Abstract
The analysis of published data on geophagy in humans, including manifestations of its variant known as “pica”, as well as information on geochemical endemics associated with geophagy and rare earth elements, together with our own research experience, allows us to conclude that geophagy in humans, as well as in animals, is primarily a natural, evolutionarily determined form of maintaining the necessary balance of rare earth elements in the neuroimmunoendocrine system. Disturbances in the composition and concentration of necessary rare earth elements (REE) in the structures of the most important protective and controlling system in the mammalian organism lead to disorders of mineral and general metabolism in the body and, as a result, to geochemical endemics. Geochemical endemics occur in landscapes with anomalous levels of biologically available REE forms, i.e., levels that differ significantly both towards deficiency and towards exceeding background levels. The imbalance in the metabolism of other chemical elements in the body seems to have a subordinate importance in the mechanism of occurrence of geochemical endemics in relation to REE.
1. Introduction
At the beginning of 2025, the journal “Geosciences” published an article entitled “Major and minor causes of geophagy-lithophagy in animals and humans”, in which we tried to summarize all the facts we had collected (the results of our own studies, as well as from literature sources), which directly or indirectly indicate the connection of most cases of geophagy-lithophagy in mammals and birds with the desire to maintain the balance of rare earth elements (REEs) in the neuroimmunoendocrine system of the body. We started from the fact that the normal functioning of this most important control and defense system in any living organism depends on the presence in it of only necessary (present in necessary amounts) and the absence of unnecessary (toxic) REE elements. The fact that humans and animals live in environments with a critical excess or deficiency of these elements in food and drinking water forces them to maintain the necessary balance of REEs with the help of natural sorbents that can be enriched or depleted of the necessary RE elements, depending on the specific situation.
We have decided to write this article as a follow-up to the previous one, and to focus it on a review of published evidence on geophagy, mainly in humans, as well as on the relationship of this phenomenon with landscape REE anomalies, which, as it turns out, are always areas of various endemic geochemically determined diseases. On the basis of these data, we intend to strengthen our previous hypothesis about the main cause of geophagy from the point of view of the idea of the incompleteness of the immunity theory and the existence of a component in the neuroimmunoendocrine system with the participation of some REE elements, which regulates the movement of all or part of the chemical elements and their spatial and temporal binding in the material structures of the body.
Before presenting the material, let us first define some terms. Geophagy is the phenomenon of regular, deliberate ingestion of earthy substances, specially selected in certain places, which is characteristic of humans and animals living for a long time in certain natural and anthropogenic landscapes. In the medical community, cases of geophagy are usually referred to as “pica” (from the Latin word “magpie”—a bird characterized by its habit of grabbing everything). Pica refers to cases in which a person in a state of stress tends to consume a variety of non-food substances: soil, chalk, coal, ash, cement mortar, etc. In other words, pica is a broader term used in medicine to describe any food disorder that occurs in a person. When researchers describe such disorders as regular consumption of predominantly earthy substances by people, we have also used publications on such cases in our analyses, identifying pica with geophagy.
In order to shorten the term “earthy substances consumed by humans”, we will sometimes refer to them more simply as “edible” earths.
2. Results
2.1. A Brief History of the Study of Geophagy in Humans; The Spatial Relationship of Geophagy in Humans and Animals
The phenomenon of geophagy has always been characteristic of humans and many animal groups. However, in mammals and humans, geophagy is and has always been far from ubiquitous. The real extent of the spread of this phenomenon among humans only became apparent in the middle of the 20th century, after all the ethnographic descriptions of the peoples of the world and their inherent food and cultural peculiarities had been generalized [1,2,3,4,5]. From these data it is clear that the phenomenon of geophagy was found not so long ago among practically all peoples of the world, but was particularly widespread in some areas of the near-equatorial zone of the Earth, including both continents of the Americas, Africa, the south of Eurasia, the islands of Oceania, Australia and New Zealand. Archaeological excavations of ancient human burials in Africa and South America have shown that the historical roots of geophagy traditions go back many thousands of years [6,7,8].
The geophagic habits of many indigenous peoples in the equatorial zone of the Earth are still preserved today. As for the middle and high latitudes, geophagy has persisted there almost exclusively in animals. In humans, this phenomenon is registered only in the form of a peak among migrants from the tropics, where geophagic traditions continue to persist.
It should be emphasized once again that geophagy in humans (as in all mammals) has never been widespread and has never covered the entire population in any area. As B. Laufer noted, “geophagy is not a universal phenomenon in any particular tribal or social group” [3], p. 103. As a factual confirmation of this conclusion, we can cite data from the work of A.S. Wiley and S.H. Katz [9]. On the basis of various ethnographic sources on Africa, the researchers identified 60 indigenous populations with different attitudes towards geophagy in pregnant women. In 10 of them, geophagy was almost universal, in 28 common, in 6 low frequent, in 7 very rare and in 9 there was no geophagy at all.
The most extensive areas where geophagy has been prevalent and where the phenomenon continues to be recorded are in the equatorial zone of the Earth. In Africa, for example, geophagy often covers thousands of square kilometers in both lowland and mountainous areas. In the middle and high latitudes, geophagy in animals is found mainly in mountainous areas and only in local territories. In this case, we are only talking about mammals; the group of cloacal animals with muscular stomachs is not considered in this article.
In assessing the prevalence of geophagy in humans, it is important to note that areas where this phenomenon was typical in the past often remain areas of mass geophagy in domestic and wild animals. As a typical example of this situation, Maasai pastoralists in Tanzania have been observed to lead their herds several times a week to places where the animals can consume earthy substances. These places are also actively visited by wild animals [10]. Often the places where wild and domestic animals go to consume earthy substances are also the places where people get “edible” earths. Descriptions of such facts are often found in studies of geophagy and manifestations of pica in humans.
Another example of spatial overlap between geophagy in humans and wildlife comes from a recent study in South India (Tamil Nadu). Exploratory surveys on the prevalence of pica were conducted with adult females from five villages [11]. It was found that unripe mango, ash and earthy substances were the most important non-food items and substances consumed by pregnant women in the area. A second study in the same area of India [12] showed that pica usually occurs long before pregnancy and is most commonly associated with childhood. In this case, children eat earthy substances and charcoal. Four interviewees stated that women of all ages living in the area were prone to geophagy.
Marakkanam Reserved Forest, also in Tamil Nadu, is home to endemic macaques (Macaca radiata), which occasionally consume soil from termite mounds. In August 1991, J. Voros et al. [13] analyzed soil samples from three termite mounds used by macaques and three samples of surrounding soils as controls. Clay minerals in all samples included halloysite, illite, smectite, vermiculite and chlorite, with a significantly higher content of smectite in the termite samples. Crystalline impurities included quartz, mica and feldspar. REE distribution spectra in samples from termite sites and in control samples had similar curve shapes with a characteristic cerium anomaly, which the researchers attribute to the presence of alluvial monazite in all local soils and subsoil horizons. The authors explained the consumption of termite clays by a possible desire of the monkeys to eliminate gastric disorders during the period of eating large quantities of ripe fruits.
An interesting fact of geophagy in local animals was also observed by a group of Indian biologists near the Sokkanathapuram village [14]. This is relatively close to the area where C.D. Placek and E.H. Hagen conducted studies [11,12]. The biologists observed the earthy substances being eaten by frugivorous bats. The researchers hypothesized that soil minerals are needed by bats to detoxify harmful organic matter contained in the ripe fruits and leaves of some trees, which make up the bulk of these animals’ diet at this time of year.
Our analysis of the geological map of Tamil Nadu (Geological map of India M 1:5 00,000,0, 1993, the map is freely available on the Internet) with georeferencing of all cases of geophagy described above has shown that all of them are confined to the outcrops of a vast massif of charnokites—metamorphic rocks enriched in REE, which are concentrated mainly in two minerals, monazite and orthopyroxene [15]. The long weathering process of these rocks in the neighboring state of Kerala with alluvial transport of monazite sands to the sea coast led to the formation of one of the world’s largest REE placer deposits in the Chavara-Neendakara area. We will return to the issue of REEs in southern India and their source—charnokites—but for now we will move on to another topic.
2.2. Mineral, Chemical and Particle Size Compositions of “Edible Earths”
As the analysis of publications on the material composition of “edible” earths in different regions of the world shows [7,16,17,18,19,20,21,22,23,24,25,26], the vast majority of them are fine-grained products of chemical weathering of a variety of rocks ranging from ultramafic to felsic. In terms of particle size distribution, “edible” earths are most often represented by clay and clay-silt particles, but sometimes there are varieties with the predominance of the fine sand fraction. Their color, according to the evaluation of a collection of 402 samples [27], can vary from bright white to light yellow, orange, red, reddish brown, purple, dark grey, black, blue and light green. In terms of mineral composition, they are most often mixtures in varying proportions of secondary clay minerals and primary igneous crystals, with quartz and feldspars usually predominant. The nature of the clay minerals depends on the material composition of the original rocks and the climatic peculiarities of their weathering. Representatives of the kaolinite (kaolinite, halloysite), smectite (smectite, nontronite) or hydrous mica (illite, vermiculite) groups may dominate in the composition of clay minerals. Calcium and magnesium carbonates, mica (biotite, muscovite), low temperature and biogenic silica (chalcedony, diatomaceous earth), iron (goethite, hematite) and aluminum (gibbsite) oxides and hydroxides, zeolites and talc are found as significant impurities, while impurities from volcanic glasses, crystals of pyroxenes and amphiboles are less common.
The chemical composition of “edible” earths is always dominated by silicon and aluminum, sometimes with comparable proportions of calcium and magnesium. There are “edible” earths with significant amounts of iron. Sodium and potassium are usually low in edible earths. Titanium, manganese, strontium, barium, zinc and copper are common trace elements. Lead, arsenic and cadmium are sometimes elevated. The levels of other trace elements are usually quite insignificant. Organic matter in “edible” earths is usually not very high either, no more than 2–3%. In some cases, however, people consume almost pure carbon in the form of charcoal as a substitute for typical “edible” earths. Why they consume charcoal will become clear in the text below.
2.3. Which Population Groups Are Characterized by Geophagy; In Which Form and in Which Quantities Are Earthy Substances Consumed?
Another study by S. Young, co-authored with J. Miller [28], in an attempt to understand the cause of geophagy, provides an analysis of extensive data from published sources as well as the Pica Literature Database. This analysis shows that geophagy is most common during pre-adolescence and pregnancy. Geophagy in children has been most extensively studied in sub-Saharan Africa. In this region of the world, the prevalence of geophagy has been estimated to be 47% among schoolchildren [29] and 74% among students [30]. In most males, the habit of geophagy declines rapidly between the ages of 5 and 18 [31]. In women, geophagy always increases dramatically during pregnancy [27]. It is most pronounced in the first trimester and gradually decreases in the second and third trimesters, with complete resolution after delivery, although there are exceptions [24,32,33]. Cases of morbid conditions in which people develop an uncontrollable desire to eat earthy substances have also been described [34].
According to studies by different authors in African countries [35,36,37], the prevalence of geophagy among pregnant women in Namibia, Uganda and Malawi ranges from 82 to 88%. The same studies showed that the main types of “edible” earths were pure clay or enriched with clay minerals, including those from termite sites. In Namibia, the average daily intake of clay soils is about 150 g; in Malawi, about the same amount is consumed per day with a frequency of intake ranging from two to several times; in Uganda, the daily dose is about 50 g.
Extremely interesting research that sheds some light on the culture of geophagy among the typically African tribe of Chaggas living on the slopes of Mount Kilimanjaro in Tanzania is given by J.W. Knudsen [38]. The researcher, a native of Tanzania and a Bantu speaker, reports that for the women of the Chaggas tribe, the culture of geophagy is part of a secret knowledge accumulated through practice. Living on the volcanic soil of Kilimanjaro for centuries, many Chaggas women have had several healthy children, even if the pregnancies are consecutive. The women the researcher spoke to claimed that such fertility results were due to the practice of geophagy, which is said to improve the blood and increase blood volume, which is essential during pregnancy. Geophagy is practiced by women regardless of social class, education or economic status. The consumption of “edible” earth is not publicly displayed, but neither is it particularly hidden. Many respondents claimed that geophagy cures morning sickness and provides a sense of well-being, especially during the first trimester of pregnancy. There is a strong emotional relief from eating earth, comparable to the satisfaction of sexual intercourse. The researcher felt that this knowledge of self-medication did not require the intervention of modern medicine.
Studies conducted in urban Europe in the context of people migrating from areas where geophagy is prevalent demonstrated that many migrant women continued to hold on to practices and beliefs about pregnancy that had been passed on to them by previous generations of relatives in their countries of origin [39,40,41].
For example, in a study with Cameroonian women in London, one participant reported that when her mother found out that her daughter was pregnant, she sent her clay, the same clay that she used to buy at the market in Cameroon and eat. She also said that she did not like the clay sold at the London markets, as it did not taste at all like the clay from Cameroon [42].
Somewhat similar, but somewhat different results were obtained in a survey of geophagy practices in northern India [43]. Among other things, it was shown that geophagy is common across all age and social groups, and that the quantities of earth consumed are sometimes quite substantial. The main motivation for its consumption in humans is a strong craving. The most common self-reported positive effect of geophagy was a sense of relief.
People extract “edible” earth from a variety of places, digging in the ledges of river terraces, in dug quarries on plains and hillsides. Where possible, they choose clays. If clay is not available nearby, people often travel many kilometers to find it.
In Africa, as elsewhere, the collected “edible” earths are specially treated before consumption. For example, D.E. Vermeer [44], p. 61 describes their preparation by the Tiv people of Nigeria as follows. First, the extracted earthy mass is placed in water; sand and iron oxide crusts are removed; then it is dried and sifted. The fine material is soaked in water again and made into a doughy mass from which clay cakes are molded. These are dried in the sun for several days. According to D.E. Vermeer, pregnant women could consume up to 300 g of this clay per day. In the three villages studied, Vermeer estimated the annual production of such cakes at around 300–400 tons.
In parts of Africa and India, “edible” earths from termite mounds are highly valued. As described in Ghana [35,45], earth from termite mounds is consumed by between 34 and 68% of rural women. A.R.P. Walker et al. [46] found that the practice of consuming earth from termite sites is also widespread among rural women in South Africa. In western Kenya, about 50% of women consume earth, with half of them preferring material from termite sites [47]. Some women interviewed in a study by Francoise [48] reported that the easiest way to consume earth from a termite site is to break off small pieces from the termite mound, chop and eat. Van Huis [45] found that pregnant women often consumed such earths at 30 g three times a day.
2.4. Review of Existing Hypotheses on the Causes of Geophagy
The causes of geophagy have been discussed by many researchers for at least two hundred years, but until recently there were no clear signs of unravelling the main cause of this phenomenon, which could unite all its manifestations throughout the world. One of the most detailed attempts to summarize everything that has been done to solve the causal question belongs to S. Young et al. [27]. The authors base their conclusions on the analysis of 367 publications on the study of the geophagy phenomenon, identifying 4 most promising hypotheses proposed at different times by different authors.
The first hypothesis, conventionally referred to as the “mineral” hypothesis, suggests that people eat soil to make up for the lack of minerals in their diet, especially iron and zinc [49,50,51], as well as calcium [9]. Analyzing the entire literature on the mineral hypothesis, S. Young et al. [27], citing data from the US Institute of Medicine, rightly pointed out that if geophagy is a response to mineral deficiency, pregnant women should consume soil in the late rather than the early stages of pregnancy, when the need for minerals is the greatest. In the early stages of pregnancy, women need less iron than they do in normal life with regular menstrual blood loss. In late pregnancy, women’s iron requirements actually increase dramatically due to fetal development. Calcium requirements are also lower in early pregnancy. The maximum requirement for this element for fetal development is also in the third trimester. Zinc requirements do not change significantly during pregnancy.
A literature review conducted by S. Young and colleagues [27] to investigate the ability of “edible” earths to supply iron, calcium and zinc to the human body [52,53,54,55,56,57,58] demonstrated that the amount of these elements in the consumed soils is highly variable and, in most cases, the ingestion of clay substances interferes with their absorption. A number of experiments on rats [56,57,59] came to a similar conclusion: clays interfere with the absorption of the cations in question, and their consumption may even cause deficiencies of these elements in the body.
The second hypothesis is the “protection” hypothesis. It suggests that clay soils, when ingested, can alleviate toxicosis associated with pregnancy or with various toxic substances in plant foods, or toxins produced by parasites and other pathogens. This hypothesis suggests two mechanisms by which “edible” clay soils can protect the body. First, they may strengthen the integrity of the intestinal mucosal layer, which acts as a biological barrier between ingested materials and the internal environment [60], and second, they may adsorb and eliminate pathogens from the intestine [61,62,63,64]. However, when both mechanisms are realized, the absorption of beneficial substances, including dietary iron, is inevitably compromised [65].
Similar protective effects against endo- and exotoxins have been attributed to charcoal, which is commonly prescribed in cases of childhood poisoning [66]. Experiments in rats show that charcoal is even more effective than kaolin in adsorbing endotoxins [67], which may explain why humans and some primates consume charcoal [68,69]. As will be shown later, charcoal is also an effective sorbent for a number of important micronutrients, as discussed below.
The third hypothesis is “therapeutic”, suggesting that geophagy should be associated with gastrointestinal diseases. In this case, smectite and kaolinite may provide protection against the development of ulcers in the gastrointestinal tract [70]. These ulcers occur when there is an imbalance between the production of substances such as gastric acid or pepsin and the mucosa with its protective layer of mucus. Smectite and kaolinite can bind free hydrogen ions as well as bile acid salts and enzymes such as pepsin. Smectite may also have an additional effect on mucosal ulcers by reducing the production of interleukin-1β by monocytes and macrophages, which may lead to a reduction in inflammation [60].
The fourth hypothesis, which S. Young et al. [27] called the “non-adaptive” one, is related to hunger. We prefer to call it the “mental” hypothesis. Geophagy may be associated with a general maladaptive state of the psyche, even in cases of starvation. As noted above, people who consume “edible” earth may develop an uncontrollable desire to eat soil and other nonfood substances [34].
It has long been known that pica, including geophagy as a variant, is common among intellectually disabled and people with mental illness in appropriate treatment hospitals. This fact seems to suggest that intellectually disabled people sometimes cannot distinguish between edible and inedible substances, but a study conducted in the United States showed that such people are quite selective about what they consume, while often being aggressive in their search for necessary inedible substances when they cannot find them [71].
It should be noted that there are currently no clinical guidelines for the assessment or treatment of Pica syndrome [72]. Known etiological theories associated with pica include gastrointestinal disorders, micronutrient deficiencies, neurological disorders, and obsessive compulsory disorder [73].
Another form of the “psychic” component of geophagy is the consumption of earthy substances associated with magical rituals, be they religious rituals, faith-based efforts to cure illness, or occasions related to the pronouncement of vows [5].
2.5. The Relationship Between Geophagy and Landscape REE Anomalies
To demonstrate convincingly that there is a link between geophagy and landscape REE anomalies, we turn to the published data on the territories of two African countries, Cameroon and Nigeria. We chose Cameroon because it is the only country in Africa for which a more or less detailed map of REE distribution in soils has been compiled. In addition, a relatively large amount of work has been done in this area to study the chemical composition and human consumption of “edible” earths. As for Nigeria, this country is adjacent to Cameroon and has similar rocks and soils, but in a different ratio. While volcanic and ancient metamorphic rocks predominate in Cameroon, Nigeria has a significant proportion of Mesozoic sedimentary rocks and modern alluvial deposits (river alluvium covers large areas in the Lake Chad region and the Niger River valley). Nigeria also has one of the richest histories of geophagy research on the African continent.
First of all, let us locate the main areas of active geophagy in Cameroon and the characteristics of the “edible” earths consumed in its territory, according to the analysis of relevant publications.
Information on the location of the main areas of geophagy and the characteristics of the “edible” earths were obtained from [26,74,75,76]. Some important nuances on the geology of Cameroon were found in [77]. The information about the distribution of rare earth elements in soils throughout the country can be found in the remarkable, one could say unique, work of J.P. Temga et al. [78].
There are three well known areas of extraction and consumption of ‘edible’ earths. The first area, called Sabga, is located 16 km east of Bamenda. The whole range of volcanics from rhyolites to basalts is distributed in this area. Clays are extracted from the weathered crust of trachyte (alkaline rocks of intermediate composition) on the slopes of Bamenda. The major clay minerals are smectite (49–60%) and kaolinite (4–6%). The primary crystals are quartz (19–34%) and feldspar (6–12%), with significant admixtures of goethite (3–6%) and hematite (1–3%). The average particle diameter varies from 2 to 9 microns. In the chemical composition of argillaceous rocks, the share of iron is significant (4.42–7.65%). Among the trace elements, the contents of Zr, Nb, LREE, Y and Zn are increased. The clay is partly consumed by local people, but more is supplied to markets in Nigeria [26,76].
The second area where people from local villages and towns extract and consume “edible” earth called Ediki. The earth is extracted from a railway excavation and is described as an overdeposited silty loam consisting of (in decreasing order of proportion) quartz, kaolinite, mica, microcline, goethite, hematite, anatase, and ilmenite crystals. Quartz of mudstone dimensions is the major component in all samples, while kaolinite content ranges from 7 to 14%. Kaolinite was formed by the weathering of potassium feldspars [74]. The chemical composition of trace elements was not described.
The third area of extraction and consumption of “edible” earths is Moco. These earths are predominantly yellowish in color, muddy and highly plastic. Their pH varies around 5. The mineral composition is in decreasing order: kaolinite, quartz, mica, microcline, goethite, followed by anatase, smectite, hematite, gibbsite in comparable proportions [75]. The clay is consumed by the local population. The chemical composition is also not studied in detail.
This areas of extraction and consumption of “edible” earths in Cameroon are limited to the area with the highest population density, and soils with anomalously high REE contents are also mapped there [78]. It should be noted that high REE contents in soils always coincide with areas of high levels of these elements in vegetation and usually in groundwater, which in Africa is often used by the population for drinking.
Let us now look at some data from [78] on the REE content of Cameroonian soils and the peculiarities of soil enrichment with them. It is important to note that the rock and soil varieties identified in Cameroon are not restricted to Cameroon, in fact, the whole set of rocks and soils characteristic of the entire tropical climatic zone is represented in this country. The soil maps presented in [78] are the result of 604 soil samples collected by thirty collectors throughout Cameroon. The total sampling area was 475,000 km2. Inductively coupled plasma mass spectrometry was used to determine the chemical element content of all samples, including all representatives of the REE group. The results show that the REE content of soils can vary from extremely low to extremely high. Total REE values vary from 0.482 to 5926 mg/kg (five orders of magnitude!) with a median value of 297.42 mg/kg. The total median values of REE, LREE and HREE sums are 297.42, 275.11 and 22.31 mg/kg respectively. Such a large variation in REE content reflects the geological and landscape-climatic diversity of the Cameroonian territory, and indeed of Africa as a whole. This means that soils with both extremely low and extremely high REE contents can be found in Africa. Furthermore, the average REE content of soils in many regions of Africa is much higher (often many times) than that of soils in any other country in the world.
For comparison, the mean values of total REE (mg/kg) in soils of China are 154.6 [79]; Malaysia, 154.51 [80]; Europe, 125.28 [81]; Australia, 104.83 [82]; Brazil, 100.81 [83]; Cuba, 74.19 [84]; Sweden, 89.60 [81]; and Russia, 98.61 [85]. In some areas of Russia, particularly in Yakutia and along the western shore of Lake Baikal, average REE levels in soils can be less than 30 mg/kg [86]. Even lower average REE levels in soils (15 mg/kg) have been found in some areas of Germany [87].
In Cameroonian soils, depending on the type of parent rock, the total REE content decreases in the following order: alkaline igneous rocks (nephelinite, syenite) > carbonatites > basic igneous rocks (alkaline basalts), which are comparable to ancient metamorphic rocks (crystalline schists, amphibolites, gneisses), followed by Quaternary alluvium and acidic igneous rocks. Regardless of the rock type, the REE content increases from fresh to highly weathered. The lowest REE contents in the soil profile occur in the upper horizons, especially in the thickest weathered crusts, which are characteristic of all humid tropical forests, regardless of the type of underlying rock. Such areas in the tropics dominated by thick weathering crusts, often tens of meters thick, can be very extensive. They include the central and lower parts of the Congo and Niger river basins in Africa and the Amazon in South America.
As will be shown further, the most important indicator of REE-related soil quality for us is the content of bioavailable forms of HREE in soils. As we know, the largest HREE deposits found in China are of the ion adsorption type and are restricted to lateritic soils [88]. In Cameroon, the highest HREE enrichment values are also confined to lateritic soils formed mainly on ancient metamorphic rocks of the charnockite series, amphibolites and organogenic limestones with total mean values of 115.60 mg/kg, 79.63 and 82.98, respectively [78].
Let us now briefly review the situation in Nigeria. Various aspects of geophagy have been very actively studied in this country since the mid-1960s, and interest in the subject has grown over time, as evidenced by the impressive but not complete list of publications [17,89,90,91,92,93,94,95,96,97,98,99,100]. According to these authors, geophagy is widespread among different populations living in Nigeria. As elsewhere in Africa, children and pregnant women are the main consumers of “edible” earths. Pregnant women, who are the main users, consume an average of 20 g per day as needed. Such soils are extracted from both natural outcrops and termite mounds. According to [101], many “edible” earth extraction sites are also visited by domestic and wild animals. Most of the clay earthy substances used for human consumption in Nigeria are mined in the river valleys. Laboratory studies of such earths show that kaolinite is the dominant clay mineral. Halloysite, nontronite, palygorskite and illite also occur. The kaolinite content varies from 38 to 72%, quartz from 8 to 40%, with the remainder being feldspars, iron and aluminum oxides. The pH of the consumed clay earths ranges from 2.76 to 7.2.
J.V. Smith et al. [102], citing other authors, reported that there are about 14 million cattle in Nigeria and they are kept in the savannah zones in a pastoral and grazing pattern. Geophagy is unusually common among the animals in the pastures, they mostly eat sand, sometimes clayly termite mounds. The researchers tried to determine the cause of geophagy through chemical analyses of samples of plant food and soil consumed by the animals. The only conclusion they came to was that the animals probably lacked phosphorus.
According to S.M.A. Adelana et al. [103], about half of Nigeria’s territory consists of metamorphic gneiss-migmatite and crystalline schists of the Archaean basement, intruded in places by Jurassic granitoids and Paleogene-Neogene volcanics of contrasting composition, similar to those described above in Cameroon. The rocks of the ancient basement are intruded in places by REE-enriched charnockites (we mentioned such rocks in India above). The vast valleys of the main rivers, the Niger and the Benue, divide the country into three roughly equal parts. Along these valleys are basins filled with Jurassic to modern sedimentary rocks. An ore-bearing zone of sulphide Sn-Ta-Nb mineralization extends along the ancient basement from the southwest through the central part to the northeast of Nigeria. Jurassic granites also contain rare metal-polymetallic sulphide mineralization associated with REE, U and Th-bearing minerals (monazite, zircon, pyrochlore, thorite, fergosonite).
Based on the geological data given, we should expect a range of soils in Nigeria with REE distributions close to those found in Cameroon. Since the climate in this country is a humid tropical one, there should be both positive and negative anomalies of REE contents in soils in this area. Moreover, the areas where there should be a severe deficiency of REE in soils should be much more extensive than in the territory of Cameroon, especially in the Niger estuary, where there are thick layers of highly weathered loose sediments washed by acidic meteoric waters. The Niger estuary and the Congo estuary are undoubtedly the most extensive areas in Africa with the least REE-rich soils. The consequences of this will be clear from the text below.
2.6. Linking Geophagy to Geochemical Endemic Diseases
According to the analysis of published data, geophagy was often associated with one or more diseases among the informants interviewed. The most common of these diseases in Africa are anemia, including sickle cell anemia, digestive disorders, and various forms of mineral metabolism disorders (rickets, osteomalacia), with fluorosis of the skeleton and podoconiosis occurring in some areas. In northern India, the desire for geophagy is often associated with blood disorders, digestive disorders, gall and kidney stones, fluorosis of the teeth, and skeletal pathologies. According to W. Laufer [3], among the cannibals of the island of New Zealand, geophagy was always combined with the consumption of human flesh. This seems to be a fact that has no connection with any disease, but only at first glance. Why this is so will become clear in the following text. Cases of compulsive eating of earthy substances, combined with depression, abdominal distention, characteristic pallor, and worsening weakness, always ending in death, were widespread among black slaves in the New World. In the United States, such a disease was called “cachexia africana” [104]. Later, F.W. Cragin [105] concluded that the tendency to earth-eating was a consequence rather than a cause of this disease. A similar symptomatic disease called endomyocardial fibrosis was not described in Africa until 1947 [106].
2.6.1. Endomyocardial Fibrosis
Endomyocardial fibrosis (EMF) is characterized by the development of fibrous tissue (collagen and elastin) in the endocardium and heart valves [107]. In some patients, fibrosis also develops in the skeletal muscles, leading to their atrophy [108]. The disease is most characteristic of the tropical forest zone, with a predominantly rural population. Most cases have been reported in Africa (Uganda, Côte d’Ivoire, Nigeria, Mozambique), India, and South America (Brazil and Colombia). A large number of reports of EMF disease have also been identified in China, in Guangxi Province [106].
More than half of all EMF cases are diagnosed within the first ten years of a person’s life [109,110], with a second peak incidence occurring in women of childbearing age [111]. The preponderance of cases in adult females was found in Uganda, but more males were affected in Mozambique. In other studies, both sexes were equally affected [106,110,112,113]. There are reports that the disease is common among migrants. For example, in Uganda, immigrants from the neighboring countries of Rwanda and Burundi were found to be the most common among patients [111], while in Mozambique, the highest incidence was found among rural inhabitants of the coastal marine zone [114]. This fact is of particular interest to us because, according to Barakos et al. [115], one of the large alluvial deposits of Congolese monazite is located on the coast of Mozambique. Why this is important will become clear in the following text.
It is believed that the cause of EMF has not been conclusively determined. In the 1980s, a group of health workers from India conducted studies of heart tissue and blood samples from diseased individuals that showed elevated cerium and low magnesium levels compared to control subjects [116,117]. After studying the prevalence of EMF in the state of Kerala in southern India, researchers found that most cases were concentrated in areas with high monazite content in the soil. As a result, it was hypothesized that endomyocardial fibrosis is caused by a combination of elevated dietary cerium levels and magnesium deficiency [116].
The geochemical hypothesis of the origin of the disease was attempted to be tested by C.C. Kartha et al. [118] in experiments on rats. The result also showed the accumulation of Ce in the heart tissue. Another important observation for us, made by Indian researchers, was that the consumption of earthy substances was common among children in Kerala [118].
It should be noted that in the southern states of India with EMF disease, there is a link with animal geophagy. In Kerala, the existence of places visited by wild animals (deer, wild boar, and elephants) to consume earthy substances has been described, particularly in the Chinnar wildlife sanctuary [119]. The wildlife sanctuary is located in the Western Ghats mountain range on the border between the states of Kerala and Tamil Nadu (Figure 1A). Chinnar wildlife sanctuary is dominated by Precambrian metamorphic rocks, predominantly charnockites, the same rocks that are enriched in REEs, most of which are concentrated in monazite [15].
As can be clearly seen in Figure 1A, rivers flow from the mountains in the Chinnar wildlife sanctuary area towards both the state of Tamil Nadu and the sea coast of Kerala. As mentioned above, thick placers of ilmenite-zircon-sillimanite sands with high monazite content are found in the coastal zone of Kerala. All the river valleys along which the weathering products of charnockites have been transported are also filled with sands containing monazite. Locations of monazite in soil samples are shown in Figure 1B. According to Kutty et al. [120], numerous cases of endomyocardial fibrosis have been reported among local residents along the banks and mouths of the transit rivers (Figure 1C). The study of the chemical composition of some forage plants, as well as a number of lianas and palms, conducted in a number of residences of EMF patients revealed elevated levels of REE in them. The content of cerium in cassava tubers was 6 times higher than the local background, and in Dioscorea liana, 15 times higher [121].
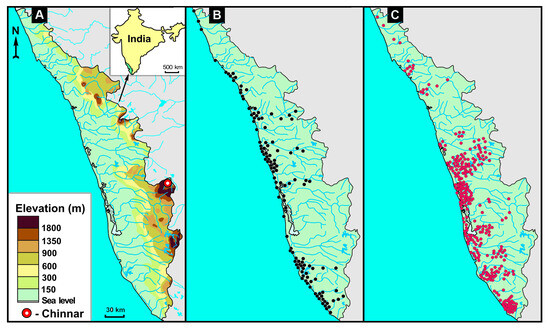
Figure 1.
(A)—Topography and river network in Kerala with the location of Chinnar sanctuary; (B)—Distribution of monazite sand (black dots); and (C)—Locations of 300 EMF patients (red dots) in Kerala (adapted from [122]).
A similar picture of EMF disease association with REE-enriched soils in Africa has only been found in Uganda [107]. Coincidently, the practice of geophagy is very common among the local population in Uganda [123]. In Uganda, W. Smith et al. 1998 [107] surveyed rural areas near the capital city of Kampala and collected soil samples, drinking water samples, and samples of the most important fodder crops grown by the local population for food. Water was collected from hand-dug wells, boreholes, and natural springs. The soils in the study area overlie Precambrian metamorphic rocks (mainly granitogneisses and crystalline schists). Unfortunately, the whole REE spectrum was not analyzed in the collected materials, but only cerium and partially lanthanum, so we can only indirectly assess the content of other REE elements in the samples. According to the data of chemical and microprobe analyses of the soils, it was found out that most of Se and La in them are contained in the fine fraction (about 20 microns). Judging by the chemical composition of individual particles examined by microprobe, the major REE minerals are not monazite, but some secondary, possibly aqueous phosphates or carbonates. In plant samples, elevated Ce concentrations were found in batata and cassava. The highest Ce concentrations were found in beans and green leaf plants, including tea. Ce concentrations in water samples ranged from less than 0.01 to 6.1 µg/L with an average of 0.29 µg/L, which is close to the average content of this element in the water of major rivers of the world [124]. The Ce indicator in water of 6 µg/L corresponds to waters with high sum of REE (from 15 to 20 µg/L), which is hundreds of times higher than the norm for drinking water. At the same time, Ce concentrations in water samples decrease in the following order: hand dug wells–springs–shallow wells–deep wells, which indicates that the main part of REEs is brought from underground horizons. The attempt made by B.G. Rawlins et al. [125] to estimate the bioavailability of cerium in the composition of local soils for humans by means of special laboratory tests showed that the highest bioavailability (24%) is observed in fractions from 1 to 20 microns. Again, the only complaint is that the researchers limited themselves to the determination of only a few RE elements. As shown by Chinese researchers [126], soils formed on the basis of mafic or metamorphic rocks with a high content of mafic minerals are often characterized by enrichment with RE elements of the heavy subgroup.
Based on the results of studies conducted in Uganda on the association of EMF with REE and geophagy, the situation is typical of many countries in Africa. Our comparison of countries in Africa where EMF diseases are prevalent according to [106] with areas where geophagy was historically prevalent in humans showed good convergence is shown in Figure 2. We used the map of geophagy prevalence in Africa from Henry and Cring [127]. We will draw conclusions from the information presented at the end of the article.
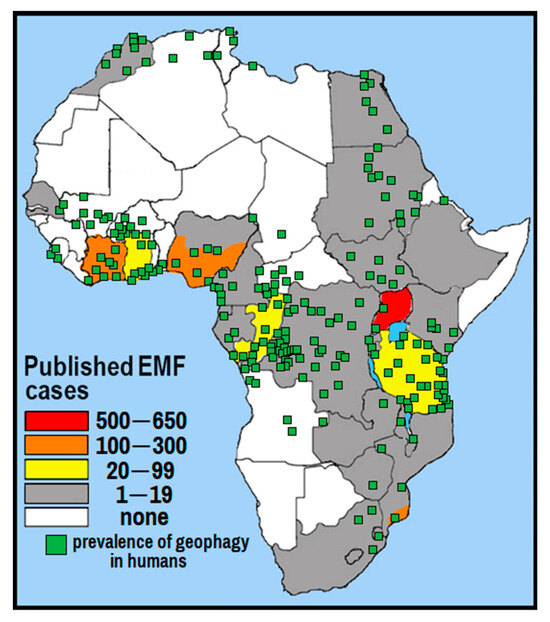
Figure 2.
The incidence of EMF diseases in African countries according to [106] and the prevalence of geophagy in humans (adapted from [127]).
2.6.2. Sickle Cell Disease
Sickle cell disease (SCD) is an inherited blood disorder in which the structure of the hemoglobin protein is disrupted, resulting in a characteristic sickle shape, hence the name. The disease is associated with a mutation in the gene encoding the β-chain of the major type of hemoglobin A, resulting in the synthesis of abnormal hemoglobin S (HbS), in which the sixth position of the β-chain contains valine instead of glutamic acid. Under hypoxic conditions, hemoglobin S polymerizes to form long sickle-shaped strands. Sickle cell anemia occurs when a person inherits two abnormal copies of hemoglobin S, one from each parent. A person with one abnormal copy usually has no symptoms of the disease; such people are called carriers of abnormal hemoglobin. The red blood cells carrying hemoglobin S have a reduced ability to carry oxygen, so SCD patients have increased destruction of red blood cells in the spleen, increased hemolysis, frequent signs of chronic hypoxia, and problems in the hematopoietic system.
It is important to note that under normal circumstances, carriers of the disease show few symptoms. Newborns are usually quite healthy, have a normal weight, and develop normally until about 3–5 months of age. The first signs of the disease in an infant are swelling and pain in the hands or feet and some developmental delay with a refusal to walk due to red blood cells clogging small capillaries. Women with SCD are able to carry and deliver normal babies, but they have an increased risk of complications during pregnancy that can lead to miscarriage, premature delivery, or worsening of the mother’s anemia.
It is almost impossible to describe a typical SCD patient, as the symptoms and their severity vary widely. The anemia that accompanies the disease is caused by an accelerated cycle of red blood cell destruction. Healthy red blood cells typically last 90–120 days, while sickle cells last only 10–20 days. This makes the patient physically less enduring and can cause yellow jaundice associated with excessive breakdown of hemoglobin, whereas periodic blockage of small capillaries in any part of the body can lead to a wide range of different symptoms.
SCD is particularly prevalent in malaria-endemic regions of the world because patients and carriers of abnormal hemoglobin have an increased innate resistance to infection by various strains of malaria plasmodium. This gives them an advantage over people with normal hemoglobin in surviving in areas where malaria is endemic.
As of 2021, SCD affects about 7.7 million people worldwide, directly and indirectly causing approximately 400,000 deaths annually. About 80% of SCD patients are believed to live in sub-Saharan Africa. The distribution of abnormal hemoglobin (HbS) in Africa is shown in Figure 3, adapted from [128].
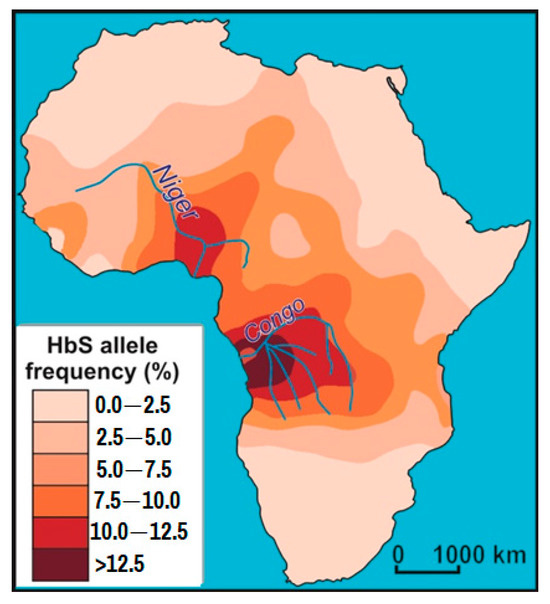
Figure 3.
Frequency of abnormal hemoglobin (HbS) in Africa (adapted from [128]).
A 2006 WHO report estimated that in Nigeria, Africa’s most populous country, sickle cell disease was found in 2% of newborns, or 150,000 children. Uganda has the fifth highest incidence of SCD in Africa. The prevalence rate is 20% of the population. Interestingly, it is Uganda that is home to the Bamba people with a 45% rate of abnormal hemoglobin carriers, which is the highest recorded rate in the world.
SCD disease occurs, although to a lesser extent, in parts of India, southern Europe, western Asia, and in natives of these areas who have migrated to other parts of the world [129].
A. Guimarães et al. [130] investigated the association between the occurrence of pica syndrome, including the consumption of earthy substances, and sickle cell anemia. A comprehensive literature search was conducted in MEDLINE (PubMed), Latin American and Caribbean Center on Health Sciences Information (Bireme), and Google Scholar databases from July 2017 to January 2024. A total of 1487 identified articles were analyzed, in which pica syndrome in the study subjects was associated with irregular dietary patterns, with increased symptoms of the disease, and with micronutrient deficiencies, particularly iron and zinc.
Most of the identified studies found a positive association between sickle cell anemia and pica syndrome, with zinc and iron deficiency identified as major contributors to unusual eating habits. However, it remains unclear whether pica is a cause or a consequence of these micronutrient deficiencies.
It is important to note that laboratory studies have consistently shown abnormalities in patients with pica syndrome. For example, F. Ahmed [131] found that hemoglobin levels were significantly lower in the pica group than in the control group (79.3 ± 18.3 g/L vs. 90.2 ± 28.0 g/L). M.N. Aloni et al. [132] and H.J. Kolthof et al. [133] noted a higher rate of hemolysis in patients with pica syndrome, which explains the lower hemoglobin and hematocrit levels in children with pica syndrome and SCD compared to those without pica syndrome.
When interviewing patients with SCD, many researchers emphasize the state of anxiety that can accompany women throughout pregnancy, potentially leading to maladaptive or dysfunctional behaviors such as pica syndrome [134,135]. It has been suggested that pica occurs during pregnancy as a way of coping with anxiety and stress. Complications in SCD patients, such as preterm birth, low fetal weight and growth, have also been reported [136]. The general conclusion is that the consumption of non-food substances can be considered as an attempt to meet the needs caused by the imbalance of some chemical elements in the body. Psychological or emotional disorders have also been mentioned as causes of pica.
These facts clearly indicate a link between sickle cell anemia and the phenomenon of geophagy. At the end of this article, we will present our view of why such a connection exists and how it relates to rare earth elements.
2.6.3. Prion Diseases (Spongiform Encephalopathies)
Prion diseases, also known as spongiform encephalopathies (SEs), are a spectrum of diseases with overlapping signs and symptoms that primarily affect the brain and nervous system of mammals, including humans. The changes in the brain are expressed in a specific pattern of neuronal death with the formation of many tiny holes, making the brain tissue sponge-like, hence the name.
The characteristics of prion diseases include:
- -
- Possibility of transmission from animal to human and vice versa;
- -
- Long incubation period from 1 year to 20 years or more;
- -
- Absence of symptoms for a long time after infection;
- -
- At the initial stage of the disease there are no signs of inflammation or changes in the blood;
- -
- Extraordinary resistance of the pathogenic agent to physical and chemical influences;
- -
- Prions are believed to be involved in the development of mental disorders and myopathy [137];
- -
- The mechanism of action of prions and their transformation from normal to pathological forms remains unclear.
SE are unique diseases because their etiology can be genetic (hereditary), sporadic (not hereditary) or infectious (through consumption of infected food or non-food substances or, for example, blood transfusion). Most cases of transmission identified to date have been infectious, with healthy humans or animals ingesting tissue from organisms with prion disease. It is important to note that conventional sterilization methods, such as boiling or irradiation of materials, do not destroy infectious prions.
Prion diseases in animals have been known for hundreds of years. For example, a disease with SE characteristics in sheep called scrapie was discussed in the British House of Commons as early as 1755 [138].
In humans, a disease with SE symptoms was first described by Alfons Maria Jakob in 1921 [139]. The most famous and massive case of human SE disease was described by the physicians V. Zigas and D.C. Gajdusek [140] on the island of New Guinea. The fatal disease, called “kuru”, affected Papuan cannibals, mostly children and women, rarely adult men. It turned out that the tribe of about 35,000 people had a tradition of ritual cannibalism in which only women and children ate the brains of enemies or relatives, which was associated with a different incidence according to sex and age.
In 1978, a disease with characteristic symptoms of SE in the brain and a fatal outcome was identified in deer and elk in Colorado (USA). The disease was called chronic wasting disease (CWD) because of its external manifestations of weight loss and general wasting [141]. Subsequent epidemiological studies have shown evidence of CWD in free-ranging ungulates not only in Colorado but also in southeastern Wyoming and western Nebraska.
The pure protein nuclein-free nature of the SE agent in the form of single species molecules was discovered by S.B. Prusiner in 1982. He named the discovered protein as “infectious prion protein” [142]. In mammals, such a protein can exist in two isoforms. One of them, known as PrPC (from Prion Protein Cell), is a normal prion protein, which has a helical shape and is most abundant in neurons and glia. In mammals this protein plays an important role in the protection of neurons and glia from oxidative stress, is involved in the regulation of intracellular calcium (Ca+) in neurons, is also involved in copper metabolism, the maintenance of normal synaptic function and signaling in nervous tissue, but its main function is related to the maintenance of diurnal rhythms in the body (in all its cellular structures).
To date, prion proteins have been found in mammals, including humans, birds and yeast; there is evidence for the existence of prion-like proteins in plants [143]. Obviously, they should be present in all microorganisms, including prokaryotic forms.
The infectious prion protein, known by the abbreviation PrPSc (from the English Scrapie), has a different external shape from the normal one; some of its helical domains are straightened. This process of shape change is called conformational change and involves a change in the spatial structure of the protein molecule, but not in its amino acid composition. In an organism infected with infectious prion protein, the process of accumulation of conformationally altered PrPSc protein molecules is triggered by the reformatting of PrPC molecules.
In the 1980s and 1990s, SE spread epidemically among cattle in the United States and several other countries. This was due to animals being fed bone meal from slaughterhouses. A decade later, as a result of human consumption of animal products containing PrPSc-contaminated tissues, symptoms previously described as Creutzfeldt-Jakob disease (CJD) began to appear en masse in humans. Research confirmed the similarity of prions isolated from CJD patients to those isolated from a cow killed by SE [144]. In the same years, it was firmly established that PrPSc protein is present in almost all organs and tissues of infected animals, with particularly high concentrations in the brain, spinal cord and bone marrow, eyeballs, blood, spleen, endocrine glands, lymph nodes, muscle, intestine, liver and lungs.
Clinical signs of SE in humans usually include psychiatric problems such as depression, confusion or memory problems, there may be persistent insomnia, lack of coordination. In the later stages of the disease, severe mental impairment (dementia) is seen, and patients lose the ability to move and speak. The problem of treating prion diseases is still unresolved [137].
In recent years, the problem of prion diseases in wild ungulates in North America has been actively studied. Among those susceptible to natural CWD infection, white-tailed (Odocoileus virginianus), mule deer (O. hemionus) and black-tailed deer (O. h. columbianus), moose (Alces alces) and introduced red deer (Cervus elaphus) have been identified [141,145]. In Europe, PrPSc-type prions have been detected in free-ranging reindeer (Rangifer tarandus), red deer and elk in Norway, Sweden and Finland [146,147,148]. South Korea reported CWD in captive deer introduced from Canada [149]. It has also been shown that transmission of infectious prions in animals can occur both vertically from mother to offspring and horizontally through direct contact with sick animals or contaminated environmental components [150]. During the preclinical, asymptomatic phase, PrPSc prions can be detected in feces, urine, and saliva as early as 6 months after infection [151]. Symptoms associated with late-stage CWD infection include wasting, excessive salivation, behavioral changes, ataxia, depression, and weakness [141,145]. There is also evidence that PrPSc prions associated with soil are more infectious, suggesting that soil may serve as both an environmental reservoir and a transmission medium for CWD [152].
Relatively recently, a number of American researchers [151,153,154,155] came up with the idea that ungulates can become infected with CWD by ingesting soil and water at natural salt licks (we call them “kudurs”), which can be contaminated with infectious prions by the secretions (saliva, urine, faeces) of sick animals. After exploring such ideas and studies to test them, it became clear to us that there are many kudurs in CWD-endemic areas in the United States. One such area of 23 km2 with 11 kudurs, according to [154], was studied in Rocky Mountain National Park (marked by us as a blue ring on the map from the Internet: “CWD distribution in wild and captive animal populations in North America as of 2023”). This map is shown in Figure 4. And since we already know that most kudurs are concentrated in REE-anomalous landscapes, the CWD endemicity of such areas should also be related, and most likely due to REE anomalies. Unfortunately, we were unable to find the necessary geological information on CWD-endemic areas in the public domain. From the article [154] we could only determine that in the studied area with kudurs, loamy soils are distributed on ancient granitoids, gneisses and shales. In addition, we found a small-scale map scheme with the location of REE deposits of different types in the world, including the territory of the USA. A fragment of such a scheme from [156] for the USA is presented in the inset of Figure 4. The presented information gives reason to consider our hypothesis as very promising. In our previous article [157] we suggested that infectious prion proteins, which can cause sporadic diseases in animals, are produced in bacterial cells as part of the microflora of the digestive tract when there is a long-term excessive intake of REE elements of the heavy subgroup. Having studied the problem of prion diseases in more detail, we have changed our view. We now believe that infectious prion proteins may well be present in the composition of the soil microflora at sites with REE anomalies. We will discuss this issue in more detail in the final section of this article.
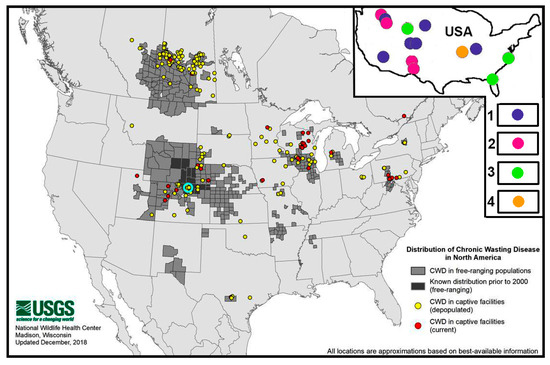
Figure 4.
Distribution of CWD in wild and captive populations in North America in 2023 (adapted from [158]), where the blue ring shows the location of the area with kudurs (adapted from [154]). The inset shows the distribution of REE deposits in the USA according to [156] associated with: 1—carbonatites; 2—alkaline rocks of various types; 3—modern and paleoslides; 4—clayey weathering crusts.
3. Discussion
On the Incompleteness of the Theory of Immunity and the Special Role of Rare Earth Elements in the Neuroimmune-Endocrine System of Animals and Humans
In the previous sections of this article, we have tried to summarize all the published information on human geophagy, on the hypotheses proposed by different authors on the causes of this phenomenon, as well as on the connection of geophagy with some areas of already identified geochemical endemics, which are directly or indirectly associated with REE-anomalous landscapes. By REE-anomalous we mean such landscapes, in the components of which (in natural waters, soils and vegetation) the REE content is higher or lower than the values at which the corresponding endemics in animals and humans caused by such a factor begin to appear.
Our research experience in various aspects of geophagy spans 50 years. For the last 10 years, our studies have been conducted in different regions of Russia: in the expanses of Yakutia [86], in the mountains of the Caucasus [159], southern Siberia and the Far East [160,161,162,163,164], and especially in the mountains of the Sikhote-Alin [165,166], which we carried out in connection with the verification of the “rare-earth hypothesis” we proposed in 2015 [167]. This finally allowed us to establish the link between cases of mass geophagy in wild and domestic animals and REE-anomalous landscapes. The analysis of published information on the effect of various REE elements on the body of animals and humans, based on experiments with laboratory animals and, in particular, on the use of REE in the practice of industrial fattening of animals, allowed us to determine with high probability the set of REE elements contained in the normal mammalian organism. It appears that only La, Se, Pr, Nd, as well as Sc and Y are vital REE for this group of animals. The remaining REEs, including representatives of the heavy subgroup, are pathogenic. Their presence in the body of mammals (including humans), depending on which elements they are and in what quantities, determines the type of endemic disease and the peculiarities of its development. The pathogenicity of HREE is due to the fact that they can replace light REE in various structures of the neuroimmunoendocrine system, but they cannot perform their functions. This leads to disease. But not only that. Diseases are not always associated with the substitution of vital REEs by their heavy analogs; there are cases when the amount of one or another vital REE element in the body is lower or higher than the thresholds, beyond which normal functioning of the neuroimmunoendocrine system and consequently the normal course of many metabolic processes in the body are impossible.
A very important and still poorly understood issue is the bioavailability of REE, i.e., the forms in which these elements can be assimilated by the body. It should be only free ions as part of liquid phases or colloidal systems, or they can only be assimilated as part of organomineral compounds. Analysis of published data on this issue shows that organomineral REE compounds have the greatest assimilative capacity, but can also be assimilated, albeit to a lesser extent, from easily soluble secondary REE minerals. Clearly, the production of easily soluble secondary minerals and organomineral REE compounds under natural conditions is only possible during long periods of chemical and biological weathering of REE-enriched rocks. This explains the lack of direct association of landscape REE anomalies with “fresh” rocks enriched in such elements and young volcanites, and at the same time the presence of numerous landscape REE anomalies in the tropical zone on ancient rocks enriched in REE-rich minerals. Since the most pathogenic of the REE are elements of the heavy subgroup, it follows that at least a part of the geochemical endemism related to geophagy is associated with them.
According to the data [78] discussed above when considering the soils of Cameroon, the most intense HREE anomalies in soil in the tropical zone are concentrated on alkaline rocks of basic and intermediate composition, on some metamorphic rocks of carbonate formation, as well as on rocks with a high content of orthopyroxenes (rocks such as charnokites and some others). The formation of landscape anomalies with an excess of bioavailable forms of HREE at high latitudes is usually confined either to ancient weathering crusts of the same rocks as in the tropics, or to local zones of hydrothermal activity of volcanics of different ages and contrasting composition, mainly in areas of rare metal ores with arsenic and sulphides. The latter is most common in mountainous areas in high latitude climates.
In REE-deficient landscapes, the introduction of vital REE elements of the light subgroup into the body seems to be possible not only from organomineral and easily soluble mineral forms, but also from poorly soluble REE minerals. The absorption of REE in the body from poorly soluble mineral compounds seems to be mediated by specific microorganisms in the digestive microflora.
The geochemical hypothesis of endomyocardial fibrosis proposed by Indian physicians finds more and more confirmation with time, but from our point of view we have managed to see only a small fragment of that “iceberg” which represents the problem of the real involvement of REE in the structures of organisms and the functions performed by them. We are convinced that the main role in the occurrence of EMF diseases is not played by cerium and magnesium, but by some elements of the HREE subgroup, which enter the body of humans with drinking water and food (mainly vegetable) in the places where they live.
The main hypothesis concerning the sickle cell anemia assumes a hereditary mechanism of its spread, but nothing clear has been proposed about the cause and circumstances of the occurrence of this disease. However, it is quite logical to assume that organisms living for a long time in the same REE-anomalous landscapes should adjust to environmental conditions at the level of genotype adaptation. That is why many migrants in Europe and the USA, descendants of indigenous peoples from some countries of the equatorial zone, often persistently retain geophagic habits in foreign lands, and in the second and third generations. And the reason for this is not only cultural and behavioral stereotypes, but first of all the requirement of the body, which is not genetically adapted to live in a new different flow of rare earth elements passing through it. Moreover, migrants may be genetically adapted to both higher and lower levels of REE intake into the body in relation to their level in the new place of residence.
Looking at a map of abnormal hemoglobin (HbS) occurrence in Africa (Figure 3), it is clear that the maximum frequency of this indicator is concentrated at the mouths of the largest rivers in Congo and Niger, both of which are located in the tropical rainforest zone. These are the areas with the thickest weathering crusts and consequently the soils with the lowest REE content. These areas have been inhabited by humans for thousands of years, judging by the fact that they are now among the most densely populated on the African continent. It is logical to assume that in these areas, due to their geochemical peculiarities in terms of REE content, a genetic mutation of hemoglobin appeared at some point, which took hold and began to spread throughout the world.
Landscape REE anomalies undoubtedly affect all types of animals, including soil microorganisms, plasmodia, and viruses. It can be assumed that some of the representatives of the most ancient life forms (most likely, these are some bacteria or archaea) initially use prion proteins of PrSc type, the structural form of which differs from normal mammalian prion proteins. If we accept this hypothesis, then the cases of sporadic forms of transmissible spongiform encephalopathies found in the United States, northern Europe, and the island of New Guinea are actually not rare. Diseases of this type are certainly widespread, and their main foci among both humans and animals should be expected in the tropics. And it is necessary to look for them first of all in areas of active geophagy. We also have no doubt that SWD-infected animals can be detected almost everywhere where there are signs of mass geophagy among animals. It can be noted here that we have repeatedly recorded external evidence characteristic of CWD in red deer using camera traps at kudurs in different areas of the Sikhote-Alin.
Why has geophagy proved to be the key to understanding the specific role of REE in living systems? First of all, because this phenomenon is widespread throughout the world and affects all groups of terrestrial animals, but especially herbivores, whose diet consists of plants—organisms with the most diverse chemical composition, also in terms of REE. To a lesser extent, geophagy is found in omnivores and even less so in carnivores. The main significance of geophagy, as we defined it earlier [157], is the regulation of the composition and concentration of REE in the neuroimmunoendocrine system, which is relevant for any living organism. In landscapes that are deficient in essential REE, this regulation takes place through the consumption of soil substances enriched in LREE. These can be not only clay soils, but also pure sandy soils enriched with REE minerals. Animals sometimes even eat LREE-enriched coal. In landscapes where there is an excess of HREE in groundwater and vegetation, the only natural evolutionary way to regulate the neuroimmunoendocrine system is to consume any natural sorbents that can remove excess HREE from the body. Such sorbents may be mineral, organomineral or purely carbon-based. Mammals most commonly use clay soils, zeolites, finely dispersed siliceous rocks, and less commonly peaty soils and charcoal, choosing sodium-enriched substances whenever possible. In REE-anomalous landscapes, sodium, like lithium, helps to alleviate the state of stress in the body caused by imbalances in the neuroimmunoendocrine system. It should be noted that in sodium-deficient landscapes, the search for sodium for animals, especially hoofed animals, can take on an independent importance and become the main object of the consumed mineral substances. Our research experience shows that cases of geophagy for the sole purpose of finding sodium are much less common than it seems.
People living in REE-anomalous landscapes prefer clays (kaolinite, smectite, hydrous mica) and choose those available to them. Sometimes charcoal, starch and other substances with a sorption effect on HREE ions are used.
In animals with muscular stomachs, the relationship with REE has its own peculiarities. It seems that herbivorous birds smaller than a crow do not suffer from a deficiency of the necessary REE in their organism, because such small animals have a negligible need for such elements. However, the problem of excessive intake of HREE most certainly exists and is practically widespread due to the small size of birds. The choice of sorbents to solve such a problem is mostly limited to quartz and high silica silicates. The mechanism of action of such sorbents in the bodies of birds has already been discussed [157]. In relatively large birds a deficiency of LREE is possible, if we take into account the known cases of their active consumption of clayey rocks in tropical forests of South America and Africa. We also considered this issue in the previous publication.
Geophagy has accompanied humans throughout their evolutionary path, and since the emergence of consciousness, a purely instinctive mechanism of regulation of the neuroimmunoendocrine system began to change under the influence of intelligence and cultural stereotypes. On the whole, geophagy in modern man is an echo, a trace of once dominant instinctive forms of restoration of normal functioning of the body living in conditions of significant pressure of an unfavorable geochemical factor.
Why is the evolution of the regulation of the chemical composition of living organisms by means of geophagy specifically directed towards rare earth elements? In order to answer this question, we need to take a look at some peculiarities of the neuroimmunoendocrine system and the role of rare earth elements in it. But before we do so, let us look at another layer of facts, this time from paleontology, which we believe are also relevant to the problem under consideration.
As early as the 1980s, the study of well-preserved carcasses of ancient animals in the permafrost of Yakutia, in particular Shandrin and Kirgilyakh mammoths and Churapchi woolly rhinoceros, revealed a significant content of fine pelitic mineral fractions in their digestive tracts [168,169]. The prevalence of geophagy among mammoths is also indicated by E.N. Mashchenko [170] on the basis of his excavations of Pleistocene fauna in the area of Shestakovsky Yar. However, the most interesting findings for us were made by S.V. Leshchinsky [171]. His detailed studies of numerous bone remains of representatives of the mammoth fauna show that in the late Pleistocene mammoths en masse showed pathological changes in bones and teeth with characteristic signs of osteodystrophy. Such signs in the bones included osteoporosis, osteofibrosis, osteomalacia, osteolysis, exostoses, fractures with the formation of false joints and lesions of the articular surfaces (cartilage atrophy, ulcers, peripheral erosions, friction grooves, etc.). In most cases, the various destructions accompany each other, which, in the opinion of the author, indirectly points to a common cause of their occurrence—mineral deficiency in the diet. It seems to us that this is not a matter of mineral deficiency. This is most likely to be the case when another ecological catastrophe occurs over vast areas, caused by powerful ashfalls as a result of volcanic emissions of atomized chemically highly active ash material with a high concentration of REE. For example, the Paektusan volcano in the northern part of Korea was characterized by ash falls that covered large areas. Volcanic glass from Paektusan magmas dispersed during eruptions, often transported over hundreds of kilometers, is mainly represented by alkaline trachyandesites and pantellerites, which are characterized by elevated REE contents [172]. The consumption by animals of vegetation with a residue of such ashes inevitably leads to a sharp REE imbalance in the animal body. As a result, the neuroimmunoendocrine system cannot withstand the load and a cascade failure of not only mineral but also general metabolism begins.
Now for our version of the main role of REE in the neuroimmunoendocrine system. At present, the main purpose of the immune system is described as the defense of the body against the invasion by foreign living entities or their components. The mechanisms of this defense are limited to the recognition and destruction of uninvited biogenic “foreigners”. However, some functions are performed by the innate component of immunity and others by the acquired component. We believe that within the neuroimmunoendocrine system, which is broadly unified and inseparable into three components, there is one more component, the role of which is reduced to the recognition of chemical elements entering the body (perhaps it is only metals), the control of their flows and their incorporation into the corresponding spatial and temporal structures of the body (temporal because there is no real space without time, any structures in the body are constantly changing in time). Let’s call this component the “system of chemical defense of the body” (SCDB). This system is most likely based on the unique physical and chemical properties of a number of chemical elements embedded in various structures of the neuroimmunoendocrine system, including membrane prion proteins. The main controlling chemical elements in the SCDB are the rare earth elements, the part of them mentioned above as vital. They probably also include selenium, iodine and a few others.
Some experimental data can be cited as facts indicating the controlling nature of the REE influence on a number of metal atoms in the central nervous system of animals. For example, it has been shown in rats that the use of water-soluble form of Y salts in the diet [173] as well as La salts in the form of a dietary supplement [174] alters the distribution of a number of trace elements (Ca, Zn, Mn, Fe, Ni, Co) in rat brain tissue, which, according to the researchers, ultimately leads to changes in physiological functions. With regard to the influence of REE on mineralization-demineralization processes, data from a US patent [175] on the use of lanthanum for the treatment of a number of bone diseases, including osteoporosis, are worth mentioning, which show the enhancing effect of this element on the processes of bone formation and bone density indicators, as well as on the differentiation of osteoblasts. In addition, C. Knebel [176] showed in his thesis that the addition of low concentrations of REE to pig feed results in a decrease in the calcium/phosphorus ratio in the bones of the animals. According to U. Wehr et al. [177], REE supplementation in rat diets can restore the loss of bone mass associated with postmenopausal osteoporosis.
Specialists in the study of prion diseases in animals have already produced data on the character of the development of the disease in relation to the characteristics of the chemical background on which it develops, which seems to be determined under the influence of REE. For example, Mn has been found to increase the infectivity of scrapie protein transplanted into mice in cultured cells [178], and a higher Mg/Cu ratio in the brains of mice significantly increases the survival time of animals after intracerebral inoculation with CWD [179].
It has already been shown that prions are proteins capable of adopting different structural conformations with significant functional differences, with at least one conformation being self-sustaining [180]. Inherited prion conformations result in profound phenotypic consequences that can be beneficial or detrimental, depending on both the genetic and chemical background. The chemical background appears to be determined by the state of the SCDB, primarily the state of the rare earth component in it, which depends on the influx of a particular set of REE into the body from food, drinking water and the dusty atmosphere.
Thus, developing our hypothesis, we once again draw attention to the fact that as long as the system of chemical defense of the body is functioning adequately, metabolic processes proceed in a normal mode. However, if the influx of rare earth elements into the body for a sufficiently long time does not allow the normal work of the SCDB, there is a failure of the flows of various chemical elements in the body; simultaneously, there are problems with their incorporation into its various structures which leads to geochemical endemics. And not just geochemical endemics. In REE-anomalous landscapes, as mentioned above, many life forms, including bacteria, plasmids and viruses, mutate. This is why REE-anomalous landscapes in Africa are characterized by various diseases due to weakened immunity caused by disruptions in the SCDB. These are not only the widespread diseases associated with impaired mineral metabolism (rickets, osteomalacia, fluoride bone pathologies, various forms of arsenicosis), but also such little-studied diseases characteristic of REE-anomalous landscapes as podoconiosis or elephantiasis, which is most likely of a fungal nature and occurs in people with reduced immunity. These include some forms of cancer, such as the endemic form of Kaposi’s sarcoma. The association of these diseases with REE-anomalous landscapes is evident in the patterns of their distribution across Africa [181]. Areas of podoconiosis disease in Africa are confined to areas where rock formations richest in REE minerals are widespread. As for the prevalence of Kaposi’s sarcoma, this disease spatially coincides well with areas of active geophagy.
If selenium and iodine are found to be involved in the composition of the SCDB, the list of geochemical endemics is significantly extended. It includes Kashin endemics and goiter. It is possible that endemics of all kinds, already identified in the context of biogeochemistry and those yet to be identified, are associated with disturbances in the composition and concentration of elements in the SCDB.
4. Conclusions
Our analysis of published data on human geophagy and manifestations of this phenomenon in the form of “pica”, as well as information on geochemical endemics associated with geophagy and rare earth elements, together with our own research experience, allow us to draw the following conclusions:
- In humans, as in animals, geophagy is a natural, evolutionary form of self-regulation by organisms using natural mineral substances.
- The main purpose of this self-regulation is to maintain the composition and concentration of rare earth elements in the neuroimmunoendocrine system, which is necessary for its normal functioning.
- Only some of the RE-elements are vital for mammals, probably La, Ce, Nd, Pr, Sc and Y, whereas most other elements exhibit pathogenicity.
- The pathogenicity of RE-elements is due to their ability to easily replace vital RE-elements in structures of the neuroimmunoendocrine system, but they cannot adequately perform the desired functions.
- The main function of REEs, which are normally found in the mammalian neuroimmunoendocrine system, and which they perform together with some other elements (possibly including Se, I and some others), is to control the flow of chemical elements (possibly only metals) in the organism, determining the place in the body’s structure for each of them.
- Disturbances in the composition and concentration of essential elements in the mammalian neuroimmunoendocrine system lead to disorders of mineral and general metabolism in the body and, ultimately, to the development of geochemical endemics.
- Most geochemical endemics occur in landscapes with anomalous, i.e., significantly different from background, levels of biologically available REE forms, either deficient or excessive.
Author Contributions
Conceptualization, A.M.P. and N.V.B.; methodology, A.M.P.; investigation, N.V.B. and K.S.G.; writing—original draft preparation, A.M.P. and D.A.S.; writing—review and editing, K.S.G. and D.A.S.; visualization, A.M.P.; funding acquisition, N.V.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the TPU development program Priority 2030.
Data Availability Statement
Data sharing is not applicable (only appropriate if no new data is generated or the article describes entirely theoretical research). No new data were created or analyzed in this study.
Acknowledgments
The author would like to thank I.Yu. Chekryzhov, I.V. Seryodkin, E.A. Vakh, V.V. Ivanov, N.Y. Popov, A.V. Vetoshkina, A.V. Ruslan, T.N. Lutsenko, R.A. Makarevich, and A.S. Kholodov, who participated in the collection and processing of factual information during the landscape geochemical studies in the areas of active geophagy in Siberia and the Russian Far East in the period from 2020 to 2024.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ross, T. Personal Narrative of Travels to the Equinoctial Regiom of America During the Years 1799–1804 by Alexander von Humholdt and Aime Bonpland; Routledge: London, UK, 1895; Volume 2. [Google Scholar]
- Hooper, D.; Mann, H.H. Earth eating and the earth eating habit in India. Mem. Asiat. Soc. Bengal Calcutta 1906, 1, 249–270. [Google Scholar]
- Laufer, B. Geophagy Publications of the Field Museum of Natural History. Anthropol. Ser. 1930, 18, 101–198. [Google Scholar]
- Sternberg, L.Y. Gilyaks, Orochs, Golds, Negidals, Ainu; Dalgiz: Habarovsck, Russia, 1933; 740p. [Google Scholar]
- Anell, B.; Lagercrantz, S. Gefagical customs. Stud. Ethnogr. Ups. 1958, 17, 98. [Google Scholar]
- Vermeer, D.E. Geophagical clays from the Benin area of Nigeria: A major source for the markets of West Africa. Nat. Geogr. Soc. Res. Rep. 1984, 16, 705–711. [Google Scholar]
- Browman, D.L.; Gundersen, J.N. Altiplano Comestible Earths: Prehistoric and Historic Geophagy of Highland Peru and Bolivia. Geoarchaeol. Int. J. 1993, 8, 413–425. [Google Scholar] [CrossRef]
- Rowland, M.J. Geophagy: An Assessment of Implications for the Development of Australian Indigenous Plant Processing Technologies. Aust. Aborig. Studies 2002, 1, 51–66. Available online: https://www.thefreelibrary.com/Geophagy:+an+assessment+of+implications+for+the+development+of...-a0127621929 (accessed on 3 July 2025).
- Wiley, A.S.; Katz, S.H. Geophagy in pregnancy: A test of a hypothesis. Curr. Anthropol. 1998, 39, 532–545. [Google Scholar]
- Mills, A.; Milewski, A. Geophagy and nutrient supplementation in the Ngorongoro Conservation Area, Tanzania, with particular reference to selenium, cobalt and molybdenum. J. Zool. 2007, 271, 110–118. [Google Scholar] [CrossRef]
- Placek, C.D.; Hagen, E.H. A Test of Three Hypotheses of Pica and Amylophagy Among Pregnant Women In Tamil Nadu, India. Am. J. Hum. Biol. 2013, 25, 803–813. [Google Scholar] [CrossRef]
- Placek, C.D. Ethnomedical and Sociocultural Factors of Pica Substances in Rural South India. Ecol. Food Nutr. 2017, 56, 238–255. [Google Scholar] [CrossRef] [PubMed]
- Voros, J.; Mahaney, W.C.; Milner, M.W. Geophagy by the Bonnet Macaques (Macaca radiata) of Southern India: A Preliminary Analysis. Primates 2001, 42, 327–344. [Google Scholar] [CrossRef]
- Mahandran, V.; Raghuram, H.; Nathan, P.T. Geophagy by the Indian short-nosed fruit bat, Cynopterus sphinx (Pteropodidae) while foraging on Madhuca latifolia (Sapotaceae) in Tamil Nadu, South India. Acta Ethologica 2016, 19, 95–99. [Google Scholar] [CrossRef]
- Anitha, J.K.; Joseph, S.; Rejith, R.G.; Sundararajan, M. Monazite chemistry and its distribution along the coast of Neendakara-Kayamkulam belt, Kerala, India. SN Appl. Sci. 2020, 2, 812. [Google Scholar] [CrossRef]
- Gebel, A.D. About earthy substances used for food in Persia. In Notes of the Imperial Academy of Sciences; Imperial Academy of Sciences: St. Petersburg, Russia, 1862; Volume 2, pp. 126–135. [Google Scholar]
- Vermeer, D.E.; Ray, E.; Ferrell, J. Nigerian Geophagical Clay: A Traditional Antidiarrheal Pharmaceutical. Science 1985, 227, 634–636. [Google Scholar] [CrossRef] [PubMed]
- Ferrell, R.E.; Vermeer, D.E.; LeBlanc, W.S. Chemical and mineralogical composition of geophagical materials. Trace Subst. Environ. Health 1985, XIX, 47–55. [Google Scholar]
- Mahaney, W.C.; Hancock, R.G.V. Geochemical analysis of African buffalo geophagic sites and dung on Mount Kenya, East Africa. Mammalia 1990, 54, 25–32. [Google Scholar] [CrossRef]
- Mahaney, W.C.; Milner, M.W.; Sunmugadas, K.; Hancock, R.G.V.; Aufreiter, S.; Wrangham, R.; Pier, H.W. Analysis of geophagy soils in Kibale Forest, Uganda. Primates 1997, 38, 159–176. [Google Scholar] [CrossRef]
- Mahaney, W.C.; Zippin, J.; Hancock, R.G.V.; Aufreiter, S.; Campbell, S.; Malloch, D.; Wink, M.; Huffman, M.A. Chemistry, mineralogy and microbiology of termite mound soils eaten by the chimpanzees of the Mahale mountains, Western Tanzania. J. Trop. Ecol. 1999, 15, 565–588. [Google Scholar] [CrossRef]
- Mahaney, W.C.; Milner, M.W.; Muliono, H.; Hancock, R.G.V.; Aufreiter, S. Mineral and chemical analyses of soil eaten by humans in Indonesia. Int. J. Environ. Health Res. 2000, 10, 93–109. [Google Scholar] [CrossRef]
- Wilson, M.J. Clay mineralogical and related characteristics of geophagic materials. J. Chem. Ecol. 2003, 29, 1525–1547. [Google Scholar] [CrossRef]
- Young, S.L.; Wilson, J.; Hillier, S.; Delbos, E.; Ali, S.M.; Stoltzfus, R.J. Differences and Commonalities in Physical, Chemical and Mineralogical Properties of Zanzibari Geophagic Soils. J. Chem. Ecol. 2010, 36, 129–140. [Google Scholar] [CrossRef]
- Ngole, V.M.; Ekosse, G.E. Physico-chemistry, mineralogy, geochemistry and nutrient bioaccessibility of geophagic soils from Eastern Cape, South Africa. Sci. Res. Essays 2012, 7, 1319–1331. [Google Scholar] [CrossRef]
- Kenne Kalguem, E.D.; Wouatong, A.S.L.; Njopwouo, D.; Ekosse, G. Physico-Chemical Characterization of Clayey Materials Consumed by Geophagism in Locality of Sabga (North-Western Cameroon): Health Implications. Int. J. Appl. Sci. Technol. 2018, 8, 57–68. [Google Scholar] [CrossRef]
- Young, S.L.; Sherman, P.W.; Lucks, J.B.; Pelto, G.H. Why on earth?: Evaluating hypotheses about the physiological functions of human geophagy. Q. Rev. Biol. 2011, 86, 97–120. [Google Scholar] [CrossRef]
- Young, S.L.; Miller, J.D. Medicine beneath your feet: A biocultural examination of the risks and benefits of geophagy. Clays Clay Miner. 2019, 67, 81–90. [Google Scholar] [CrossRef]
- Saathoff, E.; Olsen, A.; Kvalsvig, J.D.; Geissler, P.W. Geophagy and its association with geohelminth infection in rural schoolchildren from northern KwaZulu-Natal, South Africa. Trans. R. Soc. Trop. Med. Hyg. 2002, 96, 485–490. [Google Scholar] [CrossRef]
- Nchito, M.; Geissler, P.W.; Mubila, L.; Friis, H.; Olsen, A. Effects of iron and multimicronutrient supplementation on geophagy: A two-by-two factorial study among Zambian schoolchildren in Lusaka. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 218–227. [Google Scholar] [CrossRef]
- Geissler, P.W.; Mwaniki, D.; Thiong’o, F.; Friis, H. Geophagy as a risk factor for geohelminth infections: A longitudinal study of Kenyan primary schoolchildren. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 7–11. [Google Scholar] [CrossRef]
- Luoba, A.I.; Wenzel Geissler, P.; Estambale, B.; Ouma, J.H.; Alusala, D.; Ayah, R.; Mwaniki, D.; Magnussen, P.; Friis, H. Earth-eating and reinfection with intestinal helminths among pregnant and lactating women in western Kenya. Trop. Med. Int. Health 2005, 10, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.; Padilha, P.d.C.; Della Líbera, B.; Nogueira, J.L.; Oliveira, L.M.d.; Astulla, Á. Pica: Epidemiology and association with pregnancy complications. Rev. Bras. De Ginecol. E Obs. 2009, 31, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Halsted, J.A. Geophagia in man: Its nature and nutritional effects. Am. J. Clin. Nutr. 1968, 21, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Kambunga, S.N.; Candeias, C.; Hasheela, I.; Mouri, H. Review of the nature of some geophagic materials and their potential health effects on pregnant women: Some examples from Africa. Environ. Geochem. Health 2019, 41, 2949–2975. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, C.B.; Chandrajith, R. Introduction to Medical Geology; Springer science & business media: Durham, NC, USA, 2009. [Google Scholar] [CrossRef]
- Lakudzala, D.; Khonje, J. Nutritive potential of some ‘edible’soils in Blantyre city, Malawi. Malawi Med. J. 2011, 23, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.W. Kula Udongo (earth eating habits): A social and cultural practice among Chagga Women on the slopes of Mount Kilimanjaro. Indilinga Afr. J. Indig. Knowl. Syst. 2002, 1, 19–25. [Google Scholar] [CrossRef]
- Benza, S.; Liamputtong, P. Pregnancy, childbirth and motherhood: A meta-synthesis of the lived experiences of immigrant women. Midwifery 2014, 30, 575–584. [Google Scholar] [CrossRef]
- Cousik, R.; Hickey, M.G. Pregnancy and childbirth practices among immigrant women from India:“Have a healthy baby”. Pregnancy Childbirth 2016, 4, 18–2016. [Google Scholar] [CrossRef]
- Madziva, C.; Chinouya, M.J. Clay Ingestion during pregnancy among Black African Women in a North London Borough: Understanding cultural meanings, integrating indigenous and biomedical knowledge systems. Front. Sociol. 2020, 5, 20. [Google Scholar] [CrossRef]
- Madziva, C.; Chinouya, M.J. African migrant women acquisition of clay for ingestion during pregnancy in London: A call for action. Public Health 2023, 223, 110–116. [Google Scholar] [CrossRef]
- Traugott, M.T.; Singh, M.; Raj, D.K.; Kutalek, R. Geophagy in India: A qualitative exploratory study on motivation and perception of female consumers. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 123–130. [Google Scholar] [CrossRef]
- Vermeer, D.E. Geophagy among the Ewe of Ghana. Ethnology 1971, 10, 56–72. [Google Scholar] [CrossRef]
- Van Huis, A. Cultural significance of termites in sub-Saharan Africa. J. Ethnobiol. Ethnomedicine 2017, 13, 8. [Google Scholar] [CrossRef]
- Walker, A.; Walker, B.; Jones, J.; Verardi, M.; Walker, C. Nausea and vomiting and dietary cravings and aversions during pregnancy in South African women. BJOG Int. J. Obstet. Gynaecol. 1985, 92, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Luoba, A.I.; Geissler, P.W.; Estambale, B.; Ouma, J.H.; Magnussen, P.; Alusala, D.; Ayah, R.; Mwaniki, D.; Friis, H. Geophagy among pregnant and lactating women in Bondo District, western Kenya. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 734–741. [Google Scholar] [CrossRef]
- Foti, F.L. The Possible Nutritional/Medicinal Value of Some Termite Mounds Used by Aboriginal Communities of Nauiyu Nambiyu (Daly River) and Elliott of the Northern Territory, with an Emphasis on Mineral Elements. Master’s Thesis, Charles Darwin University, Brinkin, NT, Australia, 1994. [Google Scholar] [CrossRef]
- Hunter, J.M. Geophagy in Africa and in the United States: A culture-nutrition hypothesis. Geogr. Rev. 1973, 63, 170–195. [Google Scholar] [CrossRef]
- Çavdar, A.O.; Arcasoy, A.; Cin, S.; Babacan, E.; Gözdasoğlu, S. Geophagia in Turkey: Iron and zinc deficiency, iron and zinc absorption studies and response to treatment with zinc in geophagia cases. Prog. Clin. Biol. Res. 1983, 129, 71–97. [Google Scholar] [PubMed]
- Prasad, A.S. Recognition of zinc-deficiency syndrome. Nutrition 2001, 17, 67–69. [Google Scholar] [CrossRef]
- Sayers, G.; Lipschitz, D.A.; Sayers, M.; Seftel, H.C.; Bothwell, T.H.; Charlton, R. Relationship between pica and iron nutrition in Johannesburg black adults. S. Afr. Med. J. 1974, 48, 1655–1660. [Google Scholar]
- Bhalla, J.; Khanna, P.; Srivastava, J.; Sur, B.; Bhalla, M. Serum zinc level in pica. Indian Pediatr. 1983, 20, 667–670. [Google Scholar]
- Chen, X.-C.; Yin, T.-A.; He, J.-S.; Ma, Q.-Y.; Han, Z.-M.; Li, L.-X. Low levels of zinc in hair and blood, pica, anorexia, and poor growth in Chinese preschool children. Am. J. Clin. Nutr. 1985, 42, 694–700. [Google Scholar] [CrossRef]
- Lofts, R.H.; Schroeder, S.; Maier, R. Effects of serum zinc supplementation on pica behavior of persons with mental retardation. Am. J. Ment. Retard. AJMR 1990, 95, 103–109. [Google Scholar] [PubMed]
- Dreyer, M.J.; Chaushev, P.G.; Gledhill, R.F. Biochemical investigations in geophagia. J. R. Soc. Med. 2004, 97, 48. [Google Scholar] [CrossRef]
- Hooda, P.S.; Henry, C.J.K.; Seyoum, T.A.; Armstrong, L.D.M.; Fowler, M.B. The potential impact of soil ingestion on human mineral nutrition. Sci. Total Environ. 2004, 333, 75–87. [Google Scholar] [CrossRef]
- Abrahams, P.W.; Follansbee, M.H.; Hunt, A.; Smith, B.; Wragg, J. Iron nutrition and possible lead toxicity: An appraisal of geophagy undertaken by pregnant women of UK Asian communities. Appl. Geochem. 2006, 21, 98–108. [Google Scholar] [CrossRef]
- Edwards, A.A.; Mathura, C.B.; Edwards, C.H. Effects of maternal geophagia on infant and juvenile rats. J. Nat. Med. Assoc. 1983, 75, 895–902. [Google Scholar]
- González, R.; Sánchez de Medina, F.; Martínez-Augustin, O.; Nieto, A.; Gálvez, J.; Risco, S.; Zarzuelo, A. Anti-inflammatory effect of diosmectite in hapten-induced colitis in the rat. Br. J. Pharmacol. 2004, 141, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Barr, M. Adsorption studies on clays II. The adsorption of bacteria by activated attapulgite, halloysite, and kaolin. J. Am. Pharm. Assoc. 1957, 46, 490–492. [Google Scholar] [CrossRef]
- Gilardi, J.D.; Duffey, S.S.; Munn, C.A.; Tell, L. Biochemical functions of geophagy in parrots: Detoxification of dietary toxins and cytoprotective effects. J. Chem Ecol. 1999, 25, 897–922. [Google Scholar] [CrossRef]
- Lipson, S.M.; Stotzky, G. Adsorption of reovirus to clay minerals: Effects of cation-exchange capacity, cation saturation, and surface area. Appl. Environ. Microbiol. 1983, 46, 673–682. [Google Scholar] [CrossRef]
- Ngole, V.; Ekosse, G.; De Jager, L.; Songca, S. Physicochemical characteristics of geophagic clayey soils from South Africa and Swaziland. Afr. J. Biotechnol. 2010, 9, 36. [Google Scholar] [CrossRef]
- Seim, G.L.; Ahn, C.I.; Bodis, M.S.; Luwedde, F.; Miller, D.D.; Hillier, S.E.; Tako, E.C.; Glahn, R.P.; Young, S.L. Bioavailability of iron in geophagic earths and clay minerals, and their effect on dietary iron absorption using an in vitro digestion. Food Funct. 2013, 4, 1263–1270. [Google Scholar] [CrossRef]
- Levy, G. Gastrointestinal clearance of drugs with activated charcoal. N. Engl. J. Med. 1982, 307, 676–678. [Google Scholar] [CrossRef]
- Ditter, B.; Urbaschek, R.; Urbaschek, B. Ability of various adsorbents to bind endotoxins in vitro and to prevent orally induced endotoxemia in mice. Gastroenterology 1983, 84, 1547–1552. [Google Scholar] [CrossRef]
- Cooney, D.O.; Struhsaker, T.T. Adsorptive capacity of charcoals eaten by Zanzibar red colobus monkeys: Implications for reducing dietary toxins. Int. J. Primatol. 1997, 18, 235–246. [Google Scholar] [CrossRef]
- Struhsaker, T.T.; Cooney, D.O.; Siex, K.S. Charcoal Consumption by Zanzibar Red Colobus Monkeys: Its Function and Its Ecological and Demographic Consequences. Int. J. Primatol. 1997, 18, 61–72. [Google Scholar] [CrossRef]
- Kikouama, O.J.R.; Balde, L. From edible clay to a clay-containing formulation for optimization of oral delivery of some trace elements: A review. Int. J. Food Sci. Nutr. 2010, 61, 803–822. [Google Scholar] [CrossRef]
- Danford, D.E. Pica and nutrition. Annu. Rev. Nutr. 1982, 2, 303–322. [Google Scholar] [CrossRef]
- Williams, D.E.; McAdam, D. Assessment, behavioral treatment, and prevention of pica: Clinical guidelines and recommendations for practitioners. Res. Dev. Disabil. 2012, 33, 2050–2057. [Google Scholar] [CrossRef]
- Chalker, A.E. The psychopathology of Pica: Etiology, assessment, and treatment. Inq. J. 2017, 9, 2. [Google Scholar]
- Diko, M.; Ekosse, G.; Ayonghe, S.; Ntasin, E. Physical and geotechnical characterization of unconsolidated sediments associated with the 2005 Mbonjo landslide, Limbe, Cameroon. Int. J. Phys. Sci. 2012, 7, 2784–2790. [Google Scholar] [CrossRef][Green Version]
- Diko, M.; Ekosse, G. Soil ingestion and associated health implications: A physicochemical and mineralogical appraisal of geophagic soils from Moko, Cameroon. Stud. Ethno-Med. 2014, 8, 83–88. [Google Scholar] [CrossRef]
- Kenne Kalguem, E.D.; Wouatong, A.S.L.; Njopwouo, D.; Kemteu, C.S.; Ekosse, G. Geophagic Clayey Materials of Sabga Locality (North West Cameroon): Genesis and Medical Interest. Earth Sci. 2019, 8, 45–59. [Google Scholar] [CrossRef]
- Tsafack, J.P.F.; Wandji, P.; Bardintzeff, J.-M.; Bellon, H.; Guillou, H. The Mount Cameroon stratovolcano (Cameroon volcanic line, Central Africa): Petrology, geochemistry, isotope and age data. Geochem. Mineral. Petrol. 2009, 47, 65–78. [Google Scholar]
- Temga, J.P.; Sababa, E.; Mamdem, L.E.; Ngo Bijeck, M.L.; Azinwi, P.T.; Tehna, N.; Zame, P.Z.O.; Onana, V.L.; Nguetnkam, J.P.; Bitom, L.D.; et al. Rare earth elements in tropical soils, Cameroon soils (Central Africa). Geoderma Reg. 2021, 25, e00369. [Google Scholar] [CrossRef]
- Hu, Z.; Haneklaus, S.; Sparovek, G.; Schnug, E. Rare earth elements in soils. Commun. Soil Sci. Plant Anal. 2006, 37, 1381–1420. [Google Scholar] [CrossRef]
- Mohamad, H.; Rafek, A.G. The distribution of rare earth elements in tropical granitic soil: A case study from Malaysia. J. SE Asian Earth Sci. 1993, 8, 617–625. [Google Scholar] [CrossRef]
- Sadeghi, M.; Morris, G.A.; Carranza, E.J.M.; Ladenberger, A.; Andersson, M. Rare earth element distribution and mineralization in Sweden: An application of principal component analysis to FOREGS soil geochemistry. J. Geochem. Explor. 2013, 133, 160–175. [Google Scholar] [CrossRef]
- Diatloff, E.; Asher, C.; Smith, F. Concentrations of rare earth elements in some Australian soils. Soil Res. 1996, 34, 735–747. [Google Scholar] [CrossRef]
- da Silva, Y.J.A.B.; do Nascimento, C.W.A.; da Silva, Y.J.A.B.; Biondi, C.M.; Silva, C.M.C.A.C. Rare earth element concentrations in Brazilian benchmark soils. Rev. Bras. Ciência Solo 2016, 40, e0150413. [Google Scholar] [CrossRef]
- Alfaro, M.R.; do Nascimento, C.W.A.; Biondi, C.M.; da Silva, Y.J.A.B.; da Silva, Y.J.A.B.; de Aguiar Accioly, A.M.; Montero, A.; Ugarte, O.M.; Estevez, J. Rare-earth-element geochemistry in soils developed in different geological settings of Cuba. Catena 2018, 162, 317–324. [Google Scholar] [CrossRef]
- Kotelnikova, A.; Rogova, O.; Stolbova, V. Lanthanides in the soil: Routes of entry, content, effect on plants, and genotoxicity (a review). Eurasian Soil Sci. 2021, 54, 117–134. [Google Scholar] [CrossRef]
- Panichev, A.M.; Baranovskaya, N.V.; Seryodkin, I.V.; Okhlopkov, I.M.; Mamaev, N.V.; Chekryzhov, I.Y.; Zhornyak, L.V.; Vakh, E.A.; Ivanov, V.V.; Lutsenko, T.N.; et al. Geophagy among wild ungulates in Yakutia landscapes with low rare earth element contents. Bull. Tomsk. Polytech. Univ. Georesources Eng. 2025, 336, 194–216. [Google Scholar] [CrossRef]
- Markert, B.; De Li, Z. Natural background concentrations of rare-earth elements in a forest ecosystem. Sci. Total Environ. 1991, 103, 27–35. [Google Scholar] [CrossRef]
- Vieira, C.C.; Botelho, N.F.; Garnier, J. Geochemical and mineralogical characteristics of REEY occurrences in the Mocambo Granitic Massif tin-bearing A-type granite, central Brazil, and its potential for ion-adsorption-type REEY mineralization. Ore Geol. Rev. 2019, 105, 467–486. [Google Scholar] [CrossRef]
- Vermeer, D.E. Agricultural and Dietary Practices Among the Tiv, Ibo and Birom Tribes, Nigeria. Ph.D Thesis, University of Berkeley, Berkeley, CA, USA, 1964. [Google Scholar]
- Vermeer, D.E. Geophagy among the Tiv of Nigeria. Ann. Assoc. Am. Geogr. 1966, 56, 197–204. [Google Scholar] [CrossRef]
- Izugbara, C.O. The cultural context of geophagy among pregnant and lactating Ngwa women of Southeastern Nigeria. Afr. Anthropol. 2003, 10, 180–199. [Google Scholar] [CrossRef]
- Davies, T.; Lar, U.; Solomon, A.; Abraham, P. Mineralogy and geochemistry of geophagic materials consumed in Jos-Plateau State of Nigeria. Pap. Present. Int. Conf. S. Afr. 2008. [Google Scholar]
- Eigbike, C.; Nfor, B.; Imasuen, I. Physicochemical investigations and health implications of geophagial clays of Edo State, Mid-Western Nigeria. J. Geol. Geosci. 2013, 3, 2. [Google Scholar] [CrossRef]
- Odewumi, S. Mineralogy and geochemistry of geophagic clays from share area, Northern Bida Sedimentary Basin, Nigeria. J. Geol. Geosci. 2013, 2, 108. [Google Scholar] [CrossRef]
- Lar, U.; Agene, J.; Umar, A. Geophagic clay materials from Nigeria: A potential source of heavy metals and human health implications in mostly women and children who practice it. Environ. Geochem. Health 2015, 37, 363–375. [Google Scholar] [CrossRef]
- Okunlola, O.; Owoyemi, K. Compositional characteristics of geophagic clays in parts of Southern Nigeria. Earth Sci. Res. 2015, 4, 1. [Google Scholar] [CrossRef]
- Ekenedo, G.O.; Okereke, C.B. Pattern of Geophagy Among Inhabitants of Owerri, Nigeria. Int. J. Dev. Res. 2018, 8, 20157–20163. [Google Scholar]
- Asowata, I.T. Geophagic clay around Uteh-Uzalla near Benin: Mineral and trace elements compositions and possible health implications. SN Appl. Sci. 2021, 3, 569. [Google Scholar] [CrossRef]
- Olajide-Kayode, J.O.; Kolawole, T.O.; Oyaniran, O.O.; Mustapha, S.O.; Olatunji, A.S. Potentially Harmful Element toxicity in Geophagic clays consumed in parts of southeastern Nigeria. J. Trace Elem. Miner. 2023, 4, 100050. [Google Scholar] [CrossRef]
- Ojo, O.J.; Adepoju, S.A.; Awe, A.; Adedoyin, A.D.; Abdulraman, S.O.; Omoyajowo, B.T. Physicochemical and mineralogical composition studies of clays from Share and Tshonga areas, Northern Bida Basin, Nigeria: Implications for Geophagia. Open Geosci. 2024, 16, 20220507. [Google Scholar] [CrossRef]
- Ajibade, O.M.; Oladipupo, S.D.; Osobamiro, T.M.; Bankole, J.K. Geophagic clay predominance and its possible health implications in the south-east region of Nigeria. J. Sustain. Sci. Manag. 2022, 17, 97–117. [Google Scholar] [CrossRef]
- Smith, J.W.; Adebowale, E.; Ogundola, F.; Taiwo, A.; Akpavie, S.; Larbi, A.; Jabbar, M. Influence of minerals on the aetiology of geophagia in periurban dairy cattle in the derived savannah of Nigeria. Trop. Anim. Health Prod. 2000, 32, 315–327. [Google Scholar] [CrossRef]
- Adelana, S.; Olasehinde, P.; Bale, R.; Vrbka, P.; Edet, A.; Goni, I. An overview of the geology and hydrogeology of Nigeria. In Applied Groundwater Studies in Africa; CRC Press: Boca Raton, FL, USA, 2008; pp. 181–208. [Google Scholar]
- Mason, D. On atrophia a ventriculo (mal d’Estomac) or dirt-eating. Edinb. Med. Surg. J. 1833, 39, 95–289. [Google Scholar]
- Cragin, F.W. Observations on Cachexia Africana or dirt-eating. Am. J. Med. Sci. 1836, 17, 356–364. [Google Scholar] [CrossRef]
- Bukhman, G.; Ziegler, J.; Parry, E. Endomyocardial Fibrosis: Still a Mystery after 60 Years. PLoS Neglected Trop. Dis. 2008, 2, e97. [Google Scholar] [CrossRef]
- Smith, B.; Chenery, S.R.N.; Cook, J.M.; Styles, M.T.; Tiberindwa, J.V.; Hampton, C.; Freers, J.; Rutakinggirwa, M.; Sserunjogi, L.; Tomkins, A.; et al. Geochemical and environmental factors controlling exposure to cerium and magnesium in Uganda. J. Geochem. Explor. 1998, 65, 1–15. [Google Scholar] [CrossRef]
- Freers, J.; Masembe, V.; Schmauz, R.; Mayanja-Kizza, H. Endomyocardial fibrosis syndrome in Uganda. Lancet 2000, 355, 1994–1995. [Google Scholar] [CrossRef] [PubMed]
- Somers, K. Restrictive Cardiomyopathies. In Pediatric Cardiology International Congress Series 906; Pongpanich, B., Sueblinvong, V., Vongprateep, C., Eds.; Excerpta Medica: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Mocumbi, A.O.; Ferreira, M.B.; Sidi, D.; Yacoub, M.H. A population study of endomyocardial fibrosis in a rural area of Mozambique. N. Engl. J. Med. 2008, 359, 43–49. [Google Scholar] [CrossRef]
- Rutakingirwa, M.; Ziegler, J.L.; Newton, R.; Freers, J. Poverty and eosinophilia are risk factors for endomyocardial fibrosis (EMF) in Uganda. Trop. Med. Int. Health 1999, 4, 229–235. [Google Scholar] [CrossRef]
- Connor, D.H.; Somers, K.; Hutt, M.S.; Manion, W.C.; D’Arbela, P.G. Endomyocardial fibrosis in Uganda (Davies’ disease). Part I: An epidemiologic, clinical, and pathologic study. Am. Heart J. 1967, 74, 687–709. [Google Scholar] [CrossRef]
- Davies, J.; Spry, C.; Vijayaraghavan, G.; De Souza, J. A comparison of the clinical and cardiological features of endomyocardial disease in temperate and tropical regions. Postgrad. Med. J. 1983, 59, 179–183. [Google Scholar] [CrossRef][Green Version]
- Ferreira, B.; Matsika-Claquin, M.; Hausse-Mocumbi, A.; Sidi, D.; Paquet, C. Geographic origin of endomyocardial fibrosis treated at the central hospital of Maputo (Mozambique) between 1987 and 1999. Bull. Soc. Pathol. Exot. 2002, 95, 276–279. [Google Scholar] [PubMed][Green Version]
- Barakos, G.; Mischo, H.; Gutzmer, J. Status quo and future evaluations of global rare earth mining (with respect to special rare earth element-industry criteria). In Proceedings of the Third International Future Mining Conference, Sydney, Australia, 4–6 November 2015. [Google Scholar][Green Version]
- Valiathan, M.S.; Kartha, C.C.; Eapen, J.T.; Dang, H.S.; Sunta, C.M. A geochemical basis for endomyocardial fibrosis. Cardiovasc. Res. 1989, 23, 647–648. [Google Scholar] [CrossRef]
- Eapen, J.; Kartha, C.; Valiathan, M. Cerium levels are elevated in the serum of patients with endomyocardial fibrosis (EMF). Biol. Trace Elem. Res. 1997, 59, 41–44. [Google Scholar] [CrossRef]
- Kartha, C.C.; Valiathan, M.S.; Eapen, J.T.; Rathinam, K.; Kumary, T.V.; Kutty, V.R. Enhancement of cerium levels and associated myocardial lesions in hypomagnesaemic rats fed on cerium-adulterated diet. In Endomyocardial Fibrosis; Valiathan, M.S., Somers, K., Kartha, C.C., Eds.; Oxford University Press: Dehli, India, 1993; pp. 243–253. [Google Scholar]
- Ramachandran, K.K.; Balagopalan, M.; Vijayakumaran Nayr, P. Use Pattern and Chemical Characterization of the Natural Salt Licks in Chinnar Wildlife Sanctuary (Research Report 94); Kerala Forest Research Institute Peechi: Thrissur, India, 1995; 18p. [Google Scholar]
- Kutty, V.R.; Abraham, S.; Kartha, C.C. Geographical distribution of endomyocardial fibrosis in South Kerala. Int. J. Epidemiol. 1996, 25, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Eapen, J.T. Elevated Levels of Ceriumin Tubers from Regions Endemic for Endomyocardial Fibrosis (EMF). Bull. Environ. Contam. Toxicol. 1998, 60, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Valiathan, M.; Kartha, C.; Nair, R.; Shivakumar, K.; Eapen, J. Geochemical basis of tropical endomyocardial fibrosis. Curr. Sci. 1994, 67, 99–104. [Google Scholar]
- Huebl, L.; Leick, S.; Guettl, L.; Akello, G.; Kutalek, R. Geophagy in northern Uganda: Perspectives from consumers and clinicians. Am. J. Trop. Med. Hyg. 2016, 95, 1440. [Google Scholar] [CrossRef]
- Gaillardet, J.; Viers, J.; Dupré, B. Trace elements in river waters. Treatise Geochem. 2003, 5, 605. [Google Scholar] [CrossRef]
- Rawlins, B.; Cordeiro, M.; Smith, B. Exposure and Bioavailability of Cerium Through the Ingestion of Soil (Uganda); Technical Report WC/98/12; British Geological Survey: Nottinghamshire, UK, 1998. [Google Scholar]
- Chen, R. Characteristics and regularity analysis of the Chengnan rare earth ores in Ninghua County. Fujian Prov. J. Yangtze Univ. (Nat. Sci. Ed.) 2013, 10, 65–67. [Google Scholar]
- Henry, J.M.; Cring, F.D. Geophagy an Anthropological Perspective. In Soils and Human Health; Brevik, E.C., Burgess, L.C., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 179–198. [Google Scholar] [CrossRef]
- Encyclopædia Britannica. Available online: https://www.britannica.com/science/sickle-cell-anemia (accessed on 8 October 2025).
- Elzouki, A.Y.; Harfi, H.A.; Nazer, H.; Oh, W.; Stapleton, F.; Whitley, R.J. Textbook of Clinical Pediatrics, 2nd ed.; Springer: Berlin, Germany, 2011. [Google Scholar] [CrossRef]
- Guimarães, A.; Machado, C.L.; Santos, J.; Lanziani, R.; Cordovil, K. Pica in sickle cell disease: Nutritional management and implications. N. Afr. J. Food Nutr. Res. 2024, 8, 154–164. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Gaboli, H.O.; Attalla, B. Pica among Sudanese children with sickle cell anemia. Basic Res. J. Med. Clin. Sci. 2015, 4, 1–7. [Google Scholar]
- Aloni, M.N.; Lecerf, P.; Lê, P.-Q.; Heijmans, C.; Huybrechts, S.; Devalck, C.; Azzi, N.; Ngalula-Mujinga, M.; Ferster, A. Is Pica under-reported in children with sickle cell disease? A pilot study in a Belgian cohort. Hematology 2015, 20, 429–432. [Google Scholar] [CrossRef]
- Kolthof, H.; van Weel, E. ‘My daughter eats my mattress!’—A patient with pica. Tijdschr. Psychiatr. 2008, 50, 185–189. [Google Scholar] [PubMed]
- Ayeta, A.C.; da Cunha, A.C.B.; Heidelmann, S.P.; Saunders, C. Fatores nutricionais e psicológicos associados com a ocorrência de picamalácia em gestantes. Rev. Bras. Ginecol. E Obs. 2015, 37, 571–577. [Google Scholar] [CrossRef]
- Young, S.L. Pica in pregnancy: New ideas about an old condition. Annu. Rev. Nutr. 2010, 30, 403–422. [Google Scholar] [CrossRef]
- Kachani, A.T.; Cordás, T.A. Da ópera-bufa ao caos nosológico: Pica. Arch. Clin. Psychiatry 2009, 36, 162–169. [Google Scholar] [CrossRef]
- Brown, P. 1755 and all that: A historical primer of transmissible spongiform encephalopathy. BMJ 1998, 317, 1688–1692. [Google Scholar] [CrossRef][Green Version]
- Katscher, F. It’s Jakob’s disease, not Creutzfeldt’s. Nature 1998, 393, 11. [Google Scholar] [CrossRef]
- Zigas, V.; Gajdusek, D.C. Kuru: Clinical study of a new syndrome resembling paralysis agitans in natives of the Eastern Highlands of Australian New Guinea. Med. J. Aust. 1957, 2, 745–754. [Google Scholar] [CrossRef]
- Williams, E.; Young, S. Chronic wasting disease of captive mule deer: A spongiform encephalopathy. J. Wildl. Dis. 1980, 16, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Prusiner, S.B. Novel proteinaceous infectious particles cause scrapie. Science 1982, 216, 136–144. [Google Scholar] [CrossRef]
- Chakrabortee, S.; Kayatekin, C.; Newby, G.A.; Mendillo, M.L.; Lancaster, A.; Lindquist, S. Luminidependens (LD) is an Arabidopsis protein with prion behavior. Proc. Natl. Acad. Sci. USA 2016, 113, 6065–6070. [Google Scholar] [CrossRef] [PubMed]
- Health Care Providers. Clinical Overview of Creutzfeldt-Jakob Disease (CJD). Available online: https://www.cdc.gov/creutzfeldt-jakob/hcp/clinical-overview/index.html (accessed on 1 July 2025).
- Collinge, J. Prion diseases of humans and animals: Their causes and molecular basis. Annu. Rev. Neurosci. 2001, 24, 519–550. [Google Scholar] [CrossRef]
- Spraker, T.; Miller, M.; Williams, E.; Getzy, D.; Adrian, W.; Schoonveld, G.; Spowart, R.; O’Rourke, K.I.; Miller, J.; Merz, P. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. J. Wildl. Dis. 1997, 33, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Benestad, S.L.; Mitchell, G.; Simmons, M.; Ytrehus, B.; Vikøren, T. First case of chronic wasting disease in Europe in a Norwegian free-ranging reindeer. Vet. Res. 2016, 47, 88. [Google Scholar] [CrossRef]
- Chronic Wasting Disease. Moose Found Dead in Forest with Chronic Wasting Disease. Available online: https://cwd-info.org/wp-content/uploads/2018/03/Update-119.pdf (accessed on 1 July 2025).
- Vikøren, T.; Våge, J.; Madslien, K.I.; Røed, K.H.; Rolandsen, C.M.; Tran, L.; Hopp, P.; Veiberg, V.; Heum, M.; Moldal, T. First detection of chronic wasting disease in a wild red deer (Cervus elaphus) in Europe. J. Wildl. Dis. 2019, 55, 970–972. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Shon, H.-J.; Joo, Y.-S.; Mun, U.-K.; Kang, K.-S.; Lee, Y.-S. Additional cases of chronic wasting disease in imported deer in Korea. J. Vet. Med. Sci. 2005, 67, 753–759. [Google Scholar] [CrossRef]
- Escobar, L.E.; Pritzkow, S.; Winter, S.N.; Grear, D.A.; Kirchgessner, M.S.; Dominguez-Villegas, E.; Machado, G.; Townsend Peterson, A.; Soto, C. The ecology of chronic wasting disease in wildlife. Biol. Rev. 2020, 95, 393–408. [Google Scholar] [CrossRef]
- Plummer, I.H.; Johnson, C.J.; Chesney, A.R.; Pedersen, J.A.; Samuel, M.D. Mineral licks as environmental reservoirs of chronic wasting disease prions. PLoS ONE 2018, 13, e0196745. [Google Scholar] [CrossRef]
- Johnson, C.J.; Pedersen, J.A.; Chappell, R.J.; McKenzie, D.; Aiken, J.M. Oral transmissibility of prion disease is enhanced by binding to soil particles. PLoS Pathog. 2007, 3, e93. [Google Scholar] [CrossRef]
- VerCauteren, K.C.; Burke, P.W.; Phillips, G.E.; Fischer, J.W.; Seward, N.W.; Wunder, B.A.; Lavelle, M.J. Elk use of wallows and potential chronic wasting disease transmission. J. Wildl. Dis. 2007, 43, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, M.J.; Phillips, G.E.; Fischer, J.W.; Burke, P.W.; Seward, N.W.; Stahl, R.S.; Nichols, T.A.; Wunder, B.A.; VerCauteren, K.C. Mineral licks: Motivational factors for visitation and accompanying disease risk at communal use sites of elk and deer. Environ. Geochem. Health 2014, 36, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.C.; Carlson, C.M.; Cross, P.C.; Johnson, C.J.; Richards, B.J.; Russell, R.E.; Samuel, M.D.; Sargeant, G.A.; Walsh, D.P.; Walter, W.D. Chronic Wasting Disease—Research by the U.S. Geological Survey and Partners; Open-File Report 2019-1109; U.S. Geological Survey: Reston, VA, USA, 2019; 29p. [CrossRef]
- Elliott, H.; Wall, F.; Chakhmouradian, A.; Siegfried, P.; Dahlgren, S.; Weatherley, S.; Finch, A.; Marks, M.; Dowman, E.; Deady, E. Fenites associated with carbonatite complexes: A review. Ore Geol. Rev. 2018, 93, 38–59. [Google Scholar] [CrossRef]
- Panichev, A.; Golokhvast, K. Major and Minor Causes of Geophagy-Lithophagy in Animals and Humans. Geosciences 2025, 15, 75. [Google Scholar] [CrossRef]
- U.S. Geological Survey. CWD Map March 2017. Available online: https://www.usgs.gov/media/images/cwd-map-march-2017 (accessed on 8 October 2025).
- Panichev, A.; Trepet, S.; Chekryzhov, I.Y.; Seryodkin, I.; Vakh, E.; Makarevich, R.; Eskina, T.; Bibina, K.; Stolyarova, T.; Mitina, E. A study of kudurs used by wild animals located on the water sources high in REE content in the Caucasus Nature Reserve. Environ. Geochem. Health 2021, 43, 91–112. [Google Scholar] [CrossRef]
- Panichev, A.M.; Seryodkin, I.V.; Kalinkin, Y.N.; Makarevich, R.A.; Stolyarova, T.A.; Sergievich, A.A.; Khoroshikh, P.P. Development of the “rare-earth” hypothesis to explain the reasons of geophagy in Teletskoye Lake are kudurs (Gorny Altai, Russia). Environ. Geochem. Health 2018, 40, 1299–1316. [Google Scholar] [CrossRef]
- Panichev, A.M.; Baranovskaya, N.V.; Seryodkin, I.V.; Chekryzhov, I.Y.; Vakh, E.A.; Kalinkin, Y.V.; Lutsenko, T.N.; Popov, N.Y.; Ruslan, A.V.; Ostapenko, D.S.; et al. Excess of REE in plant foods as a cause of geophagy in animals in the Teletskoye Lake basin, Altai Republic, Russia. World Acad. Sci. J. 2023, 5, 6. [Google Scholar] [CrossRef]
- Panichev, A.M.; Baranovskaya, N.V.; Seryodkin, I.V.; Chekryzhov, I.Y.; Soktoev, B.R.; Ivanov, V.V.; Vakh, E.A.; Desyatova, T.V.; Lutsenko, T.N.; Popov, N.Y.; et al. The Main Cause of Geophagy According to Extensive Studies on Olkhon Island, Lake Baikal. Geosciences 2023, 13, 211. [Google Scholar] [CrossRef]
- Panichev, A.M.; Baranovskaya, N.V.; Chekryzhov, I.Y.; Kalinkin, Y.N.; Kholodov, A.S.; Spandidos, D.A.; Tsatsakis, A.; Golokhvast, K.S. Kudurs (mineral licks) on ultrabasic rocks in the Altai Mountains, Russia. World Acad. Sci. J. 2022, 5, 2. [Google Scholar] [CrossRef]
- Panichev, A.M.; Baranovskaya, N.V.; Chekrizhov, I.Y.; Ivanov, V.V.; Tsatska, A.N. An Unusual Variety of Geophagy: Coal Consumption by Snow Sheep in the Transbaikalia Mountains. Dokl. Earth Sci. 2024, 516, 1061–1066. [Google Scholar] [CrossRef]
- Panichev, A.M.; Popov, V.K.; Chekryzhov, I.Y.; Seryodkin, I.V.; Stolyarova, T.A.; Zakusin, S.V.; Sergievich, A.A.; Khoroshikh, P.P. Rare earth elements upon assessment of reasons of the geophagy in Sikhote-Alin region (Russian Federation), Africa and other world regions. Environ. Geochem. Health 2016, 38, 1255–1270. [Google Scholar] [CrossRef]
- Panichev, A.M.; Baranovskaya, N.V.; Seryodkin, I.V.; Chekryzhov, I.Y.; Vakh, E.A.; Soktoev, B.R.; Belyanovskaya, A.I.; Makarevich, R.A.; Lutsenko, T.N.; Popov, N.Y.; et al. Landscape REE anomalies and the cause of geophagy in wild animals at kudurs (mineral salt licks) in the Sikhote-Alin. Environ. Geochem. Health 2021, 44, 1137–1160. [Google Scholar] [CrossRef] [PubMed]
- Panichev, A.M. Rare Earth Elements: Review of Medical and Biological Properties and Their Abundance in the Rock Materials and Mineralized SpringWaters in the Context of Animal and Human Geophagia Reasons Evaluation. Achiev. Life Sci. 2015, 9, 95–103. [Google Scholar] [CrossRef]
- Shilo, N.A.; Lozhkin, A.V.; Titov, E.E.; Shumilov, Y.V. Kirgilyakh Mammoth; Nauka: Moscow, Russia, 1983; 209p. (In Russian) [Google Scholar]
- Bgatov, V.I.; Lazarev, P.A.; Speshilova, M.A. Lithophagy and Mammoth Fauna; Yakutsk Scientific Center: Yakutsk, Russia, 1989; 32p. (In Russian) [Google Scholar]
- Mashchenko, E.N. New data on the peculiarities of mammoth biology. Priroda 1999, 10, 41–53. (In Russian) [Google Scholar]
- Leshchinskiy, S.V. Strong evidence for dietary mineral imbalance as the cause of osteodystrophy in Late Glacial woolly mammoths at the Berelyokh site (Northern Yakutia, Russia). Quat. Int. 2017, 445, 146–170. [Google Scholar] [CrossRef]
- Popov, V.K.; Sakhno, V.G.; Kuzmin, Y.V.; Glascock, M.D.; Choi, B.-K. Geochemistry of volcanic glasses of Pektusan volcano. Dokl. Akad. Nauk. 2005, 403, 242–247. [Google Scholar]
- Liu, J.; Shen, Z.; Yang, W.; Che, J.; Xie, L.; Lei, H. Effect of Long-term Intake of Rare Earth in Drinking Water on Trace Elements in Brains of Mice. J. Rare Earths 2002, 20, 562–564. [Google Scholar]
- Feng, L.; Xiao, H.; He, X.; Li, Z.; Li, F.; Liu, N.; Zhao, Y.; Huang, Y.; Zhang, Z.; Chai, Z. Neurotoxicological consequence of long-term exposure to lanthanum. Toxicol. Lett. 2006, 165, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Atherton, N.D.; Totten, J.W.; Gaitonde, M.D. Treatment of Bone Diseases. US 2002/0051822A1, 2 May 2002. [Google Scholar]
- Knebel, C. Untersuchungen Zum Einfluss Seltener Erd-Citrate Auf Leistungsparameter Beim Schwein Und Die Ruminale Fermentation Im künstlichen Pansen (RUSITEC). Doctoral Thesis, Ludwig Maximilian University of Munich, München, Germany, 2004. [Google Scholar]
- Wehr, U.; Feldhaus, A.; Rambeck, W. Rare earth elements restore accelerated bone loss in a small animal model of postmenopausal osteoporosis. In Proceedings of the Society of Nutrition Physiology, Goöttingen, Germany, 21–23 March 2006. [Google Scholar]
- Davies, P.; Brown, D.R. Manganese enhances prion protein survival in model soils and increases prion infectivity to cells. PLoS ONE 2009, 4, e7518. [Google Scholar] [CrossRef] [PubMed]
- Nichols, T.A.; Spraker, T.R.; Gidlewski, T.; Cummings, B.; Hill, D.; Kong, Q.; Balachandran, A.; VerCauteren, K.C.; Zabel, M.D. Dietary magnesium and copper affect survival time and neuroinflammation in chronic wasting disease. Prion 2016, 10, 228–250. [Google Scholar] [CrossRef]
- Shorter, J.; Lindquist, S. Prions as adaptive conduits of memory and inheritance. Nat. Rev. Genet. 2005, 6, 435–450. [Google Scholar] [CrossRef]
- Ziegler, J.L. Endemic Kaposi’s sarcoma in Africa and local volcanic soils. Lancet 1993, 342, 1348–1351. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).