Abstract
Oat bran (OB) may be regarded as food industry by-product, with a high perspective as a bioresource in the production of high-value polyphenolic substances. On this basis, the investigation presented herein aimed at (i) using 1- and 2-propanol organosolv treatment and studying the effect of an alkali (sodium hydroxide) catalyst on releasing bound polyphenols, (ii) establishing models of polyphenol recovery by employing severity and response surface methodology, and (iii) investigating the polyphenolic profile of the extracts produced. Yield in total polyphenols as a function of treatment severity was satisfactorily described by linear models, indicating that for both 1- and 2-propanol treatments, temperature and time could be used interchangeably to adjust severity. Furthermore, the 1-propanol process was found to be more efficient at lower severity compared to the 2-propanol process. The optimization using response surface methodology revealed that, under identical condition settings (t = 300 min, T = 90 °C), the 1-propanol treatment afforded a total polyphenol yield of 17.15 ± 0.51 mg ferulic acid equivalents per g−1 dry mass, whereas the 2-propanol treatment gave a yield of 14.78 ± 1.11 mg ferulic acid equivalents per g−1 dry mass. Liquid chromatography–tandem mass spectrometry analyses showed that the extract produced from the 1-propanol treatment was significantly more enriched in ferulic acid and p-coumaric acid compared to the extract generated with the 2-propanol treatment. Moreover, the antioxidant activity of the extracts was in concurrence with the polyphenolic composition. Based on the evidence, the alkali-catalyzed, 1-propanol organosolv treatment of OB is proposed as a sustainable and efficient methodology to recover multipurpose natural antioxidants.
1. Introduction
Population growth around the world has put an unprecedented strain on how bioresources are used, leading to non-regulated overexploitation, ecosystem degradation, and serious environmental aggravation. The production of extraordinary amounts of waste is a matter of utmost importance in relation to agriculture and food production. This leftover biomass is composed of biological tissues with a high organic content; as a result, inappropriate handling and excessive disposal in landfills may pose immediate concerns to ecosystem conservation, public health, and environmental contamination [1,2]. Based on the aforementioned factors, linear economy models have, up to this point, been widely acknowledged to be both inefficient and environmentally harmful. As a result, circular economy aspects are becoming universally accepted by embracing sustainable routes of agricultural development and food manufacturing. Valorization of food wastes to produce energy, high-value molecules, and plant-based chemicals is one of the key tenets of circular economy methods [3,4].
Bioactive phytochemicals, such as polyphenols, a well-known family of phytochemicals with a variety of bioactivities, are notably abundant in plant food wastes [5]. The development of green technologies for their efficient recovery and exploitation [6,7] has thus been the focus of pertinent studies. In addition to using safe, non-toxic solvents, the development of eco-friendly polyphenol extraction entails using cutting-edge methods, such as ultrasound-assisted extraction, microwave-assisted extraction, etc., which have, in some cases, been shown to be significantly more effective than traditional extraction techniques. Organosolv treatments have also been included in recent reports, which have been proven to be high-performance extraction techniques [8].
The lignocellulosic matrix of cell walls is intended to be broken down by the organosolv procedures, which were initially designed for biomass pretreatment to enhance cellulose recovery and fermentability [9,10]. The liberation of intracellular metabolites, including polyphenols, and their entrainment into the liquid phase of the extraction solvent are both made possible by cell wall disintegration [11]. This increases mass transfer and improves extraction yields. A common solvent used in the organosolv process is ethanol, but several other alcohols have also been used for this purpose, including glycerol, butanol, and 2-propanol [12,13]. Additionally, acid catalysis, usually by including sulfuric acid in the solvent system, can significantly improve the process performance [14].
Oat (Avena sativa), also referred to as brome and wild wheat, is an annual grass, which includes naked oats and bark oats. In recent years, oat has attracted much attention for the functional properties of its bioactive substances, such as β-glucan, arabinoxylan, oligosaccharides, tocopherols, and polyphenolic substances [15]. Oat polyphenols, similar to what is observed in other cereals too, are mostly contained in the bran, where they constitute an insoluble fraction, since they are covalently attached to polysaccharide matrices [15,16]. This insoluble bound phenolic fraction may account for up to 88% of the total polyphenols [17]. The polyphenolic composition of oat bran is mainly represented by ferulic acid, accompanied by lower amounts of phenolic acids (vanillic, syringic), other hydroxycinnamates (caffeic, p-coumaric), and avenathramides [18,19].
Ferulic acid, which is by far the principal phenolic acid in oat bran [18,19], is well known for its antioxidant potency [20], and several studies have reported on its involvement in battling degenerative diseases, such as cancer and cardiovascular disorders [21]. By virtue of these properties, ferulic acid is regarded as a biomolecule with high prospects as a functional food ingredient, an efficient food antioxidant, and a high-potency precursor of bio-vanillin production [22].
The ferulic acid-bearing lignocellulosic network is a macromolecular complex of high recalcitrance, hence its disintegration dictates treatments performed under rather harsh conditions. Organosolv treatments, originally deployed for lignocellulose untangling and improvement of enzymic modification of cellulose [9,10], may be promising techniques in this regard. Such treatments actually include lignocellulosic material processing with organic solvent/water mixtures at relatively high temperatures for a predetermined resident time, which have an interdependent relationship [9,23]. Treatment efficiency may be boosted by the addition of catalysts (i.e., sulfuric acid, sodium hydroxide), which accelerate ether and ester bond cleavage. Such bonds are also involved in ferulic acid attachment onto cereal cell wall polysaccharides [24]. Therefore, the bound polyphenol fraction occurring in cereal brans cannot be easily recovered by deploying conventional solvent extraction methods, but polyphenols may be liberated upon hydrolysis using alkaline or acid catalysis [25,26].
A recent study on wheat bran ethanol organosolv treatment demonstrated that sodium hydroxide was a far more effective catalyst compared to sulfuric acid, providing extracts with an exceptionally high ferulic acid concentration [27]. The development of an organosolv treatment to recover oat bran (OB) polyphenols, using sodium hydroxide as a catalyst, was considered a challenging prospect. Thus, this work was carried out to develop an organosolv process with the objective to maximize polyphenol release/recovery from OB using 1- and 2-propanol, which are low-cost, green, and readily available solvents [28]. The evaluation of the treatment was based on both the efficiency and severity, by estimating combined severity factors and response surface optimization. Extract assessment was accomplished by tentatively identifying major polyphenols and determining their concentration. Antioxidant activity was also considered as an additional evaluation criterion. To the best of the authors’ knowledge, this is the first report on alkali-catalyzed OB organosolv treatment for antioxidant polyphenol recovery.
2. Materials and Methods
2.1. Chemicals
p-Coumaric acid (>98%), caffeic acid (≥98%), and ferulic acid (≥98%) were from Extrasynthese (Genay, France). Ethanol, sodium carbonate, 1-propanol, and 2-propanol were purchased from Honeywell/Riedel-de Haen (Seelze, Germany). HPLC grade solvents were used for all chromatographic analyses. Folin–Ciacalteu reagent was from Merck (Darmstadt, Germany). The 2,4,6-tripyridyl-s-triazine (TPTZ) and iron chloride hexahydrate (FeCl3·6H2O) were from Honeywell/Fluka (Steinheim, Germany), and 2,2-diphenylpicrylhydrazyl (DPPH) and ascorbic acid were from Sigma–Aldrich (Darmstadt, Germany).
2.2. Oat Bran
Commercially available oat bran (OB) was purchased from a local grocery store (Chania, Greece), and upon receipt, it was milled in a table blender. The milled material was sieved, and the powder, with an average particle diameter < 0.5 mm, was collected and stored in air-tight plastic vials at 4 °C for no longer than 7 days. This material was used for all organosolv treatments tested.
2.3. Solvent Assay and Acid-Catalyzed Organosolv Treatment
The solvents considered for the organosolv treatments were 1- and 2-propanol. These solvents were initially tested for their efficacy in polyphenol extraction from OB. For this purpose, an exact mass of 1 g of milled OB was combined with 10 mL of pure 1- or 2-propanol, or 20, 40, 60, and 80 (v/v) aqueous mixtures. For all extractions, a hotplate (Witeg, Wertheim, Germany) at a stirring speed of 500 rpm was used at a resident time of 210 min and a constant temperature of 70 °C [29]. Upon completion of the extraction, centrifugation at 10,000× g was employed to separate the solid residue, and the clear extract was used for any further analysis.
To accomplish the organosolv treatments, a previously published protocol was implemented [27]. Thus, mixtures with the optimum 1- or 2-propanol/water proportion were spiked with concentrated sodium hydroxide solution to provide a final sodium hydroxide concentration (CSoHy) of 1.5% (w/v). Following this, 10 mL of each of these solvent systems was mixed with 1 g OB, and treatments were carried out under continuous stirring at 500 rpm and 90 °C for 60, 180, and 300 min. After each treatment, cell debris was removed by centrifugation at 10,000× g.
2.4. Severity Determination
Taking into consideration the treatment temperature and time, severity may be appraised and used as a tool to compare the harshness of the processes, as shown below [30,31]:
SF = logRo
SF is termed as the severity factor, Ro is considered to represent the severity, and the value 100 °C is regarded as the reference temperature. The empirical factor 14.75 is related to the treatment temperature and activation energy. The combined severity factor (CSF) is a more integrated form of SF, and takes into account the pH of the treatment, which may be a critical parameter that affects biomatrix (OB) disintegration [32]:
CSF = logRo′ − pH
It has also been proposed that an alternative expression, termed as CSF′, may represent a fairer comparison of treatment severities at widely different pHs [32]:
CSF′ = logRo + |pH − 7|
2.5. Experimental Design and Treatment Optimization
Utilizing the response surface approach, the organosolv treatment was optimized. Treatment temperature, T, and residence time, t, were used as independent (process) variables, and the yield in total polyphenols (YTP), was used as the response. An 11-point central composite with three center points was used as the experimental design. Both independent variables (t, T) were codified in three levels: −1, 0, and 1, following a procedure described elsewhere [33]. Table 1 displays the actual and codified levels of each variable.

Table 1.
Information on the ranges of the process variables used and their actual and coded levels.
The ranges for each variable evaluated were chosen based on recent literature as well as preliminary research [27]. The temperature of 90 °C was selected as a safe upper limit to avoid high vapor pressure when approaching solvent boiling point, since the solvent systems used were aqueous 1- and 2-propanol mixtures. The statistical significance of each of the resulting model’s individual coefficients as well as the overall statistical significance of the models (R2, p) were evaluated using the analysis of variance (ANOVA) and lack-of-fit tests, both with a minimum 95% significance level.
2.6. Determination of Total Polyphenols and Antioxidant Properties
Total polyphenols were determined following a previously published Folin–Ciocalteu method [34]. Results were given as ferulic acid equivalents using a standard curve (50–700 mg L−1, R2 = 0.9987). The antiradical activity (AAR) and the ferric-reducing power (PR) were measured using well-established methodologies, as previously described [35].
2.7. Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS)
To identify and quantify the principal polyphenols found in OB, a TSQ Quantum Access MS/MS detector (Thermo Scientific, Waltham, MA, USA) was used in conjunction with an ACQUITY Binary Solvent management and ACQUITY Sample management (Waters, Milford, MA, USA). Separations were accomplished on a Fortis SpeedCore C18, 100 mm × 2.1 mm (2.6 μm), at 40 °C, and using a 5 μL injection volume and a flow rate of 0.3 mL min−1. A linear gradient elution program was employed with the mobile phases (A) water (1% acetic acid), and (B) methanol (1% acetic acid), by applying a linear gradient elution program as follows: 0 min, 0% B; 20 min, 100% B. Negative ionization mode was employed for mass spectra acquisition, with a sheath gas pressure of 30 (arbitrary units), capillary temperature set at 300 °C, collision pressure at 1.5 mTorr, and auxiliary gas pressure at 15 (arbitrary units). p-coumaric acid and ferulic acid quantitation was carried out by external standard calibration curves using the highest intensity fragment that was produced during the collision-induced dissociation (CID) of the selected precursor molecular ion.
2.8. Statistical Analysis
JMP™ Pro 16 (SAS, Cary, NC, USA) was employed to perform distribution analysis, set up response surface experimental designs, and calculate attendant statistics (ANOVA, lack of goodness of fit). SigmaPlot™ 15.0 (Systat Software Inc., San Jose, CA, USA) was used for all linear and nonlinear regressions with a significance level of at least 95%. Organosolv treatments were performed at least twice, and analytical measurements were performed in triplicate. The displayed values are the mean ± standard deviation (SD).
3. Results and Discussion
3.1. Solvent Testing
The initial stage in the organosolv process development was to identify a water/alcohol proportion that would enable increased recovery of oat polyphenols. For this purpose, a screening was performed using water/alcohol mixtures with variable compositions (Figure 1). For the mixtures of 1-propanol/water, a 40% (v/v) solution was shown to provide the highest YTP, which was significantly different (p < 0.05). On the other hand, the most efficient polyphenol recovery was accomplished with 2-propanol/water mixtures of 60% (v/v), whereas the 40% mixture was of significantly lower efficacy. Based on this outcome, 40% solutions of 1-propanol and 60% solutions of 2-propanol were selected for further testing.
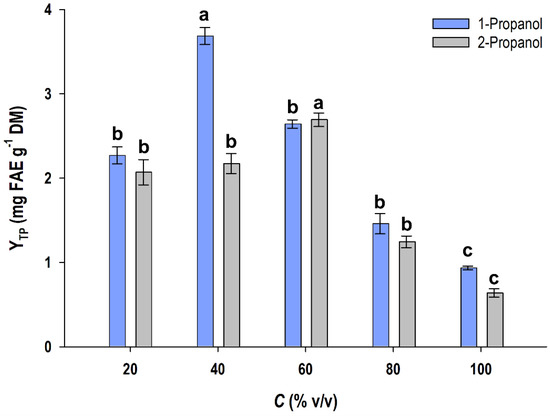
Figure 1.
Testing of alcohol/water mixtures with regard to total polyphenol recovery from OB. Extractions were accomplished at 70 °C for 210 min. Columns designated with different letters (a, b, c) display statistically different values. Bars on the columns indicate standard deviation.
3.2. Assessment of Treatment Severity
A previous recent study clearly demonstrated the high effectiveness of the alkali-catalyzed ethanol organosolv treatment in the recovery of polyphenols from wheat bran [27]. In that study, exceptionally increased yields of total polyphenols were attained using solvent systems that contained 1.5% sodium hydroxide as a catalyst. On such a basis, solvent systems composed of 40% 1-propanol/1.5% sodium hydroxide and 60% 2-propanol/1.5% sodium hydroxide were employed to carry out organosolv treatments by testing various combinations of time and temperature, which corresponded to various severity levels (Table 2). For the treatment accomplished with 1-propanol/sodium hydroxide, significantly higher YTP (p < 0.05) could be obtained at CSF higher than −10.64 and CSF higher than 7.19. Likewise, the treatment with 2-propanol/sodium hydroxide afforded a significantly higher YTP at CSF and CSF′ levels that corresponded to −10.49 and 7.41. For both treatments, the CSF and CSF′ levels required for maximum YTP were very similar to −10.85 and 7.63, respectively, which were determined for the alkali-catalyzed ethanol organosolv treatment of wheat bran [27].

Table 2.
The values determined for the combined severity factor (CSF), the alternative combined severity factor (CSF′), and yield in total polyphenols (YTP) as a function of the temperature and time settings.
As a following step in the examination of the effect of treatment severity on the polyphenol recovery performance, linear regressions were attempted between YTP and either CSF or CSF′ values to identify any possible correlation pattern. Figure 2 visualizes the outcome of the linear regressions established, which were of high statistical significance, as shown below from the linear models derived:
YTP(1-prop) = 1.80CSF1-prop + 36.01 (R2 = 0.94, p < 0.0001)
YTP(2-prop) = 3.43CSF2-prop + 49.80 (R2 = 0.93, p < 0.0001)
YTP(1-prop) = 1.80CSF′1-prop + 3.28 (R2 = 0.94, p < 0.0001)
YTP(2-prop) = 3.43CSF′2-prop − 11.54 (R2 = 0.93, p < 0.0001)
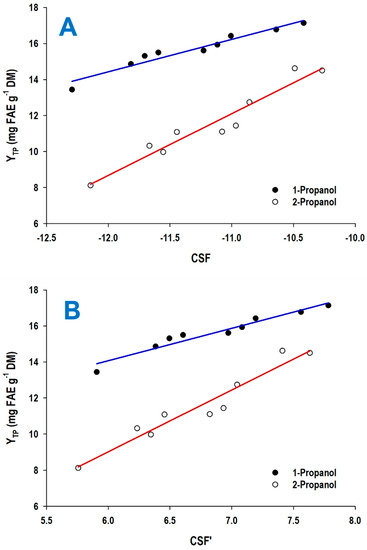
Figure 2.
Linear regressions describing the correlation of the yield in total polyphenols (YTP) with the combined severity factor (CSF) (A) and the alternative combined severity factor (CSF′) (B).
The use of Equation (6) enabled the determination of the theoretically maximum YTP that could be achieved, which was 17.25 mg FAE g−1 DM. Similarly, the use of Equation (7) gave a theoretical maximum YTP of 17.28 mg FAE g−1 DM. Both values were virtually identical to the experimental value of 17.14 mg FAE g−1 DM (Table 2). In the same manner, the theoretical maximum values of YTP computed from the Equations (8) and (9) were 14.57 and 14.63 mg FAE g−1 DM, respectively, which perfectly matched the experimental value of 14.50 mg FAE g−1 DM. These findings strongly indicated that, using the empirical models established, reliable predictions on YTP could be generated based on any combination of T and t within the ranges tested.
3.3. Response Surface Treatment Optimization
Considering the evidence that emerged from the linear models encompassing severity, 1- and 2-propanol/sodium hydroxide treatments exhibited differentiated effects on polyphenol recovery from OB. Therefore, response surface methodology was deployed to fully assess the effect of both solvents and provide alternative means of process modeling. For this purpose, temperature, T, and time, t, were used as the process variables. With such an approach, the effects of T and t on treatment performance could be further evaluated and possible cross (synergistic) functions could be revealed. Overall model appraisal and optimization of the treatments included analysis of variance (ANOVA) and lack-of-fit tests (Table 3, Table 4 and Table 5), taking into account the closeness of the measured and predicted values (Table 6).

Table 3.
Data derived from effect tests for the optimization of both treatments with 1- and 2-propanol.

Table 4.
Data pertaining to parameter estimates for the optimization of both treatments with 1- and 2-propanol.

Table 5.
Statistical data obtained after performing the lack-of-fit test for the optimization of both treatments with 1- and 2-propanol.

Table 6.
Analytical presentation of the design points included in the response surface methodology, and the corresponding measured and predicted values of response for both 1- and 2-propanol organosolv treatments.
To deliver the equations representing the models derived, non-significant terms were excluded; hence, the models (equations) contained only statistically significant terms (Table 4). The square correlations coefficients (R2) gave a measure of the total variability around the mean, as this was predicted by the models:
YTP(1-Prop) = 15.68 + 0.79X1 + 0.99X2
YTP(2-Prop) = 11.72 + 1.47X1 + 1.84X2
Both models were shown to have R2 values equal to or higher than 0.97 (Figures S1 and S2, Supplementary Material), and statistically significant p values for lack-of-fit (<0.05), considering a confidence interval of at least 95%. These findings dictated that the models derived from the response surface methodology exhibited outstanding fitting to the experimental (measured) data.
On the basis of the models, the three-dimensional plots were constructed to portray the influence of the process variables on the response (YTP), and to visualize the overall effect of the two solvents tested (Figure 3). For both the treatments with 1- and 2-propanol/sodium hydroxide, only t (X1) and T (X2) were significant, whereas no cases of significant cross or quadratic effects were observed. Therefore, the models described by Equations (10) and (11) represented linear correlations, where YTP was directly proportional to both t and T. This finding was in absolute agreement with the models established based on severity, and clearly demonstrated that increases in both process variables within the ranges tested could lead to attaining higher polyphenol recovery.
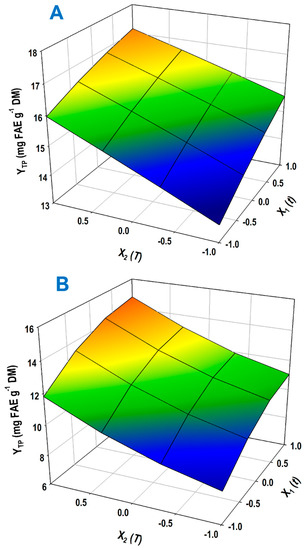
Figure 3.
Three-dimensional model portraying of the effect of treatment variables (t, T) on the response (YTP). (A) Treatment performed with 1-propanol; (B) treatment performed with 2-propanol.
With the aid of the desirability function (Figures S1 and S2, Supplementary Material), the determination of the theoretically optimal values for both t and T, as well as the maximum predicted YTP for each treatment, could be performed. For the treatment with 1-propanol/sodium hydroxide, maximum YTP (17.15 ± 0.51 mg FAE g−1 DM) could be achieved at t = 300 min and T = 90 °C. In the case of 2-propanol/sodium hydroxide treatment, the maximum YTP was determined to be 14.78 ± 1.11 mg FAE g−1 DM under the same set of conditions. On the grounds of the above data, it was clear that the organosolv treatment with 1-propanol/sodium hydroxide was almost 14% more efficient than that with 2-propanol/sodium hydroxide when identical regimes of process time and temperature were employed. The crucial role of sodium hydroxide as an alkali catalyst was revealed when treatments were carried out in its absence. The incorporation of sodium hydroxide at the optimal concentration resulted in a 132% increase in YTP in the 1-propanol-based treatment, while the corresponding 2-propanol-based treatment gave a performance increase of 373% when sodium hydroxide was used (Figure 4). This outcome underlined the undisputed importance of the alkali catalyst in recovering OB polyphenols.
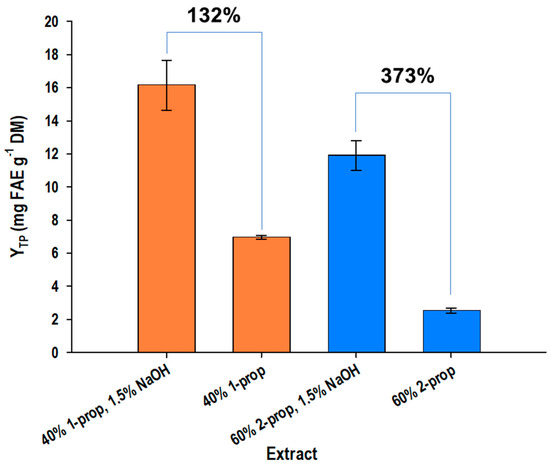
Figure 4.
Diagram highlighting the effect of sodium hydroxide as an alkali catalyst on the performance of the organosolv treatments carried out with either 1- or 2-propanol. Bars on the columns indicate standard deviation.
To have a critical assessment of the efficiency of the 1-propanol/sodium hydroxide treatment and to better illustrate the prospect of the process developed, the YTP levels attained were compared to those reported in the relevant literature. It has been shown that enzyme-assisted extraction of OB polyphenols using a mixture of carbohydrases could only afford a YTP of 4.80 mg gallic acid equivalents g−1 DM [36]. Integration of alkali-catalyzed hydrolysis did not boost polyphenol recovery to higher levels [36,37]. In another study on enzyme-catalyzed release of OB polyphenols, the yields determined were just under 2 mg gallic acid equivalents g−1 DM [18].
Conventional solvent extraction of various milling fractions of oat did not provide yields higher than 1 mg gallic acid equivalents g−1 DM [38], while extraction with supercritical fluids was of even lower efficacy, giving a maximum yield of as high as 0.89 mg gallic acid equivalents g−1 DM [39]. Comparable levels of 0.84 mg gallic acid equivalents g−1 DM were also achieved through solid-state fermentation of OB [40]. Therefore, to the best of the authors’ knowledge, the YTP achieved by the 1-propanol/sodium hydroxide treatment represents the highest level reported.
3.4. Polyphenolic Profile and Antioxidant Properties
In addition to the total yield in polyphenols, the composition of the extracts generated with 1- and 2-propanol-based treatments was also examined to ascertain whether the different treatments affected the polyphenolic profile and the relative amounts of individual constituents. The extracts from both treatments, produced under optimal conditions, were analyzed by liquid chromatography–tandem mass spectrometry (LC–MS/MS) to perform a tentative identification and quantification of the main compounds. The diagnostic fragments at m/z = 178.1 and 134.1, as well as the molecular ion at m/z = 193, were used to identify ferulic acid. Similarly, p-coumaric acid was recognized by the corresponding ions at m/z = 163 and 119.3 (Figures S3 and S4, Supplementary Material).
Irrespective of the alcohol used to perform the organosolv treatment, the role of sodium hydroxide as a catalyst was of paramount importance. Thus, while simple extraction with either 1- or 2-propanol afforded ferulic acid levels that did not exceed 5.3 μg g−1 DM, the use of sodium hydroxide boosted recoveries to 481.55 and 291.05, respectively (Table 7). Likewise, p-coumaric acid occurred at undetectable levels in extracts produced only with alcohols, but in the extracts obtained with sodium hydroxide catalysis, small amounts of p-coumaric acid accompanied ferulic acid. The organosolv treatment with 1-propanol was proven to be far more effective than that with 2-propanol, giving a ferulic acid recovery that was 91% higher. The corresponding difference for p-coumaric acid was almost 51%. In total, the 1-propanol treatment yielded a 65% higher amount of polyphenol recovery.

Table 7.
Data illustrating the composition of major polyphenols and the antioxidant activity of the extracts produced with both 1- and 2-propanol treatments under optimized conditions. Data on extracts obtained from treatments without alkali catalysts are also given for comparison. Assignments: FA, ferulic acid; p-CouA, p-coumaric acid.
Earlier investigations showed the ferulic acid and p-coumaric acid content in OB to vary from 54.7 to 123.3 μg g−1 DM and 14.3 to 20.2 μg g−1 DM, respectively [38]. Other authors reported much lower values of 19.3 to 43.3 μg g−1 DM and 6.3 to 9.8 μg g−1 DM, respectively [39], but levels of 5.7–49.4 and 247.2–1153.6 μg g−1 DM have also been found upon alkaline hydrolysis [19]. In another study, alkaline hydrolysis of OB gave a ferulic acid level of 1769.94 μg g−1 DM [37]. On the other hand, treatment with various hydrolytic enzymes was demonstrated to provide outstanding yields of 2578.6 and 55.6 μg g−1 DM, respectively [36]. In contrast, treatment of OB with cellulase afforded ferulic acid and p-coumaric acid yields that were up to 124.03 and 11.35 μg g−1 DM, respectively [18].
The extracts produced by employing treatments with 1-propanol/sodium hydroxide and 2-propanol/sodium hydroxide under optimized conditions were tested for both antiradical activity (AAR) and ferric-reducing power (PR). Based on AAR, the extract obtained with 1-propanol/sodium hydroxide displayed outstanding performance, which greatly surpassed that of the extract generated with 2-propanol/sodium hydroxide (Table 7). The results drawn from the PR test were not so pronounced, yet the 1-propanol/sodium hydroxide extract was proven once again to be the most active. Taking into account the polyphenolic composition of the extracts, as portrayed in Table 4, it would appear that the large difference in ferulic acid concentration between the two extracts was reflected in the antioxidant activity.
Such a result would not come as a surprise, considering that ferulic acid was shown to have a key role in the manifestation of antioxidant activity, being the major antioxidant contributor [26,41]. This argument was also concurred by hydrolysis studies, which illustrated that ferulic acid-enriched extracts produced with alkaline hydrolysis exhibited powerful antiradical activity [25].
4. Conclusions
The efficient recovery of oat bran (OB) polyphenols is a challenging prospect that involves deploying extraction technologies that provide a high recovery yield, purity, and stability of the target phytochemicals, as well as minimal environmental impact and the highest economic feasibility. This study demonstrated that the alkali-catalyzed organosolv treatment developed using 1-propanol may be a highly effective means of obtaining polyphenolic antioxidants from oat bran. Severity and response surface optimization modeling illustrated that the 1-propanol-based treatment was significantly more efficacious compared to 2-propanol, and equally severe. The extract produced under optimized conditions showed ferulic acid to be the major polyphenolic constituent, whereas p-coumaric acid occurred at significantly lower levels. On the basis of the data generated, it could be supported that alkali-catalyzed 1-propanol organosolv treatment could have an important perspective in the recovery of high value compounds from OB, such as ferulic acid. Based on the outcome of this investigation, it could be proposed that 1-propanol organosolv treatment of oat bran, integrated by alkaline catalysis, could be a very effective route for releasing and recovering pure ferulic acid, which may be used in the pharmaceutical, food, and cosmetics industries as a bioactive ingredient. Similar processes could be incorporated in biorefinery approaches to shape strategies that would contribute towards the establishment of wider circular economy policies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/environments10070118/s1, Figure S1: Data associated with the response surface methodology, implanted to optimize the 1-propanol organosolv treatment. A, desirability function; B, correlation between actual (measured) and predicted values. Number designated with different color in the inset tables denote statistically different values; Figure S2: Data associated with the response surface methodology, implanted to optimize the 2-propanol organosolv treatment. A, desirability function; B, correlation between actual (measured) and predicted values. Number designated with different color in the inset tables denote statistically different values; Figure S3: A representative total ion chromatogram of 1-propanol extract obtained under optimized conditions: RT 6.16 min, p-coumaric acid; RT 7.14 min, ferulic acid); Figure S4: Fragmentation patterns of p-coumaric and ferulic acids.
Author Contributions
S.C., A.G. and S.G.: Performance of experiments, data curation, and analysis; D.P.M. and S.G.: Methodology—design and supervision; S.C., A.G. and S.G.: Data curation; D.P.M., S.G.: Writing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Mediterranean Agronomic Institute of Chania (M. A. I. Ch.).
Data Availability Statement
The data is available from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bakan, B.; Bernet, N.; Bouchez, T.; Boutrou, R.; Choubert, J.-M.; Dabert, P.; Duquennoi, C.; Ferraro, V.; Garcia-Bernet, D.; Gillot, S. Circular economy applied to organic residues and wastewater: Research challenges. Waste Biomass Valor. 2022, 13, 1267–1276. [Google Scholar] [CrossRef]
- Yaashikaa, P.; Kumar, P.S.; Varjani, S. Valorization of agro-industrial wastes for biorefinery process and circular bioeconomy: A critical review. Bioresour. Technol. 2022, 343, 126126. [Google Scholar] [CrossRef] [PubMed]
- Amran, M.A.; Palaniveloo, K.; Fauzi, R.; Satar, N.M.; Mohidin, T.B.M.; Mohan, G.; Razak, S.A.; Arunasalam, M.; Nagappan, T.; Sathiya Seelan, J.S. Value-added metabolites from agricultural waste and application of green extraction techniques. Sustainability 2021, 13, 11432. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Battisti, A.P.; Valencia, G.A.; de Andrade, C.J. The Production of High-Added-Value Bioproducts from Non-Conventional Biomasses: An Overview. Biomass 2023, 3, 123–137. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Schwember, A.R.; Parada, R.; Garcia, S.; Maróstica Júnior, M.R.; Franchin, M.; Regitano-d’Arce, M.A.B.; Shahidi, F. Opinion on the hurdles and potential health benefits in value-added use of plant food processing by-products as sources of phenolic compounds. Int. J. Mol. Sci. 2018, 19, 3498. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K. Toward petroleum-free with plant-based chemistry. Curr. Opin. Green Sustain. Chem. 2021, 28, 100450. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging green techniques for the extraction of antioxidants from agri-food by-products as promising ingredients for the food industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Abdoun, R.; Grigorakis, S.; Kellil, A.; Loupassaki, S.; Makris, D.P. Process optimization and stability of waste orange peel polyphenols in extracts obtained with organosolv thermal treatment using glycerol-based solvents. ChemEngineering 2022, 6, 35. [Google Scholar] [CrossRef]
- Wei Kit Chin, D.; Lim, S.; Pang, Y.L.; Lam, M.K. Fundamental review of organosolv pretreatment and its challenges in emerging consolidated bioprocessing. Biomass Convers. Biorefinery 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Sun, C.; Song, G.; Pan, Z.; Tu, M.; Kharaziha, M.; Zhang, X.; Show, P.-L.; Sun, F. Advances in organosolv modified components occurring during the organosolv pretreatment of lignocellulosic biomass. Bioresour. Technol. 2023, 368, 128356. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: Chemistry, kinetics, and substrate structures. Biomass Convers. Biorefinery 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, J.; Wang, X.; Wang, J.; Fan, W. Alcoholysis: A promising technology for conversion of lignocellulose and platform chemicals. ChemSusChem 2017, 10, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.A.; Murton, K.D.; Smith, D.A.; Dedual, G. A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose. Biomass Convers. Biorefinery 2022, 12, 5427–5442. [Google Scholar] [CrossRef]
- Tang, Y.; Li, S.; Yan, J.; Peng, Y.; Weng, W.; Yao, X.; Gao, A.; Cheng, J.; Ruan, J.; Xu, B. Bioactive components and health functions of oat. Food Rev. Int. 2022, 1–20. [Google Scholar] [CrossRef]
- Peterson, D.M. Oat antioxidants. J. Cereal Sci. 2001, 33, 115–129. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Importance of Insoluble-Bound Phenolics to the Antioxidant Potential Is Dictated by Source Material. Antioxidants 2023, 12, 203. [Google Scholar] [CrossRef]
- Chen, D.; Shi, J.; Hu, X. Enhancement of polyphenol content and antioxidant capacity of oat (Avena nuda L.) bran by cellulase treatment. Appl. Biol. Chem. 2016, 59, 397–403. [Google Scholar] [CrossRef]
- Soycan, G.; Schär, M.Y.; Kristek, A.; Boberska, J.; Alsharif, S.N.; Corona, G.; Shewry, P.R.; Spencer, J.P. Composition and content of phenolic acids and avenanthramides in commercial oat products: Are oats an important polyphenol source for consumers? Food Chem. X 2019, 3, 100047. [Google Scholar] [CrossRef]
- Graf, E. Antioxidant potential of ferulic acid. Free Radic. Biol. Med. 1992, 13, 435–448. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Ferulic acid: Pharmacological and toxicological aspects. Food Chem. Toxicol. 2014, 65, 185–195. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Borand, M.N.; Karaosmanoğlu, F. Effects of organosolv pretreatment conditions for lignocellulosic biomass in biorefinery applications: A review. J. Renew. Sustain. Energy 2018, 10, 033104. [Google Scholar] [CrossRef]
- Khosravi, A.; Razavi, S.H. The role of bioconversion processes to enhance bioaccessibility of polyphenols in rice. Food Biosci. 2020, 35, 100605. [Google Scholar] [CrossRef]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Verma, B.; Hucl, P.; Chibbar, R. Phenolic acid composition and antioxidant capacity of acid and alkali hydrolysed wheat bran fractions. Food Chem. 2009, 116, 947–954. [Google Scholar] [CrossRef]
- Papadaki, E.S.; Palaiogiannis, D.; Lalas, S.I.; Mitlianga, P.; Makris, D.P. Polyphenol Release from Wheat Bran Using Ethanol-Based Organosolv Treatment and Acid/Alkaline Catalysis: Process Modeling Based on Severity and Response Surface Optimization. Antioxidants 2022, 11, 2457. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Morsli, F.; Grigorakis, S.; Halahlah, A.; Poulianiti, K.P.; Makris, D.P. Appraisal of the combined effect of time and temperature on the total polyphenol yield in batch stirred-tank extraction of medicinal and aromatic plants: The extraction efficiency factor. J. Appl. Res. Med. Arom. Plants 2021, 25, 100340. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar]
- Sidiras, D.; Politi, D.; Giakoumakis, G.; Salapa, I. Simulation and optimization of organosolv based lignocellulosic biomass refinery: A review. Bioresour. Technol. 2022, 343, 126158. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity–relating pH to biomatrix opening. New Biotech. 2010, 27, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, V.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I.; Makris, D.P. β-Cyclodextrin-aided aqueous extraction of antioxidant polyphenols from peppermint (Mentha× piperita L.). Oxygen 2022, 2, 424–436. [Google Scholar] [CrossRef]
- Cherif, M.M.; Grigorakis, S.; Halahlah, A.; Loupassaki, S.; Makris, D.P. High-efficiency extraction of phenolics from wheat waste biomass (bran) by combining deep eutectic solvent, ultrasound-assisted pretreatment and thermal treatment. Environ. Process. 2020, 7, 845–859. [Google Scholar] [CrossRef]
- Kaltsa, O.; Lakka, A.; Grigorakis, S.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. A green extraction process for polyphenols from elderberry (Sambucus nigra) flowers using deep eutectic solvent and ultrasound-assisted pretreatment. Molecules 2020, 25, 921. [Google Scholar] [CrossRef]
- Alrahmany, R.; Tsopmo, A. Role of carbohydrases on the release of reducing sugar, total phenolics and on antioxidant properties of oat bran. Food Chem. 2012, 132, 413–418. [Google Scholar] [CrossRef]
- Martín-Diana, A.B.; García-Casas, M.J.; Martínez-Villaluenga, C.; Frías, J.; Peñas, E.; Rico, D. Wheat and oat brans as sources of polyphenol compounds for development of antioxidant nutraceutical ingredients. Foods 2021, 10, 115. [Google Scholar] [CrossRef]
- Hitayezu, R.; Baakdah, M.M.; Kinnin, J.; Henderson, K.; Tsopmo, A. Antioxidant activity, avenanthramide and phenolic acid contents of oat milling fractions. J. Cereal Sci. 2015, 63, 35–40. [Google Scholar] [CrossRef]
- Walters, M.; Ribeiro, A.P.L.; Hosseinian, F.; Tsopmo, A. Phenolic acids, avenanthramides, and antioxidant activity of oats defatted with hexane or supercritical fluid. J. Cereal Sci. 2018, 79, 21–26. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Cătoi, A.-F.; Vodnar, D.C. Solid-state yeast fermented wheat and oat bran as a route for delivery of antioxidants. Antioxidants 2019, 8, 372. [Google Scholar] [CrossRef]
- Mateo Anson, N.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G.R.M.M. Ferulic acid from aleurone determines the antioxidant potency of wheat grain (Triticum aestivum L.). J. Agric. Food Chem. 2008, 56, 5589–5594. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).