Forced Degradation Study of Atazanavir, Emtricitabine, Nirmatrelvir, Oseltamivir, Ribavirin and Sofosbuvir
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Conducting Forced Degradation Tests
2.3. Monitoring the Conversion of Antivirals in Forced Degradation Tests
3. Results and Discussion
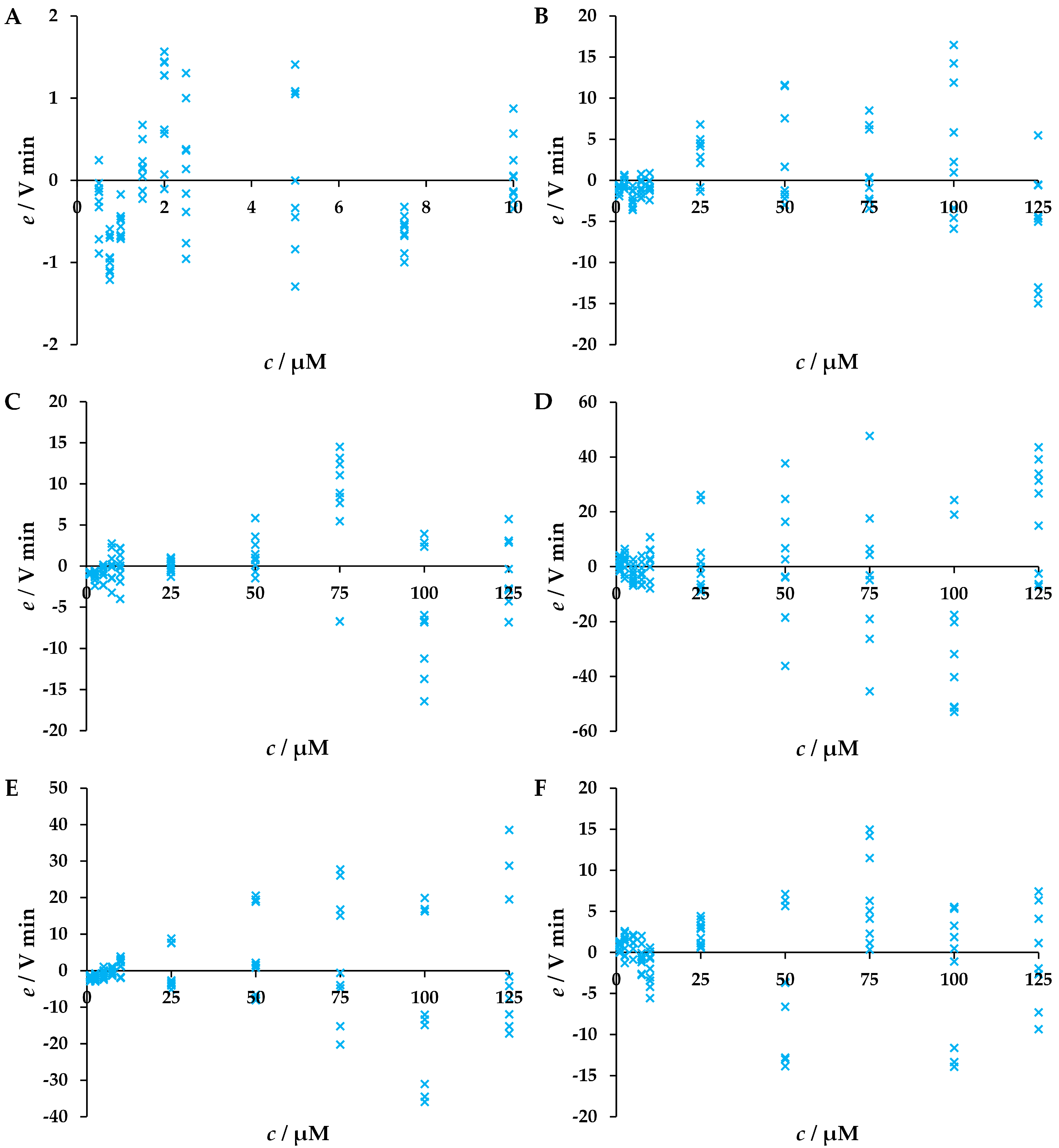
3.1. Validation of Developed LC-UV Methods
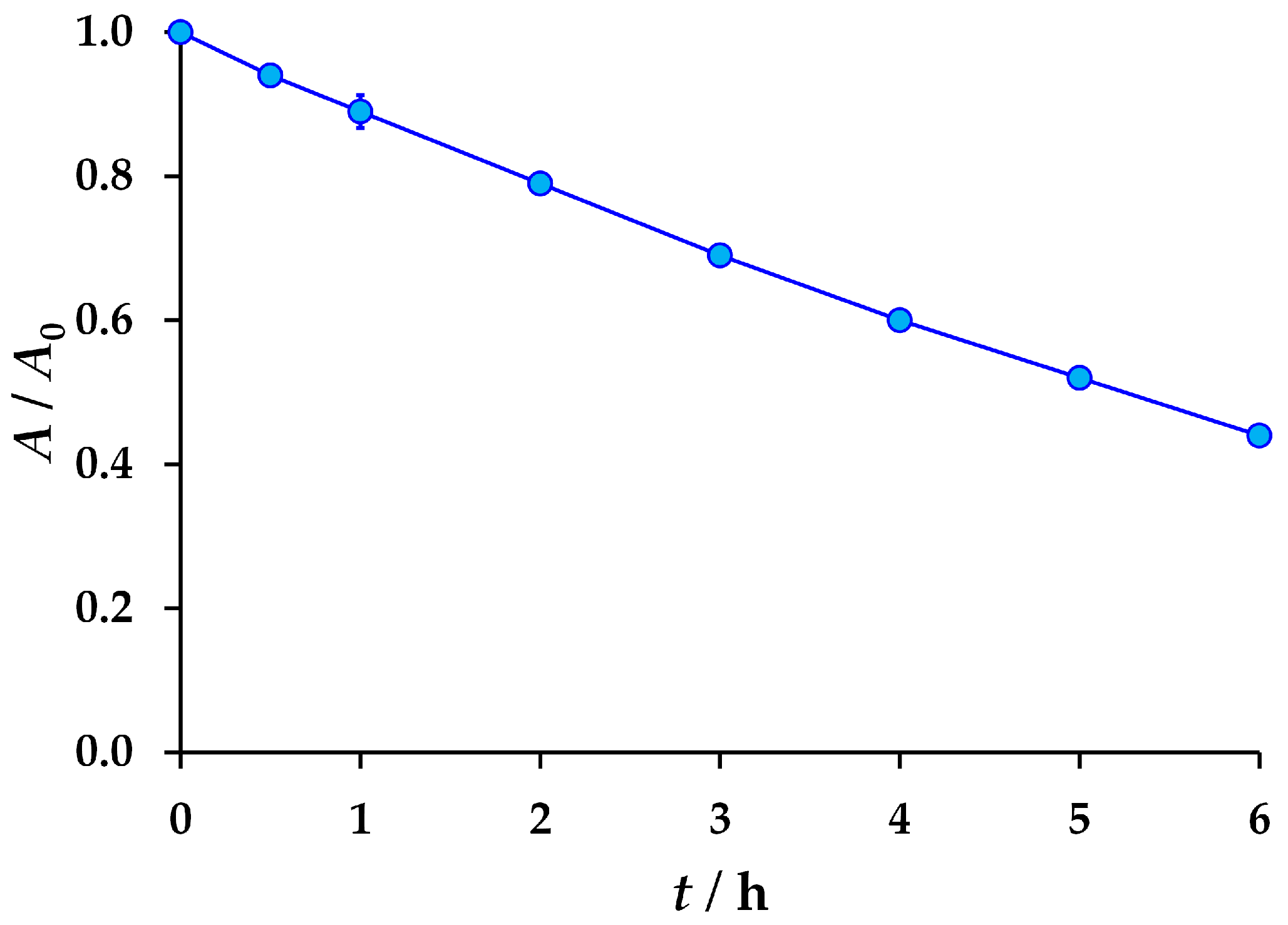
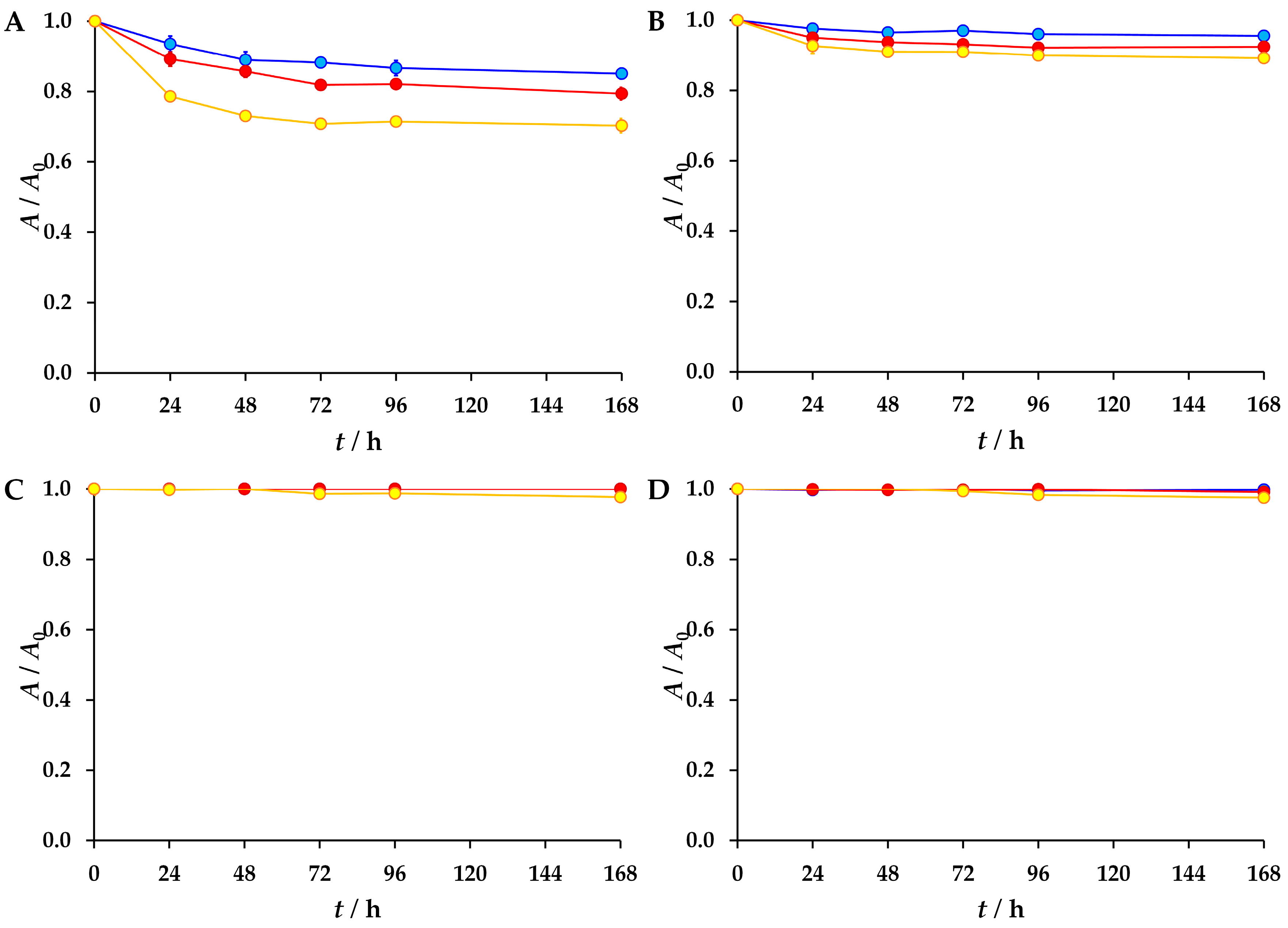
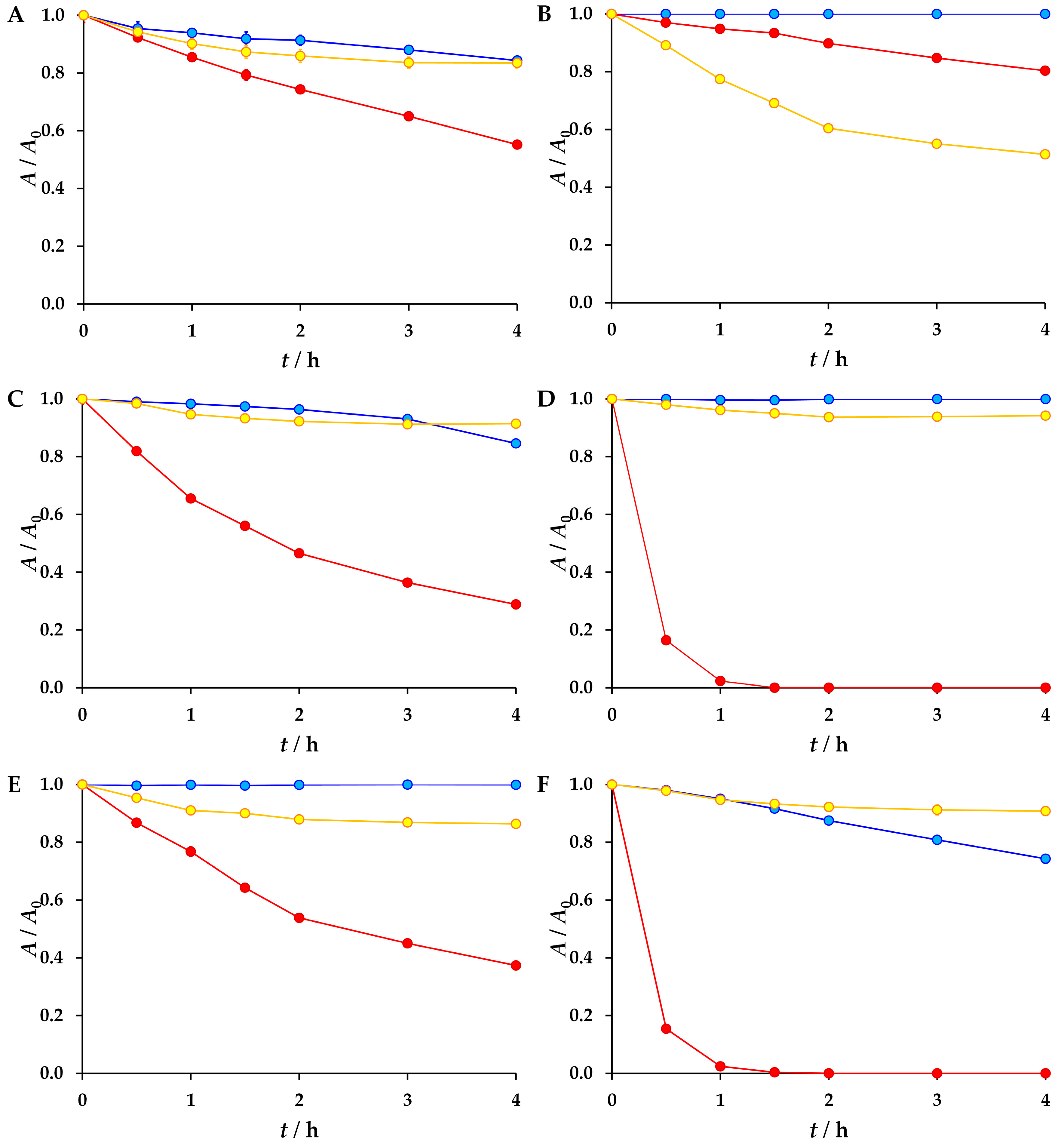
3.2. The Results of the Forced Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roemer, T.; Krysan, D.J. Antifungal Drug Development: Challenges, Unmet Clinical Needs, and New Approaches. Cold Spring Harb. Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef]
- Woods, D.J.; Williams, T.M. The challenges of developing novel antiparasitic drugs. Invert. Neurosci. 2007, 7, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Bryan-Marrugo, O.L.; Ramos-Jiménez, J.; Barrera-Saldaña, H.; Rojas-Martínez, A.; Vidaltamayo, R.; Rivas-Estilla, A.M. History and progress of antiviral drugs: From acyclovir to direct-acting antiviral agents (DAAs) for Hepatitis C. Med. Univ. 2015, 17, 165–174. [Google Scholar] [CrossRef]
- Howes, L. Why are antivirals so hard to develop? Chem. Eng. News 2021, 99. Available online: https://cen.acs.org/pharmaceuticals/drug-discovery/antiviral-drug-development-covid-19/99/i19 (accessed on 12 October 2025).
- Chow, D. Why are Viruses Hard to Kill? Virologists Explain Why These Tiny Parasites are so Tough to Treat. NBC News. Available online: https://www.nbcnews.com/science/science-news/why-are-viruses-hard-kill-virologists-explain-why-these-tiny-n1202046 (accessed on 12 October 2025).
- Race, M.; Ferraro, A.; Galdiero, E.; Guida, M.; Núñez-Delgado, A.; Pirozzi, F.; Siciliano, A.; Fabbricino, M. Current emerging SARS-CoV-2 pandemic: Potential direct/indirect negative impacts of virus persistence and related therapeutic drugs on the aquatic compartments. Environ. Res. 2020, 188, 109808. [Google Scholar] [CrossRef]
- Domínguez-García, P.; Rodríguez, R.R.; Barata, C.; Gómez-Canela, C. Presence and toxicity of drugs used to treat SARS-CoV-2 in Llobregat River, Catalonia. Spain. Environ. Sci. Pollut. Res. 2023, 30, 49487–49497. [Google Scholar] [CrossRef]
- Maremane, S.R.; Belle, G.N.; Oberholster, P.J.; Omotola, E.O. Occurrence of selected Covid-19 drugs in surface water resources: A review of their sources, pathways, receptors, fate, ecotoxicity, and possible interactions with heavy metals in aquatic ecosystems. Environ. Geochem. Health 2025, 47, 3. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010, 36, 15–26. [Google Scholar] [CrossRef]
- Giouni, E.A.; Kyriazi, M.; Malamis, D.; Loizidou, M. Design of an innovative system for the detoxification of pharmaceutical wastewater. Tech. Ann. 2023, 1, 14 pages. [Google Scholar] [CrossRef]
- Moratalla, A.; Cotillas, S.; Lacasa, E.; Cañizares, P.; Rodrigo, M.A.; Sáez, C. Electrochemical technologies to decrease the chemical risk of hospital wastewater and urine. Molecules 2021, 26, 6813. [Google Scholar] [CrossRef]
- Schwarz, S.; Gildemeister, D.; Hein, A.; Schröder, P.; Bachmann, J. Environmental fate and effects assessment of human pharmaceuticals: Lessons learnt from regulatory data. Environ. Sci. Eur. 2021, 33, 68. [Google Scholar] [CrossRef]
- Alidehi-Ravandi, R.; Abdullah, H.O. Topological Indices of Drug Molecular Structures: An Application with the Treatment and Prevention of COVID-19. J. Discrete Math. Appl. 2023, 8, 83–103. [Google Scholar]
- Ramírez-Olivencia, G.; Estébanez, M.; Membrillo, F.J.; del Carmen Ybarra, M. Use of ribavirin in viruses other than hepatitis C. A review of the evidence. Enferm. Infecc. Microbiol. Clin. 2019, 37, 602–608. [Google Scholar] [CrossRef]
- Thomas, E.; Ghany, M.G.; Liang, T.J. The application and mechanism of action of ribavirin in therapy of hepatitis C. Antivir. Chem. Chemother. 2012, 23, 1–12. [Google Scholar] [CrossRef]
- Gupta, Y.K.; Meenu, M.; Mohan, P. The Tamiflu fiasco and lessons learnt. Indian J. Pharmacol. 2015, 47, 11–16. [Google Scholar] [CrossRef]
- Sur, M.; Lopez, M.J.; Patel, P.; Baker, M.B. Oseltamivir. In StatPearls, Internet ed.; StatPearls Publishing: Treasure Island, FL, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539909/ (accessed on 3 April 2025).
- Hussar, D.A. New Drugs of 2003. J. Am. Pharm. Assoc. 2004, 44, 168–210. [Google Scholar] [CrossRef]
- Choi, J.; Horner, K.A.; Carnevale, K. Atazanavir. In StatPearls, Internet ed.; StatPearls Publishing: Treasure Island, FL, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551608/ (accessed on 3 April 2025).
- Muller, J.T.; Al Khalili, Y. Emtricitabine. In StatPearls, Internet ed.; StatPearls Publishing: Treasure Island, FL, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539853/ (accessed on 3 April 2025).
- Yoosefian, M.; Moghani, M.Z.; Juan, A. In silico evaluation of atazanavir as a potential HIV main protease inhibitor and its comparison with new designed analogs. Comput. Biol. Med. 2022, 145, 105523. [Google Scholar] [CrossRef]
- Mattino, A.; Ferrari, D.; Nozza, S.; Muccini, C.; Ripa, M.; Spagnuolo, V.; Castagna, A.; Locatelli, M.; Sabetta, E. Development, validation and clinical implementation of a HPLC-MS/MS method for the simultaneous quantification of bictegravir, emtricitabine, doravirine, cabotegravir, lenacapavir, fostemsavir, tenofovir alafenamide and the corresponding metabolites temsavir and tenofovir, in human plasma. J. Chromatogr. B 2025, 1267, 124803. [Google Scholar] [CrossRef]
- Gentile, I.; Maraolo, A.E.; Buonomo, A.R.; Zappulo, E.; Borgia, G. The discovery of sofosbuvir: A revolution for therapy of chronic hepatitis C. Expert Opin. Drug Discov. 2015, 10, 1363–1377. [Google Scholar] [CrossRef]
- Barenie, R.E.; Avorn, J.; Tessema, F.A.; Kesselheim, A.S. Public funding for transformative drugs: The case of sofosbuvir. Drug Discov. Today 2021, 26, 273–281. [Google Scholar] [CrossRef]
- Lam, C.; Patel, P. Nirmatrelvir-Ritonavir. In StatPearls, Internet ed.; StatPearls Publishing: Treasure Island, FL, USA. Available online: https://www.ncbi.nlm.nih.gov/books/NBK585126/ (accessed on 3 April 2025).
- Hashemian, S.M.R.; Sheida, A.; Taghizadieh, M.; Memar, M.Y.; Hamblin, M.R.; Baghi, H.B.; Nahand, J.S.; Asemi, Z.; Mirzaei, H. Paxlovid (Nirmatrelvir/Ritonavir): A new approach to Covid-19 therapy? Biomed. Pharmacother. 2023, 162, 114367. [Google Scholar] [CrossRef]
- Martinjak, V.; Miloloža, M.; Markić, M.; Furač, L.; Cvetnić, M.; Bolanča, T.; Kučić Grgić, D.; Ukić, Š. Revealing the Adverse Potential of Six SARS-CoV-2 Antivirals by Aliivibrio fischeri Assay: Toxicity Analysis of Single Agent Solutions and Binary Mixtures. Water 2024, 16, 3607. [Google Scholar] [CrossRef]
- Durgule, S.M.; Patil, P.M.; Kore, A.P.; Singh, M.T.; Kadam, S.D. An introduction to forced degradation studies for drug substance and drug products. J. Emerg. Technol. Innov. Res. 2021, 8, 260–269. [Google Scholar]
- Stability Testing of New Drug Substances and Products Q1A(R2). ICH Harmonised Tripartite Guideline, Step 4 Version, 6 February 2003, International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Available online: https://database.ich.org/sites/default/files/Q1A%28R2%29%20Guideline.pdf (accessed on 13 March 2025).
- Stability Testing: Photostability Testing of New Drug Substances and Products Q1B. ICH Harmonised Tripartite Guideline, Step 4 Version, 6 November 1996, International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Available online: https://database.ich.org/sites/default/files/Q1B%20Guideline.pdf (accessed on 13 March 2025).
- Nagasamy Venkatesh, D.; Shanmuga Kumar, S.D. Forced Degradation—A Review. Biomed. J. Sci. Tech. Res. 2022, 47, 38381–38394. [Google Scholar] [CrossRef]
- Keil, K.; Hochreitter, J.; DiFrancesco, R.; Zingman, B.S.; Reichman, R.C.; Fischl, M.A.; Gripshover, B.; Morse, G.D. Integration of atazanavir into an existing liquid chromatography UV method for protease inhibitors: Validation and application. Ther. Drug Monit. 2007, 29, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Rathod, S.; Chopade, J.; Mahaparale, S.; Jadhao, Y.P. Analytical Method Development and Validation for Estimation of Emtricitabine in Tablet Dosage Form by Reverse Phase High Performance Liquid Chromatography. Indian J. Pharm. Sci. 2024, 86, 294–301. [Google Scholar] [CrossRef]
- Imam, M.S.; Batubara, A.S.; Gamal, M.; Abdelazim, A.H.; Almrasy, A.A.; Remzy, S. Adjusted green HPLC determination of nirmatrelvir and ritonavir in the new FDA approved co-packaged pharmaceutical dosage using supported computational calculations. Sci. Rep. 2023, 13, 137. [Google Scholar] [CrossRef]
- Gungor, S.; Bulduk, I.; Aydın, B.S.; Sagkan, R.I. A comparative study of HPLC and UV spectrophotometric methods for oseltamivir quantification in pharmaceutical formulations. Acta Chromatogr. 2022, 34, 258–266. [Google Scholar] [CrossRef]
- Belal, F.; Sharaf El-Din, M.K.; Eid, M.I.; El-Gamal, R.M. Validated stability-indicating liquid chromatographic method for the determination of ribavirin in the presence of its degradation products: Application to degradation kinetics. J. Chromatogr. Sci. 2015, 53, 603–611. [Google Scholar] [CrossRef]
- Srawanthi, P.; Sireesha, D.; Kiran, G.; Akiful Haque, M.; Bakshi, V. Method Development and Validation for Simultaneous Estimation of Sofosbuvir and Velpatasvir by UV-Visible Spectrophotometry. Int. J. Pharm. Sci. Res. 2021, 21, 3969–3974. [Google Scholar] [CrossRef]
- Antonetti, M. Fundamentals of Chemical Hydrolysis and its Application. J. Thermodyn. Catal. 2025, 1, 445. [Google Scholar]
- Chiu, P.-H.; Yang, Y.-L.; Tsao, H.-K.; Sheng, Y.-J. Deep learning for predictions of hy-drolysis rates and conditional molecular design of esters. J. Taiwan Inst. Chem. Eng. 2021, 126, 1–13. [Google Scholar] [CrossRef]

| Conditions | Atazanavir | Emtricitabine | Nirmatrelvir | Oseltamivir | Ribavirin | Sofosbuvir |
|---|---|---|---|---|---|---|
| Mobile phase composition | 40% CH3OH & 60% HCOOH | 5% CH3OH & 95% 20 mM KH2PO4 | 38% ACN 1 & 62% 20 mM KH2PO4 | 55% CH3OH & 45% 20 mM KH2PO4 | 100% 20 mM KH2PO4 | 47% CH3OH & 53% 20 mM KH2PO4 |
| Mobile phase flow | 1 mL min−1 | 1 mL min−1 | 1 mL min−1 | 1 mL min−1 | 1 mL min−1 | 1 mL min−1 |
| Injection volume | 20 µL | 30 µL | 10 µL | 30 µL | 30 µL | 30 µL |
| Owen temperature | 40 °C | 30 °C | 40 °C | 30 °C | 30 °C | 30 °C |
| Detection wavelength | 248 nm | 280 nm | 217 nm | 215 nm | 207 nm | 261 nm |
| Retention time | 15.09 min | 13.48 min | 10.42 min | 10.39 min | 3.55 min | 8.89 min |
| Antiviral | Calibration Range/mM | Intercept | Slope | R2 |
|---|---|---|---|---|
| Atazanavir | 0.0005–0.0020 | −2.3 × 103 | 1.8 × 107 | 0.9976 |
| 0.0020–0.0100 | −4.2 × 102 | 1.7 × 107 | 0.9998 | |
| Emtricitabine | 0.0010–0.0100 | 9.2 × 102 | 1.5 × 107 | 0.9995 |
| 0.0100–0.1250 | 4.8 × 103 | 1.5 × 107 | 0.9999 | |
| Nirmatrelvir | 0.0010–0.0100 | −3.9 × 102 | 4.8 × 106 | 0.9934 |
| 0.0100–0.1250 | 2.6 × 103 | 4.7 × 106 | 0.9990 | |
| Oseltamivir | 0.0010–0.0100 | −5.6 × 102 | 1.9 × 107 | 0.9952 |
| 0.0100–0.1250 | −3.8 × 102 | 1.9 × 107 | 0.9990 | |
| Ribavirin | 0.0010–0.0100 | 81 | 2.1 × 107 | 0.9997 |
| 0.0100–0.1250 | 4.6 × 103 | 2.1 × 107 | 0.9997 | |
| Sofosbuvir | 0.0010–0.0100 | 2.3 × 103 | 1.7 × 107 | 0.9993 |
| 0.0100–0.1250 | 4.8 × 102 | 1.7 × 107 | 0.9999 |
| Antiviral | Calibration Range/mM | Intercept | Slope | R2 | Recovery ± SD 1 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Value | Lower 95% | Upper 95% | Value | Lower 95% | Upper 95% | ||||
| Atazanavir | 0.0005–0.0020 | 4.3 × 10−19 | −1.91 × 10−5 | 1.91 × 10−5 | 1.0000 | 0.9850 | 1.0150 | 0.9976 | 100.34 ± 3.25 |
| 0.0020–0.0100 | 1.7 × 10−18 | −2.70 × 10−5 | 2.70 × 10−5 | 1.0000 | 0.9956 | 1.0044 | 0.9998 | 99.93 ± 0.51 | |
| Emtricitabine | 0.0010–0.0100 | 8.7 × 10−19 | −4.07 × 10−5 | 4.07 × 10−5 | 1.0000 | 0.9934 | 1.0066 | 0.9995 | 100.14 ± 1.32 |
| 0.0100–0.1250 | 5.8 × 10−13 | −2.05 × 10−4 | 2.05 × 10−4 | 1.0000 | 0.9973 | 1.0027 | 0.9999 | 100.13 ± 0.20 | |
| Nirmatrelvir | 0.0010–0.0100 | 1.7 × 10−18 | −0.2 × 10−4 | 0.2 × 10−4 | 1.0000 | 0.9750 | 1.0250 | 0.9934 | 100.70 ± 3.34 |
| 0.0100–0.1250 | −2.8 × 10−17 | −6.59 × 10−4 | 6.59 × 10−4 | 1.0000 | 0.9913 | 1.0087 | 0.9990 | 100.02 ± 1.25 | |
| Oseltamivir | 0.0010–0.0100 | 8.7 × 10−19 | −1.32 × 10−4 | 1.32 × 10−4 | 1.0000 | 0.9786 | 1.0214 | 0.9952 | 101.09 ± 3.48 |
| 0.0100–0.1250 | −1.4 × 10−17 | −6.57 × 10−4 | 6.57 × 10−4 | 1.0000 | 0.9913 | 1.0087 | 0.9990 | 100.28 ± 0.94 | |
| Ribavirin | 0.0010–0.0100 | −1.0 × 10−12 | −3.38 × 10−5 | 3.38 × 10−5 | 1.0000 | 0.9945 | 1.0055 | 0.9997 | 100.12 ± 0.52 |
| 0.0100–0.1250 | 4.2 × 10−17 | −3.88 × 10−4 | 3.88 × 10−4 | 1.0000 | 0.9949 | 1.0051 | 0.9997 | 100.03 ± 0.32 | |
| Sofosbuvir | 0.0010–0.0100 | 1.6 × 10−12 | −4.86 × 10−5 | 4.86 × 10−5 | 1.0000 | 0.9921 | 1.0079 | 0.9993 | 99.48 ± 1.82 |
| 0.0100–0.1250 | −5.5 × 10−17 | −2.00 × 10−4 | 2.00 × 10−4 | 1.0000 | 0.9974 | 1.0026 | 0.9999 | 100.02 ± 0.42 | |
| Antiviral | Calibration Range/mM | Concentration Level/mM | SD/mM | RSD/% |
|---|---|---|---|---|
| Atazanavir | 0.0005–0.0020 | 0.0010 | 0.0000 | 0.00 |
| 0.0020–0.0100 | 0.0050 | 0.0001 | 1.66 | |
| Emtricitabine | 0.0010–0.0100 | 0.0050 | 0.0001 | 1.59 |
| 0.0100–0.1250 | 0.0750 | 0.0003 | 0.40 | |
| Nirmatrelvir | 0.0010–0.0100 | 0.0050 | 0.0002 | 3.93 |
| 0.0100–0.1250 | 0.0750 | 0.0014 | 1.77 | |
| Oseltamivir | 0.0010–0.0100 | 0.0050 | 0.0002 | 3.73 |
| 0.0100–0.1250 | 0.0750 | 0.0014 | 1.88 | |
| Ribavirin | 0.0010–0.0100 | 0.0050 | 0.0001 | 1.20 |
| 0.0100–0.1250 | 0.0750 | 0.0008 | 1.12 | |
| Sofosbuvir | 0.0010–0.0100 | 0.0050 | 0.0001 | 1.40 |
| 0.0100–0.1250 | 0.0750 | 0.0003 | 0.44 |
| Antiviral | Limit of Detection/mM | Limit of Quantification/mM |
|---|---|---|
| Atazanavir | 6.3 × 10−5 | 1.9 × 10−4 |
| Emtricitabine | 9.9 × 10−5 | 3.0 × 10−4 |
| Nirmatrelvir | 8.9 × 10−5 | 2.7 × 10−4 |
| Oseltamivir | 3.4 × 10−4 | 1.0 × 10−3 |
| Ribavirin | 8.3 × 10−5 | 2.5 × 10−4 |
| Sofosbuvir | 9.6 × 10−5 | 2.9 × 10−4 |
| Forced Degradation Treatment | |||||||
|---|---|---|---|---|---|---|---|
| Antiviral | Sunlight (6 h) | Water (168 h) | 1 M HCl (4 h) | 1 M NaOH (4 h) | 30% H2O2 (4 h) | ||
| 25 °C | 30 °C | 40 °C | |||||
| Atazanavir | 15% | 21% | 30% | 16% | 45% | 17% | |
| Emtricitabine | 56% | 20% | 49% | ||||
| Nirmatrelvir | 4% | 8% | 11% | 15% | 72% | 9% | |
| Oseltamivir | 2% | 100% | 6% | ||||
| Ribavirin | 63% | 14% | |||||
| Sofosbuvir | 1% | 2% | 26% | 100% | 6% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinjak, V.; Večenaj, L.; Hofer, R.; Tomić, F.; Lastovčić, D.; Babić Visković, B.; Ašperger, D.; Cvetnić, M.; Kučić Grgić, D.; Bolanča, T.; et al. Forced Degradation Study of Atazanavir, Emtricitabine, Nirmatrelvir, Oseltamivir, Ribavirin and Sofosbuvir. Environments 2025, 12, 417. https://doi.org/10.3390/environments12110417
Martinjak V, Večenaj L, Hofer R, Tomić F, Lastovčić D, Babić Visković B, Ašperger D, Cvetnić M, Kučić Grgić D, Bolanča T, et al. Forced Degradation Study of Atazanavir, Emtricitabine, Nirmatrelvir, Oseltamivir, Ribavirin and Sofosbuvir. Environments. 2025; 12(11):417. https://doi.org/10.3390/environments12110417
Chicago/Turabian StyleMartinjak, Viktorija, Luka Večenaj, Roberta Hofer, Filip Tomić, Dora Lastovčić, Bruna Babić Visković, Danijela Ašperger, Matija Cvetnić, Dajana Kučić Grgić, Tomislav Bolanča, and et al. 2025. "Forced Degradation Study of Atazanavir, Emtricitabine, Nirmatrelvir, Oseltamivir, Ribavirin and Sofosbuvir" Environments 12, no. 11: 417. https://doi.org/10.3390/environments12110417
APA StyleMartinjak, V., Večenaj, L., Hofer, R., Tomić, F., Lastovčić, D., Babić Visković, B., Ašperger, D., Cvetnić, M., Kučić Grgić, D., Bolanča, T., & Ukić, Š. (2025). Forced Degradation Study of Atazanavir, Emtricitabine, Nirmatrelvir, Oseltamivir, Ribavirin and Sofosbuvir. Environments, 12(11), 417. https://doi.org/10.3390/environments12110417









