Abstract
Soil contamination is a major contemporary issue. In light of increasing efforts to align seedling production with the sustainable use and preservation of soil resources, this study aimed to explore the potential of selected plant-growth-promoting bacteria as natural alternatives to mineral fertilizers, a major soil pollutant in the forestry sector. The experiment involved inoculating one-year-old sessile oak (Quercus petraea) seedlings with multiple single bacterial treatments and a consortia derived from sessile oak rhizosphere and monitoring their effects on plant physiological parameters such as chlorophyll, carotenoid, and nitrogen content, along with selected parameters of the rapid chlorophyll a fluorescence induction curve (an OJIP curve). The results indicated that the selected bacterial strains improved specific plant physiological parameters at certain points during the monitoring period; however, further research is necessary to draw statistically significant conclusions. Although these bacteria did not directly enhance photosynthetic parameters, their potential remains evident and could be harnessed through improved application methods. Future studies should focus on identifying site conditions that support the proliferation of the introduced bacterial populations.
1. Introduction
Soil contamination is a major global issue today, mainly caused by human activities [1,2,3,4,5]. Many areas are either already devastated or continually polluted, such as lands used for agriculture, forestry, or horticulture, where large amounts of fertilizers, pesticides, and other chemicals are regularly polluting the soil [6,7,8,9].
In the forestry sector, one of the pollution hotspots is forest nurseries, where there is a need for new technologies, such as sustainable seedling production adapted to modern challenges. Additionally, fertilizers are also used in young plantations to support their initial growth through targeted applications, such as in planting holes, or large–scale surface applications [10]. The loss of genetic diversity, overuse of pesticides, dependence on imported materials (peat, artificial fertilizers, etc.), and high energy consumption are common limitations of modern seedling production [11,12,13,14,15]. Problems are particularly associated with seedling transplantation and the use of sterile substrates that lack microorganisms [16,17]. One common solution to address these issues and improve seedling production is the use of mineral fertilizers. Nitrogen fertilizer use is projected to increase to 249 million metric tons by 2050 [18]. However, applying mineral fertilizers can lead to soil acidification and heavy metal accumulation [19,20,21]. This negatively affects biodiversity, microbial functions, and water quality [22,23,24,25,26,27].
A sustainable approach to seedling production that aims to enhance both physiological and morphological seedling qualities is vital for tackling global production challenges. Examples from agriculture show successful use of plant-growth-promoting bacteria (PGPBs) to boost photosynthesis, pigments, hormones, and secondary metabolites [28,29,30]. PGPBs provide several benefits to plants, such as increased growth and yield, stronger resistance to disease and stress, less reliance on fertilizers, and healthier soil [31,32,33,34]. PGPBs work by helping plants acquire essential elements and nutrients, including P and K absorption, nitrogen fixation, siderophore production, and the modulation of plant hormones like indole-3-acetic acid (IAA), ethylene, and gibberellic acid (GA). It also offers direct or indirect phytoprotection as a biocontrol agent [35].
Chlorophylls and carotenoids are vital photosynthetic pigments that influence a plant’s ability to harvest light, providing essential components for energy production through direct participation in photosynthesis or as accessory pigments that protect leaves from damage and help gather extra light. Therefore, by supporting plant nutrient levels, PGPBs stimulate biosynthetic processes in plants, including pigment formation, boosting their growth potential further. For example, Jabborova [36] reported promotion of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids by Pseudomonas koreensis IGPEB inoculation in ginger plants. There are also studies showing that PGPBs can modify photosynthetic metabolism, performance, and rate [37].
A good example of this aspect is the rapid chlorophyll fluorescence induction curve (an OJIP curve), consisting of four steps: “O” (origin, minimum fluorescence; F0, fluorescence intensity when all reaction centers of photosystem II are opened), J (the first inflection point; the reduction of quinon QA on the acceptor side of photosystem II), I (the second inflection point; reduction of the plastoquinone pool), and “P” (peak, maximum fluorescence; Fmax, fluorescence intensity when all reaction centers of photosystem II are closed). Monitoring it can detect even minor changes that indicate stress from both internal and external plant environments [38], before they appear morphologically. The OJIP transient steps reflect changes in Photosystem II and Photosystem I, as well as the efficiency of the electron transport chain in transferring electrons to the final acceptor [39]. As steps in the electron transport chain are represented by specific parameters measured with a chlorophyll fluorometer, the information on plant physiological state, both disturbances and improvements, can be obtained.
The use of PGPBs in forestry as biofertilizers is limited and still under investigation [40,41]. Application of PGPBs in woody species has shown a significant positive effect on photosynthesis, antioxidant enzyme activity, and phytohormone levels [26,29].
Sessile oak (Quercus petraea (Matt.) Liebl.) is an autochthonous European deciduous tree, recognized for its ecological adaptability and its role in establishing and maintaining ecosystems. It is valuable economically for its versatile uses and high-quality timber. Its importance is expected to grow in future climate scenarios [42,43] for reforestation and afforestation efforts due to its adaptability, drought tolerance [44], and relative resistance to storms [45,46]. As a climate-resilient hardwood species, it is also widely cultivated in forest nurseries [47,48,49].
This study aimed to explore how selected bacterial isolates with previously confirmed plant-growth promoting traits affect the physiological parameters of one-year-old sessile oak seedlings. Our null hypotheses were: i) the selected PGPBs have a positive impact on pigment and nitrogen content in sessile oak seedlings, and ii) PGPBs do not induce stress in sessile oak seedlings, estimated through the following OJIP parameters: minimum fluorescence (F0), maximum fluorescence (Fm), maximum quantum efficiency of PSII photochemistry (Fv/Fm), performance index (Pi Abs), absorption per active reaction center (ABS/RC), trapped energy flux per active reaction center (TR0/RC), electron transport flux per active reaction center (ET0/RC), and dissipation energy per active reaction center (DI0/RC).
2. Materials and Methods
2.1. Sessile Oak Seedling Production
Sessile oak seedlings were grown from acorns, collected manually in autumn of 2020 from sessile oak natural forests in Serbia. Healthy and fully developed acorns, originating from vigorous trees, were selected for seedling production. After transportation to the laboratory, acorns were stratified and kept in a refrigerator at 4 °C.
The acorns were sown in LIECO L15 containers (Lieco, Austria) filled with a mixture of white and black peat (70:30 ratio) (Free Peat BV, Vriezenveen, The Netherlands) in March 2021. The seedlings were kept in the nursery of the Institute of Forestry under half-shade conditions and watered regularly every two days.
2.2. Bacterial Treatment Preparation
The strains used in this experiment were originally isolated from the sessile oak rhizosphere of natural sessile oak forests in the Rudnik mountain (Serbia) and are part of the collection of the University of Belgrade—Faculty of Biology and the Institute of Forestry. Based on the previous partial 16S rDNA characterization and analysis using the National Center for Biotechnology Information (NCBI) server (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi accessed on 14 November 2024.) and GenBank database, the strains are identified as shown in Table 1. The strains were chosen from a bigger collection according to their in vitro proven PGP activity (Table 1). The criteria were that they exhibited at least two traits, did not show any antagonism amongst them, and had a positive effect on the morphological parameters of sessile oak seedlings’ growth in planta, which was previously shown [50,51], or in other plants [52,53,54].

Table 1.
Bacterial isolates identified based on 16S rDNA and exhibited PGP traits.
Individual bacterial isolates and three consortia were used to treat one-year-old sessile oak seedlings. For inoculum preparation, each bacterial strain was grown in Luria-Bertani (LB) broth (tryptone 10 g/L, yeast extract 5 g/L, NaCl 5 g/L) at 30 °C for 24 h, then centrifuged at 4000 rpm for 20 min. The cell pellets were dissolved in 0.01 M MgSO4 to reach a final concentration of 108 CFU/mL. Equal proportions of the appropriate bacterial strains were combined to prepare the consortia.
2.3. Sessile Oak Seedling Inoculation
In the last week of May, twenty seedlings per treatment were randomly selected and separated into eight experimental groups, as follows: five treatments (T1—Viridibacillus sp. R3.17; T2—Pseudomonas sp. R4.2.1P; T3—Pseudomonas sp. R4.29P; T4—Lysinibacillus sp. R1.4; T5—Pseudomonas sp. R4.45P) and three consortia (C2—T4 + T5; C3—T1 + T2 + T3; and C5—T1 + T2 + T3 + T4 + T5). Consortia C2 and C3 were formed on the basis of similar growth rates of the bacterial strains that constitute them. The soil of each seedling was inoculated with a sterile syringe containing 10 mL of inoculum (concentration 108 CFU/mL). A 10 mL volume of tap water was used for the control group.
2.4. Measurements of Photosynthetic Parameters
Leaf nitrogen, chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid content were determined to assess the nitrogen status and photosynthetic pigment concentrations, which are indicative of the overall plant’s health. The non-destructive SPAD-502 chlorophyll meter (Konica Minolta, Tokyo, Japan) was used to determine the relative leaf chlorophyll content, by measuring the difference in transmission of light through leaves at two wavelengths, red (650 nm) and infrared (940 nm). The obtained SPAD values were converted to relative concentrations of nitrogen, chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids based on established standard curves (Supplementary Material, Figures S1–S5), created through pigment extraction and spectrophotometry according to Bielinis et al. [55], and equations following Wellburn [56]. In short, leaf tissue was collected using a circular punch and pigments were extracted with dimethylsulfoxide (DMSO), followed by incubation in a water bath at 65 °C for one hour in the dark. Spectrophotometric measurements were performed, and final concentrations were calculated using equations. Additionally, the ratio of chlorophyll a to chlorophyll b and the total chlorophyll to carotenoid ratio were also determined. One leaf per seedling was chosen for measurement, and SPAD readings were taken four times during the growing season—June, August, September, and October. Measurements were performed in the midzone of the leaf, on the right side of the mid-vein. All measurements were taken at the same time in the morning hours, on twenty plants per treatment.
Chlorophyll fluorescence induction kinetics (the OJIP test) were measured using the PAR-FluorPen FP 110/D (Photon Systems Instruments, Brno, Czech Republic). Before each measurement, the leaves were dark-adapted for 30 min with clips to fully relax Photosystem II (PSII) reaction centers and achieve the maximum photochemical efficiency of the sampled leaves. The OJIP measurements were conducted in the morning on ten pre-selected plants per treatment (one leaf per seedling) during July, August, and October, targeting the middle region of the leaf and avoiding major veins. Among various biophysical parameters derived from the OJIP data, the following eight were selected for analysis: minimum fluorescence (F0), maximum fluorescence (Fm), maximum quantum efficiency of PSII photochemistry (Fv/Fm), performance index (Pi Abs), absorption per active reaction center (ABS/RC), electron transport flux per active reaction center (ET0/RC), trapped energy flux per active reaction center (TR0/RC), and dissipation energy per active reaction center (DI0/RC).
Both SPAD and Fluor Pen measurements were taken on intact leaves in situ during growth.
2.5. Statistical Analyses
The obtained data were tested for normal distribution using the Shapiro–Wilk test. Levene’s test was employed to assess the homogeneity of variance. To identify significant differences between treatments, a one-way ANOVA was conducted, followed by the Tukey HSD test, or the Games–Howell test if the homogeneity of variance assumption was not met. The level of statistical significance was set at p < 0.05.
All statistical analyses were conducted using the IBM SPSS Statistics for Windows, version 26 software package (IBM Corp., Armonk, NY, USA) and Microsoft Office Excel 2016 (Microsoft Corporation, Redwoods, WA, USA).
3. Results
Leaf chlorophyll, carotenoid, and nitrogen concentrations, measured four times during the growing season, are shown in Figure 1. Pigments generally exhibited a pattern of increase followed by a decrease over time. The timing of peak concentrations varied depending on the specific treatment and SPAD—derived parameters. For some treatments, peak concentrations of chlorophyll a were observed as early as June (Figure 1a), while for others, the highest concentrations occurred in August (Figure 1a). A similar trend was seen for nitrogen content (Figure 1e). In June (Figure 1a–e; Supplementary Material, Figure S6), seedlings treated with treatment T5 had the highest mean concentrations of chlorophyll a (2.62 mg/g), chlorophyll b (1.03 mg/g), total chlorophyll (3.75 mg/g), carotenoids (0.90 mg/g), and nitrogen (24.03 g/kg). Furthermore, these were the highest mean values recorded during the growing season. In contrast, the lowest mean values were found for treatment T1 for chlorophyll a (1.99 mg/g), chlorophyll b (0.77 mg/g), and total chlorophyll (2.93 mg/g), while for treatment T2, for carotenoids (0.75 mg/g) and nitrogen (20.97 g/kg). No statistically significant differences between the treatments were observed in June, according to ANOVA.
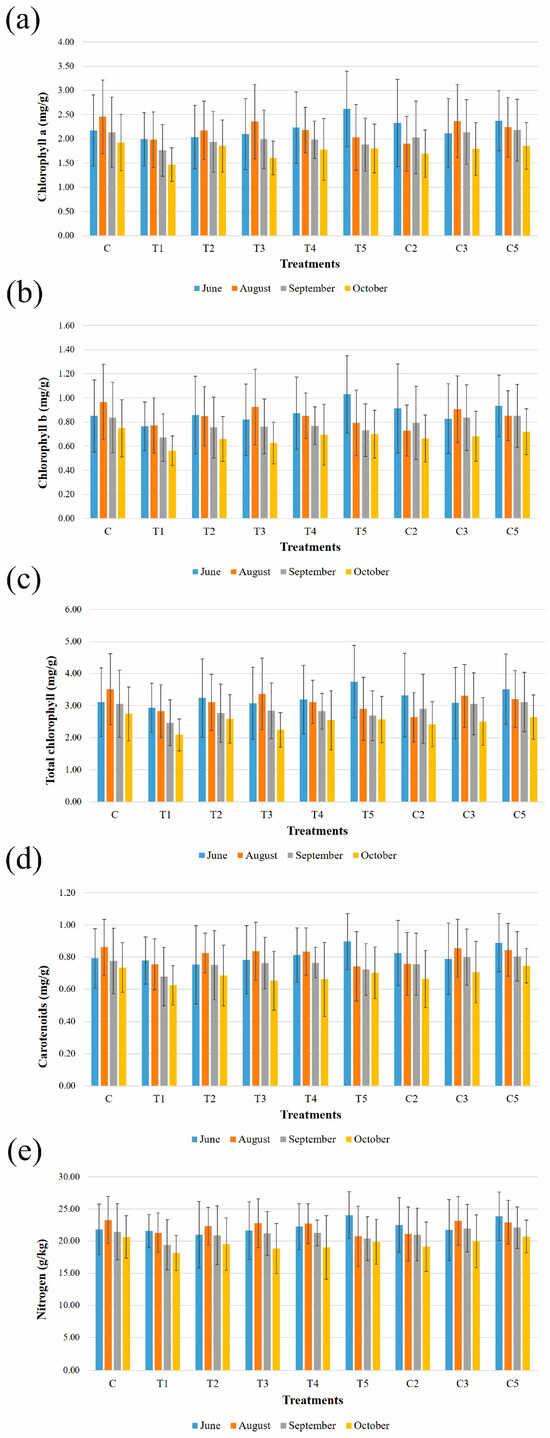
Figure 1.
Chlorophyll a, chlorophyll b, total chlorophyll, carotenoids, and nitrogen content of one-year-old sessile oak seedlings inoculated with different bacterial treatments. Mean values with the standard deviations of the measured parameters ((a) chlorophyll a content; (b) chlorophyll b content; (c) total chlorophyll content; (d) carotenoids content; (e) nitrogen content) for each treatment (C—untreated control; T1—Viridibacillus sp. R3.17; T2—Pseudomonas sp. R4.2.1P; T3—Pseudomonas sp. R4.29P; T4—Lysinibacillus sp. R1.4; T5—Pseudomonas sp. R4.45P; C2—T4 + T5; C3—T1 + T2 + T3; and C5—T1 + T2 + T3 + T4 + T5) and month (June, August, September, and October) are presented.
In August (Figure 1a–e; Supplementary Material, Figure S7), the control treatment, i.e., seedlings treated with tap water only, contained the highest mean concentrations of chlorophyll a (2.45 mg/g), chlorophyll b (0.97 mg/g), total chlorophyll (3.51 mg/g), and carotenoids (0.86 mg/g), as well as nitrogen content (23.28 g/kg). The lowest mean values of chlorophyll a, chlorophyll b, and total chlorophyll were recorded for treatment group C2 (1.90 mg/g, 0.73 mg/g, and 2.64 mg/g, respectively), while minimal carotenoid and nitrogen values were recorded for treatment T5 (0.74 mg/g and 20.75 g/kg, respectively). ANOVA followed by Tukey’s HSD test revealed statistically significant differences between the control treatment and T1 (F = 2.66; p < 0.024), and control and treatment C2 (p < 0.043) for chlorophyll a content. Differences in chlorophyll b content between the control treatment and T1 (F = 2.76; p < 0.017) and C2 (p < 0.033) were statistically significant as well, according to the ANOVA and Tukey’s HSD test. In addition, total chlorophyll content differences between the control treatment and T1 (F = 2.75; p < 0.020) and control treatment and C2 (p < 0.028) were observed by the same statistical tests.
In September (Figure 1a–e; Supplementary Material, Figure S8), the highest mean values of chlorophyll a, chlorophyll b, total chlorophyll, and nitrogen content were reported for the C5 treatment (2.18 mg/g, 0.85 mg/g, 3.11 mg/g, 22.07 mg/g, respectively). The highest carotenoid content was detected for both the C3 and C5 treatments (0.80 mg/g). Treatment T1 had the lowest mean values for each of the measured parameters (chlorophyll a, 1.76 mg/g; chlorophyll b, 0.67 mg/g; total chlorophyll, 2.47 mg/g; carotenoids, 0.68 mg/g; and nitrogen content, 19.42 g/kg). No statistically significant differences between the treatments were recorded.
The lowest mean values of each parameter measured by SPAD were reported in October (Figure 1a–e; Supplementary Material, Figure S9). The control group had the highest mean values of chlorophyll a (1.92 mg/g), chlorophyll b (0.75 mg/g), and total chlorophyll (2.74 mg/g). However, the highest mean values of carotenoids and nitrogen were noted for treatment C5. On the other hand, the lowest mean values of chlorophyll b, total chlorophyll, carotenoids, and nitrogen were reported for T1 (0.56 mg/g, 2.09 mg/g, 0.62 mg/g, 18.13 g/kg, respectively) and of chlorophyll a for T3 (1.60 mg/g). A statistically significant difference was observed in chlorophyll a content between the control treatment and T1 (F = 3.39; p < 0.001), T1 and T2 (p < 0.003), and T1 and treatment C3 (p < 0.037), according to ANOVA and the Games–Howell post hoc test. The same tests revealed statistically significant differences in chlorophyll b content between the control treatment and T1 (F = 3.121; p < 0.00). Regarding total chlorophyll content, ANOVA combined with the Games–Howell post hoc test detected statistically significant differences between the control treatment and T1 (F = 3.396; p< 0.001), control and T3 (p < 0.032), and treatments T1 and T2 (p < 0.010).
The chlorophyll a to chlorophyll b ratio remained fairly consistent throughout the growing season (Figure 2). In June, the highest ratio was found for treatment T1 (2.67), while the lowest was for T2 (2.52). By August, during mid-season, the ratio ranged from 2.62 (treatment C5) to 2.55 (control treatment). The lowest ratio in September was also for the control treatment (2.56), whereas the highest ratio was 2.58, observed for treatments T1, T3, T4, and T5. After the ratio stabilized in September, changes appeared in October, with a range from 2.55 (C2) to 2.77 (T2).
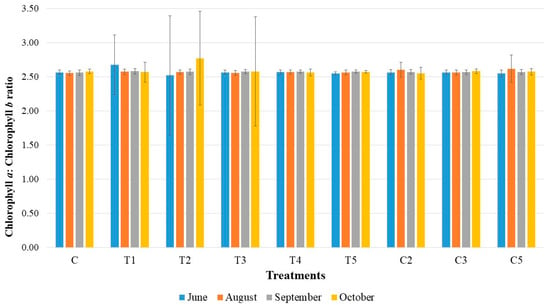
Figure 2.
Ratio of chlorophyll a to chlorophyll b with the standard deviations in the tested bacterial treatments on one-year-old sessile oak seedlings during a growing season (June, August, September, and October).
Total chlorophyll to carotenoid content for each treatment throughout the growing season is shown in Figure 3. The ratio values generally decreased toward October. The most significant drop from June to October was observed for treatment T2 (13.32%), while the smallest decline occurred in treatment C2 (6.14%). Interestingly, in treatment T4, the highest total chlorophyll to carotenoid ratio was recorded in October (3.96). The control treatment had its peak ratio in August, with an 8.27% decline observed in October.
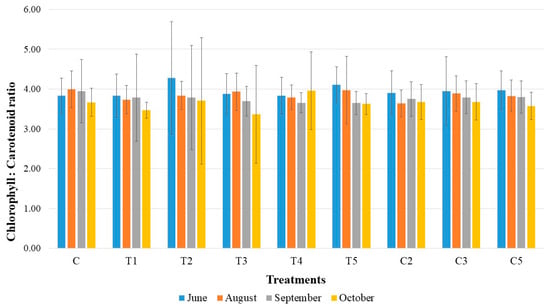
Figure 3.
Ratio of total chlorophyll to carotenoid with the standard deviations in the tested bacterial treatments on one-year-old sessile oak seedlings during a growing season (June, August, September, and October).
The chlorophyll a fluorescence OJIP transient measurements are presented in Figure 4.
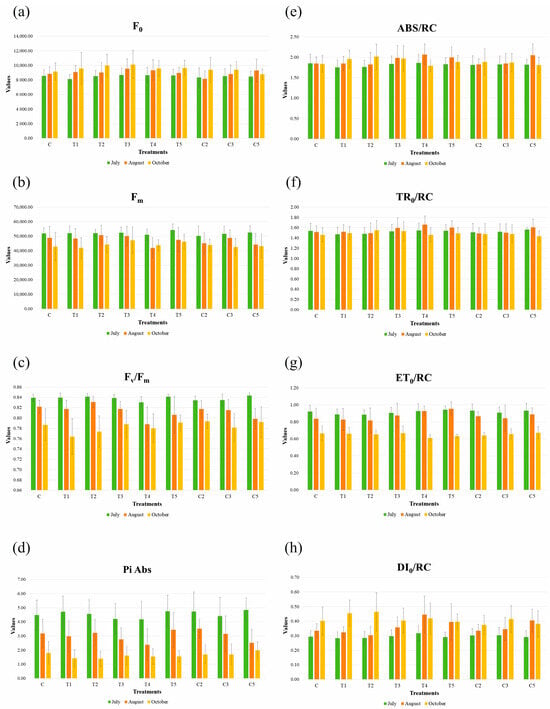
Figure 4.
Selected OJIP biophysical parameters of one-year-old sessile oak seedlings inoculated with different bacterial treatments. Mean values with the standard deviations of the measured parameters ((a) F0—minimum fluorescence; (b) Fm—maximum fluorescence; (c) Fv/Fm—maximum quantum efficiency of PSII photochemistry; (d) Pi Abs—performance index; (e) ABS/RC—absorption per active reaction center; (f) TR0/RC—trapped energy flux per active reaction center; (g) ET0/RC—electron transport flux per active reaction center; and (h) DI0/RC—dissipation energy per active reaction center) for each treatment (C—untreated control; T1—Viridibacillus sp. R3.17; T2—Pseudomonas sp. R4.2.1P; T3—Pseudomonas sp. R4.29P; T4—Lysinibacillus sp. R1.4; T5—Pseudomonas sp. R4.45P; C2—T4 + T5; C3—T1 + T2 + T3; and C5—T1 + T2 + T3 + T4 + T5) and month (July, August, and October) are presented.
Minimum fluorescence (F0) increased overall towards October (Figure 4a). The lowest value was recorded in July for T1 (8140.59), while the highest was for T3 (8694.83). In August, the value for the C2 treatment unexpectedly decreased to 8182.50. By October, minimal fluorescence increased for all treatments, except C5, which slightly decreased by 5.6% compared to the previous measurement. The highest value was observed for T3 (10,108.28).
Maximum fluorescence (Fm) decreased during the growing season (Figure 4b). The highest value was observed in July for treatment T5 (54,322). Interestingly, in August, Fm dropped in treatment T4 to its lowest observed value (41,929.10). In October, the highest Fm value was recorded in the T3 treatment (47,216.36), while the lowest was found in the T1 treatment (41,864.17).
Maximum quantum efficiency of PSII (Fv/Fm) expressed a declining trend during the growing season (Figure 4c), with maximal values in July, and the lowest values in October. While in July the values were almost without difference between treatments (0.83–0.84), in August the lowest value was detected in treatment T4 (0.79) and the highest in T2 (0.83), which was close to the control treatment value (0.82). October values were slightly lower, ranging from 0.76 (T1 treatment) to 0.79 (treatments C, T3, T5, C2, C5).
Performance index (Pi Abs) also decreased towards the growing season ending (Figure 4d). The highest value was noted in July for treatment T5 (4.85), while the lowest value in that month was for treatment T4 (4.17). For the same treatment, the lowest value was detected in August (2.38), while the highest value was for C2 (3.51). In October, the highest value was reported again for treatment T5 (1.99) and the lowest for T2 (1.39).
Absorption per active reaction center (ABS/RC) remained very stable across all three months in the control treatment (Figure 4e), while fluctuations were observed for other treatments. In July, values ranged from 1.76 (T1 treatment) to 1.86 (T4). In August, a rise was recorded, with the highest value for T4 (2.07) and the lowest for both T2 and C2 (1.82). In October, absorbed energy per reaction center ranged from 1.79 (T4) to 2.01 (T2).
Trapped energy flux per active reaction center (TR0/RC) (Figure 4f) varied across treatments and months. In July, treatment T5 was the most effective (1.56), while T1 had the lowest value (1.47). In August, however, T4 treatment was the most efficient (1.66), and T2 and C2 recorded the lowest values (1.49). A decline from July was observed for the control, C2, and C3. In October, T5 treatment had the lowest trapped energy per reaction center (1.43), while T2 was the most efficient (1.55).
The electron transport flux per active reaction center (ET0/RC) (Figure 4g) decreased as October approached, except for treatments T4 and T5, where August values were slightly higher than those in July. July values ranged from 0.89 (T1, T2) to 0.94 (T5). In August, the lowest value recorded was 0.82 (T2), while T5 had the highest at 0.95. By October, a further decline occurred, with the lowest value for T4 (0.61) and the highest for treatments C, T3, and T5 (0.67).
Dissipation energy per active reaction center (DI0/RC) (Figure 4h) represents the non-photochemical energy loss per reaction center, i.e., the energy lost through fluorescence or heat dissipation. The parameter fluctuated with leaf temporal dynamics, meaning the values increased toward the end of the growing season. In July, the lowest value was recorded for T1 (0.28), while the highest was for treatment T4 (0.32). August values ranged from 0.30 (T2) to 0.45 (T4). In October, the most efficient treatment was C2 (0.37), while the highest non-photochemical energy loss was reported for treatment T2 (0.46).
Statistically significant differences were not observed for any of these parameters at any time point.
4. Discussion
Being the central component of the photosynthesis process, chlorophyll content is consequently an indicator of a leaf’s photosynthetic capacity, which varies over time depending on seasonal changes, leaf ontogeny, and phenology. In our study, all pigments and nitrogen content generally followed a bell-shaped temporal pattern, reaching their peak values in June or August, followed by an autumn decline, consistent with the leaf’s annual cycle. This pattern is also supported by the general trend observed in the temporal dynamics of OJIP parameters.
Leaf pigment concentration varies within plant species, season, leaf age, and environmental conditions, especially light exposure. The highest pigment and nitrogen concentrations were observed in June and August, following leaf development and expansion of leaf area. As a result, a dilution effect may occur, leading to a temporary decrease in measured leaf traits. The lowest values recorded in October indicate the start of the leaf senescence process and pigment degradation.
Leaf pigment concentration is also influenced by leaf structure, such as whether it is heliomorphic (sun leaves) or sciomorphic (shade leaves). Additionally, young trees, like our one-year-old sessile oak seedlings, only produce shade leaves, which are characterized by less developed palisade tissue and higher relative chlorophyll content compared to sun leaves [57]. A higher chlorophyll content, especially chlorophyll b, indicates an adaptation to lower light availability and the plant’s need to maximize photosynthesis in limited sunlight conditions.
In this research, the idea was to check how the treatment with a bacterial preparation, for whose individual strains have been shown to have a positive effect on the morphological parameters of the growth of sessile oak seedlings [50,51] and other plants [52,53,54], affects the physiological parameters of growth of one-year-old sessile oak seedlings.
In the majority of plants, the ratio of chlorophyll a to chlorophyll b is usually 3:1 [58], whereas in shaded conditions, the ratio slightly drops [59]. In our study, chlorophyll a to chlorophyll b ratios support the outcome of shaded growth conditions and remained stable during the growing season for most treatments, with slight increases and decreases (Figure 2). Regardless of bacterial treatment, a slow decreasing trend was observed at the end of the season, from August or September to October, indicating subtly increased chlorophyll a degradation and the onset of senescence [60]. This is supported by the total chlorophyll to carotenoid ratio. The total chlorophyll to carotenoid ratio (Figure 3) declined toward the end of the growing season, indicating faster degradation of chlorophylls compared to carotenoids [61]. Interestingly, for treatment T4, the ratio increased in October to reach its maximum value during the growing season, since this treatment experienced a slower but consistent decrease in total chlorophyll from June to October, followed by a more dynamic change in carotenoid content.
Regarding the bacterial treatment effect on seedlings’ pigment and nitrogen content, the data are fairly consistent within each month (Supplementary Material, Figures S6–S9), with few statistically significant differences.
Seedlings in treatment T5 had an exceptional performance in June. The bacteria Pseudomonas koreensis has enhanced chlorophyll content and growth in various plant species [59,60,61,62,63,64,65]. Many of the studies were conducted in a stressful environment [62,63,64,65,66,67,68]. P. koreensis also demonstrated biocontrol capabilities [69]. In our study, the strain R4.45 (treatment T5) enhanced chlorophyll a, chlorophyll b, total chlorophyll, carotenoid, and nitrogen contents in sessile oak seedlings in June, although the rise was not statistically significant. These values were maximal up to the end of the growing season. From June, a decline was noted, and interestingly, in August, carotenoid and nitrogen contents were the lowest when compared to other treatments. The fluorescence data confirm stress conditions, likely caused by increased light absorption (ABS/RC and TR0/RC had higher values than in the control treatment; Figure 4e,f), which triggered higher electron transport (higher ET0/RC; Figure 4g). However, this was not followed by efficient downstream energy flow, leading to increased energy dissipation (DI0/RC; Figure 4h), along with a reduced Fv/Fm (Figure 4c) and performance index (Figure 4d).
Similar to Pseudomonas sp. R4.45P in treatment T5, seedlings treated with strain Pseudomonas sp. R4.2.1P in treatment T2 showed the highest levels of chlorophyll b and total chlorophyll in June. In contrast, chlorophyll a, carotenoids, and nitrogen content decreased in August. Chlorophyll fluorescence data confirmed higher pigment content, with increased F0 and Fm compared to the control treatment (Figure 4a,b), leading to a higher number of active reaction centers and, consequently, a lower photochemical load per reaction center (lower values of ABS/RC, TR0/RC, and ET0/RC than the control; Figure 4e–g). This indicates better energy distribution across reaction centers and overall improved photosynthetic performance (higher Fv/Fm and Pi Abs, and lower DI0/RC than the control; Figure 4c,d,h). The increase could be due to a higher light intensity, which reduced chlorophyll b as it was less needed for light gathering or converted it to chlorophyll a through chlorophyll b reductase, resulting in increased chlorophyll a and carotenoid content as a defense mechanism, i.e., photoprotection. As a result, the highest total chlorophyll to carotenoid ratio was observed. The rise in nitrogen content was a prerequisite for the increase in chlorophyll, as nitrogen is its direct building block. In October, a stress signature was noted, with Pi Abs and Fv/Fm lower than in the control, and DI0/RC higher (Figure 4d,c,h). There was energy overload in reaction centers, with higher ABS/RC and TR0/RC compared to the control (Figure 4e,f), and inefficient electron transport, with lower ET0/RC (Figure 4g), supported by the decrease in pigment and nitrogen content observed in October.
In treatment T4, seedlings were inoculated with Lysinibacillus sp. R1.4. Chlorophyll a and b, as well as their total content, were at maximal levels in June; they were higher than the control treatment, but not statistically significant. After June, the pigment levels gradually declined. By contrast, carotenoid and nitrogen content were highest in August, which can be explained by light stress and the need for photoprotection. The data from chlorophyll fluorescence are consistent with the aforementioned. In July, many inactive reaction centers were present, enhancing the minimum fluorescence (Figure 4a) but lowering maximum fluorescence (Figure 4b). The higher values of ABS/RC, TR0/RC, and ET0/RC, (Figure 4e–g, respectively) compared to the control treatment indicate more energy absorption and higher pressure on the electron-transport chain resulted in higher energy dissipation (DI0/RC; Figure 4h), which consequently lead to lower Fv/Fm (Figure 4c) and Pi Abs (Figure 4d) than in control treatment. This trend persisted in August, in addition to lower contents of chlorophyll pigments. In October, compared to August, less energy was absorbed per reaction center, but still, photosynthetic productivity decreased.
Treatment T1 was not successful in enhancing pigment or nitrogen content overall. In fact, seedlings treated with Viridibacillus sp. R3.17 demonstrated the minimal mean values for all SPAD measured parameters in September, October, and June, with the exception of carotenoid and nitrogen content, which were the second lowest content. However, the minimal values were statistically significant only in August and October for chlorophyll a, chlorophyll b, and total chlorophyll content, when compared to the control treatment. Viridibacillus arvi is a soil bacterium, reported by Jovanović et al. [50] to improve the height and root collar diameter of sessile oak seedlings. Ramakrishna et al. [68] emphasized that PGPBs are more efficient when used in less fertile, marginal soils. This is best illustrated by PGPBs used in bioremediation and recultivation of soil in post-mining areas [40,70]. V. arvi was also found in heavy metal-contaminated soil, in a uranium mining waste pile [71]. July fluorescence data indicate optimized photosynthetic performance, although more energy could have been loaded as ABS/RC, TR0/RC, ET0/RC, and DI0/RC were lower than in the control (Figure 4e–h, respectively). In contrast, data from the August measurements indicated problems in reactive centers (F0 value higher than in control treatment, Fm higher values compared to control; Figure 4a,b) and electron transport efficiency (ET0/RC lower value than in control treatment; Figure 4g), and consequently a decline in performance (Pi Abs lower values; Figure 4d). In October, the damage process continued, leading to higher absorption per active reaction center (Figure 4e), lower maximum quantum efficiency of PSII (Figure 4c), and higher levels of dissipated energy (Figure 4h). The chlorophyll to carotenoid ratio (Figure 3) indicates higher chlorophyll degradation, and the chlorophyll a to chlorophyll b ratio (Figure 2) suggests a problem with reaction centers, as they are dependent on chlorophyll a.
In treatment T3, seedlings were inoculated with Pseudomonas sp. R4.29P. While chlorophyll content was slightly lower compared to the control treatment in June, by the end of the season, this performance gap widened. The chlorophyll levels for this treatment, both individual and total, were narrow, reaching peak values in August, followed by an initial decline in September, and then another significant drop in October. In fact, in October, treatment T3 was the second lowest among all tested treatments. Carotenoid and nitrogen dynamics also showed a bell-shaped temporal pattern, with smaller fluctuations during June, August, and September, while a more pronounced decline was observed in October. The results suggest there was a need for photoprotection during the more light-intensive part of the growing season, since carotenoid content slightly fluctuated until October, when a decrease in all pigments was noted, indicating the possible onset of senescence-related processes. Our findings differ from the earlier work of Samaddar et al. [52], where inoculation of red pepper significantly increased chlorophyll levels during salinity stress. Chlorophyll a fluorescence data show mild stress in July (F0 and DI0/RC increased, while Pi Abs, ABS/RC, TR0/RC, ET0/RC decreased; Figure 4a,d–h, respectively). In August, despite the enhancement of chlorophyll content (Figure 1c), the performance index was lower compared to the control (Figure 4d), indicating less active reaction centers available and higher energy dissipation per active reaction center (Figure 4h). In October, seedlings were still below optimal photosynthetic performance (Pi Abs lower than control; Figure 4d); however, the photosynthetic system was capable of functioning without stress.
In treatment C2, two bacterial strains were combined: Lysinibacillus sp. R1.4 and Pseudomonas sp. R4.45P. Both bacteria were successful in June as individual treatments, enhancing chlorophyll content. C2 treatment also positively affected and increased levels of chlorophyll a, chlorophyll b, total chlorophyll, carotenoids, and nitrogen. The values measured were higher than in the control treatment but not statistically significant. Additionally, seedlings in treatment C2 performed better than those in treatment T4, where Lysinibacillus sp. R1.4 was applied alone. Conversely, seedlings from treatment group T5 achieved higher levels of the aforementioned parameters. In August, a decline in all parameters was observed, particularly in chlorophyll a, chlorophyll b, and total chlorophyll, which reached their lowest levels in that month. According to PAR Fluor Pen data, in July, probably due to June’s higher chlorophyll content (Figure 1c) that was still affecting the seedlings, there were more reaction centers that decreased the absorption rate per reaction center; however, photosynthetic electron transport was enhanced (increase in ET0/RC). There was a need for photoprotection through energy dissipation (higher DI0/RC). The balance was established in August, since energy dissipation equaled the control treatment (Figure 4h). However, in October, with the decline in pigment content (Figure 1c), there was a reduction in the number of reaction centers. The present ones were overloaded (ABS/RC, TR0/RC higher than in the control treatment), and the electron transport chain (ET0/RC lower than the control treatment) was not efficient.
A combination of three bacterial strains, Viridibacillus sp. R3.17, Pseudomonas sp. R4.2.1P, and Pseudomonas sp. R4.29P, was used for treatment C3. In June and August, slightly smaller values were mainly observed in SPAD-related parameters compared to the control treatment, while in September, only carotenoid and nitrogen contents were slightly higher than in the control group. A further drop in SPAD-measured values characterized October. When comparing the success of each treatment individually and in combination, C3 values were generally better than those of individual treatments; however, the observed values were very close to those in treatment T3. Nevertheless, there were instances where T3 alone outperformed the consortia, and cases where T2 treatment proved more successful than both C3 and T3. This could imply that Pseudomonas sp. R4.29P outnumbered the other two bacterial strains in the consortia, resulting in an effect most similar to the T3 treatment alone. The chlorophyll a fluorescence data indicate stress conditions in July, potentially from higher irradiation, since it was followed by carotenoid content enhancement in August. In addition, the system improved, although the performance index and energy dissipation per reaction center still indicated imbalance. In October, a decrease in pigment content was recorded, and consequently, a new destabilization of the photosynthetic system occurred, which could have been related to the upcoming senescence process.
C5 combined all five bacterial strains, and its performance in June regarding SPAD-related parameters was the second best, after the T5 treatment. This is supported by chlorophyll a fluorescence data, with more active reactive centers (ABS/RC lower than in control), better energy usage (higher TR0/RC, ET0/RC), and no additional dissipation (DI0/RC equal to the control treatment). In August, however, there was a decline, and C5’s performance was lower than that of the control treatment and similar to T2 and T4 (chlorophyll a, chlorophyll b, carotenoids, and nitrogen content). The chlorophyll a fluorescence data confirm the stress condition (Pi Abs, Fm, and Fv/Fm were lower than the control, while DI0/RC and F0 were higher). In September, another comparative rise was observed regarding the maximum measured SPAD-related parameter values at that time. Additionally, in October, treatment T5 exhibited the highest nitrogen and carotenoid content, and in other parameters, it was also very successful. Although it was not higher than the control treatment, it was close to the maximum detected value. This is corroborated by the chlorophyll a fluorescence data. The enhancement was noted in the minimum fluorescence and performance index; the lower energy was absorbed in the reaction center due to the higher number of available centers. The electron transport chain was efficient, as in the control treatment, while the dissipated energy was lower. The achievement of treatment C5 was noted twice during the growing season, at the beginning and at the end. Good seedling performance at the beginning of the season is important for boosting plant growth, accumulating essential nutrients, and establishing a strong foundation for overall plant productivity. On the other hand, chlorophyll retention longer in autumn prolongs photosynthetic activity, which in turn provides the plant with larger amounts of nutrients, better health status, greater resistance to different stressors, etc. Our results indicate that PGPBs, when acting synergistically, provide plants with multiple benefits at once, making this a reliable approach for producing healthy seedlings. The C5 treatment helped seedlings by aiding phosphate acquisition, producing IAA, producing ACC (which modulates ethylene production), and facilitating the uptake of metal ions.
Notably, sessile oak seedlings from the control treatment reached high levels of tested physiological parameters during the growing season. In August, all maximum values related to SPAD-derived parameters were observed in the control group, while in October, only carotenoids and nitrogen were higher in the C5 treatment group. Additionally, in September, measurements for the control group were close to the highest values recorded.
In our study, the early positive impact of plant inoculation with PGPBs on chlorophyll a, chlorophyll b, total chlorophyll, carotenoid, and nitrogen content was observed, for example, in June for treatments T4, T5, C2, and C5. In September, chlorophyll a, chlorophyll b, total chlorophyll, carotenoid, and nitrogen content were also higher in the C5 treatment than in the control group, and the C3 treatment had values equal to the control. Finally, in October, carotenoid and nitrogen contents were higher in the C5 treatment than in the control. However, all the aforementioned differences were not statistically significant. Nevertheless, the bacterial strains demonstrated potential for increasing pigments and nitrogen content. Nitrogen is one of the most important and often limiting elements for plant growth [72], since it is a major structural component of various plant cell metabolites [73]. If exposed to low-N stress, plants respond by degrading their own proteins, decreasing photosynthesis, accelerating leaf senescence, and reducing biosynthetic processes [73]. The higher content of nitrogen at the beginning of the growing season is important for optimal growth and development of seedlings. On the other hand, higher content observed in September and October, i.e., late in the growing season, could indicate prolonged metabolic activity and nutrient accumulation, which in the next growing season could provide a better starting point and growth due to larger nutrient deposition. The larger chlorophyll content also contributes to the aforementioned observations.
The natural environment is unpredictable, with frequent situations that can be perceived as stressful. Therefore, numerous studies report improved efficiency of PGPBs under different abiotic or biotic stress conditions. However, in our study, no stress was intentionally induced; instead, we investigated whether bacterial treatments themselves could act as a potential stress factor for the plant and reflect on the seedlings’ physiological and photosynthesis-related parameters.
Treatment of sessile oak seedlings with PGPBs did not induce stress in sessile oak seedlings, as no statistically significant differences in evaluated stress parameters between treated and untreated plants were clearly discerned. Sessile oak seedlings were kept in the nursery of the Institute of Forestry, in semi-controlled conditions, i.e., the temperature and solar irradiance were not controlled. Hence, detected stress symptoms during the growing season could be caused by other disturbing agents. In order to have a comprehensive analysis of seedling physiological activities during the growing season, we tried to connect the results of pigment content dynamics to chlorophyll a kinetics and provide an extensive explanation for the detected stress situation.
The most pronounced effects of plant inoculation with PGPBs are often observed shortly after inoculation, but due to abiotic or biotic factors, such as the re-establishment of native microbial communities, these effects may diminish over time [74,75]. Therefore, the most probable evidence for plant stress induced by bacteria is a poor physiological condition soon after inoculation, i.e., as early in June/July, based on comprehensive data acquired during the experiment. In addition, in our experiment in July (the earliest chlorophyll kinetics measurement), treatments T3 and C3 showed a clearer indication of poorer physiological condition (lower Pi Abs, ABS/RC, TR0/RC, ET0/RC values, while larger F0 and DI0/RC values). However, further investigation would be needed to demonstrate that the observed effects result from bacterial treatments, since pigment content data did not exclude solar irradiance as a possible stress inducer.
We did not observe statistically significant effects of applied plant-growth-promoting bacteria and/or consortia on the photosynthesis parameters of sessile oak seedlings. However, in the study by Jovanović et al. [50], Viridibacillus sp. R3.17, a member of the applied bacterial consortium, increased the height of one-year-old sessile oak seedlings in the Košutnjak provenance by 9.55%. At the same time, the bacterial strain Pseudomonas sp. R4.2.1P (also a member of the consortium) increased the root collar diameter and height of the seedlings from the Košutnjak by 4.79% and 6.40% respectively, and Avala provenances by 14.73% and 7.14% respectively. Moreover, Viridibacillus sp. R3.17 increased the root collar diameter (29.68%) and root collar diameter increment (44.74%) of two-year-old sessile oak seedlings compared to untreated controls [51].
The relationship between enhanced morphological parameters and photosynthetic improvement is dependent on the mechanisms of plant growth promotion. Barrusio et al. [76] reported that an indole acetic acid (IAA)-producing bacterial strain only increased the fresh weight of Arabidopsis thaliana, with no impact on the Fv/Fm ratio. This could explain the lack of effect of confirmed PGP bacterial strains used in this study, since they were all characterized as IAA producers (Table 1).
These findings prove the in planta growth-promoting potential of selected bacteria, and justify the need for further research of PGP mechanisms and interactions with native microbiota, as well as for optimization of application frequencies. Monitoring of bacterial strains in the rhizosphere is necessary for determining their persistence and environmental fate in the rhizosphere and for proving the causality of effect with the particular bacterial treatment. Additionally, the broader environmental challenge associated with soil contamination underscores the need to adopt a natural, sustainable, and eco-friendly approach to address long-term environmental pollution and soil degradation in the forestry sector.
5. Conclusions
Soil contamination is one of the major global environmental issues that is likely to increase in the future. In the forestry sector, soil quality is crucial for successful seedling production, and there is a need for a sustainable approach, where environmentally friendly alternatives, such as biofertilizers based on natural microorganisms, replace conventional agricultural practices involving artificial fertilizers and pesticides due to the degradation of soil and the surrounding environment and biodiversity loss. In this study, we aimed to assess the impact of five selected bacterial strains and three consortia on the physiological parameters related to photosynthesis in one-year-old sessile oak seedlings. While the results showed slightly elevated values in some inoculated treatments compared to the control, the differences were subtle and not statistically significant. However, no adverse effect on photosynthesis and growth of the seedling was detected. In the future, optimizing bacterial inoculum density and application timing should be studied to enhance the observed effects. Additionally, introducing controlled stress conditions in selected treatment groups may provide better insight into the role of PGPBs under suboptimal growth conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments12110409/s1, Figure S1: Chlorophyll a standard curve; Figure S2: Chlorophyll b standard curve; Figure S3: Total chlorophyll standard curve; Figure S4: Carotenoids standard curve; Figure S5: Nitrogen standard curve; Figure S6: SPAD-related parameters measured in June with their standard deviations; Figure S7: SPAD-related parameters measured in August with their standard deviations; Figure S8: SPAD-related parameters measured in September with their standard deviations; and Figure S9: SPAD-related parameters measured in October with their standard deviations.
Author Contributions
Conceptualization, V.P. and T.B.; methodology, S.L.; validation, O.M. and A.V.; formal analysis, A.L. and S.S.; investigation, S.L.; resources, S.S., L.R., S.M., and A.L.; data curation, A.L., L.R., O.M., and A.V.; writing—original draft preparation, S.L.; writing—review and editing, T.B., V.P., and S.M.; visualization, S.L. and A.V.; supervision, T.B. and V.P.; project administration, V.P. and T.B.; and funding acquisition, S.S., L.R.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agreement on realization and funding of scientific research activity of scientific research organizations in 2025 funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia [grant number 451-03-137/2025-03/200178 and 451-03-136/2025-03/200027].
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dror, I.; Yaron, B.; Berkowitz, B. Microchemical contaminants as forming agents of anthropogenic soils. Ambio 2017, 46, 109–120. [Google Scholar] [CrossRef]
- Frazer-Williams, R.; Sankoh, A. Soil contamination resulting from inefficient solid waste management. In Environmental Pollution and Public Health; Frazer-Williams, R., Ogundiran, M.B., Unuabonah, E.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 251–264. [Google Scholar] [CrossRef]
- Haghighizadeh, A.; Rajabi, O.; Nezarat, A.; Hjyani, Z.; Haghmohammadi, M.; Hedayatikhah, S.; Asl, S.D.; Beni, A.A. Comprehensive Analysis of Heavy Metal Soil Contamination in Mining Environments: Impacts, Monitoring Techniques, and Remediation Strategies. Arab. J. Chem. 2024, 17, 105777. [Google Scholar] [CrossRef]
- Melnychenko, V. Phytoremediation of Soils Contaminated as a Result of Military and Anthropogenic Impact. Nauk. Dopovìdì Nacìonalʹnogo Unìversitetu Bìoresursiv ì Prir. Ukraïni 2024, 20, 72–84. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Bahar, M.M.; Naidu, R. Diffuse Soil Pollution from Agriculture: Impacts and Remediation. Sci. Total Environ. 2024, 962, 178398. [Google Scholar] [CrossRef] [PubMed]
- Breś, W.; Politycka, B. Contamination of Soils and Substrates in Horticulture. In Soil Contamination—Current Consequences and Further Solutions; Larramendy, M.L., Soloneski, S., Eds.; InTechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Sun, S.; Sidhu, V.; Rong, Y.; Zheng, Y. Pesticide Pollution in Agricultural Soils and Sustainable Remediation Methods: A Review. Curr. Pollut. Rep. 2018, 4, 240–250. [Google Scholar] [CrossRef]
- Pahalvi, H.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A. Chemical Fertilizers and Their Impact on Soil Health. In Soil Health; Springer: Cham, Switzerland, 2021; pp. 1–20. [Google Scholar] [CrossRef]
- Wu, Q.; Cao, Y.; Yu, T.; Yang, J.; Fan, S.; Feng, C.; Liu, Z.; Huang, C. A Scientometric Analysis and Visualization of Forest Soil Contamination Research from Global Perspectives. Forests 2024, 15, 1068. [Google Scholar] [CrossRef]
- Baláš, M.; Kuneš, I.; Podrázský, V.; Gallo, J.; Lopot, F. Chemical Forest Amelioration: Experience from the Czech Republic and Other Selected Countries—A Review. J. For. Sci. 2024, 70, 103–121. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; MacDonald, J.E. Seedling Production and the Field Performance of Seedlings. Forests 2018, 9, 740. Available online: https://www.mdpi.com/1999-4907/9/12/740 (accessed on 8 September 2025). [CrossRef]
- Barros, N.F.; Dedecek, R.A.; Schumacher, M.V. The Production Chain of Tree Seedlings in the Context of Climate Change. Forests 2022, 14, 1693. Available online: https://www.mdpi.com/1999-4907/14/9/1693 (accessed on 8 September 2025).
- Yousaf, A.; Hussain, M.; Ahmad, S.; Riaz, A.; Shaukat, S.; Shaukat, S.W.A.; Mishr, R.S.; Akram, S.; Majeed, M.; Tabassum, A.; et al. Environmental Sustainability Assessment of Softwood and Hardwood Seedlings Production in Forest Nurseries: A Case Study from Pakistan. Braz. J. Biol. 2022, 84, e260615. [Google Scholar] [CrossRef]
- Rügner, H.; Forster, B. A Global Review on Innovative, Sustainable, and Effective Materials Composing Growing Media for Forest Seedling Production. Curr. For. Rep. 2023, 9, 148–167. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Wang, L.; Li, Y. Environmental Risk Substances in Soil on Seed Germination: A Review. J. Hazard. Mater. 2024, 460, 132805. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0304389424010975 (accessed on 9 September 2025).
- Pyoabalo, A.; Tchabi, A.; Abotsi, K.; Adjonou, K.; Segla, K.; Kokutse, A.; Kokou, K. Effect of Nursery Substrate on the Growth of Pterocarpus erinaceus Poir. Seedlings. Int. J. Curr. Res. Biosci. Plant Biol. 2021, 8, 17–27. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Xu, S. Selective Biotic Stressors’ Action on Seed Germination: A Review. Plant Sci. 2024, 341, 111601. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0168945224001833 (accessed on 10 September 2025).
- Ahmed, M.; Rauf, M.; Mukhtar, Z.; Saeed, N.A. Excessive Use of Nitrogenous Fertilizers: An Unawareness Causing Serious Threats to Environment and Human Health. Environ. Sci. Pollut. Res. 2017, 24, 26983–26987. [Google Scholar] [CrossRef] [PubMed]
- Stekolnikov, K.E.; Gasanova, E.S.; Stekolnikova, N.V. Agrogenic Transformation (Degradation) of Chernozems of the Central Chernozem Region. BIO Web Conf. 2021, 36, 03021. [Google Scholar] [CrossRef]
- Khatun, J.; Intekhab, A.; Dhak, D. Effect of Uncontrolled Fertilization and Heavy Metal Toxicity Associated with Arsenic (As), Lead (Pb) and Cadmium (Cd), and Possible Remediation. Toxicology 2022, 479, 153274. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Chen, B.; Wang, L.; Xie, Z.; Wang, J.; Yang, Z. Fertilization Restructures Nematode Assemblages by Modifying Soil pH in Croplands of Northeast China. Front. Environ. Sci. 2023, 11, 1207379. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K. Long-Term Effects of Mineral Fertilizers on Soil Microorganisms—A Review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Guo, Z.; Han, J.; Li, J.; Xu, Y.; Wang, X. Effects of Long-Term Fertilization on Soil Organic Carbon Mineralization and Microbial Community Structure. PLoS ONE 2019, 14, e0211163. [Google Scholar] [CrossRef]
- Daws, M.; Blackburn, C.; Standish, R.; Tibbett, M. Canary in the Coal Mine: Lessons from the Jarrah Forest Suggest Long-Term Negative Effects of Phosphorus Fertilizer on Biodiverse Restoration after Surface Mining. Front. For. Glob. Change 2022, 5, 786305. [Google Scholar] [CrossRef]
- Petraityte, D.; Arlauskienė, A.; Cesevičienė, J. Use of Digestate as an Alternative to Mineral Fertilizer: Effects on Soil Mineral Nitrogen and Winter Wheat Nitrogen Accumulation in Clay Loam. Agronomy 2022, 12, 402. [Google Scholar] [CrossRef]
- Liu, F.; Ma, H.; Liu, B.; Du, Z.; Ma, B.; Jing, D. Effects of Plant Growth-Promoting Rhizobacteria on the Physioecological Characteristics and Growth of Walnut Seedlings under Drought Stress. Agronomy 2023, 13, 290. [Google Scholar] [CrossRef]
- Keyhani, A.; He, W.; Teng, M.; Yan, Z.; Xu, J.; Fayaz, M.; Zhou, C.; Wei, P.; Wang, P. Effect of Mineral Fertilizers on Microorganisms Community Characteristic during Leaf Litter Decomposition under Pinus massoniana in a Subtropical Forest. Appl. Soil Ecol. 2024, XX, 105421. [Google Scholar] [CrossRef]
- Çığ, F.; Sönmez, F.; Nadeem, M.A.; Sabagh, A.E. Effect of Biochar and PGPR on the Growth and Nutrients Content of Einkorn Wheat (Triticum monococcum L.) and Post-Harvest Soil Properties. Agronomy 2021, 11, 2418. [Google Scholar] [CrossRef]
- Liu, F.; Ma, H.; Peng, L.; Du, Z.; Ma, B.; Liu, X. Effect of the Inoculation of Plant Growth-Promoting Rhizobacteria on the Photosynthetic Characteristics of Sambucus williamsii Hance Container Seedlings under Drought Stress. AMB Express 2019, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hua, M.; Guo, D.; Xue, Y.; Chen, X.; Rui, L.; Zhou, N. Effects of Plant Growth-Promoting Rhizobacteria on Growth Indicators and Physiological Characteristics of Peucedanum praeruptorum Dunn Leaves. Plant Signal. Behav. 2023, 18, 2203571. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Święcicka, I.; Thijs, S.; Vangronsveld, J. Beneficial Features of Plant Growth-Promoting Rhizobacteria for Improving Plant Growth and Health in Challenging Conditions: A Methodical Review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Saeed, Q.; Wang, X.; Haider, F.; Kučerík, J.; Mumtaz, M.; Holátko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere Bacteria in Plant Growth Promotion, Biocontrol, and Bioremediation of Contaminated Sites: A Comprehensive Review of Effects and Mechanisms. Int. J. Mol. Sci. 2021, 22, 10529. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Mishra, A.; Jan, S.; Bhat, M.; Kamal, M.; Rahman, S.; Shah, A.; Jan, A. Plant Growth Promoting Rhizobacteria in Plant Health: A Perspective Study of the Underground Interaction. Plants 2023, 12, 629. [Google Scholar] [CrossRef]
- Khoso, M.; Wagan, S.; Alam, I.; Hussain, A.; Ali, Q.; Saha, S.; Poudel, T.; Manghwar, H.; Liu, F. Impact of Plant Growth-Promoting Rhizobacteria (PGPR) on Plant Nutrition and Root Characteristics: Current Perspective. Plant Stress 2023, 11, 100341. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Jabborova, D. The effects of Pseudomonas koreensis IGPEB 17 and arbuscular mycorrhizal fungi on growth and physiological properties of ginger. Turk. J. Agric. For. 2022, 46, 488–495. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, L.; Liu, Q.; Sikder, M.; Vestergård, M.; Zhou, K.; Wang, Q.; Yang, X.; Feng, X. Pseudomonas fluorescens Promote Photosynthesis, Carbon Fixation and Cadmium Phytoremediation of Hyperaccumulator Sedum alfredii. Sci. Total Environ. 2020, 726, 138554. [Google Scholar] [CrossRef] [PubMed]
- Küpper, H.; Benedikty, Z.; Morina, F.; Andersen, E.; Mishra, A.; Trtílek, M. Analysis of OJIP Chlorophyll Fluorescence Kinetics and QA Reoxidation Kinetics by Direct Fast Imaging. Plant Physiol. 2019, 179, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papadogeorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Karličić, V.; Golubović Ćurguz, V.; Raičević, V. The Alleviation of Reforestation Challenges by Beneficial Soil Microorganisms. Reforesta 2016, 1, 238–260. [Google Scholar] [CrossRef]
- Mohan, E.; Rajendran, K. Sustainable Development of Horticulture and Forestry through Bio-Inoculants. In Sustainable Crop Production; Hasanuzzaman, M., Fujita, M., Filho, M.C.M.T., Nogueira, T.A.R., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Lindner, M.; Fitzgerald, J.B.; Zimmermann, N.E.; Reyer, C.; Delzon, S.; van Der Maaten, E.; Schelhaas, M.J.; Lasch, P.; Eggers, J.; van Der Maaten-Theunissen, M.; et al. Climate change and European forests: What do we know, what are the uncertainties, and what are the implications for forest management? J. Environ. Manag. 2014, 146, 69–83. [Google Scholar] [CrossRef]
- Schelhaas, M.-J.; Nabuurs, G.-J.; Hengeveld, G.; Reyer, C.; Hanewinkel, M.; Zimmermann, N.E.; Cullmann, D. Alternative forest management strategies to account for climate change-induced productivity and species suitability changes in Europe. Reg. Environ. Change 2015, 15, 1581–1594. [Google Scholar] [CrossRef]
- Perkins, D.; Uhl, E.; Biber, P.; Du Toit, B.; Carraro, V.; Rötzer, T.; Pretzsch, H. Impact of climate trends and drought events on the growth of oaks (Quercus robur L. and Quercus petraea (Matt.) Liebl.) within and beyond their natural range. Forests 2018, 9, 108. [Google Scholar] [CrossRef]
- Kunz, J.; Löffler, G.; Bauhus, J. Minor European broadleaved tree species are more drought-tolerant than Fagus sylvatica but not more tolerant than Quercus petraea. For. Ecol. Manag. 2018, 414, 15–27. [Google Scholar] [CrossRef]
- Modrow, T.; Kuehne, C.; Saha, S.; Bauhus, J.; Pyttel, P.L. Photosynthetic performance, height growth, and dominance of naturally regenerated sessile oak (Quercus petraea [Mattuschka] Liebl.) seedlings in small-scale canopy openings of varying sizes. Eur. J. For. Res. 2020, 139, 41–52. [Google Scholar] [CrossRef]
- Ministère de l’Agriculture. Résultats de l’enquête statistique annuelle MAAF/IRSTEA sur les ventes en France de plants forestiers pour la campagne de plantation 2015–2016. In 2017-229 NdSD.; Ministère de l’Agriculture: Paris, France, 2017; p. 32. Available online: https://info.agriculture.gouv.fr/gedei/site/bo-agri/instruction-2017-229/telechargement (accessed on 14 September 2025).
- Popović, V.; Lučić, A.; Rakonjac, L.; Kerkez-Janković, I. Analysis of morphological quality parameters of one-year old bare root sessile oak (Quercus petraea (Matt.) Liebl) seedlings. Sustain. For. 2019, 79–80, 59–64. [Google Scholar]
- Girard, Q.; Ducousso, A.; De Gramont, C.; Louvet, J.; Reynet, P.; Musch, B.; Kremer, A. Provenance variation and seed sourcing for sessile oak (Quercus petraea (Matt.) Liebl.) in France. Ann. For. Sci. 2022, 79, 27. [Google Scholar] [CrossRef]
- Jovanović, S.; Popović, V.; Lučić, A.; Rakonjac, L. Bacterial Treatment Impact on Morphological Traits of One-Year Old Sessile Oak Seedlings of Two Serbian Provenances. Sustain. For. 2023, 87–88, 79–87. [Google Scholar] [CrossRef]
- Jovanović, S.; Lučić, A.; Rakonjac Lj Popović, V. Uticaj bakterijskih tretmana na dvogodišnje sadnice hrasta kitnjaka (Quercus petraea (Matt.)Liebl). Šumarstvo 2023, 3–4, 127–137. [Google Scholar]
- Samaddar, S.; Chatterjee, P.; Choudhury, A.; Ahmed, S.; Sa, T. Interactions between Pseudomonas spp. and their role in improving the red pepper plant growth under salinity stress. Microbiol. Res. 2019, 219, 66–73. [Google Scholar] [CrossRef]
- Ahsan, N.; Shimizu, M. Lysinibacillus Species: Their Potential as Effective Bioremediation, Biostimulant, and Biocontrol Agents. Rev. Agric. Sci. 2021, 9, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Dasila, H.; Rana, A.; Maithani, D.; Rana, A.; Sahgal, M.; Tewari, S. Interaction between Dalbergia sissoo Roxb. and Pseudomonas koreensis AS15 Strain is Cultivar Specific. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 297–306. [Google Scholar] [CrossRef]
- Bielinis, E.; Jóźwiak, W.; Robakowski, P. Modelling of the relationship between the SPAD values and photosynthetic pigments content in Quercus petraea and Prunus serotina leaves. Dendrobiology 2015, 73, 125–134. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Petković, B.; Merkulov, L.; Duletić-Laušević, S. Anatomija i Morfologija Biljaka sa Praktikumom; Stanković, S., Ed.; Biološki fakultet Univerziteta u Beogradu: Beograd, Srbija, 2014; ISBN 978-86-7078-084-2. [Google Scholar]
- Hall, D.O.; Rao, K.K. Photosynthesis; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Willows, R.D. Chlorophylls. In Plant Pigments and Their Manipulation; Davies, K.M., Ed.; Blackwell Publishing: Oxford, UK, 2004; pp. 23–46. [Google Scholar]
- Mattila, H.; Valev, D.; Havurinne, V.; Khorobrykh, S.; Virtanen, O.; Antinluoma, M.; Mishra, K.B.; Tyystjärvi, E. Degradation of chlorophyll and synthesis of flavonols during autumn senescence—The story told by individual leaves. AoB Plants 2018, 10, ply028. [Google Scholar] [CrossRef]
- Donnelly, A.; Yu, R.; Rehberg, C.; Meyer, G.; Young, E.B. Leaf chlorophyll estimates of temperate deciduous shrubs during autumn senescence using a SPAD-502 meter and calibration with extracted chlorophyll. Ann. For. Sci. 2020, 77, 30. [Google Scholar] [CrossRef]
- Babu, A.; Shea, P.; Sudhakar, D.; Jung, I.; Oh, B. Potential use of Pseudomonas koreensis AGB-1 in association with Miscanthus sinensis to remediate heavy metal(loid)-contaminated mining site soil. J. Environ. Manag. 2015, 151, 160–166. [Google Scholar] [CrossRef]
- Adhikari, A.; Khan, M.; Lee, K.; Kang, S.; Dhungana, S.; Bhusal, N.; Lee, I. The Halotolerant Rhizobacterium—Pseudomonas koreensis MU2 Enhances Inorganic Silicon and Phosphorus Use Efficiency and Augments Salt Stress Tolerance in Soybean (Glycine max L.). Microorganisms 2020, 8, 1256. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Sun, Y.; Shi, M.; Han, X.; Jing, Y.; Li, Y.; Li, H.; Lai, H. Pseudomonas koreensis promotes tomato growth and shows potential to induce stress tolerance via auxin and polyphenol-related pathways. Plant Soil 2021, 462, 141–158. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, C.; Jing, Y.; Yang, S.; Li, H.; Xue, Q.; Lai, H. Pseudomonas koreensis Culture Filtrate Alleviates Tomato Drought Stress: Modulation of Antioxidant Systems Coupled with the Porphyrin and Chlorophyll–Photosynthesis–Fructose and Mannose Axis. Plant Soil 2022, 484, 237–256. [Google Scholar] [CrossRef]
- Kalleku, J.; Ihsan, S.; Al-Azzawi, T.; Khan, M.; Hussain, A.; Chebitok, F.; Das, A.; Moon, Y.; Mun, B.; Lee, I.; et al. Halotolerant Pseudomonas koreensis S4T10 Mitigates Salt and Drought Stress in Arabidopsis thaliana. Physiol. Plant. 2024, 176, e14258. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, M.; Moon, Y. Synergistic Effect of Serratia fonticola and Pseudomonas koreensis on Mitigating Salt Stress in Cucumis sativus L. Curr. Issues Mol. Biol. 2025, 47, 194. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Rathore, P.; Kumari, R.; Yadav, R. Brown Gold of Marginal Soil: Plant Growth Promoting Bacteria to Overcome Plant Abiotic Stress for Agriculture, Biofuels and Carbon Sequestration. Sci. Total Environ. 2020, 711, 135062. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.; Li, H.; Guo, D.; Sharma, A.; Verma, K.; Solanki, M.; Upadhyay, S.; Lakshmanan, P.; Yang, L.; et al. Nitrogen Fixation and Phytohormone Stimulation of Sugarcane Plant through Plant Growth Promoting Diazotrophic Pseudomonas. Biotechnol. Genet. Eng. Rev. 2023, 40, 15–35. [Google Scholar] [CrossRef]
- Wang, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Natarajan, D.; Ma, Y. Plant Growth-Promoting Bacteria in Metal-Contaminated Soil: Current Perspectives on Remediation Mechanisms. Front. Microbiol. 2022, 13, 966226. [Google Scholar] [CrossRef] [PubMed]
- Suhr, M.; Lederer, F.L.; Günther, T.J.; Raff, J.; Pollmann, K. Characterization of Three Different Unusual S-Layer Proteins from Viridibacillus arvi JG-B58 That Exhibits Two Super-Imposed S-Layer Proteins. PLoS ONE 2016, 11, e0156785. [Google Scholar] [CrossRef][Green Version]
- Ye, J.; Tian, W.; Jin, C. Nitrogen in plants: From nutrition to the modulation of abiotic stress adaptation. Stress Biol. 2022, 2, 4. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Li, J.; Huang, D. The Utilization and Roles of Nitrogen in Plants. Forests 2024, 15, 1191. [Google Scholar] [CrossRef]
- Kong, Z.; Wu, Z.; Glick, B.; He, S.; Huang, C.; Wu, L. Co-occurrence patterns of microbial communities affected by inoculants of plant growth-promoting bacteria during phytoremediation of heavy metal-contaminated soils. Ecotoxicol. Environ. Saf. 2019, 183, 109504. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, H.; Kuroda, M.; Inoue, D.; Morikawa, M.; Ike, M. Community dynamics of duckweed-associated bacteria upon inoculation of plant growth-promoting bacteria. FEMS Microbiol. Ecol. 2020, 96, fiaa101. [Google Scholar] [CrossRef]
- Barriuso, J.; Solano, B.R.; Gutiérrez Mañero, F.J. Biological Control Protection Against Pathogen and Salt Stress by Four Plant Growth-Promoting Rhizobacteria Isolated from Pinus sp. on Arabidopsis thaliana. Phytopathology 2008, 98, 666–672. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).