nucleation in the presence of other LAP in higher concentration and water-soluble materials in the untreated samples. Since no further chemical analysis of the extract or the residue sample was done, we can only speculate on what leads to this observation. One explanation could be that IN active organic materials are adsorbed on mineral particles and oriented in a way—possibly through H-bonds—that their IN active sites are not available. It is however not possible to make any conclusions since data on the participating compounds is not available. Further research with more focus on detailed chemical and mineralogical analysis is needed to shed light on the factors leading to enhanced INA by methanol-soluble organic aerosols.
4. Conclusions
Svínafellsjökull black carbon and high-latitude dust concentrations in surface snow samples were 16.16 ± 27.09 ng g−1 and 33.45 ± 66.97 × 106 ng g−1, respectively. We have measured the ice-nucleating ability of these deposited LAPs in surface snow. Ice nucleation experiments reveal that the average HLD and BC MFT (−36.19 °C) and T50 (−35.99 °C) are close to homogeneous nucleation of our system (MFT −35.71 °C, T50 −35.83 °C). We observe that freeze drying does not significantly aid in enhancing INA unless LAP concentrations are high. Thus, this concentration step did not show significant improvement for our low concentration samples. Previous studies have demonstrated that Icelandic dust deposition represents 30% of the total atmospheric suspended particle concentration []. This study’s analysis of snow samples shows that HLD and BC might not play an important role in Arctic ice nucleation, considering LAP collected from the surface, in general, show higher INA than airborne INP []. These results differ from recent aircraft studies, which reported larger HLD concentrations and used a different sampling period and INA analysis method [] yet they remain consistent with the broader evidence of conflicting results for volcanic HLD’s INA [,,,,,].
However, we found that ice nucleation activity of snow samples was enhanced after extracting filters with methanol (MeOH) suggesting methanol-soluble organic aerosols, have a more prominent INA role than BC and dust. We found ice nucleation temperatures that ranged from TO −24.98 ± 4.03 °C to T50 −34.30 ± 0.65 °C. The variability in methanol-soluble organic aerosols TO values points to the potential difference in active sites. Further analysis needs to characterize the organic aerosol fraction to understand their relevance for ice nucleation in the Arctic. Most importantly, the effect on cloud microphysics from enhanced TO should be investigated. The INA enhancement after separation could prove that methanol-soluble organic aerosol INA are likely to be inhibited by the presence of HLD and BC. Current immersion freezing models include BC as an INP and do not distinguish between LLD and HLD. Our study suggests that it is important to distinguish between LLD and HLD since our results suggest that they can have very different INA. Given that our samples show similar concentration patterns for HLD and BC it is difficult to deconvolute their role. More INA experiments linking BC and HLD morphologies, surface chemistry, and formation/volatilization mechanisms should be deployed to improve immersion freezing parameterizations in climate models and improve the prediction of INA.
Author Contributions
I.M.C.-R. spearheaded the conceptualization of the research theme and field campaign, conducted the bulk of the experimental work and data collection in Iceland, and led the writing of the original manuscript draft. H.G. provided supervision throughout the project, ensuring scientific integrity and direction, while also managing the project’s administration and securing essential funding. H.G. validated the experimental methods and results, and contributed significantly to the manuscript’s review and editing process. P.B. was instrumental in supporting the conceptualization of the research and assisted in the manuscript’s review and editing, ensuring clarity and precision in the presentation of the findings. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Austrian Science Fund (FWF), Project number GRW 6, GROW-Graduate Research Opportunities Worldwide.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
I.M.C.-R. would like to acknowledge NSF GRFP for providing coordination and funding to perform this study. I.M.C.-R thanks the Icelandic Mountain Guides for their openness and service in ensuring our safety during the sampling campaign. I.M.C.-R. and H.G. would like to thank Thomas Konegger for providing access to the freeze-drying equipment. I.M.C.-R, P.B., and H.G. would also like to thank Anne Kasper-Giebl for providing access to perform gravimetric analysis and Peter Kregsammer for providing access to ED-XRF equipment for Fe measurements.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| BC | Black Carbon |
| ED-XRF | Energy-Dispersive X-ray Fluorescence |
| HEPA | High-Efficiency Particulate Air |
| HLD | High Latitude Dust |
| INPs | Ice Nucleating Particles |
| INA | Ice Nucleation Activity |
| LLD | Low Latitude Dust |
| MFT | Mean Freezing Temperature |
| TO | Onset Ice Nucleation Temperature |
| PTFE | Polytetrafluoroethylene |
| rBC | Refractory Black Carbon |
| SP2 | Single Particle Soot Photometer |
| VODCA | Vienna Optical Droplet Crystallization Analyzer |
| WBF | Wegener–Bergeron–Findeisen process |
| Glossary | |
| Aerosols | Tiny solid or liquid particles suspended in the atmosphere, which can come from natural sources or human activities. |
| Black Carbon (BC) | A component of particulate matter derived from the incomplete combustion of fossil fuels, biofuel, and biomass, characterized by its strong light-absorbing properties. |
| Energy-Dispersive X-ray Fluorescence (ED-XRF) | A technique used for elemental analysis of a sample by measuring the characteristic X-rays emitted when the sample is bombarded with high-energy X-rays or gamma rays. |
| High-Efficiency Particulate Air (HEPA) | A type of air filter that meets standards of efficiency set by the United States Department of Energy by removing at least 99.97% of dust, pollen, mold, bacteria, and any airborne particles with a size of 0.3 microns (µm). |
| High Latitude Dust (HLD) | Dust originating from high latitude regions, often with unique chemical and physical properties compared to dust from other regions. |
| Ice Nucleating Particles (INPs) | Particles that act as nuclei for the formation of ice crystals in the atmosphere, critical for the process of cloud formation and precipitation. |
| Ice Nucleation Activity (INA) | The ability of certain particles or surfaces to facilitate the formation of ice, typically under supercooled conditions. |
| Low Latitude Dust (LLD) | Dust that originates from low latitude arid regions such as deserts. |
| Mean Freezing Temperature (MFT) | The average temperature at which a sample starts to freeze in a controlled laboratory setting. |
| Onset Ice Nucleation Temperature (TO) | The temperature at which the initial formation of ice occurs in a supercooled liquid. |
| Polytetrafluoroethylene (PTFE) | A synthetic fluoropolymer known for its non-reactive, hydrophobic, and friction-reducing properties, often used in applications where a non-stick surface is essential. |
| Refractory Black Carbon (rBC) | A form of black carbon that is resistant to heat and does not decompose or react chemically at high temperatures. |
| Single Particle Soot Photometer (SP2) | An instrument used to measure the black carbon content of individual particles in the air by detecting the light emitted by these particles when heated. |
| Vienna Optical Droplet Crystallization Analyzer (VODCA) | A laboratory instrument used to study the freezing behavior of droplets under controlled environmental conditions to assess their ice nucleation activity. |
| Wegener–Bergeron–Findeisen (WBF) process | A meteorological process that describes the rapid growth of ice crystals at the expense of supercooled water droplets in mixed-phase clouds, leading to precipitation. |
References
- Turner, D.D. Arctic Mixed-Phase Cloud Properties from AERI Lidar Observations: Algorithm and Results from SHEBA. J. Appl. Meteorol. 2005, 44, 427–444. [Google Scholar] [CrossRef]
- Pinto, J.O. Autumnal mixed-phase cloudy boundary layers in the Arctic. J. Atmos. Sci. 1998, 55, 2016–2038. [Google Scholar] [CrossRef]
- Mülmenstädt, J.; Sourdeval, O.; Delanoë, J.; Quaas, J. Frequency of occurrence of rain from liquid-, mixed-, and ice-phase clouds derived from A-Train satellite retrievals. Geophys. Res. Lett. 2015, 42, 6502–6509. [Google Scholar] [CrossRef]
- Korolev, A. Limitations of the Wegener–Bergeron–Findeisen mechanism in the evolution of mixed-phase clouds. J. Atmos. Sci. 2007, 64, 3372–3375. [Google Scholar] [CrossRef]
- Pruppacher, H.R.; Klett, J.D. (Eds.) Microphysics of Clouds and Precipitation, 2nd ed.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Turnball, D.; Fisher, J.C. Rate of nucleation in condensed systems. J. Chem. Phys. 1949, 17, 71–73. [Google Scholar] [CrossRef]
- Cantrell, W.; Heymsfield, A. Production of ice in tropospheric clouds: A review. Bull. Am. Meteorol. Soc. 2005, 86, 795–807. [Google Scholar] [CrossRef]
- Kanji, Z.A.; Ladino, L.A.; Wex, H.; Boose, Y.; Burkert-Kohn, M.; Cziczo, D.J.; Kräme, M. Overview of Ice Nucleating Particles. Meteorol. Monogr. 2017, 58, 1.1–1.33. [Google Scholar] [CrossRef]
- Dorsey, N.E. The freezing of supercooled water. Trans. Am. Philos. Soc. 1948, 38, 247–328. [Google Scholar] [CrossRef]
- Bond, T.C.; Doherty, S.J.; Fahey, D.W.; Forster, P.M.; Berntsen, T.; DeAngelo, B.J.; Flanner, M.G.; Ghan, S.; Kärcher, B.; Koch, D.; et al. Bounding the role of black carbon in the climate system: A scientific assessment. J. Geophys. Res. Atmos. 2013, 118, 5380–5552. [Google Scholar] [CrossRef]
- Johnson, B.T.; Shine, K.P.; Forster, P.M. The semi-direct aerosol effect: Impact of absorbing aerosols on marine stratocumulus. Q. J. R. Meteorol. Soc. 2004, 130, 1407–1422. [Google Scholar] [CrossRef]
- Brioude, J.; Cooper, O.R.; Feingold, G.; Trainer, M.; Freitas, S.R.; Kowal, D.; Ayers, J.K.; Prins, E.; Minnis, P.; McKeen, S.A.; et al. Effect of biomass burning on marine stratocumulus clouds off the California coast. Atmos. Chem. Phys. 2009, 9, 8841–8856. [Google Scholar] [CrossRef]
- Wilcox, E.M.; Thomas, R.M.; Praveen, P.S.; Pistone, K.; Bender, F.A.; Ramanathan, V. Black carbon solar absorption suppresses turbulence in the atmospheric boundary layer. Proc. Natl. Acad. Sci. USA 2012, 109, 11699–11704. [Google Scholar] [CrossRef]
- Fan, J.; Yuan, T.; Comstock, J.M.; Ghan, S.; Khain, A.; Leung, L.R.; Li, Z.; Martins, V.J.; Ovchinnikov, M. Dominant role by vertical wind shear in regulating aerosol effects on deep convective clouds. J. Geophys. Res. 2009, 114, D22206. [Google Scholar] [CrossRef]
- Heintzenberg, J.; Charlson, R.J. Earth’s Climate: The Ocean-Atmosphere Interaction; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Twomey, S. The nuclei of natural cloud formation part II: The supersaturation in natural clouds and the variation of cloud droplet concentration. Geofis. Pura E Appl. 1959, 43, 243–249. [Google Scholar] [CrossRef]
- Bauer, S.E.; Menon, S.; Koch, D.; Bond, T.C.; Tsigaridis, K. A global modeling study on carbonaceous aerosol microphysical characteristics and radiative effects. Atmos. Chem. Phys. 2010, 10, 7439–7456. [Google Scholar] [CrossRef]
- Ferek, R.J.; Garrett, T.; Hobbs, P.V.; Strader, S.; Johnson, D.; Taylor, J.P.; Nielsen, K.; Ackerman, A.S.; Kogan, Y.; Liu, Q.; et al. Drizzle suppression in ship tracks. J. Atmos. Sci. 1998, 55, 1740–1750. [Google Scholar] [CrossRef]
- Ackerman, A.S.; Kirkpatrick, M.P.; Stevens, D.E.; Toon, O.B. The impact of humidity above stratiform clouds on indirect aerosol climate forcing. Nature 2004, 432, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Sandu, I.; Brenguier, J.L.; Geoffroy, O.; Thouron, O.; Masson, V. Aerosol impacts on the diurnal cycle of marine stratocumulus. J. Atmos. Sci. 2008, 65, 2705–2718. [Google Scholar] [CrossRef]
- Lohmann, U.; Feichter, J. Global indirect aerosol effects: A review. Atmos. Chem. Phys. 2005, 5, 715–737. [Google Scholar] [CrossRef]
- Lohmann, U. A glaciation indirect aerosol effect caused by soot aerosols. Geophys. Res. Lett. 2002, 29, 1052. [Google Scholar] [CrossRef]
- Girard, E.; Blanchet, J.P.; Dubois, Y. Effects of arctic sulphuric acid aerosols on wintertime low-level atmospheric ice crystals, humidity and temperature at Alert, Nunavut. Atmos. Res. 2005, 73, 131–148. [Google Scholar] [CrossRef]
- Storelvmo, T.; Kristjansson, J.E.; Muri, H.; Pfeffer, M.; Barahona, D.; Nenes, A. Cirrus cloud seeding has potential to cool climate. Geophys. Res. Lett. 2008, 35, L18818. [Google Scholar]
- Hoose, C.; Kristjansson, J.E.; Burrows, S.M. How important is biological ice nucleation in clouds on a global scale? Environ. Res. Lett. 2010, 5, 024009. [Google Scholar] [CrossRef]
- Paramonov, M.; David, R.; Kretzschmar, R.; Kanji, Z. A laboratory investigation of the ice nucleation efficiency of three types of mineral and soil dust. Atmos. Chem. Phys. 2018, 18, 16515–16536. [Google Scholar] [CrossRef]
- Kanji, Z.A.; Welti, A.; Corbin, J.C.; Mensah, A.A. Black carbon particles do not matter for immersion mode ice nucleation. Geophys. Res. Lett. 2020, 46, e2019GL086764. [Google Scholar] [CrossRef]
- Harrison, A.D.; O’Sullivan, D.; Adams, M.P.; Porter, G.C.E.; Blades, E.; Brathwaite, C.; Chewitt-Lucas, R.; Gaston, C.; Hawker, R.; Krüger, O.O.; et al. The ice-nucleating activity of African mineral dust in the Caribbean boundary layer. Atmos. Chem. Phys. 2022, 22, 9663–9680. [Google Scholar] [CrossRef]
- Boose, Y.; Baloh, P.; Plötze, M.; Ofner, J.; Grothe, H.; Sierau, B.; Lohmann, U.; Kanji, Z.A. Heterogeneous ice nucleation on dust particles sourced from nine deserts worldwide—Part 2: Deposition nucleation and condensation freezing. Atmos. Chem. Phys. 2019, 19, 1059–1076. [Google Scholar] [CrossRef]
- Atkinson, J.D.; Murray, B.J.; Woodhouse, M.T.; Whale, T.F.; Baustian, K.J.; Carslaw, K.S.; Dobbie, S.; O’Sullivan, D.; Malkin, T.L. The importance of feldspar for ice nucleation by mineral dust in mixed-phase clouds. Nature 2013, 498, 355–358. [Google Scholar] [CrossRef]
- Häusler, T.; Witek, L.; Felgitsch, L.; Hitzenberger, R.; Grothe, H. Freezing on a chip—A new approach to determine heterogeneous ice nucleation of micrometer-sized water droplets. Atmosphere 2018, 9, 140. [Google Scholar] [CrossRef]
- Sanchez-Marroquin, A.; Arnalds, O.; Baustian-Dorsi, K.J.; Browse, J.; Dagsson-Waldhauserova, P.; Harrison, A.D.; Maters, E.C.; Pringle, K.J.; Vergara-Temprado, J.; Burke, I.T.; et al. Iceland is an episodic source of atmospheric ice-nucleating particles relevant for mixed-phase clouds. Sci. Adv. 2020, 6, eaba8137. [Google Scholar] [CrossRef]
- Cook, S.J.; Swift, D.A.; Graham, D.J.; Midgley, N.G. Origin and significance of ‘dispersed facies’ basal ice: Svínafellsjökull, Iceland. J. Glaciol. 2011, 57, 710–720. [Google Scholar] [CrossRef]
- Hannesdóttir, H.; Björnsson, H.; Pálsson, F.; Aðalgeirsdóttir, G.; Guðmundsson, S. Changes in the southeast Vatnajökull ice cap, Iceland, between ~1890 and 2010. Cryosphere 2015, 9, 565–585. [Google Scholar] [CrossRef]
- Magnússon, E.; Pálsson, F.; Björnsson, H.; Guðmundsson, S. Removing the ice cap of Öraefajokull central volcano, SE-Iceland: Mapping and interpretation of bedrock topography, ice volumes, subglacial troughs and implications for hazards assessments. Jökull 2012, 62, 131–150. [Google Scholar] [CrossRef]
- Adhikari, S.; Marshall, S.J. Influence of high-order mechanics on simulation of glacier response to climate change: Insights from Haig Glacier, Canadian Rocky Mountains. Cryosphere 2013, 7, 1527–1541. [Google Scholar] [CrossRef]
- Wendl, I.A.; Menking, J.A.; Färber, R.; Gysel, M.; Kaspari, S.D.; Laborde, M.J.; Schwikowski, M. Optimized method for black carbon analysis in ice and snow using the Single Particle Soot Photometer. Atmos. Meas. Tech. 2014, 7, 2667–2681. [Google Scholar] [CrossRef]
- Drab, E.; Gaudichet, A.; Jaffrezo, J.L.; Colin, J.L. Mineral particles content in recent snow at Summit (Greenland). Atmos. Environ. 2002, 36, 5365–5376. [Google Scholar] [CrossRef]
- Ryan, J.C.; Smith, L.C.; van As, D.; Cooley, S.W.; Cooper, M.G.; Pitcher, L.H.; Hubbard, A. Greenland Ice Sheet surface melt amplified by snowline migration and bare ice exposure. Sci. Adv. 2019, 5, eaav3738. [Google Scholar] [CrossRef]
- Dumont, M.; Brun, E.; Picard, G.; Michou, M.; Libois, Q.; Petit, J.R.; Geyer, M.; Morin, S.; Josse, B. Contribution of light-absorbing impurities in snow to Greenland’s darkening since 2009. Nat. Geosci. 2014, 7, 509–512. [Google Scholar] [CrossRef]
- Bisiaux, M.M.; Edwards, R.; McConnell, J.R.; Curran, M.A.J.; Van Ommen, T.D.; Smith, A.M.; Neumann, T.A.; Pasteris, D.R.; Penner, J.E.; Taylor, K. Changes in black carbon deposition to Antarctica from two high-resolution ice core records, 1850–2000 AD. Atmos. Chem. Phys. 2012, 12, 4107–4115. [Google Scholar] [CrossRef]
- Creamean, J.; Barry, K.; Hill, T.; Hume, C.; DeMott, P.; Shupe, M.; Dahlke, S.; Willmes, S.; Schmale, J.; Beck, I.; et al. Annual cycle observations of aerosols capable office formation in central Arctic clouds. Nat. Commun. 2022, 13, 3537. [Google Scholar] [CrossRef]
- Marquetto, L.; Kaspari, S.; Gysel-Beer, M. The import of black carbon from lower latitudes to the Arctic: An overlooked source of climate forcing. Environ. Res. Lett. 2020, 15, 124042. [Google Scholar]
- Zender, C.S.; Bian, H.; Newman, D. Mineral Dust Entrainment and Deposition (DEAD) model: Description and 1990s dust climatology. J. Geophys. Res. 2003, 108, 4416. [Google Scholar] [CrossRef]
- McConnel, J.R.; Edwards, R.; Kok, G.L.; Flanner, M.G.; Zender, C.S.; Saltzman, E.S.; Banta, J.R.; Pasteris, D.R.; Carter, M.M.; Kahl, J.D.W. 20th-century industrial black carbon emissions altered arctic climate forcing. Science 2007, 317, 1381–1384. [Google Scholar] [CrossRef]
- Schwarz, J.P.; Gao, R.S.; Fahey, D.W.; Thomson, D.S.; Watts, L.A.; Wilson, J.C.; Reeves, J.M.; Darbeheshti, M.; Baumgardner, D.G.; Kok, G.L.; et al. Single-particle measurements of midlatitude black carbon and light-scattering aerosols from the boundary layer to the lower stratosphere. J. Geophys. Res. 2006, 111, D16207. [Google Scholar] [CrossRef]
- Nagorski, S.A.; Kaspari, S.D.; Hood, E.; Fellman, J.B.; Skiles, S.M.K. Radiative forcing by dust and black carbon on the Juneau Icefield, Alaska. J. Geophys. Res. Atmos. 2019, 124, 3943–3959. [Google Scholar] [CrossRef]
- Si, M.; Irish, V.E.; Mason, R.H.; Vergara-Temprado, J.; Hanna, S.J.; Ladino, L.A.; Yakobi-Hancock, J.D.; Schiller, C.L.; Wentzell, J.J.B.; Abbatt, J.P.D.; et al. Ice-nucleating ability of aerosol particles and possible sources at three coastal marine sites. Atmos. Chem. Phys. 2019, 19, 15287–15306. [Google Scholar] [CrossRef]
- Huang, S.; Hu, W.; Chen, J.; Wu, Z.; Zhang, D.; Fu, P. Overview of biological ice nucleating particles in the atmosphere. Environ. Int. 2021, 146, 106197. [Google Scholar] [CrossRef]
- Hoose, C.; Möhler, O. Heterogeneous ice nucleation on atmospheric aerosols: A review of results from laboratory experiments. Atmos. Chem. Phys. 2012, 12, 9817–9854. [Google Scholar] [CrossRef]
- Van Curren, K.J.; Lamsal, L.N.; Duncan, B.N.; Beirle, S.; Theys, N.; de Smedt, I. Improved ground-level NO2 concentrations over the United States. Atmos. Chem. Phys. 2012, 12, 10637–10650. [Google Scholar]
- Bory, A.J.M.; Biscaye, P.E.; Svensson, A.; Grousset, F.E. Seasonal variability in the origin of recent atmospheric mineral dust at NorthGRIP, Greenland. Earth Planet. Sci. Lett. 2002, 196, 123–134. [Google Scholar] [CrossRef]
- Groot Zwaaftink, C.D.; Grythe, H.; Skov, H.; Stohl, A. Substantial contribution of northern high-latitude sources to mineral dust in the Arctic. J. Geophys. Res. Atmos. 2016, 121, 13678–13697. [Google Scholar] [CrossRef]
- Arnalds, O.; Olafsson, H.; Dagsson-Waldhauserova, P. Quantification of iron-rich volcanogenic dust emissions and deposition over the ocean from Icelandic dust sources. Biogeosciences 2014, 11, 6623–6632. [Google Scholar] [CrossRef]
- Dagsson-Waldhauserova, P.; Arnalds, O.; Olafsson, H. Long-term variability of dust events in Iceland (1949–2011). Atmos. Chem. Phys. 2014, 14, 13411–13422. [Google Scholar] [CrossRef]
- Prospero, J.M.; Bullard, J.E.; Hodgkins, R. High-latitude dust over the North Atlantic: Inputs from Icelandic proglacial lake sediments. Science 2012, 335, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Dörnbrack, A.; Stachlewska, I.S.; Ritter, C.; Neuber, R. Aerosol distribution around Svalbard during intense easterly winds. Atmos. Chem. Phys. 2010, 10, 1473–1490. [Google Scholar] [CrossRef]
- Crusius, J.; Schroth, A.W.; Gassó, S.; Moy, C.M.; Levy, R.C.; Gatica, M. Glacial flour dust storms in the Gulf of Alaska: Hydrologic and meteorological controls and their importance as a source of bioavailable iron. Geophys. Res. Lett. 2011, 38, L06602. [Google Scholar] [CrossRef]
- Bullard, J.E.; Austin, M.J. Dust generation on a proglacial floodplain, West Greenland. Aeolian Res. 2011, 3, 43–54. [Google Scholar] [CrossRef]
- Meinander, O.; Dagsson-Waldhauserova, P.; Amosov, P.; Aseyeva, E.; Atkins, C.; Baklanov, A.; Baldo, C.; Barr, S.L.; Barzycka, B.; Benning, L.G.; et al. Newly identified climatically and environmentally significant high-latitude dust sources. Atmos. Chem. Phys. 2022, 22, 11889–11930. [Google Scholar] [CrossRef]
- Bullard, J.E. The distribution and biogeochemical importance of high-latitude dust in the Arctic and Southern Ocean-Antarctic regions. J. Geophys. Res. Atmos. 2017, 122, 3098–3103. [Google Scholar] [CrossRef]
- Kaspari, S. Accelerated glacier melt on Snow Dome, Mount Olympus, Washington, USA, due to deposition of black carbon and mineral dust from wildfire. J. Geophys. Res. Atmos. 2014, 119, 2793–2803. [Google Scholar] [CrossRef]
- Kaspari, S.; Mayewski, P.A.; Handley, M.; Osterberg, E.; Kang, S.; Sneed, S.; Hou, S.; Qin, D. Recent increases in atmospheric concentrations of Bi, U, Cs, S and Ca from a 350-year Mount Everest ice core record. J. Geophys. Res. 2009, 114, D04302. [Google Scholar] [CrossRef]
- EPA. Method 160.2: Total Suspended Solids (TSS) (Gravimetric, Dried at 103–105 °C); Environmental Protection Agency: Washington, DC, USA, 1998.
- Felgitsch, L.; Baloh, P.; Burkart, J.; Mayr, M.; Momken, M.; Seifried, T.; Winkler, P.; Schmale, D.; Grothe, H. Birch leaves and branches as a source of ice-nucleating macromolecules. Atmos. Chem. Phys. 2018, 18, 16063–16079. [Google Scholar] [CrossRef]
- Pummer, B.G.; Bauer, H.; Bernardi, J.; Bleicher, S.; Grothe, H. Suspendable macromolecules are responsible for ice nucleation activity of birch and conifer pollen. Atmos. Chem. Phys. 2012, 12, 2541–2550. [Google Scholar] [CrossRef]
- Hauptmann, A.; Handle, K.; Baloh, P.; Grothe, H.; Loerting, T. Does the emulsification procedure influence freezing and thawing of aqueous droplets? J. Chem. Phys. 2016, 145, 211923. [Google Scholar] [CrossRef]
- DeMott, P.J.; Möhler, O.; Cziczo, D.J.; Hiranuma, N.; Petters, M.D.; Petters, S.S.; Belosi, F.; Bingemer, H.G.; Brooks, S.D.; Budke, C.; et al. The Fifth International Workshop on Ice Nucleation phase 2 (FIN-02): Laboratory intercomparison of ice nucleation measurements. Atmos. Meas. Tech. 2018, 11, 6231–6257. [Google Scholar] [CrossRef]
- Murray, B. Enhanced formation of cubic ice in aqueous organic acid droplets. Environ. Res. Lett. 2008, 3, 025008. [Google Scholar] [CrossRef]
- Baloh, P.; Hanlon, R.; Anderson, C.; Dolan, E.; Pacholik, G.; Stinglmayr, D.; Burkart, J.; Felgitsch, L.; Schmale, D.; Grothe, H. Seasonal ice nucleation activity of water samples from alpine rivers and lakes in Obergurgl, Austria. Sci. Total Environ. 2021, 800, 149442. [Google Scholar] [CrossRef]
- Felgitsch, L.; Bichler, M.; Burkart, J.; Fiala, B.; Häusler, T.; Hitzenberger, R.; Grothe, H. Heterogeneous Freezing of Liquid Suspensions Including Juices and Extracts from Berries and Leaves from Perennial Plants. Atmosphere 2019, 10, 37. [Google Scholar] [CrossRef]
- Diehl, K.; Wurzler, S. Heterogeneous drop freezing in the immersion mode: Model calculations considering soluble and insoluble particles in the drops. J. Atmos. Sci. 2004, 61, 2063–2072. [Google Scholar] [CrossRef]
- Khvorostyanov, V.I.; Curry, J.A. The theory of ice nucleation by heterogeneous freezing of deliquescent mixed CCN. part II: Parcel model simulation. J. Atmos. Sci. 2005, 62, 261–285. [Google Scholar] [CrossRef]
- Bergeron, T. On the physics of clouds and precipitation. In Proceedings of the 5th Assembly U.G.G.I., Lisbon, Portugal, 17–24 September 1933; Volume 2, pp. 156–178. [Google Scholar]
- Findeisen, W. Kolloid-meterologische Vorgänge bei der Neiderschlagsbildung. Meteorol. Z. 1938, 55, 121–133. [Google Scholar]
- Kärcher, B.; Lohmann, U. A parameterization of cirrus cloud formation: Heterogeneous freezing. J. Geophys. Res. 2003, 108, 4402. [Google Scholar] [CrossRef]
- Jensen, E.J.; Pfister, L.; Ackerman, A.S.; Tabazadeh, A.; Toon, O.B. A conceptual model of the dehydration of air due to freeze-drying by optically thin, laminar cirrus rising slowly across the tropical tropopause. J. Geophys. Res. 2001, 106, 17237–17252. [Google Scholar] [CrossRef]
- Yan, C.; Zheng, M.; Bosch, C.; Andersson, A.; Desyaterik, Y.; Sullivan, A.P.; Collett, J.L.; Zhao, B.; Wang, S.; He, K.; et al. Important fossil source contribution to brown carbon in Beijing during winter. Sci. Rep. 2018, 8, 43182. [Google Scholar] [CrossRef]
- Lin, P.; Aiona, P.K.; Li, Y.; Shiraiwa, M.; Laskin, J.; Nizkorodov, S.A.; Laskin, A. Molecular characterization of brown carbon in biomass burning aerosol particles, Environ. Sci. Technol. 2016, 50, 11815–11824. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Bergin, M.; Guo, H.; King, L.; Kotra, N.; Edgerton, E.; Weber, R.J. Size-resolved measurements of brown carbon in water and methanol extracts and estimates of their contribution to ambient fine-particle light absorption. Atmos. Chem. Phys. 2013, 13, 12389–12404. [Google Scholar] [CrossRef]
- Chen, Y.; Bond, T.C. Light absorption by organic carbon from wood combustion. Atmos. Chem. Phys. 2010, 10, 1773–1787. [Google Scholar] [CrossRef]
- Meriander, O. Light-Absorbing Impurities in European Arctic and Seasonal Snow: Identification, Variation and Effects on Albedo; Finnish Meteorological Institute: Helsinki, Finland, 2017. [Google Scholar]
- Dragosics, M.; Meinander, O.; Jónsdóttir, T.; Dürig, T.; De Leeuw, G.; Pálsson, F.; Dagsson-Waldhauserová, P.; Thorsteinsson, T. Insulation effects of Icelandic dust and volcanic ash on snowand ice. Arab. J. Geosci. 2016, 9, 126. [Google Scholar] [CrossRef]
- Wittmann, M.; Groot, C.; Steffensen, L.; Guðmundsson, S.; Pálsson, F.; Arnalds, O.; Björnsson, H.; Thorsteinsson, T.; Stohl, A. Impact of dust deposition on the albedo of Vatnajökull ice cap, Iceland. Cryosphere 2017, 11, 741–754. [Google Scholar] [CrossRef]
- Rogers, D.C.; DeMott, P.J.; Kreidenweis, S.M. Airborne measurements of tropospheric ice-nucleating aerosol particles in the Arctic spring. Geophys. Res. 2001, 106, 15053–15063. [Google Scholar] [CrossRef]
- Borys, R.D. Studies of ice nucleation by Arctic aerosol on AGASP-II. J. Atmos. Chem. 1989, 9, 169–185. [Google Scholar] [CrossRef]
- Vergara-Temprado, J.; Holden, M.A.; Orton, T.R.; O’Sullivan, D.; Umo, N.S.; Browse, J.; Reddington, C.; Baeza-Romero, M.T.; Jones, J.M.; Lea-Langton, A.; et al. Is black carbon an unimportant ice-nucleating particle in mixed-phase clouds? J. Geophys. Res. Atmos. 2018, 123, 4273–4283. [Google Scholar] [CrossRef] [PubMed]
- Jahn, L.; Fahy, W.; Williams, D.B.; Sullivan, R.C. The role of feldspar and pyroxene minerals in the ice nucleating ability of three volcanic ashes. ACS Earth Space Chem. 2019, 3, 626–636. [Google Scholar] [CrossRef]
- Diao, Y.; Myerson, A.; Hatton, T.; Trout, B. Surface design for controlled crystallization: The role of surface chemistry and nanoscale pores in heterogeneous nucleation. Langmuir 2011, 27, 5324–5334. [Google Scholar] [CrossRef]
- Asanithi, P. Surface porosity and roughness of micrographite film for nucleation of hydroxyapatite. J. Biomed. Mater. Res. A 2014, 102, 2590–2599. [Google Scholar] [CrossRef]
- Chayen, N.; Saridakis, E.; Sear, R. Experiment and theory for heterogeneous nucleation of protein crystals in a porous medium. Proc. Natl. Acad. Sci. USA 2006, 103, 597–601. [Google Scholar] [CrossRef]
- Isono, K.; Ikebe, Y. On the ice-nucleating ability of rock-forming minerals and soil particles. J. Meteorol. Soc. Jpn. 1960, 38, 213–230. [Google Scholar] [CrossRef]
- Porter, G.C.E.; Adams, M.P.; Brooks, I.M.; Ickes, L.; Karlsson, L.; Leck, C.; Salter, M.; Schmale, J.; Siegel, K.; Sikora, S.; et al. Highly active ice-nucleating particles at the summer North Pole. J. Geophys. Res. Atmos. 2022, 127, e2021JD036059. [Google Scholar] [CrossRef]
- Yun, J.; Evoy, E.; Worthy, S.; Fraser, M.; Veber, D.; Platt, A.; Rawlings, K.; Sharma, S.; Leaitch, W.; Bertram, A. Ice nucleating particles in the Canadian High Arctic during the fall of 2018. Environ. Sci. Atmos. 2022, 2, 279–290. [Google Scholar] [CrossRef]
- Zolles, T.; Burkart, J.; Häusler, T.; Pummer, B.; Hitzenberger, R.; Grothe, H. Identification of Ice Nucleation Active Sites on Feldspar Dust Particles. J. Phys. Chem. A 2015, 119, 2692–2700. [Google Scholar] [CrossRef]
- Klier, K.; Shen, J.H.; Zettlemoyer, A.C. Water on Silica and Silicate Surfaces. I. Partially Hydrophobic Silicas. J. Phys. Chem. 1973, 77, 1458–1465. [Google Scholar] [CrossRef]
- Welti, A.; Lüönd, F.; Stetzer, O.; Lohmann, U. Influence of Particle Size on the Ice Nucleating Ability of Mineral Dusts. Atmos. Chem. Phys. 2009, 9, 6705–6715. [Google Scholar] [CrossRef]
- Connolly, P.J.; Möhler, O.; Field, P.R.; Saathoff, H.; Burgess, R.; Choularton, T.; Gallagher, M. Studies of heterogeneous freezing by three different desert dust samples. Atmos. Chem. Phys. 2009, 9, 2805–2824. [Google Scholar] [CrossRef]
- Petters, M.D.; Parsons, M.T.; Prenni, A.J.; DeMott, P.J.; Kreidenweis, S.M.; Carrico, C.M.; Sullivan, A.P.; McMeeking, G.R.; Levin, E.; Wold, C.E.; et al. Ice nuclei emissions from biomass burning. J. Geophys. Res. Atmos. 2009, 114. [Google Scholar] [CrossRef]
- Möhler, O.; Linke, C.; Saathoff, H.; Schnaiter, M.; Wagner, R.; Schneider, S.; Walter, V.; Ebert, V.; Wagner, S. The effect of organic coating on the heterogeneous ice nucleation efficiency of mineral dust aerosols. Environ. Res. Lett. 2008, 3, 025007. [Google Scholar] [CrossRef]
- Möhler, O.; Büttner, S.; Linke, C.; Schnaiter, M.; Saathoff, H.; Stetzer, O.; Wagner, R.; Krämer, M.; Mangold, A.; Ebert, V.; et al. Effect of sulfuric acid coating on heterogeneous ice nucleation by soot aerosol particles. J. Geophys. Res. 2005, 110, D11210. [Google Scholar] [CrossRef]
- Boose, Y.; Kanji, Z.A.; Kohn, M.; Sierau, B.; Zipori, A.; Crawford, I.; Lloyd, G.; Bukowiecki, N.; Herrmann, E.; Kupiszewski, P.; et al. Ice nucleating particle measurements at 241 K during winter months at three different sites in the Swiss Alps. J. Atmos. Sci. 2016, 73, 2203–2228. [Google Scholar] [CrossRef]
- Mangan, T.P.; Atkinson, J.D.; Neuberg, J.W.; O’Sullivan, D.; Wilson, T.W.; Whale, T.F.; Neve, L.; Umo, N.S.; Malkin, T.L.; Murray, B.J. Heterogeneous ice nucleation by Soufriere Hills volcanic ash immersed in water droplets. PLoS ONE 2017, 12, e0169720. [Google Scholar] [CrossRef] [PubMed]
- Schill, G.P.; Genareau, K.; Tolbert, M.A. Deposition and immersion-mode nucleation of ice by three distinct samples of volcanic ash. Atmos. Chem. Phys. 2015, 15, 7523–7536. [Google Scholar] [CrossRef]
- Maters, E.C.; Dingwell, D.B.; Cimarelli, C.; Müller, D.; Whale, T.F.; Murray, B.J. The importance of crystalline phases in ice nucleation by volcanic ash. Atmos. Chem. Phys. 2019, 19, 5451–5465. [Google Scholar] [CrossRef]
- Klett, J.D.; Pruppbacher, H.R. Microphysics of Clouds and Precipitation; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).