Simple Summary
The aim of this study was to identify correlations between testicular histomorphology, fresh semen parameters, and the expression level of key spermatogenesis genes—TGFB2 and DMRT1—in roosters. Analysis of TGFB2 and DMRT1 gene expression in fresh semen demonstrated a close relationship between molecular genetic mechanisms and histomorphometric parameters. Thus, the expression level of the DMRT1 gene, which is key in determining sex in birds during embryogenesis, showed a number of negative correlations with parameters such as testicular weight, total/progressive sperm motility, and viability. TGFB2 gene expression in fresh semen showed no significant association with the studied parameters, but correlation analysis revealed a moderate positive association with DMRT1 gene expression. The results of this study support the need for an integrated approach to assessing the reproductive performance of males and the quality of sperm produced.
Abstract
The study of the relationship between testicular morphology and sperm quality is a pressing issue, for which molecular genetic approaches, including quantitative analysis of gene expression, are being implemented. The aim of this study was to identify correlations between the histomorphological structure of the testes, fresh sperm parameters, and the expression level of key spermatogenesis genes—TGFB2 and DMRT1—in roosters. The experiment was conducted on 10 Russian Snow White roosters aged 28–32 weeks. Sperm quality was assessed by volume, sperm concentration, total and progressive motility, and viability; histological analysis of the rooster testes was performed. The relative expression of the TGFB2 and DMRT1 genes in sperm was analyzed. Multiple correlation analysis of the data was conducted. A positive correlation was found between ejaculate volume and the number of spermatogonia (p = +0.651), a negative correlation between ejaculate volume and the number of second-order spermatocytes (p = −0.704), a negative correlation between the total cross-sectional area of the seminiferous tubules of the testes and sperm viability (p = −0.782), a negative correlation between the number of seminiferous tubules and the average diameter of their cross-section (p = −0.685), and a positive correlation between total and progressive sperm motility (p = +0.794). Analysis of TGFB2 and DMRT1 gene expression in sperm demonstrated a certain relationship between molecular genetic mechanisms and histomorphometric parameters. The expression level of the DMRT1 gene, which plays a key role in sex determination in birds during embryogenesis, had a number of negative correlations with such parameters as testicle weight (r = −0.782), total/progressive sperm motility (r = −0.552; r = −0.612), and viability (r = −0.552). Expression of the TGFB2 gene had no significant relationship with the studied parameters, but correlation analysis revealed a moderate positive relationship (r = +0.321) with DMRT1 gene expression. The data obtained indicate the expediency of integrating morphometric, cellular, and molecular analysis for an objective assessment of rooster reproductive function.
Keywords:
roosters; histology; sperm; testes; spermatogenesis; morphometry; correlation analysis; DMRT1; TGFB2; gene expression 1. Introduction
The reproductive system of male poultry, in particular, Gallus gallus domesticus, is a determining factor in their breeding worth, since it is on its structural and functional integrity that the success of spermatogenesis, the quality of the resulting sperm production and the fertility of the semen depend [1,2]. Unlike mammals, the testicles of birds are paired internal organs located in the abdominal cavity, which causes them to function at high temperatures—about 41–42 °C [3,4,5]. Spermatogenesis occurs in the seminiferous tubules, where all its stages are consistently realized: from the proliferation of primary germ cells, spermatogonia, to the formation of mature germ cells, spermatozoa [6]. Their full-fledged development is directly related to the quality of the semen obtained, which is the most important condition determining the effectiveness of artificial insemination in breeding programs [7]. According to scientific studies devoted to the assessment of fresh and cryopreserved semen in birds, it was found that indicators such as ejaculate volume, sperm concentration, motility and viability directly affect semen fertility, which, as a result, affects egg fertilization [7,8].
The seminiferous tubules (tubulus seminiferous) are a structural and functional unit of the testicle, which is a convoluted tubular structure lined with spermatogenic epithelium [9]. The seminiferous tubules are surrounded by a basement membrane and peritubular myoid cells, which provide the contractile function necessary for the movement of spermatozoa to the outflow ducts of the tubule [10]. The spermatogenic epithelium is formed by several cell populations at various stages of differentiation: spermatogonia, spermatocytes of the first and second orders, and spermatids, from which mature spermatozoa are formed [11,12]. Morphometric parameters of the seminiferous tubules, such as diameter, cross-sectional area, height of the spermatogenic epithelium and the inner diameter (lumen) of the tubule, are quantitative indicators characterizing the intensity and effectiveness of spermatogenesis [9,13,14]. Studies conducted on chickens have shown significant variability of these parameters depending on the age, breed, physiological state and direction of poultry productivity [9,15,16]. For example, in a study by Chinese scientists, it was found that in broiler chickens at the age of 1 week, the diameter of the seminal tubules was 40–50 µm and increased to 90–120 µm by 4 months, which corresponds to the age of puberty of roosters and the beginning of active spermatogenesis. In adult roosters, this indicator varies from 150 to 305 µm [15]. In the active reproductive phase, as a rule, the presence of all stages of spermatogenic cells with a predominance of spermatids is observed in the seminiferous tubules, which indicates the normal state of the male’s reproductive system [17,18]. In addition, Sertoli cells are an essential structural component of the spermatogenic epithelium. These cells are involved in the formation of the hematotesticular barrier and support developing gametes, so their nuclei can extend from the basement membrane to the lumen of the seminal tubule [19]. It is known that the concentration of Sertoli cells has a strong positive correlation with the testicles’ weight in roosters, which also confirms the fundamental importance of these cells in spermatogenesis in birds [20].
The search for links between the morphological structures of the testicles and the quality of the semen is the subject of many scientific studies. For example, it has been found that in mammals, testicle weight positively correlates with ejaculate volume and the total number of spermatozoa, and indicators such as diameter and cross-sectional area of the seminiferous tubules are associated with sperm concentration and motility [21]. It is also known that various disorders of the histological structure, such as a decrease in the height of the spermatogenic epithelium, an increase in the diameter of the tubule lumen, and germ cell apoptosis, are accompanied by a decrease in semen quality, and, as a result, a deterioration in its fertility [22]. In addition to traditional methods of assessing fertility in sires, for example, through spermograms, as well as microscopic and histological analysis of the gonads, molecular genetic approaches have been increasingly introduced in recent years. These include quantitative analysis of gene expression using real-time PCR (qPCR), genome-wide sequencing, analysis of polymorphisms in regulatory genes, and the use of CRISPR/Cas technology for the functional study of the role of individual genes in reproduction [23]. In our study, we focus on two genes, DMRT1 and TGFB2, which are key regulators of spermatogenesis and the development of male reproductive organs in vertebrates, including birds. The DMRT1 gene (doublesex- and mab-3-related transcription factor 1) is a conserved transcription factor localized on the Z chromosome in birds, which is considered critically important for determining the male sex and maintaining testicle function at all stages of ontogenesis [24]. In chickens, DMRT1 acts dose-dependently: individuals with the ZZ genotype (males) have a sufficient level of its expression for testicle development, whereas if the gene expression is disrupted, their partial or complete feminization is possible [25,26]. At the molecular level, the DMRT1 gene regulates Sertoli cell differentiation, supports proliferation, and prevents premature entry of spermatogonia into meiosis, ensuring constant replenishment of the stem cell population [27,28]. Violation of its expression leads to a decrease in sperm quality and, in extreme cases, to complete infertility of the male [29]. Another gene, TGFB2 (transforming growth factor beta 2), which belongs to the family of TGF-β signaling molecules, also plays an important role in the regulation of spermatogenesis and [30]. Mammalian studies have shown that TGF-β signaling is involved in the maintenance of spermatogonial stem cells, regulates their differentiation and apoptosis, thus influencing the dynamics of spermatogenesis [30,31]. Also, in one study in mice, correlations were found between the expression levels of TGF-β family genes and sperm quality, including sperm motility and viability [32]. Studies of complete transcriptomics of the testicle and appendage in roosters have revealed that the TGF-β pathway is one of the key signaling pathways regulating spermatogenesis, which interacts with pathways such as MAPK and mTOR [33]. This indicates that TGFB2 is integrated into complex regulatory networks that control sperm production in males. In addition, it was found that the TGFB2 gene is expressed in the seminal fluid of roosters, where it presumably plays an immunomodulatory role [34].
Our study is devoted to the study of multilevel mechanisms of regulation of reproductive function in roosters (Gallus gallus domesticus). Understanding the relationships between the morphology of the testicles, the expression level of key regulatory genes such as DMRT1 and TGFB2, as well as the functional parameters of rooster sperm, may open up new opportunities for improving reproductive performance in poultry farming by using molecular genetic methods in breeding and developing new biotechnological approaches.
2. Materials and Methods
For the study, roosters of the Russian Snow White breed (n = 10) aged 28–32 weeks of life were selected from the bioresource collection “Genetic Collection of Rare and Endangered Chicken breeds” (Russian Research Institute of Farm Animal Genetics and Breeding—Branch of the L.K. Ernst Federal Research Center for Animal Husbandry (RRIFAGB), St. Petersburg, Russia) according to the reaction to abdominal massage and qualitative indicators of fresh semen—ejaculate volume (mL), sperm concentration (billion/mL), total and progressive motility (%), viability (%). The chickens were kept in individual cages with the same feeding, watering and light conditions, which correspond to the direction of productivity of the breed.
2.1. Collection and Processing of the Data
Starting from the age of puberty, the roosters were accustomed to abdominal massage and were used in the semen extraction mode 2 times a week. The semen was selected in accordance with GOST 27267-2017 [35]. Ejaculate volume (mL) was measured with a graduated pipette, and sperm concentration (billion/mL) was measured using an Accuread Photometer (photometer Accuread® IMV Technologies, L’Aigle, France). Total and progressive motility (TM and PM, %) were determined using the CASA ArgusSoft-Poultry imaging system (Motic BA410E, Motic, Xiamen, China; ArgusSoft software-1, St. Petersburg, Russia) at magnification ×100. The viability of the frozen/thawed semen was evaluated by staining with eosin/nigrosin (EN) dye, visualized on a Motic BA410E phase contrast microscope (Motic BA410E, Motic, Xiamen, China) at ×1000 magnification under immersion oil. At least 200 cells were evaluated in each sample. The pink-colored cells were considered damaged (dead). A two-component medium was used to dilute the semen: 1.8 g of glucose, 2.8 g of monosodium glutamate per 100 mL of distilled water (RRIFAGB, patent No. 2485816 of the Russian Federation in 2013). The semen quality was assessed in 4 repetitions for each individual.
2.2. Preparation and Analysis of Histological Sections
Euthanasia of roosters was performed by cervical dislocation. The abdominal cavity was opened, and the right testicle was extracted. The testicles were weighed on digital scales, fixed in a 10% solution of neutral formalin (pH 7.2–7.4), purified with xylene, and then treated with paraffin. Serial sections with a thickness of ~4 µm were prepared from waxed samples on an RMD-3000 rotary microtome (MedTechnicaPoint Techology, St. Petersburg, Russia), which were stained with hematoxylin/eosin and analyzed under a microscope BIOSCOPE-4 (LOMO PLC, St. Petersburg, Russia) (magnification ×100, ×200, ×400) with an imaging system and photo/video software for the fixations. Morphohistological analysis of the composition of the spermatogenic epithelium was determined on 10 randomly selected cross-sections on selected fields measuring 100 × 100 µm2, and the main types of cells of the spermatogenic epithelium were calculated. The average values (M) and standard errors (±SE) were calculated for all indicators. The histological analysis included the calculation and evaluation of the following indicators: number of seminiferous tubules in the field of view (magnification ×100); average diameter of the cross sections of the seminiferous tubules (µm) (magnification ×200); total cross-sectional area of the seminiferous tubules in the field of view (S, µm2); number of cells of the spermatogenic epithelium: spermatogonia, spermatocytes of the first and second order, spermatids and Sertoli cells (increase ×400).
2.3. Isolation of RNA
Aliquots with a volume of 100–150 µL were selected from each individual semen sample for further RNA isolation. Total RNA samples were isolated from germ cells (sperm).
RNA isolation from rooster sperm was performed according to the following protocol. Total RNA isolation from rooster sperm was performed using the commercial Lira-Karib kit (Biolabmix LLC, Novosibirsk, Russia) according to the manufacturer’s recommendations, with individual modificationns.
2.3.1. Preliminary Purification of Sperm from Somatic Cells
The ejaculate (100–200 µL) was washed twice in 1–1.5 mL of saline (0.9% NaCl) at 800× g for 5 min at 4 °C. The supernatant containing sperm was carefully transferred to a new tube, avoiding disturbing the sediment enriched with somatic cells. After the second wash, the sperm were pelleted by centrifugation at 600× g for 5 min at 4 °C. The supernatant was removed, and the sediment was used for lysis.
2.3.2. Cell Lysis and RNA Extraction
A total of 1 mL of Lira-Carib reagent was added to the sperm sediment (per 10–50 μL of original semen). 20 μL of β-mercaptoethanol (final concentration ~0.2%) was added to ensure effective RNase inhibition. The samples were thoroughly homogenized by vortexing for 30–60 s until the sediment was completely dissolved (using ceramic beads). The samples were incubated at room temperature for 5 min to completely dissociate the nucleoprotein complexes.
2.3.3. RNA Precipitation
In total, 200 µL of chloroform per 1 mL of Lira-Carib reagent was added to the lysate. The tubes were vigorously shaken for 15–30 s, then incubated at room temperature for 2–3 min. The tubes were centrifuged at 12,000× g for 15 min at 4 °C. The upper (aqueous) phase, containing the RNA, was carefully transferred to a new RNase-free tube, avoiding the interphase layer and the organic phase. Then 0.5 mL of isopropanol was added to the aqueous phase per 1 mL of the original Lira-Carib reagent. The mixture was incubated for 10 min at room temperature. The mixture was centrifuged at 12,000× g for 10 min at 4 °C. The supernatant was carefully removed, leaving the white RNA sediment at the bottom. The sediment was washed with 1 mL of 75% ethanol (in RNase-free water). The sediment was vortexed or pipetted to resuspend. The sediment was centrifuged at 7500× g for 5 min at 4 °C. The supernatant was removed, and the sediment was allowed to dry for 1–2 min.
2.3.4. RNA Dissolution
Purified RNA was dissolved in 20–50 µL of RNase-free water or TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.0–8.0). The mixture was incubated at 55–60 °C for 10 min to completely dissolve. The dissolved RNA was stored at −80 °C.
2.3.5. RNA Quality and Quantity Control
RNA concentration and purity were assessed spectrophotometrically using a NanoDrop2000c instrument (Thermo Fisher Scientific, Philadelphia, PA, USA). Samples with an A260/A280 ratio in the range of 1.9–2.1 and an A260/A230 ratio > 2.0 were considered suitable for subsequent qPCR analysis. The average sample concentration was 400 μg/mL.
2.4. Analysis of Relative Gene Expression
The level of relative expression was determined by a two-step method, which included (1) cDNA synthesis with reagents from the M–MuLV-RH Reverse Transcriptase kit (Biolabmix LLC, Novosibirsk, Russia) and (2) PCR-RV using BioMaster RT-PCR SYBR Blue (2×) (Biolabmix LLC, Novosibirsk, Russia) on the QuantStudio™ 5 Real Time PCR System (Thermo Fisher Scientific, Philadelphia, PA, USA). RT-PCR reactions for each sample were performed in three repeats. The arithmetic mean was used for subsequent calculations. Relative quantification of gene expression was performed using the ΔΔCt method. As no validated housekeeping genes were available for chicken spermatozoa at the time of the experiment, we used GAPDH as a reference gene, with its expression measured in kidney tissue; this approach allowed for intragroup comparisons within sperm samples. The selection of the primer sequence for the DMRT1, TGFB2, and GAPDH genes was performed using the BLAST program (https://www.ncbi.nlm.nih.gov/, accessed on 20 November 2025). The primers were synthesized by IH-BFM SB RAS (Institute of Chemical Biology and Fundamental Medicine, Siberian Branch of the Russian Academy of Sciences, Novosibirsk, Russia) (Table 1).

Table 1.
The nucleotide sequence of primers for RT-PCR in real time.
2.5. Statistical Processing of Results
To identify possible links between the morphometric, cellular and functional parameters of the testicles, a correlation analysis was performed using the software Statistica 10. (StatSoft, Inc., Tulsa, OK, USA). The statistical significance of the indicators was calculated using Spearman’s coefficient (p < 0.05). The visualization of the correlation matrix was carried out in the form of a “heat map” graph created using the Python 3.12 (Python Software Foundation, Wilmington, DE, USA) scripting programming language. Statistical significance of Spearman’s rank correlation coefficients was assessed at α = 0.05. For a sample size of n = 10, the critical value of |rs| is 0.648; therefore, correlations with |rs| ≥ 0.648 were considered statistically significant (p < 0.05).
3. Results
3.1. Analysis of Rooster Semen
To evaluate the obtained semen, 4 samples of fresh ejaculate were taken from each rooster (n = 10). The average values of qualitative indicators are presented in Table 2.

Table 2.
Indicators of fresh semen in roosters aged 28–30 weeks.
The volume of ejaculate produced ranged from 0.20 mL to 0.79 mL, which corresponds to the norm for mature roosters of egg breeds. The sperm concentration ranged from 1.08 billion/mL to 3.26 billion/mL, which is also within the normal range. The indicators of total and progressive motility of spermatozoa in fresh semen had significant individual variability: TM ranged from 65% to 91%, and PM—from 57% to 79%. Both indicators had rather high values, which is typical for males in the phase of active puberty. The average value for viability was 76.5%, which indicates a high survival rate of germ cells and the suitability of sperm for artificial insemination (at least 70% according to GOST 27267-2017 [35]).
The weight of the roosters’ testicles ranged from 7.16 g to 25.47 g, which indicates individual characteristics in males, which are obviously related to hormonal, genetic or other factors (Table 3). Histological analysis of the testicle structure revealed a typical organization for birds—a multitude of densely packed seminiferous tubules lined with multilayer spermatogenic epithelium. Seminiferous tubules of small and large size, separated by interstitial tissue, are clearly visible on the analyzed samples. At the same time, almost all sections have seminiferous tubules having an elongated and sinuous shape, which indicates a complex anatomical arrangement of the seminal tubules in the testis (Figure 1 and Figure 2).

Table 3.
Cellular composition of the spermatogenic epithelium of the seminiferous tubules of roosters (Gallus gallus domesticus) at the age of 32 weeks of life.
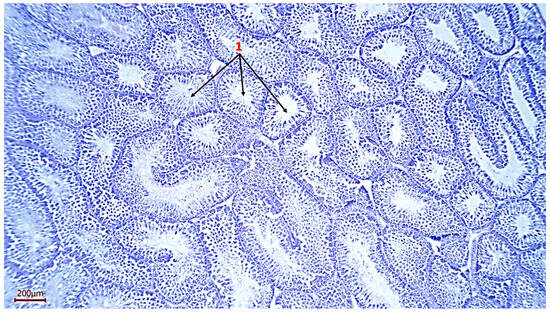
Figure 1.
Histological section of the testicle. 1—seminiferous tubules of a rooster (magnification ×100, scale 200 µm).
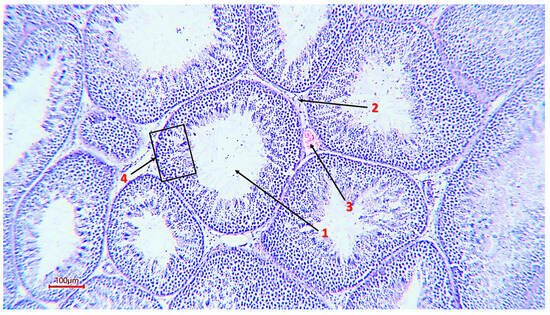
Figure 2.
The general structure of the rooster’s seminiferous tubule (magnification ×200, scale 100 µm), 1—tubule lumen; 2—interstitial tissue; 3—capillary; 4—spermatogenic epithelium.
The micrographs obtained show cells at all stages of spermatogenesis: from basally developed spermatogonia to mature spermatozoa located in the lumen of the tubule. Sertoli cells were present in all sections, which confirms the preservation of the hematotesticular barrier and trophic support of gametes. In the samples, these cells had an elongated shape, an oval light nucleus and extended from the basement membrane to the lumen of the tubule (Figure 3).
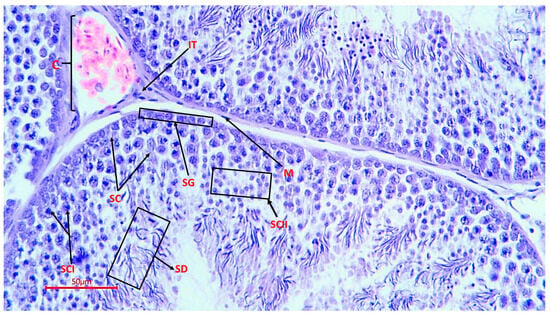
Figure 3.
Spermatogenic epithelium of the seminiferous tubule of a rooster (magnification ×400, scale 50 µm), SG—spermatogonia; SC—Sertoli cells; SCI—spermatocytes of the 1st order; SCII—spermatocytes of the 2nd order; SD—spermatids; M—myoid cells; C—capillary; IT—interstitial tissue with endocrinocytes.
3.2. Morpho-Histology and Morphometry of Testes
In cross-sections on selected fields measuring 100 × 100 µm, the main types of spermatogenic epithelial cells were counted on 10 random sections of each testis (Table 3). For each indicator, the average values (M ± SE) were calculated. The epithelium of the seminiferous tubules contained cells at all stages of spermatogenesis: spermatogonia (13.9 ± 0.9 cells), spermatocytes of the first order (20.3 ± 4.2 cells), spermatocytes of the second order (51.0 ± 13.9 cells), spermatids (65.7 ± 18.9 cells), as well as Sertoli cells (3.6 ± 1.2 cells) (Table 3). The interstitial tissue contained weakly expressed Leydig cells, which corresponds to the typical histological picture of the reproductively active period in roosters at the age of 32 weeks of life.
The average number of seminiferous tubules in the field of view at magnification × 100 was 52.8 ± 9.0 (Table 3). The results of measuring the diameter of the cross sections of the seminiferous tubules showed values from 212.4 µm to 444.4 µm (MEAN ± SE 305.4 ± 8.7 µm). The variability of the diameter of the seminiferous tubules, estimated by the coefficient of variation (CV), was in the range of 17.9–20.4%, which indicates a moderate individual heterogeneity of morphometric parameters. The minimum value of the cross-sectional area of the seminiferous tubules was 44,638.6 µm2 (preparation from male No. 9), and the maximum was 115,027.6 µm2 (preparation from male No. 7); thus, the individual variability of the indicator (CV) was 40.8%.
3.3. Correlation Analysis Between Histological Parameters and Ejaculate Quality
As a result of the Spearman correlation analysis, several significant relationships were revealed between the morphometric parameters of the testicles and the indicators of fresh ejaculate in roosters (at p < 0.05) (Table 4). At n = 10 and the significance level p < 0.05, the critical value of the Spearman coefficient was |p| = 0.648; the relationships with |p| ≤ 0.648 were interpreted as statistically significant. Thus, a correlation was considered statistically significant if its absolute value was ≤0.05. To visualize the strength of correlations, a thermal correlation matrix with a rank scale was constructed (Figure 4).

Table 4.
Significant correlations between histomorphological parameters, gene expression, and ejaculate quality in roosters (n = 10, p ≤ 0.05).
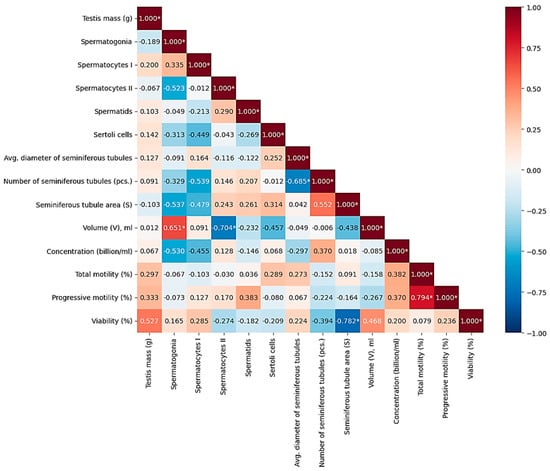
Figure 4.
Correlations between morphometric and physiological parameters of rooster testicles and macro- and microscopic indicators of semen quality. Color scale: red—positive correlation, blue—negative, white—lack of correlation. Note: * significant correlations (p < 0.05).
According to the results of the evaluation of histological samples of rooster testicles by the composition of the spermatogenic epithelium, a positive correlation (r = 0.335) was noted between the number of spermatogonia and spermatocytes of the first order, which relate to the period of germ cell growth during spermatogenesis. Evaluating the quantitative indicators of the number of spermatocytes of the second order and spermatids, which belong to the next stage of spermatogenesis—the transition to the haploid state and the maturation period—a moderate positive correlation was also noted (r = 0.290). The connections were not reliable, but they were logical and predictable. There was no relationship between testicle weight (g) and sperm concentration (billion/mL) and/or ejaculate volume (ml), while the indicator of sperm concentration had a positive relationship with the indicator of the number of seminiferous tubules of the testis (r = 0.370, p < 0.05). The strongest positive reliable (p < 0.05) correlations were found between the number of mature spermatids in the spermatogenic epithelium of the seminiferous tubules and the indicators of sperm motility: the correlation coefficient with progressive motility (PM) was +0.794; between the number of spermatids and sperm viability (r = +0.761). These results indicate that the degree of completion of spermiogenesis, reflected by the number of spermatids, directly determines the quality of sperm. Significant positive associations were also found for other histological parameters. Thus, the number of spermatogonies positively correlated with the volume of ejaculate (r = +0.651), which may indicate that the activity of stem cell proliferation affects the productivity of the seminiferous tubule. In addition, the number of Sertoli cells demonstrated a moderately high and statistically significant relationship with sperm viability (r = +0.678), which emphasizes their role in providing trophic support and ensuring the integrity of the hematotesticular barrier. The revealed high negative correlation between the volume of ejaculate and the number of spermatocytes of the second order (p = −0.704) may indicate a shift in the balance of spermatogenesis with an increase in semen volume. A strong negative relationship was also found between the total cross-sectional area of the seminiferous tubules and sperm viability (p = −0.782). The negative correlation between the number of seminiferous tubules and their average diameter (p = −0.685) probably reflects a compensatory mechanism: as the number of tubules increases, their individual size decreases, and vice versa.
3.4. Analysis of TGFB2 and DMRT1 Gene Expression
Analysis of the relative expression of the TGFB2 and DMRT1 genes in fresh semen of Russian Snow White roosters revealed significant interindividual variability both in terms of the absolute level of transcripts and the expression ratio between the genes (Figure 5 and Figure 6). Thus, the expression level of the TGFB2 gene ranged from 0.12 to 18.49 (in relative units, RQ). The majority of roosters (8 out of 10) had low or moderate expression of TGFB2 (RQ < 1.0). Only one male, No. 2, had a sharply increased expression level of 18.49 RQ, which exceeded the group average by more than 10 times. The average expression value of the TGFB2 gene was 2.59 ± 1.78 RQ. At the same time, the expression of the DMRT1 gene also showed high variability: from 0.006 to 17.20 RQ. In 4 roosters, the expression level of DMRT1 was extremely low (RQ < 0.1), while in male No. 2, it reached a maximum value of 17.20 RQ. The average DMRT1 expression level in the sample was 2.91 ± 1.55 RQ. Interestingly, most roosters with low expression of TGFB2 (RQ < 0.5) also had low or moderate expression of DMRT1 (RQ < 1.0). The exception was sample No. 9, in which the expression of TGFB2 was minimal (0.12 RQ), while the expression of DMRT1 reached 2.21 RQ, one of the highest values in the sample.
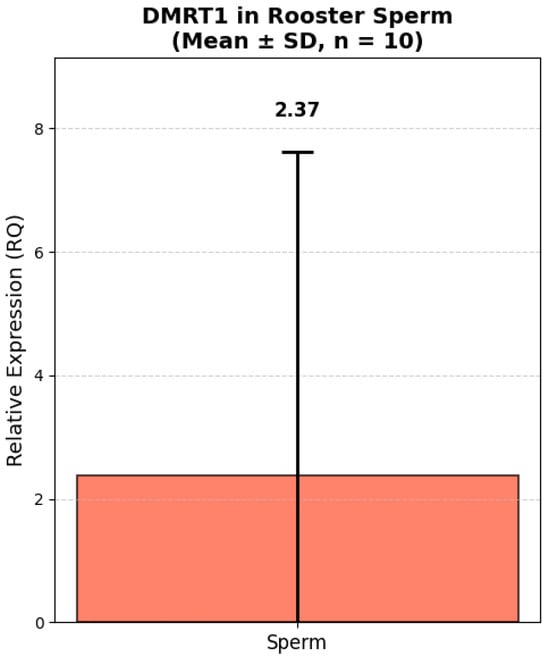
Figure 5.
Relative expression of DMRT1 in rooster spermatozoa (Mean ± SD, n = 10). Note: Relative expression was calculated using the ΔΔCt method with GAPDH as a reference gene. GAPDH expression was measured in kidney tissue due to lack of validated housekeeping genes for sperm in this study. All values are presented as mean ± standard deviation (SD) from 10 roosters.
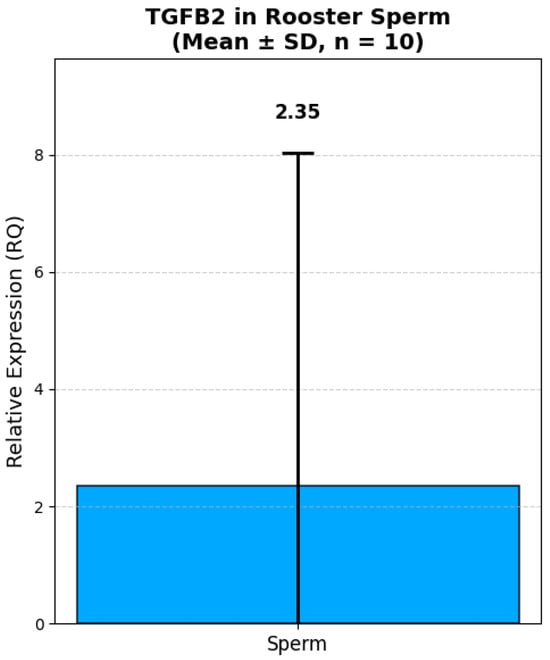
Figure 6.
Relative expression of TGFB2 in rooster spermatozoa (Mean ± SD, n = 10). Note: Relative expression was calculated using the ΔΔCt method with GAPDH as a reference gene. GAPDH expression was measured in kidney tissue due to lack of validated housekeeping genes for sperm in this study. All values are presented as mean ± standard deviation (SD) from 10 roosters.
The data obtained demonstrate that both genes are characterized by comparable levels of relative expression in the fresh semen. The proximity of the average values indicates a possible synchronized expression of these genes, which is consistent with their functional relationship in the regulation of spermatogenesis. High values of the standard error (SE > 1.6) reflect significant variability in expression levels between individuals, which may be due to individual differences in the physiological state and reproductive status of roosters.
The analysis of correlations between the relative expression of the TGFB1 and DMRT1 genes in fresh semen and the morphological/functional parameters of semen in 10 roosters revealed a number of statistically significant and biologically important relationships. The most pronounced and significant was the negative correlation between the level of DMRT1 gene expression and sperm quality. It was found that increased DMRT1 expression is significantly associated with a decrease in sperm motility and viability:
with progressive motility (r = −0.612, p < 0.05),
with total motility (r = −0.552, p < 0.05),
with viability (r = −0.552, p < 0.05).
In addition, the expression level of the DMRT1 gene showed a strong negative correlation with testicle weight (r = −0.782, p < 0.05), which indicates that in roosters with larger testicles, the expression level of this gene in semen is significantly lower. At the same time, the expression of the TGFB1 gene did not show statistically significant correlations with any of the studied parameters of sperm quality (motility, viability, concentration) or histological characteristics of the testicles (tubule diameter, number of cells, etc.). The only observed relationship is a weak positive correlation with DMRT1 expression (r = 0.321), which also did not reach the level of statistical significance (Figure 7).
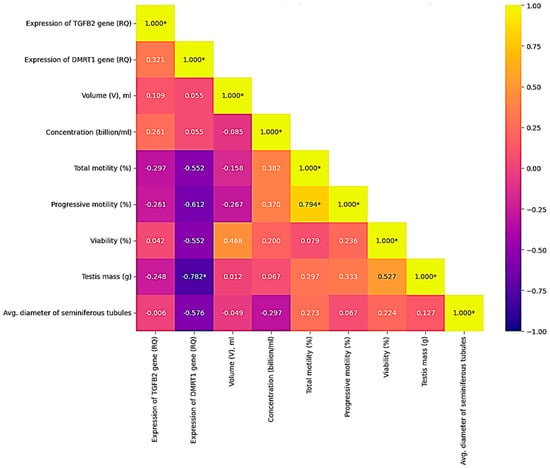
Figure 7.
Correlations between the quality of fresh rooster semen and the expression levels of the TGFB2 and DMRT1 genes (p < 0.05). Color scale: yellow—positive correlation; blue—negative; blue—lack of correlation. Note: * significant correlations (p < 0.05).
4. Discussion
Despite the homogeneous conditions of detention and the identical mode of semen sampling, significant variability in the morphometric parameters of the testicles was observed in our study—their weight ranged from 7.16 g to 25.47 g, which can be explained by individual differences in the level of circulating gonadotropins and androgens in individuals. According to Behnamifar et al. (2025), testosterone levels in the blood serum of roosters affect the morphology of the testicles and the quality of sperm production, including sperm concentration and viability [36]. Also, according to Lengyel et al. (2024), a violation of androgen signaling leads to impaired fertility even while maintaining the morphological integrity of the generative tissue, which confirms the importance of hormonal regulation in spermatogenesis [37]. In addition, our study demonstrated a significant variability (CV) in the size of the diameter of the cross–section of the seminiferous tubules by 20.4% (min diameter of the tubules is 212.7 µm; max is 444.4 µm), which is consistent with the data of other authors who studied the morphometry of reproductive organs in male poultry. For example, in a study by Mfoundou et al. (2022) in broiler chickens, the diameter of the seminiferous tubules before puberty at the age of 4 months was 110–130 µm [9]. Similar results were obtained in another study aimed at studying adaptive changes in the testicles of broilers aged 20–28 days of life (Islam et al., 2021 [14]). It was found that the diameter of the seminiferous tubules of the studied individuals was 135–145 µm, depending on the age of the chickens [14]. In a study by Razi M. Kugler P. (2010), the diameter of the seminiferous tubules of sexually mature roosters with highly active spermatogenic epithelium was 162 µm [38]. This observation is consistent with the well-known morphological principle, according to which an increase in the diameter of the seminiferous tubule is accompanied not only by an increase in its cross-sectional area, but also by the laying of more layers of spermatogenic epithelium [39]. Olawuyi et al. (2019) described that it is this morphometric dependence that allows the organ to adapt to the functional loads associated with spermatogenesis [39]. One of the key results was the detection of a strong negative correlation between the total cross-sectional area of the seminiferous tubules and sperm viability (p = −0.782). At first glance, this contradicts expectations, since an increase in the area of the epithelium is often associated with increased productivity. However, such an observation may reflect the functional heterogeneity of the tubules: in the presence of a large number of large tubules, microcirculation may be impaired, hormonal regulation may be unbalanced, or the effectiveness of supporting Sertoli cells may decrease. Similar results have been obtained in other studies conducted on mammals. For example, in a study by Crean et al. (2023), histological analysis of mouse testicles revealed that the average cross-sectional area of the seminiferous tubules negatively correlates with their number (r = −0.39, p = 0.002) [40]. The authors interpreted this as a compensatory mechanism in which a sufficient number of sperm can be produced either by increasing the diameter of the tubules (with a correspondingly smaller number on the cross-section) or by increasing the number of tubules of a smaller diameter [41]. A similar compensatory mechanism is also observed in the negative relationship between the number of seminiferous tubules and their average diameter (p = −0.685, p < 0.05). It is known that with a fixed volume of testicular tissue, there is a balance between the number of seminiferous tubules and their diameter, due to the need for optimal packaging and distribution of cells inside the gonads [42,43]. This allows the testicle to effectively use space to maximize the area of the spermatogenous epithelium while limiting the total volume of the parenchyma. Compensatory mechanisms in spermatogenesis can manifest themselves in changes in the speed of passage of germ cells through various stages of development. Thus, increased proliferation of spermatogonia in birds with a large volume of ejaculate may be accompanied by accelerated passage of second-order spermatocytes through the stages of meiosis II and early spermatidiation, which explains the negative correlation between the volume of ejaculate and the number of second-order spermatocytes [44]. At the same time, the revealed positive correlation between the volume of ejaculate and the number of spermatogonies (r = 0.651) may indicate that males with a large semen volume have more intense initial stages of spermatogenesis, in particular, the proliferation of spermatogonies. A study by O’Donnell et al., 2017, described that this stage is a hormone-modulated process that can be activated when the demand for sperm production increases [41]. This is consistent with the concept of “demand-driven spermatogenesis”, according to which its intensity adapts to the physiological needs of the body. With increased activity of the accessory glands, sperm production increases, thereby intensifying the proliferation of spermatogonia. It is noteworthy that in our study, such a significant indicator as the concentration of spermatozoa did not have significant correlations with most morphometric parameters of the testicles. Similar results were described in a study by Sun et al. (2019), which also revealed complex, nonlinear relationships between testicle morphometry and sperm quality parameters in Beijing-You roosters aged 40–44 weeks [22]. It was found that males with low sperm motility had low semen volume and sperm viability; however, correlations between the morphometric parameters of the testicles and the concentration of ejaculate were either weak or absent. The authors explained this by the fact that semen quality depends not so much on the structural parameters of the generative tissue of the reproductive organs as on the intensity of germ cell apoptosis, the functional state of the testicle appendage, and other factors [22]. In addition, Liu et al. (2023) demonstrated in their study that the sperm DNA fragmentation index (DFI) negatively correlated with the viability, concentration, and progressive motility of germ cells, but had no relationship with ejaculate volume [44]. This confirms that the various parameters of sperm production are regulated independently of each other and do not always correlate with the morphometric parameters of the testicles.
The development of male reproductive organs, starting from the period of postnatal development to puberty, is characterized by intensive proliferation of Sertoli cells and their subsequent differentiation [45]. The low number of Sertoli cells found in our study (3.6 ± 1.2 in the ×400 field of view) with a sufficiently high number of developing germ cells fully corresponds to the data from the scientific literature, according to which one Sertoli cell is capable of supporting approximately 30–50 gametes [38,45,46]. The low number of Sertoli cells in roosters in relation to basally oriented germ cells, compared with other species, is explained by their high cytochemical activity of diaphorase, lactate dehydrogenase, succinate dehydrogenase, cytochrome oxidase, which are metabolically more independent of Sertoli cells [39]. In a meta-analysis conducted by Rebourcet et al. (2017), they found that there is a strong correlation between the number of Sertoli cells formed during the active reproductive period and the total number of gametes in adulthood (r = 0.800; p < 0.001), which once again confirms the importance of these cells in spermatogenesis [47]. However, a study by Wilson et al. (2018) revealed that in roosters that have reached puberty (~29 weeks), there is a gradual decrease in the concentration of Sertoli cells in the spermatogenic epithelium, which is associated with the onset of active spermatogenesis and the completion of the formation of reproductive organs [20]. The negative correlation observed in our study between the number of Sertoli cells and first-order spermatocytes (p = −0.449) did not reach the threshold of statistical significance; however, it may reflect the phase dynamics of the spermatogenic cycle: in the areas of the seminiferous tubules dominated by the meiotic stage, the relative density of Sertoli cells decreases due to an increase in the number of germinal cells. Alternatively, this may be due to a methodological limitation—the partial overlap of Sertoli cell nuclei with a high density of spermatocytes in the visual field [48].
Of particular interest in our study is the analysis of the relationship between the expression level of key genes involved in the regulation of spermatogenesis and indicators of both the histological structure of the testicles and the functional quality of fresh semen. The most significant results relate to the negative relationship between DMRT1 gene expression and key indicators of reproductive function, which requires special attention and interpretation. For the gene DMRT1, our results showed a mean relative expression (RQ) of 2.38 ± 5.44 in spermatozoa. Although this value is relatively low compared to other tissues, it is biologically significant given that DMRT1 is a master regulator of male sexual development and is known to be highly expressed in testicular tissue. Indeed, Bgee data (https://www.bgee.org/, accessed on 17 November 2020) confirm that DMRT1 exhibits its highest expression rate (91.86) in testicular tissue, followed by spermatogenic epithelial cells of the testes—spermatocytes (87.47) and spermatids (85.20). The presence of detectable DMRT1 transcripts in mature spermatozoa suggests that residual mRNA from earlier stages of spermatogenesis may persist, potentially playing a role in posttesticular maturation and capacitation. Also, published data from Bgee on extremely low or undetectable levels of DMRT1 expression in somatic tissues such as the kidney (25.83) and heart (17.92) indicate its modest role in the regulation of these organs.
Firstly, the expression level of the DMRT1 gene significantly negatively correlates with testicle weight (r = −0.782, p < 0.05). This means that in roosters with larger testicles, the activity of the DMRT1 gene in spermatozoa is reduced. This seemingly paradoxical result may be due to the fact that DMRT1 is a key regulator of the early stages of spermatogenesis and sex determination in birds [49]. In mature testicles with high productivity, where spermatogenesis proceeds intensively, the main expression of DMRT1 is localized in testicle tissue cells (spermatogonia, Sertoli cells), whereas in mature spermatozoa, where the residual transcriptome is active, the level of DMRT1 may be reduced. Thus, the obtained value may reflect not the expression of a gene in semen, but the productive activity of the tissue, where the regulatory function of DMRT1 has already been implemented at previous stages of differentiation. Secondly, the level of DMRT1 gene expression in spermatozoa is negatively related to the functional quality of sperm: negative correlations were found with total motility (r = −0.552), progressive motility (r = −0.612) and viability (r = −0.552). This suggests that an increased DMRT1 level in mature spermatozoa may be a marker of untimely or impaired spermatogenesis. Normally, as spermatids transform into spermatozoa, histones are globally replaced by protamines, and most of the nuclear RNA is displaced [50]. Maintaining a high level of the DMRT1 gene transcript, which is active in the early stages, may indicate the incompleteness of this process, which is likely to negatively affect the morphofunctional maturity and viability of gametes [24]. In addition, the expression level of the DMRT1 gene in spermatozoa and the average diameter of the cross-section of the seminiferous tubules had a negative correlation (p = −0.576), which, although it does not reach the threshold of statistical significance, is consistent with the well-known role of DMRT1 as a regulator of the early stages of germinal tissue differentiation. A decrease in the expression of this gene in adulthood probably reflects the transition of the testicles from the phase of active differentiation to the phase of functional support of spermatogenesis, accompanied by an increase in the morphometric parameters of the tubules.
Unlike DMRT1, the expression of the TGFB2 gene showed no statistically significant correlations with either histological or semen quality indicators. The gene TGFB2 displayed a similar mean RQ (2.35 ± 5.33) in sperm, but its expression pattern across tissues is markedly different. Bgee data show that TGFB2 is expressed at high levels in many tissues, such as skeletal muscle (74,76), brain (63,20), lung (54,57), and at moderate levels in testicular (44,20) and kidney (30,16) tissues. This is logical, since this gene is involved in the TGF-β growth factor signaling pathway. The detection of TGFB2 mRNA in spermatozoa, while not unexpected, raises intriguing questions about its potential role in sperm function or fertilization. Given that TGF-β signaling has been implicated in sperm capacitation and embryo-maternal communication, even low-level expression in sperm could have functional relevance. This is consistent with the well-known role of the TGF-β signaling pathway as a regulator of proliferation, differentiation, and apoptosis of germ and somatic cells directly in the spermatogenic epithelium of the seminiferous tubules, rather than in mature spermatozoa [6,51]. Our study, which focuses on the expression of these genes in semen, cannot reflect the activity of this pathway at the testicle tissue level, which probably explains the lack of identified connections. Interestingly, no direct correlation was found between the TGFB2 and DMRT1 genes themselves (r = 0.321), which indicates an independent regulation of their expression in mature spermatozoa. This result highlights the need to consider each of these genes as a separate molecular marker. The data obtained complement the known information that the quality of sperm is determined not only by quantitative characteristics (volume, concentration), but also by the molecular maturity of germ cells. Our study demonstrates that the level of DMRT1 expression in fresh semen can serve as a prognostic marker of the functional quality of spermatozoa: its low level is associated with high mobility and viability, while increased expression indicates potential disorders in the process of spermiogenesis. Further research should be aimed at studying the mechanisms of compensatory regulation at the molecular level, including analysis of the expression of genes regulating apoptosis and germ cell proliferation, as well as assessment of circulating hormone levels in birds and their relationship to various morphometric parameters of the testicles.
5. Conclusions
As a result of a comprehensive study, key morphometric, cellular, and molecular parameters that affect semen quality in roosters (Gallus gallus domesticus) were identified. It has been shown that the maturity of the spermatogenic epithelium and the structural organization of the seminiferous tubules are closely related to the parameters of sperm motility and viability. It was also found that high expression of the DMRT1 gene in rooster semen may be a marker of immaturity of the spermatogenic process, while the TGFB2 gene performs background regulatory functions (the regulation of spermatogenesis and testicle development) at the molecular level under non-selective conditions. The data obtained indicate the expediency of integrating morphometric, cellular and molecular analysis for an objective assessment of rooster reproductive function.
Author Contributions
Conceptualization, A.I. and O.S.; methodology, M.P.; validation, A.I., Y.S., M.P., I.M. and E.F.; formal analysis, A.I., Y.S. and M.P.; investigation, A.I.; resources, E.F.; data curation, A.I.; writing—original draft preparation, A.I.; writing—review and editing, Y.S. and O.S.; visualization, Y.S. and I.M.; supervision, O.S.; project administration, O.S.; funding acquisition, O.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 24-16-00259.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of Russian Research Institute of Farm Animal Genetics and Breeding—Branch of the L.K. Ernst Federal Research Center for Animal Husbandry (RRIFAGB) (protocol code 1 on 13 January 2025).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the management and staff of the National Center for Farm Animals Genetic Resources (Russian Research Institute of Farm Animal Genetics and Breeding—Branch of the L.K. Ernst Federal Research Center for Animal Husbandry) for their assistance in conducting the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Volkova, N.A.; Kotova, T.O.; Vetokh, A.N.; Larionova, P.V.; Volkova, L.A.; Romanov, M.N.; Zinovieva, N.A. Genome-wide association study of testes development indicators in roosters (Gallus gallus L.). Agric. Biol. 2024, 59, 649–657. [Google Scholar] [CrossRef]
- Mohan, J.; Sharma, S.; Kolluri, G.; Dhama, K. History of artificial insemination in poultry, its components and significance. J. World’s Poult. Sci. 2018, 74, 475–488. [Google Scholar] [CrossRef]
- Beaupre, C.E.; Tressler, C.J.; Beaupré, S.J.; Morgan, J.L.; Bottje, W.G.; Kirby, J.D. Determination of testis temperature rhythms and effects of constant light on testicular function in the domestic fowl (Gallus domesticus). Biol. Reprod. 1997, 56, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Estermann, M.A.; Major, A.T.; Smith, C.A. Genetic Regulation of Avian Testis Development. Genes 2021, 12, 1459. [Google Scholar] [CrossRef]
- Padhi, A.; Ghaly, M.M.; Ma, L. Testis-enriched heat shock protein A2 (HSPA2): Adaptive advantages of the birds with internal testes over the mammals with testicular descent. Sci. Rep. 2016, 6, 18770. [Google Scholar] [CrossRef]
- Guo, H.; Liu, J.; Xu, M.; Zhu, T.; Liu, S.; Liu, P.; Liu, H.; Tang, S.; Li, Z.; Jin, W. Single-cell RNA sequencing uncovers dynamic roadmap during chicken spermatogenesis. BMC Genom. 2025, 26, 746. [Google Scholar] [CrossRef]
- Juiputta, J.; Loengbudnark, W.; Koedkanmark, T.; Chankitisakul, V.; Boonkum, W. Determining the priority semen characteristics and appropriate age for genetic improvement in Thai native roosters. PLoS ONE 2025, 20, e0319537. [Google Scholar] [CrossRef]
- Tesfay, H.H.; Sun, Y.; Li, Y.; Shi, L.; Fan, J.; Wang, P.; Zong, Y.; Ni, A.; Ma, H.; Mani, A.I.; et al. Comparative studies of semen quality traits and sperm kinematic parameters in relation to fertility rate between 2 genetic groups of breed lines. Poult. Sci. 2020, 99, 6139–6146. [Google Scholar] [CrossRef]
- Mfoundou, J.D.L.; Guo, Y.; Yan, Z.; Wang, X. Morpho-histology and morphometry of chicken testes and seminiferous tubules among yellow-feathered broilers of different ages. Vet. Sci. 2022, 9, 485. [Google Scholar] [CrossRef]
- Fleck, D.; Kenzler, L.; Mundt, N.; Strauch, M.; Uesaka, N.; Moosmann, R.; Bruentgens, F.; Missel, A.; Mayerhofer, A.; Merhof, D. ATP activation of peritubular cells drives testicular sperm transport. Elife 2021, 10, e62885. [Google Scholar] [CrossRef]
- Abdul-Rahman, I.I.; Obese, F.Y.; Robinson, J.E. Spermatogenesis and cellular associations in the seminiferous epithelium of Guinea cock (Numida meleagris). Can. J. Anim. Sci. 2016, 97, 241–249. [Google Scholar] [CrossRef]
- Beloglazova, E.V.; Kotova, T.O.; Volkova, N.A.; Volkova, L.A.; Zinovieva, N.A.; Ernst, L.K. Age dynamics of spermatogenesis in cocks in connection with optimization of bioengineering manipulation time. Agric. Biol. 2011, 6, 60–64. [Google Scholar]
- Osman, D. The connection between the seminiferous tubules and the rete testis in the domestic fowl (Gallus domesticus) morphological study. Int. J. Androl. 1980, 3, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Sultana, N.; Ayman, U.; Akter, A.; Afrose, M.; Haque, Z. Morphological and morphometric adaptations of testes in broilers induced by glucocorticoid. Vet. Med. 2021, 66, 520–529. [Google Scholar] [CrossRef]
- Mfoundou, J.D.L.; Guo, Y.J.; Liu, M.M.; Ran, X.R.; Fu, D.H.; Yan, Z.Q.; Li, M.N.; Wang, X.R. The morphological and histological study of chicken left ovary during growth and development among Hy-line brown layers of different ages. Poult. Sci. 2021, 100, 101191. [Google Scholar] [CrossRef]
- Inyawilert, W.; Rungruangsak, J.; Chanthi, S.; Liao, Y.; Phinyo, M.; Tang, P.; Nfor, O. Age-related difference changes semen quality and seminal plasma protein patterns of Thai native rooster. IJAT 2019, 15, 287–296. [Google Scholar]
- Iwasaki, Y.; Ohkawa, K.; Sadakata, H.; Kashiwadate, A.; Takayama-Watanabe, E.; Onitake, K.; Watanabe, A. Two states of active spermatogenesis switch between reproductive and non-reproductive seasons in the testes of the medaka, Oryzias latipes. Dev. Growth Differ. 2009, 51, 521–532. [Google Scholar] [CrossRef]
- Zakariah, M.; Majama, Y.B.; Gazali, Y.A.; Musa, E.Z.; Dasa, J.J.; Molele, R.A.; Mahdy, M.A. Ultrastructural changes in the spermatogenic cells of domestic chicken (Gallus gallus domesticus) observed at different reproductive stages. Micron 2024, 187, 103717. [Google Scholar] [CrossRef]
- Savel’eva, A. Morfology of immature domestic Japanese quail testiculles. Bull. KrasSAU 2017, 3, 36–41. [Google Scholar]
- Wilson, F.D.; Johnson, D.I.; Magee, D.L.; Hoerr, F.J. Testicular histomorphometrics including Sertoli cell quantitation for evaluating hatchability and fertility issues in commercial breeder-broiler roosters. Poult. Sci. 2018, 97, 1738–1747. [Google Scholar] [CrossRef]
- Arai, T.; Kitahara, S.; Horiuchi, S.; Sumi, S.; Yoshida, K. Relationship of testicular volume to semen profiles and serum hormone concentrations in infertile Japanese males. Int. J. Fertil. Womens Med. 1998, 43, 40–47. [Google Scholar]
- Sun, Y.; Xue, F.; Li, Y.; Fu, L.; Bai, H.; Ma, H.; Xu, S.; Chen, J. Differences in semen quality, testicular histomorphology, fertility, reproductive hormone levels, and expression of candidate genes according to sperm motility in Beijing-You chickens. Poult. Sci. 2019, 98, 4182–4189. [Google Scholar] [CrossRef]
- Korshunova, L.G.; Karapetyan, R.V. The Use of the Genetic Methods Based on the DNA Markers of the Productive Traits in the Selection of Chicken. Ptisevodstvo 2021, 5, 4–7. [Google Scholar]
- Smith, C.A.; Roeszler, K.N.; Ohnesorg, T.; Cummins, D.M.; Farlie, P.G.; Doran, T.J.; Sinclair, A.H. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 2009, 461, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, L.S.; Raymond, C.S.; Roeszler, K.N.; Kuroiwa, A.; Nakata, T.; Zarkower, D.; Smith, C.A. Over-expression of DMRT1 induces the male pathway in embryonic chicken gonads. Dev. Biol. 2014, 389, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Katz, M.; Sinclair, A.H. DMRT1 is upregulated in the gonads during female-to-male sex reversal in ZW chicken embryos. Biol. Reprod. 2003, 68, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Zarkower, D.; Murphy, M.W. DMRT1: An ancient sexual regulator required for human gonadogenesis. Sex. Dev. 2022, 16, 112–125. [Google Scholar] [CrossRef]
- Malolina, E.; Kulibin, A.Y. Rete testis and the adjacent seminiferous tubules during postembryonic development in mice. Russ. J. Dev. Biol. 2017, 48, 385–392. [Google Scholar] [CrossRef]
- Augstenová, B.; Ma, W.-J. Decoding Dmrt1: Insights into vertebrate sex determination and gonadal sex differentiation. J. Evol. Biol. 2025, 38, 811–831. [Google Scholar] [CrossRef]
- Young, J.C.; Wakitani, S.; Loveland, K.L. TGF-β superfamily signaling in testis formation and early male germline development. Semin. Cell Dev. Biol. 2015, 45, 94–103. [Google Scholar] [CrossRef]
- Sarraj, M.A.; Escalona, R.M.; Western, P.; Findlay, J.K.; Stenvers, K.L. Effects of TGFbeta2 on wild-type and Tgfbr3 knockout mouse fetal testis. Biol. Reprod. 2013, 88, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, H.; Wu, Z.; Zhang, X.; Sun, Y.; Gao, L.; Zhang, W.; Su, Y.-Q.; Zhang, M. The oocyte cumulus complex regulates mouse sperm migration in the oviduct. Commun. Biol. 2022, 5, 1327. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Cong, B.; Zhu, L.; Zhang, Y.; Yang, Y.; Qi, X.; Wang, X.; Xiao, L.; Long, C.; Xu, Y.; et al. Whole transcriptome sequencing of testis and epididymis reveals genes associated with sperm development in roosters. BMC Genom. 2024, 25, 1029. [Google Scholar] [CrossRef] [PubMed]
- Atikuzzaman, M.; Sanz, L.; Pla, D.; Alvarez-Rodriguez, M.; Rubér, M.; Wright, D.; Calvete, J.J.; Rodriguez-Martinez, H. Selection for higher fertility reflects in the seminal fluid proteome of modern domestic chicken. Comp. Biochem. Physiol. Genom. Proteom. 2017, 21, 27–40. [Google Scholar] [CrossRef] [PubMed]
- GOST 27267-2017; Product for Reproduction:Non-Diluted Fresh Sperm of Cocks and Turkeys-Cock: Specifications. Russian State Standard: Moscow, Russia, 2017.
- Behnamifar, A.; Rahimi, S.; Torshizi, M.A.K.; Sharafi, M.; Grimes, J.L. Physiological impacts of overweight on semen parameters and fertility in male broiler breeders. Poult. Sci. 2025, 104, 104904. [Google Scholar] [CrossRef]
- Lengyel, K.; Rudra, M.; Berghof, T.V.L.; Leitão, A.; Frankl-Vilches, C.; Dittrich, F.; Duda, D.; Klinger, R.; Schleibinger, S.; Sid, H.; et al. Unveiling the critical role of androgen receptor signaling in avian sexual development. Nat. Commun. 2024, 15, 8970. [Google Scholar] [CrossRef]
- Razi, M.; Hassanzadeh, S.; NAJAFI, G.R.; Feyzi, S.; Amin, M.; Moshtagion, M.; Janbaz, H. Histological and anatomical study of the White Rooster of testis, epididymis and ductus deferens. Iran. J. Vet. Med. 2010, 4, 229–236. [Google Scholar]
- Olawuyi, T.S.; Ukwenya, V.O.; Jimoh, A.G.A.; Akinola, K.B. Histomorphometric evaluation of seminiferous tubules and stereological assessment of germ cells in testes following administration of aqueous leaf-extract of Lawsonia inermis on aluminium-induced oxidative stress in adult Wistar rats. JBRA Assist. Reprod. 2019, 23, 24–32. [Google Scholar] [CrossRef]
- Crean, A.J.; Afrin, S.; Niranjan, H.; Pulpitel, T.J.; Ahmad, G.; Senior, A.M.; Freire, T.; Mackay, F.; Nobrega, M.A.; Barrès, R.; et al. Male reproductive traits are differentially affected by dietary macronutrient balance but unrelated to adiposity. Nat. Commun. 2023, 14, 2566. [Google Scholar] [CrossRef]
- O’Donnell, L.; Stanton, P.; de Kretser, D.M. Endocrinology of the Male Reproductive System and Spermatogenesis. In Endotext; Feingold, K.R., Adler, R.A., Ahmed, S.F., Anawalt, B., Blackman, M.R., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://pubmed.ncbi.nlm.nih.gov/25905260/ (accessed on 17 November 2025).
- Kugler, P. Histological and histochemical studies on testis, epididymis and ductus defrens of the rooster (Gallus domesticus). Gegenbaurs Morphol. Jahrb. 1975, 121, 257–288. [Google Scholar]
- Rocha, L.F.; Santana, A.L.A.; Souza, R.S.; Machado-Neves, M.; Oliveira Filho, J.C.; Santos, E.S.C.d.; Araujo, M.L.d.; Cruz, T.B.d.; Barbosa, L.P. Testicular morphometry as a tool to evaluate the efficiency of immunocastration in lambs. Anim. Reprod. 2022, 19, e20210041. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Mao, X.; Pan, F.; Chen, Y.; An, R. Correlation analysis of sperm DNA fragmentation index with semen parameters and the effect of sperm DFI on outcomes of ART. Sci. Rep. 2023, 13, 2717. [Google Scholar] [CrossRef] [PubMed]
- Pastor, L.M.; Zuasti, A.; Ferrer, C.; Bernal-Mañas, C.; Morales, E.; Beltrán-Frutos, E.; Seco-Rovira, V. Proliferation and apoptosis in aged and photoregressed mammalian seminiferous epithelium, with particular attention to rodents and humans. Reprod. Domest. Anim. 2011, 46, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A.; França, L.R. Structure of the Sertoli Cell. In Sertoli Cell Biology; Elsevier Inc.: New York, NY, USA, 2005; pp. 19–40. [Google Scholar]
- Rebourcet, D.; Darbey, A.; Monteiro, A.; Soffientini, U.; Tsai, Y.T.; Handel, I.; Pitetti, J.L.; Nef, S.; Smith, L.B.; O’Shaughnessy, P.J. Sertoli Cell Number Defines and Predicts Germ and Leydig Cell Population Sizes in the Adult Mouse Testis. Endocrinology 2017, 158, 2955–2969. [Google Scholar] [CrossRef]
- Alves, M.G.; Rato, L.; Carvalho, R.A.; Moreira, P.I.; Socorro, S.; Oliveira, P.F. Hormonal control of Sertoli cell metabolism regulates spermatogenesis. Cell. Mol. Life Sci. 2013, 70, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.; Taylor, G.; Zhao, D.; Liu, L.; Idoko-Akoh, A.; Gong, D.; Lovell-Badge, R.; Guioli, S.; McGrew, M.J.; Clinton, M. Primary sex determination in birds depends on DMRT1 dosage, but gonadal sex does not determine adult secondary sex characteristics. Proc. Natl. Acad. Sci. USA 2021, 118, e2020909118. [Google Scholar] [CrossRef]
- Bozhedomov, V.A.; Lipatova, N.A.; Sporish, E.A.; Rokhlikov, I.M.; Vinogradov, I.V. The role of structural abnormalities of sperm chromatin and DNA in the development of infertility. Androl. Genit. Surg. 2012, 3, 82–92. [Google Scholar]
- Ni, F.-D.; Hao, S.-L.; Yang, W.-X. Multiple signaling pathways in Sertoli cells: Recent findings in spermatogenesis. Cell Death Dis. 2019, 10, 541. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.