Abstract
Tree-rings are recorders of environmental signals and are therefore often used to reconstruct past environmental conditions. In this paper, we present four annually resolved, multi-centennial tree-ring isotope series from the southeastern Tibetan plateau. The investigation site, where juniper and spruce trees jointly occur, is one of the highest known tree-stands in the world. Tree ring cellulose oxygen (δ18O) and carbon (δ13C) isotopes were analyzed for a common period of 1685–2007 AD to investigate climate–isotope relationships. Therefore, various climate parameters from a local meteorological station and from the CRU 4.02 dataset were used. Tree-ring δ18O of both species revealed highly significant sensitivities with a high degree of coherence to hydroclimate variables during the growing season. The obtained δ18O–climate relationships can even be retained using a species mean. In contrast, the individual δ13C series indicated a weaker and non-uniform response to the tested variables. Underlying species-specific responses and adaptations to the long-term trend in atmospheric CO2 bias even after a trend correction identified dominant environmental factors triggering the tree-ring δ13C at our site. However, analysis of individual intrinsic water-use efficiency in juniper and spruce trees indicated a species-specific adaptation strategy to climate change.
1. Introduction
Often-quoted in a broader context as the “Third Pole” [1], an important part of the “Asian Water Towers” [2], or the “Roof of the World” [3], the Tibetan plateau (TP) exerts a key role for the climatic, hydrological and biological conditions of its highlands, mountain ranges as well as for the adjoining Asian lowland areas. Beyond the fact of its substantial role within the global climate system as the key driver of the Asian monsoonal systems [4], the current changes within the regional cryosphere, hydrosphere, biosphere and associated responses of environmental systems on the TP are subjects of intensive research. Especially, the responses, threats and adaptations of different (socio-)ecosystems to the substantial rise in temperature during the recent decades and associated changes of water availability underline the necessity of a precise quantification of these changes [5,6,7,8,9]. Contrastingly, for High Asia in general and for the Tibetan plateau in particular, only a sparse climate station network with relatively short time-series of high-resolution meteorological data is available. Therefore, existing datasets, which are mainly available at the earliest since the 1950s, hamper a substantial quantification and classification of the recent climate change within a retrospective context and underline the needs for paleoclimatic datasets [4,10,11,12].
Besides studies focusing on tree-ring width (TRW), more and more studies display the advantages of using stable isotopes in tree-rings for ecological as well as paleoclimate research on the Tibetan plateau. During the last decade, the analysis and use of tree-ring δ18O has become one of the main proxies used for paleoclimate research in High Asia. Recent works on the Tibetan Plateau, in particular, have demonstrably contributed to a better understanding of regional climate and environmental history [10,11,12,13,14,15,16,17]. In comparison, studies on the δ13C variations in tree-rings are still underrepresented due to more complex isotope fractionation effects affecting the δ13C in tree-rings. In addition, multi-century δ13C tree-ring time series usually have to undergo a trend correction procedure primarily due to the rising atmospheric CO2 concentrations (CO2 ATM) since the beginning of the industrialization (the “Suess-effect”). Especially the respective correction factor that has to be considered due to plant-internal reactions addressing rising CO2 ATM has considerable impact on the adjustment of carbon isotope time series [18,19] and may strongly modify trends of δ13C tree-ring data after correction.
Recently, progress has been made in studies on analyzing both stable carbon and oxygen isotopes, pointing to the paleoclimatic potential inherent to such an approach [20,21,22,23]. In addition, studies underline the potential and benefit within the analysis of multiple stable isotopes across species and across sites [24,25]. The reported advantage is mainly based on the possibility of a common assessment and interpretation of stored climate signals with coupled ecophysiological reactions across tree species. However, up to now, the mentioned studies focus either on the analysis of two tree species, but only for over a rather short time period of a few decades, or the establishment of longer (mutli-century to millennial) time series for two isotopes, but only investigating a single tree species. The main reason for this desideratum is presumably caused by the comparably high laboratory and time expenditure inherent to tree-ring isotope studies, although many sites on the TP possibly bear the potential of additionally analyzing several tree species. For a profound retrospective assessment, classification and categorization of the recently ongoing environmental changes and tree-specific adaptations, longer isotope time-series across species are, however, urgently required.
When considering existing studies on tree-ring δ13C and δ18O from the TP, their spatial representativity and probable inter-isotope dependencies sometimes remain difficult. Due to the lack of other existing, closely situated tree-ring isotope series especially for carbon isotope series, it remains often challenging to corroborate a wider regional representativity. Moreover, the derived, detected and stored environmental signal in the tree-ring proxy is reported to reflect a more site- and species-related physiological response to the ongoing environmental change [26,27]. This includes in particular observed adaptations in different tree species with regard to changes in their intrinsic water-use efficiency [25,28]. The sometimes heterogenous picture with regard to the (reconstructed) environmental signal stored in δ13C and δ18O series from the TP, the different levels in signal strength and differing spatial representativities of the time series still contain challenges for actual and future research.
By analyzing multi-centennial variations of different stable isotopes (δ13C and δ18O) in two different tree species from a common site on the southeastern part of the TP, our study evaluated the potential, benefit, and limitations inherent in such an approach by:
- (i)
- deciphering and explaining common and contrasting long-term isotopic variations of δ13C and δ18O between two tree species from high-altitude, climate-sensitive tree-stands;
- (ii)
- assessing the added value for an analysis of δ13C and δ18O from two local tree species; and
- (iii)
- testing if common/differing signals of the proxy series from one site can be used to better assess, decipher, and explain diverging responses of tree species to recent environmental change.
The main aim of this study was therefore a calibration study on the potential of multiple isotope parameters derived from tree-rings from a subalpine tree-stand in High Asia.
2. Materials and Methods
2.1. Study Site
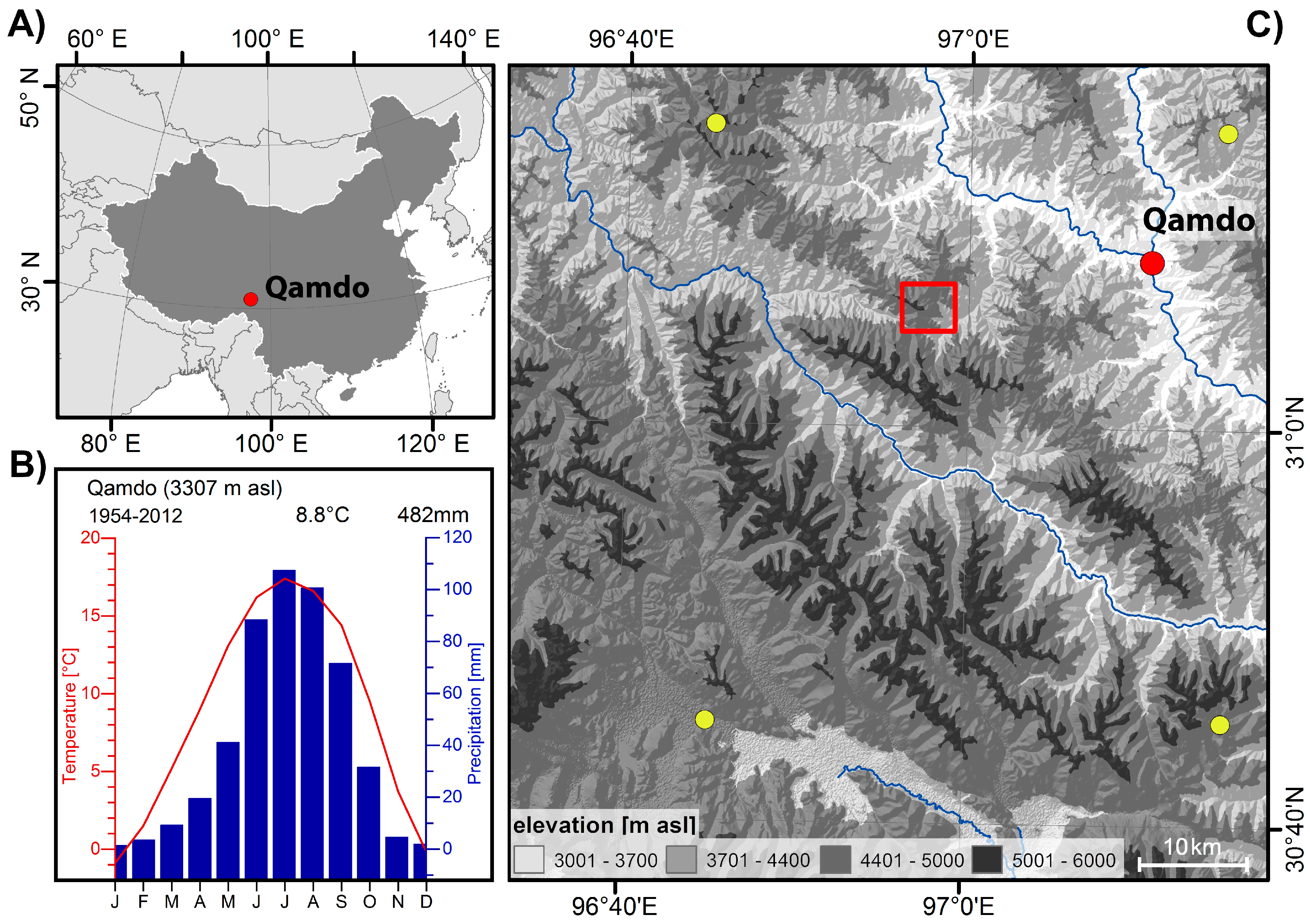
The study site is situated around 20 km southwest to the municipality of Qamdo on the upper reaches of the Mekong River on the southeastern Tibetan plateau (TP) (Figure 1). The local climate as recorded by the nearby climate station in the municipality of Qamdo (31°09′ N/97°10′ E; available period 1954–2012) is characterized by a mean annual temperature of 8.8 °C and an annual precipitation sum of 482 mm, indicating a sub-humid continental climate (Figure 1). The main summer season lasts from June to August and represents the period with highest temperatures (TJJA = 16.7 °C). Summer months with the maximum monthly temperatures are July (TJuly = 17.4 °C) and August (TAug = 16.6 °C), respectively. The summer months July and August have the highest amounts of precipitation (PrecipJJA = 296 mm; PrecipJuly = 107 mm, PrecipAug = 101 mm). In contrast, winter months are cold and show little to no rainfall/snow (TDec = 0 °C, PrecipDec = 2mm). Overall, local climate conditions display a typical summer monsoon climate with warm and humid summers contrasted by cold and dry winter conditions [4].
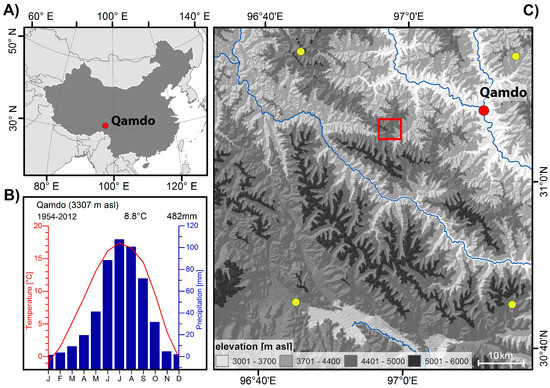
Figure 1.
Overview of the investigation site: (A) location of the site Qamdo within China; (B) climate diagram of the climate station in Qamdo; and (C) digital elevation map of the Qamdo region with the investigation site (red box), location of the city and climate station of Qamdo (red dot). Yellow points indicate locations of the used 0.5° CRU TS 4.02 grid points.
The investigated tree-stand is located at the local subalpine vegetation belt between 4400 m and 4600 m asl. on a convex slope with slightly changing southerly expositions (Figure 2). Developed from debris material, predominant soils are well-drained and shallow without influence of stagnating groundwater [12,29,30]. The upper timberline on southerly exposed slopes is formed by open, steppe-forest-like formations of Tibetan juniper (Juniperus tibetica Kom.), being one of the highest known tree-stands of the world at 4600 m asl. [31]. On average, juniper tree individuals reach heights of up to 12 m, with some individuals exceeding 20 m. As being typical for steppe forests, the juniper tree stand is characterized by a canopy cover of 10%. On eastern and southeastern expositions, denser stands of 20–25 m high Balfour spruce trees (Picea balfouriana) with a canopy cover of maximum 25% are dominant, reaching their maximum upper distribution limit at 4500 m asl. In contrast to the juniper stands, spruce trees are in parts covered with lichens of the species Alectoriella ssp., pointing to more humid microclimatic conditions presumably caused by lower radiation and higher humidity (cf. Figure 2).

Figure 2.
Overview on the sampling site near Qamdo: (I) area of predominant Juniperus tibetica tree-stands on southerly expositions at 4400–4600 m asl.; and (II) vegetation belt with the Picea balfouriana forest on southeasterly expositions.
2.2. Sampling, Dating and Sample Preparation
During several field campaigns, undisturbed and healthy mature tree-individuals of Picea and Juniperus species were sampled. We collected discs from dead trees and increment cores from living trees using an increment borer (Suunto). In the laboratory, samples were surfaced and tree ring widths were measured with an accuracy of 0.01 mm using a Lintab 6 system (Rinntech, Heidelberg/Germany). Subsequently, samples were cross-dated and validated against an existing regional master chronology [29,30]. For the analysis of the ratios of stable oxygen and carbon isotopes from tree-ring cellulose (thereafter expressed as δ18OTRC and δ13CTRC), at least 5–7 cores from both juniper and spruce individuals were selected. The obtained minimum sample depth of five trees was within the generally accepted threshold of analyzed trees per species per sample site for isotope studies [32] and was consistently available throughout the common period 1685–2007 AD covered by both species (Table 1).

Table 1.
General site information and chronology statistics for the common period 1685–2007.
All samples chosen for isotope analysis met the following criteria: (i) similar tree ages for juniper and spruce trees; (ii) no missing rings or occurrence of problematic plateaus with extremely depressed growth and narrow rings to obtain sufficient α-cellulose for the analysis of both δ13CTRC and δ18OTRC; and (iii) the analytical exclusion of the innermost 50 years of each sample to avoid a non-climatic juvenile trend of the most recent δ13CTRC values [33]. Especially Criterion (ii) was difficult to meet within multi-century old junipers known for lobate growth and in parts extremely narrow ring widths [11,12]. To obtain sufficient material for the analysis of both isotopes from such samples, it was therefore inevitable to pool material of corresponding calendar years prior to the subsequent laboratory steps, as reported and successfully applied within existing studies [11,12,13,14,17,32,33]. To avoid a mass-related over-representation of a single tree individual within our pooled samples, cut wood samples of every tree-ring were weighed individually prior to cellulose extraction. Subsequently, same amounts of each calendar year were combined for the final pooled sample year. In regard to the aim of this study of an inter tree-species comparison with the precondition of a comparable analytical setting and subsequent sound statistical analyses, sample pooling was also performed for the spruce samples.
2.3. Evaluation of δ18OTRC and δ13CTRC
In a first analytical step, dated growth rings of both species were cut under a binocular using a razor blade. Calendar year-wise pooled wholewood samples of juniper and spruce were processed to α-cellulose following the method in [34]. After the extraction process, the obtained cellulose samples were homogenized using an ultrasonic sound system and finally freeze-dried in a lyophilization unit [35]. Cellulose samples of each species of 200 µg were weighed using a micro balance and individually packed in tin (for carbon isotope analyses) and silver capsules (for oxygen isotope analyses), respectively. Measurements of the 12C/13C ratio (expressed as δ13C) and 16O/18O ratios (expressed as δ18O) of spruce and juniper samples were determined using a continuous flow DELTA V Advantage Isotope Ratio Mass Spectrometer (IRMS, Thermo Fisher Scientific Inc.) (Waltham, MA, USA). To avoid a hygroscopically caused exchange with ambient air, all samples for the assessment of oxygen analyses were stored prior to the IRMS analysis for at least 48 hours in a vacuum oven.
For the analysis of δ18OTRC isotopes, samples were pyrolyzed to CO in a HEKATech pyrolysis reactor coupled to the IRMS at 1080 °C. For the measurements of the δ13C variations in tree-ring cellulose, samples were combusted at 1020 °C under an excess of oxygen to CO2. All measurements were calibrated by periodically interposing internal and international laboratory standards resulting in an overall analytical precision for the δ18OTRC measurements of <0.2‰ and for the δ13CTRC measurements of <0.1‰, respectively.
As a result, we were able to set up for both investigated stable isotopes juniper chronologies with periods reaching from 489 to 2011 AD. At this point, it has to be noted that the Juniperus δ18OTRC time series was already used for a climate reconstruction [12]. However, by using new material, we were able to update the series until 2011 AD. Thereto, pooled samples of the new individuals were analyzed with an overlap of 20 years to the existing series. Due to their lower maximal biological age, the obtained spruce isotope chronologies cover the period from 1685 to 2007 AD. Hence, the common and further considered period covered by both species lasts from 1685 to 2007 AD.
2.4. Examination of Isotope–Climate Relationships
As being a crucial step for the calibration of tree-ring proxy data in remote high elevation areas, we conducted a thorough survey of available and representative climate datasets. For a first examination of climate–proxy relationships, instrumental data from the nearby climate station in Qamdo (available since 1954) were used. The dataset includes a wide variety of climate parameters including precipitation, temperature (Tmin, Tmax, and Tmean), wind speed (ws), vapor pressure deficit (vp), sunshine hours (sh), and relative humidity (rH). Especially in high mountain areas such as the TP with steep topoclimatic gradients within short distances, precipitation data from climate stations located in valley bottoms often show a limited representativity for study sites located at the upper tree line. Therefore we additionally used gridded means of climate variables from the four nearest 0.5° grid points of the CRU TS 4.02 dataset (Figure 1; https://crudata.uea.ac.uk/cru/data/hrg/cru_ts_4.02/cruts.1811131722.v4.02/) [36]. Therein, provided climate parameters are precipitation, temperature (Tmax, Tmean, and Tmin), diurnal temperature range (dtr), wet day frequencies (wet), cloud cover (cld), frost day frequency (frs), and potential evapotranspiration (pet). When using gridded datasets from this area, however, it must always to be taken into account that the early periods are interpolated from a rather low number of available climate stations. Therefore, we truncated the first five decades and only used the CRU data for the common period with stationary data for 1954–2007 (n = 53) where highly significant relationships between both datasets appear (p < 0.01).
For the quantification of the statistical relationships, we computed Pearson’s correlation coefficients (r) for all available variables in both datasets. The resultant correlations are visualized in a correlogram [37] where correlation coefficients are colored according to their positive/negative value including the respective level of significance. To determine the influence of the applied CO2 trend correction on the correlations with climate, time series of all isotopes and tree species were filtered using a multiple local regression filter with windows set to 1/10 of the time series length. Wavelet analysis was employed to explore the existence and significance of high- and low-frequency dynamics and the threshold for the high-pass filter (R package WaveletComp). Running correlations for all isotopes and tree species were computed using a 50-year moving window.
2.5. δ13CTRC Detrending and iWUE Calculation
As reported by many other studies, δ13CTRC time-series are often characterized by a significant decreasing trend. This decline is mainly caused by the steadily increasing combustion of fossil fuels since the beginning of the industrialization after ca. 1850 AD [18,33]. As a result, the absolute amount of the atmospheric CO2 concentration has risen steadily since 1800 from 270 ppm up to presently 410 ppm ([38], https://www.esrl.noaa.gov/gmd/ccgg/trends/data.html). Through primarily released lighter 12C isotopes, fossil fuel combustion has led to a coupled shift in the atmospheric 12CO2/13CO2 relationship resulting in a depletion of the atmospheric δ13CO2 values. As a result, plants have and do still respond with plant physiological responses to the increased atmospheric CO2 which in turn are biasing the environmental signals stored in tree-ring cellulose [19,32]. To minimize this anthropogenic bias and to extract the environmental signal incorporated within the δ13CTRC, a trend-correction of the δ13CTRC raw values has to be performed prior to the assessment of climate–δ13CTRC-relationships. Within our study, we used the rather conservative non-linear δ13C-trend correction for CO2 ATM, as introduced in [18].
To initially test a possible individual response of our two studied tree species towards changing CO2 concentrations, we calculated for the period 1900−2007 their respective intrinsic water-use efficiencies (iWUE). As reported in [39], the iWUE is defined as the ratio of net photosynthetic assimilation rate (A) to stomatal conductance (gs) for water vapor. It is calculated as:
where Ci and Ca are the CO2 concentrations in the intercellular air space of the leaves and in the atmosphere, respectively. Ci can be obtained by calculating the carbon isotope discrimination (Δ) for C3 plants, as introduced in [40]. The Δ is expressed therein as
where δ13Ca is the stable isotope value of the ambient air (in ‰) and δ13Cp the stable isotope value of plant cellulose (in ‰). Values for δ13Ca (including Ca) for the period 1900−2007 were derived from the datasets in [18,28]; the respective δ13Cp denote the measured δ13CTRC values from our study.
iWUE = A/gs = Ca × [(1 − Ci/Ca)/1.6],
Δ = (δ13Ca − δ13Cp)/(1 + [δ13Cp/1000]),
Since Δ is also a function of the difference between the intercellular and the atmospheric CO2, it can be additionally calculated as:
where a reflects the isotopic discrimination during diffusion of CO2 from the atmosphere into the intercellular space (~4.4‰.), and b denotes the isotopic discrimination caused by the discrimination against CO2 in the chloroplasts by the enzyme RuBisCO (~27‰.).
Δ = a + (b − a) × (Ci/Ca),
3. Results and Discussion
3.1. Species Specific Characteristics of δ18OTRC
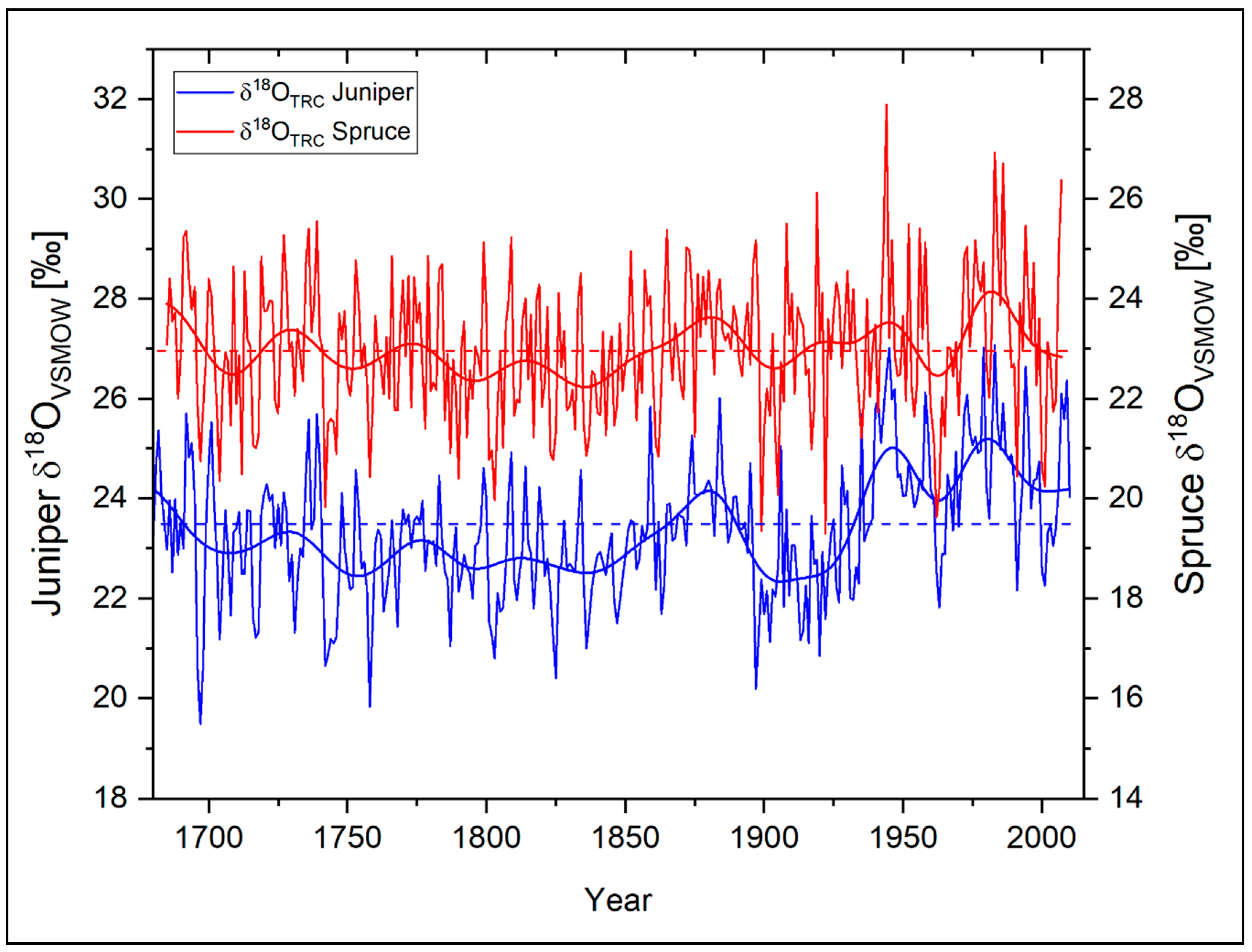
Figure 3 shows the resulting δ18OTRC time series of the two studied tree species at Qamdo during 1685−2007. For a better comparability of the low-frequency variations between the δ18OTRC time series, the scale of the individual series was adjusted by an offset of 4‰. A first quantitative examination of the two time-series already reveals a high visual similarity (Table 2). The mean values of the oxygen isotope series differ slightly within a range of about 0.6‰ (δ18OTRC Juniper = 23.43‰, δ18OTRC Spruce = 22.98‰). Since both tree-species are situated within a short elevational range, this is assumed to be caused by slightly different plant-internal fractionations during cellulose synthesis and/or—on a microclimatic scale—a slightly differing moisture availability during the vegetation period. Probably caused by a higher proportion of earlywood [11,12], the juniper chronology is characterized by a higher autocorrelation (AC1 = 0.61) than the spruce time-series (AC1 = 0.26). It is apparent that the inter-annual variability of the individual time-series is considerably high (Figure 3). In particular, during the recent decades, the number of positive and negative excursions in both time series exceeding a deviation of more than 1.5 SD increased compared to their long-term mean.
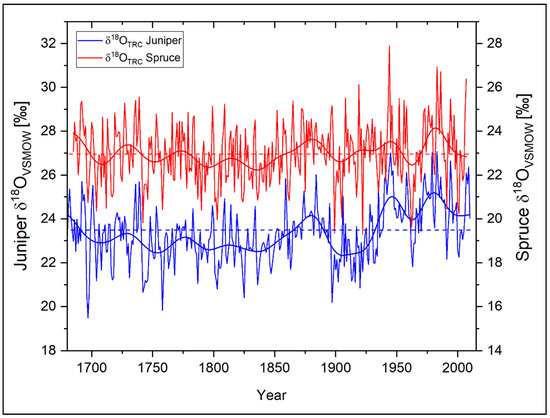
Figure 3.
Annual δ18OTRC variations of Juniperus tibetica (blue) and Picea balfouriana (red) at Qamdo for the period 1685−2010. Bold lines represent the respective 11-year Fast Fourier Transform (FFT) filter for each time-series, dotted horizontal lines the individual species mean. Note: Individual y-axes are shifted against each other with an offset of 4‰ to better show the high synchronicity between both time-series.

Table 2.
Correlation coefficients (r) and Gleichläufigkeit (GLK) between stable carbon and oxygen isotope time-series of Juniperus tibetica and Picea balfouriana on different frequency scales for the common period 1685–2007. All given values are highly significant at p < 0.01.
The highly significant (p < 0.01) correlation coefficient of r = 0.60 and Gleichläufigkeit (sign test) of 70% between the isotope chronologies underline their strong common signal (Table 2). The similarity between the series is also shown by highly consistent variations on lower frequencies (11-year and 21-year Fast Fourier Transform (FFT) filter). While regarding the respective low frequency domains of the two series, the statistical relationships are even higher and stable within their respective levels of significance with p < 0.01 (11-year FFT filtered: r = 0.73, GLK = 91%; 21-year FFT filtered: r = 0.75, GLK 78%). This leads to the assumption that both species are probably controlled by the same environmental influences and therefore show a very similar temporal variability and environmental sensitivity.
3.2. Species Specific Characteristics of δ13CTRC
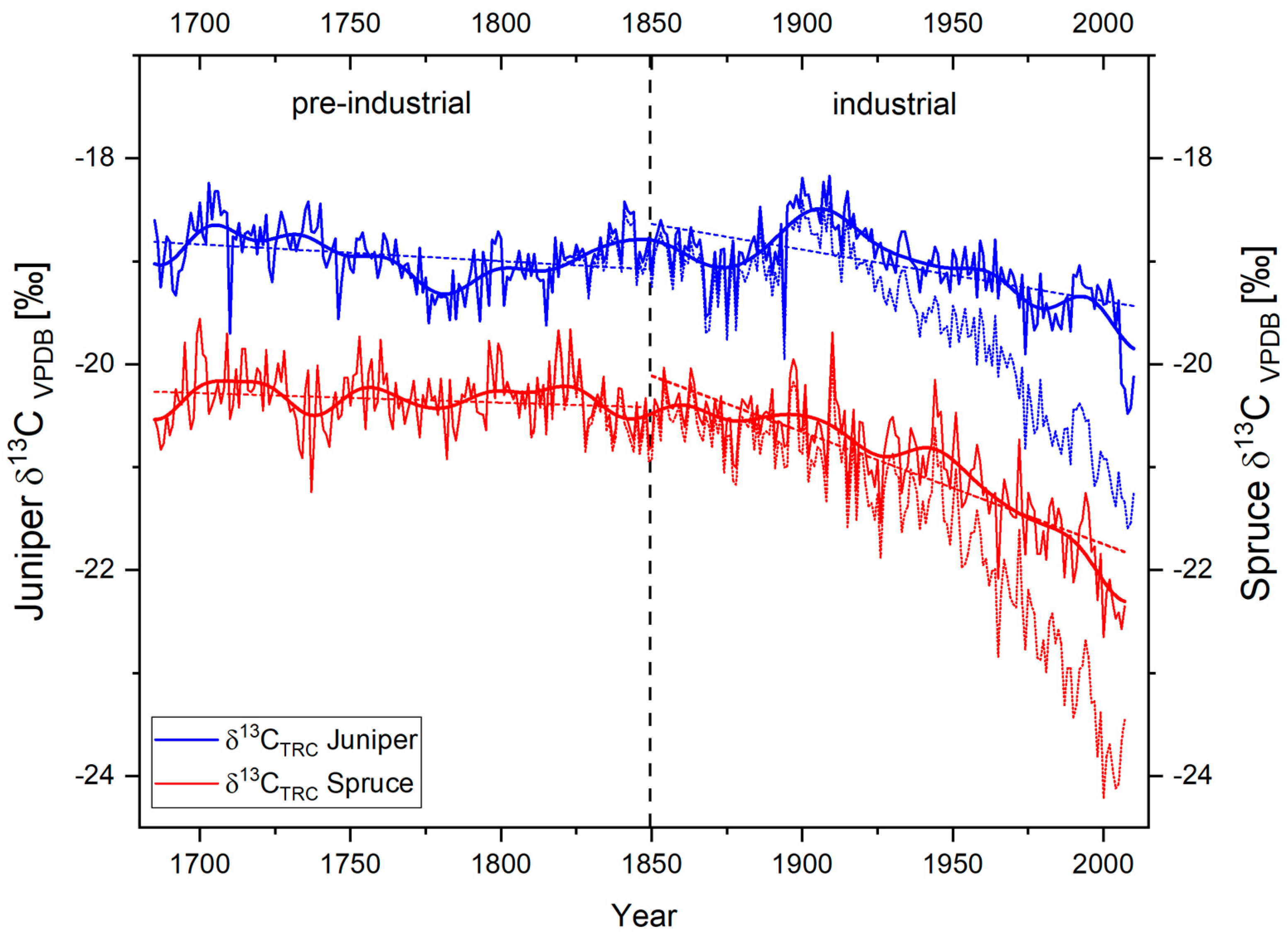
In contrast to the δ18OTRC-variations, the courses of the δ13CTRC time series highly differ between species. In general, both time series show an offset of around 2‰ in their individual mean values (mean δ13CTRC juniper = −18.83‰, mean δ13CTRC Spruce = −20.92‰). Mainly caused by the steadily rising influence of 13C-depleted atmospheric CO2, both series are highly influenced by a markedly negative trend since the end of the 19th century. After 1900 AD, this anthropogenic trend becomes more apparent for the juniper trees, resulting in an absolute minimum of the δ13CTRC juniper series in 2010 at −21.59‰ (Figure 4). For the spruce trees, a corresponding trend can be observed, resulting in the lowest overall value of −24.21‰ in 2005. As mentioned, this non-climatic trend has to be corrected before evaluating the respective climate–proxy relationship. Figure 4 displays the results of the performed δ13C trend correction on the two isotope chronologies. By regarding the common period (1685–2007) of both CO2-corrected time series, it is noticeable that both tree species exhibit an overall negative trend (Figure 4), which is more prominent for spruce. During the pre-industrial period, long-term linear trends in both species display a similar, only slightly negative trend. After 1850, these trends are further enhanced with different timing and magnitude between the species. Compared to the juniper trees, the linear long-term trend of spruce δ13CTRC indicate a more pronounced decrease after 1850 AD. While regarding the 11-year FFT filter of both species (Figure 4), this can be explained by an apparent positive trend in the juniper δ13CTRC until the early 20th century. Thereafter, the junipers also show a comparable negative trend to the absolute minimum of the time series in 2007. Presumably, these differing trends point to species-specific physiological strategies against the rising atmospheric CO2 content.
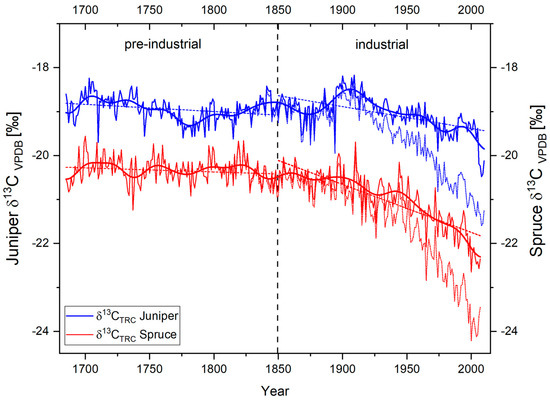
Figure 4.
Annual δ13CTRC variations of Juniperus tibetica (blue) and Picea balfouriana (red) at Qamdo for the period 1685−2011. Bold lines represent δ13CTRC CO2-corrected series and associated 11-year Fast Fourier Transform (FFT) filters, bright dotted time series indicate the species specific uncorrected δ13CTRC raw series, and dotted lines represent species-specific linear trends of the corrected δ13CTRC – series over the pre-industrial (1685−1849) and industrial period (1850−2007), respectively.
Compared to the δ18OTRC time-series, the correlation between the species-specific corrected δ13CTRC chronologies is lower (r = 0.44, GLK = 52%; Table 2). Although the statistical interrelation increases in the low frequency domains (11-year FFT filtered: r = 0.64, GLK = 64%; 21-year FFT filtered: r = 0.69, GLK = 65%), this is assumable mainly caused by—despite the applied CO2-correction—the still apparent negative trend since 1800 AD. The high influence and species-specific bias of this trend is additionally displayed in Table 2 by calculations with the uncorrected δ13CTRC raw values.
3.3. Climate–δ18OTRC Relationships
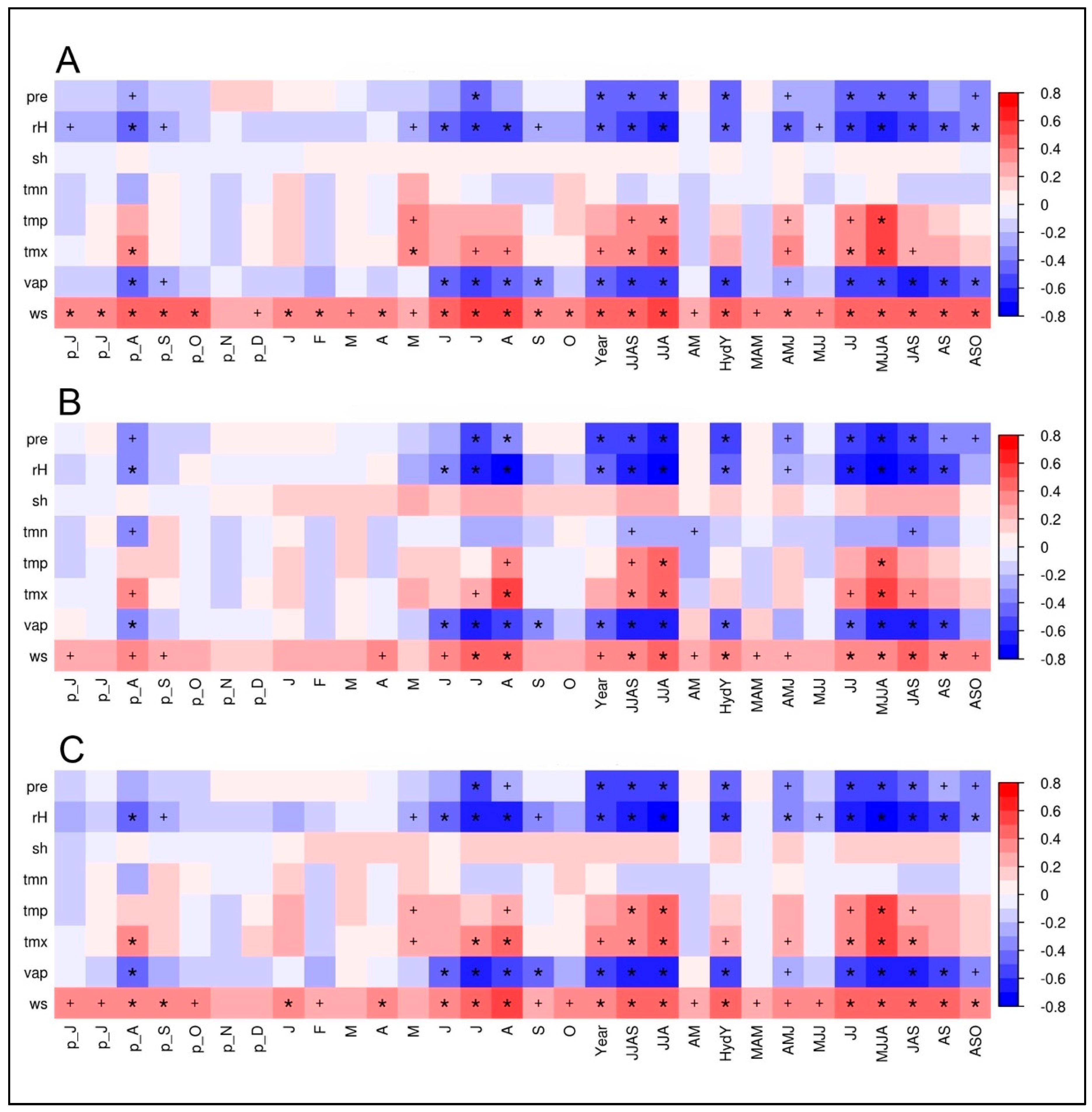
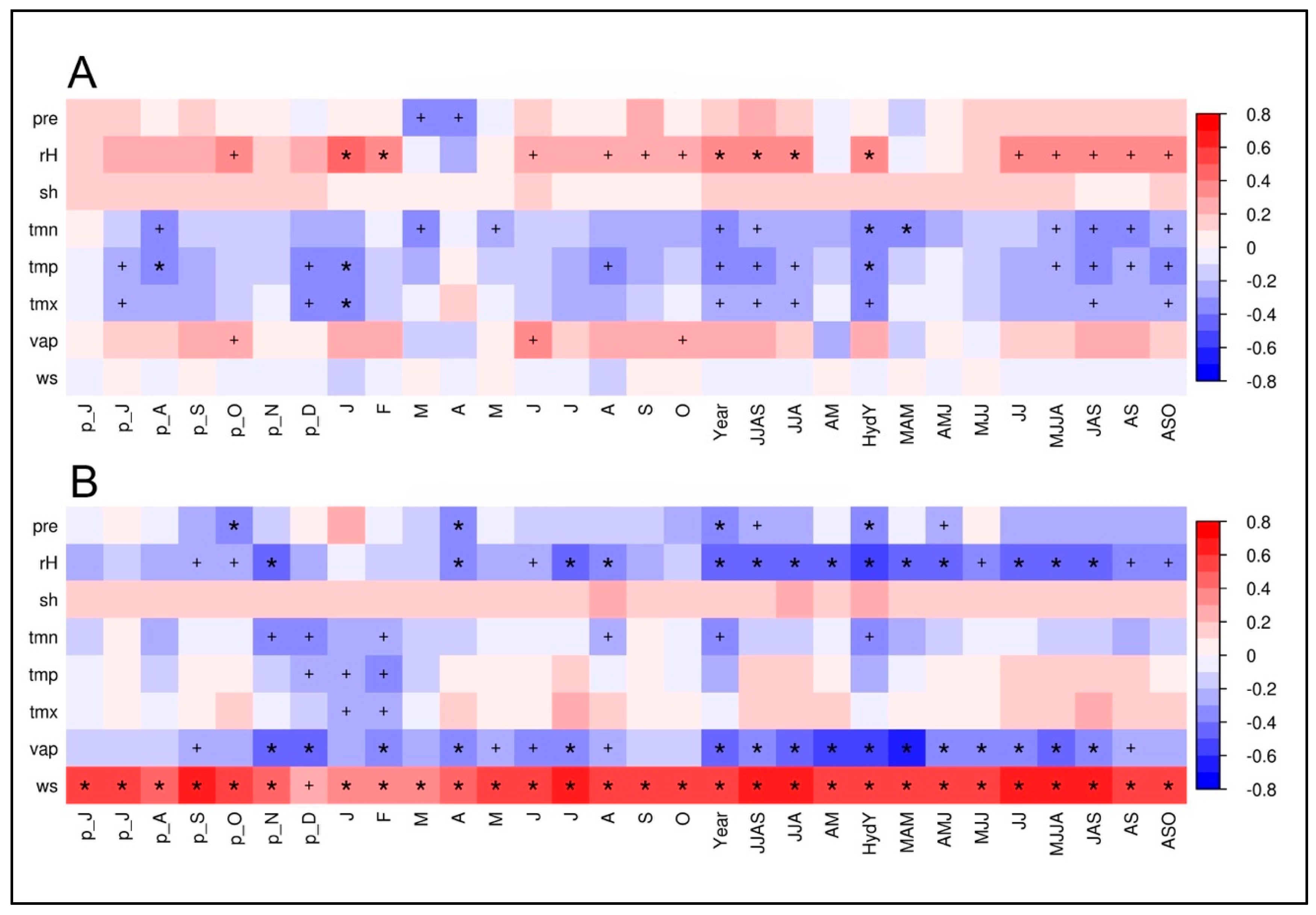
The climate–isotope relationships for the two investigated tree species based on calculations with the climate station at Qamdo are displayed in the Figure 5A,B. According to the high similarity between the δ18OTRC-series of both species, it was expected that the resulting climate correlations are highly synchronous. To check the potential benefit and additional information inherent in a combination of tree species, we tested the applicability of a mean isotope series. To evaluate if the climate signal is dominant over species-specific plant-physiological growth characteristics, juniper and spruce δ18OTRC chronologies were averaged. Additionally, the first principal components (PC) of the two tree species for both isotopes were computed, with prior centering and scaling applied, as δ13CTRC values differ significantly in their mean. Since for two centered and standardized variables, the correlation matrix always has the same eigenvectors, the correlation between its first PC and the mean is 1. Therefore, we used the calculated species δ18OTRC mean and performed the same correlation analyses as for the individual species (Figure 5C).
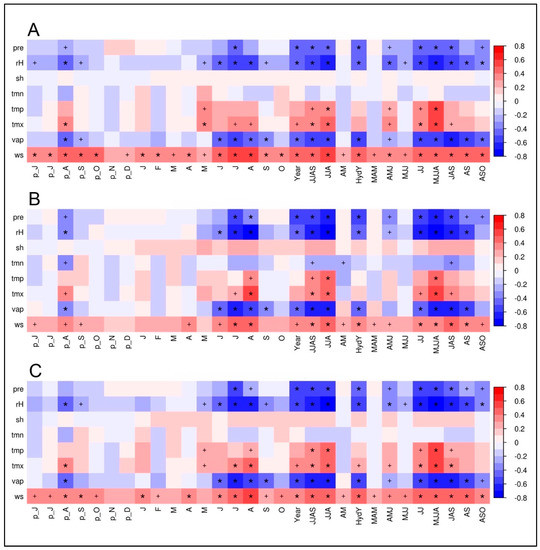
Figure 5.
Climate–proxy relationships between δ18OTRC of: juniper trees (A); spruce trees (B); and the site-internal δ18OTRC mean (C) plotted against monthly and seasonal averages of different climate parameters (pre, precipitation; rH, relative humidity; sh, sunshine hours; tmn, minimum temperature; tmp, mean temperature; tmx, maximum temperature; vap, vapor pressure deficit; ws, wind speed) from Qamdo climate station for the period 1954–2007 (n = 54). The prefix “p” indicates relationships to the previous years’ monthly means. Red/blue colors indicate positive/negative correlation coefficients (r). The respective level of significance is displayed by a plus sign (p < 0.05) or an asterisk (p < 0.01).
It is apparent that hydroclimatic parameters including precipitation, vapor pressure deficit and relative humidity (rH) have the highest negative influence on the δ18O variations in spruce and juniper tree-rings. Values of juniper δ18OTRC reveal highly significant correlations with different monthly and seasonal means during the vegetation period (lasting from May to September) of different hydroclimatic variables (Figure 5A). Within those, correlations with relative humidity averaged over the complete vegetation period have the strongest (negative) impact on δ18OTRC (rMJJAS = −0.67, p < 0.01. Considering the high autocorrelation of the juniper δ18OTRC series, the implementation of the previous year’s vegetation period even slightly increases correlations with rH (rMJJAS + pyMJJAS = −0.68, p < 0.01). Precipitation (r Precip MJJAS = −0.46, p> 0.01), nd vapor pressure (rMJJAS = −0.59, p < 0.01) are also significantly correlated to δ18OTRC during the summer season. Positive significant correlations occur with maximum temperature during May to September (rMJJAS = 0.45, p < 0.01). Results for single months display similar sensitivities, although showing continuously lower correlation strength.
Nearly identical climate–proxy relationships are apparent for spruce and the Qamdo climate station (Figure 5B). The corresponding correlogram reveals nearly the same pattern as for juniper, although the individual absolute values of the correlations are mostly higher. Highly significant relationships occur with relative humidity and vapor pressure deficit during the summer months (rH: rMJJA = −0.74, p < 0.01, vp: rMJJA = −0.62, p < 0.01), precipitation during the summer monsoon season (rJJA = −0.62, p < 0.01), and maximum temperatures in August (rAug = 0.52, p < 0.01). It is apparent that the hydroclimatic conditions during the vegetation period are decisive for the δ18OTRC variations in the tree-ring.
The combined species’ mean chronology showed largely identical results as displayed for the individual species’ correlations (Figure 5C). Interestingly, statistical relationships and significance levels mainly represent mainly the levels of the results from the spruce trees (e.g., rH: rMJJA = −0.74; vp: rMJJA = −0.62, p < 0.01), or are even increase in contrast to the species-specific results (rH: rJJA = −0.75, p < 0.01). The climate–isotope relationships are kept stable within such a combination of inter-species δ18OTRC series, clearly indicating the strong influence of the same external/climate factor(s) on both species. This result underlines the use of an inter-species approach for using δ18OTRC to reconstruct past climate variability and enhancing the representativity of tree-sites.
Proxy–climate calculations with climate variables from the CRU dataset also reflect the proposed general high similarities of responses among species (Figure S1). Interestingly, differences occur concerning the respective parameter and season the investigated species react most sensitive. Whereas the juniper δ18OTRC is highly sensitive to climate parameters related to drought or moisture stress during the summer monsoon period (pet: rMJJA = −0.54, p < 0.01, TMax: rMJJA = −0.51 p < 0.01, precipitation rMJJA = 0.53, p < 0.01), spruce δ18OTRC is more related to precipitation variations (rMJJA = −0.71, p < 0.01). Especially for the junipers, the location on a southerly exposed slope results in a more drought and insolation related sensitivity. In general, the correlations for junipers are more consistent than those of the spruce trees (Figure S1A,B). Presumably, the CRU dataset: (i) better reflects climate conditions at the upper tree-line; and (ii) is more representative for juniper than for spruce.
The assessment of the CRU–δ18OTRC relationship with a species’ mean exceeds the already strong results revealed with local instrumental climate data. It is obvious that, by using a species’ mean δ18OTRC value, the species-specific correlations coefficients can be further increased (Figure S1C). For example, the correlation with potential evapotranspiration during the summer season (May to August) increases up to an r-value of rMJJA= 0.60 (p < 0.01), while species-specific results are noticeably lower (pet juniper: rMJJA = 0.50; pet spruce: rMJJA = 0.54). Similar effects can be obtained for maximum temperature during the same season, with a species-mean r-value of rMJJA = 0.52 in comparison to the δ18OTRC of juniper (rMJJA = 0.50, p < 0.01) or spruce (rMJJA = 0.47, p < 0.01). Apparently, by averaging the δ18OTRC values of individual species, biases within the δ18OTRC variations related to species-specific responses are dampened in favor to a common site signal.
3.4. Climate–δ13CTRC Relationships
As expectable from the rather low inter-series correlation between the δ13CTRC time series, the two conifer species show only minor similarities in their respective climate–proxy relationships (Figure 6). For junipers, the correlations with climate station data reveal mostly weak statistical relationships and therefore a surprisingly weak storage of environmental signals in the δ13CTRC (Figure 6A). Highly significant positive correlations with climate parameters and juniper δ13CTRC-variations can only be obtained for different temperature variables during spring season (Tmin; rMAM = 0.36, p < 0.01), and the hydrological year (mean Temperature tmp: rHydY = 0.35, p < 0.01). It is apparent that all displayed correlations stay on much lower significance levels than the δ18OTRC–juniper relationships.
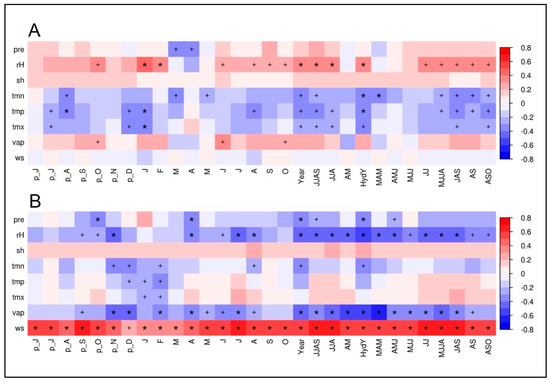
Figure 6.
Climate–proxy relationships between δ13CTRC of juniper trees (A) and spruce trees (B) against different monthly and seasonal means of climate parameters (pre, precipitation; rH, relative humidity; sh, sunshine hours; tmn, minimum temperature; tmp, mean temperature; tmx, maximum temperature; vap, vapor pressure deficit; ws, wind speed) from the Qamdo climate station for the period 1954–2007 (n = 54). “p” shows relationships to the previous years’ monthly means. Red/blue color indicates a positive/negative correlation coefficient (r). The respective level of significance is indicated by a plus sign (p < 0.05) or an asterisk (p < 0.01).
Figure 6B reveals the correlations between δ13CTRC values of spruce trees and the climate station in Qamdo. Interestingly, the results are in parts opposite to the correlations for the juniper trees. Especially the change of the sign in the correlation, e.g., for hydroclimate variables such as relative humidity and precipitation, is remarkable. This justifies and confirms that, in the case of δ13CTRC, the use of a cross-species mean for evaluating proxy–isotope relationships is not appropriate. Highest positive correlations were obtained for wind speed throughout the year, indicating the strong influence of wind on transpiration and gas exchange on leaf level. Significantly negative relationships are apparent for vapor pressure during the hydrological year (November to October) and spring (rHydY = −0.54, p < 0.01; rMAM = −0.58, p < 0.01), and for relative humidity during the hydrological year and the vegetation period (rHydY = −0.53, p < 0.01; rMJJA = −0.50, p < 0.01). It can be concluded that only the spruce δ13CTRC time series at our study site fulfills the preconditions to reconstruct past environmental history and variability.
As for the δ18OTRC, we tested climate–δ13CTRC relationships with the CRU TS 4.02 dataset for the period 1901–2007 (Figure S2). Interestingly, correlation coefficients as well as the respective significance levels for juniper are more stable throughout the year than for spruce. This agrees with the results obtained by the analyses with δ18OTRC (cf. Section 3.3), however, with considerably lower correlation coefficients. For juniper δ13CTRC, highest correlations are manifested with different temperature variables and potential evaporation (Figure S2A). Highly significant positive correlations of spruce δ13CTRC occur with diurnal temperature range (dtr), and negative correlations with minimum temperatures (tmn) (Figure S1B).
3.5. Implications of Species-Specific Proxy–Climate Responses of Multi-Centennial Isotope Series
Within the subalpine setting and design of our study, across-species δ18OTRC variations show a very uniform picture in their individual relationships to the tested environmental parameters. The derived climate–proxy correlations clearly indicate that, despite minor differences in exposure and altitude, the same climate and environmental signals are stored in the individual δ18OTRC variations. The investigated species are regardless of the used climate dataset (climate station or CRU 4.02 dataset) most sensitive to humidity related climate parameters during the vegetation period (May to August), clearly underlining a common external forcing. Among those, relative humidity, precipitation, and maximum temperature have the highest impact. These relationships can be directly linked to the influence of the Asian summer monsoon system, which leads via high amounts of precipitation during the vegetation period season to a relative depletion of δ18O in precipitation (=amount effect) [41]. As a result, negative relationships of δ18OTRC with hydroclimate parameters such as rH and precipitation occur and are in accordance with plant-physiological models [42,43]. Our findings are corroborated through similar results from numerous studies from the TP and the surrounding areas [10,11,12,13,14,15,16,17,27]. This underlines the straightforward and reasonable fingerprints of summer monsoon signals in δ18OTRC time-series from the summer monsoon-influenced area.
Minor tree-specific differences are apparent in the absolute values of the derived correlation coefficients, where spruce trees in general show higher values. However, the displayed correlation patters are very similar in terms of seasonality and signs. The higher sensitivities of spruce trees point to a higher general climate sensitivity compared to the long-living junipers. Since it is not possible to carry out separate analyses of earlywood (EW) and latewood (LW) due to narrow annual rings in junipers with only few rows of latewood, it remains open whether varying climate sensitivities of EW and LW are responsible for the lower signal strength in juniper. An indication of such different sensitivities was found for instance in other studies and species, e.g., from southwestern and southeastern China [23,25]. It has to be noted, however, that these studies are based on significantly shorter time periods of only few years to decades, and use species with significantly wider annual rings compared to ours (Abies and Picea).
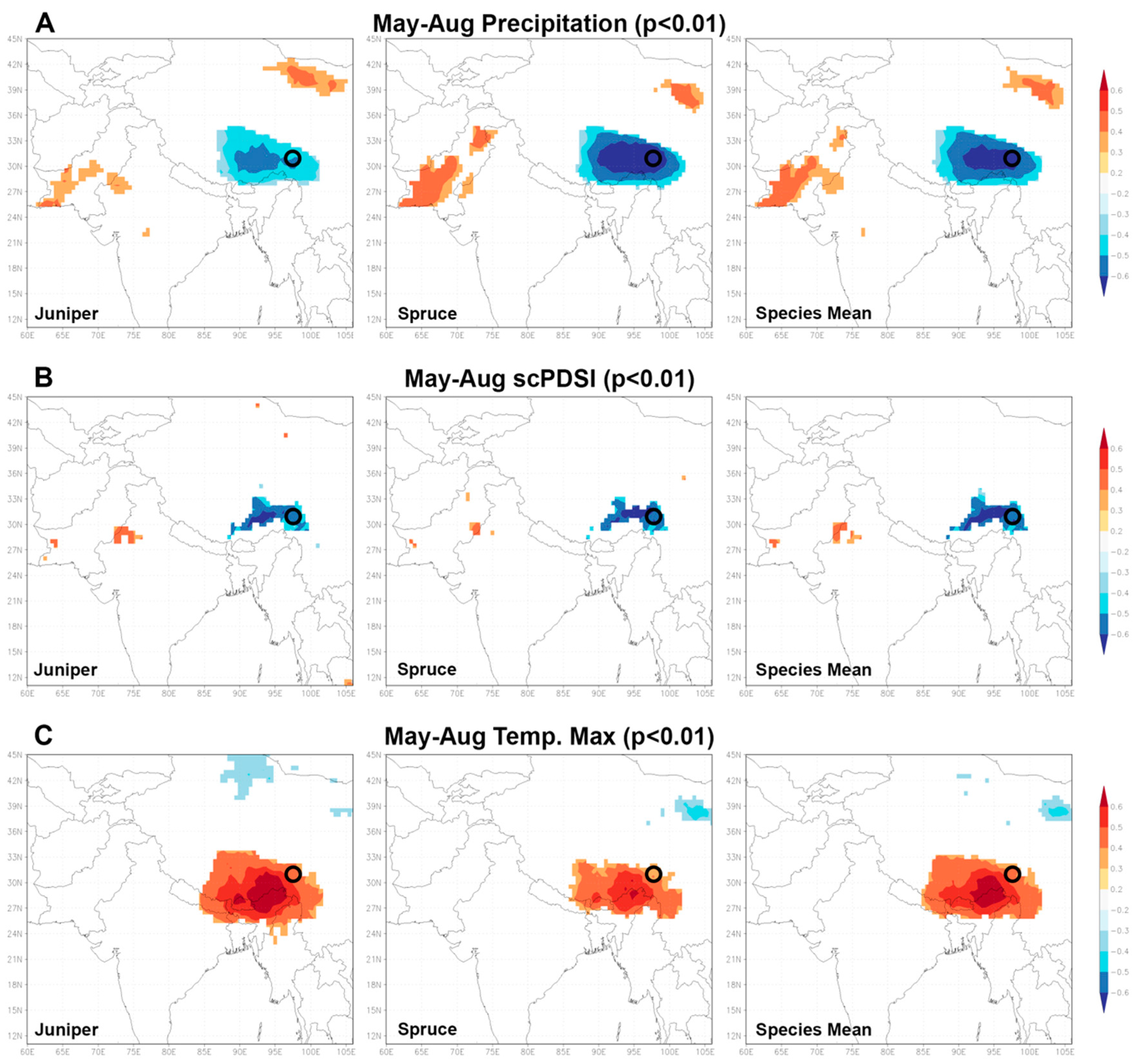
A striking result of our study is that both tree species indicate over the full common period of approximately 400 years a very high conformity in different frequency ranges. Hereby, a very high agreement can be found for single positive and negative extreme years as well as for lower frequency ranges, underlining the uniform response to a common forcing. This is confirmed by the climate–proxy relationship of the cross-species mean isotope chronology compared to the individual species chronologies. When analyzing the climate–isotope response of the average value, species-specific correlations are retained highly significant in both, their response and temporal linkage (individual months and seasons). Consequently, the spatial representativity of both oxygen time series should be expectedly similar. In fact, the analysis of spatial correlations with the three different δ18OTRC datasets using the KNMI climate explorer (https://climexp.knmi.nl) allows this relationship to be further quantified (Figure 7). The discussed higher sensitivity of spruce trees compared to junipers also results in consistently higher spatial correlations with different humidity and drought parameters. These are insofar as from a theoretical perspective of isotope fractionation and fixation reasonable, since they display an often reported negative (MJJA precipitation amd MJJA scPDSI) or positive relationship (increased evaporation during the summer season via TMax) mainly caused by a precipitation amount or temperature effect during the summer monsoon season. Interestingly, Figure 7 displays that the high spatial correlations remain stable with the calculation of a species’ mean and thus validates a general high common sensitivity to hydroclimate variables and large spatial representativity of multi-century oxygen isotope time-series across species. This uniform behavior across species further underlines the suitability and applicability of annually resolved, tree-ring based stable oxygen isotope time series to serve as an independent dating control for other archives (e.g., ice cores), as suggested in [44].
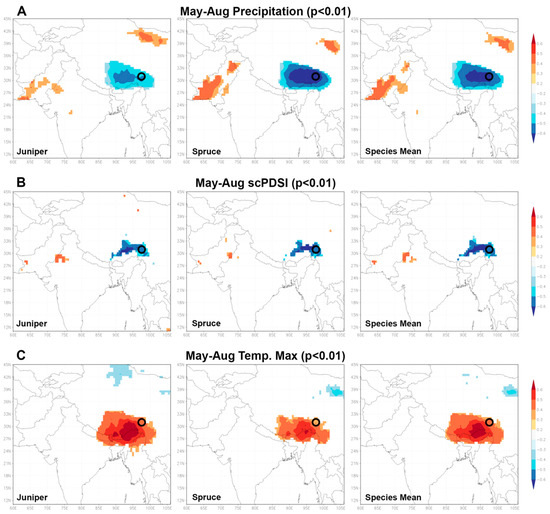
Figure 7.
Spatial correlations (p < 0.01) of δ18O variations in juniper, spruce and the species’ mean (vertically arranged) with climate variables using the Climate Explorer. (A–C) Selected climate variables for the individual species (juniper and spruce) and the calculated δ18O mean of our study site for the summer monsoon season (May to August).
It is striking that, in contrast to the investigated oxygen isotope series, the derived δ13CTRC series reveal a low common signal across species combined with a missing uniform response to the tested environmental parameters. This confirms results of previous studies on δ13CTRC from China, where no common cross-regional proxy signal has been found [25,44,45,46,47,48]. Instead, these studies suggest that the respective δ13CTRC series rather display more site-related ecophysiological responses on a changing environment. This can be underlined by the variety of interpretations according to which the respective δ13CTRC series serves as a proxy for changes in the plant-internal water-use strategy [25,28], as a proxy for temperature during the vegetation period [45], and for summer temperatures [46]. Only few studies indicate a dependency of δ13CTRC variations on moisture conditions (including precipitation and rH) [47,48]. This is probably connected to a generally drier setting at the investigation site and therefore a higher drought limitation at these sites compared to our location.
Although we can infer for both studied species at our study site similar macro- and mesoclimatic conditions and soil characteristics, differing physiological factors and processes seem to overprint or bias the detection of dominant environmental signals in our tree-ring δ13C. As mentioned in [33,38,39,40], 13C variations in tree-rings are on the one hand controlled by external environmental factors that regulate the uptake of atmospheric CO2 via the stomata of the leaves. Stomatal conductivity is in turn mainly determined by factors such as rH, temperature, moisture and wind. On the other hand, the carbon isotopes of CO2 are subject to a fractionation at the enzyme RUBISCO dependent on the respective CO2 concentration within the leaves. This, e.g., results in a discrimination in favor to the lighter 12C and against the heavier 13C isotopes during photosynthesis. Therefore, sensitivities and responses of the individual δ13CTRC to humidity parameters like rH and maximum temperature would be expectable. However, this is not straightforward displayed in our results. Instead, results of climate–isotope correlations with relative humidity indicate an indistinct, species-specific response during the vegetation period (positive for junipers, negative for spruce).
The displayed negative results for correlations between δ13CTRC of spruce and rH are in so far expectable and reasonable since a higher rH during the vegetation period linked to more open stomata leads to a depletion in δ13CTRC. This relationship stays stable for different seasonal means and single months during the vegetation period. Therefore. a seasonally higher moisture availability during the summer monsoon season apparently does not seem to be decisive for the regulations of the stomata. Presumably, moisture is not the main limiting factor for spruce during the summer monsoon season, although monsoonally triggered higher temperatures and humidity are then predominant. A higher moisture availability during the summer season leads to a further increase in the discrimination against 13C. For juniper, the explanation of the positive relationship between δ13CTRC and rH remains at this point challenging. As mentioned, conditions with higher humidity during the summer monsoon season would expectably be associated to more favorable conditions resulting in wider opened stomata. This will in turn result in lower δ13CTRC values. Thus, a negative correlation between δ13CTRC and the relative humidity would be expected as consequently displayed by the spruce δ13CTRC. Since correlations with a more radiation related parameter such as sunshine hours for the junipers do not display any significant result, possible short-range differences in humidity between both tree stands can be excluded as the reason for this. At this stage, explanations for such differences between species cannot be evaluated without knowledge of possibly underlying and possibly biasing plant-internal factors.
It remains striking that the climate δ13CTRC relationships are compared to the results derived from the δ18OTRC significantly lower and somehow inconsistent in terms of a uniform (site and species) signal and representativity. In addition, the underlying long-term trend especially apparent for the spruce trees clearly hampers the decoding of climate signals in our δ13CTRC series. To evaluate the influence of low-frequency variations on the different species and isotopes, high-pass filtered versions of all time-series were correlated against the climate station data. In the δ18OTRC series, the removal of the low-frequency variations which can be visualized through wavelets (Figure S3) results in slightly less strong, but still consistent correlation patterns. For the δ13CTRC series, the high-pass filtering removes the significance in the juniper–climate relationships almost completely. Contrasting, the correlations of the spruce δ13CTRC series with climate are enhanced in the positive (temperature) as well as negative (precipitation, rH, and vapor pressure) directions. Although correlations vary over time, this suggests that species-specific correlations with climate data since the 1960s are highly dependent on the chosen trend correction method. This is additionally corroborated by the difference displayed in moving correlations between the raw δ13CTRC series and the CO2-corrected δ13CTRC series (Figure S4). Such an influence of the chosen trend correction is in line with the results of [45].
3.6. Long-Term Trends in Carbon Isotope Ratios and their Implications for Recording Climate Signals
As displayed in Figure 4, even the corrected δ13CTRC time series do not indicate a uniform signal between species. In contrast to δ18OTRC, the δ13CTRC series reveal for both species a highly individual year-to-year variability as well as negative long-term trends. In addition, since 1900, a significantly more negative trend in spruce δ13CTRC values as compared to the juniper values is evident. By taking into account approximately comparable site conditions for both studied species, only certain internal physiological drivers can probably lead to the stronger depletion of the heavier 13C isotopes in the spruce trees. Thus, we assume that species-specific ecophysiological responses to increasing atmospheric CO2 or to increasing temperatures cause differences in the uptake of heavier 13C in the tree-rings. The impact of this changing external forcing and the resulting different response of the two tree species become even more impressive when considering the δ13CTRC in a multi-centennial perspective.
Global and regional studies show that trees tend to respond to an increase in atmospheric CO2 through various ecophysiological adaptations, such as modulations in the response of photosynthesis, stomatal conductance, or a response in the water-use efficiency (iWUE) [49,50,51,52,53,54]. It is likely that the substantial disparities and trends in our δ13CTRC series display species-specific ecophysiological reactions to a rising atmospheric CO2 concentration and/or to a substantial altitude-dependent increase in temperature on the TP [55,56]. Recently, it is reported that even within a sampling site such reactions can be species-specific [57]. Consequently, such plant-internal adaptations will in turn lead to a blurred picture of climatic signals stored in the respective δ13CTRC values. The apparent negative δ13CTRC after correction for atmospheric CO2 change can be therefore one reason for the weak correlations of δ13CTRC with climate parameters. In addition, the choice of the suitable correction factor can have an influence that should not be underestimated, as reported in [43]. Probably our carbon isotope time-series indicate an individual response of the investigated tree species towards the changing CO2 concentrations rather than recording an environmental signal.
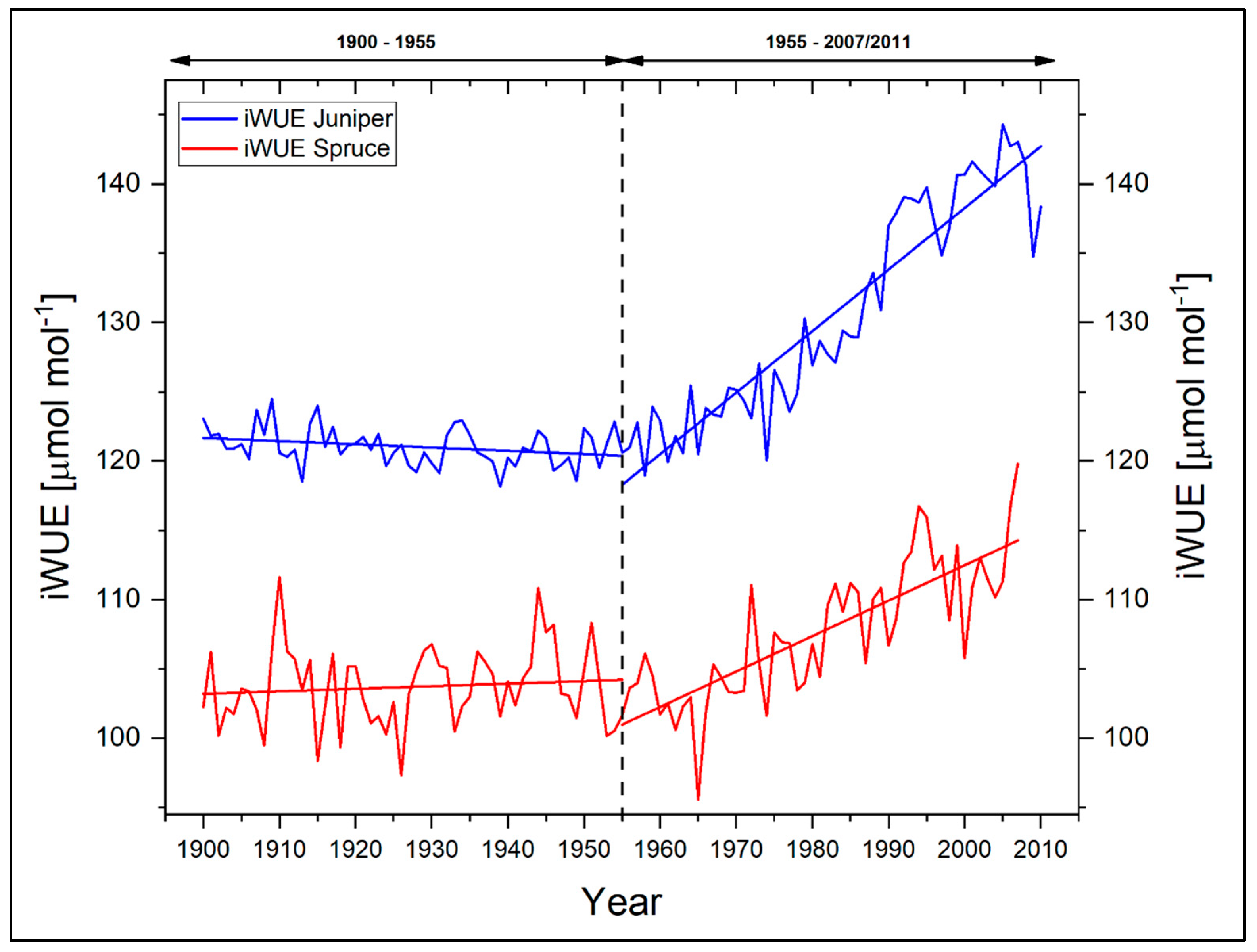
Figure 8 shows the iWUE of the two investigated tree species for the period 1900–2007. With respect to the reported environmental change in terms of a general warming trend since the 1950s, we additionally split the investigation period into two individual segments with a threshold in 1955. It is apparent that the displayed individual iWUEs differ in their absolute amounts as well as in their long-term trends for the full period between the species. This points to different species-specific physiological strategies and adaptations either with regard to changes in climate and/or changes in the atmospheric CO2 concentration. Whereas the iWUE of juniper trees is characterized by a more or less stable phase throughout the early period (−2% for the period 1900–1954), a steady and strong increase in the iWUE is apparent since the 1960s (+20%), reaching a maximum in 2005. With regard to the full period from 1900 to 2007, the relative increase in iWUE for juniper is 17%. In comparison, spruce tree iWUE is characterized by a slight increase (+1%) during 1900–1954. Similar to the juniper trees, spruce trees show a remarkably positive trend towards a higher iWUE for the recent six decades and therefore an adaptation for reducing water loss through transpiration. However, the absolute increase in iWUE of spruce since 1955 is markedly lower (+14%) and also evolves in its long-term rate during 1900–2007 generally slower than in juniper (+12%). Juniperus as a more drought tolerant species can probably better respond and react to the ongoing environmental changes with rising temperatures and lower humidity with a faster acceleration within their respective iWUE. On the other hand, more favorable microclimatic conditions with higher humidity at the spruce stands might help this species to better cope with the increase in temperature. The different trends and responses in iWUE indicate that plant physiological adaptations to changing external environmental parameters can be detected for both tree species. These plant physiological adaptations aggravate a more straightforward interpretation of our climate–proxy relationships. A necessary deciphering need in turn further analyses on the responsible plant-internal drivers. The future use of our δ13CTRC time series for climate reconstruction appears at this stage therefore challenging. Although admittedly reflecting an initial stage, our results on the iWUE are in good accordance to existing studies from the Tibetan Plateau dealing with Pinus and Abies species, where similar positive trends in iWUE were reported for the last decades [28,58]. In addition, on a global scale, such responses can be observed in various tree species, e.g., from the tropics [59,60] or more temperate zones [53,61,62,63,64,65,66].
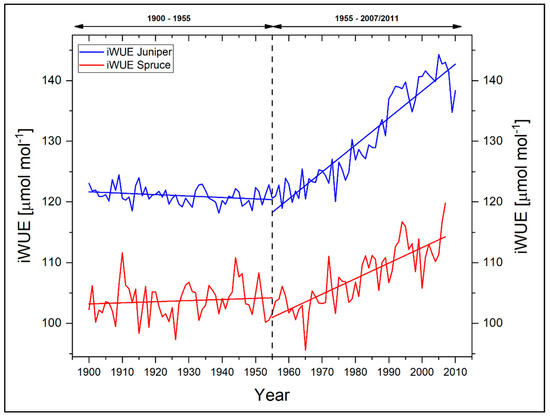
Figure 8.
Intrinsic water-use efficiencies for Juniperus tibetica and Picea balfouriana at Qamdo. Dashed line indicates dividing line between the investigation periods, while old lines represent the individual linear trends of both species for the periods 1900–1954 and 1955–2007, respectively.
We are aware that substantial ecophysiological investigations on and interpretations of iWUE need more knowledge about the corresponding changes in controlling plant physiological and environmental factors (e.g., ci, ca, and the carbon isotope discrimination Δ). Since this study mainly targeted a first estimation of the usability and the included potentials and limitations of a dual isotope and dual species approach at a single site, we focused on a first qualitative description of ecophysiological findings. Therefore, the identification and quantification of the proportion or the combination of, e.g., temperature rise and CO2 concentration, on the two tree species clearly exceeds the scope of the presented manuscript. More complex and further comprehensive ecophysiological analyses of the obtained results will be subjects of an upcoming study.
4. Conclusions
This study represents the first substantial comparison of annually resolved, multi-centennial tree-ring δ13C and δ18O isotope time series from a high mountain site at the southeastern Tibetan Plateau. Although the external forcings and general site characteristics can be regarded as highly comparable, a thorough evaluation on responsible environmental factors causing the respective δ13CTRC and δ18OTRC variations in the tree species revealed in part substantial differences.
The displayed δ18OTRC variations of juniper and spruce trees show a congruent response to the analysed climate parameters, indicating a uniform response across species to a common forcing. As best results were obtained for various hydroclimatic parameters during the vegetation period, notably a summer monsoon signal was herein recorded. By combining and averaging the δ18OTRC time series of our investigated species, existing highly significant proxy–climate relationships on selected hydroclimatic variables could be in most cases retained. Microclimatic influences and/or species-specific differences in the individual tree species are presumably reduced in favor to a more representative and common site signal. Thus, when analyzing δ18O in tree-rings, the expressiveness of δ18OTRC-time series can be enhanced by studying two tree species at one location.
In contrast, only weak relationships occur between the δ13CTRC series and tested environmental parameters. This is all the more astonishing as the locations of both tree stands are close to each other. Consequently, mainly plant-internal strategies and species-specific effects or adaptations are biasing the recording of climate signals. In combination with a—still strongly preserved—long-term trend after atmospheric CO2 trend correction in spruce, several questions remain open for an unambiguous interpretation of the δ13CTRC variations. Both studied species apparently reflect an individual response to environmental change. Juniper trees situated at higher elevations and southerly exposed react more sensitively with regards to the increasing irradiation and decrease in cloud cover and rH. This is for example evident from the changes in the iWUE. For spruce trees, the microclimatic advantage of a comparably higher humidity may be favorable, resulting in a delayed, lowered and lagged increase in iWUE. A verification of this assumption, however, requires a separate investigation, which should also include studies on seasonal isotope variations and coupled plant-internal effects.
Nevertheless, such species-specific investigations on similar settings might help to predict the influence and impact of future climate change on the adaptation ability of dominant tree species in the area of the treeline ecotone on the Tibetan plateau. For a quantification of such plant-internal responses, future studies should evaluate the complex interaction on the impact of a probable CO2-fertilization effect on iWUE, a general rise in temperature on the photosynthetic rate, and the interplay of growth and iWUE.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3263/9/4/151/s1, Figure S1. Climate–proxy correlation of: juniper δ18OTRC (A); spruce δ18OTRC (B); and the site-internal δ18OTRC mean (C) against monthly and seasonal means of different gridded climate parameters from the CRU TS4.01 dataset for the period 1954–2007 (cld, cloud cover; dtr, diurnal temperature range; frs, number of frost days; pet, potential evapotranspiration; tmn, minimum temperature; tmp, mean temperature; tmx, maximum temperature; wet, wet days frequency). “p” shows relationships to the previous years’ monthly means. Red/blue colous indicate positive/negative correlation coefficients (r). The respective level of significance is indicated by a plus sign (p < 0.05) or an asterisk (p < 0.01); Figure S2. Climate–proxy correlation between juniper δ13CTRC (A) and spruce δ13CTRC (B) against different monthly and seasonal means of gridded climate parameters from the CRU TS4.01 dataset for the period 1954–2007 (cld, cloud cover; dtr, diurnal temperature range; frs, number of frost days; pet, potential evapotranspiration; tmn, minimum temperature; tmp, mean temperature; tmx, maximum temperature; wet, wet days frequency). “p” shows relationships to the previous years’ monthly means. Red/blue colors indicate positive/negative correlation coefficients (r). The respective level of significance is indicated by a plus sign (p < 0.05) or an asterisk (p < 0.01); Figure S3. Wavelet analyses (Morlet) for both isotopes and species during the common period (1685–2007); (left) original values; and (right) high-pass filtered version. Periods of significant periodicities are framed by white lines; Figure S4. Moving correlations (50-year windows) between the raw δ13C series of spruce and juniper (in violet) and the CO2-corrected δ13C series of the investigated two tree species (in orange).
Author Contributions
Conceptualization, J.G.; analysis and visualization, J.G., W.J.-H.M., and P.H.; methodology, writing and original draft preparation, all authors; and funding acquisition, J.G., G.H.S., and A.B.
Funding
This research was partly funded by the German Research Foundation DFG, grant number BR1895/21-1.
Acknowledgments
The authors are grateful to the laboratory staffs from the German Research Centre of Jülich and of the Institute of Geography of the Friedrich-Alexander-University of Erlangen-Nürnberg for their assistances during the analytical analyses. We thank the three reviewers for their substantial help to improve the manuscript and R. Huang for sharing his data. The presented datasets are available after acceptance throughout the PANGAEA online platform (www.pangaea.de).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Yao, T.; Thompson, L.; Yang, W.; Yu, W.; Gao, Y.; Guo, X.; Yang, X.; Duan, K.; Zhao, H.; Xu, B.; et al. Different glacier status with atmospheric circulations in Tibetan plateau and surroundings. Nat. Clim. Chang. 2012, 2, 663–667. [Google Scholar] [CrossRef]
- Immerzeel, W.; van Beek, L.; Bierkens, M. Climate Change will affect the Asia Water Towers. Science 2010, 328, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Royden, L.H.; Burchfiel, B.C.; van der Hilst, R.R. The geological evolution of the Tibetan plateau. Science 2008, 321, 1054–1058. [Google Scholar] [CrossRef]
- Böhner, J. General climatic controls and topoclimatic variations in Central and High Asia. Boreas 2006, 35, 279–295. [Google Scholar] [CrossRef]
- Yang, K.; Wu, H.; Qin, J.; Lin, C.; Tang, W.; Chen, Y. Recent climate changes over the Tibetan plateau and their impacts on energy and water cycle: A review. Glob. Planet. Chang. 2014, 112, 79–91. [Google Scholar] [CrossRef]
- Yang, K.; Ye, B.; Zhou, D.; Wu, B.; Foken, T.; Qin, J.; Zhou, Z. Response of the hydrological cycle to recent climate changes in the Tibetan plateau. Clim. Chang. 2011, 109, 517–534. [Google Scholar] [CrossRef]
- Cheng, G.; Wu, T. Responses of permafrost to climate change and their environmental significance, Qinghai-Tibet plateau. J. Geophys. Res. 2007, 112, F02S03. [Google Scholar] [CrossRef]
- Kang, S.; Xu, Y.; You, Q.; Flügel, W.A.; Pepin, N.; Yao, T. Review of climate and cryospheric change in the Tibetan plateau. Environ. Res. Lett. 2010, 5, 01510. [Google Scholar] [CrossRef]
- Yang, B.; He, M.; Shishov, V.; Tychkov, I.; Vaganov, E.; Rossi, S.; Ljungqvist, F.C.; Bräuning, A.; Grießinger, J. New perspective on spring vegetation phenology and global climate change based on Tibetan Plateau tree-ring data. Proc. Natl. Acad. Sci. USA 2017, 114, 6966–6971. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Daux, V.; Zhang, Q.-B.; Risi, C.; Hou, S.-G.; Stievenard, M.; Pierre, M.; Li, Z.; Masson-Delmotte, V. Reconstruction of southeast Tibetan plateau summer climate using tree ring 18O: Moisture variability over the past two centuries. Clim. Past 2012, 8, 205–213. [Google Scholar] [CrossRef]
- Grießinger, J.; Bräuning, A.; Helle, G.; Thomas, A.; Schleser, G.H. Late Holocene Asian summer monsoon variability reflected by δ18O in tree-rings from Tibetan junipers. Geophys. Res. Lett. 2011, 38, L03701. [Google Scholar] [CrossRef]
- Grießinger, J.; Bräuning, A.; Helle, G.; Hochreuther, P.; Schleser, G.H. Late Holocene relative humidity history on the southeastern Tibetan plateau inferred from a tree-ring δ18O record: Recent decrease and conditions during the last 1500 years. Quat. Int. 2017, 430, 52–59. [Google Scholar] [CrossRef]
- Wernicke, J.; Hochreuther, P.; Grießinger, J.; Zhu, H.; Wang, L.; Bräuning, A. Multi-century humidity reconstructions from the southeastern Tibetan Plateau inferred from tree-ring δ18O. Glob. Planet. Chang. 2016, 149. [Google Scholar] [CrossRef]
- Wernicke, J.; Grießinger, J.; Hochreutherm, P.; Bräuning, A. Variability of summer humidity during the past 800 years on the eastern Tibetan Plateau inferred from δ18O of tree-ring cellulose. Clim. Past 2015, 11, 327–337. [Google Scholar] [CrossRef]
- Hochreuther, P.; Wernicke, J.; Grießinger, J.; Mölg, T.; Zhu, H.; Wang, L.; Bräuning, A. Influence of the Indian Ocean Dipole on tree-ring δ18O of monsoonal Southeast Tibet. Clim. Chang. 2016, 137, 217–230. [Google Scholar] [CrossRef]
- Xu, G.; Liu, X.; Trouet, V.; Treydte, K.; Wu, G.; Chen, T.; Sun, W.; An, W.; Wang, W.; Zeng, X.; et al. Regional shifts (1710–2019) in East Central Asia and linkages with atmospheric circulation recorded in tree-ring δ18O. Clim. Dyn. 2018. [Google Scholar] [CrossRef]
- Liu, X.; Xu, G.; Grießinger, J.; An, W.; Wang, W.; Zeng, X.; Wu, G.; Qin, D. A shift in cloud cover over the southeastern Tibetan plateau since 1600, evidence from regional tree-ring 18O and its linkages to tropical oceans. Quat. Sci. Rev. 2014, 88, 55–68. [Google Scholar] [CrossRef]
- McCarroll, D.; Gagen, M.H.; Loader, N.J.; Robertson, I.; Anchukaitis, K.J.; Los, S.; Young, G.H.F.; Jalkanen, R.; Kirchhefer, A.; Waterhouse, J.S. Correction of tree ring stable carbon isotope chronologies for changes in the carbon dioxide content of the atmosphere. Geochim. Cosmochim. Acta 2009, 73, 1539–1547. [Google Scholar] [CrossRef]
- Loader, N.J.; McCarroll, D.; Gagen, M.; Robertson, I.; Jalkanen, R. Extracting climatic informations from stable isotopes in tree rings. In Stable Isotopes as Indicators of Ecological Change; Dawson, T.E., Siegwolf, R.T.W., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 27–48. [Google Scholar]
- Xu, G.; Liu, X.; Qin, D.; Chen, T.; Sun, W.; An, W.; Wang, W.; Wu, G.; Zeng, X.; Ren, J. Drought history inferred from tree ring 13C and 18O in the central Tianshan Mountains of China and linkage with the North Atlantic Oscillation. Theor. Appl. Climatol. 2014, 116, 385–401. [Google Scholar] [CrossRef]
- Xu, G.; Liu, X.; Belmecheri, S.; Chen, T.; Wu, G.; Wang, B.; Zeng, X.; Wang, W. Disentangling Contributions of CO2 Concentration and Climate to Changes in intrinsic water-use efficiency in the arid boreal forest in China’s Altay Mountains. Forests 2018, 9, 642. [Google Scholar] [CrossRef]
- Xu, G.; Liu, X.; Sun, W.; Chen, T.; Zhang, X.; Zeng, X.; Wu, G.; Wang, W.; Qin, D. Application and verification of simultaneous determination of cellulose δ13C and δ18O in Picea shrenkiana tree rings from northwestern China using the high-temperature pyrolysis method. J. Arid Land 2018, 10, 864–876. [Google Scholar] [CrossRef]
- An, W.; Liu, X.; Leavitt, S.W.; Ren, J.; Sun, W.; Wang, W.; Wang, Y.; Xu, G.; Chen, T.; Qin, D. Specific climatic signals recorded in earlywood and latewood 18O of tree rings in southwestern China. Tellus B 2012, 64, 18703. [Google Scholar] [CrossRef]
- Hartl-Meier, C.; Zang, C.; Büntgen, U.; Esper, J.; Rothe, A.; Göttlein, A.; Dirnböck, T.; Treydte, K. Uniform climate sensitivity in tree-ring stable isotopes across species and sites in a mid-latitude temperate forest. Tree Physiol. 2014, 35, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Grießinger, J.; Gebrekirstos, A.; Fan, Z.-X.; Bräuning, A. Earlywood and Latewood Stable Carbon and Oxygen Isotope Variations in Two Pine Species in Southwestern China during the Recent Decades. Front. Plant Sci. 2017, 7, 2050. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, X.; Treydte, K.; Evans, M.N.; Wang, W.; An, W.; Sun, W.; Xu, G.; Wu, G.; Zhang, X. Climate signals in tree-ring δ18O and δ13C from southeastern Tibet: Insights from observations and forward modelling of intra- to interdecadal variability. New Phytol. 2017, 216, 1104–1118. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yang, B.; Bräuning, A.; Rossi, S.; Ljungqvist, F.C.; Shishov, V.; Grießinger, J.; Wang, J.; Liu, J.; Qin, C. Recent advances in dendroclimatology in China. Earth-Sci. Rev. 2019. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, H.; Liu, X.; Liang, E.; Grießinger, J.; Wu, G.; Li, X.; Bräuning, A. Does increasing intrinsic water use efficiency (iWUE) stimulate tree growth at natural timberline on the southeastern Tibetan plateau? Glob. Planet. Chang. 2017, 148, 217–226. [Google Scholar] [CrossRef]
- Bräuning, A. Climate history of the Tibetan plateau during the last 1000 years derived from a network of Juniper chronologies. Dendrochronologia 2001, 19, 127–137. [Google Scholar]
- Zhu, H.; Shao, X.; Yin, J.; Huang, L. Early summer temperature reconstruction in the eastern Tibetan plateau since AD 1440 using tree-ring width of Sabina tibetica. Theor. Appl. Clim. 2011, 106, 45–53. [Google Scholar] [CrossRef]
- Miehe, G.; Miehe, S.; Vogel, J.; La, D. Highest tree-line in the northern hemisphere found in southern Tibet. Mt. Res. Dev. 2007, 27, 169–173. [Google Scholar] [CrossRef]
- Leavitt, S. Tree-ring C-H-O isotope variability and sampling. Sci. Total Environ. 2010, 408, 5244–5253. [Google Scholar] [CrossRef]
- McCarroll, D.; Loader, N.J. Stable isotopes in tree rings. Quat. Sci. Rev. 2004, 23, 771–801. [Google Scholar] [CrossRef]
- Wieloch, T.; Helle, G.; Heinrich, I.; Voight, M.; Schyma, P. A novel device for batch-wise isolation of a-cellulose from small-amount wholewood samples. Dendrochronologia 2011, 29, 115–117. [Google Scholar] [CrossRef]
- Laumer, W.; Andreau, L.; Helle, G.; Schleser, G.H.; Wieloch, T.; Wissel, H. A novel approach for the homogenization of cellulose to use micro-amounts for stable isotope analyses. Rapid Commun. Mass Spectrom. 2009, 23, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations—The CRU TS 3.10 dataset. J. Clim. 2014, 34, 623–642. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2013. Available online: www.R-project.org/ (accessed on 1 March 2019).
- MacFarling Meure, C.; Etheridge, D.; Trudinger, C.; Steele, P.; Langenfelds, R.; Van Ommen, T.; Smith, A.; Elkins, J. Law Dome CO2, CH4 and N2O ice core records extended to 2000 years BP. Geophys. Res. Lett. 2006, 33, L14810. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Hall, A.E.; Farquhar, G.D. Stable Isotopes and Plant Water Relations; Elsevier: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Farquhar, G.D.; O’Leary, M.; Berry, J. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Funct. Plant Biol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 4, 436–468. [Google Scholar]
- Barbour, M. Stable oxygen isotope composition of pant tissue: A review. Funct. Plant Biol. 2007, 34, 83–94. [Google Scholar] [CrossRef]
- Gessler, A.; Ferrio, J.P.; Hommel, R.; Treydte, K.; Werner, R.A.; Monson, R.K. Stable isotopes in tree rings e toward a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiol. 2014, 34, 796–818. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhu, H.; Liang, E.; Grießinger, J.; Wernicke, J.; Yu, W.; Hochreuther, P.; Risi, C.; Zeng, Y.; Fremme, A.; et al. Temperature signals in tree-ring oxygen isotope series from the northern slope of the Himalaya. Earth Planet. Sci. Lett. 2018, 506, 455–465. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Shao, X.; Leavitt, S.; Xu, G.; An, W.; Qin, D. A 200 year temperature record from tree ring δ13C at the Qaidam Basin of the Tibetan Plateau after identifying the optimum method to correct for changing atmospheric CO2 and δ13C. J. Geophys. Res. Biogeosci. 2011, 116, G04022. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Li, Q.; Song, H.; Linderholm, H.W.; Leavitt, S.W.; Wang, R.; An, Z. Tree-ring stable carbon isotope-based May–July temperature reconstruction over Nanwutai, China, for the past century and its record of 20th century warming. Quat. Sci. Rev. 2014, 93, 67–76. [Google Scholar] [CrossRef]
- Liu, Y.; Ta, W.; Li, Q.; Song, H.; Sun, C.; Cai, Q.; Liu, H.; Wang, L.; Hu, S.; Sun, J.; et al. Tree-ring stable carbon isotope-based April–June relative humidity reconstruction since AD 1648 in Mt. Tianmu, China. Clim. Dyn. 2017, 50, 1733–1745. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.; Leavitt, S.W.; Cai, Q.; Liu, W. A preliminary seasonal precipitation reconstruction from tree-ring stable carbon isotopes at Mt. Helan, China, since AD 1804. Glob. Planet. Chang. 2004, 41, 229–239. [Google Scholar] [CrossRef]
- Körner, C.; Paulsen, J. A world wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Hyvönen, R.; Agren, G.I.; Linder, S.; Persson, T.; Cotrufo, M.F.; Ekblad, A.; Freeman, M.; Grelle, A.; Janssens, I.A.; Jarvis, P.G.; et al. The likely impact of elevated CO2, nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: A literature review. New Phytol. 2007, 173, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Drake, B.G.; Gonzalez-Meier, M.A.; Long, S.P. More efficient plants: A consequence of rising atmospheric CO2? Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 609–639. [Google Scholar] [CrossRef]
- Battipaglia, G.; Saurer, M.; Cherubini, P.; Calfapietra, C.; McCarthy, H.R.; Norby, R.J.; Cotrufo, M.F. Elevated CO2 increases tree-level intrinsic water-use efficiency. Insights from carbon and oxygen isotopes analyses in tree rings across three forest FACE sites. New Phytol. 2013, 197, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Giammarchi, F.; Cherubini, P.; Pretzsch, H.; Tonon, G. The increase of atmospheric CO2 affects growth potential and intrinsic water-use efficiency of Norway spruce forests: Insights from a multiple-stable isotope analysis in tree rings of two Alpine chronosequences. Trees 2017, 31, 503–515. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Tschaplinski, T.J.; Norby, R.J. Plant water relations at elevated CO2—Implications for water-limited environments. Plant Cell Environ. 2002, 25, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yan, Z.; Zhao, P.; Zhu, Y.; Yu, Y.; Tang, G.; Jones, P. Climatic warming in China during 1901–2015 based on an extended dataset of instrumental temperature records. Environ. Res. Lett. 2012, 12, 064005. [Google Scholar] [CrossRef]
- Qin, J.; Yang, K.; Liang, S.; Guo, X. The altitudinal dependence of recent rapid warming over the Tibetan plateau. Clim. Chang. 2009, 97, 321–327. [Google Scholar] [CrossRef]
- Altieri, S.; Mereu, S.; Cherubini, P.; Castaldi, S.; Sirignano, C.; Lubritto, C.; Battipaglia, G. Tree-ring carbon and oxygen isotopes indicate different water-use strategies in three Mediterranean shrubs at Capo caccia (Sardinia, Italy). Trees 2015, 29, 1593–1603. [Google Scholar] [CrossRef]
- Xu, G.; Liu, X.; Qin, D.; Chen, T.; An, W.; Wang, W.; Wu, G.; Zeng, X.; Ren, J. Climate warming and increasing atmospheric CO2 have contributed to increased water use efficiency on the northeastern Tibetan plateau since 1850. Trees Struct. Funct. 2013, 27, 465–475. [Google Scholar] [CrossRef]
- Hietz, P.; Wanek, W.; Duenisch, O. Long-term trends in cellulose delta C-13 and water-use efficiency of tropical Cedrela and Swietenia from Brazil. Tree Physiol. 2005, 25, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Wils, T.H.G.; Roberston, I.; Woodborne, S.; Hall, G.; Kaprowski, M.; Eshetu, Z. Anthropogenic forcing increases the water-use efficiency of African trees. J. Quat. Sci. 2016, 31, 386–390. [Google Scholar] [CrossRef]
- Urrutia-Jalabert, R.; Malhi, Y.; Barichivivh, J.; Lara, A.; Delgado-Huertas, A.; Rodríguez, C.G.; Cuq, E. Increased water use efficiency but contrasting tree growth patterns in Fitzroya cupressoides forests of southern Chile during recent decades. J. Geophys. Res. Biogeosciences 2015, 120, 2505–2524. [Google Scholar] [CrossRef]
- Keenan, T.F.; Hollinger, D.Y.; Bohrer, G.; Dragoni, D.; Munger, J.W.; Peter, H. Richardson, Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 2013, 499, 324–327. [Google Scholar] [CrossRef]
- Feng, X. Trends in intrinsic water-use efficiency of natural trees for the past 100-200 years: A response to atmospheric CO2 concentration. Geochim. Cosmochim. Acta 1999, 63, 1891–1903. [Google Scholar] [CrossRef]
- Penuelas, J.; Hunt, J.M.; Ogaya, R.; Jump, A.S. Twentieth century changes of tree ringδ13C at the southern range-edge of Fagus sylvatica: Increasing water use efficiency does not avoid the growth decline induced by warming at low altitudes. Glob. Chang. Biol. 2008, 14, 1076–1088. [Google Scholar] [CrossRef]
- Frank, D.; Poulter, B.; Saurer, M.; Esper, J.; Huntingford, C.; Helle, G.; Treydte, K.; Zimmermann, N.E.; Schleser, G.H.; Ahlström, A.; et al. Water-use eciency and transpiration across European forests during the Anthropocene. Nat. Clim. Chang. 2015, 5, 579–583. [Google Scholar] [CrossRef]
- Silva, L.C.R.; Anand, M.; Oliveira, J.M.; Pillar, V.D. Past century changes in Araucaria angustifolia (Bertol.) Kuntze water use efficiency and growth in forest and grassland ecosystems of southern Brazil: Implications for forest expansion. Glob. Chang. Biol. 2009, 15, 2387–2396. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).