Abstract
The leaching behavior of arsenic (As), lead (Pb), nickel (Ni), iron (Fe), and manganese (Mn) was investigated from subsurface core sediment of marine and nonmarine depositional environments in central Kanto Plain, Japan. A four-step sequential extraction technique was adopted to determine the chemical speciation, potential mobility, and bioavailability of metals under natural conditions in variable depositional environments. In addition, a correlation of these properties with pore water and total metal content was carried out. The concentration of As in pore water was found to be 2–3 times higher than the permissible limit (10 µg/L) for drinking water and leachate in fluvial, transitional, and marine environments. The trend of potential mobile fractions of As, Pb, and Ni showed Fe–Mn oxide bound > carbonate bound > ion exchangeable bound > water soluble in the fluvial environment. However, in the marine environment, it showed Fe–Mn oxide bound > water soluble > carbonate bound > ion exchangeable bound for As. The leaching of As in this fluvial environment is due to the organic matter-mediated, reductive dissolution of Fe–Mn oxide bound, where Mn is the scavenger. The amount of total content of As and sulfur (S) in transitional sediment reflects an elevated level of leachate in pore water, which is controlled by S reduction. However, the leaching of As in marine sediment is controlled by pH and organic matter content.
1. Introduction
Soil and sediment, which play a key role in geochemical cycling, are widely known as the sink and source of potentially hazardous trace metals [1]. Metals are bound with soil or sediment particles by a variety of mechanism with different metal phases. As a result, assessments of toxicity and bioavailability cannot be comprehensively undertaken by measuring only the total metal content [2]. It is important to perform specific speciation analyses. Sequential extraction analysis has become the most widely used method to determine precise chemical speciation, leaching behavior, and the potential mobility of metals in sediment [3,4,5,6]. It is also known as “operationally defined” when selected chemical reagents are used to extract metal from different phases or bindings of the soil/sediment particles [7,8,9,10,11].
As and Pb are recognized as highly toxic metalloids. These elements show persistent and toxic characteristics, even at very low concentrations, if they are ingested by humans and animals through air, water, or food intake [12]. There are many examples of the carcinogenic effects of As and Pb [13,14]. Groundwater is one of the main sources of drinking water due to freshwater scarcity and geomorphological differences in certain places. On the other hand, soil/sediment has a ubiquitous influence on human life. Soil, sediment, and groundwater can be polluted due to the leaching of metals, and it may become harmful for living beings and for the environment. The leaching behavior of As and Pb, along with other metals from sediment, also depends on various influencing factors such as pH, electrical conductivity, oxidation reduction potential, organic matter content, the adsorption capacity of some minerals, and type of clay mineral, along with different metal phases [15,16]. It is necessary to consider changes in any natural environmental condition after the direct or indirect influence of anthropogenic activities. The role of the depositional environment on the leaching behavior of metals cannot be ignored.
Several investigations were performed on the chemical speciation, potential mobility, and bioavailability of As and Pb in the core sediment of various characteristics and different depositional environments [7,17,18,19,20,21]. However, the sediment of different depositional environments (marine, nonmarine, and peat) and their correlation with pore water concentration in a single profile have not been largely considered by researchers. Understanding and assessing the toxicity of these metals will be very helpful for future soil, groundwater, and city development, where similar geological distribution of marine sediment exists at shallow depths. Moreover, the influence of marine sediment on the nonmarine shallow aquifer has not yet been properly investigated. Sediment was often investigated after oxidation or aeration due to improper handling and preservation after collection. In such a way, the original state of the metal phases changes and produces a vague or incorrect picture of the toxicity and availability of these metals. Therefore, a special preservation method is required to avoid strong oxidation and to keep its properties in place. However, it is impossible to maintain the original subsurface conditions in a laboratory set up. Partial or semi-oxidation takes place at the time of sample preparation and processing.
In this research, attempts were made to keep sediment core samples in their near original condition. A special chemical was added with an indicator to avoid oxidation, and samples were kept sealed before preparation. Non-air dried or semi-oxidized core sediments of different depositional environments (marine, nonmarine, and peat) were investigated after special preservation technique. Pore water chemistry was also compared with the total content and potential mobility of metals. The objective of this work was to understand the leaching behavior of the selected metals (As, Pb, Ni, Fe, and Mn) after assessing the chemical speciation, bioavailability, potential mobility. It is also aimed to assess the pollution risk from marine and nonmarine sediment to the environment under natural conditions.
For this study, one sediment core was selected from a low land valley between the Omiya upland area of Saitama City, Japan (Figure 1). This area is in Kanto lowland, which is the largest plain in Japan. The Kanto Plain is in the Neogene sedimentary basin [22]. The basin is filled with Neogene and Quaternary sediments, where the maximum thickness is greater than 3000 m [23,24,25]. The uplands are well distributed in the central Kanto plain, and are composed of mainly upper Pleistocene strata [26,27,28]. Holocene alluvial lowland/valleys occupy the space between the Pleistocene upland. Geologically, marine sediments are widely distributed at shallow depths beneath a thin layer of nonmarine fluvial and alluvial sediment [29,30].
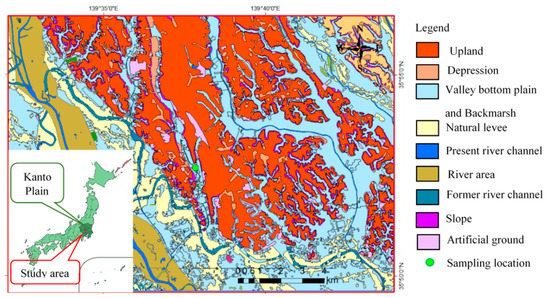
Figure 1.
Location map of sampling site.
2. Materials and Methods
2.1. Sediment Sample Collection and Site Description
The sampling site is located at 35°52′40.74″ N and 139°37′50.35″ E at Yono, Saitama City, in the central Kanto plain, Japan (Figure 1). The area of Saitama City is 217.43 km2, and the population density is 5830 per km2. Saitama City is only about 15 to 30 km from the capital; therefore, this city is strongly affected by the increased population and development of the past decades. Soil dredging has become a common phenomenon in this area for different kinds of underground development. Regarding the Saitama City municipal water supply, 86% comes from treated river water and 13% from groundwater (Saitama prefecture 2017). Thus, groundwater and subsurface soil pollution are becoming an environmental concern for further development. Groundwater is often used to water gardens or for agricultural activities when surface water scarcity occurs in the dry season. Therefore, a borehole location was selected inside Saitama City for this research. The borehole location is in a park beside a small river, the Konuma; this place was undisturbed from development activities, and it is assumed that the original depositional environment still exists in its original form. The borehole depth was extended from 2 to 27 m.
The study area is in the incised valley system near the Omiya upland area, where Kioroshi formation is distributed. Kioroshi formation under the Shimousa group of the Pleistocene age was formed at marine isotope stage (MIS) 5.5 sea-level highstand. This formation is comprised of some parts of the lower incised valley system and the upper barrier island system of the Kanto plain [31,32,33]. Thus, it was easier to obtain naturally occurring sediment samples of marine and nonmarine environments from a single vertical profile. In total, 25 sediment samples were collected at intervals of 1 m. Borehole samples were collected by applying the rotary drilling method. Lithologic reconstruction was carried out by correlating with nearby borehole environments and observing the physicochemical properties of the obtained samples.
2.2. Sediment Sample Preservation and Preparation
Sediment samples were collected from the inner part of the boring core just after drilling. Each sample was preserved in a plastic bag and sealed with an oxygen absorbent solution pack (RP System TM; Mitsubishi Gas Chemical Company, Inc., Japan). This solution pack helps to prevent oxidation and to keep moisture content at its original level. A pink tablet was used in each plastic bag as an indicator of oxidization; a change in color from pink to purple indicates a change of oxidation. It was ensured that the indicators in all samples showed pink color before sample analyses.
2.3. Physicochemical Property Analysis
The water content, organic matter content, pH, and electrical conductivity (EC) of the leachate of preserved soil/sediment were measured in the laboratory. Samples were preserved with the RP System TM just after sample collection from the borehole area, and leachate was collected from preserved samples at the laboratory. It was assumed that the physicochemical properties remained in their original state, i.e., without the influence of oxidation. The leachate was prepared by mixing the soil/sediment with distilled water at a ratio of 1:10. The leachate was centrifuged to avoid contamination with the electrode of the pH and EC meter. The leachate was analyzed for pH, EC, and oxidation-reduction potential (ORP). The percentage of total organic matter (OM) was determined by applying the LOI method, where temperatures of 110 °C and 600 °C were chosen.
2.4. Pore Water Collection
Pore water was extracted using Rhizon soil moisture samplers (Rhizosphere Research Products, Netherlands) just after sample collection. The pH and EC were measured immediately after collection; the samples were preserved in a refrigerator before testing.
2.5. Total Metal Content Analysis
For total metal and S content in the sediment, about 10 g of sample was taken from the packets and dried in an oven overnight at 60 °C. Then, the dried samples were crushed using an automatic magnetic grinder to obtain homogenized powdered samples. The Ringaku x-ray fluorescence technique was used to measure the total chemistry at the Center for Environmental Science in Saitama (CESS). For this machine, 3 g of dried powdered samples were mixed with 0.3 g of cellulose powder to bind and make a circular pellet. Three different types of standard samples from the geological survey of Japan were used to measure the unknown samples.
2.6. Chemical Speciation Using Sequential Extraction Method
A four-step modified Wenzel’s and BCR sequential extraction method was applied to measure the potential mobile fractions and chemical speciations of sediment. Water soluble (step 1), ion exchangeable (step 2), carbonate bound (step 3), and Fe–Mn oxide bound (reducible, step 4) phases are, together, considered as potential mobile phases/fractions. These inorganic phases are loosely bound to the sediment which can easily leach into the environment [34,35,36,37,38]. On the other hand, residual and organic (org.) bound/oxidizable phases are complex and much stronger than the potential mobile phases. In this research, organic matter bound and residuals were together considered as residuals. This four-step method was adopted in this research work. For each step, samples were thoroughly mixed with distilled water and other analytical grade chemical solutions at a ratio of 1:12.5. Finally, leachates were sequentially extracted in centrifuge tubes. After each extraction, samples were measured to determine metal concentrations using an inductively coupled plasma mass spectrometer (ICP-MS) and an inductively coupled plasma atomic emission spectrometer (ICP-AES). The leachates were extracted in the following order:
Step-1 Water soluble phase/speciation: Distilled water was mixed and shaken for 4 h.
Step-2 Ion exchangeable phase/speciation: 0.05 M (NH4)2SO4 was mixed with the residue from step 1 and shaken for 6 h.
Step-3 Carbonate bound phase/speciation: 0.11 M acetic acid was mixed with the residue from step 2 and shaken for 16 h.
Step-4 Fe–Mn oxide bound phase/speciation: 0.2 M of ammonium oxalate and oxalic acid solution were mixed with the residue from step 3 and shaken for 4 h.
2.7. Risk Assessment Code
The application of the risk assessment code (RAC) is useful to assess the bioavailability of metals in sediment. The sum of water soluble, ion exchangeable, and carbonate bound fractions are considered as the total bioavailability of the metals. Metals of these phases are weakly bonded, which may equilibrate with the aqueous phase and leach easily [39,40,41]. According to the RAC guidelines, any metal from soil/sediment can leach into the bioavailable fraction; less than 1% of total metal will be considered safe for the environment, and 11–30% is considered to present a medium risk for the environment. A bioavailable fraction of more than 50% of the total metal content in soil/sediment is considered a very high risk for the environment (Table 1) [39].

Table 1.
Criteria of risk assessment code.
2.8. Correlation Matrix
A Pearson correlation analysis was conducted on the metal content in sediment, pore water, and leachate, with physical parameters to identify the predominant factors from one data set to another. This relation helps to determine the interactive relationship between metals and other physicochemical parameters. The statistical software, PSPP (GNU PSPP 1-2.0), was used, where p < 0.01 and p < 0.05 were set as the level of significance.
3. Results and Discussion
3.1. Lithologic Reconstruction
A lithological column was performed after correlating with the relevant references of nearby areas and collecting physicochemical data of the sediment samples, e.g., visual observations of the color, particle size distribution, electrical conductivity, and S content (Figure 2). The lithostratigraphic reconstruction and landform evolution in the Kanto Plain have been studied by different parties [29,32,42,43,44], and research there is ongoing. For this research, the sediments of different depositional environments were identified and described, along with the chemical characteristics. Marine sediment thickness was confirmed from the EC value, S content, the presence of marine shell fragments, and correlation with other references [30,32,43,44]. Marine sediment is separated from nonmarine sediment by 3 m of transitional sediment (Figure 2). The upper part consists of nonmarine sediment; it includes 2–3 m of organic matter-rich, alluvial soil, underlain by about 1.5 m of peat and organic-rich clayey silt. From a depth of 6.2 to 7.8 m, tuff mixed clays are distributed over fluvial sediment.
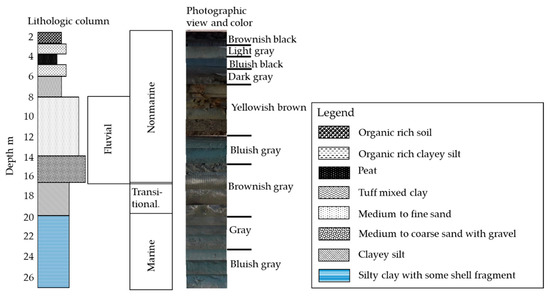
Figure 2.
Lithological column of the borehole profile.
Fluvial sandy sediments occur from about 7.8 to 16 m in depth. They consist of yellowish-brown, medium to fine sand, which is distinct from the 3 m thick, bluish gray, medium to coarse sand and 1 m thick, light gray, fine sand. A yellowish-brown color is an indication of an oxidizing environment for sand, which is clearly distinct from the reduced, bluish-gray sand.
This thick, fluvial sandy layer can be considered as the shallow aquifer for this area. Clayey silt is present from a depth of 16 to 20 m; it could be described as transitional sediment after confirming its physicochemical data from Figure 3 and with reference [30]. Marine sediment is distributed from 20 to 27 m.
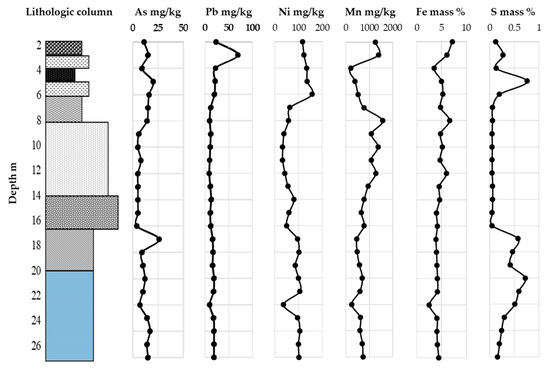
Figure 3.
Total content of As, Pb, Ni, Mn, Fe, and S in core sediment.
The marine sediment consisted of light gray-colored, silty clay with fragments of marine shells. At a depth of 22 m, the content of the marine shell fragment was relatively higher than in the other part. The sediment color was bluish gray from a depth of 24 to 27 m, which is an indication of more reductive condition than here than elsewhere.
3.2. Physical and Chemical Characteristics of Soil and Sediment Leachate
The pH, EC, ORP, and OM content (%) of sediment leachate were determined. The pH ranged from a minimum of 6.37 to a maximum of 8.04. The marine sediment showed a relatively lower pH than the nonmarine sediment. In the marine sediment, the pH ranged from 6.55 to 6.95. In the fluvial sediment, the pH ranged from 6.65 to 8.04. The pH was 6.65 in peat, which was relatively lower than that of the upper and lower alluvial sediment layers. The marine sediments showed several times higher EC values than the nonmarine fluvial, tuffaceous, and alluvial clays. The EC was relatively higher than that in other part in topsoil up to a depth of 3 m. Though it is difficult to get the ORP under laboratory conditions, an attempt was made to measure it from the leachate. Marine sediment showed a relatively lower ORP value (less than 200 mV) than nonmarine sediment.
Soil Eh (ORP) less than 300 mV is generally considered to be anaerobic [45]. As the measurement was performed on the leachate, it does not actually represent the soil/sediment ORP. The value of the ORP of the upper fluvial sediment was relatively higher (299–307 mV). The color of these samples was yellowish-brown, which is also an indication of relatively oxidized conditions compared to the other samples. The OM content was very low in the fluvial sediments. Upper alluvial and peat showed relatively higher percentages, i.e., 21% and 67%, respectively. Tuffaceous clay at a depth of 6.2 m showed a relatively higher value, i.e., 31%, whereas the range in the fluvial sediment was 1–2%. In the marine and transitional sediment, the OM content showed a range of 3–8%. However, sediment at depths of 25 m and 26 m showed relatively higher values, i.e., 23% and 16%, respectively.
3.3. Total Content of As and other Elements in Vertical Profile
The total concentration of As, Pb, Ni, Fe, Mn, and S in soil and sediment of the vertical profile is plotted in Figure 3. The concentration range of As in the vertical profile was far below that of the general soil standard limit (150 mg/kg) [46]. The concertation range indicates natural origin, although it is relatively higher than the world soil average content of As, i.e., 6.83 mg/kg [45]. In the transitional sediment, the As content was 26 mg/kg at a depth of 17 m, which was higher than any other layer in the vertical profile. In the marine sediment, the range of As concentration was 7 to 17 mg/kg. It has been reported that As in lowland sites often exists in sediment, as it is adsorbed on the surface of clay minerals, iron oxyhydroxide, or arsenopyrite (FeAsS) as an impurity in framboidal pyrite [47].
The distribution of Pb is similar throughout the vertical profile, except in alluvial clay. The concentration of Pb was 69 mg/kg at 3 m, which was much higher than that of the other part in the profile. The lowest value in the marine sediment was 10 mg/kg at a depth of 22 m, where the marine shell fragment content was relatively high. The world average soil content of Pb is 27 mg/kg [45]. The average concentration in the vertical profile did not exceed the world average, except in the surface alluvial clay at a depth of 3.2–3.4 m [45]. The Pb content in the surface soil, alluvial clay, peat, and tuffaceous clay was higher than in any other part, i.e., 117 to 158 mg/kg. The highest concentration in the profile was 158 mg/kg in the tuffaceous clay at 6.2–6.4 m. The occurrence of As and Pb in sediment in lowland sites occurs due to the adsorption on iron oxyhydroxide and clay minerals [48]. The total Ni content in the vertical profile exceeded world soil average concentrations, i.e., 29 mg/kg, as well as the average agricultural soil concentration of Japan [45].
The concentrations of Mn and Fe were high at a depth of 8 to 12 m. The color of the sediment at this depth is brownish gray to yellowish brown. It is different from other portions of the vertical profile, which were assumed to be oxidized. In the marine sediment, Mn ranged from 248 mg/kg to 728 mg/kg. The content of Mn was 395 mg/kg in peat, which is below the world average value of 488 mg/kg [45]. The content of Mn was 1255–1386 mg/kg at a depth of 2–3 m in the surface soil and sediment, which is higher than the world average value.
The distribution of Fe in the vertical profile ranged from 3 to 7%. In the fluvial sediment, Fe content was 4 to 7%, whereas in the marine and transitional sediment, it was about 4%. At a depth of 22.2–22.4 m, the Fe content was about 3%, which was the lowest value in the profile.
The S content was 0.16 to 0.72% and 0.41 to 0.57% in the marine and transitional sediment, respectively. At a depth of 22–27 m, the content was relatively lower than the upper part of the marine and transitional sediment. In the fluvial sediment, the range was from 0.4 to 0.6%, which was much lower than the other part in the vertical profile. Generally, the S content in the terrestrial sediment was lower than 0.3%. Terrestrial sediment can be differentiated from marine sediment when it displays a range of S mass % of 0.3–3% [49,50]. However, the S content was 0.76% in the peat, which was higher than any other nonmarine deposit.
3.4. Chemical Speciation and Potential Mobility of As and other Elements (Pb, Ni, Fe & Mn) in Marine and Nonmarine Sediment
In this study, four inorganic chemical speciations were determined using a four-step sequential extraction analysis. The residual concentration (residual, sulfide, and organic matter bound) was calculated by subtracting all four inorganic speciations from the total content and calculating the percentage with potential mobile fractions (Figure 4 and Figure 5). The percentages of all four steps is shown in Figure 4 and Figure 5. The residual and oxidizable bound percentages were more than 97–99% for As in the marine sediment. In the marine sediment, the speciation followed a trend, i.e., Fe–Mn oxide bound > water soluble > carbonate bound > ion exchangeable bound. However, in some parts, e.g., at a depth of 21 m, the trend was Fe–Mn oxide bound > carbonate bound > water soluble > ion exchangeable bound. On the other hand, in fluvial (nonmarine) sediment, the trend was Fe–Mn oxide bound > carbonate bound > ion exchangeable > water soluble. Reducible (Fe–Mn oxide) bound seemed to be the most dominant fraction after the oxidizable and residual fractions for As; this is in agreement with other studies, as the reductive dissolution or desorption of iron oxide is the main mechanism for the leaching of As in nonmarine/terrestrial environments [51,52,53].
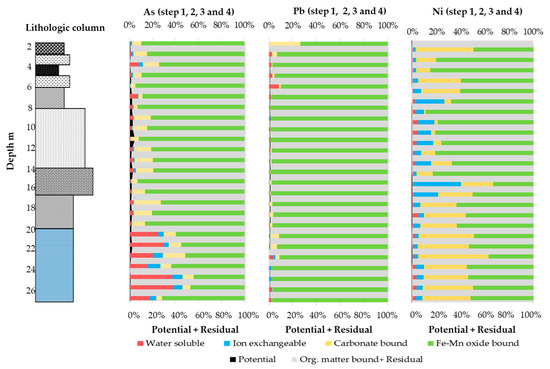
Figure 4.
Percentage of water soluble (step 1), ion exchangeable (step 2), carbonate bound (step 3), Fe–Mn oxide bound (step 4), potential (all four steps together), and residual (Org. matter bound + Residual) fractions for As, Pb, and Ni.
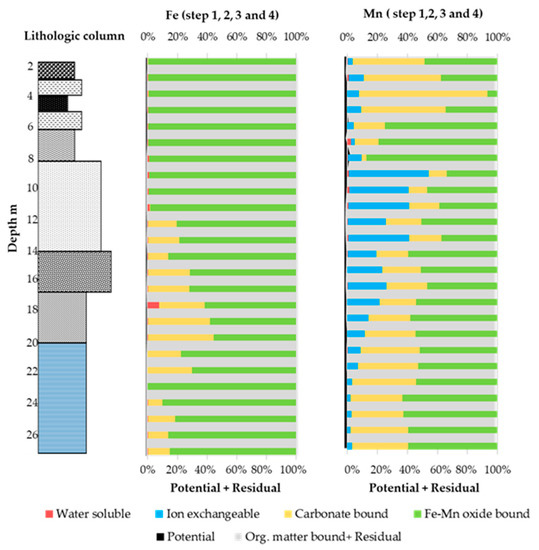
Figure 5.
Percentage of water soluble (step 1), ion exchangeable (step 2), carbonate bound (step 3), Fe–Mn oxide bound fractions (step 4), potential and residual (Org. matter bound + Residual) speciation fractions for Fe and Mn.
Potential mobile fractions were up to 5% at a depth of 11 m; in contrast, they were less than 1 to 3% of the total content in other parts. Fe–Mn oxide bound was the main dominating speciation between the potential mobile fractions in peat, alluvial clay, and tuffaceous clay. These were less than 2% of the total concentration. The potential mobile fractions in the fluvial environment showed maxima of 375.6 µg/L at a depth of 11.2–11.4 m, 297. 49 µg/L in tuffaceous clay at 7.8–8 m, and 34.42 µg/L in peat, which was relatively far lower than elsewhere. The highest concentration was 427.52 µg/L in the transitional environment at 17.2–17.4 m, and 234.7 µg/L in the marine environment at 21.2–21.4 m.
The potential mobile fraction of Pb was mainly Fe–Mn oxide bound in the fluvial and transitional sediment. The percentage was less than 1% of the total concentration. This indicates a lower possibility of Pb leaching from sediment, as 99% of the total content comprised residual and oxidizable fractions. On the other hand, the potential mobile fractions of Pb in marine sediment were negligible, i.e., less than 0.1%. So, the leaching possibilities of Pb from marine sediment are negligible under natural conditions.
The trend of the potential mobile fractions of Ni was different to those of As and Pb, i.e., carbonate bound ≥ Fe–Mn oxide bound > water soluble > ion exchangeable bound in the marine, transitional, and lower part of the fluvial sediment. The potential mobile fractions were less than 1% in all of the vertical profile. The percentage of the potential mobile fractions was relatively higher (0.5%) at a depth of 22 m in the marine sediment and 14 m in the fluvial sediment compared to other parts of the vertical profile. Residual and organic matter bound fractions comprised more than 99% of the total content, which indicates a low probability of leaching from sediment.
For Fe, the fractions in the core sediment followed the trend Fe–Mn oxide bound > carbonate bound, whereas water soluble and ion exchangeable fractions were unavailable from the potential mobile fractions. Residual and organic matter bound were the main parts, i.e., more than 99% of the total concentration. The higher amount of Fe in the residual and organic matter bound reflects its strong immobile bonds in crystalline structures (e.g., magnetite, goethite, and hematite) [54]. Moreover, the speciation trend implies very poor leaching behavior of both marine and nonmarine sediment under various environments (neutral, oxidizable or reducible, etc.). The Fe–Mn oxide bound percentage was relatively high (up to 1%) in the upper part of the core, where alluvial, tuffaceous clay and peat were distributed up to 8 m from the top (Figure 5).
The speciation trend of Mn was different from that of Fe in the peat; the carbonate bound was the dominating fraction after the residual fractions. The trend was Fe–Mn oxide bound ≥ carbonate bound in the marine sediment; in contrast, water solubility and ion exchanges are negligible among the potential mobile fractions. In the fluvial sediment for Mn, ion exchange was the dominant speciation; this indicates easy leaching of Mn into the environment; this trend reflects the possible mobility and leaching behavior in soil or groundwater under acidic or reducible environments. The potential mobile fractions were lower in percentage (~1%) for the fluvial sediment compared to the marine, upper alluvial, peat, and tuffaceous clay (1–2%). The presence of carbonate bound percentage in the alluvial, peat, lower fluvial, marine, and transitional sediment implies the possibility of some Mn leaching under low pH values.
3.5. Risk Assessment Code (RAC)
Bioavailable fractions are a combination of very weakly-bonded chemical fractions, so it is easy, compared to other chemical speciation fractions, to leach them into the environment. According to the RAC percentage, the possible risk of leaching of bioavailable fractions to the subsurface environment is presented in Figure 6. When the RAC category results in a value between 0 to 1%, it is considered as being of no risk or safe. On the contrary, 1% to 10% is considered as showing a low risk of leaching. Figure 6 shows that all five metals fall under 2%. Most of the samples showed no risk of leaching as their bioavailable percentages were below 1%. However, the low risk of leaching of Mn was at the depth of 4.2–4.4 m from the peat and 10.2–10.4 m in the fluvial sediment under natural subsurface environmental conditions. The risk of Fe and Pb leaching was negligible, as the RAC percentage is almost 0 for all sediments. As, Ni, and Mn showed relatively similar risk levels of leaching behavior for both marine and nonmarine sediment.
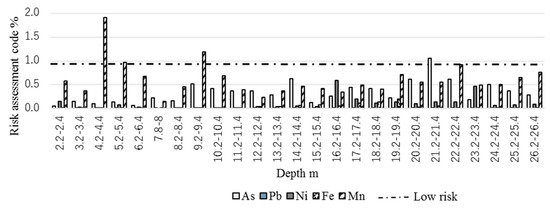
Figure 6.
Calculated risk assessment code for As, Pb, Ni, Fe, and Mn.
3.6. Chemical Properties of Pore Water
The pore water concentrations of As and other elements are plotted in Figure 7. The concentration of As exceeded the permissible limit of 10 µg/L [55,56] for leachate and drinking water in some samples. A concentration was 29.79 µg/L was observed in the nonmarine tuffaceous clay at a depth of 7.8–8 m, which was about 3 times the permissible limit. In fluvial sediment, which was just below the tuffaceous clay, the concentrations were 23.99 µg/L and 15.95 µg/L at a depth of 8.2 and 9.2 m, respectively. The concentration exceeded the permissible limit.
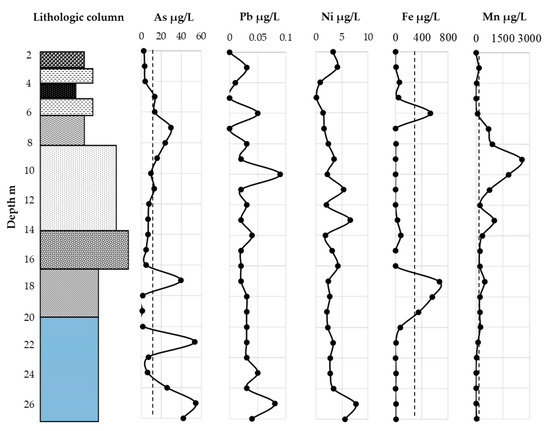
Figure 7.
Measured pore water concentrations for As, Pb, Ni, Fe, and Mn. Dots show permissible limits for leachate and drinking water.
Sediments at this depth might be influenced by leaching from tuffaceous clay. The sediment pore water concentration above the tuffaceous clay also exceeded the permissible limit. This might have influenced the elevated value at a depth of 11.2 m. The pore water concentrations of all other nonmarine sediment were below the permissible limits. However, the As concentration of the pore water was 39.44 µg/L at a depth of 17.2 m in the transitional sediment, i.e., about 4 times higher than the permissible limit. At this depth, the Fe concentration was also relatively higher than in other parts. Therefore, it is possible that Fe acts as scavenger for As leaching in transitional sediment, although this must be cross checked.
The concentrations of As in the pore water of the marine sediment were 53.62, 25.59, 54.5, and 41.85 µg/L at depths of 21, 24, 25, and 26 m, respectively. These concentrations were several times higher than the permissible limit. However, there was no clear relationship between the Fe and Mn concentrations for the elevated concentration of As. In this case, sulfate concentrations or other factors, such as pH or EC might play an important role in As leaching from marine sediment [48]. The Pb concentrations in the pore water for all sediments were much lower than the permissible limit (10 µg/L). Therefore, the leaching behavior of Pb and As was different, according to their chemical characteristics. The Ni, Fe, and Mn concentrations in pore water were not so high, and no fixed permissible limits have been set for Fe and Mn in primary drinking water. However, in the tuffaceous clay and transitional sediment at depths of 6.2–6.4 m and 17.2–19.4 m showed higher concentrations than the permissible limit for Fe (300 µg/L) for secondary drinking water. The Mn concentration in the pore water showed several times higher values in fluvial, transitional, upper 2 m of marine, and tuffaceous clay than the permissible limits for secondary drinking water (50 µg/L) [56].
3.7. Correlation
A Pearson correlation was observed among As and other metals in pore water in terms of the total content. Other physicochemical parameters were measured in the sediment of the fluvial, transitional, and marine depositional environments (Table 2, Table 3 and Table 4). The number of observation points for peat, tuffaceous clay, and alluvial sediment in the nonmarine depositional environment were very few; as a result, they were not considered for Pearson correlation. Another set of correlations was measured between the pore water of As and other metals with potential mobile fractions of different depositional environments (Table 5, Table 6 and Table 7).

Table 2.
Correlation of As and other metals in pore water and sediment with physical parameters of (a) fluvial environment.

Table 3.
Correlation of As and other metals in pore water and sediment with physical parameters of (b) transitional environment.

Table 4.
Correlation of As and other metals in pore water and sediment with physical parameters of (c) the marine environment.

Table 5.
Correlation of potential mobile fractions, pore water concentration, and some parameters of As and other metals in (a) the fluvial environment.

Table 6.
Correlation of potential mobile fractions, pore water concentration, and some parameters of As and other metals in (b) the transitional environment.

Table 7.
Correlation of potential mobile fractions, pore water concentration, and some parameters of As and other metals in (c) the marine environment.
As content in the pore water showed a significant positive correlation with total As content in the fluvial and transitional sediment. However, these didn’t exhibit any correlation in the marine sediment (Table 2, Table 3 and Table 4). Moreover, a significant (p < 0.05) positive correlation was observed with the total Fe, Mn, and pH of the leachate (R2 = 0.71, R2 = 0.76 and R2 = 0.76) and the OM % in the fluvial environment (p < 0.01). This result indicates that the leaching of As in the terrestrial fluvial environment depends on Fe, Mn, and organic matter content. The main binding of potential mobile fractions (Figure 3) in the fluvial sediments was also Fe–Mn oxide bound; this suggests that the leaching of As occurred in the fluvial sediment due to the change of the oxidation-reduction environment of amorphous binding with Fe and Mn oxide. The organic matter content influenced the environmental conditions. This result shows good agreement with other research, especially concerning fluvial environments such as the Bengal delta [57,58,59,60]. In the transitional and marine environment, the As in the pore water did not exhibit any correlation with Fe and Mn. However, the As in the pore water showed a positive correlation with total S content (R2 = 0.95) and ORP of leachate (R2 = 0.94) in the transitional environment. In contrast, it showed a positive correlation with pH (R2 = 0.63) and significant positive (p < 0.05) correlation with the Ni concentration in the pore water (R2 = 0.75) in the marine environment. Moreover, the total As content showed significant (p < 0.01) positive correlation with the EC of leachate in the marine environment (Table 4).
The As content in the pore water exhibited a significant (p < 0.05) positive correlation with potential mobile fractions in the fluvial and transitional environment. The As in the pore water also showed a significant (p < 0.01) positive correlation with the potential mobile fractions of Mn (R2 = 0.9) in the fluvial environment. However, there was no significant correlation between the pore water and the potential mobile fractions of As in the marine environment. The potential mobile fractions of As showed a significant (p < 0.05) positive correlation with the Mn content in the pore water and a positive correlation with the total S and pore water Fe concentration in the transitional environment.
In the transitional sediment, it seems that the leaching of As was controlled by S content and ORP. S reduction, along with As enrichment, may play important roles in leaching from the transitional sediment to environments such as pore water. In contrast, Mn plays the role of the scavenger in the fluvial and transitional environment for As. It is difficult to understand the leaching behavior of As in the marine environment, as it is different from that in the nonmarine fluvial and transitional environment. The potential mobile fractions of As had a significant (p < 0.01) positive correlation with the fraction of Pb in the marine environment. However, this research showed that pH and EC had important effects on the leaching of As from the marine sediment (Table 6 and Table 7). There was a significant (p < 0.01) positive correlation between the total Pb, Ni, Fe, and Mn in the marine sediment, as most of the parts were strongly bound with residual and organic matter (Figure 3 and Figure 4).
Pb had a significant positive correlation with Ni in the fluvial and transitional sediment and did not show a good relationship with total Fe and Mn content. The potential mobile fractions of Pb and Ni showed a positive correlation with the pore water concentration (R2 = 0.97 and R2 = 0.92, respectively) in the transitional sediment. Pb also had a positive correlation with the total content in the marine environment.
Pb in the pore water showed a significant (p < 0.05) positive correlation (R2 = 0.87) with the OM content (%) in marine environment. There were only a few data in the transitional sediment which affected the significance level of the correlation. Therefore, it seems that the reductive dissolution of Fe–Mn oxide bound of Pb is the main process associated with its leaching in the fluvial and transitional environments. However, a significant positive correlation with OM content (%) and total content of Pb in the marine environment implies a tendency for a low level of leaching in the pore water.
Ni in the pore water had a positive correlation without any significance with total Pb, Ni, OM content (%), and the potential mobile fractions of Ni in the transitional sediment. Ni showed a significant (p < 0.05) positive correlation with the potential mobile fractions of Mn, pH of the leachate, pore water As, and Pb; it exhibited a significant (p < 0.01) correlation (R2 = 0.96) with pore water OM content (%) in the marine environment (Table 6 and Table 7).
This implies that the leaching of As, Ni, and Pb in the marine environment is controlled by pH and OM content (%), where organic matter plays an important role in lowering pH or reducing conditions. It is also evident that a medium degree of mobility of Ni, Mn, and As occurs under acidic or reducing environments with variable potentials [45].
4. Conclusions
The leaching behavior of As, Pb, and Ni from soil/sediment is different, due to their individual chemical characteristics in response to various potentials in different depositional environments. Though the total contents of As and other metals were within the ranges of soil standards, the pore water concentration of As exceeded the permissible limit in some layers of the fluvial (aquifer), transitional, and marine environment. The chemical characteristics of the upper part of the fluvial sediment were influenced by the overlaying tuffaceous clay, and by oxidation. The leaching of As, Pb, and Fe in the fluvial environment was mainly controlled by the oxidation-reduction process of the Fe–Mn oxide bound percentage, which was influenced by the organic matter content. However, the leaching of As was relatively high in the transitional environment due to As enrichment, and was controlled by sulfur reduction. Mn played an important role, i.e., that of the scavenger, to leach As in the fluvial and transitional environment. On the other hand, the leaching of As, Pb, and Ni in the marine environment was controlled by pH and organic matter content. The pore water As content in peat indicated poor leaching due to a higher amount of organic matter content. The overall risk of bioavailable percentage of all metals was less than 1%, except for Mn (RAC > 1%) in the peat layer; this indicates that there is no risk of these metals leaching into the subsurface environment. In the fluvial environment, the Mn content in the pore water was several times the secondary drinking water permissible limit; this indicated poor water quality in terms of Mn in the shallow aquifer. There was no/low risk of pollution from these metals in the subsurface environment, as most of the metals are bound to residual and complex binding processes with organic matter. However, a change of oxidation/reduction or acidic conditions may leach considerable amounts of As to the environment from the peat, transitional, and marine environments. Special measures should be taken if these sediments are excavated for underground development.
Author Contributions
S.H. (Sushmita Hossain), T.I., and S.H. (Shoichi Hachinohe) acquired data and carried out the analysis. S.H. (Sushmita Hossain) interpreted the data and prepared the original draft. C.T.O. supervised overall research work.
Funding
Research work is partially funded by MEXT, Japan. This work was supported by JSPS KAKENHI Grant Number JP26281065.
Acknowledgments
This research work is supported by the Center for Environmental Science in Saitama (CESS), Geological survey of Kanto area, and Saitama University, Japan.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Wang, S.; Jia, Y.; Wang, S.; Wang, X.; Wang, H.; Zhao, Z.; Liu, B. Fractionation of heavy metals in shallow marine sediments from Jinzhou Bay, China. J. Environ. Sci. 2010, 22, 23–31. [Google Scholar] [CrossRef]
- Ure, A.M.; Davidson, C.M. (Eds.) Chemical Speciation in the Environment, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2001. [Google Scholar]
- Kraus, U.; Wiegand, J. Long-term effects of the Aznalcóllar mine spill—Heavy metal content and mobility in soils and sediments of the Guadiamar river valley (SW Spain). Sci. Total. Environ. 2006, 367, 855–871. [Google Scholar] [CrossRef]
- Ho, H.H.; Swennen, R.; Cappuyns, V.; Vassilieva, E.; Van Gerven, T.; Van Tran, T. Speciation and Mobility of Selected Trace Metals (As, Cu, Mn, Pb and Zn) in Sediment with Depth in Cam River-Mouth, Haiphong, Vietnam. Aquat. Geochem. 2013, 19, 57–75. [Google Scholar] [CrossRef]
- Race, M.; Nabelkova, J.; Fabbricino, M.; Pirozzi, F.; Raia, P. Analysis of Heavy Metal Sources for Urban Creeks in the Czech Republic. Water Air Soil Pollut. 2015, 226, 226. [Google Scholar] [CrossRef]
- Morillo, J.; Usero, J.; Gracia, I. Heavy metal distribution in marine sediments from the southwest coast of Spain. Chemosphere 2004, 55, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Lund, W. Speciation Analysis? Why and How? Fresenius. J. Anal. Chem. 1990, 337, 557–564. [Google Scholar]
- Shan, X.; Chen, B. Evaluation of sequential extraction for speciation of trace metals in model soil containing natural minerals and humic acid. Anal. Chem. 1993, 65, 802–807. [Google Scholar] [CrossRef]
- Qiang, T.; Xiao-Quan, S.; Zhe-Ming, N. Evaluation of a sequential extraction procedure for the fractionation of amorphous iron and manganese oxides and organic matter in soils. Sci. Total. Environ. 1994, 151, 159–165. [Google Scholar] [CrossRef]
- Ryan, P.C.; Hillier, S.; Wall, A.J. Stepwise effects of the BCR sequential chemical extraction procedure on dissolution and metal release from common ferromagnesian clay minerals: A combined solution chemistry and X-ray powder diffraction study. Sci. Total. Environ. 2008, 407, 603–614. [Google Scholar] [CrossRef]
- Mahurpawar, M. Effects of Heavy Metals on Human Health. Int. J. Res. 2015, 3, 7234. [Google Scholar]
- Smith, A.H.; Lingas, E.O.; Rahman, M. Contamination of drinking-water by arsenic in Bangladesh: A public health emergency. Bull. World Heal. Organ. 2000, 78, 1093–1103. [Google Scholar]
- Martine, S.; Griswold, W. Human Health Effect of Heavy Metals. Environ. Sci. Technol. Riefs Citizens 2009, 15, 1–6. [Google Scholar]
- Farrah, H.; Pickering, W.F. Factors influencing the potential mobility and bioavailability of metals in dried lake sediments. Chem. Speciat. Bioavailab. 1993, 5, 81–96. [Google Scholar] [CrossRef][Green Version]
- Huang, J.-H.; Ilgen, G. Factors affecting arsenic speciation in environmental samples: Sample drying and storage. Int. J. Environ. Anal. Chem. 2006, 86, 347–358. [Google Scholar] [CrossRef]
- Esslemont, G. Heavy metals in seawater, marine sediments and corals from the Townsville section, Great Barrier Reef Marine Park, Queensland. Mar. Chem. 2000, 71, 215–231. [Google Scholar] [CrossRef]
- Guevarariba, A.; Sahuquillo, A.; Rubio, R.; Rauret, G. Assessment of metal mobility in dredged harbour sediments from Barcelona, Spain. Sci. Total. Environ. 2004, 321, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.M.; Eltayeb, M.; Potgieter-Vermaak, S.S.; Van Grieken, R.; Potgieter, J. Assessment of heavy metals pollution in Sudanese harbours along the Red Sea Coast. Microchem. J. 2007, 87, 104–112. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, J.J.; Zhang, K.; Zhou, Y. Enrichment and mechanisms of heavy metal mobility in a coastal quaternary groundwater system of the Pearl River Delta, China. Sci. Total. Environ. 2016, 545, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Le Pape, P.; Morin, G.; Asta, M.P.; King, G.; Bártová, B.; Suvorova, E.; Frutschi, M.; Ikogou, M.; Pham, V.H.C.; et al. Arsenic Speciation in Mekong Delta Sediments Depends on Their Depositional Environment. Environ. Sci. Technol. 2018, 52, 3431–3439. [Google Scholar] [CrossRef]
- Seno, T.; Ogawa, Y.; Tokuyama, H.; Nishiyama, E.; Taira, A. Tectonic evolution of the triple junction off central Honshu for the past 1 million years. Tectonophys. 1989, 160, 91–116. [Google Scholar] [CrossRef]
- Ito, M.; Katsura, Y. Inferred glacio-eustatic control for high-frequency depositional sequences of the Plio-Pleistocene Kazusa Group, a forearc basin fill in Boso Peninsula, Japan. Sediment. Geol. 1992, 80, 67–75. [Google Scholar] [CrossRef]
- Takahashi, M.; Hayashi, H.; Kasahara, K.; Kimura, H. Geologic interpretation of the seismic reflection profile along the western margin of the Kanto Plain-With special reference to the Yoshimi metamorphic rocks and western extension of the Tonegawa Tectonic Line-. J. Geol. Soc. Jpn. 2006, 112, 33–52. [Google Scholar] [CrossRef]
- Suzuki, H. Underground Geological Structure beneath the Kanto Plain, Japan. Natl. Res. Inst. Earth Sci. Disaster Prev. Jpn. 2002, 63, 1–19, (In Japanese, with English abstract). [Google Scholar]
- Kaizuka, S. Landform evolution of the kantô plain. Geogr. Rev. Jpn. 1958, 31, 59–85, (In Japanese, with English abstract). [Google Scholar] [CrossRef][Green Version]
- Sugihara, S.; Takahara, I.; Hosono, M. On the Kanto Loam Bed and Topography of Musashino Upland, Kanto Plain. Quat. Res. 1972, 11, 29–39, (In Japanese, with English abstract). [Google Scholar] [CrossRef][Green Version]
- Nakazawa, T.; Tanabe, S. Geology of the Noda District. Geol. Surv. Jpn. Natl. Inst. Adv. Ind. Sci. Technol. 2011, 4, 72. [Google Scholar]
- Matsuda, I. Distribution of the Recent Deposits and Buried Landforms in the Kanto Lowland, Central Japan. Geogr. Reports Tokyo Metrop. Univ. 1974, 9, 1–36. [Google Scholar]
- Komatsubara, J.; Kimura, K.; Fukuoka, S.; Ishihara, Y. Sedimentary Facies and Physical Properties of the Latest Pleistocene to Holocene Sediment Core (GS-SSS-1) in the Arakawa Lowland, Saitama City, Central Japan. J. Sedimentol. Soc. Jpn. 2010, 69, 3–15. [Google Scholar] [CrossRef]
- Murakoshi, N.; Masuda, F. Estuarine, barrier-island to strand-plain sequence and related ravinement surface developed during the last interglacial in the Paleo-Tokyo Bay, Japan. Sediment. Geol. 1992, 80, 167–184. [Google Scholar] [CrossRef]
- Nakazawa, T.; Nakashima, R.; Ueki, T.; Tanabe, S.; Oshima, H.; Horiuchi, S. Sequence Stratigraphy of the Pleistocene Kioroshi Formation, Shimousa Group beneath the Omiya Upland, Central Kanto Plain, Central Japan. J. Geol. Soc. Jpn. 2006, 112, 349–368, (In Japanese, with English abstract). [Google Scholar] [CrossRef]
- Ishihara, T.; Sugai, T.; Hachinohe, S. Buried surfaces during the Last Glacial Age in the middle and upper part of the Arakawa Lowland and the Menuma Lowland, Central Japan. Quat. Res. (Daiyonki-Kenkyu) 2011, 50, 113–128. [Google Scholar] [CrossRef][Green Version]
- Okoro, H.K.; Fatoki, O.S. A Review of Sequential Extraction Procedures for Heavy Metals Speciation in Soil and Sediments. J. Environ. Anal. Toxicol. 2012, 1, 1–9. [Google Scholar] [CrossRef]
- Ahnstrom, Z.; Parker, D. Development and Assessment of a Sequential Extraction Procedure for the Fractionation of Soil Cadmium. Soil Sci. Soc. Am. J. 1999, 63, 1650. [Google Scholar] [CrossRef]
- Narwal, R.P.; Singh, B.R.; Salbu, B. Association of cadmium, zinc, copper, and nickel with components in naturally heavy metal-rich soils studied by parallel and sequential extractions. Commun. Soil Sci. Plant Anal. 1999, 30, 1209–1230. [Google Scholar] [CrossRef]
- Beck, J.N.; Gauthreaux, K.; Sneddon, J. Abstracts of Papers. In Proceedings of the 221st National ACS Meeting, San Diego, CA, USA, 1–5 April 2001. [Google Scholar]
- Gleyzes, C.; Tellier, S.; Astruc, M. Fractionation studies of trace elements in contaminated soils and sediments: A review of sequential extraction procedures. TrAC Trends Anal. Chem. 2002, 21, 451–467. [Google Scholar] [CrossRef]
- Perin, G.; Craboledda, L.; Lucchese, M.; Cirillo, R.; Dotta, L.; Zanetta, M.L.; Oro, A.A. Heavy Metal Speciation in the Sediments of Northern Adriatic Sea. A New Approach for Environmental Toxicity Determination. In Heavy Metals in the Environment; Lekkas, T.D., Ed.; CEP Consultants: Edinburgh, UK, 1985; Volume 2, pp. 454–456. [Google Scholar]
- Pardo, R.; Barrado, E.; Lourdes, P.; Vega, M. Determination and speciation of heavy metals in sediments of the Pisuerga river. Water Res. 1990, 24, 373–379. [Google Scholar] [CrossRef]
- Singh, K.P.; Mohan, D.; Singh, V.K.; Malik, A. Studies on distribution and fractionation of heavy metals in Gomti river sediments—a tributary of the Ganges, India. J. Hydrol. 2005, 312, 14–27. [Google Scholar] [CrossRef]
- Ishihara, T.; Sugai, T.; Hachinohe, S. Fluvial response to sea-level changes since the latest Pleistocene in the near-coastal lowland, central Kanto Plain, Japan. Geomorphology 2012, 147, 49–60. [Google Scholar] [CrossRef]
- Ishihara, T.; Sugai, T. Eustatic and regional tectonic controls on late Pleistocene paleovalley morphology in the central Kanto Plain, Japan. Quat. Int. 2017, 456, 69–84. [Google Scholar] [CrossRef]
- Sugai, T.; Matsushima, H.; Ishihara, T. Late Quaternary Landform Development of the Kanto Plain. In The Agglomeration of the Animation Industry in East Asia; Springer: Gateway East, Singapore, 2018; pp. 1–18. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Ministry of Environment Japan. Guideline of investigation and measure based on soil contamination countermeasures act. Available online: http://www.env.go.jp/water/dojo/gl_ex-me/index.html (accessed on 20 May 2019).
- Shimada, N. The Essence of Problems on Groundwater and Soil Pollutions Caused by Naturally Occurring Heavy Metals and Harmful Elements: Arsenic. Oyo Tech. Rep. 2009, 29, 31–59. [Google Scholar]
- Ueshima, M.; Takemura, T.; Saito, T.; Ito, Y.; Hamamoto, S.; Saito, H.; Komatsu, T. Relationship between trace elements and depositional environments in shallow sediments: A case study from Southern Kanto Plain, Central Japan. Environ. Earth Sci. 2017, 76, 633. [Google Scholar] [CrossRef]
- Keith, L.M. Geochemical Indicators of Marine and Freshwater Sedimenta. Res. Geochem. 1959, 38–61. [Google Scholar]
- Yoshida, M.; Hoyanagi, K.; Urabe, A.; Yamazaki, A.; Yamagishi, M.; Omura, A. Reconstruction of Sedimentary Environments on the Basis of Sedimentary Facies, TOC, TN, and TS Contents; Examples from the Latest Pleistocene and Holocene Sediments in the Niigata Plain, Central Japan. Mem. Geol. Soc. Jpn. 2006, 59, 93–109, (In Japanese, with English abstract). [Google Scholar]
- Bhattacharya, P.; Chatterjee, D.; Jacks, G. Occurrence of Arsenic-contaminatedGroundwater in Alluvial Aquifers from Delta Plains, Eastern India: Options for Safe Drinking Water Supply. Int. J. Water Resour. Dev. 1997, 13, 79–92. [Google Scholar] [CrossRef]
- Peters, S.L.; Dowling, C.B.; Poreda, R.J.; Basu, A.R.; Aggarwal, P.K. Geochemical study of arsenic release mechanisms in the Bengal Basin groundwater. Water Resour. Res. 2002, 38, 12-1. [Google Scholar]
- Stüben, D.; Berner, Z.A.; Chandrasekharam, D.; Karmakar, J. Arsenic enrichment in groundwater of West Bengal, India: Geochemical evidence for mobilization of As under reducing conditions. Appl. Geochem. 2003, 18, 1417–1434. [Google Scholar] [CrossRef]
- Massolo, S.; Bignasca, A.; Sarkar, S.K.; Chatterjee, M.; Bhattacharya, B.D.; Alam, A. Geochemical fractionation of trace elements in sediments of Hugli River (Ganges) and Sundarban wetland (West Bengal, India). Environ. Monit. Assess. 2012, 184, 7561–7577. [Google Scholar] [CrossRef]
- USFJ. Japan Environmental Governing Standards. Available online: https://www.usfj.mil/Portals/80/Documents/Other/2016 JEGS.pdf (accessed on 21 May 2019).
- EPA. National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#Inorganic (accessed on 21 May 2019).
- Nickson, R.; McArthur, J.; Burgess, W.; Ahmed, K.M.; Ravenscroft, P.; Rahmanñ, M.; Rahman, M. Arsenic poisoning of Bangladesh groundwater. Nature 1998, 395, 338. [Google Scholar] [CrossRef]
- Nickson, R.T.; McArthur, J.M.; Ravenscroft, P.; Burgess, W.G.; Ahmed, K.M. Mechanism of Arsenic Release to Groundwater, Bangladesh and West Bengal. Appl. Geochem. 2000, 15, 403–413. [Google Scholar] [CrossRef]
- Stuckey, J.W.; Schaefer, M.V.; Kocar, B.D.; Benner, S.G.; Fendorf, S. Arsenic Release Metabolically Limited to Permanently Water-Saturated Soil in Mekong Delta. Nat. Geosci. 2016, 9, 70–76. [Google Scholar] [CrossRef]
- Islam, F.S.; Gault, A.G.; Boothman, C.; Polya, D.A.; Charnock, J.M.; Chatterjee, D.; Lloyd, J.R. Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature 2004, 430, 68–71. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).