Abstract
This study aimed to evaluate the incorporation of graphene-based additives, graphene nanoplatelets (GNPs) and graphene oxide quantum dots (GOQDs), into polymeric fiber matrices used as biofilm supports in anaerobic digestion systems, determining additive specific effects by benchmarking the impregnated matrices against the same nylon carrier without additives under identical operational conditions. Modified matrices were assessed through BMP assays using the liquid fraction of fruit and vegetable waste (LF-FVW) as substrate. Intermediate GNP and GOQD loadings (FM50 and FMDOT50) achieved the highest methane yields (317.9 ± 20.2 and 348.4 ± 20.0 mL CH4/g COD(rem)) compared with the control fiber matrix (301.0 ± 20.1 mL CH4/g COD(rem)). Scanning electron microscopy (SEM) and Fourier transform infrared (FTIR) analyses confirmed nanomaterial retention on the matrix surface and interaction with microbial aggregates. Embedding the nanostructures within the fiber enhanced biofilm formation and methane yield while minimizing nanomaterial washout. Future work will focus on advanced physicochemical characterization (XRD, XPS, BET, and EDX mapping), leaching tests to assess long term stability, and scale up evaluation for full scale anaerobic digestion applications.
1. Introduction
Anaerobic digestion (AD) is a biological process that converts organic matter into biogas under oxygen-free conditions, mainly producing methane and carbon dioxide [1]. It plays a crucial role in sustainable waste management and renewable energy production, contributing to the reduction in greenhouse gas emissions and the valorization of organic residues into valuable bioenergy products. Its performance depends on factors such as biomass retention and microbial activity and has been optimized through different reactor configurations. However, the efficiency of this process is often limited by operational instabilities, biomass washout, and inhibition phenomena caused by substrate fluctuations or toxic intermediates [2,3,4]. Therefore, developing strategies that enhance process stability, improve mass transfer, and increase microbial retention is essential for achieving consistent and scalable biogas generation. Recent research has explored innovative strategies to improve efficiency by integrating biofilms, membranes, and polymers into various reactor designs to enhance biogas yield [5,6]. Different reactor configurations such as completely stirred tank reactors (CSTRs), up flow anaerobic sludge blanket (UASB), expanded granular sludge bed (EGSB), and fixed-bed reactors have been designed to improve contact between microorganisms and substrates. Among these, fixed-bed systems are particularly advantageous for promoting biofilm formation on support materials, thereby enhancing microbial density and process resilience [7]. In biofilm based systems, microorganisms grow attached to solid surfaces, forming complex structures surrounded by extracellular polymeric substances that stabilize the community and protect cells from environmental stress. This configuration not only prevents biomass washout but also improves the overall metabolic activity of the microbial consortium, especially under high organic loading rates. Microbial supports made from activated carbon or ceramics have been used in fixed-bed reactors, improving biomass retention [8]. Similarly, natural zeolite has been reported to increase biogas production from dairy sludge by providing a stable environment for microbial growth and activity. Granular activated carbon has also been shown to promote direct interspecies electron transfer (DIET), a microbial mechanism in which electrons are directly exchanged between syntrophic species without the need for soluble intermediates, enhancing methane generation efficiency [9].
Recently, the use of graphene based supports in AD has proven effective in increasing organic matter retention and enhancing overall biogas production performance by providing a larger surface area for microbial adhesion and activity [8]. It has been demonstrated that the addition of graphene nanoplatelets (GNPs) significantly influences the metabolic activity of microbial communities involved in AD. For instance, the incorporation of conductive materials such as graphene has been reported to enhance the methanogenesis pathway, resulting in up to a 25% increase in methane production and a 19% increase in methane yield due to improved electron transfer capacities [9]. Similarly, it has been highlighted that the presence of graphene in AD systems significantly enhances biomethane production by promoting DIET, shortening the lag phase typically associated with anaerobic processes, accelerating system startup, and improving stability [10].
Graphene based nanomaterials, particularly GNPs and GOQDs, possess unique structural and electrical properties that make them attractive for bioelectrochemical and anaerobic systems. These quantum dots are nanoscale fragments of graphene oxide typically smaller than 10 nm, containing abundant oxygenated functional groups such as hydroxyl, carboxyl, and carbonyl. These functionalities confer high hydrophilicity, excellent aqueous dispersibility, and strong electron transfer ability, enabling efficient participation in redox processes. Their small size and high surface to volume ratio facilitate close interaction with microbial cells and the extracellular matrix, improving metabolic activity and biofilm formation in anaerobic systems [11]. Their large specific surface area, high electrical conductivity, and chemical stability facilitate DIET between syntrophic bacteria and methanogenic archaea, which is a key mechanism in efficient methanogenesis. Their ability to mediate electron transfer can accelerate the conversion of intermediates such as volatile fatty acids into methane [12].
However, graphene based materials also present certain limitations. In most studies, they are used in powder form, which leads to material losses or requires additional pretreatments for recovery. Moreover, the release of nanoparticles through the digestate can contaminate the environment and exert toxic effects on organisms inhabiting terrestrial and aquatic ecosystems [13,14,15]. Recent regulatory frameworks have emphasized the need for standardized risk assessments and leaching protocols to evaluate the environmental safety of engineered nanomaterials. Current mitigation strategies include immobilization within polymeric supports and surface functionalization to prevent nanoparticle release and minimize ecological impact [16,17,18,19,20]. In addition, the processing of these materials still involves high costs, which could compromise their economic feasibility at larger scales [21]. Accordingly, there is a clear need for alternative support materials that minimize nanoparticle leaching, mitigate environmental risks, and reduce the processing and recovery costs associated with powdered graphene based materials [13,14,15,16].
In this context, the development of composite materials, such as polymeric matrices or nanocomposites based on glass/epoxy, polymers, and sponges, has attracted increasing interest to mitigate these limitations. These supports can enhance graphene retention, reduce nanoparticle leaching, and improve the AD process [22]. However, the proper design of such materials poses important challenges, including ensuring strong adhesion between nanoparticles and the matrix, preventing migration through the support pores, and achieving homogeneous graphene dispersion within the material, which are crucial aspects to maintain good performance and fully exploit its properties [14,15]. Several strategies have been developed to address these challenges, such as the surface functionalization of graphene with carboxyl, amine, or epoxy groups to enhance polymer compatibility, the use of ultrasonic and solvent assisted dispersion techniques to achieve uniform nanomaterial distribution, and the application of chemical crosslinking agents or compatibilizers to reduce nanoparticle migration during operation [23,24,25,26]. Achieving homogeneous dispersion and strong interfacial adhesion between the polymer and the nanomaterial are essential for maintaining mechanical stability and preventing nanoparticle migration during operation. These physicochemical interactions determine the durability, reproducibility, and environmental safety of biofilm carriers [25,26,27,28].
For instance, Megahed et al. [29] investigated the dispersion effect of micro- and nanosized aluminum particles on the mechanical properties of glass/epoxy composites as well as graphene nanoplatelet/polymer nanocomposites. In these cases, the filler and the matrix exhibited weak van der Waals interfacial interactions, which resulted in a large exposed surface area and sufficient strength for adequate stress transfer between phases [30]. Proper dispersion, combined with a large contact surface, promotes the interaction between GNPs and the matrix, thereby contributing to greater retention and material stability.
Despite these advances, most studies have focused on dispersed or powdered nanomaterials rather than immobilized systems. This limitation restricts the practical scalability of nanomaterial assisted AD, as recovery and long term stability remain problematic under continuous operation. Embedding nanomaterials within polymeric supports represents a promising strategy to overcome these challenges, allowing controlled retention, reusability, and reduced environmental risks. Nevertheless, few studies have evaluated hybrid nanocomposite supports incorporating both conductive GNPs and functionalized GOQDs graphene derivatives, even though their synergistic combination could enhance both electrical conductivity and biocompatibility [31].
This polymer nanomaterial configuration ensures sufficient microbial contact even after the formation of a primary biofilm layer. The fibrous architecture of the nylon matrix provides a large surface area and interconnected microvoids that allow microbial colonization to extend beyond the outermost biofilm layer. Moreover, the uniform distribution of GNPs and GOQDs within the polymeric fibers creates conductive and hydrophilic domains that remain accessible for microbial attachment and electron exchange. These conductive pathways also help maintain metabolic activity by minimizing diffusional resistance and the shielding effects typically caused by dense biofilm layers. Consequently, this composite structure promotes sustained biofilm development and effective mass and electron transfer over time [32,33,34].
In addition to structural stability, supports in AD must promote biofilm growth by providing shapes and surfaces, such as branched structures, that facilitate microbial adhesion. This phenomenon, based on colonization processes and the secretion of extracellular substances, enables the formation of stable communities that reinforce the digestion process [35]. In this context, GNPs, owing to their remarkable physicochemical properties, emerge as a promising alternative for incorporation into polymeric fiber matrices. This integration not only structurally reinforces the supports but also promotes the development of anaerobic biofilms and enhances biogas production [36]. Furthermore, these characteristics can strengthen mechanisms such as DIET, thereby increasing the efficiency of AD processes [37]. Compared to other carbon based nanomaterials, GNPs are more economically accessible and easier to produce, making them attractive for large-scale applications in waste treatment technologies [38,39,40,41].
The combination of GNPs and GOQDs integrates the conductivity and structural stability of GNPs with the high dispersibility and biocompatibility of GOQDs, offering a balanced and efficient alternative for enhancing AD performance [42,43,44]. Among the different polymers used as biofilm carriers such as polyurethane foams, polyethylene rings, or ceramic rings nylon has proven particularly suitable for anaerobic systems due to its mechanical strength, chemical resistance, and long-term stability under oxygen-free conditions [45,46,47]. Commercial nylon fibers (3M®, Maplewood, MN, USA; 5 × 1 cm) are widely used as support matrices in anaerobic systems because their microrough and hydrophilic surface facilitates microbial attachment and stable biofilm formation. The 5 × 1 cm strip format provides a uniform surface area that can be easily standardized and handled during experimental runs, improving reproducibility.
Moreover, the incorporation of GNPs and GOQDs into nylon fibers offers a lightweight, conductive, and cost-effective alternative to conventional carbonaceous supports such as granular activated carbon or graphite granules, combining mechanical robustness with enhanced electron transfer capacity. Additionally, these composite fibers may offer potential advantages in terms of improved biomass retention and possible reusability during multiple operation cycles; however, these aspects were not experimentally evaluated in the present work.
The approach proposed in this study embedding GNPs and GOQDs into commercial nylon fibers addresses these limitations by improving nanomaterial retention, ensuring mechanical stability, and reducing the potential for environmental release. The incorporation of conductive nanostructures directly into the fiber improves mechanical stability, minimizes nanomaterial loss, and provides a continuous conductive environment that favors microbial attachment and facilitates potential electron exchange among syntrophic communities. This hybrid configuration promotes robust biofilm formation and sustained methane production, addressing current limitations such as poor nanomaterial retention, aggregation, and possible environmental release previously reported by Igarashi et al. [48], Baek et al. [49], and Valentin et al. [50].
The objective of this study was to evaluate, as a preliminary approach, an immobilization strategy in which GNPs and GOQDs were embedded within a commercial nylon fiber matrix used as a biofilm carrier for AD. The feasibility and performance of the nanomaterial impregnated fibers were assessed in terms of biofilm formation and methane production during single cycle operation. Beyond the experimental scale, this approach proposes a practical and scalable strategy for improving AD by enhancing microbial retention and stability through conductive, mechanically robust, and biocompatible supports. The surface morphology and distribution of GNPs within the fibers were examined by SEM, which was selected because it provides high resolution imaging that allows direct visualization of fiber structure, nanomaterial dispersion, and microbial attachment essential for evaluating surface modification and biofilm development. Beyond the laboratory scale, this approach offers potential benefits for large scale AD systems by improving process stability and extending the operational lifespan of biofilm carriers. The use of low cost commercial nylon and small nanomaterial loadings enhances the economic feasibility of implementation in existing wastewater treatment infrastructures. Moreover, by immobilizing GNPs and GOQDs within the polymeric matrix, the risk of nanomaterial release into the environment is minimized, contributing to safer and more sustainable applications of nanotechnology in anaerobic biotechnology. The findings of this work contribute to expanding the current understanding of nanomaterial assisted anaerobic systems and pave the way for the development of next-generation biofilm reactors with higher efficiency, stability, and environmental safety.
2. Materials and Methods
2.1. Preparation of Graphene Nanoplatelets (GNPs) from Conventional Graphite
The process was divided into two stages:
Stage 1. Two hundred grams of conventional graphite (CG) powder (Química Meyer, Mexico City, Mexico) were mixed with 4000 mL of distilled water and introduced into a steam explosion unit. The process was carried out at 170 °C and 130 PSI for 45 min. Subsequently, the chamber was depressurized, and the steam-treated solid was decanted to remove excess water and then dried at 70 °C. The resulting dried powder corresponded to steam-exploded graphite (C-EG), which was stored.
Stage 2. The C-EG obtained in Stage 1 was exfoliated by dispersing 500 mg of C-EG in 50 mL of distilled water. The mixture was subjected to ultrasonication using a CP Ultrasonic Processor CP750 (Cole-Parmer, Vernon Hills, IL, USA) at 750 W power, 20 kHz frequency, and 50% amplitude for 80 min. The resulting dispersion was dried at 70 °C to obtain steam-exploded and exfoliated graphite (GNPs) [51]. The resulting graphene nanoplatelets exhibited a laminar morphology with a mean lateral size ranging from 1 to 5 µm and a thickness below 10 nm, corresponding to few-layer graphene structures. These morphological characteristics are consistent with previous reports using the same steam explosion and ultrasonic exfoliation route. The synthesis process yielded approximately 90.5% of GNPs based on the initial graphite mass.
2.2. Synthesis of Graphene Oxide Quantum Dots (GOQDs)
The synthesis of GOQDs was carried out following the method reported by [52]. For this purpose, 100 mg of graphene oxide were weighed and placed in a container, and 12 mL of N,N-dimethylformamide (DMF, Sigma-Aldrich®, St. Louis, MO, USA) were added. The mixture was sonicated in an ultrasonic bath for 15 min. It was then transferred to a 100 mL Teflon-lined stainless-steel autoclave (BAOSHISHAN®, Zhengzhou, Henan, China), which was heated in a muffle furnace at 200 ± 2 °C for 8 h. After the reaction, the solution was filtered through a Büchner funnel and washed with distilled water until a neutral pH was reached. The solid obtained was dried in an oven at 65 °C for 4 h. The synthesis yield of GOQDs was approximately 69.3%, and their particle size was in the range of 5–20 nm, consistent with values reported in the literature for this method [52]. These details enhance the reproducibility of the synthesis process.
2.3. Preparation and Impregnation of GNPs and GOQDs into the Fiber Matrix
Commercial nylon fibers matrices (3M®, Maplewood, MN, USA) were selected as the biofilm carrier due to their mechanical strength, chemical stability, and suitability for anaerobic systems. The fibers were cut into 5 × 1 cm strips to provide a reproducible surface area for microbial attachment and easy handling during experimental assays.
The fibers were dried in an oven at 65 °C for 24 h to remove residual moisture. Subsequently, suspensions of GNPs and GOQDs were prepared at concentrations of 30, 50, and 100 mg/mL and sonicated in 100 mL of distilled water for 30 min. concentrations previously optimized to ensure uniform dispersion and avoid aggregation. Both nanomaterials were selected based on their complementary properties: GNPs provide mechanical reinforcement and electrical conductivity, whereas GOQDs offer high dispersibility, hydrophilicity, and biocompatibility. These characteristics were considered relevant for improving microbial attachment and stability within the nylon fiber matrix. The fibers were then immersed in 35 mL vials and subjected to an ultrasonic bath (JPS-A, VEVOR®, 40 kHz, Shanghai, China) at 40 °C for 15 min to promote nanomaterial dispersion on the fiber surface. Finally, the fibers were dried in an oven at 65 ± 2 °C for 24 h. The resulting materials were labeled as FM30, FM50, and FM100, corresponding to the GNP concentrations used, and as FMDOT30, FMDOT50, and FMDOT100 for the equivalent GOQD concentrations. A schematic representation of the synthesis and impregnation process of GNPs and GOQDs into the nylon fiber matrix is presented in Figure 1. The resulting nanocomposite fiber matrices were designated as FM (fiber matrix impregnated with graphene nanoplatelets) and FMDOT (fiber matrix impregnated with graphene oxide quantum dots), followed by the respective concentration codes (30, 50, and 100). These designations were used consistently in Table 1, Table 2 and Table 3 to describe the experimental treatments.
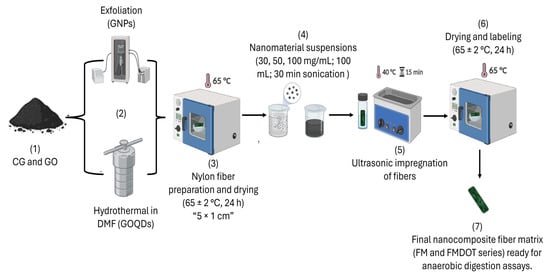
Figure 1.
Schematic representation of the synthesis and impregnation process of graphene nanoplatelets (GNPs) and graphene oxide quantum dots (GOQDs) into the nylon fiber matrix. The workflow includes: (1) raw materials (CG and GO), (2) nanomaterial synthesis by exfoliation (GNPs) and hydrothermal treatment in DMF (GOQDs), (3) nylon fiber preparation and drying, (4) preparation of nanomaterial suspensions, (5) ultrasonic impregnation of fibers, (6) drying and labelling, and (7) final nanocomposite fiber matrix (FM and FMDOT series) ready for anaerobic digestion tests.
Non impregnated fiber matrices were used as the control matrix (CFM). The control fiber consisted of the same commercial nylon fibers (3M®, Maplewood, MN, USA) without nanomaterial addition and was inoculated under the same conditions as the impregnated fibers. Nylon-based materials have been widely employed as biofilm supports in anaerobic systems due to their mechanical resistance, stability under anoxic conditions, and surface roughness that facilitates microbial attachment [53,54]. This standardized preparation ensured reproducibility among all assays.
2.4. Biofilm Growth on the Fiber Matrix
For the development of bacterial biofilm on the fiber matrix, a laboratory-scale sludge anaerobic biofilm reactor (LASR) with a working volume of 1.5 L was used. Inside the reactor, the fibers impregnated with GNPs and GOQDs were mounted on a stainless steel support. The LASR was fed with the liquid fraction of fruit and vegetable waste (LF-FVW), collected from the municipal market in Orizaba, Veracruz, Mexico. This fraction was processed according to the method described by Alvarado-Vallejo et al. [55] to obtain the raw liquid fraction, which was used during the startup phase of the LASR.
Subsequently, inoculum was collected from a pilot-scale fixed-bed reactor operated at ambient temperature at the Instituto Tecnológico de Orizaba. The inoculum was incubated under mesophilic conditions (35 ± 2 °C) and previously characterized by the following physicochemical parameters: total solids (TS), total volatile solids (TVS), pH, total chemical oxygen demand (TCOD), and soluble chemical oxygen demand (SCOD). The TVS parameter represents the organic fraction of the sample, determined as the loss on ignition at 550 °C, and should not be confused with volatile matter, which refers to thermally released compounds in solid fuels under inert conditions. The exact bacterial composition of the inoculum was not determined at the beginning of the experiment. However, the inoculum originated from a stable anaerobic sludge previously acclimated under mesophilic conditions, containing a representative microbial consortium composed of hydrolytic, acidogenic, acetogenic, and methanogenic microorganisms commonly found in anaerobic digestion systems. This ensured reproducible microbial activity for biofilm development and methane production.
The LF-FVW was adjusted to an initial concentration of 5 g COD/L for inoculation, startup, and conditioning of the bioreactor [56,57,58], using the fiber matrix impregnated with GNPs and GOQDs in the LASR. The operating conditions were a hydraulic retention time (HRT) of 24 h and a pH ranging from 5.23 to 7.5. During this phase, the reactor was operated in a semi-continuous mode, with periodic feeding every two days and partial effluent withdrawal prior to refeeding. This setup favored progressive biofilm colonization on the fibers under stable conditions. Once the biofilm was visually confirmed and the system reached steady performance, the inoculated fibers were removed and prepared for subsequent methane potential assays.
2.5. Analytical Techniques
Throughout all experimental phases, daily sampling of influent and effluent was performed, collecting 30 mL samples to evaluate the removal percentages of pH, TCOD, SCOD, TS, and TVS, according to the procedures established in the Standard Methods for the Examination of Water and Wastewater [59]. pH was measured using a pH meter, model pH700 (OAKTON®, Vernon Hills, IL, USA). TCOD and SCOD were determined by the colorimetric method using a high-range photometer, model V-2000 (CHEMetrics®, Midland, VA, USA), with a detection range of 0–1500 ppm. TS and TVS were quantified by the gravimetric method using an oven, model HCF (Riossa®, Mexico City, Mexico), and a muffle furnace, model J-01 (Marla®, Zapopan, Jalisco, Mexico). All samples were processed immediately after collection.
2.6. Anaerobic Digestion Experiments
Biochemical Methane Potential (BMP) tests were performed to evaluate the methane production potential of the inoculated fiber matrices. Batch experiments were conducted over a period of 28 days, in triplicate, using 120 mL glass serum bottles with a working volume of 75 mL. The substrate was LF-FVW at a concentration of 5 g COD/L and an initial pH between 7.12 and 7.30. In this phase, the fibers matrices previously impregnated with GNPs or GOQDs and inoculated in the LASR were transferred to the BMP bottles to evaluate methane yield under batch conditions. All bottles were tightly sealed with butyl rubber stoppers and incubated at 35 ± 2 °C until daily methane production was less than 1% of the cumulative volume for three consecutive days.
Biogas volume was measured at regular intervals until production ceased, using 5–60 mL glass syringes with Luer-Lock shut-off valves. Methane content was determined by gas chromatography (GC) using a Buck Scientific 310 gas chromatograph (Buck Scientific®, East Norwalk, CT, USA). Blank tests containing only the fiber matrices, without GNPs, GOQDs, or substrate, were included to account for endogenous methane production [60,61]. Methane production obtained with the control fiber matrix (CFM) was used as a reference to compare the methanogenic performance of the matrices modified with GNPs and GOQDs.
2.7. Material Characterization
The functional groups of CG, C-EG, GNPs, GOQDs, and the fiber matrix were identified by Fourier transform infrared spectroscopy (FT-IR) using a Vectro 33 FT-IR spectrometer (Bruker®, Billerica, MA, USA) with a resolution of 1 cm−1.
The morphology of the biofilm developed on the fiber matrix in response to graphene-based material was observed using a scanning electron microscope, model Nova NanoSEM 200 (FEI®, Hillsboro, OR, USA). Fiber matrix samples were fixed with 2.5% (v/v) glutaraldehyde at 4 °C, washed, gradually dehydrated in a series of ethanol solutions with increasing concentrations, and then dried with CO2 at the critical point. Finally, the samples were gold-coated and analyzed by SEM.
Statistical Analysis
Descriptive statistical analyses were performed to ensure the reliability of the experimental results. All measurements related to the type of nanomaterial GNPs and GOQDs and their concentrations (0, 30, 50, and 100 mg/g support) were conducted in triplicate. The mean values and standard deviations of each treatment were calculated. In addition, methane production and yield data were compared based on the values calculated from the removed COD.
Subsequently, inferential statistical analysis was performed using a two-way analysis of variance (ANOVA) with replication, considering the type of fixed material and the applied concentration as factors. Prior to performing the ANOVA, the assumptions of normality and homogeneity of variances were verified. Normality was assessed using the Shapiro–Wilk test, and homogeneity of variances was evaluated with Levene’s test. Both analyses confirmed that the data met the required assumptions (p > 0.05). This analysis aimed to evaluate the effect of both factors on methane production and yield. All statistical calculations were performed using Microsoft Excel® version 2504 (Microsoft Corporation, Redmond, WA, USA), with a significance level set at p < 0.05.
2.8. Scope and Control Design
The objective of this study was to isolate the additive effect of GNPs and GOQDs on a commercial polymeric fiber matrix used as a biofilm support in anaerobic systems. Two configurations were evaluated under identical operational conditions: (i) the control fiber matrix without nanomaterials (CFM) and (ii) the modified fiber matrices impregnated with GNPs or GOQDs. This comparative setup allowed direct evaluation of the influence of nanomaterial addition on biofilm activity and methane production. The study is explicitly framed as a preliminary evaluation, and no advanced physicochemical or leaching analyses were conducted at this stage. A true blank control (without any fiber or additive) was not included in this work; however, its inclusion is planned for future experiments to strengthen the comparative framework and isolate the independent contribution of the fiber matrix itself. Future work will also incorporate extended characterization (e.g., XRD, XPS, BET, EDX mapping) and standardized leaching tests to evaluate the long-term stability of the nanomaterial fiber system.
3. Results and Discussion
This section presents a preliminary evaluation of the additive effect of graphene nanoplatelets and graphene oxide quantum dots incorporated into a commercial polymeric fiber matrix used as biofilm support in anaerobic systems. The objective is to identify the potential influence of these nanomaterials on biofilm fiber matrices with and without nanomaterial addition under identical experimental conditions, in accordance with the defined scope of this study (Section 2.8). The following subsections describe: (i) the impregnation and physicochemical characterization of the modified fiber matrices, (ii) reactor performance and methane yield results, and (iii) biofilm morphology, which together provide initial insight into the contribution of GNPs and GOQDs to anaerobic biofilm behavior. These comparisons were made between the CFM and the nanomaterial-impregnated matrices under identical conditions, allowing an isolated assessment of the additive effect of GNPs and GOQDs. A full control without fiber or additives was not included in this study but is acknowledged as an important element for future validation.
3.1. Fixation of GNPs and GOQDs in the Fiber Matrix
Table 1 presents the results of GNPs and GOQD fixation in the fiber matrix. After drying each matrix with its respective concentration, the highest amounts of nanomaterial were retained in FM100 and FMDOT100, corresponding to 1.84% and 3.52% of fixed material, respectively. Additionally, the retention percentage relative to the initial nanomaterial concentration was calculated and included in Table 1 to improve comparability across treatments, as suggested by the reviewer. The retention ranged between 7% and 14%, indicating effective impregnation of GNPs and GOQDs onto the fiber matrices. These small variations among treatments reflect the adsorption capacity limit of the fiber surface at higher nanomaterial loadings. According to the applied concentration, an increase in the amount of nanomaterial fixed in the polymeric fiber matrix was observed. Specifically, the GNP content in FM100 was 16% higher compared to FM30, and a similar trend was observed in FMDOT100. This effect is attributed to the higher initial concentration and the dispersion achieved by sonication. In the case of the control fiber matrix (FMC), the difference between the Dry matrix weight (330.02 ± 0.03 mg) and the Fixed weight (328.0 ± 0.03 mg) corresponds to the removal of residual moisture after 24 h of drying at 65 °C, which explains the slight reduction. Regarding the variability observed in dry matrix weights among FM and FMDOT fibers, although all fibers were cut to the same nominal size (5 × 1 cm), small variations in fiber thickness, nylon density, and cutting irregularities led to minor differences in the measured dry weights. This inherent variability is typical of polymeric fibers and does not affect the interpretation of nanomaterial impregnation, which was the main parameter under evaluation. Additionally, the trend in which FM50 > FM100 and FMDOT30 > FMDOT100 indicates that the relationship between nanomaterial concentration and retained mass is not strictly linear. At higher concentrations, aggregation and partial detachment of weakly bound particles during drying may occur, slightly reducing the final mass retained on the fiber surface.

Table 1.
Fiber matrix weights at each concentration of GNPs and GOQDs, as well as the nanomaterial fixed on the fiber surface.
Table 1.
Fiber matrix weights at each concentration of GNPs and GOQDs, as well as the nanomaterial fixed on the fiber surface.
| Fiber Matrix | Dry Matrix Weight (mg) | Fixed Weight (mg) | Retention (%) |
|---|---|---|---|
| FMC | 330.02 ± 0.03 | 328.0 ± 0.03 | - |
| FM30 | 347.07 ± 0.01 | 2.2 ± 0.22 | 7.3 ± 0.7 |
| FM50 | 372.32 ± 0.02 | 4.15 ± 0.25 | 8.3 ± 0.5 |
| FM100 | 360.32 ± 0.01 | 14.42 ± 0.58 | 14.4 ± 0.6 |
| FMDOT30 | 392.5 ± 0.05 | 3.9 ± 0.31 | 13.0 ± 1.0 |
| FMDOT50 | 360.57 ± 0.02 | 6.62 ± 1.08 | 13.2 ± 2.1 |
| FMDOT100 | 352.85 ± 0.01 | 12.42 ± 1.25 | 12.4 ± 1.3 |
Each fiber was tested in triplicate, and the values are expressed as mean ± standard error.
3.1.1. Physicochemical Characterization of the Materials
Figure 2 shows the FTIR spectra of the carbon-based materials used in the fiber matrix: CG, C-EG, GNPs, and GOQDs. For CG, after the steam explosion, two characteristic bands were observed at approximately 905 cm−1 and 967 cm−1, attributed to the stretching vibration of epoxy groups [62,63] and the stretching vibration of oxygenated groups (C–O) [64], respectively. This confirms that CG contains a certain amount of oxygen from the beginning. After the exfoliation process for 80 min to obtain GNPs, the band at 967 cm−1 became more intense, without the appearance of additional oxygenated groups. This behavior indicates that the generation of oxygenated groups was not influenced by pressure, explosion time, or temperature during the steam explosion stage, considering CG as the starting material, in agreement with the report by Pérez-Ramírez et al. [64].
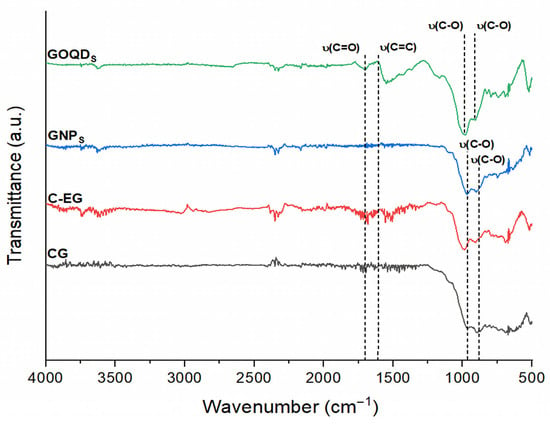
Figure 2.
FTIR spectra of the carbon-based materials used in the fiber matrix (CG = conventional graphite, C-EG = steam-exploded graphite, GNPs = graphene nanoplatelets, and GOQDs = graphene oxide quantum dots).
It is worth highlighting the importance of using pure water in the preparation of GNPs, as it eliminates stabilizers and avoids the need for purification after fixation in the fiber matrix [64]. By avoiding surfactants or stabilizing agents, no residual chemicals remain that could disrupt cell–surface interactions or inhibit microbial adhesion; this preserves biocompatibility and favors rapid colonization and stable biofilm formation, consistent with the higher methane yields observed in the BMP assays.
For the GOQDs, an increase in the C–O stretching band (≈1050–1150 cm−1) was observed, indicating a redistribution of oxygenated functional groups rather than a complete loss. The absence of a well-defined hydroxyl stretching band around 3200–3500 cm−1 suggests that surface –OH groups were either present in low concentration or overlapped with adsorbed water signals. This variation is consistent with partial reduction and structural rearrangement of the oxygen containing groups during the hydrothermal treatment in DMF, in accordance with reports for similar GOQD syntheses [52].
3.1.2. Fiber Matrix Characterization
Figure 3 shows the FTIR spectra of the CFM (control), FM30, FM50, and FM100 matrices fixed with GNPs, as well as FMDOT30, FMDOT50, and FMDOT100 matrices modified with GOQDs. In the CFM spectra, characteristic Nylon bands were observed, including those associated with N–H stretching (~3300 cm−1), CH2 (~2913 cm−1), and amide I (~1650 cm−1) and amide II (~1553 and 1467 cm−1) bands, corresponding to C=O and N–H bonds, respectively. The assignment of these absorption bands was confirmed by previous reports on Nylon 6,6 and graphene-based nanomaterials. The characteristic N–H stretching vibration near 3300 cm−1 and the CH2 stretching at ~2913 cm−1 are typical of polyamide structures [65,66]. The amide I (~1650 cm−1) and amide II (~1553–1467 cm−1) bands correspond to C=O and N–H vibrations, respectively, in agreement with the spectra of pristine Nylon 6,6 [65]. For GOQDs, the bands at ~1730 cm−1 (C=O stretching) and ~1050–1150 cm−1 (C–O stretching) are consistent with oxygenated functional groups reported for graphene oxide and graphene oxide quantum dots [67,68]. These assignments support the interpretation that the nanomaterials were successfully incorporated into the polymeric matrix through non-covalent and hydrogen-bond interactions.
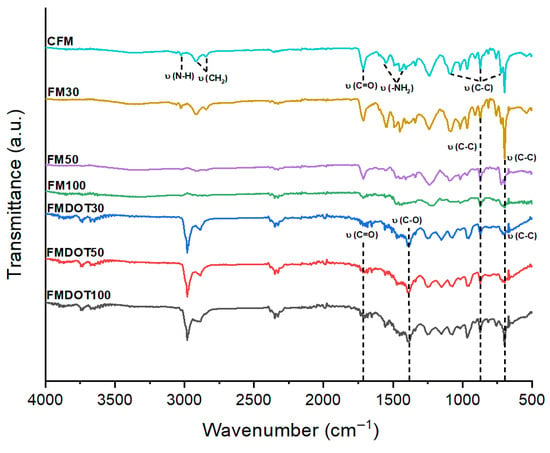
Figure 3.
FTIR spectra of the different concentrations in the fiber matrix: CFM = control fiber matrix, FM30 = fiber matrix 30, FM50 = fiber matrix 50, FM100 = fiber matrix 100, FMDOT30 = fiber matrix dot 30, FMDOT50 = fiber matrix dot 50, and FMDOT100 = fiber matrix dot 100.
Comparison among the different GNP concentrations revealed a progressive disappearance of these bands in FM30 and FM50, an effect more evident in FM100, where the bands assigned to –NH2 and –NH– groups vanished in the region from 3027 to 1450 cm−1. This behaviour can be attributed to the increased GNP concentration and its interaction with the matrix. Graphene nanoplatelets, due to their laminar structure and low surface functionalization, promote physical interactions such as π–π stacking and van der Waals forces with polymer chains. These interactions may restrict the vibrational mobility of the Nylon functional groups, thereby reducing the intensity of their characteristic bands and even leading to their disappearance in the FTIR spectra. Moreover, optical shielding resulting from the strong absorbance of GNPs may contribute to this phenomenon. In FM100, no bands associated with oxygenated groups (C–O) were detected, which is consistent with the low functionalization expected from the steam explosion process, where no extreme pressure or temperature conditions were applied to promote oxygen incorporation. In contrast, the spectra of FMDOT30, FMDOT50, and FMDOT100 showed an opposite trend: an increase in the intensity of certain bands, particularly those related to C=O (~1730 cm−1) and C–O (~1050 cm−1) vibrations, attributable to oxygenated functional groups present in GOQDs. Unlike GNPs, GOQDs exhibit higher surface functionalization with groups such as –OH, –COOH, and C=O, enabling the formation of hydrogen bonds and other noncovalent interactions with the Nylon matrix. These interactions not only fail to suppress the molecular vibrations of the polymer but also intensify them, reflecting greater compatibility and homogeneous dispersion within the matrix.
These findings are consistent with previous reports showing that the incorporation of carbon nanomaterials can induce changes in the intensity of spectral peaks associated with modifications in the crystallinity of the polymeric matrix. Specifically, the presence of GOQDs caused shifts and increases in N–H band intensities, suggesting the formation of hydrogen bonds or even covalent interactions between Nylon and GOQDs [69,70]. Overall, the FTIR data indicate that the chemical nature and surface functionalization of the nanomaterial play a crucial role in the interactions with the matrix: more disruptive in the case of GNPs and more cooperative in the case of GOQDs. This supports the hypothesis that the oxygenated groups of GOQDs do not negatively alter the polymer structure but may even improve its compatibility [71]. Importantly, the FTIR spectra also provide direct evidence of the successful integration of GNPs and GOQDs within the nylon matrix through the appearance and intensity variation of characteristic vibrational bands (C=O, C–O, and N–H). The spectral shifts observed confirm interfacial interactions and chemical compatibility between the nanomaterials and the polymer backbone, reinforcing the effectiveness of the impregnation and fixation procedure. Although FTIR offers indirect chemical confirmation rather than spatially resolved mapping, its results when combined with SEM morphological data strongly support the stable incorporation of nanomaterials into the fiber structure. Future studies should include quantitative assessments of the mechanical reinforcement provided by the nanomaterial fiber interaction and the biofilm adhesion strength to better understand the structural compatibility and stability of the modified matrices under operational conditions. Additionally, complementary spectroscopic analyses (e.g., Raman and XPS) are recommended to verify the bonding environment and confirm the chemical states of the nanocomposites [72], thereby strengthening the interpretation of the nanomaterial matrix interaction.
3.1.3. Substrate Characterization
Table 2 presents the physicochemical characterization of LF-FVW and inoculum, performed beforehand to use them in the sludge reactor and carry out the inoculation of nanomaterial-impregnated fibers. LF-FVW showed a TCOD of 36.78 ± 1.3 g/L, an SCOD of 27.89 ± 1.7 g/L, a TS content of 1.56 ± 0.1%, and a TVS percentage of 75.85 ± 0.4%. The temperature was 25 ± 0.2 °C and the recorded pH was 4.01 ± 0.3. Additionally, the COD/TS ratio was calculated and included in Table 2 to provide a better understanding of the relationship between organic matter and total solids, which is often used as an indicator of substrate biodegradability. The inoculum presented a TCOD of 84.2 ± 1.2 g/L, an SCOD of 68 ± 1.3 g/L, a TS content of 4.49 ± 0.01%, and a TVS percentage of 41.51 ± 2.39%. The temperature was 24 ± 0.2 °C and the pH was 8.93 ± 0.64. This characterization was essential to establish the initial conditions in the reactor and to promote the interaction between the nanomaterials and the fiber matrix.

Table 2.
Physicochemical composition and COD/TS ratio of LF-FVW and inoculum.
Table 2.
Physicochemical composition and COD/TS ratio of LF-FVW and inoculum.
| Parameter | LF-FVW | Inoculum | COD/TS Ratio |
|---|---|---|---|
| TCOD (g/L) | 36.78 ± 1.3 | 84.2 ± 1.2 | 23.6 |
| SCOD (g/L) | 27.89 ± 1.7 | 68 ± 1.3 | |
| TS (%) | 1.56 ± 0.1 | 4.49 ± 0.01 | |
| TVS (%) | 75.85 ± 0.4 | 41.51 ± 2.39 | |
| Temperature (°C) | 25 ± 0.2 | 24 ± 0.2 | |
| pH | 4.01 ± 0.3 | 8.93 ± 0.64 |
Values are expressed as mean ± standard error (n = 3). COD/TS ratio was calculated to indicate substrate biodegradability.
3.2. LASR Monitoring
The LASR was operated for a total of 75 days, using 5 g COD/L of LF-FVW as feed and an initial inoculum of 100 mL. During this period, three distinct phases describing the system’s behaviour were identified, as shown in Figure 4. All fiber matrices (CFM, FM30, FM50, FM100, FMDOT30, FMDOT50, and FMDOT100) were installed simultaneously in the LASR to allow parallel inoculation and biofilm development. Therefore, Figure 4 represents the overall reactor performance (TCOD and SCOD removal) rather than the individual behavior of each matrix. A schematic timeline was incorporated within the same figure to visually represent the experimental sequence and summarize the operational phases as follows: Phase I (0–54 days) microbial adaptation with full inoculum; Phase II (55–64 days) stabilization and evaluation with 50% reduced suspended biomass to assess biofilm activity; and Phase III (65–75 days) operation with the fiber matrices only, confirming biofilm-based methanogenic activity. These phases were intentionally designed to evaluate the progressive adaptation and methanogenic performance of the nanomaterial-impregnated fiber matrices under identical substrate conditions. The only variable among them was the gradual reduction in suspended inoculum, allowing the assessment of the biofilm’s contribution to COD removal and methane generation.
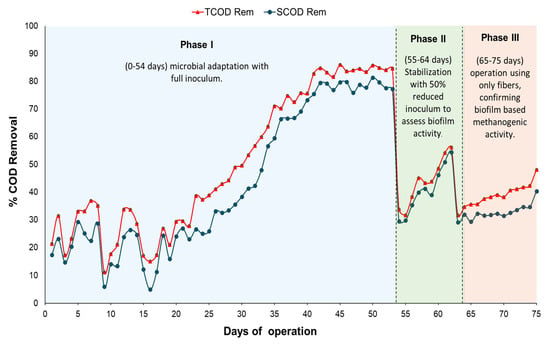
Figure 4.
Monitoring of COD removal (%) in the LASR during 75 days of operation, showing the three operational phases with all fiber matrices (control and nanomaterial-impregnated) co-immobilized in the reactor. The color-coded schematic highlights Phase I (0–54 days) microbial adaptation with full inoculum; Phase II (55–64 days) stabilization and evaluation with 50% reduced suspended biomass; and Phase III (65–75 days) operation with the fiber matrices only, confirming biofilm-based methanogenic activity.
In the first phase (0–54 days), moderate removals of 26.97% TCOD and 24.39% SCOD were observed, attributable to the microbial adaptation process to the new substrate and the initial colonization of the fiber surface. During this stage, hydrolytic and acidogenic bacteria were predominant, initiating the degradation of easily biodegradable compounds in the LF-FVW and forming the first biofilm layers on the nanomaterial-impregnated fibers. From day 20 to day 53, within phase I, the system achieved significantly higher substrate removal, with maximum values of 84.70% TCOD and 77.18% SCOD. This improvement is associated with the progressive establishment of a stable biofilm and the transition to acetogenic and methanogenic activity, where the microbial community became better adapted to the substrate and the operational conditions of the LASR. These results are consistent with previous studies indicating that the use of supports, such as granular activated carbon (GAC) or biofilm systems in anaerobic reactors, enhances removal efficiency after the acclimation period. For instance, Lin and Ho [41] observed COD removals of up to 82% in a biological activated carbon (BAC) reactor treating textile effluents, while moving bed biofilm reactor (MBBR) systems achieved efficiencies above 90% in SCOD removal after 60 days of operation [72,73]. In addition, two-stage anaerobic treatment configurations with LF-FVW residues have shown removals close to 84% [74]. Moreover, from an operational perspective, the nylon fiber matrix offers a more cost-effective and scalable alternative compared with conventional BAC and MBBR systems. While these systems require the periodic replacement of activated carbon or plastic carriers leading to higher operational and maintenance costs the nylon fibers can be reused for multiple cycles without significant structural degradation. Their flexible geometry and modular configuration also facilitate scale-up and integration in different reactor designs, supporting the economic feasibility and long-term applicability of this approach [7,54].
In the second phase (55–64 days), the initial inoculum volume (100 mL) increased to 130.5 mL due to microbial growth during operation. In this stage, half of this volume was removed, leaving only 65.2 mL in the reactor, with the aim of evaluating whether the biofilm formed on the fiber matrix could sustain methanogenic activity with a reduced amount of suspended biomass. Under these conditions, 56.19% TCOD and 54.36% SCOD removals were achieved, confirming that the attached biofilm maintained the metabolic stability of the system even after reducing the suspended fraction.
Finally, in the third phase (65–75 days), the residual inoculum volume (65.2 mL) was completely removed, and the reactor was operated solely with 1.5 L of LF-FVW. During this stage, 51.65% TCOD and 46.47% SCOD removals were achieved, attributable to the effect of the fiber matrix as a support for biofilm development. At this point, methanogenic activity was maintained primarily by the attached biomass, with the biofilm acting as a self-sustaining microbial consortium capable of balancing hydrolysis, acetogenesis, and methanogenesis. The porous and rough structure of the nanomaterial-impregnated fibers favored microbial adhesion and nutrient transfer, allowing stable biogas production even without suspended sludge. Previous studies have shown that the porous and rough structure of biocarriers favors microbial adhesion, enhances cell retention, and enables efficient treatment of high-strength effluents under conditions of low HRT and high OLR [75,76]. At the end of this stage, the organic load in the effluent was 2.40 g COD/L. These results confirm the effectiveness of the LASR system coupled with nanomaterial-impregnated fibers as a viable alternative for the anaerobic treatment of liquid organic waste.
3.2.1. Effect of the Fiber Matrix on Biogas and Biomethane Production
Figure 5 shows the effects of different matrices with varying concentrations of GNPs and GOQDs on cumulative methane production. For matrices with GNPs (FM30, FM50, and FM100), methane production was 108.0 ± 7.40, 110.20 ± 5.28, and 112.72 ± 7.55 mL CH4/g COD(rem), respectively. In contrast, for matrices with GOQDs (FMDOT30, FMDOT50, and FMDOT100), cumulative methane production values were 119.49 ± 7.15, 126.72 ± 9.83, and 116.10 ± 7.06 mL CH4/g COD(rem), respectively. The CFM, without nanomaterial addition, recorded a methane production of 106.58 ± 6.82 mL CH4/g COD(rem). These results indicate that incorporating GNPs and GOQDs into the matrix enhanced methane production. In particular, FMDOT50 exhibited a 19% increase compared to CFM, while FM100 showed a 6% increase. This performance can be attributed to the fact that the addition of quantum dots in anaerobic systems optimizes nutrient balance and microbial activity in the substrate, which are crucial factors for efficient methane production. Previous studies have shown that the addition of co-substrates, such as agricultural residues and food waste, improves methane yield by enhancing microbial interactions and nutrient availability [77,78]. In this context, the presence of GOQDs could strengthen these interactions by providing a more favourable environment for microbial growth and activity. Furthermore, their conductive nature enables more efficient interactions among microorganisms involved in anaerobic digestion, accelerating organic matter decomposition and, consequently, methane production.
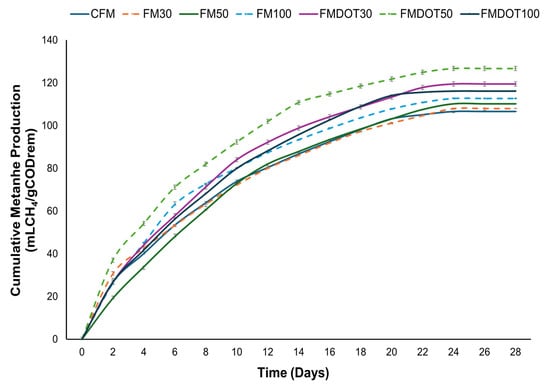
Figure 5.
Specific methane production from the fiber matrix after 28 days of incubation: CFM = control fiber matrix, FM30 = fiber matrix 30, FM50 = fiber matrix 50, FM100 = fiber matrix 100, FMDOT30 = fiber matrix dot 30, FMDOT50 = fiber matrix dot 50, and FMDOT100 = fiber matrix dot 100. Error bars represent the standard deviation of the mean (n = 3).
The small size and structural properties of GOQDs also contribute to their effectiveness as additives. These quantum dots, ranging from 1 to 4 nm, exhibit a high degree of surface functionalization, which improves their solubility and interaction with microbial cells [79]. Therefore, FMDOT30 also showed a 12% increase in methane production compared to the control matrix. This observation is consistent with that reported by Wang et al. [15], who demonstrated the positive effect of graphene with an addition of 20.0 g/L, resulting in a 17% increase in methane production rate. Similarly, Lin et al. [72] observed improvements in methane production when incorporating graphene-based materials. In addition, Park et al. [76] reported that carbon-based conductive materials such as granular activated carbon (GAC) and powdered activated carbon (PAC) enhanced methane production on different substrates (acetic acid and ethanol), achieving values between 139.2 and 189.4 mL CH4/g COD(rem). A possible contribution of DIET cannot be excluded; however, it should be regarded only as a plausible hypothesis in our system. Recent studies have shown that conductive carbon-based materials, including graphene derivatives, can facilitate DIET by enhancing electron exchange between syntrophic partners during anaerobic digestion [11,48]. In the present work, DIET was not directly verified, and confirming this mechanism would require electrochemical and microbial analyses.
The daily methane production rate is shown in Figure 6. On day 2, FMDOT50 exhibited a remarkable increase in production, reaching a maximum value of 37.02 ± 0.4 mL/d, compared to the control matrix, which recorded 26.71 ± 0.6 mL/d. On the other hand, FM30 reached a maximum rate of 30.62 ± 0.8 mL/d, a value comparable to that reported by Lin et al. [13,80], who observed production rates in the range of 15.8 to 23.3 mL/d when using powdered activated carbon on substrates such as acetic acid and ethanol.
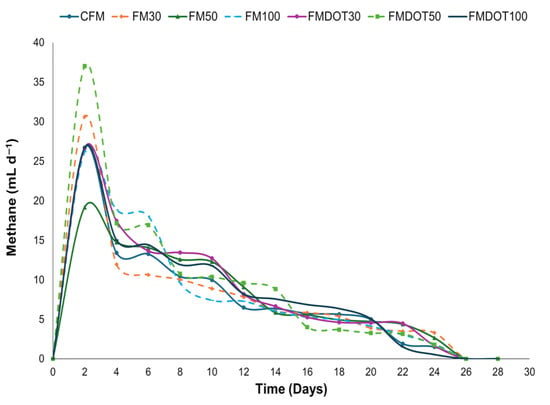
Figure 6.
Daily methane production rate from the fiber matrix after 28 days of incubation: CFM = control fiber matrix, FM30 = fiber matrix 30, FM50 = fiber matrix 50, FM100 = fiber matrix 100, FMDOT30 = fiber matrix dot 30, FMDOT50 = fiber matrix dot 50, and FMDOT100 = fiber matrix dot 100. Error bars represent the standard deviation of the mean (n = 3).
The biogas and methane yield values after the BMP tests are shown in Figure 7. The highest methane yields were obtained with the FMDOT50 and FMDOT30 matrices, with values of 348.36 ± 20.01 and 342.33 ± 20.03 mL CH4/g COD(rem), respectively. These values represented increases of 16% and 14% compared to CFM (301.02 ± 20.5 mL CH4/g COD(rem)).
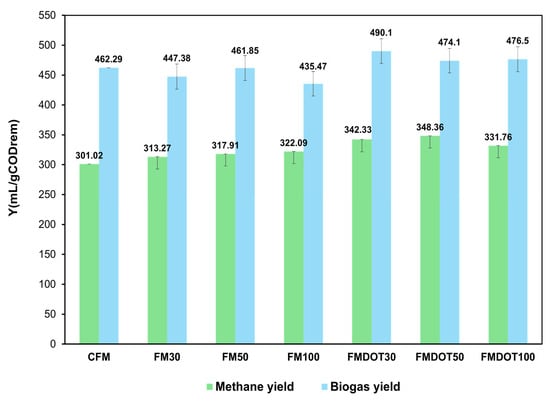
Figure 7.
Methane and biogas yields from the fiber matrix after 28 days of incubation: CFM = control fiber matrix, FM30 = fiber matrix 30, FM50 = fiber matrix 50, FM100 = fiber matrix 100, FMDOT30 = fiber matrix dot 30, FMDOT50 = fiber matrix dot 50, and FMDOT100 = fiber matrix dot 100. Error bars represent the standard deviation of the mean (n = 3).
In the case of FM100, a yield of 322.09 ± 20.60 mL CH4/g COD(rem) was achieved, corresponding to a 7% increase compared to CFM. According to the two-way ANOVA results, both the type of nanomaterial (p = 0.0048) and the applied concentration (p = 0.0078) had significant effects on methane production, confirming that these factors independently influenced the observed differences among treatments. The GOQD-modified matrices (FMDOT30 and FMDOT50) exhibited statistically higher methane yields (p < 0.05) than both the control (CFM) and the GNP only matrices (FM30, FM50, FM100), validating the positive impact of GOQDs on methanogenic activity. These significant differences demonstrate that the enhancement in methane yield was not a random variation but a reproducible effect attributable to the conductive and electron-mediating properties of GOQDs.
These results, expressed as a function of the effectively degraded substrate, indicate an improvement in conversion efficiency attributable to the presence of GOQDs in the matrices. It has been reported that methane production increases by 10% to 24% through the addition of carbon nanomaterials such as nitrogen doped GOQDs [81,82,83], nanographene, or graphene oxide [84], which validates that the behavior observed in this work falls within a range supported by the literature. This reinforces the hypothesis that quantum dots can facilitate mechanisms such as direct interspecies electron transfer among microbial species, promoting higher methanogenic activity and more efficient substrate conversion. Although DIET was not confirmed in this study, similar effects have been reported when conductive carbon based materials were incorporated into anaerobic systems, improving methane yield through possible DIET related pathways [11,48]. Therefore, DIET is proposed here only as a possible explanation for the enhanced biofilm activity and methane generation observed in the GNP and GOQD-modified matrices, rather than a confirmed mechanism. Further electrochemical or microbial community analyses will be required to verify this hypothesis. Although this study provides indirect evidence of enhanced electron transfer and microbial activity through the incorporation of conductive nanomaterials, no direct electrochemical measurements were performed to quantify these effects. To strengthen the mechanistic understanding of the observed enhancements, future investigations should include electrochemical analyses such as chronoamperometry (CA), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS) to evaluate electron transfer kinetics within the biofilm. Additionally, the quantification of extracellular redox mediators and the assessment of microbial activity through respiration rates or gene expression of electron transfer proteins (e.g., mtrA, omcZ) would provide more definitive evidence linking the nanomaterial incorporation to bioelectrochemical performance. These approaches would allow correlating electron transfer efficiency with methanogenic activity, thereby validating the proposed DIET related mechanisms with quantitative electrochemical support [85,86].
A schematic conceptual figure illustrating the proposed DIET mechanism facilitated by conductive nanomaterials GNPs and GOQDs within the nylon fiber matrix was added to visually represent microbial interactions and electron flow pathways (Figure 8).
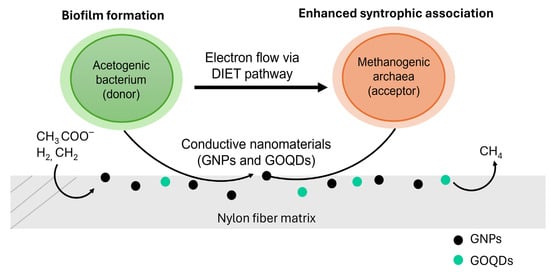
Figure 8.
Schematic representation of the DIET mechanism facilitated by GNPs and GOQDs within the nylon fiber matrix. The figure illustrates microbial syntrophic interactions between acetogenic (donor) and methanogenic (acceptor) microorganisms through conductive nanomaterials that enhance electron transfer and biofilm formation.
The two-way ANOVA results showed that both the type of nanomaterial and the applied concentration significantly affected methane production, while their interaction was not significant (p = 0.1031). Specifically, the type of nanomaterial (p = 0.0048) and concentration (p = 0.0078) had independent effects on the dependent variable, indicating that each factor acts separately. This suggests that methane production is primarily determined by the type of nanomaterial incorporated, while concentration modulates the magnitude of the effect without altering the relationship between material and yield. The specific methane yields obtained in the BMP tests confirmed this behaviour. The FM30, FM50, and FMDOT100 matrices reached values of 313.27 ± 20.02, 317.27 ± 20.02, and 331.76 ± 20.60 mL CH4/g COD(rem), respectively, all higher than the value recorded for CFM (301.02 ± 20.50 mL CH4/g COD(rem)). The improvement was more evident in treatments with GOQDs, which outperformed GNPs in methane production and yield.
Nevertheless, in the case of FMDOT100, a possible inhibition was observed, attributed to an overdose of nanomaterial, suggesting the existence of an optimal concentration threshold to maximize methanogenic efficiency. The complete statistical analysis results are presented in Table 3.

Table 3.
Results of the two-way analysis of variance (ANOVA) applied to methane production. A result was considered statistically significant when p < 0.05.
Table 3.
Results of the two-way analysis of variance (ANOVA) applied to methane production. A result was considered statistically significant when p < 0.05.
| Source of Variation | Sum of Squares | df | Mean Square | F | p-Value | Significant (p < 0.05) |
|---|---|---|---|---|---|---|
| Type of nanomaterial | 1060.848 | 1 | 1060.848 | 14.96 | 0.0048 | Yes |
| Concentration | 1757.389 | 3 | 585.796 | 8.26 | 0.0078 | Yes |
| Interaction between Nanomaterial and Concentration | 612.049 | 3 | 204.016 | 2.88 | 0.1031 | No |
Note: A result is considered statistically significant when p < 0.05. df = degrees of freedom.
It has been indicated that although GOQDs can facilitate certain metabolic pathways, they may also alter the composition of microbial communities under specific conditions, thereby reducing methane yields [78]. It is noteworthy that in this study, GNPs derived from conventional graphite were used, processed only with water. This represents an advantage compared to other commercial nanomaterials that require harsh chemicals for their synthesis and often end up mixed with the digestate, posing an environmental risk. It has been demonstrated that carbon-based materials such as graphite, biochar, and carbon cloth can promote methanogenic fermentation and COD removal [79]. Additionally, it has been reported that sludge electrical conductivity improves in the presence of carbon nanotubes, which could favour DIET between fermentative bacteria and methanogens during AD [80,82]. Muratçobanoğlu et al. [87], reported that during AD of the organic fraction of municipal solid waste (OFMSW), the addition of materials such as reduced graphene oxide (rGO) under fully mixed, semicontinuous, mesophilic conditions enhanced CH4 production. This was attributed to an increase in archaea and bacteria compatible with DIET, particularly at the end of the acetoclastic pathway. It has been confirmed that DIET can also occur with different conductive materials [81]. Due to their high electrical conductivity and mechanical strength, GOQDs can reinforce the structural integrity of microbial biofilms, a key aspect for improving the efficiency of anaerobic digestion [82].
3.2.2. COD Removal and pH Monitoring in BMP Tests
The TCOD(rem) for CFM was 95.79%, a high value attributed to biofilm formation on the fibers. However, the matrices with fixed nanomaterials achieved slightly higher values (FM100 = 96.29%; FMDOT50 = 96.27%), indicating that the incorporation of GNPs and GOQDs enhanced organic matter degradation. Although modest in percentage terms, this improvement translated into a more noticeable increase in methane yield: CFM produced 301.02 mL CH4/g COD(rem), whereas FMDOT30 and FMDOT50 reached 342.33 and 348.36 mL CH4/g COD(rem), corresponding to 14% and 16% increases, respectively.
Figure 9 shows the final COD removal efficiency for each matrix, evidencing that nanomaterial functionalized fibers consistently outperformed the control. This suggests that, even with high substrate degradation, methane conversion was less efficient in CFM, potentially reflecting the absence of nanostructured conductors that may enable conductive interactions consistent with DIET-like pathways [11,48]. Despite the natural variability of LF-FVW, the substrate showed a stable and high organic content, consistent with previous reports [83,84], supporting the effectiveness of the physical pretreatment (grinding and filtration) applied to urban organic solid waste (UOSW) before digestion. Furthermore, GOQDs have been shown to accelerate substrate degradation and enhance overall biogas production [88]. Recent studies have highlighted synergistic effects when combined with conductive materials such as biochar or activated carbon, suggesting that a multifactorial approach could further optimize process efficiency [89]. Although no specific statistical test was performed for COD removal, the observed increases in FM100 and FMDOT50 were consistent with the statistically significant differences (p < 0.05) confirmed by ANOVA for methane yield, supporting the reliability of these trends.
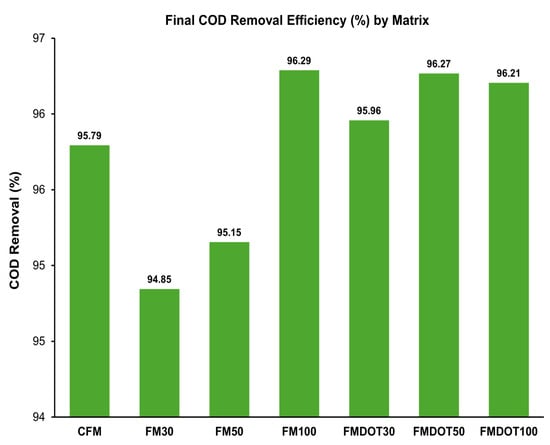
Figure 9.
COD removal efficiency during LASR operation with CFM and nanomaterial-impregnated fiber matrices. Error bars represent the standard deviation of the mean (n = 3).
In anaerobic digestion, pH is a critical parameter for system stability, as values close to neutrality favor the activity of methanogenic archaea. In the present experiment, the initial pH was adjusted to 7.5 to optimize biomethane production. During the tests, pH remained within a stable range, indicating favorable conditions for microbial activity [90,91]. In particular, matrices containing GNPs and GOQDs contributed to greater pH stability, suggesting an additional buffering effect in the system. This behavior is consistent with previous reports. Goodarzi et al. [92] reported that GNPs with different degrees of oxidation allow better control of digester conditions by reducing pH fluctuations. Ma et al. [93] observed that GOQDs act as nucleation centers for microbial communities, facilitating more stable metabolic pathways and reducing the accumulation of inhibitory metabolites. In addition, studies on hybrid GO systems with metal ions have demonstrated higher resistance to pH shocks, maintaining both COD removal and biogas production under adverse conditions [94].
Furthermore, Florentino et al. [95] and Lü et al. [96] demonstrated that the use of conductive materials such as pyrochar and granular activated carbon helps inhibit ammonia accumulation. Zhao et al. [97] reported that carbon cloth mitigates acidification and accelerates methanogenesis through DIET mechanisms. Overall, these findings reinforce that the incorporation of nanomaterials such as GNPs and GOQDs not only stabilizes the pH of the medium but also optimizes the system’s metabolic efficiency by reducing inhibitors and promoting a more robust and sustained methanogenesis [98].
3.2.3. Biofilm Morphology in the Fiber Matrix
SEM analysis was used to characterize the morphology of the nanomaterials and their distribution on the fiber matrices (Figure 10a–i). Pure graphene nanoplatelets (Figure 10a) exhibited a laminar and wrinkled sheet-like morphology typical of exfoliated graphene structures obtained by steam explosion and ultrasonic treatment. The control fiber matrix (CFM, Figure 10b) displayed a smooth and homogeneous surface, while the FM100 matrix (Figure 10c,d) showed the presence of nanoplatelet stacks adhered to the polymeric surface. These laminar sheets are consistent with the morphology of graphene nanoplatelets with a lateral size of 1–5 µm and a thickness below 10 nm, resulting from the sonication and drying processes applied for fixation.
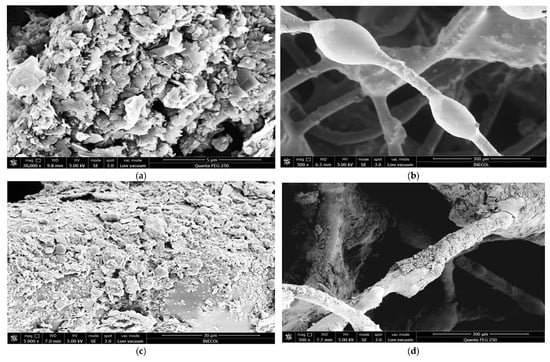
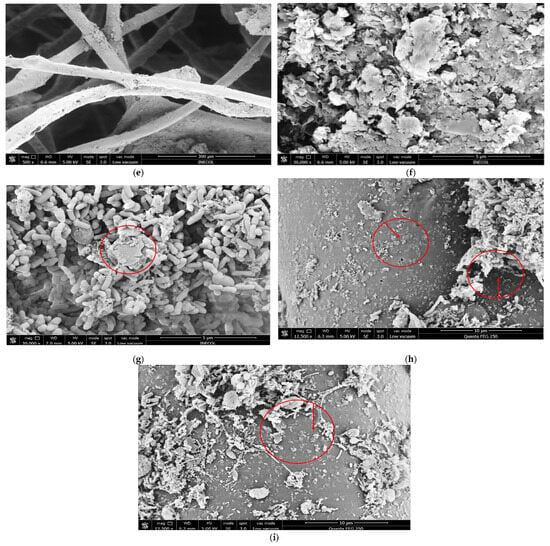
Figure 10.
SEM images of fiber matrices before and after inoculation. (a) Pure graphene nanoplatelets. (b) Control fiber matrix without nanomaterials (CFM). (c) Fiber matrix with GNPs fixed at a concentration of 100 mg/mL (FM100). (d) Fiber matrix showing laminar and irregular structures consistent with GNPs stacked on the nylon surface. (e,f) Fiber matrices modified with GOQDs (FMDOT50): (e) 500× showing homogeneous surface coating and nanomaterial deposition; (f) 30,000× showing fine aggregated regions and increased surface roughness consistent with GOQD fixation after the water heat process. (g–i) Fiber matrices with GNPs after inoculation, evidencing the interaction between nanomaterials and the microbial biofilm. Red annotations indicate representative regions of nanomaterial retention and microbial colonization.
In contrast, the FMDOT50 matrices (Figure 10e,f) exhibited a rougher and more irregular surface morphology compared with FM100, with fine aggregated regions and heterogeneous texture. These features are consistent with the deposition of GOQDs fixed onto the fiber matrix through the water–heat process. Although individual GOQDs (2–10 nm) could not be directly visualized due to their nanometric scale, their presence was inferred from the observed micro-roughness and localized contrast variations in the SEM images. Such morphological characteristics suggest an increased surface area favorable for microbial attachment and biofilm development, as further evidenced in the inoculated matrices (Figure 10g–i). The branched surface promoted colonization by adsorption and the formation of a structured biofilm that behaved as a “dynamic membrane,” acting as both a barrier and a catalytic surface for substrate degradation [99]. Morphological observations of the biofilm under SEM revealed coccoid and rod shaped cells compatible with Clostridium, Methanococcus, and Methanobacterium spp., which are commonly reported in mesophilic anaerobic systems operating at 35–36 °C and pH 6.5–7.5 [100,101]. Although the SEM images suggested morphological similarities with these genera, specific taxonomic confirmation was beyond the scope of this study and would require molecular identification.
In the case of GOQDs impregnated fiber matrices, no clear morphological features could be distinguished by SEM due to their nanometric size (typically < 20 nm) [102] and their homogeneous distribution on the fiber surface. Therefore, only representative micrographs of GNPs are shown, as they provided clearer evidence of nanoparticle retention. SEM observations and biomass quantification revealed distinct types of interaction between the nanomaterials and the biofilm. Graphene nanoplatelets promoted microbial adhesion and provided conductive surfaces that favored the establishment and growth of dense biofilms. In contrast, graphene oxide quantum dots contributed to structural integration within the fiber matrix, improving the uniformity and connectivity of the biofilm. These interactions are consistent with the enhanced biomass retention and methane yields obtained in the BMP assays. The presence of GOQDs in the matrices was instead corroborated indirectly by FTIR signals associated with oxygenated functional groups and by the enhanced biofilm growth and methane yields observed in the reactors.
The interaction between GNPs and the biofilm indicates that exposed nanoplatelets serve as a platform for adhesion and biofilm development. GNPs were covered by a matrix of extracellular polymeric substances (EPS), mainly composed of polysaccharides, proteins, and nucleic acids, forming a hydrated gel capable of stabilizing bacterial cells and facilitating their communication [103,104]. Since the GNPs were synthesized solely in water, they did not cause rejection or toxicity within the biofilm. In contrast, graphene oxide (GO) and reduced graphene oxide (rGO), obtained through chemical agents, have been reported to inhibit bacterial growth or biofilm formation due to oxidative stress and physical damage to the cell membrane [7,72,73,74,75]. Adhered biomass results showed that FM100 reached 3.34 mg VS/cm2, while FM30 and FM50 recorded 2.28 and 2.08 mg VS/cm2, and CFM 2.22 mg VS/cm2. The FMDOT30, FMDOT50, and FMDOT100 matrices (with quantum dots) presented 2.61, 2.30, and 4.21 mg VS/cm2, respectively. These values fall within the typical range for biofilms on porous supports (2–4 mg/cm2), with the highest value (4.21 mg/cm2) approaching densities reported for mature biofilms [105,106]. In the literature, the addition of graphene nanoplatelets has been shown to increase biogas production by 20–36%, mainly due to their role in improving cell retention and promoting more efficient microbial interactions [11,107]. Some authors have attributed these improvements to enhanced conductive connections among syntrophic partners, possibly compatible with DIET [11,32]. In our case, DIET was not demonstrated and remains a plausible hypothesis. Although statistical analysis was not conducted for attached biomass, the observed variations among the matrices followed the same significant pattern (p < 0.05) observed for methane yield, indicating consistent improvement in microbial retention in the fiber matrix impregnated with nanomaterials.
Although enhanced retention and potential recovery of the impregnated fibers were identified as theoretical advantages of this composite configuration, this study was limited to a single operational cycle. Therefore, the reuse and long term recovery of the fibers were not experimentally assessed and should be addressed in future studies to validate their potential for continuous or cyclic operation. Our FM100 and FMDOT100 matrices, which exhibited the highest biofilm densities, also achieved the greatest COD removals (96.29% and 96.21%) and elevated methane production (112.72 and 116.10 mL). However, FMDOT50, with intermediate biomass (2.30 mg VS/cm2), generated the maximum methane yield (126.72 mL). Similar results have been reported with carbon quantum dots, which increased CH4 production by approximately 24% by reducing electron transfer resistance and enriching active methanogenic archaea [80]. Likewise, Liu et al. [108], observed a 23% improvement in CH4 with doped CQDs at doses of 0.5 g/L. This supports the hypothesis that, beyond density, the functional quality of the biofilm enhanced in FM100 by GNPs and in FMDOT by quantum dots governs methanogenic efficiency. This effect has also been reported by Liu et al. [109], who explained that nanomaterials can act as redox centers or conductive bridges between syntrophic microorganisms, thereby enhancing the metabolic efficiency of the system.
Overall, our results are consistent with evidence showing that graphene and quantum dot based nanomaterials [11,44,110] can act as biofilm enhancers and microbial catalysts, improving biogas yield even in systems with intermediate biomass. The areas highlighted in Figure 10e–g show laminar elements or irregular groupings morphologically consistent with graphene nanoplatelets. These structures, partially embedded in the microbial matrix, reinforce the hypothesis that GNPs not only act as a physical support but also as promoters of bioelectrochemical synergies. This structural integration aligns with recent studies reporting that GNPs not only remain attached after bacterial colonization but also become incorporated into the biofilm architecture, enhancing its stability and functionality as a conductive platform [111,112,113]. To improve interpretability, Figure 10 was reprocessed at higher resolution and annotated with red circles and arrows to indicate regions of nanomaterial retention and biofilm growth. Nevertheless, it is important to recognize that SEM analysis presents inherent limitations in accurately resolving the biofilm nanomaterial interfaces. The sample dehydration and gold sputtering steps required for imaging can introduce morphological artifacts, and the spatial resolution of conventional SEM is insufficient to distinguish between nanoscale graphene layers, extracellular polymeric substances, and microbial cells. Therefore, the images should be interpreted as qualitative evidence of surface colonization and nanomaterial retention rather than definitive confirmation of molecular-level interactions. Future work will include complementary microscopic techniques such as confocal laser scanning microscopy (CLSM) and transmission electron microscopy (TEM) to validate these interpretations and quantitatively assess the three-dimensional architecture of biofilm nanomaterial assemblies [114]. However, we acknowledge that distinguishing microbial cells, fibers, and nanomaterials solely from SEM images presents certain challenges. To complement the morphological interpretation, Figure 10g–i were explicitly annotated with red circles highlighting regions consistent with GNP biofilm interactions. Future studies will include complementary imaging analyses such as fluorescent or confocal microscopy with differential staining to confirm microbial nanomaterial associations more rigorously. Although the present study focused on the structural and functional evaluation of the modified fiber matrices, it did not include leaching or degradation analyses. These aspects are crucial to ensure the environmental safety and long-term stability of nanomaterial based supports in anaerobic systems. Therefore, future work will incorporate standardized tests (e.g., TCLP and continuous flow leaching assays) to quantify nanoparticle release and assess the mechanical and chemical stability of the polymeric matrix under operational conditions. This step will be essential to validate the environmental feasibility and durability of the GNP and GOQD modified matrices for potential scale-up applications. In addition to these technical findings, the results of this study also have important environmental and practical implications. Enhancing the retention of graphene based nanomaterials within the polymeric fiber matrix not only improves biofilm stability and methane yield but also supports waste to energy valorization by converting food residues into renewable biogas. Furthermore, the stable immobilization of nanomaterials minimizes the risk of environmental release and promotes sustainability, reinforcing the feasibility of scaling up fixed-bed anaerobic digestion systems for practical applications in the treatment of organic waste.
3.3. Limitations of the Study
This study represents a preliminary evaluation of the additive effect of graphene-based nanomaterials (GNPs and GOQDs) incorporated into a commercial polymeric fiber matrix used as biofilm support in anaerobic systems. The experimental design focused on assessing the combined performance of the composite material under batch conditions; therefore, an isolated evaluation of the fiber substrate without nanomaterials was not included. Future work will incorporate a blank control and extend the physicochemical characterization through advanced techniques (e.g., XRD, XPS, BET, EDX mapping) to further validate the stability and reproducibility of the nanocomposite system.
Electrochemical and microbial community analyses were beyond the scope of this preliminary study but will be essential in future research to confirm the role of conductive nanomaterials in direct interspecies electron transfer (DIET). Likewise, complementary imaging and molecular techniques (e.g., confocal microscopy, 16S rRNA sequencing, FISH) will be applied to validate biofilm–nanomaterial interactions and microbial composition with higher resolution.
Finally, long-term operation, fiber recovery, and reusability tests were not addressed at this stage. These aspects will be considered in subsequent studies to evaluate the mechanical integrity and environmental safety of the modified fibers under continuous anaerobic operation.
4. Conclusions
This study provides preliminary evidence of the additive effect of GNPs and GOQDs incorporated into a commercial polymeric fiber matrix used as biofilm support in anaerobic systems. Intermediate nanomaterial loadings (FM50 and FMDOT50) achieved the highest methane yields, 317.91 ± 20.18 and 348.36 ± 20.01 mL CH4/g COD(rem), respectively, representing increases of 6% and 16% compared with the CFM. COD removal efficiencies remained high (96.29% for FM100 and 96.27% for FMDOT50), and the attached biomass ranged from 2.30 to 4.21 mg VS cm−2, indicating favorable microbial colonization. These tendencies suggest that incorporating graphene based nanomaterials may enhance biofilm activity and organic matter conversion under identical operational conditions. While a possible contribution of DIET could be involved in these enhancements, this mechanism remains unconfirmed in our system and should be addressed in future work through electrochemical and microbial analyses [11,48].
SEM and FTIR analyses supported the presence of GNPs and GOQDs on the fiber matrix and their interaction with microbial aggregates, consistent with the improved methane yield. However, since no physicochemical or leaching analyses were conducted, these results should be interpreted as an initial step toward understanding the behavior and stability of nanomaterial modified fiber matrices. From an environmental standpoint, the long-term use of nanomaterials in wastewater treatment requires careful assessment of their stability and potential release during operation. In this regard, the nylon fiber matrix acted not only as an efficient microbial carrier but also as a physical barrier that effectively retained the GNPs and GOQDs within the biofilm. This immobilization minimizes the risk of nanoparticle discharge into the effluent or sludge, contributing to safer operation and environmental protection.
Moreover, the inherent stability of carbon-based nanomaterials under anaerobic conditions suggests that their functionality can be maintained over extended operational periods. Nevertheless, future studies should evaluate possible leaching behavior under continuous flow conditions and assess the environmental fate of these nanomaterials after reactor decommissioning. Finally, the scalability of this retention strategy appears feasible, since polymeric fiber carriers are low cost, easy to handle, and compatible with fixed-bed and hybrid reactor configurations, which facilitates their adaptation to pilot and full-scale anaerobic systems.
Beyond these laboratory findings, the outcomes of this research highlight relevant environmental and practical implications. The improved biofilm stability and methane yield achieved with GNPs and GOQDs modified fiber matrices contribute to waste-to-energy valorization by converting organic residues into renewable biogas. Additionally, the stable retention of nanomaterials supports sustainable reactor operation and minimizes environmental risks, strengthening the feasibility of scaling up fixed-bed anaerobic digestion systems for real world applications.
Author Contributions
Conceptualization, A.M.S.-A.; methodology, A.M.S.-A., A.A.-L. and C.V.-S.; validation, A.M.S.-A. and A.A.-L.; formal analysis, A.A.-L. and J.M.M.-C.; investigation, A.M.S.-A., A.M.-S. and A.A.-L.; writing—original draft preparation, A.M.S.-A. and E.S.R.-M.; writing—review and editing, A.A.-L., A.M.-S., J.M.M.-C. and E.S.R.-M.; visualization, A.M.S.-A.; supervision, A.A.-L. and C.V.-S.; project administration, A.A.-L. and N.A.V.-C.; funding acquisition, A.A.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by the Tecnológico Nacional de México under project number 16811.23-P.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
Alexa Mariana Salgado-Arreguín acknowledges the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI), Mexico, for the doctoral scholarship (CVU 1040873). The authors acknowledge the Tecnológico Nacional de México, Campus Querétaro, for providing access to its research facilities and technical assistance in the characterization of materials. The authors also acknowledge the Instituto de Ecología, A.C. (INECOL), Unidad de Microscopía Avanzada, Campus Xalapa, for the SEM characterization support. During the preparation of this manuscript, the authors used ChatGPT (GPT-5, OpenAI, 2025 version) and Scite Assistant (Scite Inc., 2025 version) to improve the clarity, grammar, and citation consistency of the English text. Figures were created using BioRender.com. The authors reviewed and edited all content generated with these tools and take full responsibility for the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Anaerobic digestion |
| BAC | Biological activated carbon |
| ANOVA | Analysis of variance |
| BMP | Biochemical methane potential |
| C-EG | Steam-exploded graphite |
| CFM | Control fiber matrix |
| COD | Chemical oxygen demand |
| CQDs | Carbon quantum dots |
| DIET | Direct interspecies electron transfer |
| DMF | N,N-dimethylformamide |
| EPS | Extracellular polymeric substances |
| FM | Fiber matrix with graphene nanoplatelets |
| FMDOT | Fiber matrix with graphene oxide quantum dots |
| FMP | Polymeric fiber matrix |
| FTIR | Fourier transform infrared spectroscopy |
| GAC | Granular activated carbon |
| GC | Gas chromatography |
| GNPs | Graphene nanoplatelets |
| GO | Graphene oxide |
| GOQDs | Graphene oxide quantum dots |
| HRT | Hydraulic retention time |
| LASR | Lab-scale anaerobic sludge reactor |
| LF-FVW | Liquid fraction of fruit and vegetable waste |
| OFMSW | Organic fraction of municipal solid waste |
| PAC | Powdered activated carbon |
| pH | Potential of hydrogen |
| rGO | Reduced graphene oxide |
| SCOD | Soluble chemical oxygen demand |
| SEM | Scanning electron microscopy |
| TCOD | Total chemical oxygen demand |
| TS | Total solids |
| TVS | Total volatile solids |
| UOSW | Urban organic solid waste |
| Vu | Working volume |
References
- Darmey, J.; Ahiekpor, J.C.; Narra, S.; Achaw, O.W.; Ansah, H.F. Municipal solid waste generation trend and bioenergy recovery potential: A review. Energies 2023, 16, 7753. [Google Scholar] [CrossRef]
- Ebrahimian, F.; Denayer, J.F.; Mohammadi, A.; Khoshnevisan, B.; Karimi, K. A critical review on pretreatment and detoxification techniques required for biofuel production from the organic fraction of municipal solid waste. Bioresour. Technol. 2023, 368, 128316. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.S.; Sridhar, A.; Vishali, S. Utilization of fruit and vegetable waste to produce value-added products: Conventional utilization and emerging opportunities—A review. Chemosphere 2022, 287, 132221. [Google Scholar] [CrossRef] [PubMed]
- Orduña-Gaytán, F.; Vallejo-Cantú, N.A.; Alvarado-Vallejo, A.; Rosas-Mendoza, E.S.; Sandoval-Herazo, L.C.; Alvarado-Lassman, A. Evaluation of the removal of organic matter and nutrients in the co-treatment of fruit and vegetable waste using a bioreactor-constructed wetlands system. Processes 2022, 10, 278. [Google Scholar] [CrossRef]
- Alvarado-Lassman, A.; Sandoval-Ramos, A.; Flores-Altamirano, M.G.; Vallejo-Cantú, N.A.; Méndez-Contreras, J.M. Strategies for the startup of methanogenic inverse fluidized-bed reactors using colonized particles. Water Environ. Res. 2010, 82, 387–391. [Google Scholar] [CrossRef]
- Huang, X. The Promotion of Anaerobic Digestion Technology Upgrades in Waste Stream Treatment Plants for Circular Economy in the Context of “Dual Carbon”: Global Status, Development Trend, and Future Challenges. Water 2024, 16, 3718. [Google Scholar] [CrossRef]
- Abera, G.B.; Trømborg, E.; Solli, L.; Walter, J.M.; Wahid, R.; Govasmark, E.; Horn, S.J.; Aryal, N.; Feng, L. Biofilm Application for Anaerobic Digestion: A Systematic Review and an Industrial Scale Case. Biotechnol. Biofuels Bioprod. 2024, 17, 145. [Google Scholar] [CrossRef]
- Marín-Peña, O.; Alvarado-Lassman, A.; Vallejo-Cantú, N.A.; Juárez-Barojas, I.; Rodríguez-Jarquín, J.P.; Martínez-Sibaja, A. Electrical conductivity for monitoring the expansion of the support material in an anaerobic biofilm reactor. Processes 2020, 8, 77. [Google Scholar] [CrossRef]
- Rosas-Mendoza, E.S.; Méndez-Contreras, J.M.; Martínez-Sibaja, A.; Vallejo-Cantú, N.A.; Alvarado-Lassman, A. Anaerobic digestion of citrus industry effluents using an anaerobic hybrid reactor. Clean Technol. Environ. Policy 2018, 20, 1387–1397. [Google Scholar] [CrossRef]
- Ajay, C.M.; Mohan, S.; Dinesha, P.; Rosen, M.A. Review of impact of nanoparticle additives on anaerobic digestion and methane generation. Fuel 2020, 277, 118234. [Google Scholar] [CrossRef]
- Kang, H.J.; Lee, S.H.; Lim, T.G.; Park, J.H.; Kim, B.; Buffière, P.; Park, H.D. Recent advances in methanogenesis through direct interspecies electron transfer via conductive materials: A molecular microbiological perspective. Bioresour. Technol. 2021, 322, 124587. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, J.; Feng, Y.; Ding, J. Improvement of direct interspecies electron transfer via adding conductive materials in anaerobic digestion: Mechanisms, performances, and challenges. Front. Microbiol. 2022, 13, 860749. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Gan, R.; Jia, H.; Yong, X.; Yong, Y.C.; Zhou, J. Enhanced biogas production from swine manure anaerobic digestion via in-situ formed graphene in an electromethanogenesis system. Chem. Eng. J. 2020, 389, 124510. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, Y.; Lin, P.; Li, J.; Dong, H.; Yu, H.; Qi, L.; Ren, L. Effects of graphite, graphene, and graphene oxide on the anaerobic co-digestion of sewage sludge and food waste: Attention to methane production and the fate of antibiotic resistance genes. Bioresour. Technol. 2021, 339, 125585. [Google Scholar] [CrossRef]
- Longhin, E.M.; Rios-Mondragon, I.; Mariussen, E.; Zheng, C.; Busquets, M.; Gajewicz-Skretna, A.; Hofshagen, O.-B.; Gómez Bastus, N.; Franco Puntes, V.; Cimpan, M.R.; et al. Hazard Assessment of Nanomaterials: How to Meet the Requirements for (Next Generation) Risk Assessment. Part. Fibre Toxicol. 2024, 21, 54. [Google Scholar] [CrossRef]
- Schwirn, K.; Voelker, D.; Galert, W.; Quik, J.; Tietjen, L. Environmental Risk Assessment of Nanomaterials in the Light of New Obligations Under the REACH Regulation: Which Challenges Remain and How to Approach Them? Integr. Environ. Assess. Manag. 2020, 16, 706–717. [Google Scholar] [CrossRef]
- Bleeker, E.A.J.; Swart, E.; Braakhuis, H.; Fernández-Cruz, M.L.; Friedrichs, S.; Gosens, I.; Herzberg, F.; Jensen, K.A.; von der Kammer, F.; Kettelarij, J.; et al. Towards harmonisation of testing of nanomaterials for EU regulatory requirements on chemical safety—A proposal for further actions. Regul. Toxicol. Pharmacol. 2023, 139, 105360. [Google Scholar] [CrossRef]
- Chávez-Hernández, J.A.; Velarde-Salcedo, A.J.; Navarro-Tovar, G.; González, C. Safe Nanomaterials: From Their Use, Application, and Disposal to Regulations. Nanoscale Adv. 2024, 6, 1583–1610. [Google Scholar] [CrossRef]
- Environment and Climate Change Canada. Framework for the Risk Assessment of Manufactured Nanomaterials Under the Canadian Environmental Protection Act (CEPA). Government of Canada. 2022. Available online: https://www.canada.ca/en/environment-climate-change/services/evaluating-existing-substances/framework-risk-assessment-manufactured-nanomaterials-cepa-draft.html (accessed on 10 October 2025).
- Kundu, D.; Banerjee, S.; Karmakar, S.; Banerjee, R. A new insight on improved biomethanation using graphene oxide from fermented Assam lemon waste. Fuel 2022, 309, 122195. [Google Scholar] [CrossRef]
- Abdelwahab, T.A.M.; Pan, J.; Ni, J.-Q.; Yang, C.; Mohanty, M.K.; Darwish, E.A.; Desoky, S.H.M.; Yang, H.; Fodah, A.E.M. Nanoparticles for improving biogas production and effluent biofertilizer. Sci. Rep. 2025, 15, 19233. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, R.; Ren, Z.; Zhang, B.; Lu, Q.; Yi, M.; Luo, Y. Quantitative Relationship Between Surface Chemistry of Graphene and Compatibility with Rubbers Established by Two-Dimensional Solubility Parameters. RSC Adv. 2024, 14, 39081–39093. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, X.; Wang, S.; Chen, L.; Li, H. The functional graphene/epoxy resin composites prepared by a novel two-phase extraction method. Front. Chem. 2024, 12, 1433727. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Atchudan, R.; Cheong, I.W. Recent studies on dispersion of graphene–polymer composites. Polymers 2021, 13, 2375. [Google Scholar] [CrossRef]
- Dehnou, K.H.; Shariati, A.; Morsali, A.; Alipour, M.; Ghasemi, S. A review: Studying the effect of graphene nanoparticles on polymers. In J. Nanomater.; 2023; 2023, pp. 1–17. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9891084/ (accessed on 10 October 2025).
- Wang, Y.; Li, H.; Zhang, X.; Xu, C. Optimizing graphene dispersion via polymer grafting for enhanced interface compatibility. Macromolecules 2025, 58, 2421–2433. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, M.; Liu, Y.; Zhao, D.; Liu, Y.; Tian, Z.; Liu, P.; Wang, L.; Li, L.; Yan, M. Interface-engineered functionalized graphene oxide reinforced polyamide 6 composites with enhanced mechanical and thermal properties. Polymer 2025, 335, 128849. [Google Scholar] [CrossRef]
- Megahed, M.; Fathy, A.; Morsy, D.; Shehata, F. Mechanical performance of glass/epoxy composites enhanced by micro- and nanosized aluminum particles. J. Ind. Text. 2021, 51, 68–92. [Google Scholar] [CrossRef]
- Guo, Z.; Xie, C.; Zhang, P.; Zhang, J.; Wang, G.; He, X.; Zhang, Z. Toxicity and transformation of graphene oxide and reduced graphene oxide in bacteria biofilm. Sci. Total Environ. 2017, 580, 1300–1308. [Google Scholar] [CrossRef]
- Ohemeng-Ntiamoah, J.; Datta, T. Biomethane potential test reveals microbial adaptation and increased methane yield during anaerobic co-digestion. Bioresour. Technol. Rep. 2021, 15, 100754. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Feng, Y.; Li, Q.; Wang, Q. Enhanced performances of anaerobic digestion processes: Role of nanomaterials in improving stability and electron transfer. J. Hazard. Mater. 2023, 459, 132249. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, O.; Kozlova, E.; Bredikhin, M.; Gubin, S.; Kolesnikov, E. Conductive polymers and their nanocomposites: Structure, properties, and applications. Polymers 2023, 15, 3783. [Google Scholar] [CrossRef] [PubMed]
- Usevičiūtė, L.; Baltrėnas, P.; Baltrėnaitė-Gedienė, E. Surface modification of polymeric media coated with conductive polyaniline to enhance methane production for anaerobic low-strength wastewater treatment. Int. J. Environ. Res. Public Health 2021, 18, 11493. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, D.; Huang, W.; Yang, Y.; Ji, M.; Nghiem, L.D.; Trinh, Q.T.; Tran, N.H. Insights into biofilm carriers for biological wastewater treatment processes: Current state-of-the-art, challenges, and opportunities. Bioresour. Technol. 2019, 288, 121619. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.M.A.; Othman, M.H.D.; Tai, Z.S.; Rabuni, M.F.; Amhamed, A.O.A.; Puteh, M.H.; Jaafar, J.; Rahman, M.A.; Kurniawan, T.A. Overcoming challenges in water purification by nanocomposite ceramic membranes: A review of limitations and technical solutions. J. Water Process Eng. 2024, 57, 104613. [Google Scholar] [CrossRef]
- Khan, T.; Aslam, M.; Basit, M.; Raza, Z.A. Graphene-embedded electrospun polyacrylonitrile nanofibers with enhanced thermo-mechanical properties. J. Nanopart. Res. 2023, 25, 78. [Google Scholar] [CrossRef]
- -Kady, M.M.; Ansari, I.; Arora, C.; Rai, N.; Soni, S.; Verma, D.K.; Mahmoud, A.E.D. Nanomaterials: A comprehensive review of applications, toxicity, impact, and fate to environment. J. Mol. Liq. 2023, 370, 121046. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Buendia, I.M.; Bak, J.; Baun, A. Degradability of aged aquatic suspensions of C60 nanoparticles. Environ. Pollut. 2011, 159, 3134–3137. [Google Scholar] [CrossRef]
- Lead, J.R.; Batley, G.E.; Alvarez, P.J.; Croteau, M.N.; Handy, R.D.; McLaughlin, M.J.; Judy, J.D.; Schirmer, K. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects—An updated review. Environ. Toxicol. Chem. 2018, 37, 2029–2063. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Wu, F.; Sajjad, W.; Zhang, Q.; Khan, I.; Ullah, K.; Wang, W. Long-term exposure to high-concentration silver nanoparticles induced toxicity, fatality, bioaccumulation, and histological alteration in fish (Cyprinus carpio). Environ. Sci. Eur. 2021, 33, 1–11. [Google Scholar] [CrossRef]
- Kang, G.S.; Park, S.H.; Lee, J.H.; Lee, Y.S. Graphene quantum dots with nitrogen and oxygen co-doping: Tailored surface functionalization and enhanced redox performance. Chem. Eng. J. 2020, 389, 124465. [Google Scholar] [CrossRef]
- Usevičiūtė, L.; Kriauciunas, D.; Vaitkiene, A.; Rybakova, D.; Stankevičius, M.; Kliopova, I. Effects of graphene-based nanomaterials on anaerobic digestion: Biogas yield, microbial community, and potential environmental impact. Materials 2025, 18, 3561. [Google Scholar] [CrossRef]
- Ponzelli, M.; Lombardo, M.; Sacchi, R.; Santoro, C.; Frattini, D. The ambivalent role of graphene oxide in anaerobic environments: From microbial toxicity to bioelectrochemical stimulation. J. Hazard. Mater. 2024, 472, 134253. [Google Scholar] [CrossRef]
- Tan, X.M.; Lin, C.; Fugetsu, B. Studies on toxicity of multi-walled carbon nanotubes on suspension rice cells. Carbon 2009, 47, 3479–3487. [Google Scholar] [CrossRef]
- Ghoto, K.; Simon, M.; Shen, Z.J.; Gao, G.F.; Li, P.F.; Li, H.; Zheng, H.L. Physiological and root exudation response of maize seedlings to TiO2 and SiO2 nanoparticles exposure. BioNanoScience 2020, 10, 473–485. [Google Scholar] [CrossRef]
- Ramos-Galicia, L.; Reyes-Vázquez, C.D.; Martínez-Hernández, A.L.; Rodríguez-González, J.A.; Rubio-González, C.; Almendarez-Camarillo, A.; Velasco-Santos, C. Performance of graphene derivatives produced by chemical and physical methods as reinforcements in glass fiber composite laminates. Appl. Compos. Mater. 2021, 28, 923–949. [Google Scholar] [CrossRef]
- Igarashi, K.; Miyako, E.; Kato, S. Direct interspecies electron transfer mediated by graphene oxide-based materials. Front. Microbiol. 2020, 10, 3068. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Kim, J.; Lee, C. Role and potential of direct interspecies electron transfer: A review on conductive materials in anaerobic digestion and related challenges. Processes 2018, 6, 159. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Wang, Y.; Chen, Y.; Zhao, Z.; Sun, C.; Zhang, J.; Li, S.; Zhou, Q.; Liu, H.; et al. Insights into Microbial Interactions and Electron Transfer Pathways in Anaerobic Digestion Systems: Implications for Process Optimization. Biotechnol. Biofuels Bioprod. 2023, 16, 391. [Google Scholar] [CrossRef]
- Yao, X.; Li, C.; Zhang, Y.; Wang, L.; Liu, Y.; Guo, Z. Fabrication and mechanical performance of graphene nanoplatelet/glass fiber reinforced polymer (GFRP) hybrid composites. Front. Mater. 2021, 8, 773343. [Google Scholar] [CrossRef]
- de la Luz-Asunción, M.; Pérez-Ramírez, E.E.; Martínez-Hernández, A.L.; García-Casillas, P.E.; Luna-Bárcenas, J.G.; Velasco-Santos, C. Adsorption and kinetic study of Reactive Red 2 dye onto graphene oxides and graphene quantum dots. Diam. Relat. Mater. 2020, 109, 108002. [Google Scholar] [CrossRef]
- Chaiprasert, P.; Vitidsant, T.; Tanticharoen, M.; Bhumiratana, S. Nylon fibers as supporting media in anaerobic hybrid reactors: Its effects on system performance and microbial distribution. Water Res. 2003, 37, 4605–4612. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Li, Y.; Zhang, W.; Zhang, X.; Liu, J.; Xu, J. Biofilm reactor with permeable materials as carriers for enhanced wastewater treatment performance. Water 2023, 15, 2415. [Google Scholar] [CrossRef]
- Alvarado-Vallejo, A.; Marín-Peña, O.; Rosas-Mendoza, E.S.; Méndez-Contreras, J.M.; Alvarado-Lassman, A. The valorization of fruit and vegetable wastes using an anaerobic fixed biofilm reactor: A case of discarded tomatoes from a traditional market. Processes 2024, 12, 1923. [Google Scholar] [CrossRef]
- Almendrala, M.C.; Valenzuela, K.A.T.; Santos, S.M.N.B.; Avena-Ardeta, L.G.S. Enhanced Biogas Production via Anaerobic Co-Digestion of Slaughterhouse and Food Waste Using Ferric Oxide as a Sustainable Conductive Material. arXiv 2025, arXiv:2505.04635. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, H.; Liu, L.; Yan, Z.; Chui, C.; Yang, N.; Wang, C.; Shen, G.; Chen, Q. Enhancing methane production in anaerobic digestion of food waste using co-pyrolysis biochar derived from digestate and rice straw. Molecules 2025, 30, 1766. [Google Scholar] [CrossRef]
- Sahil, S.; Nanda, S. Process Intensification of Anaerobic Digestion of Biowastes for Improved Biomethane Production: A Review. Sustainability 2025, 17, 6553. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Ortiz, M.; Gaviria, V.; Tamayo, A.; Chejne, F. Enhancing biomethane production from organic waste by incorporating biochar and activated carbon during anaerobic digestion. J. Mater. Cycles Waste Manag. 2025, 27, 924–938. [Google Scholar] [CrossRef]
- Ohemeng-Ntiamoah, J.; Datta, T. Can microbial acclimation work to avert inhibition during FOG co-digestion? In Residuals and Biosolids Conference 2021; Water Environment Federation: Alexandria, VA, USA, 2021. [Google Scholar]
- Acik, M.; Lee, G.; Mattevi, C.; Pirkle, A.; Wallace, R.M.; Chhowalla, M.; Chabal, Y. The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J. Phys. Chem. C 2011, 115, 19761–19781. [Google Scholar] [CrossRef]
- Pérez-Ramírez, E.E.; Ramos-Galicia, L.; de la Luz-Asunción, M.; Saucedo-Rivalcoba, V.; Martínez-Hernández, A.L.; Rubio-Rosas, E.; Velasco-Santos, C. A green and easy large-scale method for obtaining graphene nanoplatelets by steam explosion and ultrasonic exfoliation. ChemistrySelect 2022, 7, e202202425. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, L.; Wang, B.; Ruoff, R.S. Chemical vapor deposition of graphene on thin-metal films. Cell Rep. Phys. Sci. 2021, 2, 100372. [Google Scholar] [CrossRef]
- Rodríguez, A.; Alvarado, M.; Pérez, E.; Torres, J. Surface modification of nylon 6,6 by plasma treatment: Effect on chemical structure and adhesion properties. Coatings 2021, 11, 197. [Google Scholar] [CrossRef]
- Tech, J.E.T. A Combination of Waste Biomass Activated Carbon and Nylon Nanofiber for Removal of Triclosan from Aqueous Solutions. J. Environ. Treat. Tech. 2020, 8, 1036–1045. Available online: https://www.researchgate.net/publication/343180322_A_Combination_of_Waste_Biomass_Activated_Carbon_and_Nylon_Nanofiber_for_Removal_of_Triclosan_from_Aqueous_Solutions (accessed on 10 October 2025). [CrossRef]
- Melo, J.P.; Ríos, P.L.; Povea, P.; Morales-Verdejo, C.; Camarada, M.B. Graphene Oxide Quantum Dots as the Support for the Synthesis of Gold Nanoparticles and Their Applications as New Catalysts for the Decomposition of Composite Solid Propellants. ACS Omega 2018, 3, 7278–7287. [Google Scholar] [CrossRef]
- Jin, S.H.; Kim, D.H.; Jun, G.H.; Hong, S.H.; Jeon, S. Tuning the Photoluminescence of Graphene Quantum Dots through the Charge Transfer Effect of Functional Groups. ACS Nano 2013, 7, 1239–1245. [Google Scholar] [CrossRef]
- Menchaca-Campos, C.; García-Pérez, C.; Castañeda, I.; García-Sánchez, M.A.; Guardián, R.; Uruchurtu, J. Nylon/graphene oxide electrospun composite coating. Int. J. Polym. Sci. 2013, 2013, 621618. [Google Scholar] [CrossRef]
- Li, X.; Gu, X.; Zhang, S.; Li, H.; Feng, Q.; Sun, J.; Zhao, Q. Improving the fire performance of nylon-6,6 fabric by chemical grafting with acrylamide. Ind. Eng. Chem. Res. 2013, 52, 2290–2296. [Google Scholar] [CrossRef]
- Lim, J.V.; Bee, S.T.; Sin, L.T.; Ratnam, C.T.; Abdul Hamid, Z.A. Fabrication and conductivity of graphite nanosheet/nylon-610 nanocomposites using graphite nanosheets treated with supercritical water at different temperatures. Polymers 2022, 14, 4660. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.I.; Newbury, D.E.; Joy, D.C.; Lyman, C.E.; Echlin, P.; Lifshin, E.; Sawyer, L.; Michael, J.R. Scanning Electron Microscopy and X-Ray Microanalysis, 4th ed.; Springer: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Li, R.; Tan, W.; Zhao, X.; Dang, Q.; Song, Q.; Xi, B.; Zhang, X. Evaluation on the methane production potential of wood waste pretreated with NaOH and co-digested with pig manure. Catalysts 2019, 9, 539. [Google Scholar] [CrossRef]
- Wilkins, D.; Rao, S.; Lü, X.; Lee, P. Effects of sludge inoculum and organic feedstock on active microbial communities and methane yield during anaerobic digestion. Front. Microbiol. 2015, 6, 1114. [Google Scholar] [CrossRef]
- Hoang, T.; Pham, H.; Tran, Q. A facile microwave-assisted hydrothermal synthesis of graphene quantum dots for organic solar cell efficiency improvement. J. Nanomater. 2020, 2020, 3207909. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.H.; Seong, H.J.; Sul, W.J.; Jin, K.H.; Park, H.D. Metagenomic insight into methanogenic reactors promoting direct interspecies electron transfer via granular activated carbon. Bioresour. Technol. 2018, 259, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Al-Samet, M.; Goto, M.; Mubarak, N.; Al-Muraisy, S. Evaluating the biomethane potential from the anaerobic co-digestion of palm oil mill effluent, food waste, and sewage sludge in Malaysia. Environ. Sci. Pollut. Res. 2021, 28, 67632–67645. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Xia, Z.; Sun, J.; Dai, X.; Chen, X.; Ni, B. The inhibitory impacts of nano-graphene oxide on methane production from waste activated sludge in anaerobic digestion. Sci. Total Environ. 2019, 646, 1376–1384. [Google Scholar] [CrossRef]
- Li, H.; Xu, D.; Li, Y.; Feng, H.; Liu, Z.; Li, X.; Gu, T.; Yang, K. Extracellular electron transfer is a bottleneck in the microbiologically influenced corrosion of C1018 carbon steel by the biofilm of sulfate-reducing bacterium Desulfovibrio vulgaris. PLoS ONE 2015, 10, e0136183. [Google Scholar] [CrossRef]
- Mohamad, A.N.; Aziz, F.; Yusof, N.; Jaafar, J.; Wan Salleh, W.N.; Jye, L.W.; Ismail, A.F. Recent advances in heavy metal removal by thin film nanocomposite membrane. Water Supply 2023, 23, 3635–3659. [Google Scholar] [CrossRef]
- Deena, S.R.; Kumar, G.; Vickram, A.S.; Rani Singhania, R.; Dong, C.D.; Rohini, K.; Anbarasu, K.; Thanigaivel, S.; Ponnusamy, V.K. Efficiency of various biofilm carriers and microbial interactions with substrate in moving bed-biofilm reactor for environmental wastewater treatment. Bioresour. Technol. 2022, 359, 127421. [Google Scholar] [CrossRef]
- Gahlot, P.; Ahmed, B.; Brat, S.; Aryal, N. Conductive material engineered direct interspecies electron transfer (DIET) in anaerobic digestion: Mechanism and application. Environ. Technol. Innov. 2020, 20, 101056. [Google Scholar] [CrossRef]
- Nusair, A.; Barber, M.; Pramanik, A.; Ethridge, C.; William, C.; Alkhateb, H.; Ucak-Astarlioglu, M.; Ray, P.C.; D’Alessio, M. Graphene-coated sand for enhanced water reuse: Impact on water quality and chemicals of emerging concern. Sci. Total Environ. 2024, 945, 174078. [Google Scholar] [CrossRef]
- Claes, A.; Melchi, L.; Uludağ-Demirer, S.; Demirer, G. Supplementation of carbon-based conductive materials and trace metals to improve biogas production from apple pomace. Sustainability 2021, 13, 9488. [Google Scholar] [CrossRef]
- Van Steendam, C.; Smets, I.; Skerlos, S.; Raskin, L. Improving anaerobic digestion via DIET requires development of suitable characterization methods. Curr. Opin. Biotechnol. 2019, 57, 183–190. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Zhang, Y.; Lovley, D.R. Sparking anaerobic digestion: Promoting DIET to enhance methane production. iScience 2020, 23, 101794. [Google Scholar] [CrossRef] [PubMed]
- Muratçobanoğlu, H.; Gökçek, Ö.B.; Muratçobanoğlu, F.; Mert, R.A.; Demirel, S. Biomethane enhancement using reduced graphene oxide in anaerobic digestion of municipal solid waste. Bioresour. Technol. 2022, 354, 127163. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Jiang, Z.; Feng, L.; Pan, J.; Zhu, M.; Ma, C.; Jing, Z.; Jiang, H.; Zhou, H.; et al. Bio-based carbon materials with multiple functional groups and graphene structure to boost methane production from ethanol anaerobic digestion. Bioresour. Technol. 2022, 344, 126353. [Google Scholar] [CrossRef] [PubMed]
- Akshaya, N.B.; Jacob, S. Unification of waste management from fish and vegetable markets through anaerobic co-digestion. Waste Biomass Valorization 2020, 11, 1941–1951. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Demirer, G. Upgrading anaerobic sludge digestion by using an oil refinery by-product. Sustainability 2022, 14, 15693. [Google Scholar] [CrossRef]
- Martins, G.; Salvador, A.; Pereira, L.; Alves, M. Methane production and conductive materials: A critical review. Environ. Sci. Technol. 2018, 52, 10241–10253. [Google Scholar] [CrossRef]
- Goodarzi, M.; Arjmand, M.; Eskicioglu, C. Nanomaterial-amended anaerobic sludge digestion: Effect of pH as a game changer. Environ. Res. 2024, 240, 117463. [Google Scholar] [CrossRef]
- Ma, S.; Wang, H.; Gao, X.; Bian, C.; Zhu, W. Mitigating ammonia inhibition in anaerobic digestion with lignin-based carbon materials synthesized by hydrothermal carbonization. Carbon Res. 2025, 4, 20. [Google Scholar] [CrossRef]
- Li, Y.; Ma, C.; Ma, J.; Guo, W.; Liu, Y.; Jing, Z.; Xu, Q. Promoting potential direct interspecies electron transfer and methanogenesis with nitrogen- and zinc-doped carbon quantum dots. J. Hazard. Mater. 2021, 410, 124886. [Google Scholar] [CrossRef]
- Gao, F.; Xue, Y.; Deng, P.; Cheng, X.; Yang, K. Removal of aqueous ammonium by biochars derived from agricultural residuals at different pyrolysis temperatures. Chem. Speciat. Bioavailab. 2015, 27, 92–97. [Google Scholar] [CrossRef]
- Lü, F.; Wang, J.; Shao, L.; He, P. Granular activated carbon for ammonia removal from aqueous solutions: Adsorption characteristics and mechanisms. Water Res. 2016, 100, 1–10. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, L.; Zhong, G.; Huang, Y.; Zhang, X.; Chu, C.; Wang, M. Titanium dioxide nanoparticles induce mitochondrial dynamic imbalance and damage in HT22 cells. J. Nanomater. 2019, 2019, 4607531. [Google Scholar] [CrossRef]
- Wu, F.; Xie, J.; Xin, X.; He, J. Effect of activated carbon/graphite on enhancing anaerobic digestion of waste activated sludge. Front. Microbiol. 2022, 13, 999647. [Google Scholar] [CrossRef]
- Charitidis, P.J. Iron-based nanoparticles for enhanced biogas production: Comparative performance analysis and optimization strategies for sustainable anaerobic digestion. Int. Res. J. Innov. Eng. Technol. 2025, 9, 24–38. [Google Scholar] [CrossRef]
- Velasco-Maldonado, P.; Pat-Espadas, A.; Cházaro-Ruiz, L.; Cervantes, F.; Hernández-Montoya, V. Cold oxygen plasma induces changes on the surface of carbon materials enhancing methanogenesis and N2O reduction in anaerobic sludge incubations. J. Chem. Technol. Biotechnol. 2019, 94, 3367–3374. [Google Scholar] [CrossRef]
- Tian, T.; Qiao, S.; Li, X.; Zhang, M.; Zhou, J. Nano-graphene induced positive effects on methanogenesis in anaerobic digestion. Bioresour. Technol. 2017, 224, 41–47. [Google Scholar] [CrossRef]
- Balkanloo, P.G.; Sharifi, K.M.; Marjani, A.P. Graphene quantum dots: Synthesis, characterization, and application in wastewater treatment—A review. Mater. Adv. 2023, 4, 4272–4293. [Google Scholar] [CrossRef]
- Ersahin, M.E.; Tao, Y.; Ozgun, H.; Gimenez, J.B.; Spanjers, H.; van Lier, J.B. Impact of anaerobic dynamic membrane bioreactor configuration on treatment and filterability performance. J. Membr. Sci. 2017, 526, 387–394. [Google Scholar] [CrossRef]
- Laiq Ur Rehman, M.; Iqbal, A.; Chang, C.C.; Li, W.; Ju, M. Anaerobic digestion. Water Environ. Res. 2019, 91, 1253–1271. [Google Scholar] [CrossRef]
- Jelinkova, S.; Bacova, J.; Rousarova, E.; Nyvltova, P.; Knotek, P.; Capek, J.; Juklíková, M.; Strakova, N.; Apektov, L.; Janka, J. TiO2 P25 nanoparticles induce mitochondrial damage and increased glutathione synthesis in SH-SY5Y neural cells. Food Chem. Toxicol. 2025, 202, 115496. [Google Scholar] [CrossRef] [PubMed]
- Colunga, A.; Rangel-Mendez, J.R.; Celis, L.B.; Cervantes, F.J. Graphene oxide as electron shuttle for increased redox conversion of contaminants under methanogenic and sulfate-reducing conditions. Bioresour. Technol. 2015, 175, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Ci, S.; Cai, P.; Wen, Z.; Li, J. Graphene-based electrode materials for microbial fuel cells. Sci. China Mater. 2015, 58, 496–509. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Wang, H.; Zhao, S.; Li, Y.; Feng, L.; Pan, J.; Zhou, H.; Xu, C. High-efficient synthesis of straw-derived carbon quantum dots for facilitating methanogenic performance in high-load co-digestion of food waste and excess sludge. Chem. Eng. J. 2024, 500, 156792. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, S.; Zhang, H.; Feng, L.; Zhou, H.; Pan, J.; Li, Y.; Xu, C. N-doped carbon quantum dots enhance anaerobic digestion under light condition: The performances and potential mechanisms. Chem. Eng. J. 2023, 475, 146359. [Google Scholar] [CrossRef]
- Tan, G.; Yu, H.Q. Benefits of conductive additive for direct interspecies electron transfer in anaerobic digestion. Front. Environ. Sci. Eng. 2025, 19, 170. [Google Scholar] [CrossRef]
- Chang, H.; Wu, H. Graphene-based nanocomposites: Preparation, functionalization, and energy and environmental applications. Energy Environ. Sci. 2013, 6, 3483–3507. [Google Scholar] [CrossRef]
- Sousa-Cardoso, F.; Teixeira-Santos, R.; Campos, A.F.; Lima, M.; Gomes, L.C.; Soares, O.S.; Mergulhão, F.J. Graphene-based coating to mitigate biofilm development in marine environments. Nanomaterials 2023, 13, 381. [Google Scholar] [CrossRef]
- Romeu, M.J.; Gomes, L.C.; Sousa-Cardoso, F.; Morais, J.; Vasconcelos, V.; Whitehead, K.A.; Pereira, M.F.R.; Soares, O.S.G.P.; Mergulhão, F.J. How do graphene composite surfaces affect the development and structure of marine cyanobacterial biofilms? Coatings 2022, 12, 1775. [Google Scholar] [CrossRef]
- Lin, L.; Hou, C.; Zhang, X.; Wang, Y.; Chen, Y.; He, T. Highly efficient visible-light-driven photocatalytic reduction of CO2 over g-C3N4 nanosheets/tetra(4-carboxyphenyl) porphyrin iron(III) chloride heterogeneous catalysts. Appl. Catal. B Environ. 2018, 221, 312–319. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).