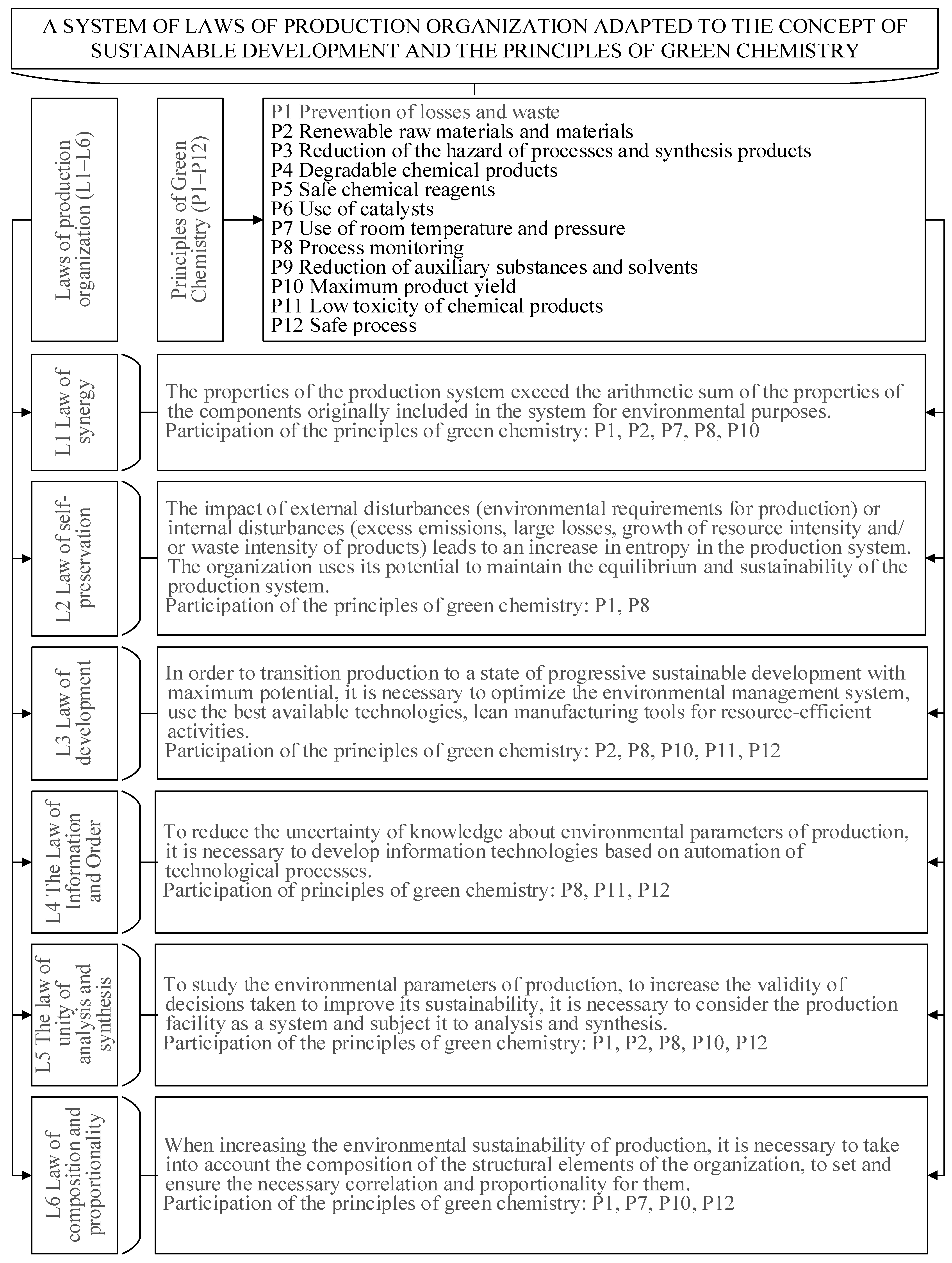
4.1. Solutions for Organizing Sustainable Chemical Industries Based on the Principles of Green Chemistry
Production organization as a science is based on six basic principles or laws: synergy, self-preservation, development, information orderliness, unity of analysis and synthesis, composition, and proportionality. Observance of these laws in the organization of production allows the formation of an “ideal”, effectively functioning industrial object. When creating sustainable industries, following the laws of production organization is mandatory and important to achieve the maximum possible design goals and results.
Interpretation of the laws of production organization in the projection of “green” chemistry allows the adaptation of the system of laws to the principles of formation of sustainable industries (
Figure 1).
The law of synergy says that the properties of a sustainable production system as a whole exceed the sum of the properties of its elements. For example, investing resources in the ecologization of production will significantly strengthen the potential of the production system. The law of self-preservation takes place when there is an impact on production of external or internal factors that lead to increasing uncertainty. To maintain the sustainability of the production system, the organization uses its potential by adopting green technologies. Throughout its life cycle, chemical production is continuously improving, increasing environmental sustainability, which is regulated on the basis of the law of development. The law of awareness and orderliness is necessary to reduce the uncertainty of the environmental parameters of production. In order to study environmental parameters and improve the validity of decisions on greening production, it is necessary to consider the object as a system and subject it to analysis and synthesis. Such a system approach, based on the law of unity of analysis and synthesis, allows us to make a deep analysis of the elements or subsystems of the production system, identify problem areas, and find effective solutions. Finally, the law of composition and proportionality says that it is necessary to take into account the composition of structural elements of production when carrying out environmental engineering.
Using the logical content method, let us consider which of the 12 principles of green chemistry can be observed and developed within the framework of the laws of production organization. As stated earlier, achieving sustainable production is possible by changing the approaches to the synthesis and technology of chemical products, methods of cleaning pollutants, and methods of production organization—the re-engineering of main, auxiliary, and service production processes.
To visualize the relationships, we used the adjacency matrix RL, where the principles of green chemistry P1–P12 are represented vertically, and the laws of production organization L1–L6 are represented horizontally (
Table 2).
We believe that it is reasonable to choose vectors of sustainable production creation with PL values > 4.0 (PLmax) or high strength of interrelation between the law of production organization and the principle of green chemistry. PLmax is observed for six items—principles of green chemistry:
P1 Loss and waste prevention (PL = 4.4);
P2 Renewable raw materials and materials (PL = 4.2);
P7 Use of room temperature and pressure (PL = 4.0);
P8 Process monitoring (PL = 5.2);
P10 Maximization of product yield (PL = 4.4);
P12 Safe process (PL = 4.4).
Certain laws of production organization can have a greater regulatory impact in the creation of sustainable production and give high development efficiency: PL criterion values > 0.8. The laws of production organization with PL criterion value < 0.8, with the same organizational efforts, will show a relatively lower environmental result.
Thus, the system of vectors of engineering of sustainable chemical production based on the principles of green chemistry will have the following form:
where S is a system of vectors of engineering of sustainable chemical production.
B1, … B6—vectors of engineering with high strength of interrelation of the law of production organization and the principle of green chemistry.
The set includes synthesized vectors of the organization of sustainable productions, which, according to the results of logical and content analysis, have a high potential for the implementation of environmental projects.
The interpretation of the sign representation of engineering vectors (B1–B6) in the decisions on the organization of sustainable production is presented in
Figure 2.
In order to control the parameters on the solutions of sustainable production organization B1–B6, it is necessary to monitor such indicators (relative indicators) as the following:
Volume of losses of material resources in production chains relative to the normative value (B1);
Share of by-products and waste products returned to the production cycle (B2);
Consumption of fuel and energy resources per unit of production (B3);
Duration of the production cycle relative to its critical path (B4);
Consumption of material resources per unit of production (B5);
Emissions into the atmosphere, discharges into water bodies, and solid waste per unit of production (B6).
The above indicators are logically consistent with the metric of “green” chemistry development—the E-factor, which is the ratio of the mass of by-products (waste) to the mass of the obtained target product.
An example of the practical application of vector B3 can be shown using a real industrial facility—a gas condensate stabilization unit at an oil refinery. Here, thermos ream integration was applied to optimize the heat exchange system through pinch analysis. The results revealed a pinch point for the technology in question of 24 °C. This can lead to a reduction in fuel consumption of up to 30%, which translates into savings of up to USD 3 million per year.
4.2. Priority on the Useful Use of Waste and Circular Resource Chains
In one way or another, all synthesized solutions for the organization of sustainable chemical production are aimed at increasing the closed-loop production and organization of circular resource chains [
35]. The return of waste into the production cycle is possible both within their own enterprise and by transferring waste to third-party organizations for recycling purposes.
According to state statistics, in the primary production and in the process of recovery of rubber tires, tires, and other rubber products at Russian enterprises in 2024, more than 50 types of waste were generated (FKKO code 3 31 200 00 00 0) [
36,
37]. For the analysis, 48 waste items were selected where the volume of accumulated waste materials exceeded 1 ton per year. Of the 48 waste items, 33 were returned to the production cycle within their own enterprise or sold to other organizations for beneficial use. Accordingly, 15 types of waste were not sent for recycling, but were disposed of in landfills (
Figure 3 and
Figure 4).
Analysis of the list of 15 types of waste sent for storage or disposal in landfills shows the presence of such materials as the following:
Paper packaging contaminated with reagents;
Waste of contaminated polyethylene and ferrous metal containers;
Waste of gaskets made of sheet rubber;
Waste of cotton fabric during the manufacture of cord;
Bag filters used in gas purification and others.
The listed types of waste have a potential for recycling into associated or other products, which is justified by the availability of appropriate technology and global practices. However, at this stage of production, the listed waste items are not utilized but sent to landfills. In general, statistics show that in developed countries, industrial waste is recycled almost completely, whereas in Russia, the share of the useful use of industrial waste is about 40%.
Waste returned to the production cycle is predominantly recycled within the framework of its own production, as well as sold to other enterprises for reuse. Many enterprises combine their own recycling and organization of external resource chains in various proportions.
In our own production, four types of waste are fully recycled, which make up 3.19% of the mass of all waste (
Table 3). These are mainly residues of bitumen–rubber mastic, textile samples in the production of rubber products. These waste items can be processed using the main equipment of the enterprise, which is the reason for their own use.
A combination of in-house recycling and waste sales to third parties in various proportions is observed for 17 types of waste, which is 92.84% of the volume of usefully used waste (
Table 4). Five types of waste are used at 50% or more of their mass: these are the readily recyclable waste of rubber mixtures and cord trimmings in the production of automobile tires. Less than 20% of waste from rubber–fabric products and rubber–metal shavings is returned to the production cycle, which is due to the more complex technology of their secondary use. The choice of in-house recycling or waste sales is also made by taking into account the availability of equipment and technology at the enterprise for the secondary use of materials. Mainly, rubber mixture waste without significant impurities is processed at in-house production facilities (more than 70%).
Twelve types of waste are sold for recycling to third-party organizations, which is 3.97% of the volume of usefully used waste (
Table 5). Subject to sale on the side is the waste of contaminated paper packaging and gasket fabric, as well as the waste of latex and rubber shock-absorbing cords and the waste of rubber glue that requires special equipment.
The high level of natural resources in Russian industry has a somewhat restraining effect on the level of waste recycling. In this regard, the waste recycling industry is poorly developed both in organizational and technical terms and from an economic point of view. There are also administrative difficulties, consisting of the lack of a legislative base and state support.
4.3. Modeling the Dependence of the Waste Recycling Level on the Model of Waste Management Organization for Rubber Tires and Covers
In Russian industry, as in the industry of any country, a general model of waste management organization is formed under the influence of legislation and other factors [
36]. The general model includes three priorities in a certain ratio: the first priority is for waste recycling in own production, the second priority is for waste recycling in third-party production, and the third priority is for waste storage and disposal. The task is to build a mathematical model of usefully used waste in circular resource chains. The data array covers 48 types of waste from the production of rubber tires, covers, and other rubber products.
For modeling, we use the method of creating mathematical models based on artificial neural networks. The fact that the problem is nonlinear is beyond doubt, which is why preference is given to neural networks [
38,
39,
40]. Neural networks are capable of learning from examples when the type and structure of the relationships between input and output data are unknown, and of identifying hidden patterns [
41,
42].
Most classical regression models assume that the relationship between the features and the target variable can be easily transformed into a linear relationship using polynomial features. If the actual relationship resembles a spiral or a complex curve, regression will provide a rough approximation [
43]. A neural network, thanks to its activation functions and multiple layers, is inherently capable of approximating a continuous function of any complexity. The network architecture automatically identifies and models complex interactions between multiple features in hidden layers [
44].
At the input to the neural network, we accept the following variables:
Dependent variable y is the specific weight of usefully used waste in circular resource chains;
Independent variable x1 is the mass of waste returned to its own production cycle;
Independent variable x2 is the mass of waste returned to the production cycle of another enterprise;
Independent variable x3 is the mass of waste sent for storage or disposal at landfills.
To achieve a balance between overfitting and underfitting of the network, we split the dataset into subsamples in the ratio: training—70%, test—30%. We use a universal function approximator for regression models—a direct propagation neural network, MLP. We train the network based on the iterative numerical optimization algorithm BFGS. The resulting neural network with a training performance of 0.87 has a three-layer MLP 3-3-1 architecture with three hidden neurons, where each neuron of the first layer is connected to each neuron of the hidden layer. The activation functions of the hidden neurons and the output neuron are logistic. Synaptic weights for a more accurate approximation of the function y = f (x) are presented in the
Table 6.
The adders of the input variables x1, x2, x3 and the synaptic weights t1, t2, t3, respectively, will have the following form:
The activation function of hidden neurons transforms adders t1, t2, t3 into the output signal of the hidden layer neuron (σ1, σ2, σ3):
The adder of the output variable y and the synaptic weights t4 will have the following form:
The activation function of the output neuron y or the model of usefully used waste in circular resource chains will have the following form:
Based on the obtained model, the specific gravity of usefully used waste in circular resource chains was predicted (
Table 7). Three scenarios of waste production and turnover models were selected:
Scenario 1—development of the model towards the priority of waste storage and disposal at landfills (ratio x1:x2:x3 = 0.25:0.35:0.40);
Scenario 2—development of the model in equal ratios (ratio x1:x2:x3 = 0.33:0.33:0.33);
Scenario 3—development of the model towards the priority of waste return to the production cycle (ratio x1:x2:x3 = 0.45:0.35:0.20).
The table shows that the transition from one scenario to another is not accompanied by a polar change in the specific gravity of usefully used waste, and the range of y_(t) values varies in the range of 50–58%. The ternary graph confirms the relative stability of the dependent variable when changing x1–x3 (
Figure 5).
It is logical that the highest value of the specific weight of usefully used waste in circular resource chains during forecasting (y = 57.489) is obtained in the third scenario with the development of the model towards the priority of returning waste to the production cycle (ratio x1:x2:x3 = 0.45:0.35:0.20).