Simple Summary
Norfloxacin nicotinate (NOR-N), a derivative and effective substitute of norfloxacin (NOR), has been widely used in livestock production and aquaculture sectors. However, the adverse impacts of NOR-N on non-target animals and their associated biological response mechanisms are still not completely elucidated. This study revealed that exposure to the high doses of NOR-N induced apoptosis, developmental neurotoxicity and aberrant DNA methylation in zebrafish (Danio rerio) larvae. These findings contribute to uncovering the response mechanisms of fish to NOR-N exposure and enhancing understanding of the potential hazards that NOR-N may pose to aquaculture ecosystems.
Abstract
This study aimed to evaluate the response mechanisms of zebrafish larvae to Norfloxacin nicotinate (NOR-N) exposure. Embryos were exposed to NOR-N from 4 h post-fertilization (hpf) until 96 hpf. The exposure concentrations included 0.002, 0.2, 1, and 5 mg/L (simulating both normal and exceptionally high environmentally relevant levels of NOR), as well as a high dose of 25 mg/L. Subsequent analyses focused on apoptosis, neurodevelopment, and DNA methylation in the resulting zebrafish larvae. The results showed that high-dose NOR-N (≥5 mg/L) induced obvious apoptotic cell death in zebrafish larvae, accompanied by increased activities of Cas3 and Cas9, up-regulated P53, Bax, Puma, Apaf1, Cas3 and Cas9 genes expression, and reduced Mdm2 levels and Bcl2/Bax ratio. Moreover, exposure to 5 and/or 25 mg/L NOR-N resulted in a significant up-regulation of neurodevelopment-related genes (Sox2, Sox3 and Sox19a), concomitantly with a marked decline in the transcription of DNA methylation genes, including Dnmt1, Dnmt3a1, Dnmt3b1, Dnmt3b2 and Dnmt3b4. Overall, our findings demonstrated that NOR-N exposure could induce apoptosis, developmental neurotoxicity and aberrant DNA methylation in zebrafish larvae. These findings provide insights to guide the safe application of NOR-N in aquaculture and support the assessment of its potential ecological risks to aquatic ecosystems.
1. Introduction
Norfloxacin (NOR), a broad-spectrum antibacterial fluoroquinolone (FQ), is widely used in human and veterinary medical fields, and also serves as a growth promoter in livestock farming and aquaculture practices [1]. However, due to its adverse effects and potential environmental risks to organisms and human health, the use of NOR has been restricted in aquaculture and livestock husbandry in China [2]. Despite these regulations, structurally related alternatives continue to be introduced into the market. One such compound is norfloxacin nicotinate (NOR-N), a chemical adduct formed from nicotinic acid and NOR, which exhibits superior water solubility and bioavailability compared to NOR [3]. These properties have facilitated its widespread use as a replacement for NOR in aquaculture and livestock production. The standard therapeutic regimen for NOR-N in fisheries involves oral administration at 15–30 mg/kg body weight (calculated as NOR) over 3 to 5 days [4]. Nevertheless, upon entering the bloodstream, NOR-N rapidly dissociates into NOR and nicotinic acid, ultimately exerting its biological effects and undergoing metabolism in the form of NOR [5]. Hence, NOR has been used as the target analyte in studies investigating the pharmacokinetics of NOR-N in animal blood and tissues [3,6]. Consequently, the extensive application of NOR-N has raised concerns regarding the accumulation of NOR in aquatic ecosystems, as well as the associated risks of harm to non-target biota such as fish.
Recent studies have detected NOR residues in global surface water bodies, with concentrations typically spanning the ng/L to µg/L range. In China, levels measured in major river basins fall between 0.03 and 1.38 µg/L, while those in shallow groundwater range from 0.44 to 35.3 μg/L [7]. Even higher concentrations have been reported in other regions, including 52.60 μg/L in Kenya [8] and 470 μg/L in India [9]. Notably, an exceptionally high concentration of 6.06 mg/L was documented in mangrove-associated shrimp aquaculture ponds in Vietnam [10]. While such environmental levels may fall below thresholds of immediate concern for human health, they can nonetheless induce adverse effects in non-target aquatic organisms. Accumulating evidence further indicates that NOR exposure induces multifaceted toxic effects on fish species [11,12,13,14]. For instance, NOR has been shown to induce oxidative stress by altering antioxidative enzyme activities including glutathione peroxidase (Gpx), catalase (CAT) and superoxide dismutase (SOD) in species including male goldfish (0.4–10 mg/L) [15], juvenile zebrafish (Danio rerio) (0.0001–30 mg/L) [16] and catfish (0.0025–160 mg/L) [13]. Moreover, NOR exposure could lead to immunotoxicity in crayfish (3 μg/L) [17] and common carp (1 mg/L) [10,18], as well as developmental neurotoxicity and apoptosis in zebrafish embryos (600–1200 mg/L) [19]. Specifically, studies have reported the up-regulation of neurodevelopment-related genes (enolase 2 (Eno2) and sex determining region Y-box 2 (Sox2)) and elevated apoptotic signals, including activated Caspase 3 (Cas3) and an increased the expression ratio of bcl2-associated X protein (Bax) to B-cell lymphoma 2 (Bcl2), in the brain of exposed embryos [19]. Furthermore, as a representative β-diketone antibiotic (DKA), NOR is also associated with genotoxic effects on fish, such as DNA damage and aberrant DNA hypermethylation [20,21]. While the developmental toxicity, apoptosis, oxidative stress, immunotoxicity, genotoxicity and neurotoxicity of NOR have been relatively well-characterized in aquatic animals [22,23,24], the responses of non-target aquatic animals to its widely used derivative, NOR-N, remain largely unexplored.
The zebrafish is not only a popular ornamental fish but also a favored model organism, possessing a well-characterized genome that enables the investigation of toxicity mechanisms of drugs (e.g., antibiotics) on its physiological functions [25,26]. For instance, FQ antibiotics are known to provoke cardiac functional abnormalities and cardiovascular toxicity in zebrafish, and the intensity of these toxic responses shows a positive relationship with the antibiotic exposure dose [25]. Both chronic exposure to low concentrations and acute exposure to high levels of FQs have been shown to impair the zebrafish motor system, including cartilage tissues and skeletal muscle [27]. Studies have also reported that FQ antibiotics can trigger oxidative stress, epigenetic effects and immunotoxicity in zebrafish [28,29]. Moreover, apoptosis, developmental neurotoxicity and DNA methylation have been recognized as core mechanisms underlying drug-induced toxicity in zebrafish embryos [30,31,32]. For example, apoptosis functions as a cellular response mechanism to exogenous stress. Prior investigations have documented that chemical exposures can trigger apoptotic cell death in zebrafish embryos, accompanied by elevated activities of Cas3 and caspase 9 (Cas9), as well as altered expression levels of apoptosis-related genes such as Cas9, tumor protein p53 (P53) and p53 up-regulated modulator of apoptosis (Puma) [31,33,34,35]. Furthermore, toxicity investigations carried out on zebrafish embryos have revealed that the expression profiles of Sox genes (e.g., Sox2, Sox3 and Sox19a) can serve as effective indicators for the rapid assessment of potential developmental neurotoxicity caused by environmental toxicant stress [19,36]. Additionally, DNA methylation, a key epigenetic mechanism governing gene expression across all eukaryotic kingdoms, serves a vital function in mediating the defense response to environmental stressors throughout organismal development [37]. Alterations in the mRNA expression of DNA methyltransferase (Dnmts) genes, such as Dnmt1 and Dnmt3, in zebrafish are now regarded as valuable biomarkers for signaling early-stage abnormal DNA methylation triggered by environmental stimuli, including drug abuse or toxins [32,35,38]. Research indicates that FQ antibiotic-induced oxidative stress may alter the action of Dnmts, thereby triggering hypermethylation in zebrafish larvae [28].
In our previous study, we established five NOR-N exposure concentrations (0.002, 0.2, 1, 5, and 25 mg/L) to evaluate the toxicological impacts of NOR-N on zebrafish embryos over a 96 h post-fertilization (hpf) exposure period. The selected exposure concentrations and durations reflected a combination of relevant exposure scenarios: measured environmental residues of NOR, previously documented effect levels of NOR associated with adverse organismal outcomes, and typical treatment durations used in aquaculture with NOR N. This experimental design demonstrated that NOR-N induced immunotoxicity, oxidative stress and developmental toxicity in zebrafish embryos [39]. Building on these preliminary findings, the present study further investigates NOR-N’s impact on apoptosis, developmental neurotoxicity, and DNA methylation in zebrafish embryos, with the goal of gaining a more thorough comprehension of the response mechanisms of fish to this drug. The specific objectives were: (1) to assess apoptotic responses through multiple indicators, including apoptotic cell death, two key caspase activities (Cas3 and Cas9) and the expression patterns of eight apoptosis-related genes (P53, Bax, Puma, Apaf1 (apoptotic protease activating factor-1), Mdm2 (murine double minute 2), Bcl2, Cas3 and Cas9); and (2) to evaluate the transcriptional changes in three neurodevelopment-associated genes (Sox2, Sox3 and Sox19a) and seven Dnmts (e.g., Dnmt1, Dnmt3a1, Dnmt3a2, Dnmt3b1, Dnmt3b2, Dnmt3b3 and Dnmt3b4) in NOR-N-exposed zebrafish larvae. The findings are expected to provide insights for guiding the safe application of NOR-N in fish farming and assessing its potential ecological risks to aquatic ecosystems.
2. Materials and Methods
2.1. Zebrafish Maintenance
Healthy adult zebrafish, with a mean weight of 0.46 ± 0.08 g and a standard length of 3.58 ± 0.12 cm, were sourced from the Pearl River Fisheries Research Institute (Guangzhou, China). These zebrafish were maintained in an automated rearing system filled with dechlorinated tap water under controlled environmental conditions: temperature 28 ± 1 °C, pH 7.2 ± 0.1, dissolved oxygen 6.3 ± 0.2 mg/L, total water hardness 122.7 ± 3.5 mg/L as CaCO3, and a 14 h light/10 h dark photoperiod. They were provided with brine shrimp as feed twice per day. Prior to spawning induction, adult zebrafish (1 female per 2 males) were housed in separate compartments of spawning tanks overnight. The next morning, spawning was triggered by exposure to full-spectrum LED light (1000 Lux) for 3–4 h; embryos were then gathered within 30 min post-spawning and washed with the system water. At 4 hpf, fertilized embryos exhibiting normal development were identified under an Olympus microscope (Tokyo, Japan) and used in the exposure assay. The animal experimental protocol used in this research was examined and granted approval by Jiangxi Agricultural University’s Committee for the Care and Use of Experimental Animals.
2.2. Experimental Design
NOR-N (purity > 98%, obtained from J&K Scientific, Beijing, China) was dissolved in dechlorinated tap water to prepare a stock solution at 1000 mg/L. Test solutions of varying concentrations (0.002, 0.2, 1, 5, and 25 mg/L) were subsequently prepared by performing serial dilutions of this stock solution. The conditions and protocols for exposure were consistent with those described in our prior research [39]. Briefly, the selection of exposure concentrations and durations was based on three key considerations: (1) the concentrations of 0.002, 0.2, 1, and 5 mg/L were chosen to mimic the actual NOR concentrations reported in surface waters and aquaculture ponds, which span 0.03 µg/L to 6.06 mg/L—where 0.002 and 0.2 mg/L correspond to normal environmentally relevant levels, while 1 and 5 mg/L represent exceptional environmental concentrations; (2) the high concentration of 25 mg/L was designated as a biologically effective level, as it has been shown to induce significant adverse effects on organisms in preceding investigations; and (3) the 96 hpf exposure duration was designed to align with the relevant fishery industry guidelines for NOR-N, which specify a recommended oral dosage of 15–30 mg/kg body weight (expressed as NOR equivalents) administered over a 3–5 day period. Sixty normally developing 4 hpf (sphere-stage) embryos were randomly allocated to each treatment. Using a static-renewal exposure protocol, the embryos were maintained in 100 mL glass beakers containing 60 mL of the respective exposure solutions until 96 hpf. Each experimental group, including the dechlorinated tap water control and all NOR-N treatments, was conducted with three replicates. All exposures were conducted under the following conditions: temperature 28 ± 1 °C, pH 7.3 ± 0.1, dissolved oxygen 6.3 ± 0.2 mg/L, total water hardness 129.4 ± 4.1 mg/L as CaCO3, and a 14 h light/10 h dark cycle. Daily maintenance involved replacing half of the test solution in each beaker to maintain stable exposure concentrations, and monitoring embryo survival twice daily, with immediate removal of any dead individuals. Embryo mortality across experimental groups was low (5–8%), providing a viable sample size of larvae in each group for the following analytical stages. Upon termination of the experiment at 96 hpf, samples were collected from three independent replicate beakers per group (n = 3). Approximately five newly hatched larvae per beaker were sampled for the analysis of apoptotic cells. Additionally, fifteen larvae per beaker were pooled as a single sample for caspase activity assays, and another fifteen were pooled for gene transcription analysis.
2.3. Measurements of Apoptotic Cells
Apoptotic cells in live zebrafish larvae were visualized using acridine orange (AO), a fluorescent dye that selectively stains nucleic acids and serves as a marker for apoptosis [40]. Following three rinses with PBS, the larvae were placed in 5 μg/mL AO at 28 °C in the dark for 30 min. After staining, they were subjected to another three PBS washes [41], briefly anesthetized with 0.03% MS-222, and immediately examined under an Olympus fluorescent microscope (Japan). The semi-quantitative analysis of apoptotic cells was conducted by two blinded researchers independently, yielding consistent results between observers.
2.4. Determination of Caspase Enzymatic Activity
Upon termination of the experiment at 96 hpf, a pool of fifteen larvae per replicate (n = 3) was homogenized on ice in 200 µL of the provided kit lysis buffer. The homogenate underwent centrifugation at 10,000× g for 15 min at 4 °C, and the resulting supernatant was collected to assay Cas3 and Cas9 activities, as well as the total protein content, using commercial kits (Beyotime, Shanghai, China) according to the manufacturer’s protocols. Moreover, to enhance clarity and facilitate direct comparison, caspase activities (expressed in % of control) were calculated as a percentage in relation to the control group.
2.5. Gene Expression Analysis
Trizol reagent (Invitrogen, Carlsbad, CA, USA) was used to isolate total RNA from pools of 15 zebrafish larvae. The integrity and concentration of RNA were, respectively, validated by 1% agarose gel electrophoresis and UV spectrophotometry. Subsequently, 500 ng of total RNA was transcribed reversely into cDNA using the RT reagent Kit (PrimeScript®, Takara Biotechnology, Dalian, China), and the produced cDNA was preserved at −20 °C for subsequent real-time quantitative PCR (qPCR) analysis.
Expression analysis of the P53, Bax, Puma, Apaf1, Mdm2, Bcl2, Cas3, Cas9, Sox2, Sox3, Sox19a, Dnmt1, Dnmt3a1, Dnmt3a2, Dnmt3b1, Dnmt3b2, Dnmt3b3 and Dnmt3b4 genes was performed via qPCR. Reactions were performed in a 20 μL mixture including SYBR Green Premix (Takara Biotechnology, China) and gene-specific primers (Table 1), employing a qPCR System (ABI 7500, Applied Biosystems, Foster City, CA, USA). The cycling program was: 95 °C for 30 s, followed by 40 cycles of 95 °C for 10 s and 60 °C for 34 s, with a melting curve step to verify amplicon specificity. The primers demonstrated amplification efficiencies ranging from 90% to 105%, with melting curve analyses showing a single sharp peak specific to the target amplicon. All reactions were run in triplicate. Relative gene expression was normalized to β-actin and quantified using the 2−△△Ct method.

Table 1.
Sequences of primers used for real-time quantitative PCR.
2.6. Statistical Analysis
Data from all assays were expressed as mean ± standard deviation (SD). To compare treatment groups with the control, one-way analysis of variance (ANOVA) was performed, followed by Dunnett’s post hoc test for multiple comparisons (SPSS 22.0, IBM, Chicago, IL, USA). Levene’s test was conducted to verify variance homogeneity prior to parametric analysis. Significance was accepted at p < 0.05.
3. Results
3.1. Observation of Apoptotic Cells in Live Zebrafish Larvae Following NOR-N Exposure
AO staining of zebrafish larvae at 96 hpf revealed a dose-dependent increase in the number of apoptotic cells within the brain and heart regions. This increase was particularly notable in larvae exposed to 5 and 25 mg/L NOR-N (Figure 1).
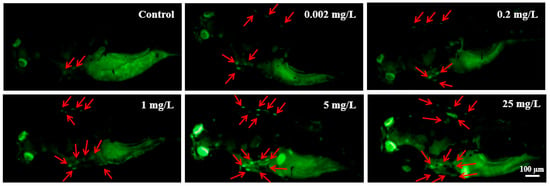
Figure 1.
Apoptotic cells in live zebrafish larvae following exposure to different doses of NOR-N. Red arrows indicate apoptotic cells accumulated in the brain and heart regions of NOR-N exposed larvae.
3.2. Changes in Caspase Enzymatic Activity in Zebrafish Larvae Exposed to NOR-N
Exposure to NOR-N led to a dose-dependent increase in the activities of Cas3 and Cas9 (Figure 2). While the lowest dose (0.002 mg/L) only caused a non-significant rise (p > 0.05) compared to the control, a statistically marked induction was observed at higher doses (≥1 mg/L for Cas3 and ≥5 mg/L for Cas9, p < 0.05).
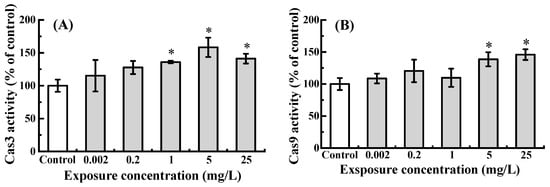
Figure 2.
Cas3 (A) and Cas9 (B) activities in zebrafish larvae following embryonic exposure to NOR-N. Activity levels are expressed as a percentage of the control. Values are presented as mean ± SD (n = 3; 15 larvae for each n). Asterisks (*) indicate significant differences from the control (p < 0.05).
3.3. Responses of Apoptosis-Related Gene Expression in Zebrafish Larvae to NOR-N Exposure
The expression levels of P53, Bax, Puma, Apaf1, Mdm2, Bcl2, Cas3, Cas9 and Bcl2/Bax ratio were profiled in zebrafish embryos following exposure to NOR-N until 96 hpf (Figure 3).
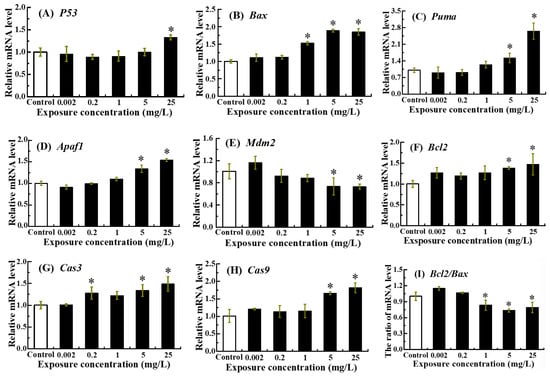
Figure 3.
Expression levels of apoptosis-related genes in zebrafish larvae following embryonic exposure to NOR-N. Relative mRNA levels of (A) P53, (B) Bax, (C) Puma, (D) Apaf1, (E) Mdm2, (F) Bcl2, (G) Cas3, (H) Cas9 and (I) Bcl2/Bax ratio were assessed. Values are presented as mean ± SD (n = 3; 15 larvae for each n). Asterisks (*) indicate significant differences from the control (p < 0.05).
Relative to the control group, a significant increase in P53 mRNA was detected exclusively in the 25 mg/L treatment group (p < 0.05) (Figure 3A). Transcriptional levels of Bax, Puma, Apaf1, Bcl2, Cas3 and Cas9 exhibited a general dose-dependent upward trend, with marked elevations observed following treatment with 5 and 25 mg/L NOR-N (p < 0.05) (Figure 3B–D,F–H). In contrast, both Mdm2 mRNA expression and the Bcl2/Bax ratio displayed clear dose-dependent reductions (Figure 3E,I). Specifically, Mdm2 transcripts were significantly decreased in the 5 and 25 mg/L treatment groups (p < 0.05), while the Bcl2/Bax ratio showed significant declines in the 1, 5 and 25 mg/L treatment groups (p < 0.05).
3.4. Neurodevelopment-Related Gene Expression Patterns in Zebrafish Larvae Under NOR-N Exposure
The mRNA expression of neurodevelopment-related genes including Sox2, Sox3 and Sox19a was presented in Figure 4. Sox2, Sox3 and Sox19a mRNA expression levels exhibited a gradual upward trend as the exposure dose of NOR-N increased, with significant up-regulation detected in the 5 and 25 mg/L NOR-N treatment groups (p < 0.05).
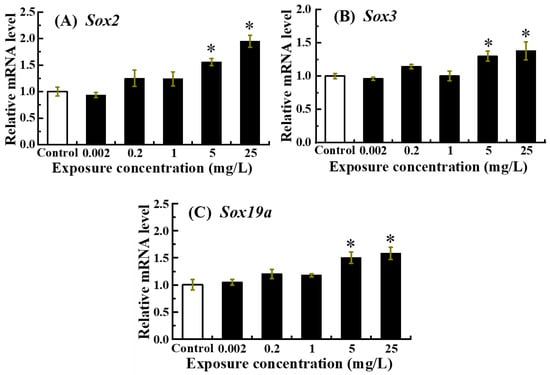
Figure 4.
Relative expression levels of neurodevelopment-related genes in zebrafish larvae following embryonic exposure to NOR-N. Transcript levels of (A) sox2, (B) sox3, and (C) sox19a were measured. Values are presented as mean ± SD (n = 3; 15 larvae for each n). Asterisks (*) indicate significant differences from the control (p < 0.05).
3.5. Transcriptional Responses of DNA Methylation-Associated Genes in Zebrafish Larvae to NOR-N Exposure
The transcriptional responses of DNA methylation-associated genes to NOR-N exposure were shown in Figure 5. A dose-dependent decrease was observed for Dnmt1, Dnmt3a1, Dnmt3b1, Dnmt3b2 and Dnmt3b4, with expression reaching its lowest point at 25 mg/L (p < 0.05) (Figure 5A,B,D,E,G). Conversely, Dnmt3a2 and Dnmt3b3 mRNA levels showed no significant variation compared to the control (p > 0.05) (Figure 5C,F).
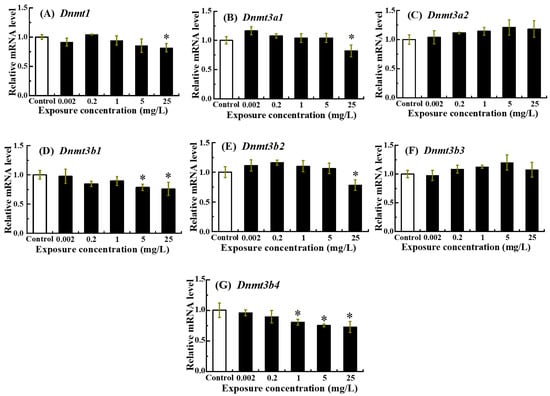
Figure 5.
Transcription levels of DNA methylation-associated genes in zebrafish larvae following embryonic exposure to NOR-N. Relative mRNA levels of (A) Dnmt1, (B) Dnmt3a1, (C) Dnmt3a2, (D) Dnmt3b1, (E) Dnmt3b2, (F) Dnmt3b3 and (G) Dnmt3b4 were measured. Values are presented as mean ± SD (n = 3; 15 larvae for each n). Asterisks (*) indicate significant differences from the control (p < 0.05).
4. Discussion
Prior investigations have indicated that NOR possesses the ability to trigger developmental toxicity, apoptosis, oxidative stress, immunotoxicity, genotoxicity and neurotoxicity in non-target aquatic organisms, such as various fish species [13,16,19,22]. Consequently, considering that NOR-N serves as an adduct and substitute for NOR, the potential harmful impacts of this substance on aquatic organisms warrant attention. Our previous study found that NOR-N exposure could induce immunotoxicity, oxidative stress and developmental toxicity in zebrafish embryos [39]. Nevertheless, to gain a more profound understanding of the fish response mechanisms under NOR-N exposure, it is worthwhile to conduct further investigations into the additional endpoints, including apoptosis, developmental neurotoxicity, and DNA methylation in zebrafish embryos.
Apoptosis serves as an essential mediator of growth and development in organisms including fish, and it usually participates in response to diverse environmental stressors during the early development stage [30,40,42,43]. In the current investigation, exposure to NOR-N led to a dose-dependent increase in apoptotic cells within the brain and heart regions of live zebrafish larvae, indicating that significant apoptosis induction occurred, particularly in the high dose groups (≥5 mg/L).
As is well known, the mitochondria-dependent apoptotic pathway serves as a key regulatory mechanism for cell fate [44]. P53 not only promotes the mitochondrial release of cytochrome c into the cytosol through the activation of pro-apoptotic genes, such as Puma and Bax, but also directly upregulates the transcription of its key negative regulator, Mdm2 [45,46]. Once in the cytoplasm, cytochrome c forms a complex with Apaf1, and this complex induces the sequential activation of Cas9 and then Cas3 [47]. Additionally, during apoptosis, a decrease in the Bcl-2/Bax ratio is a critical event that facilitates cytochrome c release from mitochondria, serving as a decisive indicator of cell death commitment [48]. The current study revealed that exposure to NOR-N caused a dose-dependent increase in P53, Bax, Puma, Apaf1, Cas3 and Cas9 mRNA levels, along with elevated enzymatic activities of Cas3 and Cas9. The parallel elevation in both transcript abundance and catalytic function of these caspases implies that their activity is regulated at the transcriptional level via an autogenous mechanism, thereby implicating the caspase signaling pathway in NOR-N-induced apoptosis in zebrafish larvae [48]. Concurrently, a decrease was observed in anti-apoptotic indicators, including Mdm2 expression and the Bcl2/Bax ratio in the high dose groups (≥5 mg/L). As evidenced by severe apoptosis in Mdm2-knockdown embryos, P53 has been shown to be essential for DNA damage-induced apoptosis in developing zebrafish following environmental insults (e.g., irradiation) [49]. Consistent with this, we found that NOR-N exposure triggered a marked increase in P53 expression and a concurrent significant decrease in Mdm2 expression, indicating that both genes play a role in mediating the apoptotic response. Overall, these results implied that high doses of NOR-N (5 and/or 25 mg/L) activated the mitochondria-dependent pathway to induce apoptotic cell death in zebrafish larvae. Consistent with this, prior investigations have indicated that antibiotics, fungicides and other xenobiotics induced apoptosis in zebrafish larvae, which was associated with the activation of Cas3, the induction of mRNA levels of P53, Bax, Apaf1, Cas3 and Cas9, and the reduction in the Bcl2/Bax ratio [19,31,50].
The proper expression of Sox genes is pivotal for normal nervous system formation in zebrafish embryos, and any alteration may result in adverse developmental outcomes [36]. Among Sox genes, Sox2 is critical for maintaining pluripotency, cellular differentiation, and neuronal formation in zebrafish [51], while Sox3, often alongside Sox1 and Sox2, acts as a key transcription factor in early vertebrate neurodevelopment and neural stem cell maintenance [52]. Sox19a, recognized as one of the earliest markers of the central nervous system of vertebrates, further underscores the importance of this gene family [53]. Consistent with these roles, knockdown of Sox19a, Sox2 or Sox3 in zebrafish embryos led to severe central nervous system abnormalities [54]. Prior investigations have also indicated that exogenous chemicals could disrupt nervous system development by interfering with the transcription of Sox genes [32,55,56]. Importantly, Sox2 is re-expressed in mature neural tissues post-injury and is essential for initiating repair and neural plasticity mechanisms, often as part of a glial or progenitor cell response aimed at counteracting damage [57]. This implied that Sox expression may represent a double-edged sword, serving both as a marker of neurotoxic insult and a potential indicator of the brain’s innate repair mechanisms being activated. In the current study, NOR-N exposure exhibited a dose-dependent increase in Sox2, Sox3, and Sox19a mRNA levels in zebrafish larvae. This up regulation may reflect an attempt by the larvae to mobilize intrinsic resilience mechanisms against the neurotoxic challenge posed by high doses of NOR-N (5 and 25 mg/L). These findings align with earlier reports showing that NOR caused developmental neurotoxicity in larval zebrafish, as evidenced by up-regulating Sox2 and Eno2 (a mature neuron marker) gene expressions [19].
Additionally, it is noteworthy that the developmental neurotoxicity caused by NOR-N in this study was evidenced by the obvious apoptotic cell death in the brain region of larval zebrafish, which is recognized as a late-stage neurotoxicity biomarker [58]. The parallel increases in brain apoptosis and the transcript levels of Sox2, Sox3, and Sox19a suggested that the NOR-N-induced overexpression of these Sox genes might represent a key molecular event underlying NOR-N-induced developmental neurotoxicity. Given these findings, the potential hazards of NOR-N to the developing nervous system in fish warrant serious consideration, and further investigation is needed to fully elucidate the precise response mechanisms in fish induced by this drug.
DNA methylation functions as a key epigenetic mechanism implicated in a variety of biological processes such as genomic imprinting, X-chromosome inactivation and cellular differentiation. Alterations in DNA methylation represent a major non-genotoxic effect caused by exogenous toxic chemicals [37]. This process is regulated by DNA methyltransferases, which establish and maintain genome-wide methylation patterns [59]. As is widely recognized, Dnmt1 functions to maintain the global DNA methylation patterns during DNA replication, whereas Dnmt3b and Dnmt3a regulate de novo methylation, critical for establishing new patterns during early embryogenesis [60,61]. In the current research, following NOR-N exposure, the expression of Dnmt3a2 and Dnmt3b3 remained unchanged, whereas the mRNA expression levels of Dnmt1, Dnmt3a1, Dnmt3b1, Dnmt3b2, and Dnmt3b4 were downregulated at 5 and/or 25 mg/L. This bifurcated pattern suggests a targeted cellular adaptation, in which the sustained expression of Dnmt3a2 and Dnmt3b3 may serve a compensatory or housekeeping role, ensuring precise methylation at genomic loci critical for cellular homeostasis or for the controlled execution of apoptosis [62]. Conversely, the broad suppression of multiple Dnmts could facilitate a programmed reduction in global DNA methylation, a change often linked to the activation of stress response and pro apoptotic genes [63]. Together, these results imply suggested that high doses of NOR-N might disrupt epigenetic regulation by impairing both the DNA methylation maintenance and de novo methylation processes during early zebrafish development. This finding aligns with previous reports that attribute pollutant-induced alterations in DNA methylation to the dysregulated expression of Dnmt3 and Dnmt1 genes [32,38,64]. For example, Pb exposure has been shown to decrease the expression of Dnmt3b and Dnmt1 and the level of global DNA methylation in zebrafish [35,65]. Similarly, Benzo[a]pyrene was observed to generally suppress Dnmt expression and lower global methylation during the development of zebrafish [66], and atrazine exposure was demonstrated to inhibit both the activity and expression of Dnmts, leading to reduced global methylation [67]. These examples illustrate that high doses of NOR-N might similarly regulate global DNA methylation patterns by down-regulating Dnmt expression in larval zebrafish. However, the extent to which NOR-N exposure affects genome-wide methylation or specific gene-level methylation in fish remains unclear, necessitating further research to clarify the detailed mechanisms for epigenetic disruption in fish under NOR-N exposure.
This study demonstrated that exposure to high doses (5 and/or 25 mg/L) of NOR-N led to apoptosis, developmental neurotoxicity, and aberrant DNA methylation in zebrafish larvae at 96 hpf. We recognize certain limitations in translating these findings to environmental contexts. First, current environmental monitoring focuses on the metabolite NOR, with no available data on environmental levels of the parent compound NOR-N. Consequently, our exposure concentrations were based on NOR data and may not accurately reflect real-world NOR-N levels, and we could not distinguish between the biological effects of NOR-N and its active metabolite, NOR. Second, while the zebrafish embryo-larval model is a robust and standardized system for early-life toxicity screening, our conclusions are inherently limited to this specific model and developmental stage. To address these gaps, future work should focus on several key directions. Environmental monitoring must be expanded to specifically quantify NOR-N in aquatic environments, establishing realistic exposure ranges for toxicological testing. Comparative toxicology studies are needed to disentangle the distinct effects of NOR-N and NOR through parallel exposure experiments, supported by analytical methods to track their fate and bioaccumulation. Expanding biological models is crucial; incorporating additional test species (e.g., Daphnia magna or commercially relevant fish) and life stages (e.g., adults) would better define ecological risks and sensitive windows of toxicity. Finally, mechanistic investigations using in vitro systems—such as piscine (e.g., ZFL) or mammalian (e.g., HEK293T) cell lines—could efficiently elucidate direct molecular actions (e.g., on Dnmt activity) [68,69] and provide preliminary insight into potential human health implications, especially given exposure routes via water and food chains.
5. Conclusions
In conclusion, this study showed that exposing zebrafish larvae to high doses of NOR-N (5 and/or 25 mg/L) triggered apoptotic cell death, enhanced Cas9 and Cas3 activities, altered transcript levels of mitochondria-dependent apoptotic pathway genes, up-regulated key neurodevelopmental genes, and suppressed genes associated with DNA methylation. These results indicated that NOR-N induced apoptosis, developmental neurotoxicity, and aberrant DNA methylation in zebrafish larvae. The findings offer valuable insights into the response mechanisms of fish to NOR-N exposure and provide a scientific basis for guiding NOR-N’s safe application in fish farming. Nevertheless, deepening our understanding of NOR-N’s molecular and cellular toxic mechanisms requires additional studies employing diverse aquatic species and in vitro models.
Author Contributions
Writing—original draft, H.F.; Investigation, H.F., R.W., H.L. and X.L.; Methodology, R.W. and X.L.; Visualization, F.W., K.L. and T.S.; Resources, F.W. and K.L.; Formal analysis: H.L., J.R. and L.W.; Writing-review and editing: H.F., F.L. and X.L.; Project administration: F.L. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Science and Technology Project of Jiangxi Provincial Education Department (GJJ180186), the National Natural Science Foundation of China (42367062), the Natural Science Foundation of Jiangxi Province (20202BABL205006), and the Key Technology Research and Development Project of Jiangxi Provincial Department of Agriculture and Rural Affairs (2023yyzygg-05, 2022yyzygg-03, and 2025yyzygg-05).
Institutional Review Board Statement
The animal experimental protocol used in this research was examined and granted approval by Jiangxi Agricultural University’s Committee for the Care and Use of Experimental Animals (Approval Code: JXAULL-2024-04-16; Approval Date: April 2024). All experimental operations were performed in full compliance with China’s Guide for the Care and Use of Laboratory Animals [70].
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We acknowledge and thank the reviewers and the editor of this paper for their constructive feedback that helped improve the quality of our work.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NOR | Norfloxacin |
| FQ | Fluoroquinolone |
| NOR-N | Norfloxacin nicotinate |
| Gpx | Glutathione peroxidase |
| CAT | catalase |
| SOD | superoxide dismutase |
| Eno2 | enolase 2 |
| Sox | sex determining region Y-box |
| Sox2 | sex determining region Y-box 2 |
| Cas3 | Caspase 3 |
| Bax | bcl2-associated X protein |
| Bcl2 | B-cell lymphoma 2 |
| DKA | β-diketone antibiotic |
| Cas9 | caspase 9 |
| P53 | tumor protein p53 |
| Puma | p53 up-regulated modulator of apoptosis |
| Dnmts | DNA methyltransferase |
| Apaf1 | apoptotic protease activating factor-1 |
| Mdm2 | murine double minute 2 |
| hpf | hours post-fertilization |
| AO | acridine orange |
| qPCR | real-time quantitative PCR |
| SD | standard deviation |
| ANOVA | one-way analysis of variance |
References
- Drlica, K.; Malik, M.; Kerns, R.J.; Zhao, X. Quinolone-mediated bacterial death. Antimicrob. Agents Chemother. 2008, 52, 385–392. [Google Scholar] [CrossRef]
- MAPRC (Ministry of Agriculture and Rural Affairs of the People’s Republic of China). Announcement No. 2292 of the Ministry of Agriculture of the People’s Republic of China; Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2015.
- Xu, N.; Ai, X.; Liu, Y.; Yang, Q. Comparative pharmacokinetics of norfloxacin nicotinate in common carp (Cyprinus carpio) and crucian carp (Carassius auratus) after oral administration. J. Vet. Pharmacol. Ther. 2015, 38, 309–312. [Google Scholar] [CrossRef]
- Liu, F. Fishery Antibiotic Drugs (Part 6). Hunan Agric. 2016, 6, 26. [Google Scholar]
- Soback, S.; Gips, M.; Bialer, M.; Bor, A. Effect of lactation on single-dose pharmacokinetics of norfloxacin nicotinate in ewes. Antimicrob. Agents Chemother. 1994, 38, 2336. [Google Scholar] [CrossRef]
- Park, S.C.; Yun, H.I.; Oh, T.K. Comparative pharmacokinetic profiles of two norfloxacin formulations after oral administration in rabbits. J. Vet. Med. Sci. 1998, 60, 661–663. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.G.; Singer, A.C.; Zhu, Y.G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Muriuki, C.W.; Home, P.G.; Raude, J.M.; Ngumba, E.K.; Munala, G.K.; Kairigo, P.K.; Gachanja, A.N.; Tuhkanen, T.A. Occurrence, distribution, and risk assessment of pharmerciuticals in wastewater and open surface drains of peri-urban areas: Case study of Juja town, Kenya. Environ. Pollut. 2020, 267, 115503. [Google Scholar] [CrossRef]
- Ranjan, N.; Singh, P.K.; Maurya, N.S. Pharmaceuticals in water as emerging pollutants for river health: A critical review under Indian conditions. Ecotoxicol. Environ. Saf. 2022, 247, 114220. [Google Scholar] [CrossRef]
- Zhang, J.M.; Li, P.; Chen, C.Z.; Liu, B.; Liu, L.; Li, Z.H. Network-based investigation of norfloxacin-induced nasal immune-related inflammatory responses in carp (Cyprinus carpio) and its cross-species implications for olfactory immune disturbance. Fish Shellfish Immunol. 2025, 166, 110623. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, L.; Ou, R.; Nie, X.; Yang, Y.F.; Wang, F.; Li, K. Effects of norfloxacin on hepatic genes expression of P450 isoforms (CYP1A and CYP3A), GST and P-glycoprotein (P-gp) in Swordtail fish (Xiphophorus Helleri). Ecotoxicology 2015, 24, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Fang, M.; Magnuson, J.T.; Greer, J.B.; Chen, Q.; Zheng, Y.; Xiong, Y.; Luo, S.; Zheng, C.; Schlenk, D. Maternal exposure to environmental antibiotic mixture during gravid period predicts gastrointestinal effects in zebrafish offspring. J. Hazard Mater. 2020, 399, 123009. [Google Scholar] [CrossRef] [PubMed]
- Chandni; Vaseem, H. Investigation of antioxidant defense system, digestive enzymes and histopathological alterations in intestine of catfish, Hetropneustes fossilis exposed to antibiotic norfloxacin. Ecol. Front. 2024, 44, 839–849. [Google Scholar] [CrossRef]
- Bian, C.Q.; Xun, Z.B.; Fu, Y.Y.; Wang, Z.L.; Wang, J.J.; Wu, L. Maternal exposure to norfloxacin induces impairment of cardiac development in zebrafish offspring. Comp. Biochem. Physiol. C 2025, 297, 110263. [Google Scholar] [CrossRef]
- Liu, J.; Lu, G.; Wu, D.; Yan, Z. A multi-biomarker assessment of single and combined effects of norfloxacin and sulfamethoxazole on male goldfish (Carassius auratus). Ecotoxicol. Environ. Saf. 2014, 102, 12–17. [Google Scholar] [CrossRef]
- Bartoskova, M.; Dobsikova, R.; Stancova, V.; Pana, O.; Zivna, D.; Plhalova, L.; Blahova, J.; Marsalek, P. Norfloxacin-toxicity for zebrafish (Danio rerio) focused on oxidative stress parameters. BioMed Res. Int. 2014, 2014, 560235. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, F.; Jin, Q. Antibiotics and chemical disease-control agents reduce innate disease resistance in crayfish. Fish Shellfish Immunol. 2019, 86, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Li, L.X.; Zhang, S.Q.; Yin, M.H.; Li, T.Z.; Zeng, B.H.; Liu, L.; Li, P.; Li, Z.H. Tissue-specific toxicity in common carp (Cyprinus carpio) caused by combined exposure to triphenyltin and norfloxacin. Fishes 2024, 9, 415. [Google Scholar] [CrossRef]
- Xi, J.; Liu, J.; He, S.; Shen, W.; Wei, C.; Li, K.; Zhang, Y.; Yue, J.; Yang, Z. Effects of norfloxacin exposure on neurodevelopment of zebrafish (Danio rerio) embryos. Neurotoxicology 2019, 72, 85–94. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Liu, J.; Yin, X.; Zhang, Z.; Wang, C.; Li, Y.; Wang, H. Reproductive toxicity of β-diketone antibiotic mixtures to zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2017, 141, 160–170. [Google Scholar] [CrossRef]
- Yin, X.; Wang, H.; Zhang, Y.; Dahlgren, R.A.; Zhang, H.; Shi, M.; Gao, M.; Wang, X. Toxicological assessment of trace beta-diketone antibiotic mixtures on zebrafish (Danio rerio) by proteomic analysis. PLoS ONE 2014, 9, e102731. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Mukherjee, S.; Mandal, S.M. Fluoroquinolone antibiotics show genotoxic effect through DNA-binding and oxidative damage. Spectrochim. Acta A 2020, 227, 117634. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. A review of the toxicity in fish exposed to antibiotics. Comp. Biochem. Phys. C 2020, 237, 108840. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Li, P.; Zhao, X.L.; He, S.W.; Xing, S.Y.; Cao, Z.H.; Zhang, H.Q.; Li, Z.H. Hepatotoxicity in carp (Cyprinus carpio) exposed to environmental levels of norfloxacin (NOR): Some latest evidences from transcriptomics analysis, biochemical parameters and histopathological changes. Chemosphere 2021, 283, 131210. [Google Scholar] [CrossRef]
- Shen, R.; Yu, Y.; Lan, R.; Yu, R.; Yuan, Z.; Xia, Z. The cardiovascular toxicity induced by high doses of gatifloxacin and ciprofloxacin in zebrafish. Environ. Pollut. 2019, 254, 112861. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ma, Y.; Yao, S.; Zhang, J.; Hu, C. In vivo and in silico evaluations of survival and cardiac developmental toxicity of quinolone antibiotics in zebrafish embryos (Danio rerio). Environ. Pollut. 2021, 277, 116779. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Zeng, X.; Cong, X.; Yu, K.; Qiu, Y.; Yu, X.; Zhang, L.; Wu, Y.; Zhang, W.; Huang, L. The effects of environmental fluoroquinolones on the motor system in zebrafish: Reversible? Ecotoxicol. Environ. Saf. 2025, 304, 119116. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Pinto, G.; Correia, B.; Nunes, B. Embryonic development, locomotor behavior, biochemical, and epigenetic effects of the pharmaceutical drugs paracetamol and ciprofloxacin in larvae and embryos of Danio rerio when exposed to environmental realistic levels of both drugs. Environ. Toxicol. 2019, 34, 1177–1190. [Google Scholar] [CrossRef]
- Li, F.; Wang, H.; Liu, J.; Lin, J.; Zeng, A.; Ai, W.; Wang, X.; Dahlgren, R.A.; Wang, H. Immunotoxicity of β-diketone antibiotic mixtures to zebrafish (Danio rerio) by transcriptome analysis. PLoS ONE 2016, 11, e0152530. [Google Scholar] [CrossRef]
- Iftikhar, N.; Konig, I.; English, C.; Ivantsova, E.; Souders II, C.L.; Hashmi, I.; Martyniuk, C.J. Sulfamethoxazole (SMX) alters immune and apoptotic endpoints in developing zebrafish (Danio rerio). Toxics 2023, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Chen, J.; Cao, X.; Zhang, J.; Luo, J.; Wang, Y. Roxithromycin exposure induces motoneuron malformation and behavioral deficits of zebrafish by interfering with the differentiation of motor neuron progenitor cells. Ecotoxicol. Environ. Saf. 2024, 276, 116327. [Google Scholar] [CrossRef]
- Santos, D.; Luzio, A.; Bellas, J.; Monteiro, S.M. Microplastics- and copper-induced changes in neurogenesis and DNA methyltransferases in the early life stages of zebrafish. Chem. Biol. Interact. 2022, 363, 110021. [Google Scholar] [CrossRef] [PubMed]
- Hrubik, J.; Glisic, B.; Samardzija, D.; Stanic, B.; Pogrmic-Majkic, K.; Fa, S.; Andric, N. Effect of PMA-induced protein kinase C activation on development and apoptosis in early zebrafish embryos. Comp. Biochem. Phys. C 2016, 190, 24–31. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, Y.; Yu, R.; Zhao, X.; Wang, Q.; Cai, L. Pretilachlor has the potential to induce endocrine disruption, oxidative stress, apoptosis and immunotoxicity during zebrafish embryo development. Environ. Toxicol. Pharmacol. 2016, 42, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Han, E.J.; Ahn, G.; Kwak, I.S. Effects of thermal stress-induced lead (Pb) toxicity on apoptotic cell death, inflammatory response, oxidative defense, and DNA methylation in zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2020, 224, 105479. [Google Scholar] [CrossRef]
- Desai, K.; Spikings, E.; Zhang, T. Use of methanol as cryoprotectant and its effect on sox genes and proteins in chilled zebrafish embryos. Cryobiology 2015, 71, 1–11. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Yao, H.; Zhao, F.; Wang, L.; Wang, X.; Xing, H.; Xu, S. Effects of atrazine and chlorpyrifos on DNA methylation in the liver, kidney and gill of the common carp (Cyprinus carpio L.). Ecotoxicol. Environ. Saf. 2014, 108, 142–151. [Google Scholar] [CrossRef]
- Aluru, N.; Kuo, E.; Helfrich, L.W.; Karchner, S.I.; Linney, E.A.; Pais, J.E.; Franks, D.G. Developmental exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin alters DNA methyltransferase (dnmt) expression in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 2015, 284, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, F.; Li, K.; Nie, X.; Fang, H. Effects of norfloxacin nicotinate on the early life stage of zebrafish (Danio rerio): Developmental toxicity, oxidative stress and immunotoxicity. Fish Shellfish Immunol. 2020, 96, 262–269. [Google Scholar] [CrossRef]
- Deng, J.; Yu, L.; Liu, C.; Yu, K.; Shi, X.; Yeung, L.W.Y.; Lam, P.K.S.; Wu, R.S.S.; Zhou, B. Hexabromocyclododecane-induced developmental toxicity and apoptosis in zebrafish embryos. Aquat. Toxicol. 2009, 93, 29–36. [Google Scholar] [CrossRef]
- Duan, J.; Yu, Y.; Shi, H.; Tian, L.; Guo, C.; Huang, P.; Zhou, X.; Peng, S.; Sun, Z. Toxic effects of silica nanoparticles on zebrafish embryos and larvae. PLoS ONE 2013, 8, e74606. [Google Scholar] [CrossRef]
- Dong, W.Q.; Sun, H.J.; Zhang, Y.; Lin, H.J.; Chen, J.R.; Hong, H.C. Impact on growth, oxidative stress, and apoptosis-related gene transcription of zebrafish after exposure to low concentration of arsenite. Chemosphere 2018, 211, 648–652. [Google Scholar] [CrossRef]
- Park, K.; Han, E.J.; Ahn, G.; Kwak, I.S. Effects of combined stressors to cadmium and high temperature on antioxidant defense, apoptotic cell death, and DNA methylation in zebrafish (Danio rerio) embryos. Sci. Total Environ. 2020, 716, 137130. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef]
- Gopisetty, G.; Ramachandran, K.; Singal, R. DNA methylation and apoptosis. Mol. Immunol. 2006, 43, 1729–1740. [Google Scholar] [CrossRef]
- Chua, J.S.; Liew, H.P.; Guo, L.; Lane, D.P. Tumor-specific signaling to p53 is mimicked by Mdm2 inactivation in zebrafish: Insights from mdm2 and mdm4 mutant zebrafish. Oncogene 2015, 34, 5933–5941. [Google Scholar] [CrossRef]
- Santos, N.M.S.D.; Vale, A.D.; Reis, M.I.R.; Silva, M.T. Fish and apoptosis: Molecules and pathways. Curr. Pharm. Des. 2008, 14, 148–169. [Google Scholar] [CrossRef]
- Jin, Y.; Zheng, S.; Fu, Z. Embryonic exposure to cypermethrin induces apoptosis and immunotoxicity in zebrafish (Danio rerio). Fish Shellfish Immunol. 2012, 30, 1049–1054. [Google Scholar] [CrossRef]
- Langheinrich, U.; Hennen, E.; Stott, G.; Vacun, G. Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr. Biol. 2002, 12, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Wu, P.; Huang, L.; Li, H.; Qian, L.; Pang, S.; Qiu, L. Short-term developmental effects and potential mechanisms of azoxystrobin in larval and adult zebrafish (Danio rerio). Aquat. Toxicol. 2018, 198, 129–140. [Google Scholar] [CrossRef]
- Gong, J.; Hu, S.Q.; Huang, Z.G.; Hu, Y.B.; Wang, X.N.; Zhao, J.X.; Qian, P.P.; Wang, C.; Sheng, J.J.; Lu, X.F.; et al. The requirement of Sox2 for the spinal cord motor neuron development of zebrafish. Front. Mol. Neurosci. 2020, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Archer, T.C.; Jin, J.; Casey, E.S. Interaction of Sox1, Sox2, Sox3 and Oct4 during primary neurogenesis. Dev. Biol. 2011, 350, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Vriz, S.; Joly, C.; Boulekbache, H.; Condamine, H. Zygotic expression of the zebrafish Sox-19, an HMG box-containing gene, suggests an involvement in central nervous system development. Mol. Brain Res. 1996, 40, 221–228. [Google Scholar] [CrossRef]
- Okuda, Y.; Ogura, E.; Kondoh, H.; Kamachi, Y. B1 SOX coordinate cell specification with patterning and morphogenesis in the early zebrafish embryo. Dev. Biol. 2010, 344, 490–491. [Google Scholar] [CrossRef]
- Li, B.; Chen, J.; Du, Q.; Wang, B.; Qu, Y.; Chang, Z. Toxic effects of dechlorane plus on the common carp (Cyprinus carpio) embryonic development. Chemosphere 2020, 249, 126481. [Google Scholar] [CrossRef]
- Wang, B.; Dong, J.; Xiao, H.; Li, Y.; Jin, Y.; Cui, M.; Zhang, S.Q.; Fan, S.J. Metformin fights against radiation-induced early developmental toxicity. Sci. Total Environ. 2020, 732, 139274. [Google Scholar] [CrossRef] [PubMed]
- Balint, V.; Peric, M.; Dacic, S.; Stanisavljevic Ninkovic, D.; Marjanovic, J.; Popovic, J.; Stevanovic, M.; Lazic, A. The role of SOX2 and SOX9 transcription factors in the reactivation-related functional properties of NT2/D1-derived astrocytes. Biomedicines 2024, 12, 796. [Google Scholar] [CrossRef]
- Liu, F.; Han, X.; Li, N.; Liu, K.; Kang, W. Aconitum alkaloids induce cardiotoxicity and apoptosis in embryonic zebrafish by influencing the expression of cardiovascular relative genes. Toxicol. Lett. 2019, 305, 10–18. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Qiu, Q.; Qi, Y.; Huang, D.; Zhang, Y. 2,4-Dichlorophenol induces global DNA hypermethylation through the increase of S-adenosylmethionine and the upregulation of DNMTs mRNA in the liver of goldfish Carassius auratus. Comp. Biochem. Phys. C 2013, 160, 54–59. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Jiang, M.; Gao, J.; Zhou, X.; Zhong, H.; Zhang, S.; Xu, J.; Yu, F.; Lai, X.; Yan, B.; Gao, H. Differentiation of mtDNA methylation in tissues of ridgetail white prawn, Exopalaemon carinicauda. Animals 2025, 15, 2037. [Google Scholar] [CrossRef]
- Chen, T.P.; Ueda, Y.; Dodge, J.E.; Wang, Z.J.; Li, E. Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Mol. Cell. Biol. 2003, 23, 5594–5605. [Google Scholar] [CrossRef]
- Saini, M.; Selokar, N.L.; Agrawal, H.; Singla, S.K.; Palta, P. Treatment of buffalo (Bubalus bubalis) donor cells with trichostatin A and 5-aza-2′-deoxycytidine alters their growth characteristics, gene expression and epigenetic status and improves the in vitro developmental competence, quality and epigenetic status of cloned embryos. Reprod. Fertil. Dev. 2014, 28, 824. [Google Scholar]
- Gyimah, E.; Xu, H.; Fosu, S.; Mensah, J.K.; Dong, X.; Akoto, O.; Issaka, E.; Zhang, Z. Gene expression patterns and DNA methylation of neuron and pancreatic β-cell developments in zebrafish embryos treated with bisphenol F and AF. Heliyon 2024, 10, e33805. [Google Scholar] [CrossRef]
- Sanchez, O.F.; Lee, J.; Hing, N.Y.K.; Kim, S.-E.; Freeman, J.L.; Yuan, C. Lead (Pb) exposure reduces global DNA methylation level by non-competitive inhibition and alteration of dnmt expression. Metallomics 2017, 9, 149–160. [Google Scholar] [CrossRef]
- Knecht, A.L.; Truong, L.; Marvel, S.W.; Reif, D.M.; Garcia, A.; Lu, C.; Simonich, M.T.; Teeguarden, J.G.; Tanguay, R.L. Transgenerational inheritance of neurobehavioral and physiological deficits from developmental exposure to benzo[a]pyrene in zebrafish. Toxicol. Appl. Pharmacol. 2017, 329, 148–157. [Google Scholar] [CrossRef]
- Wirbisky-Hershberger, S.E.; Sanchez, O.F.; Horzmann, K.A.; Thanki, D.; Yuan, C.; Freeman, J.L. Atrazine exposure decreases the activity of DNMTs, global DNA methylation levels, and dnmt expression. Food Chem. Toxicol. 2017, 109, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Sae-Lee, C.; Barrow, T.M.; Colicino, E.; Choi, S.H.; Rabanal-Ruiz, Y.; Green, D.; Korolchuk, V.I.; Mathers, J.C.; Byun, H.-M. Genomic targets and selective inhibition of DNA methyltransferase isoforms. Clin. Epigenet. 2022, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Blanc, M.; Rüegg, J.; Scherbak, N.; Keiter, S.H. Environmental chemicals differentially affect epigenetic-related mechanisms in the zebrafish liver (ZF-L) cell line and in zebrafish embryos. Aquat. Toxicol. 2019, 215, 105272. [Google Scholar] [CrossRef]
- National Research Council. Guide for the Care and Use of Laboratory Animals—Chinese Version; The National Academies Press: Washington, DC, USA, 2015.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.