Simple Summary
Keratin-associated proteins (KAPs) are fundamental components of wool and hair fibres. They are split into three broad groups: the high sulphur (HS), the ultra-high sulphur (UHS) and the high glycine-tyrosine (HGT) KAPs. KRTAP25-1 encodes a HS-KAP protein and the gene has recently been identified in humans. Here, we report the absence of a KRTAP25-1 in sheep, and we describe a new HS-KRTAP (named KRTAP28-1) in the chromosome region where KRTAP25-1 was expected to be found. Six variants (A−F) of KRTAP28-1 containing eight single nucleotide polymorphisms (SNPs) and a TG dinucleotide repeat polymorphism were detected. One SNP was located upstream of the start codon and all the others were non-synonymous SNPs, including a nonsense SNP. The TG repeat polymorphism would lead to a reading frame shift at the carboxyl-terminal end. The association of KRTAP28-1 with wool traits was investigated with 383 Southdown × Merino-cross lambs from seven sire lines. Of the four genotypes with a frequency over 5%, lambs of genotypes AB and BD produced wool of a smaller mean fibre diameter (MFD) than lambs of genotype BC. This shows that KRTAP28-1 is associated with a key wool trait, and variation in this gene might therefore have value as a marker for improving that trait.
Abstract
Keratin-associated proteins (KAPs) are a diverse group of proteins and form a matrix that cross-links keratin intermediate filaments in hair and wool fibres. From over 100 KAP genes (KRTAPs) identified in mammalian species, KRTAP25-1 is a high sulphur (HS)-KAP gene, which has recently been described in humans. Here, we report the absence of KRTAP25-1 in sheep, and describe a new HS-KRTAP (named KRTAP28-1) in the chromosome region where KRTAP25-1 was expected to be found. Six variants (A−F) of KRTAP28-1 containing eight single nucleotide polymorphisms (SNPs) and a TG repeat polymorphism were detected. One was positioned 30 bp upstream of the transcription start codon and all the others were non-synonymous SNPs, including a nonsense SNP. The TG repeat polymorphism would lead to a reading frame shift at the carboxyl-terminal end. The effect of KRTAP28-1 on wool traits was investigated with 383 Southdown × Merino-cross lambs from seven sire lines. Of the four genotypes with a frequency of over 5%, lambs of genotypes AB and BD produced wool of a smaller MFD than lambs of genotype BC. This shows that KRTAP28-1 is associated with wool fibre diameter, and that variation in this gene might have potential for use as a gene marker for reducing wool fibre diameter.
1. Introduction
Keratin-associated proteins (KAPs) are found in wool and hair fibres. They form a cross-linked network with the keratin intermediate filaments, and they are believed to play a central role in defining the physico-mechanical properties of these fibres. They have a high content of either cysteine, or both glycine and tyrosine, and traditionally they have been divided into three broad groups based on their amino acid composition: the high glycine–tyrosine (HGT; 35−60 mol% glycine and tyrosine), the high sulphur (HS; ≤30 mol% cysteine), and the ultra-high sulphur (UHS; >30 mol% cysteine) groups [1].
To date, over 100 KAP genes have been identified in mammalian species, including genes from sheep, rabbits, mice, and humans [1,2]. They have been positioned in 27 families (KAP1–KAP27), based on similarities in their encoded proteins, with each family comprising 1–12 members [1,3,4]. Bioinformatics analysis of the human genome sequence has led to the identification of 89 functional human KRTAPs and these have been placed into 25 KAP families: KAP1–KAP13, KAP15–KAP17 and KAP19–KAP27 [4,5].
The sequences of the KRTAPs appear to be conserved across species and based on sequence similarity; many of the human KRTAP orthologs have been identified in sheep and goats. These include ovine KRTAP8-2 [6], ovine KRTAP11-1 [7], ovine KRTAP13-3 [8], ovine KRTAP15-1 [9], ovine KRTAP20-1 [10], ovine KRTAP20-2 [11], ovine KRTAP22-1 [12], ovine KRTAP24-1 [13], ovine KRTAP26-1 [14], caprine KRTAP13-3 [15], caprine KRTAP20-2 [16] and caprine KRTAP24-1 [17]. Variation in KRTAP26-1 has been recently reported to associate with a number of wool traits [14]. It would be interesting to identify other KRTAPs near KRTAP26-1 and investigate whether they too are variable and how that variation, should it exist, affects wool traits.
KRTAP25-1 is a HS-KAP gene that has been located in a position between KRTAP24-1 and KRTAP26-1 in humans [5]; but the gene has not been reported in any other species. In this study, we report our failed attempt to find a KRTAP25-1 ortholog in the sheep genome, and report an apparently new KRTAP (named ovine KRTAP28-1) that was identified in a chromosomal region that should harbour KRTAP25-1, were it to exist. We report nucleotide sequence variation in the sequence identified, and its association with wool traits is also investigated.
2. Materials and Methods
This research involved animals and was undertaken in accordance with the Animal Welfare Act 1999 (New Zealand Government). The collection of blood drops from sheep by the nicking of their ears is covered by Section 7.5 Animal Identification, in: Code of Welfare: Sheep and Beef Cattle (2016). According to this Act, we are allowed to collect blood and wool as these are considered to be part of normal day-to-day farming activities.
2.1. Sheep Blood and Wool Samples
Three hundred and eighty-two Southdown × Merino-cross lambs from seven sire lines were investigated. The lambs were tagged with an identification number within 12 hours of birth. Their date of birth, weight at birth, birth rank (i.e., whether they were a single, twin or triplet), gender, and dam and sire were recorded. The ewes and lambs remained together until weaning. At tailing (lambs varied from 2–6 weeks of age), blood samples from all the lambs were collected onto TFN paper (Munktell Filter AB, Sweden), and genomic DNA was purified using the approach described by Zhou et al. [18].
Wool samples were collected at first shearing (12 months of age) from the mid-side of the lambs. Greasey fleece weight (GFW) was measured at shearing; and subsequently, mean fibre diameter (MFD), fibre diameter standard deviation (FDSD), coefficient of variation of fibre diameter (CVFD), mean staple length (MSL), mean fibre curvature (MFC), mean staple strength (MSS), prickle factor (PF) and wool yield (Yield) were measured using International Wool Textile Oganisation (IWTO) standardized methods by the New Zealand Wool Testing Authority Ltd (Ahuriri, Napier, NZ). Clean fleece weight (CFW) was then calculated from the GFW and Yield measurements.
2.2. Bioinformatic Analysis of the Sheep Genome Sequence
The human KRTAP25-1 coding sequence (GenBank NM_001128598.1) was used to search the Ovine Genome Assembly Oar_v4.0 using the Basic Local Alignment Search Tool (BLAST) in order to ascertain whether there were any homologous sequences in the sheep genome. In humans, KRTAP25-1 is located between KRTAP24-1 and KRTAP26-1 on chromosome 21 [5]. To further look for the presence of sequences homologous to human KRTAP25-1, the ovine genome sequence (approximately 37 kb in length) spanning KRTAP24-1 and KRTAP26-1 was retrieved from NCBI and specifically aligned with the human KRTAP25-1 sequence. Open Reading Frame Finder (ORF Finder; www.ncbi.nlm.nih.gov/orffinder/) was used to detect any open reading frame (ORF) that could encode a cysteine-rich protein in the region between these two genes.
2.3. PCR Amplification and Polymorphism Screening
The DNA sequences flanking the ORF detected above were utilised to design a pair of PCR primers to amplify a fragment containing the entire ORF. These primers were 5′-TAGACAAGCCATTCTCTGTTG-3′ and 5′-CATTCCAGTATTCCTGCCTG-3′, and they were synthesised by Integrated DNA Technologies (Coralville, IA, USA).
Amplification was performed in a 15-µL reaction containing the genomic DNA on one 1.2-mm punch of TFN paper, 150 µM of each deoxyribonucleotide triphosphate (dNTP; Bioline, London, UK), 2.5 mM of Mg2+, 0.25 µM of each primer, 0.5 U of Taq DNA polymerase (Qiagen, Hilden, Germany) and 1× reaction buffer supplied with the enzyme. The thermal profile consisted of an initial denaturation for 2 min at 94 °C, followed by 40 cycles of 30 s at 94 °C, 30 s at 58 °C and 40 s at 72 °C, concluding with a final extension incubation of 5 min at 72 °C. Amplification was carried out in S1000 thermal cyclers (Bio-Rad, Hercules, CA, USA).
The PCR amplicons were vetted for sequence variation by means of single-stranded conformational polymorphism (SSCP) analysis. Each amplicon (0.7 µL) was mixed with 7 µL of loading dye (98% formamide, 10 mM EDTA, 0.025% bromophenol blue, and 0.025% xylene cyanol). After denaturation at 90 °C for 5 min, the samples were promptly cooled on wet ice and then loaded on 16 cm × 18 cm, 10% acrylamide: bisacrylamide (37.5:1) (Bio-Rad) gels with 1% glycerol. Electrophoresis was performed using Protean II xi cells (Bio-Rad), at 320 V for 18 h at 14 °C in 0.5× Tris/Borate/EDTA (TBE) buffer. Gels were silver-stained according to the method of Byun et al. [19].
2.4. Sequencing of Variants and Sequence Analyses
PCR amplicons representing different banding patterns from sheep that seemed to be homozygous were sequenced in both directions by the Lincoln University (New Zealand) DNA sequencing service. Variants that were only found in sheep that seemed to be heterozygous were sequenced using an approach described by Gong et al. [20]. In this method, a band corresponding to the variant was removed as a gel sliver from the polyacrylamide gel, macerated and then used as a template for re-amplification with the original primers. This second amplicon was then sequenced.
Sequence translation, alignments and comparisons were undertaken using DNAMAN (version 5.2.10, Lynnon BioSoft, Vaudreuil, QC, Canada). A phylogenetic tree was constructed based on the predicted amino acid sequence of the ORF using MEGA version 7.0. The BLAST algorithm was used to search the NCBI GenBank (www.ncbi.nlm.nih.gov/) databases for homologous sequences.
2.5. Statistical Analyses
Hardy-Weinberg equilibrium (HWE) was calculated using POPGENE version 1.32 (Molecular Biology and Biotechnology Centre, University of Alberta, Edmonton, AB, Canada).
Statistical analyses were undertaken using Minitab version 16 (Minitab Inc., State College, PA, USA). General Linear Mixed-Effect Models (GLMMs) were used to test the association between variation in KRTAP28-1 and variation in ten wool traits. In these models, only sheep with genotypes that occurred in the population at a frequency over 5% were analysed using multiple pairwise comparisons with Bonferroni corrections. Sire and gender were found to affect all the wool traits (p < 0.05) and were thus incorporated into all the models as a random factor and a fixed factor, respectively. Birth rank was not found to affect wool traits and it was thus not incorporated into the models.
All p-values were considered statistically significant when p < 0.05 unless otherwise indicated. Trends were noted when 0.05 ≤ p < 0.1.
3. Results
3.1. The Absence of the KRTAP25-1 Homologue and the Presence of KRTAP28-1 in the Sheep Genome
A BLAST search of the Ovine Genome Assembly Oar_v4.0 using the human KRTAP25-1 coding sequence did not reveal any homologous sequence in the genome of the sheep. Alignment of the sheep genome sequence between KRTAP24-1 and KRTAP26-1 with human KRTAP25-1 confirmed the apparent absence of a KRTAP25-1 ortholog in sheep. However, bioinformatics analysis of this genome region identified a 408-bp ORF that would encode a protein containing 8.2 mol% cysteine. The most common amino acid residue found in this protein was serine (16.3 mol%). Evolutionary analysis revealed that this protein appeared to be different to human KAP25-1 and all other known HS-KAPs, and the most closely related KAPs were KAP3-n and KAP23-1 (Figure 1). Further sequence comparison with KAP3-n, KAP23-1 and KAP25-1 did not reveal any obvious sequence similarity for this protein (Supplementary Figure S1).
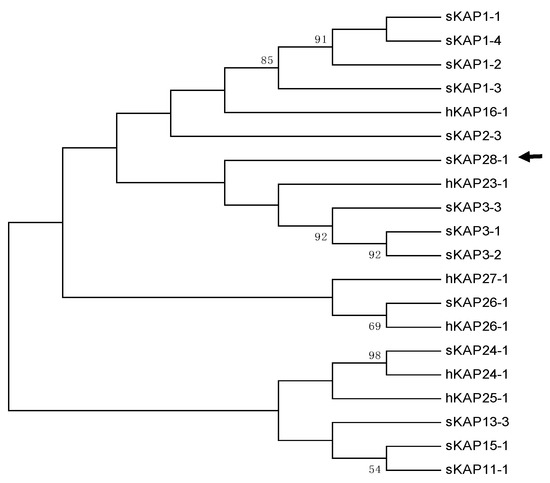
Figure 1.
Phylogenetic tree for high sulphur keratin-associated proteins (HS-KAPs) that have been identified in sheep, along with human HS-KAPs. The tree is assembled using amino acid sequences. The sheep KAPs are specified with an “s” prefix, while the human KAPs are specified with an “h”. The numbers at the forks show the bootstrap confidence values, with only those equal to or greater than 50% being shown. The newly identified sheep KAP28-1 gene is marked with a horizontal arrow. The GenBank accession numbers for all other sheep KAPs are: NM_001159760 (sKAP1-1), HQ897975 (sKAP1-2), NM_001159761 (sKAP1-3), GQ507741 (sKAP1-4), P02443 (sKAP2-1), U60024 (sKAP2-3), M21099 (sKAP3-1), M21100 (sKAP3-2), M21103 (sKAP3-3), HQ595352 (sKAP11-1), JN377429 (sKAP13-3), KX817979 (sKAP15-1) and JX112014 (sKAP24-1). The accession numbers for the human KAPs are: NM_181623 (hKAP15-1), NM_001146182 (hKAP16-1), NM_181624 (hKAP23-1), NM_001085455 (hKAP24-1), NM_001128598 (hKAP25-1), NM_203405 (hKAP26-1) and NM_001077711 (hKAP27-1).
Taken together, this suggests that this ORF presents a newly identified HS-KAP gene, and one that would belong to a new family. It was therefore named SHEEP-KRTAP28-1 according to the updated KAP nomenclature [2]. The chromosomal location of this newly identified KRTAP28-1 sequence is displayed in Figure 2.
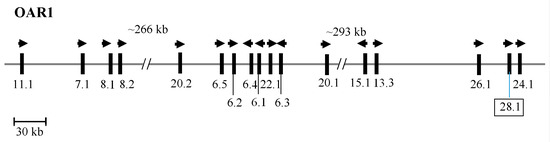
Figure 2.
The KAP genes identified to date on sheep chromosome 1. The newly identified ovine KRTAP28-1 is shown in a box. Vertical bars signify the location of the KRTAPs and the arrowheads specify the direction of transcription. The numbers below the bars are the names of the KAP genes (i.e., 11.1 is KRTAP11-1). The nucleotide distances are inexact and refer to NC_019458.2.
3.2. Variation in Ovine KRTAP28-1
PCR-SSCP analysis revealed six different banding patterns for the notional ovine KRTAP28-1, with either one or a blend of two patterns being detected for each sheep (Figure 3). This is consistent with there being homozygous and heterozygous individuals, and it suggests that the amplicons were derived from a single gene.
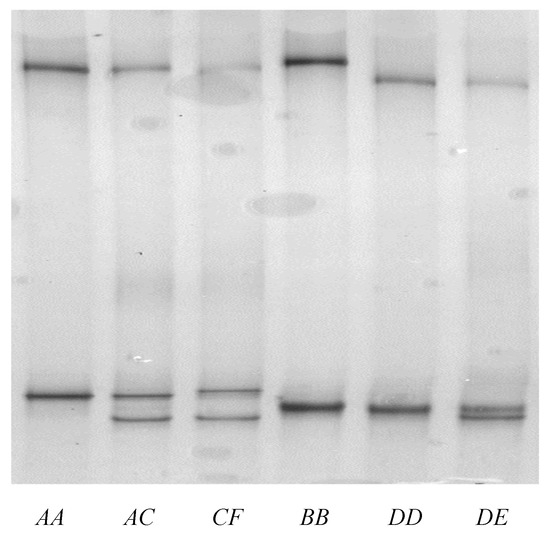
Figure 3.
PCR-single-stranded conformational polymorphism of the ovine KAP28-1 gene. Five unique banding patterns corresponding to five variant sequences (A–E) are shown in either homozygous or heterozygous forms.
The sequencing of PCR amplicons representative of these banding patterns revealed eight single nucleotide polymorphisms (SNPs) and a TG dinucleotide repeat (Figure 4). One SNP (c.-30T/G) was located upstream of the start codon, whereas all of the other SNPs were located in the coding region. These were c.7T/C, c.95T/C, c.117T/G, c.136C/T, c.167c/T, c.176G/A, and c.211G/A. All of the coding region SNPs were non-synonymous, and one of the non-synonymous SNPs could create a premature stop codon at position 46 of the putative polypeptide (Figure 5). There were 11 TG repeats found in variants A and F, 14 TG repeats in variants C and E, and 16 TG repeats in variants B and D. This TG dinucleotide repeat polymorphism would lead to a reading frame shift in the carboxyl terminus of the protein, and create a novel in-frame stop codon (Figure 5).
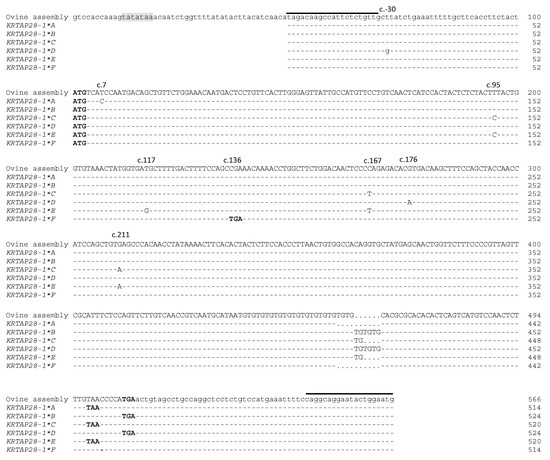
Figure 4.
Alignment of the ovine KRTAP28-1 variant sequences together with the ovine genome assembly sequence. Eight single nucleotide polymorphisms and a dinucleotide repeat polymorphism were detected in ovine KRTAP28-1. Nucleotides in the coding region are presented in upper case letters and those in the non-coding regions are in lower case. Dashes designate nucleotides indistinguishable to the ovine assembly, and dots have been introduced to increase the alignment. A putative TATA box is shaded, and the start and stop codons are presented in bold. The TG dinucleotide repeat region is boxed, and the horizontal lines designate the primer binding regions.

Figure 5.
Predicted amino acid sequence alignment of the ovine KRTAP28-1 variants, together with the ovine genome-derived assembly. Dashes signify amino acids identical to the assembly sequence, and dots have been introduced to increase the alignment.
In total, ten different genotypes were observed (Table 1). The common genotypes were AB, BB, BC and BD, and the remaining six genotypes (AA, AC, AD, BF, DD and DE) were rare with a frequency of less than 5%. These gave frequencies of 14.9%, 57.5%, 9.1%, 16.4%, 1.8% and 0.3% for variants A, B, C, D, E and F, respectively. The genotype data were not in the Hardy–Weinberg Equilibrium for the sheep population investigated (Table 1).

Table 1.
Genotype count and Hardy–Weinberg Equilibrium (HWE) test for ovine KRTAP28-1.
3.3. Effect of KRTAP28-1 on Wool Traits
As genotypes AA, AC, AD, BF, DD and DE were detected at a low frequency (less than 5%), the sheep with these genotypes were not included in the association analysis. Associations were therefore only tested for the sheep with the four genotypes: AB, BB, BC and BD.
The KRTAP28-1 genotype was found to affect MFD. Sheep with genotypes AB and BD produced wool with smaller MFD than sheep of genotype BC (Table 2). No effects on other wool traits were detected.

Table 2.
Association of the KRTAP28-1 genotype and wool traits.
4. Discussion
This study reports the identification of what appears to be a new KAP gene in sheep. The low sequence similarity to all of the other known ovine HS-KAP genes, along with the high level of sequence variability between these genes, suggests that this novel gene should be assigned to a new family. The protein encoded by this new KAP gene would contain a lower than expected content of cysteine (8.2 mol%), compared to typical HS-KAPs, although a comparably low content of cysteine has been reported for KRTAP24-1 [13] and KRTAP26-1 [14]. This supports the assignment of this gene to the HS-KAP group. Given that up to 27 KAP families have been identified across mammalian species [2], this new KAP family was therefore named KAP28, and the gene was named KRTAP28-1.
This putative ovine KRTAP28-1 sequence is located between ovine KRTAP26-1 and ovine KRTAP24-1 on sheep chromosome 1, and in a corresponding region to where KRTAP25-1 is found in this group of KAP genes in humans [5]. While it could therefore be argued that the ovine and human sequences are homologues, the sequence differences between human KRTAP25-1 and the notional ovine KRTAP28-1 would suggest otherwise (see Supplementary Figure S1).
The expression of KRTAP25-1 has not been detected in Caucasians; but it remains a possibility that the expression may be individual or population-specific, with it perhaps being weakly expressed in Caucasians but strongly expressed in Orientals [21]. This would suggest that KRTAP25-1 may contribute to racial differences in the human hair fibre. In this context, the apparent absence of KRTAP25-1 and its substitution with KRTAP28-1 in these sheep might possibly suggest that KRTAP28-1 plays a role in defining a unique characteristic or breed-specific trait in wool fibres; but the occurrence of this gene in other breeds needs to be confirmed beforehand.
The detection of six variant sequences for ovine KRTAP28-1 is consistent with the observation that KRTAPs are polymorphic. There are, however, some unique characteristics of this variation. First is the presence of a dinucleotide repeat sequence variation in the coding region. While nucleotide repeat polymorphisms that do not result in reading frame shifts have been reported in other KRTAPs, including KRTAP1-1 [22], KRTAP5-4 [23], KRTAP6-1 [24], and KRTAP6-3 [25], a frame shift nucleotide repeat polymorphism has not been reported previously. The dinucleotide repeat polymorphism would potentially lead to changes in the carboxyl terminal sequence and the length of the protein. This may have a major effect on the structure and/or function of the protein, assuming all the variants are expressed. Second is the seemingly high frequency of occurrence of SNPs. The presence of eight SNPs in the 473–483 bp fragment amplified (excluding the primer-binding regions) would give a density of 16.6–16.9 SNPs per kb. This is more than three times the average density of 4.9 SNPs per kb across the sheep genome described by Kijas et al. [26]. This provides further evidence that KRTAPs have come about via unique evolutionary processes, including such things as gene conversion, as has been suggested for KRTAP1-n [22,27]. Third, is the predominance of non-synonymous SNPs. While SNPs are commonly found in KRTAPs, the nature of the SNPs observed in different KRTAPs appears to be different. In some KRTAPs, such as KRTAP1-2 [28,29], KRTAP6-2 [25], KRTAP8-1 [30], KRTAP11-1 [7] and KRTAP26-1 [14], synonymous SNPs prevail, whereas in other KRTAPs such as KRTAP1-4 [27], KRTAP5-4 [23], KRTAP6-5 [25] and KRTAP13-1 [8], non-synonymous SNPs are found to predominate. Having a very high level of non-synonymous SNPs, as would appear to be the case with the putative KRTAP28-1, would appear to be quite unique. A high non-synonymous SNP rate would suggest that selective pressure may have been exerted on KRTAP28-1. Lastly is the unusual presence of a nonsense SNP. Nonsense SNPs are rare in KRTAPs and have only been reported for KRTAP20-2 [11]. In KRTAP20-2, the nonsense SNP is located near the stop codon, while in KRTAP28-1 the nonsense SNP is located early in the coding sequence and it would result in the loss of approximately two-thirds of the protein. If expressed, this would have a major effect on the structure and/or function of the protein and hence it would potentially have a major effect on fibre traits. However, the effect of this SNP on wool traits could not be investigated here due to a low frequency of the variant in the sheep investigated in this study. Further studies are required to investigate the effect of this SNP in a large sheep population, together with the expression of KRTAP28-1 variants.
The deviations of genotypes from HWE may be due to a variety of causes, such as genotyping errors, outbreeding or inbreeding, sampling bias, natural selection, and sequence mutation/exchange. As the DNA samples were genotyped using PCR-SSCP, which is a reliable technique for determining variants, it is unlikely that the deviation from HWE for the KRTAP28-1 variation is caused by genotyping errors. Outbreeding was not possible for these sheep, and inbreeding is unlikely to be a cause, as inbreeding might lead to increased homozygosity, which is not apparent given the diversity of the heterozygous genotypes that were observed. All of the progeny from the seven sire lines were investigated in this study, suggesting that sampling bias is unlikely to be a cause. Considering the functional significance of KRTAP28-1, natural selection is unlikely to be a cause as well. Given that KRTAPs undergo a high level of sequence variation [1] and DNA recombination events have been suggested for some KRTAPs [21,26], the deviation of the KRTAP28-1 genotype data from HWE is possibly caused by DNA mutation and/or DNA recombination; but further investigations are required to confirm this.
The finding that wool of the AB and BD genotypes had a smaller MFD than wool of the BC genotype suggests that variation in KRTAP28-1 is associated with MFD, and that A and D are associated with a decrease in MFD while C is associated with an increase in MFD. This association with KRTAP28-1 is somewhat different to that reported for nearby KRTAP26-1 [14]. Despite there being an association with MFD, variation in KRTAP26-1 also affects FDSD, PF and MSL [14]. It would therefore seem unlikely that the effect detected for KRTAP28-1 is due to the close linkage to KRTAP26-1 on ovine chromosome 1. Given that fifteen other KRTAPs have been identified in a cluster with KRTAP28-1 and KRTAP26-1 [1,10,11,12,14], and that all of these KRTAPs are polymorphic and potentially expressed in the wool fibre, it is potentially very difficult to unravel the effects for individual KRTAPs. Nevertheless, the association detected in this study suggests that variation in KRTAP28-1 should be further investigated with regards to its effect on wool fibre diameter.
5. Conclusions
This study identified a new KAP gene in sheep and this gene was found to belong to a previously identified KAP family. We reported variation in the gene and this variation was found to be associated with wool fibre diameter. These results may be useful in the development of gene markers for reducing wool fibre diameter.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2615/9/4/142/s1, Figure S1. Comparison of the predicated amino acid sequences of the newly identified ovine KAP28-1 gene and other similar KAP genes.
Author Contributions
Conceptualization, H.Z., J.T. and J.G.H.H.; Formal analysis, L.B., J.W., H.Z. and H.G.; Investigation, L.B., J.W. and H.G.; Methodology, L.B., H.Z. and H.G.; Supervision, H.Z., J.T. and J.G.H.H.; Writing—original draft, L.B. and H.Z.; Writing—review and editing, H.Z., J.T. and J.G.H.H.
Funding
This research was financially supported by a Ningxia Agricultural Breeding Program (2013NYYZ0402), the AGMARDT Postdoctoral Fellowship to HG and the New Zealand Guardian Trust for the Vernon Willey Trust Fellowship to HZ and the Lincoln University Gene-Marker Laboratory.
Acknowledgments
We would like to thank Qian Fang, Yunhai Li and Wenhao Li for technical assistance and Andrea Hogan for managing the wool data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gong, H.; Zhou, H.; Forrest, R.H.; Li, S.; Wang, J.; Dyer, J.M.; Luo, Y.; Hickford, J.G. Wool keratin-associated protein genes in sheep—A review. Genes 2016, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhou, H.; McKenzie, G.W.; Yu, Z.; Clerens, S.; Dyer, J.M.; Plowman, J.E.; Wright, M.W.; Arora, R.; Bawden, C.S. An updated nomenclature for keratin-associated proteins (KAPs). Int. J. Biol. Sci. 2012, 8, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Langbein, L.; Praetzel-Wunder, S.; Winter, H.; Schweizer, J. Human hair keratin-associated proteins (KAPs). Int. Rev. Cytol. 2006, 251, 209–263. [Google Scholar] [PubMed]
- Rogers, M.A.; Schweizer, J. Human KAP genes, only the half of it? Extensive size polymorphisms in hair keratin-associated protein genes. J. Invest. Dermatol. 2005, 124, vii–ix. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Winter, H.; Langbein, L.; Wollschläger, A.; Praetzel-Wunder, S.; Jave-Suarez, L.F.; Schweizer, J. Characterization of human KAP24.1, a cuticular hair keratin-associated protein with unusual amino-acid composition and repeat structure. J. Invest. Dermatol. 2007, 127, 1197–1204. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, H.; Dyer, J.M.; Hickford, J.G. The sheep KAP8-2 gene, a new KAP8 family member that is absent in humans. SpringerPlus 2014, 3, 528. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, H.; Dyer, J.M.; Hickford, J.G. Identification of the ovine KAP11-1 gene (KRTAP11-1) and genetic variation in its coding sequence. Mol. Biol. Rep. 2011, 38, 5429–5433. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhou, H.; Dyer, J.M.; Plowman, J.E.; Hickford, J.G.H. Identification of the keratin-associated protein 13-3 (KAP13-3) gene in sheep. Open J. Genet. 2011, 1, 60–64. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Zhu, J.; Hu, J.; Liu, X.; Li, S.; Luo, Y.; Hickford, J.G. Identification of the ovine keratin-associated protein 15-1 gene (KRTAP15-1) and genetic variation in its coding sequence. Small Rumin. Res. 2017, 153, 131–136. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, H.; Bai, L.; Li, W.; Li, S.; Wang, J.; Luo, Y.; Hickford, J.G.H. Associations between variation in the ovine high glycine-tyrosine keratin-associated protein gene KRTAP20-1 and wool traits. J. Anim. Sci. 2018, 97, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Gong, H.; Zhou, H.; Tao, J.; Hickford, J.G.H. A nucleotide substitution in the ovine KAP20-2 gene leads to a premature stop codon that affects wool fibre curvature. Anim. Genet. 2018, 49, 357–358. [Google Scholar] [CrossRef]
- Li, S.; Zhou, H.; Gong, H.; Zhao, F.; Wang, J.; Liu, X.; Luo, Y.; Hickford, J.G. Identification of the ovine keratin-associated protein 22-1 (KAP22-1) gene and its effect on wool traits. Genes 2017, 8, 27. [Google Scholar] [CrossRef]
- Zhou, H.; Gong, H.; Yan, W.; Luo, Y.; Hickford, J.G.H. Identification and sequence analysis of the keratin-associated protein 24–1 (KAP24-1) gene homologue in sheep. Gene 2012, 511, 62–65. [Google Scholar] [CrossRef]
- Li, S.; Zhou, H.; Gong, H.; Zhao, F.; Hu, J.; Luo, Y.; Hickford, J.G. Identification of the ovine keratin-associated protein 26-1 gene and its association with variation in wool traits. Genes 2017, 8, 225. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Wang, J.; Li, S.; Luo, Y. Molecular characterization of caprine KRTAP13-3 in Liaoning cashmere goat in China. J. Appl. Anim. Res. 2014, 42, 140–144. [Google Scholar] [CrossRef]
- Wang, J.; Che, L.; Hickford, J.G.; Zhou, H.; Hao, Z.; Luo, Y.; Hu, J.; Liu, X.; Li, S. Identification of the caprine keratin-associated protein 20-2 (KAP20-2) gene and its effect on cashmere traits. Genes 2017, 8, 328. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Zhao, M.; Gong, H.; Hao, Z.; Luo, Y.; Hickford, J.G.H. Variation in the caprine KAP24-1 gene affects cashmere fibre diameter. Animals 2019, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hickford, J.G.H.; Fang, Q. A two-step procedure for extracting genomic DNA from dried blood spots on filter paper for polymerase chain reaction amplification. Anal. Biochem. 2006, 354, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.O.; Fang, Q.; Zhou, H.; Hickford, J.G.H. An effective method for silver-staining DNA in large numbers of polyacrylamide gels. Anal. Biochem. 2009, 385, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhou, H.; Hickford, J.G.H. Diversity of the glycine/tyrosine-rich keratin-associated protein 6 gene (KAP6) family in sheep. Mol. Biol. Rep. 2011, 38, 31–35. [Google Scholar] [CrossRef]
- Rogers, M.A.; Langbein, L.; Praetzel Wunder, S.; Giehl, K. Characterization and expression analysis of the hair keratin associated protein KAP26.1. Br. J. Dermatol. 2008, 159, 725–729. [Google Scholar] [CrossRef]
- Rogers, G.R.; Hickford, J.G.H.; Bickerstaffe, R. Polymorphism in two genes for B2 high sulphur proteins of wool. Anim. Genet. 1994, 25, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhou, H.; Plowman, J.E.; Dyer, J.M.; Hickford, J.G. Analysis of variation in the ovine ultra-high sulphur keratin-associated protein KAP5-4 gene using PCR-SSCP technique. Electrophoresis 2010, 31, 3545–3547. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gong, H.; Li, S.; Luo, Y.; Hickford, J.G. A 57-bp deletion in the ovine KAP6-1 gene affects wool fibre diameter. J. Anim. Breed. Genet. 2015, 132, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gong, H.; Wang, J.; Dyer, J.M.; Luo, Y.; Hickford, J.G. Identification of four new gene members of the KAP6 gene family in sheep. Sci. Rep. 2016, 6, 24074. [Google Scholar] [CrossRef] [PubMed]
- Kijas, J.W.; Townley, D.; Dalrymple, B.P.; Heaton, M.P.; Maddox, J.F.; McGrath, A.; Wilson, P.; Ingersoll, R.G.; McCulloch, R.; McWilliam, S. A genome wide survey of SNP variation reveals the genetic structure of sheep breeds. PLoS ONE 2009, 4, e4668. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, H.; Hickford, J.G. Polymorphism of the ovine keratin-associated protein 1-4 gene (KRTAP1-4). Mol. Biol. Rep. 2010, 37, 3377–3380. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, H.; Yu, Z.; Dyer, J.; Plowman, J.E.; Hickford, J.G.H. Identification of the ovine keratin-associated protein KAP1-2 gene (KRTAP1-2). Exp. Dermatol. 2011, 20, 815–819. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, H.; Hodge, S.; Dyer, J.M.; Hickford, J.G. Association of wool traits with variation in the ovine KAP1-2 gene in Merino cross lambs. Small Rumin. Res. 2015, 124, 24–29. [Google Scholar] [CrossRef]
- Gong, H.; Zhou, H.; Plowman, J.E.; Dyer, J.M.; Hickford, J.G.H. Search for variation in the ovine KAP7-1 and KAP8-1 genes using polymerase chain reaction–single-stranded conformational polymorphism screening. DNA Cell Biol. 2011, 31, 367–370. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).