Abstract
Characterizing the mechanisms by which natural microorganisms in soil decompose gasoline hydrocarbons is of fundamental importance for a better understanding of natural attenuation and/or for predicting contaminant transport and fate in soils. To examine whether and how gasoline hydrocarbons can be decomposed under general environmental conditions, the decomposition of 10 major components generally contained in commercially available gasoline was analyzed in three arbitrarily selected Japanese soil samples. Gasoline hydrocarbons, especially aromatic hydrocarbons, are easily adsorbed by the tested Japanese soils, with straight chain hydrocarbons decomposing faster than branched hydrocarbons. Saturated monocyclic hydrocarbons were less easily decomposed than unsaturated monocyclic hydrocarbons. Enhancement of microbial decomposition of gasoline hydrocarbons requires a continuous supply of oxygen together with nutrients for the microorganisms.
1. Introduction
A current major environmental issue is the contamination of soils and/or groundwater by petroleum hydrocarbons. Many sites, such as oil refining plants and abandoned gas stations, are polluted by petroleum hydrocarbons, including gasoline resulting from leaks and accidental spills. A 1990 report of the US Environmental Protection Agency [1] stated that approximately 25% of all underground storage tanks were leaking. Regulation of Underground Storage Tanks storing either petroleum or certain hazardous substances was amended in 2015 [2], and thus oil leakage remains an issue of high interest. Similar situations are likely in other developed countries, including Japan.
The Agency for Natural Resources and Energy [3] reported that, at the end of 2011, there were 15,078 registered dealers of volatile oils and 31,467 gas stations throughout Japan, a marked decrease occurred from the 31,599 dealers and 60,421 gas stations in Japan at the end of 1994. An amendment to the Fire Service Act [4] requires that, to prevent possible leakage, underground storage tanks constructed 40 years ago should be improved, either by coating the inner surfaces of these tanks with fiber-reinforced plastic (FRP) or by utilizing an electrochemical method to minimize corrosion. Some dealers and gas stations may not be able to afford the expenses associated with these improvements and may cease operation within several years. In addition, the widespread use of hybrid vehicles, and increased use of electrically-powered vehicles, as well as hydrogen fuel cell vehicles throughout Japan has decreased the consumption of gasoline, with fewer gas stations required throughout the country. Thus, many sites potentially polluted by gasoline hydrocarbons have become a social problem and require appropriate countermeasures in the near future.
Although some active remediation techniques, such as dig and haul, pump and treat, heating and evaporation and soil washing, can be used to treat soils polluted by petroleum hydrocarbons, these techniques are generally expensive and can lead to incomplete decomposition of contaminants. Fortunately, however, gasoline hydrocarbons are biologically decomposable, with several cost-effective and environmentally friendly techniques applicable for the treatment of sites polluted by hydrocarbons, such as monitored natural attenuation (MNA). A MNA program was started [5], and has been conducted continuously in some sites in the U.S. [6]. U.S. Environmental Protection Agency (US EPA) also provided a guide on how to deal with the MNA to citizens [7]. It’s applicability has also been investigated in Japan [8,9]. MNA may include processes related to biodegradation, dispersion, dilution, sorption, volatilization, decay, chemical or biological stabilization, transformation, or destruction of contaminants [10]. When other processes are not dominant, MNA is mainly based on intrinsic and/or passive bioremediation e.g., [10]. Perry [11] reported that normal alkanes have higher decomposability, followed by branched alkanes, low molecular weight aromatic hydrocarbons and cycloalkanes. Jobson et al. [12] reported that straight chain saturated hydrocarbons, i.e., n-alkanes and n-paraffins, have higher decomposition rates than that of longer chain alkanes, aromatic hydrocarbons and cycloalkanes. The success of a bioremediation process basically depends on the ability whether the biodegradability of pollutant can continue. Several factors affect bioremediation in soil ecosystems [13]. Decomposition rates are known to be affected by soil types [14]. A few reviews on, or which cover microbial decomposition of petroleum hydrocarbons are also available [15,16,17]. Although anaerobic bio-decomposition of petroleum hydrocarbons can be possible [18], it is a common knowledge that aerobic bio-decomposition is faster compared with anaerobic bio-decomposition.
To perform a preliminary study that aims to examine whether gasoline hydrocarbons are decomposable and the differences among decomposition of different compounds in Japanese soils as natural attenuation systems by aerobic microbes, the decomposition of ten major components contained in commercially available gasoline was tested in three arbitrarily selected Japanese soil samples.
2. Materials and Methods
2.1. Test Materials
Gasoline is a mixture of multiple hydrocarbons and components, with each having different decomposition behavior. In this study, a synthetic gasoline was prepared by mixing equal volumes of ten major components generally contained in a commercially available gasoline by ourselves. These components included aliphatic and aromatic hydrocarbons, with carbon numbers ranging from 6 to 9 (Table 1). Table 1 also shows the structural characteristics of individual hydrocarbons.

Table 1.
Hydrocarbon components used to prepare a synthetic gasoline.
Three arbitrarily selected Japanese soil samples were used for the experiments of preliminary study. Surface soil (0–5 cm in depth) and sub-surface soil (10–15 cm in depth) were taken from the campus of the National Institute of Advanced Industrial Science and Technology, Tsukuba-city, Japan (Table 2). The sample of polluted soil was taken from a vacant lot of a former oil refining plant with a history of contamination by petroleum hydrocarbons, mostly heavy oils (Table 2). Surface soil was covered by leaves and twigs but removed from the surface soil during sampling. Because of that, we thought the surface soil contained more organic matters than subsurface soil. The polluted soil contained compounds of heavy oil and had been polluted for several decades. All apparatus and glass vials used for sampling were sterilized in an autoclave at 120 °C for 20 min before use. To utilize soil microorganisms in the decomposition of petroleum hydrocarbons, the water content of soil samples was not adjusted.

Table 2.
Soil samples and test conditions used for microbial decomposition tests.
2.2. Test Method and Analytical Conditions
Ten g of each soil sample was added to a 110 ml glass vial; and the air trapped in the head space was used as the oxygen source. Using a small capacity micro-syringe, 3 µL of synthetic gasoline was injected into each capped and sealed glass vial. Polytetrafluoroethylene (PTFE)/butyl septum was set between an aluminum cap and the vials for preventing the sorption of petroleum. The tightness of sealing was verified in the laboratory. The synthetic gasoline was mixed with the soil sample by shaking each glass vial for 5 min, with shaking repeated daily during the testing. To prevent or eliminate possible leakage from evaporation, the capped and sealed glass vials were placed upside-down in a water bath set within an incubator, with the temperature of the water bath maintained at 30 °C throughout the test (Figure 1). Each sample was tested by an independent experiment for examining the reproducibility.
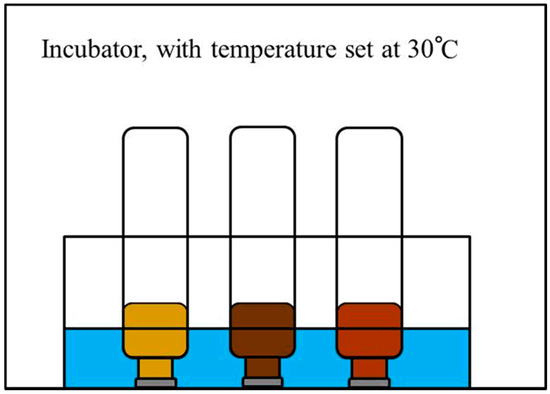
Figure 1.
Experimental set-up used to assess hydrocarbon decomposition within a temperature controlled incubator.
To examine the mechanism and process of aerobic bio-decomposition, time-dependent changes in concentration of the 10 petroleum hydrocarbons, together with inorganic gases including oxygen (O2) and carbon dioxide (CO2) were monitored, but not necessarily on the same days. The consumption of O2 is usually used as an index of aerobic bio-decomposition and production of CO2 is due to oxidation of hydrocarbons. Organic components, including gasoline hydrocarbons, were analyzed by GC-FID (Shimadzu Co., GC-2010, Kyoto, Japan), using a 15 m ZB-5m (Zebron) column with an inner diameter of 0.25 mm and a film thickness of 0.5 µm. The temperatures of the SPL injector and FID detector were set at 300 and 250 °C, respectively. The temperature of the column was initially maintained at 40 °C for 10 min and then increased to 220 °C at a speed of 5 °C per min. Calibration tests were performed which cover the range of maximum concentrations to be measured with self-adjusted standards. A 2 µL aliquot of headspace gas was withdrawn from each glass vial for analyses of gasoline hydrocarbons.
Inorganic components, including O2 and CO2 were analyzed by GC-QMS (Agilent Technologies, Agilent 6890, Santa Clara, CA, USA), using a CP-PoraBONDQ (Varian) capillary column 25 m long, with an inner diameter of 320 µm and an outer diameter of 500 µm. Inlet temperature was set at 80 °C. The column temperature was maintained at 80 °C for 1.3 min, increased to 100 °C at a speed of 80 °C per min, and maintained at 100 °C for 0.25 min. A 50 µL aliquot of headspace gas was withdrawn from each glass vial for the analyses of inorganic gaseous components.
The experiments lasted for about 4 months with a denser sampling (every one to three days) during the early stage to capture the changes in concentrations of both organic and inorganic components. Sampling intervals were adjusted with consideration of monitored results associated with changes in concentration of gasoline hydrocarbons.
3. Results and Discussion
Time-dependent changes in the concentrations of petroleum hydrocarbons during the microbial decomposition tests were assessed in surface soil (Figure 2a), subsurface soil (Figure 3a) and polluted soil (Figure 4a). The concentrations were normalized relative to the initial concentrations of the individual petroleum hydrocarbons within the same sized glass vials that had been injected with the same volume of mixed hydrocarbons. Normalized concentrations of individual petroleum hydrocarbons were much lower than 1 just after the start of experiments. In addition, the decomposition rates of straight chain hydrocarbons were faster than those of other types of hydrocarbons (Figure 2a, Figure 3a and Figure 4a).
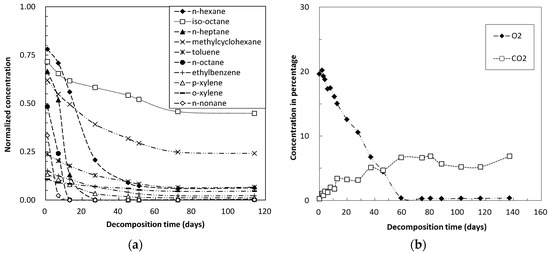
Figure 2.
Concentrations over time of (a) organic and (b) inorganic gases during decomposition tests in surface soil.
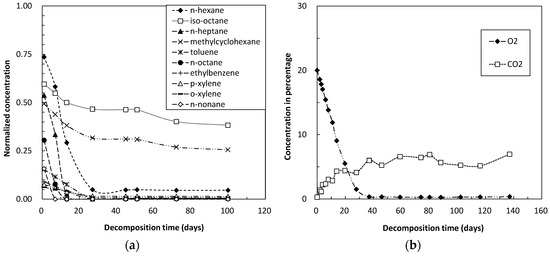
Figure 3.
Concentrations over time of (a) organic and (b) inorganic gases during decomposition tests in subsurface soil.
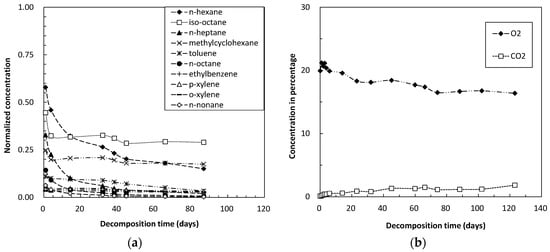
Figure 4.
Concentrations over time of (a) organic and (b) inorganic gases during decomposition tests in polluted soil.
Time-dependent changes in the concentrations of inorganic gases, especially those of O2 and CO2, during the microbial decomposition tests were also analyzed in surface soil (Figure 2b), subsurface soil (Figure 3b) and polluted soil (Figure 4b).
3.1. Effects of Adsorption
The sudden decreases in concentrations (Figure 2a, Figure 3a and Figure 4a) are likely due to the adsorption of individual hydrocarbons by organic matter within the soil samples. Sorption of organic contaminants can be significantly influenced by soil organic matters [19]. Overall, aromatic hydrocarbons are more adsorptive than aliphatic hydrocarbons, whereas, within the same hydrocarbon type, those with higher carbon numbers have more easily adsorbed than those with lower carbon numbers. This phenomenon is consistence with the report indicating that sorption is positively related to molecular weights of chemicals [20]. The sorption may delay the process of volatilization, but can affect the availability of nutrients for the microorganisms and thus the process of biological decomposition.
Compared with the surface and subsurface soils (Figure 2a and Figure 3a), the polluted soil (Figure 4a) containing components of heavy oil showed higher adsorptivity, a difference likely due to the lipophilic effects of petroleum hydrocarbons. Components of heavy oil are also organic matter, with the amount of organic matter showing a positive correlation with soil adsorptivity of volatile petroleum hydrocarbons. These findings support our previous results on the effects of soil organic matter content on volatilization properties of gasoline components in soils [21]. Although the effects of soil type on biodegradation kinetics are not yet fully understood in detail, the highly adsorption of clay and organic matter likely limits the bioavailability of petroleum hydrocarbons to soil microorganisms [22,23].
3.2. Decomposition Rate
The decomposition rates of straight chain hydrocarbons, specifically n-hexane, n-heptane, n-octane and n-nonane, were faster than those of other types of hydrocarbons (Figure 2a, Figure 3a, and Figure 4a). Normal alkanes decompose faster than branched alkanes and aromatic hydrocarbons. These observations are in agreement with previous findings e.g., [11,24,25].
Although polluted soil (Figure 4a) showed greater adsorptivity of gasoline hydrocarbons than the other two soil samples, the decomposition rates of straight chain hydrocarbons were lower than in surface and subsurface soils (Figure 2a and Figure 3a). In addition, the decomposition rate of straight chain hydrocarbons was lower in surface (Figure 2a) than in subsurface soil (Figure 3a). Many factors that affect bio-decomposition of hydrocarbons have been reviewed [15,26], the most important factor is the presence of microorganisms with the appropriate metabolic capabilities. If these microorganisms are present, then optimal rates of growth and hydrocarbon bio-decomposition can be sustained by ensuring that adequate concentrations of nutrients and oxygen are present. The differences observed in this study may be due to differences in microorganisms in the soil samples, but further study is required for confirmation in the future. In addition, identification and quantification of microbial communities in soil samples are also of fundamental necessity.
3.3. Persistence of Hydrocarbons
Corresponding with the adsorptivity and degradability of individual petroleum hydrocarbons, the concentrations of iso-octane remained high in all three soil samples (Figure 2a, Figure 3a and Figure 4a), indicating that branched chain hydrocarbons are relatively difficult to decompose [11,22]. Methylcyclohexane concentration also remained relatively high in these soil samples, due to saturated, monocyclic hydrocarbons being relatively difficult to decompose when compared with unsaturated, monocyclic hydrocarbons. In addition, toluene concentration remained relatively high compared with other types of aromatic hydrocarbons.
Hydrocarbons may persist in soil samples due to difficulties in decomposition and/or degradation, the depletion of oxygen, limited numbers of useful microorganisms, and the consumption of nutrients for these microorganisms.
3.4. Microbial Activity
The decrease in O2 and the increase in CO2 concentration during decomposition tests reflect the aerobic microbial activity. Microbial activity for the decomposition of gasoline hydrocarbons was higher in surface and subsurface soils (Figure 2b and Figure 3b) than in polluted soil (Figure 4b), and was higher in subsurface (Figure 3b) than in surface soil (Figure 2b). Higher microbial activity resulted in faster consumption of oxygen within the glass vials, with oxygen used up within 1 to 2 months. This may explain, at least in part, the decrease in decomposition rates after a certain period of time. Injection of additional oxygen into the glass vials would result in further decomposition of the residual hydrocarbon components within these soils.
4. Concluding Remarks
The number of abandoned gas stations in Japan will keep increasing in the near future due to the amendment to the Fire Service Act, which requires improvements to old fuel storage tanks, the widespread use of hybrid vehicles, and increased use of electric vehicles as well as hydrogen fuel cell vehicles, which require less gasoline. Soil contamination by petroleum hydrocarbons such as gasoline is an emerging social problem, and countermeasures are needed to clean up and/or reduce possible risks associated with petroleum hydrocarbons. To obtain fundamental information about the natural attenuation and bioremediation of petroleum hydrocarbons in Japanese soils, experiments were performed to investigate the microbial decomposition of petroleum hydrocarbons in 3 arbitrarily selected Japanese soil samples. The results obtained in this study can be summarized as follows:
- Aromatic hydrocarbons are easier adsorbed by organic matter in soil than aliphatic hydrocarbons. Hydrocarbons with higher carbon numbers are more adsorptive than hydrocarbons with lower carbon numbers.
- Decomposition properties are dependent on types of hydrocarbon. Straight chain hydrocarbons decompose faster than branched hydrocarbons.
- Compared with unsaturated monocyclic hydrocarbons, saturated monocyclic hydrocarbons are relatively difficult to decompose.
- Microbial activity depends on sites because different sites have different soil conditions. Higher microbial activity consumes oxygen more quickly. Aerobic decomposition may be accelerated by continuous injection of oxygen at appropriate concentrations.
To obtain systematic information on natural attenuation of gasoline hydrocarbons in Japanese soils, further detailed studies are required. Additional studies incorporating analyses of residual compositions remaining within soil samples as well as a characterization of soil microorganisms are underway in our laboratory. More experiments on more soil samples together with identification and quantification of microbial communities within soils are necessary for a better understanding of the microbial decomposition properties of petroleum hydrocarbons in soils.
Acknowledgments
The authors thank Koichi Shiomi of Shimadzu Co. for technical advice on chemical analyses. We are also grateful to Keiko Ogawa of AIST, Japan, for assistance with experiments.
Author Contributions
All the authors have actively contributed to this study, and confirmed and approved the final version for submission. The research plan and laboratory experiments were planned by the four authors. In addition, Junko Nishiwaki contributed to both experimental works and preparation of the draft; Yoshishige Kawabe contributed to development of analytical methods and data analysis; Takeshi Komai contributed to discussion; and Ming Zhang contributed to examination of data analysis, discussion and finalization of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. This study was performed using the general grant delivered by Japanese government to national laboratories for fundamental studies. The Japanese government had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- USEPA. Musts for USTs, a Summary of the Regulations for Underground Storage Tank Systems. EPA/530/UST-88/008; 1990. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=2000DA8S.PDF (accessed on 28 November 2017).
- USEPA. 40 CFR Parts 280 and 281 Revising Underground Storage Tank Regulations—Revisions to Existing Requirements and New Requirements for Secondary Containment and Operator Training. Final Rule, Federal Register; 2015. Available online: https://www.gpo.gov/fdsys/pkg/FR-2015-07-15/pdf/2015-15914.pdf (accessed on 23 November 2017).
- Ministry of Economy, Trade and Industry. Changes in the Numbers of Dealers of Volatile Oils and Gas Stations. 2017. Available online: http://www.meti.go.jp/press/2017/07/20170704007/20170704007-1.pdf (accessed on 28 November 2017). (In Japanese)
- Fire and Disaster Management Agency (FDMA). Uses an Existing Storage Tank under the Ground against Leakage. 2010. Available online: http://www.fdma.go.jp/html/data/tuchi2207/pdf/220708_ki144.pdf (accessed on 28 November 2017). (In Japanese)
- National Research Council. Natural Attenuation for Groundwater Remediation; The National Academies Press: Washington, DC, USA, 2000. Available online: https://toxics.usgs.gov/highlights/nat_attenuation.html (accessed on 28 November 2017).
- USGS. Natural Attenuation Strategy for Groundwater Cleanup Focuses on Demonstrating Cause and Effect. 2001. Available online: https://toxics.usgs.gov/pubs/eos-v82-n5-2001-natural/ (accessed on 23 November 2017).
- USEPA. A Citizen’s Guide to Monitored Natural Attenuation. 2012. Available online: https://www.epa.gov/sites/production/files/2015-04/documents/a_citizens_guide_to_monitored_natural_attenuation.pdf (accessed on 23 November 2017).
- Hattori, N.; Inoue, Y.; Tsuru, Y.; Katayama, A. Microbiology community structure changing in petroleum polluted groundwater under natural attenuation. In Proceedings of the Japan Society of Civil Engineers Annual meeting, Tokyo, Japan, 7–9 September 2005; Japan Society of Civil Engineers: Tokyo, Japan, 2005. Available online: http://library.jsce.or.jp/jsce/open/00035/2005/60-7/60-7-0059.pdf (accessed on 28 November 2017). (In Japanese).
- Takahata, Y.; Kasai, Y.; Hoaki, T.; Watanabe, K. Rapid intrinsic biodegradation of benzene, toluene, and xylenes at the boundary of a gasoline-contaminated plume under natural attenuation. Appl. Microbiol. Biotechnol. 2006, 73, 713–722. [Google Scholar] [CrossRef] [PubMed]
- USEPA OSWER. Use of Monitored Natural Attenuation at Superfund, RCRA Corrective Action, and Underground Storage Tank Sites. 1999. Available online: https://www.epa.gov/sites/production/files/2014-02/documents/d9200.4-17.pdf (accessed on 28 November 2017).
- Perry, J.J. Microbial metabolism of cyclic alkanes. In Petroleum Microbiology, 1st ed.; Atlas, R.M., Ed.; Macmillan Publishing Co.: New York, NY, USA, 1984; pp. 61–98, ISBN-13: 978-0029490006. [Google Scholar]
- Jobson, A.; Cook, F.D.; Westlake, D.W.S. Microbial utilization of crude oil. Appl. Microbiol. 1972, 23, 1082–1089. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC380511/pdf/applmicro00047-0068.pdf (accessed on 28 November 2017). [PubMed]
- Atlas, R.M. Microbial degradation of petroleum hydrocarbons: An environmental perspective. Microbiol. Rev. 1981, 45, 180–209. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC281502/pdf/microrev00009-0186.pdf (accessed on 28 November 2017). [PubMed]
- Bartha, R.; Bossert, I. The treatment and disposal of petroleum wastes. In Petroleum Microbiology, 1st ed.; Atlas, R.M., Ed.; Macmillan Publishing Co.: New York, NY, USA, 1984; pp. 553–578, ISBN-13: 978-0029490006. [Google Scholar]
- Leahy, J.G.; Colwell, R.R. Microbial degradation of hydrocarbons in the environment. Microbiol. Rev. 1990, 54, 305–315. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC372779/ (accessed on 27 December 2017). [PubMed]
- Olajire, A.A.; Essien, J.P. Aerobic degradation of petroleum components by microbial consortia. J. Pet. Environ. Biotechnol. 2014, 5, 1–22. Available online: http://dx.doi.org/10.4172/2157-7463.1000195 (accessed on 27 December 2017). [CrossRef]
- Yoshikawa, M.; Zhang, M.; Toyota, K. Biodegradation of volatile organic compounds and their effects on biodegradability under co-existing conditions. Microbes Environ. 2017, 32, 188–200. Available online: https://www.jstage.jst.go.jp/browse/jsme2 (accessed on 27 December 2017). [CrossRef] [PubMed]
- Salminen, J.M.; Tuomi, P.M.; Suortti, A.M.; Jorgensen, K.S. Potential for aerobic and anaerobic biodegradation of petroleum hydrocarbons in boreal subsurface. Biodegradation 2004, 15, 29–39. Available online: https://doi.org/10.1023/B:BIOD.0000009954.21526.e8 (accessed on 27 December 2017). [CrossRef] [PubMed]
- Zytner, R.G. Sorption of benzene, toluene, ethylbenzene and xylenes to various media. J. Hazard. Mater. 1994, 38, 113–126. [Google Scholar] [CrossRef]
- Brookman, G.T.; Flanagan, M.; Kebe, J.O. Literature Survey: Hydrocarbon Solubilities and Attenuation Mechanisms; American Petroleum Institute: Washington, DC, USA, 1985. [Google Scholar]
- Nishiwaki, J.; Kawabe, Y.; Sakamoto, Y.; Komai, T.; Zhang, M. Volatilization properties of gasoline components in soils. Environ. Earth Sci. 2011, 63, 87–95. Available online: https://link.springer.com/content/pdf/10.1007%2Fs12665-010-0671-7.pdf (accessed on 28 November 2017). [CrossRef]
- Huesemann, M.H. Guidelines for land-treating petroleum hydrocarbon contaminated soils. J. Soil Contam. 1994, 3, 299–318. Available online: http://www.tandfonline.com/doi/pdf/10.1080/15320389409383471 (accessed on 28 November 2017). [CrossRef]
- Tang, J.; Carroquino, M.J.; Robertson, B.K.; Alexander, M. Combined effect of sequestration and bioremediation in reducing the bioavailability of polycyclic aromatic hydrocarbons in soil. Environ. Sci. Technol. 1998, 32, 3586–3590. Available online: http://pubs.acs.org/doi/pdf/10.1021/es9803512 (accessed on 28 November 2017). [CrossRef]
- Kennicutt, M.C., II. The effect of biodegradation on crude oil bulk and molecular composition. Oil Chem. Pollut. 1988, 4, 89–112. [Google Scholar] [CrossRef]
- Singer, M.E.; Finnerty, W.R. Microbial metabolism of straight-chain and branched alkanes. In Petroleum Microbiology, 1st ed.; Atlas, R.M., Ed.; Macmillan Publishing Co.: New York, NY, USA, 1984; pp. 1–59. [Google Scholar]
- Atlas, R.M.; Bartha, R. Hydrocarbon biodegradation and oil spill bioremediation. Adv. Microb. Ecol. 1992, 12, 287–338. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).