Abstract
In this work we propose a method of geochemical feed zone (FZ) analysis based on the assumption of thermochemical equilibrium between geothermal fluids and hydrothermal minerals, for each FZ contributing to well discharge. Using our method, it is possible to calculate the mass fraction and the chemistry of each FZ fluid, namely (1) the pH and the concentrations of SiO2, CO2, Na, K, Ca, Mg, HCO3, SO4, F, and Cl of FZ liquids, and (2) the concentrations of SiO2 and CO2 of FZ vapors. The method can be applied to wells with two single-phase FZs and to wells with either three single-phase FZs or two FZs, one single-phase and the other two-phase, with different temperature and fluid chemistry.
1. Introduction
Almost all geothermal wells receive fluids, either liquid water or water vapor or a two-phase mixture, by multiple production zones or feed zones (FZs), either two or three or even more, separated by generally long nonproductive sections [1]. FZs are connected to distinct geothermal aquifers or layers, each one having different characteristics, such as temperature, pressure, flow, fluid state, and fluid chemistry.
Several physical measurements are performed, at different times, to characterize the FZs of geothermal wells [2,3]. Depth (location), temperature, and pressure of FZs are obtained through the completion test, involving temperature and pressure surveys in the well while injecting cold water, and in the subsequent heating stage, when temperature and pressure profiles are measured under shut-in conditions. Afterwards, location and characteristics of FZs are confirmed during the production test by means of downhole surveys with well flowing, the most common of which are pressure-temperature-spinner (PTS) logs.
Wellbore simulators are usually used to simulate one or two-phase fluid flow in a geothermal well with multiple FZs in order to provide a consistent interpretation of all measured physical data. Bjornsson [1] developed the first multiple FZ wellbore simulator. It solves the governing flow equations by the finite difference method, assuming steady state flow in the well. It adopts either well-head or well-bottom flowrates, enthalpies and pressures as boundary conditions, and handles well geometry, properties of FZs, injection and production, as well as internal flow.
In contrast, the chemistry of fluids from different FZs is still an unsolved problem since downhole samples are often affected by gas loss and, even more important, are mixtures of fluids coming from different FZs, similarly to the samples collected at the surface. Consequently, WATCH (https://en.isor.is/software/ (accessed on 20 January 2025)), the speciation-saturation code specifically developed for the geochemical modeling of geothermal fluids [4], provides reliable results for wells with a single FZ or with multiple FZs with similar temperature, pressure, and fluid chemistry. In contrast, when WATCH is applied to geothermal wells with multiple FZs, choosing the quartz-saturation option to fix the reference temperature, the computed aquifer temperature results in a weighted average of the temperatures of liquid-producing FZs, enthalpy turns out to be a weighted average of the enthalpies of all FZs, and the concentrations of solutes refer to the mixture of aquifer fluids.
The reconstruction of the chemical characteristics of the fluids coming from different FZs is certainly a topic of considerable importance both for improving the conceptual model of the geothermal system of interest and to identify the processes causing mineral precipitation and scaling, which has a considerable negative impact on the use of geothermal fluids. However, to the best of our knowledge, no study has been performed on this topic so far. To try to fill, at least in part, this gap, this innovative work aims to reconstruct the chemistry of fluids coming from different FZs, based on the hypothesis of thermochemical equilibrium between hydrothermal minerals and geothermal fluids. In other words, it is a theoretical approach applicable within the limits of this hypothesis, which does not pretend to resolve all the chemical complexities of geothermal fluids. In the application part of this work, we present two examples referring to two deep wells of the high-enthalpy geothermal fields of Krafla and Hellisheiði, both in Iceland. Nevertheless, our methodological approach is usable not only for the high-enthalpy geothermal fields of Iceland, but has a worldwide applicability, provided that the geothermal fluids and quartz are in thermochemical equilibrium, which is a ubiquitous condition in high-enthalpy geothermal aquifers, as recognized long ago by Morey et al. [5].
2. Methods
Geochemical FZ analysis involves the enthalpy balance and the SiO2 mass balance. For geothermal wells with two single-phase FZs, the enthalpy balance takes the following form:
and the SiO2 mass balance is written as follows:
where and = 1 − are the mass fractions of the fluids from FZs A and B, respectively, in the unit mass of mixed fluids, is silica concentration, and TD, A, and B identify the total discharge fluid, the fluid of FZ A, and the fluid of FZ B, respectively.
For geothermal wells with three single-phase FZs, the enthalpy balance is written as follows:
the SiO2 mass balance takes the following form:
and the mass balance is as follows:
In Equations (3)–(5), is the mass fraction of the fluids from FZ C, which is identified by the subscript C, whereas other symbols are as above. The following two equations are obtained rearranging Equations (3)–(5):
This approach for wells with three single-phase FZs can also be applied to wells with a single-phase FZ and a two-phase FZ, splitting the latter into a virtual liquid FZ and a virtual vapor FZ.
In order to solve either Equations (1) and (2), for geothermal wells with two FZs, or Equations (5)–(7), for geothermal wells with three single-phase FZs, it is necessary to know both the temperature and the state of water of each FZ and to assume saturation (equilibrium) with quartz. Knowing the temperature of an FZ contributing saturated liquid water, its enthalpy is obtained from the Steam Tables [6] or is computed by means of the following polynomial equation (T in °C, in kJ/kg):
which approximates the Steam Tables, whereas the SiO2 concentration of the saturated pure liquid water in equilibrium with quartz is calculated using the following polynomial equation (from Fournier and Potter [7]; T in °C, in mg/kg):
Hl = 4.523218 ⋅ 10−12 · T6 − 5.422310 ⋅ 10−9 · T5 + 2.680270 ⋅ 10−6 · T4 − 6.870220 ⋅ 10−4 · T3
+ 9.700676 ⋅ 10−2 · T2 − 2.928824 · T + 211.0268,
+ 9.700676 ⋅ 10−2 · T2 − 2.928824 · T + 211.0268,
CSiO2,l = −1.141189 ⋅ 10−11 · T6 + 1.325216 ⋅ 10−8 · T5 − 6.505939 ⋅ 10−6 · T4 + 1.707502 ⋅ 10−3 · T3
− 0.2373024 · T2 + 17.53198 · T − 511.5682.
− 0.2373024 · T2 + 17.53198 · T − 511.5682.
Similarly, knowing the temperature of an FZ contributing saturated vapor water, its enthalpy is obtained from the Steam Tables or is calculated using the following polynomial equation (T in °C, in kJ/kg):
which approximates the Steam Tables, whereas the silica concentration of the saturated water vapor in equilibrium with quartz, , is calculated using the following polynomial equation (from Fournier and Potter [7]; T in K, in mg/kg):
Hv = −7.753171 ⋅ 10−12 · T6 + 9.223014 · 10−9 · T5 − 4.522373 · 10−6 · T4 + 1.146901 · 10−3 · T3
− 0.1622219 · T2 + 13.68471 · T + 2149.589,
− 0.1622219 · T2 + 13.68471 · T + 2149.589,
Log CSiO2,v = −1.468678 ⋅ 109 · T3 + 8.931339 ⋅ 106 · T2 − 2.409290 ⋅ 104 · T
+ 23.26751.
+ 23.26751.
Equations (8) and (10) are used to calculate the enthalpies HA and HB of FZs A and B in Equation (1) and the enthalpies HA, HB, and HC of FZs A, B, and C in Equation (3), as well as in the relationships derived from them, whereas the total discharge enthalpy HTD is obtained by measuring the flowrate of the separated liquid and vapor phases at known pressure, temperature conditions, and their enthalpies or using a different approach, such as the James lip pressure method [8], the tracer flow test [9], the gas method of [10], and the chloride method of [11].
Similarly, Equations (9) and (11) are utilized to compute the SiO2 concentrations of FZs A and B in Equation (2) and the SiO2 concentrations of FZs A, B, and C in Equation (4), as well as in the relations obtained from them. It should be noted that the SiO2 concentration on a total discharge basis, , is obtained by multiplying the aqueous (undissociated) SiO2 concentration of the aquifer liquid mixture times the aquifer liquid fraction, both computed using WATCH. Alternatively, the SiO2 concentration on a total discharge basis is calculated by multiplying the aqueous (undissociated) SiO2 concentration of the separated water sample times the liquid fraction at collection. However, speciation calculations are needed to obtain the aqueous (undissociated) SiO2 concentration of the separated water sample.
The generalized enthalpy balance and SiO2 mass balance are
and
respectively, in which is the mass fraction of each liquid-producing FZ and is the mass fraction of each vapor-producing FZ. Equations (1) and (3) are special cases of Equation (12), while Equations (2) and (4) are special cases of Equation (13).
Knowing the mass fraction of each FZ, both liquid- and vapor-producing, the next step is to calculate the CO2 molalities of FZ fluids, and . For liquid-producing FZs the relation of Arnórsson and Gunnlaugsson [12] (T in K) is used:
For vapor-producing FZs, the vapor–liquid distribution coefficient of CO2, , is first computed utilizing the following equation ([13]; T in °C):
and second, CO2 molality is obtained by means of the simple relation
Then, the total discharge CO2 molality, , is computed using the mass balance:
In general, the value is different from the measured total discharge CO2 molality, , because of possible deviations from the condition described by Equation (14). Therefore, the CO2 molality of the i-th FZ fluid (either liquid or vapor) is re-calculated as follows:
For the liquid-producing FZs, the CO2 partial pressure, (in bar), is computed utilizing the simple relation
where is the Henry’s law constant of CO2, which is given by the following polynomial (T in °C; in bar/(mol/kg)):
KH,CO2= −1.27017 ⋅ 10−8 · T4 + 2.498309 ⋅ 10−5 · T3 − 1.438044 ⋅ 10−2 · T2 + 2.87047 · T − 73.80178.
Another needed variable, for the liquid-producing FZs, is the decimal logarithm of total ionic salinity, which is defined as follows:
where is the charge of the k-th ionic solute. Initially, the is estimated as a function of using a regression equation obtained by regressing the against the for the mixtures of aquifer liquids of the geothermal system of interest.
Temperature, , and are the three independent variables involved in the geothermometric relations of Chiodini et al. [14]. Since these three parameters are now known for all liquid-producing FZs, the geothermometric relations of Chiodini et al. [14] (Table 1) are used to calculate the pH and the total molalities of dissolved compatible constituents (Na, K, Ca, Mg, HCO3, SO4, and F) of each FZ liquid.

Table 1.
Geothermometric relations of Chiodini et al. [14] expressing the pH and the decimal logarithms of the total molalities of Na, K, Ca, Mg, HCO3, SO4, and F as a function of temperature (in K), (in log bar), and (in log eq/kg).
Then, the following mass balance is utilized to compute the total molality of each dissolved compatible constituent in the mixture of FZ liquids, :
In general, the computed values are different from the total molalities computed by WATCH based on analytical data, , because of possible deviations from the conditions described by the geothermometric relations of Chiodini et al. [14]. Therefore, the molalities of the k-th compatible dissolved constituent in the l-th FZ liquid are re-calculated using the following relation:
The approximated molality of chloride, the major conservative (mobile) dissolved constituent, of the l-th FZ liquid is obtained by difference using the electroneutrality equation written as follows:
The obtained result is improved using Equation (23). Relation (24) must be suitably changed, including other constituents present in high or relatively high concentrations. This is the case of the high-pH geothermal waters containing considerable amounts of HSiO3− ion and related complexes.
Owing to the considerable uncertainties on the values initially computed as a function of by means of the regression equation derived from available data, it is advisable to iterate on it, recomputing values from the obtained concentrations of all ionic solutes.
3. Results
In this section, two application examples are presented and discussed, also including the enthalpy vs. SiO2 diagram, which is an effective graphical tool for deriving the FZs mass fractions. The two examples refer to two deep wells of the high-enthalpy geothermal fields of Krafla and Hellisheiði, both in Iceland.
3.1. General Features of the Krafla Geothermal Field
The Krafla high-enthalpy geothermal system is found in the Northern Volcanic Zone, within the 8–10 km-wide Krafla caldera, which formed ca. 110–115 ka BP, during a mixed basalt–rhyolite event, known as the Halarauður eruption [15]. Recent magmatic activity has been mainly basaltic, but both basaltic and rhyolitic eruptions occurred in the past. The last intracaldera fissure eruptions took place during the 1974–1989 rifting episode, emitting basaltic products, and are known as the Krafla Fires [16].
An active geothermal system is hosted in the central part of the Krafla caldera. A total of 43 geothermal wells were drilled in the Krafla geothermal area since 1974. At present, seventeen high-pressure production wells and five low-pressure production wells feed the Krafla geothermal power plant consisting of two 30 MWe units [17].
Permeability is higher in the upper kilometer of the caldera filling, made up of hyaloclastite and lava flows, whereas it is lower in the intrusive rocks occurring at greater depths [18]. Permeability is mainly controlled by the tectonic trend of the fissure swarm, striking NNE-SSW, and the tectonic trend of WNW-ESE direction, as confirmed by recent geothermal boreholes [17].
The Krafla geothermal area has been divided into the seven sub-areas of Leirbotnar, Sudurhlídar, Vesturhlídar, Sandabotnar, Hvítholar, Vestursvædi, and Leirhnúkur [19]. In the Leirbotnar subarea the reservoir comprises an upper liquid-dominated zone down to 1000–1400 m depth, at temperatures of 190–220 °C and with sulfate as the main anion, and a lower two-phase zone at ca. 300 °C, with chloride as the major anion. The Hvítholar subarea, along the southern caldera margin, hosts a two-phase system to about 1000 m depth, with chloride as the major anion of the liquid phase, overlying a liquid-dominated cooler zone where sulphate is the main anion. Two-phase fluids with chloride as the main anion are hosted in a reservoir at ca. 300 °C at Sandabotnar, Sudurhlídar, and Vesturhlídar. All reservoir waters have low TDS and close to neutral pH.
3.2. Hydrothermal Alteration Minerals at Krafla
According to Gudmundsson and Arnórsson [20], the most important hydrothermal alteration minerals identified in the Krafla geothermal wells are quartz, calcite, epidote, smectite, mixed-layer clays, chlorite, albite, and pyrite. Anhydrite, adularia, prehnite, actinolite, wollastonite, garnet, pyrrhotite, and various zeolites, including wairakite, have also been identified. Some of these minerals display a depth–zonal distribution which is related to their temperature stability.
3.3. Well K-28 of the Krafla Geothermal Field
As a first application example of geochemical FZ analysis, we considered well K-28 of the Krafla geothermal field. This well has two FZs, at depths of 500 and 800 m, with temperatures of 230 and 240 °C, respectively, and total discharge enthalpy of 1015 kJ/kg [21]. The calculations performed using the code WATCH for the sample collected on 26 October 1997 [21] (see Supplementary Materials K-28 26.10.1997.in and K-28 26.10.1997.out) indicate the absence of a separated vapor phase in the aquifer, for an aquifer temperature of 235 °C, constrained by quartz saturation. It should be noted that an enthalpy value of 1014 kJ/kg, slightly lower than the reported value, was assumed in WATCH calculations in order to avoid an unrealistic, very small aquifer mass steam fraction, y. Using the enthalpy balance, i.e., Equation (1), the mass fractions of both FZs resulted in 0.5000, whereas the SiO2 mass balance, i.e., Equation (2), gives moderately different outcomes, namely, 0.5476 and 0.4524 for the FZs at 500 and 800 m depth, respectively. These discrepancies are probably due to uncertainties in SiO2 and enthalpy measurements. Thus, the average values, 0.5238 and 0.4762 were considered for the FZs at 500 and 800 m depth, respectively.
Alternatively, instead of using Relations (1) and (2), a graphical approach, i.e., the enthalpy vs. SiO2 concentration diagram (Figure 1), can be adopted to calculate the mass fractions of the two FZs of well K-28. Knowing the enthalpy of the two FZ liquids, their SiO2 concentration is found along the liquid branch of the quartz saturation curve. In the case of well K-28, due to the absence of a separate vapor phase in the geothermal reservoir, the point of the reservoir liquid mixture coincides with the total discharge point and is defined by the intersection of the liquid branch of the quartz saturation curve with the straight line (dashed black line in Figure 1) connecting the liquid and vapor samples collected at the pressure separator. Having positioned the two FZ liquids and the reservoir liquid mixture in the diagram, the lever rule is applied to obtain the mass fractions of the two FZs.
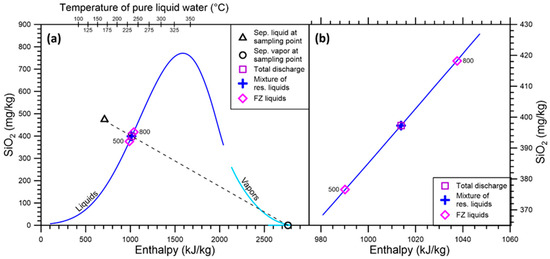
Figure 1.
(a) Diagram of enthalpy vs. SiO2 concentration for well K-28 of the Krafla geothermal field, showing the separated liquid and the separated vapor at the sampling point, the total discharge, coinciding with the mixture of reservoir liquids, in this case, and the two FZ liquids, whose label indicates the FZ depth in meters. Also shown are the liquid and vapor branches of the quartz saturation curve of Fournier and Potter [7]. (b) Zoomed view of the diagram centered on the two FZ liquids, the total discharge, and the mixture of reservoir liquids.
After having computed or graphically derived the mass fractions of the two liquid-producing FZs of well K-28, we followed the procedure described in Section 2, Methods, to compute the CO2 concentration of the two FZs, as well as the , the , and the concentrations of major ionic solutes of each FZ. The results obtained are listed in Table 2.

Table 2.
Results of the geochemical FZ analysis for well K-28 of the Krafla geothermal field; yFZ is the mass steam fraction of each FZ.
In particular, the was initially computed as a function of using the following linear regression equation:
Equation (25) was obtained from the 36 analyses of Krafla aquifer liquid mixtures computed by Cioni and Marini [22] based on the data reported by Gudmundsson and Arnórsson [21], Arnórsson et al. [23], Ping [24], Giroud [25], and Heřmanská et al. [26]. Then, we iterated on , recomputing it based on the concentrations of all ionic solutes obtained in each iteration, until the absolute value of the difference in between two successive iterations was ≤0.001 log meq/kg.
3.4. General Features of the Hellisheiði Geothermal Field
The Hellisheiði geothermal field is part of the larger Hengill geothermal field together with Nesjavellir, Bitra, and Hverahlid [27]. Overall, 50 wells were drilled in the Hellisheiði geothermal field, at depths ranging from 1 to over 3.3 km. The Hellisheiði power plant is the eighth-largest station in the world and the largest in Iceland with a current installed capacity of 303 MWe and a hot water production of 200 MWt [19].
According to Ármannsson [19], the borehole geology of Hellisheiði wells reveals the presence of various 50–300 m thick hyaloclastite units, comprising breccia, tuff, and pillow lava subunits, as well as minor scoria and tuffaceous deposits. These rocks are chiefly made up of more or less altered glass, olivine, plagioclase, and augitic clinopyroxene with accessory magnetite and ilmenite. Groups of subaerial lava flows, erupted during interglacial periods, are intercalated with the hyaloclastite units.
Three types of intrusions were recognized: (i) 10–12 m thick intermediate-to-acid dykes; (ii) <6 m thick, altered basaltic dykes, which make up most of the intrusions below 1.5 km depth; and (iii) relatively unaltered basaltic intrusions. Below 400 m b.s.l., permeability is mainly controlled by the fracture networks created by the emplacement of dioritic dykes and fresh basaltic intrusions whereas permeability is lower around the altered basalt intrusions [19]. Intrusions are very common below 1500–1600 m b.s.l. They consist of sub-vertical dyke complexes, mainly made up of fine-grained basalts.
The Hellisheiði geothermal wells discharge Na-Cl-HCO3 waters of low salinity, with TDS ranging from 1000 to 1500 mg/kg and Cl usually below 200 mg/kg [28]. Water isotopes indicate that the Hellisheiði geothermal reservoir is probably recharged by local meteoric waters [28].
3.5. Hydrothermal Alteration Minerals at Hellisheiði
Helgadóttir et al. [29] described the depth–zonal distribution of major temperature- dependent alteration minerals in the Hellisheiði geothermal field with quartz at T > 180 °C, epidote at T > 230–250 °C, wollastonite at T > 260 °C, and Al-free amphibole at T > 280 °C.
Although much of the data acquired for the Hellisheiði geothermal field is in Icelandic or not open to the public, several studies of the reservoir geology of individual Hellisheiði wells to 800 or 1200 m depth, including hydrothermal alteration mineralogy, are available as reports of the United Nations University Geothermal Training Program. According to one of these reports [30], in well HE-11, the geothermal reservoir is characterized by T > 250 °C and extends from 950 to 1192 m depth, where total circulation loss occurred. It is marked by the presence of epidote and chlorite, accompanied by quartz, calcite, sphene, pyrite, wairakite, prehnite, albite, and wollastonite.
3.6. Well HE-12 of the Hellisheiði Geothermal Field
As a second application example of geochemical FZ analysis, we took into account well HE-12 of the Hellisheiði geothermal field. Similar to the previous case (Section 3.3), this well also has two FZs. They are located at depths of 900 and 1250 m and have temperatures of 270 and 295 °C, respectively, while the total discharge enthalpy of the well is 1746 kJ/kg [27].
We ran the code WATCH for sample 08-3002, obtained on 08/10/2008 [27,28,29,30,31] (see Supplementary Materials HE-12 08.10.2008.in and HE-12 08.10.2008.out). Assuming quartz saturation, the aquifer temperature turns out to be 283.4 °C. At this aquifer temperature, the enthalpy of pure saturated liquid water is 1254.8 kJ/kg, which is 491.2 kJ/kg lower than total discharge enthalpy. This means that HE-12 is an excess enthalpy well and a separated vapor phase, with y = 0.3228, is present in the aquifer.
Since steam caps are common in geothermal aquifers [32], we assumed that the uppermost FZ delivers a saturated two-phase (liquid + vapor) fluid to the well and the lowermost FZ produces a single saturated liquid phase. (Nevertheless, also conceivable are other cases, such as (i) the production of two-phase fluids from both FZs, a case which cannot be modeled using our approach, or (ii) the production of two-phase fluids from the lowermost FZ and a single saturated liquid phase from the uppermost FZ.) We also split the uppermost FZ into two virtual FZs, a liquid one and a vapor one. Thus, well HE-12 was treated as a geothermal well with three FZs and Equations (5)–(7) were used to calculate the FZ mass fractions, which resulted in 0.3202 for the virtual vapor FZ at 900 m depth, 0.3234 for the virtual liquid FZ at the same depth, and 0.3564 for the real liquid FZ at 1250 m depth. Thus, the real FZ at 900 m depth delivers a two-phase fluid with y = , a liquid steam fraction equal to the complement to one, i.e., 0.5025, and an enthalpy of 1983.5 kJ/kg.
Again, the enthalpy vs. SiO2 concentration diagram (Figure 2) can be used to estimate the mass fractions of the two FZs of well HE-12 as well as the mass steam fraction and the mass liquid fraction of the FZ at 900 m depth instead of Relations (5)–(7). First, all available SiO2 and enthalpy data are used to position in the diagram the points representative of the sampled liquid and vapor phases, the total discharge, the real liquid FZ at 1250, as well as the virtual liquid and vapor FZs at 900 m depth; the last two points are connected by a straight line (dashed sky-blue line in Figure 2). Second, the liquid and vapor samples are connected by a straight line (dashed black line in Figure 2), along which are situated both the total discharge point and the point representative of the mixture of reservoir liquids, which is found at the intersection with the liquid branch of the quartz saturation curve. Third, the straight line passing through the point of the real liquid FZ at 1250 and the total discharge point is drawn (dashed purple line in Figure 2). The intersection of this straight line with the straight line connecting the virtual liquid and vapor FZs at 900 m depth defines the point representative of the real two-phase FZ at 900 m depth. Fourth, the lever rule is applied both (i) to the three points located along the dashed sky-blue line to obtain the mass steam fraction and the mass liquid fraction of the FZ at 900 m depth and (ii) to the three points located along the dashed purple line to obtain the mass fractions of the two FZs at 900 and 1250 m depth.
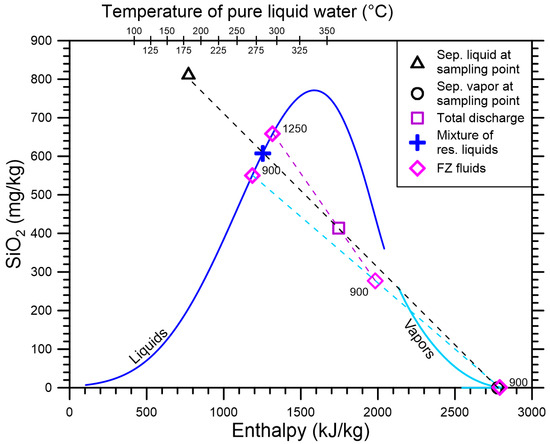
Figure 2.
Diagram of enthalpy vs. SiO2 concentration for well HE-12 of the Hellisheiði geothermal field, showing the separated liquid and the separated vapor at the sampling point, the total discharge, the mixture of reservoir liquids, and the FZ fluids, whose label indicates the FZ depth in meters. Also shown are the liquid and vapor branches of the quartz saturation curve of Fournier and Potter [7].
Knowing the mass fractions of the FZs of well HE-12, the procedure described in Section 2, Methods, was adopted to calculate the CO2 concentration of the liquid-producing and vapor-producing FZs, as well as the , the , and the concentrations of major ionic solutes of the liquid-producing FZs. The outcomes obtained are reported in Table 3.

Table 3.
Results of the geochemical FZ analysis for well HE-12 of the Hellisheiði geothermal field; yFZ is the mass steam fraction of each FZ.
Again, the was first calculated as a function of by means of the following linear regression equation:
Equation (26) was obtained from the 27 analyses of Hellisheiði aquifer liquid mixtures computed by Cioni and Marini [22] based on the data of Scott [27] and Scott et al. [31]. Afterwards, we iterated on , recalculating it from the concentrations of all ionic solutes obtained in each iteration, until the absolute value of the difference in between two successive iterations was ≤0.003 log meq/kg.
4. Discussion
The geochemical FZ analysis is based on the hypothesis of thermochemical equilibrium between the typical hydrothermal minerals occurring in active geothermal systems (quartz, albite, adularia, chlorite, muscovite, anhydrite, fluorite, calcite, and/or a Ca-Al-silicate) and geothermal fluids, representing the theoretical foundation of the used geothermometers, i.e., the quartz solubility of Fournier and Potter [7], the CO2 geothermometer of Arnórsson and Gunnlaugsson [12], and the geothermometric relations of Chiodini et al. [14]. Thus, as a general consideration, the obtained results may be affected by more or less important uncertainties depending on the more or less significant deviations from the thermochemical equilibrium condition. Therefore, it is reasonable to expect that the geochemical FZ analysis provides reasonable results for the geothermal systems in natural state conditions (i.e., before they are put into production and the spent fluids are re-injected), when they are probably close to the thermochemical equilibrium condition between hydrothermal minerals and geothermal fluids. Conversely, since re-injection causes a departure from this condition, it is reasonable to suppose that the geochemical FZ analysis provides increasingly less reliable results during the exploitation of the geothermal systems, when the impact of the reinjected fluids becomes progressively more important and the reactions between the aqueous solution and rock-forming (primary) minerals are unable to re-establish the equilibrium condition between the aqueous solution and the hydrothermal minerals due to the slow kinetics.
Erroneous mass fractions of the FZs contributing to well discharge might be computed if the geothermal fluid experiences loss of SiO2 upstream of the sampling point, whereas loss of other chemical components of interest upstream of the sampling point can cause uncertainties on their concentrations of FZ fluids. For example, calcite precipitation may determine uncertainties in the concentrations of Ca an HCO3 of FZ fluids. The error on Ca concentration might be important for Ca-poor waters, whereas the uncertainty on HCO3 concentration might be considerable for HCO3-poor waters.
For what concerns the FZ liquid chemistry of the considered wells, namely K-24 of Krafla and HE-12 of Hellisheiði, the concentrations of SiO2, CO2, K, and HCO3 increase with increasing depth and temperature, whereas pH and the concentrations of Na, Ca, Mg, SO4, and F decrease with increasing temperature, as dictated by the used geothermometric relations which, in turn, reflect the temperature dependence of the solubility of relevant hydrothermal minerals. Therefore, even if these considerations refer only to these two wells, strictly speaking, it is reasonable to expect that they have general validity, i.e., that they are also valid for other wells and other geothermal systems, provided that the chemistry of the fluids is controlled by the thermochemical equilibrium condition between hydrothermal minerals and geothermal fluids. In contrast, geochemical FZ analysis is likely to give erroneous results if one or more FZs behave as entry pathways into the geothermal aquifer for fluids in disequilibrium, such as marginal recharge fluids or downflowing shallow acidic SO4-rich fluids.
If a sufficient number of analytical data are available for a given geothermal well, geochemical FZ analysis can be performed in a different way. First, the concentration of each chemical component is regressed against SiO2 concentration and, second, the obtained regression equations are used instead of the CO2 geothermometer of Arnórsson and Gunnlaugsson [12] and the geothermometric relations of Chiodini et al. [14]. This alternative approach to geochemical FZ analysis has proven to be very interesting in the case of an Indonesian geothermal field whose data are confidential at this stage.
5. Conclusions
In this work we propose a method of geochemical FZ analysis based on the hypothesis of thermochemical equilibrium between hydrothermal minerals and geothermal fluids, for each FZ contributing to well discharge. The method allows one to compute the mass fraction and the chemistry of FZ fluids, that is, (1) the pH and the concentrations of SiO2, CO2, Na, K, Ca, Mg, HCO3, SO4, F, and Cl of FZ liquids, and (2) the concentrations of SiO2 and CO2 of FZ vapors.
The FZ mass fractions are computed solving the enthalpy balance and the mass balance of SiO2, assuming quartz saturation for the liquid and vapor phases present in the geothermal aquifer. The method also involves the CO2 geothermometer of Arnórsson and Gunnlaugsson [12] and the geothermometric relations of Chiodini et al. [14], which are used to calculate the approximate concentrations of the chemical components of interest for each FZ fluid. These approximate concentrations are then adjusted and made consistent with the concentrations for the mixtures of reservoir fluids computed by WATCH. The method is applicable to wells with two single-phase FZs and to wells with either three single-phase FZs or two FZs, one single-phase and the other two-phase, with different temperature and fluid chemistry. Erroneous FZ mass fractions might be calculated if SiO2 is lost by the geothermal fluid upstream of the sampling point, whereas uncertain concentrations of the considered chemical components might be computed if they are lost upstream of the sampling point.
Two application examples are presented and discussed, also comprising the enthalpy vs. SiO2 plot, which is a useful graphical tool to derive the FZs mass fractions, at least for wells with two or three FZs. The two examples are as follows:
(i) Well K-28 of the Krafla geothermal field, which has two liquid FZs, at depths of 500 and 800 m, with temperatures of 230 and 240 °C, respectively, and a total discharge enthalpy of 1015 kJ/kg [21].
(ii) Well HE-12 of the Hellisheiði geothermal field, which has two FZs, at depths of 900 and 1250 m, with temperatures of 270 and 295 °C, respectively, and a total discharge enthalpy of 1746 kJ/kg [27]. This well has excess enthalpy, meaning that at least one of the two FZs delivers two-phase fluids.
Geochemical FZ analysis shows that the chemical characteristics of the FZ fluids of these two wells exhibit significant differences, which are not evident based on the fluid mixtures sampled at the surface. Since the mixing of liquids with different characteristics can cause precipitation of solid phases and scaling in the aquifer and/or in the well and/or in the surface equipment, geochemical FZ analysis can be useful to understand these processes and related detrimental effects. Furthermore, geochemical FZ analysis can be useful to improve the conceptual model of the geothermal system of interest.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/geosciences15020052/s1, WATCH input files: K-28 26.10.1997.in; HE-12 08.10.2008.in; WATCH output files: K-28 26.10.1997.out; HE-12 08.10.2008.out.
Author Contributions
Conceptualization, L.M.; methodology, L.M.; software, L.M.; validation, S.O.; formal analysis, L.M. and S.O.; investigation, L.M.; resources, C.A. and G.V.; data curation L.M. and S.O.; writing—original draft preparation, L.M.; writing—review and editing, L.M., C.A., S.O. and G.V.; visualization, L.M.; supervision, C.A.; project administration, C.A. and G.V.; funding acquisition, C.A. and G.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by PRIN 2022 PNRR-Thermal model of Aeolian Islands for new perspectives of sustainable exploitation of geothermal resources, CUP H53D23011420001.
Data Availability Statement
The data presented in this study are available in reference numbers [21,22,27,31].
Acknowledgments
The Editor and the anonymous reviewers are acknowledged for their constructive comments that helped improve a previous version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Bjornsson, G. A Multi-Feedzone Geothermal Wellbore Simulator. Master’s Thesis, LBL-23546. Lawrence Berkeley National Laboratory, University of California, Earth Sciences Division, California, CA, USA, 1987; 102p. [Google Scholar]
- Marini, L.; Vespasiano, G.; De Rosa, R.; Viccaro, M.; Principe, C.; Bloise, A.; Fuoco, I.; Lelli, M.; La Russa, M.; Caruso, C.G.; et al. The geothermal resources of Vulcano Island (Aeolian Archipelago, Italy). Earth Sci. Rev. 2025. [Google Scholar]
- Grant, M.A.; Bixley, P.F. Geothermal Reservoir Engineering, 2nd ed.; Academic Press-Elsevier: Amsterdam, The Netherlands, 2011; 378p. [Google Scholar]
- Arnórsson, S.; Sigurdsson, S.; Svavarsson, H. The chemistry of geothermal waters in Iceland. I. Calculation of aqueous speciation from 0 to 370 °C. Geochim. Cosmochim. Acta 1982, 46, 1513–1532. [Google Scholar] [CrossRef]
- Morey, G.W.; Fournier, R.O.; Rowe, J.J. The solubility of quartz in water in the temperature interval from 25° to 300 °C. Geochim. Cosmochim. Acta 1962, 26, 1029–1043. [Google Scholar] [CrossRef]
- Wagner, W.; Kretzschmar, H.J. International Steam Tables—Properties of Water and Steam Based on the Industrial Formulation IAPWS-IF97; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Fournier, R.O.; Potter, R.W., II. An equation correlating the solubility of quartz in water from 25 to 900 C at pressures up to 10,000 bars. Geochim. Cosmochim. Acta 1982, 46, 1969–1973. [Google Scholar] [CrossRef]
- James, R. Measurement of steam-water mixtures discharging at the speed of sound to the atmosphere. N. Z. Eng. 1966, 21, 437–441. [Google Scholar]
- Hirtz, P.N.; Kunzman, R.J.; Broaddus, M.L.; Barbitta, J.A. Developments in tracer flow testing for geothermal production engineering. Geothermics 2001, 30, 727–745. [Google Scholar] [CrossRef]
- Mahon, W.A.J. A method for determining the enthalpy of a steam/water mixture discharged from a geothermal drillhole. N. Z. J. Sci. 1966, 9, 791–800. [Google Scholar]
- Marini, L.; Cioni, R. A chloride method for the determination of the enthalpy of steam/water mixtures discharged from geothermal wells. Geothermics 1985, 14, 29–34. [Google Scholar] [CrossRef]
- Arnórsson, S.; Gunnlaugsson, E. New gas geothermometers for geothermal exploration—Calibration and application. Geochim. Cosmochim. Ac. 1985, 49, 1307–1325. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Geothermal gas equilibria. Geochim. Cosmochim. Ac. 1980, 44, 2021–2032. [Google Scholar] [CrossRef]
- Chiodini, G.; Cioni, R.; Guidi, M.; Marini, L. Chemical geothermometry and geobarometry in hydrothermal aqueous solutions: A theoretical investigation based on a mineral-solution equilibrium model. Geochim. Cosmochim. Acta 1991, 55, 2709–2727. [Google Scholar] [CrossRef]
- Rooyakkers, S.M.; Stix, J.; Berlo, K.; Barker, S.J. Emplacement of unusual rhyolitic to basaltic ignimbrites during collapse of a basalt-dominated caldera: The Halarauður eruption, Krafla (Iceland). GSA Bull. 2020, 132, 1881–1902. [Google Scholar] [CrossRef]
- Einarsson, P. Plate boundaries, rifts and transforms in Iceland. Jökull 2008, 58, 35–58. [Google Scholar] [CrossRef]
- Mortensen, A.K.; Gudmundsson, Á.; Steingrímsson, B.; Sigmundsson, F.; Axelsson, G.; Ármannsson, H.; Hauksson, T. The Krafla Geothermal System: Research Summary and Conceptual Model Revision (Tech. Rep. No. LV-2015-098); Landsvirkjun: Reykjavík, Iceland, 2015. [Google Scholar]
- Scott, S.W.; O’Sullivan, J.P.; Maclaren, O.J.; Nicholson, R.; Covell, C.; Newson, J.; Guðjónsdóttir, M.S. Bayesian calibration of a natural state geothermal reservoir model, Krafla, North Iceland. Water Resour. Res. 2022, 58, e2021WR031254. [Google Scholar] [CrossRef]
- Ármannsson, H. The fluid geochemistry of Icelandic high temperature geothermal areas. Appl. Geochem. 2016, 66, 14–64. [Google Scholar] [CrossRef]
- Gudmundsson, B.T.; Arnórsson, S. Secondary mineral–fluid equilibria in the Krafla and Námafjall geothermal systems, Iceland. Appl. Geochem. 2005, 20, 1607–1625. [Google Scholar] [CrossRef]
- Gudmundsson, B.T.; Arnórsson, S. Geochemical monitoring of the Krafla and Námafjall geothermal areas, N-Iceland. Geothermics 2002, 31, 195–243. [Google Scholar] [CrossRef]
- Cioni, R.; Marini, L. A Thermodynamic Approach to Water Geothermometry; Springer Geochemistry Series; Springer Nature: Cham, Switzerland, 2020; 415p. [Google Scholar] [CrossRef]
- Arnórsson, S.; Gunnlaugsson, E.; Svavarsson, H. The chemistry of geothermal waters in Iceland. II. Mineral equilibria and independent variables controlling water compositions. Geochim. Cosmochim. Acta 1983, 47, 547–566. [Google Scholar] [CrossRef]
- Ping, Z. Gas Geothermometry and Chemical Equilibria of Fluids from Selected Geothermal Fields; UNU-GTP Report 1991-14; United Nations University, Geothermal Training Programme: Reykjavik, Iceland, 1991; 46p. [Google Scholar]
- Giroud, N. A chemical Study of Arsenic, Boron and Gases in High-Temperature Geothermal Fluids in Iceland. Ph.D. Thesis, Faculty of Science, University of Iceland, Reykjavik, Iceland, 2008; 110p. [Google Scholar]
- Heřmanská, M.; Stefánsson, A.; Scott, S. Supercritical fluids around magmatic intrusions: IDDP-1 at Krafla, Iceland. Geothermics 2019, 78, 101–110. [Google Scholar] [CrossRef]
- Scott, S.W. Gas Chemistry of the Hellisheidi Geothermal Field. Master’s Thesis, REYST/Faculty of Science, University of Iceland, Reykjavik, Iceland, 2011; p. 81. [Google Scholar]
- Mutonga, M.W.; Sveinbjornsdottir, A.; Gislason, G.; Amannsson, H. The isotopic and chemical characteristics of geothermal fluids in Hengill Area, SW-Iceland (Hellisheiði, Hveragerdi and Nesjavellir Fields). In Proceedings of the World Geothermal Congress, Bali, Indonesia, 25–30 April 2010; pp. 1–13. [Google Scholar]
- Helgadóttir, H.M.; Snaebjörnsdottir, S.O.; Níelsson, S.; Gunnarsdóttir, S.H.; Matthíasdóttir, T.; Hardarson, B.S.; Einarsson, G.M.; Franzson, H. Geology and hydrothermal alteration in the reservoir of the Hellisheiði high temperature system, SW-Iceland. In Proceedings of the World Geothermal Congress, Bali, Indonesia, 25–30 April 2010; pp. 25–29. [Google Scholar]
- Hartanto, D.B. Borehole Geology and Alteration Mineralogy of Well HE-11, Hellisheidi Geothermal Field, SW-Iceland; The United Nations University, Geothermal Training Programme: Reykjavík, Iceland, 2005; Reports 2005, Number 8; pp. 83–109. [Google Scholar]
- Scott, S.; Gunnarsson, I.; Arnórsson, S.; Stefánsson, A. Gas chemistry, boiling and phase segregation in a geothermal system, Hellisheidi, Iceland. Geochim. Cosmochim. Acta 2014, 124, 170–189. [Google Scholar] [CrossRef]
- Nicholson, K. Geothermal Fluids—Chemistry and Exploration Techniques; Springer: Berlin/Heidelberg, Germany, 1993; 263p. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).