Simple Summary
Chlorella vulgaris (CLV) is a green microalga with significant potential as a sustainable animal feed supplement due to its rich nutritional composition. The nutritional profile of CLV, which includes fatty acids, amino acids, vitamins, and minerals, can vary significantly depending on cultivation conditions such as nutrient availability, light intensity, temperature, water pH, and strain. Studies across various livestock species, such as ruminants, poultry, pigs, rabbits, and fish, suggest that CLV supplementation can lead to several benefits. These benefits include improved growth performance, enhanced nutrient digestibility, better product quality, and overall improvements in animal health and welfare. However, the effects of CLV supplementation are dose-dependent and can vary across different animal species. Therefore, determining optimal inclusion levels is crucial, and further species-specific research is necessary to fully understand the long-term implications of CLV in animal diets.
Abstract
This review explores the potential of Chlorella vulgaris (CLV) as an alternative supplement in animal feed. CLV is rich in essential nutrients including fatty acids, amino acids, vitamins, and minerals, as well as bioactive compounds such as antioxidants, which contribute to its health-promoting properties. The nutritional composition of CLV can vary depending on factors such as cultivation methods, nutrient availability, light intensity, temperature, water pH, strain, and processing techniques. The rigid cell wall of the microalga limits nutrient accessibility, particularly in monogastric animals. However, processing techniques such as enzymatic treatments can disrupt the cell wall, enhancing nutrient bioavailability and improving its utility as a feed ingredient. Research across livestock species has demonstrated the positive effects of CLV supplementation. For instance, CLV has improved milk production and composition in ruminants, modulated rumen microbiota, enhanced lamb growth, and elevated blood immunoglobulin levels. Moreover, the impact of CLV on ruminal fermentation is dose-dependent, with higher inclusion rates exhibiting more pronounced effects, and it may also play a role in mitigating methane emissions. In poultry, CLV supplementation leads to better growth, feed conversion ratios, immune responses, and meat and egg quality. Similarly, studies on pigs suggest that CLV can benefit immune response and fatty acid profiles, while in rabbits, CLV has been found to reduce oxidative stress and improve immune responses. Additionally, CLV has shown promise in aquaculture, improving feed utilization, immunity, and disease resistance in various fish species. While CLV shows considerable potential, the variability in animal responses and the need for optimized inclusion levels necessitate further species-specific research to elucidate the long-term implications of its inclusion in animal diets.
1. Introduction
Global demand for meat, milk, and eggs is projected to surge by 60–70% by 2050, driven by population growth, rising incomes, and dietary shifts in developing countries. Meeting this growing demand requires more efficient and environmentally sustainable methods of livestock feeding, as traditional feed production is often resource-intensive and competes with other agricultural needs [1,2]. To address these challenges, feed supplements have been widely incorporated into animal diets to optimize health, growth, reproduction, and overall production efficiency [3,4,5]. Among these supplements, those derived from the green microalgae Chlorella vulgaris (CLV) are gaining recognition for their multifaceted benefits, including high nutrient density and the potential to replace conventional feed ingredients [3,4,5,6]. For instance, microalgae can serve as alternatives to synthetic vitamins, amino acids, and antibiotics in conventional feeds, enhancing nutrient bioavailability and supporting animal health without contributing to antimicrobial resistance [4,5,6]. Chlorella vulgaris stands out as a promising alternative due to its high nutritional value and potential benefits for animal performance, product quality, and welfare [3,4,5,6]. Studies demonstrate that CLV supplementation enhances innate immunity in poultry, improves omega-3 fatty acid profiles in ruminant-derived products, and increases antioxidant levels in eggs and milk, positioning it as a multifunctional supplement for diverse livestock systems [4,5,6]. Microalgae, including CLV, are photosynthetic organisms that thrive in either photoautotrophic (utilizing sunlight, CO2, and water) or heterotrophic environments [5,6]. They require minimal resources to convert into nutrient-rich biomass [7,8,9]. This biomass is packed with essential nutrients such as fatty acids, amino acids, carotenoids, carbohydrates, pigments, vitamins, minerals, and antioxidants, making it a highly valuable feed ingredient (Figure 1) [10,11,12,13,14].
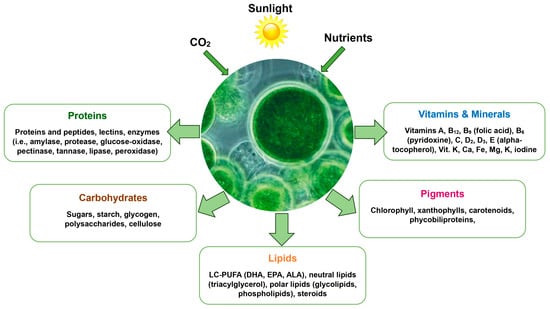
Figure 1.
The nutritional value of Chlorella vulgaris as an animal feed supplement. Adapted from Saadaoui et al. [13].
The cultivation of CLV has a relatively lower environmental impact compared to traditional agriculture. It requires less land and can be grown in controlled environments, reducing the need for pesticides and fertilizers [15]. CLV is known for its rapid growth, with a doubling time of less than 20 h [16], allowing for efficient and potentially large-scale production. This suggests that CLV can contribute to sustainable animal feed production by offering a nutrient-rich alternative to traditional feed sources while requiring fewer resources and minimizing competition with other agricultural sectors.
Studies suggest CLV supplementation can improve animal growth performance, nutrient digestibility, product quality, and overall health and welfare. This paper discusses the potential implications of dietary CLV supplementation as a sustainable animal feed. It examines the nutrient composition of CLV and reviews the results of studies conducted both in living animals and in laboratory settings using rumen fermentation techniques. The research explores how CLV impacts animal physiology and performance, and its implications for livestock production, including its potential to improve nutrient breakdown and mitigate methane emissions, which is significant for environmental sustainability.
2. Materials and Methods
2.1. Literature Search
A systematic and comprehensive literature search was conducted to evaluate the potential of Chlorella vulgaris (CLV) as a sustainable and effective animal feed supplement. The search spanned multiple academic databases, including Google Scholar, ScienceDirect, Scopus, PubMed, and Web of Science, to ensure a broad and inclusive coverage of relevant studies. The primary objective was to identify peer-reviewed, primary research articles that provided original experimental data on the efficacy of CLV in animal diets. This focus on primary studies was critical to ensure methodological rigor, data accuracy, and the ability to directly assess outcomes such as methane reduction, growth performance, nutrient digestibility, and physiological effects in animals. Both in vitro and in vivo studies were included to evaluate the reproducibility of findings and their relevance to real-world farming scenarios. Peer-reviewed journal articles formed the core of the synthesis. However, conference papers were occasionally referenced to capture emerging insights, provided they offered novel and credible data.
2.2. Searching Criteria
The search strategy employed a combination of keywords and phrases related to Chlorella vulgaris and its application in animal nutrition. Key terms included “Chlorella vulgaris nutrient composition”, “Chlorella vulgaris protein content”, “Chlorella vulgaris fatty acid profile”, “Chlorella vulgaris methane reduction”, “Chlorella vulgaris ruminal fermentation”, “Chlorella vulgaris animal growth performance”, and “Chlorella vulgaris immune response”, among others. Boolean operators (AND/OR) were used to refine the search and maximize the retrieval of relevant studies. Additional filters were applied to focus on studies involving ruminants, poultry, aquaculture, swine, and rabbits, aligning within the scope of this review. To ensure comprehensiveness, no restrictions were placed on publication years, allowing the inclusion of foundational studies as well as recent advancements. Reference lists of retrieved articles were manually screened to identify additional relevant publications, which were then sourced from the aforementioned databases. Non-academic sources, such as commercial websites or unverified reports, were excluded to maintain scientific rigor. Studies focusing on other strains of Chlorella or non-feed applications of algae were also omitted to ensure a focused and high-quality synthesis.
3. The Evolution and Global Trends of Chlorella vulgaris Production
Chlorella vulgaris, a single-celled green microalga, holds a significant place in evolutionary history as one of Earth’s most ancient life forms, with origins dating back to the Precambrian epoch approximately 2.5 billion years ago [10,17]. The name ‘Chlorella’ derives from the Greek ‘chlorós’, meaning ‘green’, and the diminutive Latin suffix ‘ella,’ meaning ‘small’ [10]. Discovered in 1890 by Dutch microbiologist and botanist Martinus Willem Beijerinck, CLV is characterized by a robust cell wall, ranging from 100 to 200 nm in thickness, providing substantial mechanical and chemical defense [10]. Taxonomically, CLV is classified as follows: Domain: Eukaryota; Kingdom: Protista; Phylum: Chlorophyta; Class: Trebouxiophyceae; Order: Chlorellales; Family: Chlorellaceae; Genus: Chlorella; and Species: Chlorella vulgaris [10].
The production of CLV has evolved significantly since its discovery. Large-scale cultivation began in the early 1960s in Japan, where it became a popular food source [18]. Today, over 70 companies worldwide are involved in its production (Table 1). Taiwan leads in production, with the Taiwan Chlorella Manufacturing Company in Taipei producing over 400 tons of dried biomass annually. Other major producers include Japan and Germany, with the Klötze facility in Germany producing 130 to 150 tons of dry biomass annually [8]. By 2009, annual production of Chlorella biomass had surpassed 2000 metric tons (dry weight) [14,19,20].

Table 1.
Some Chlorella vulgaris (CLV) production companies as reported in various studies.
4. Chemical Composition of Chlorella vulgaris
The chemical composition of CLV shows significant variations based on the growth conditions used in different studies (Table 2). This variation is evident in components such as dry matter (DM), organic matter (OM), crude protein (CP), ether extract (EE), neutral detergent fiber (NDF), acid detergent fiber (ADF), and ash content. Additional components, such as starch, crude fiber (CF), available carbohydrate (CHO), and non-structural carbohydrate (NSC), were included to understand the nutritional value and potential applications of CLV as animal feed.
The reported DM values range from 92.7% to 96.1%, indicating a relatively consistent DM content under different growth conditions. CLV demonstrates a high CP content, ranging from 22.7% to 67.7% of dry matter, reinforcing its potential as a valuable protein source for food and feed applications [21,50]. Similarly, the EE, representing lipid content, shows considerable variation, ranging from 2.4% to 14.2% [21,27]. This variation indicates the impact of growth conditions on lipid accumulation in CLV, potentially affecting its energy content and fatty acid profiles.

Table 2.
Chemical composition (% DM) of Chlorella vulgaris under different growth conditions.
Table 2.
Chemical composition (% DM) of Chlorella vulgaris under different growth conditions.
| DM | OM | CP | EE | NDF | ADF | Ash | Energy | Reference |
|---|---|---|---|---|---|---|---|---|
| 93.1 | 81.3 | 42.8 | - | 32.2 | 19.4 | 11.8 | - | [3] |
| 93.1 | - | 46.0 | 9.4 | - | - | 12.7 | 4586 a | [51] |
| 95.5 | - | 22.7 | 14.2 | - | - | - | 434 b | [21] |
| 93.2 | 94.2 | 57.9 | 13.9 | 11.8 | 4.3 | - | - | [52] |
| 95.4 | - | 48.4 | 9.2 | 20.2 | - | 11.1 | 18.8 c | [44] |
| 94.6 | - | 60.8 | 9.5 | 0.0 | - | 5.7 | - | [53] |
| 94.2 | 90.5 | 51.5 | 12.2 | 9.2 | - | - | - | [22,23] |
| 92.7 | 84.8 | 67.7 | 10.5 | 12.8 | 4.2 | - | - | [50] |
| 94.6 | - | 60.6 | 12.8 | - | - | 4.5 | - | [54] |
| NR | - | 25.0 | 2.4 | - | - | - | - | [27] |
| 96.1 | - | 47.8 | 13.3 | - | - | 6.3 | 1427 d | [55] |
| Others | Amount | Reference | ||||||
| Starch | 4.4 | [44] | ||||||
| 4.3 | [53] | |||||||
| CF | 8.8 | [3] | ||||||
| 13.0 | [54] | |||||||
| 5.4 | [27] | |||||||
| CHO | 8.1 | [55] | ||||||
| NSC | 10.6 | [52] | ||||||
| 11.9 | [22,23] |
- = not reported; a GE, gross energy (Cal/g); b Kcal; c GE, gross energy (MJ/kg DM); d kJ; DM, dry matter; OM, organic matter; CP, crude protein; EE, ether extract; NDF, neutral detergent fiber; ADF, acid detergent fiber; CF, crude fiber; CHO, available carbohydrate; NSC, non-structural carbohydrate.
The fiber content, reported as NDF and ADF, also shows variability. NDF values range from 0.0% to 32.2%, while ADF values range from 4.2% to 19.4%, which may have implications for gut health and digestion in animals. Ash content, indicative of mineral content, ranges from 4.5% to 12.7%. The reported energy values fluctuate, likely due to variations in measurement methods. Data on carbohydrate composition are more limited but highlight the presence of various carbohydrates, including starch, CF, available carbohydrates (CHO), and non-structural carbohydrates (NSC). Starch content varies from 4.3% to 4.4%, and CF ranges from 5.4% to 13.0%. These components are crucial for understanding the potential digestibility and energy value of CLV as an animal feed supplement.
The observed variations in the nutritional content of CLV can be attributed to several factors, including differences in cultivation methods, nutrient availability, light intensity, temperature, pH, and processing techniques [10,56,57]. Although the specific growth conditions were not explicitly stated in this paper, we inferred that these conditions play a significant role in shaping the chemical composition of this microalga. Additionally, variations in sample preparation, extraction techniques, and analytical instruments can lead to differences in measured nutrient levels. From an animal nutrition perspective, CLV has promising potential as a feed component. However, research is necessary to determine the ideal conditions for maximizing specific nutrient levels and to optimize processing techniques to ensure consistent and desirable nutritional content for effective utilization as an animal feed supplement.
4.1. Fatty Acid Profile of Chlorella vulgaris
Chlorella vulgaris has a diverse fatty acid (FA) composition, including saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) (Table 3). The specific FA profile can vary significantly depending on growth conditions and extraction methods. Palmitic acid (C16:0) is often the most abundant SFA in CLV, with one study reporting it at 59.85% of total FAs [58]. Other significant SFAs include myristic acid (C14:0) and stearic acid (C18:0) [44,56,58]. Lauric acid (C12:0) is present at 0.87% and 6.78% in different studies, and stearic acid (C18:0) ranges from 1.35% to 15.27% (Table 3). Oleic acid (C18:1) is the major MUFA, with reported percentages ranging from 2–51% across different studies [44]. Other MUFAs like palmitoleic acid (C16:1) and hexadecadienoic acid (C16:2) are also present. MUFAs contribute to cell membrane fluidity, which is essential for overall health and metabolic functions [56].

Table 3.
Fatty acid profile of Chlorella vulgaris.
Linoleic acid (C18:2n-6), an omega-6 fatty acid, and alpha-linolenic acid (C18:3n-3), an omega-3 fatty acid, are the predominant PUFAs in CLV (Table 3). CLV is a source of omega-3 fatty acids, including alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) [44,55]. However, the levels of these fatty acids vary greatly; for example, DHA content ranges from 0.30% to 20.94% across different studies. Linoleic acid ranges from 9.73% to 26.37%, and elaidic acid (C18:1n-9) at 33.14%. The ratio of omega-3 to omega-6 fatty acids in CLV was found to be 3.00, which is considered beneficial for cardiovascular health and reducing inflammation [55,56]. The fatty acid profile of CLV is highly variable due to factors such as cultivation conditions, harvesting time, and analytical methods. Specific growth conditions like light intensity, temperature, and nutrient availability significantly influence the types and amounts of FAs produced. Further research is needed to optimize the cultivation of Chlorella vulgaris to maximize the yield of beneficial fatty acids, especially the essential omega-3s.
4.2. Mineral Composition of Chlorella vulgaris
Chlorella vulgaris exhibits significant potential as a nutritious feed supplement for animals. However, studies reveal considerable variation in its mineral composition (Table 4), highlighting the need for more research and a deeper understanding of the factors influencing its nutritional content [5]. Phosphorus and potassium are among the most abundant minerals found in CLV. Reported values for phosphorus range from 6500 to 27,080 mg/kg. Potassium levels show even greater variability, ranging from 499.2 to 132,950 mg/kg (Table 4). Sodium content also varies considerably, with reported values between 3820 and 16,450 mg/kg. Tokuşoglu and Üunal [55] reported the following mineral concentrations in mg/kg: sodium (Na) 13,464, phosphorus (P) 17,615, calcium (Ca) 5937, and potassium (K) 499.2. Zheng et al. [27] found phosphorus at 6500 mg/kg and calcium at 2000 mg/kg. Sodium, potassium, magnesium, and iron were not reported in this study. Sucu [48] reported the following mineral concentrations in mg/kg: sodium 16,450, phosphorus 27,080, calcium 940, potassium 132,950, magnesium 12,360, and iron 5400. Martins et al. [3] found the following mineral concentrations in mg/kg: sodium 3820, phosphorus 20,400, calcium 7030, and potassium 29,200. Magnesium and iron were not reported in this study. These variations in mineral content can be attributed to different factors such as the cultivation conditions, analytical techniques used, and the specific strain of Chlorella. These factors directly influence the nutritional value of CLV as a feed supplement.

Table 4.
Analysis of Chlorella vulgaris mineral content across different studies.
5. In Vitro Ruminal Fermentation Parameters
Chlorella vulgaris demonstrates a dose-dependent influence on in vitro ruminal fermentation, with higher inclusion levels generally leading to more pronounced effects. The impact of CLV on ruminal parameters varies across studies (Table 5), highlighting the importance of dosage and other factors such as basal substrate composition. Studies using lower CLV inclusion levels (0.5–10%) typically report no significant effects on key ruminal fermentation parameters (Table 5). These parameters include total gas production, methane production, total volatile fatty acid (VFA) concentration, and molar proportions of individual VFAs such as acetate, propionate, and butyrate. For example, Gadzama et al. [6] and Vargas et al. [43] observed no effect of CLV on total gas production at these low inclusion rates. However, Kholif et al. [60] reported a significant increase in gas production with 1% CLV inclusion, suggesting variability in responses even at low dosages. Conversely, higher CLV inclusion levels, such as 25%, can lead to decreases in gas production, total VFA concentration, and acetate proportions (Table 5). Sucu [48] observed a decrease in gas production with 25% CLV inclusion. While some studies using low inclusion levels show no effect, both low (1%) and high (25%) CLV inclusion levels have been shown to reduce methane production. Gadzama et al. [6] observed no significant effect on methane production with CLV inclusion at 0.5 and 1%. Vargas et al. [43] also reported no significant effect on methane production, or VFA proportions with 1, 5, and 10% CLV. Kholif et al. [60] found that 1% CLV reduced methane production, and increased ammonia-N and NDF digestibility. Sucu [48] found that 25% CLV decreased methane production, total VFA concentration, and acetate proportions while increasing ammonia-N concentrations.

Table 5.
Studies on the effects of Chlorella vulgaris (CLV) on in vitro ruminal fermentation.
Differences in basal substrate composition, particularly crude protein (CP) and neutral detergent fiber (NDF) content, may contribute to variations in CLV effects across studies. For example, Kholif et al. [60] used a substrate with 16% CP and 33% NDF, while Vargas et al. [43] used a substrate containing 10% CP and 38% NDF. The dose-dependent nature of CLV’s impact is a critical factor, with low and high inclusion levels producing contrasting outcomes. Recent studies have demonstrated that the inclusion of Moringa oleifera can enhance the positive effects of CLV, such as methane reduction. Kholif et al. [60] showed that a combination of CLV (up to 3%) and Moringa oleifera reduced methane emissions and protozoal populations, although a minor decrease in overall gas production was also observed. More research is needed to fully understand the effects of CLV on ruminal fermentation, the mechanisms of action, optimal dosages, and potential interactions with different dietary substrates. Future studies should focus on in vivo trials to validate these in vitro findings and assess the practical applicability of CLV as a feed supplement in ruminant nutrition.
6. Chlorella vulgaris as Feed Supplement for Cattle
Supplementing cattle diets with CLV has demonstrated inconsistent effects on growth and health across different life stages and breeds (Table 6), with some studies showing decreased daily feed intake in Holstein calves [61], while others report increased feed intake in multiparous Friesian cows [62]. CLV supplementation has been observed to impact milk composition, with some studies reporting increases in milk protein and non-fat solids [54], and in other cases, increases in protein, fat, and iodine concentrations [63]. However, it is important to note that there have been no significant changes in milk production or fat content in some studies [54,63]. The inclusion of CLV in cattle diets may lead to a higher ciliate protozoa population in the rumen [64], with increases in specific genera such as Isotricha, Dasytricha, Charonina, Buetschlia, Ostracodinium, and Ophryoscolex [64]. However, a study by Kuzmaitė et al. [65] found no significant difference in the microflora of neonatal calves. It is crucial to recognize that the effects of CLV supplementation on cattle performance may vary depending on factors such as dosage, form of CLV, and breed of cattle. More so, CLV’s impact on feed intake and efficiency requires further investigation, as studies have shown varying results (Table 6). For example, Shams et al. [62] noticed a decrease in feed efficiency per kg of 4% fat-corrected milk in cows, along with a significant decrease in DMI, total digestible nutrients, crude protein, and digestible crude protein. The overall effects of CLV on cattle performance appear to be complex, thus requiring more research to fully understand its benefits and limitations.

Table 6.
Studies on the impact of Chlorella vulgaris (CLV) on Cattle.
7. Impact of Chlorella vulgaris on Sheep
There is a limited number of in vivo studies focused on CLV supplementation in sheep diets, making it challenging to draw firm conclusions about its overall efficacy in this species. However, the few studies reported on the in vivo effects of CLV supplementation in sheep, as summarized in Table 7, reveal some potential benefits of CLV supplementation in sheep, though the specific outcomes vary. Gadzama et al. [5] investigated the effects of fresh CLV at 0, 0.5, and 1% of dry matter in 4-month-old lambs, finding a 15.91% decrease in butyrate levels but no significant difference in dry matter intake, feed conversion efficiency, average daily gain, and total weight gain. In contrast, Rabee et al. [66] examined a combination of CLV with yeast (25% S. cerevisiae, 50% S. platensis, and 25% CLV) at 0 and 1% of dry matter in 5-year-old rams, reporting a 31% increase in dry matter intake (DMI), a 24% decrease in acetate, an 88% increase in propionate, a 16% decrease in butyrate, a 109% increase in isobutyrate, and a 14% increase in neutral detergent fiber (NDF) digestibility. Meanwhile, Slyusarenko et al. [67] studied the effects of CLV suspension at 0, 3, 5, 7, and 9 mL/kg body weight in lactating ewes, with performance measured in lambs, and observed a 66% increase in DMI and a two-fold increase in average daily gain (ADG). Supplementation with fresh CLV microalgae has been shown to enhance the nutritional profile of lamb meat, primarily by increasing beneficial omega-3 fatty acids, with potentially positive implications for human health (Figure 2) [5]. Specifically, the study revealed that incorporating 0.5% dry matter of CLV into the feedlot diet of lambs significantly elevated the concentration of alpha-linolenic acid (ALA) and total omega-3 long-chain fatty acids in the longissimus lumborum et thoracis (LTL) muscle. This finding is particularly significant because humans cannot synthesize omega-3 polyunsaturated fatty acids de novo and must obtain them from dietary sources such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which are known to reduce the risk of coronary heart disease, neurodegenerative disorders, and metabolic syndromes [5,13,56]. Although the study did not observe a significant increase in EPA and DHA levels in the lamb meat, the elevated ALA content—a precursor to EPA and DHA—still contributes to a more favorable fatty acid profile, which is beneficial for human health (Figure 2) [5,56]. These findings indicate that while CLV supplementation can enhance various performance parameters, the specific effects are influenced by the form and dosage of CLV, as well as the animal’s stage, necessitating further research to optimize its use across different sheep demographics [5,66,67]. Future research should prioritize bridging the gaps between in vitro and in vivo findings through standardized methodologies, longer-term trials, and exploration of synergistic effects. Additionally, it should focus on the potential to further elevate EPA and DHA levels in meat, thereby maximizing its health benefits for consumers.

Table 7.
Studies on in vivo effects of Chlorella vulgaris (CLV) supplementation in sheep.
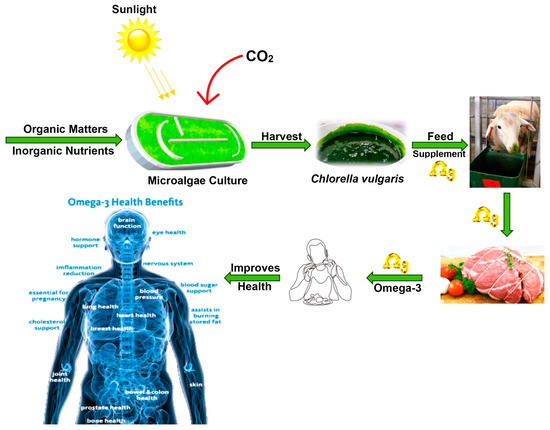
Figure 2.
Chlorella vulgaris supplementation is a promising strategy to produce value-added meat products for consumers looking to increase their omega-3 intake. Adapted from Gadzama [56].
8. Chlorella vulgaris in Goat Diets
Supplementation with CLV in goat diets has revealed several beneficial effects on milk production, nutrient digestibility, and reproductive performance (Table 8). A 10 g/day dose of CLV, in fresh, dried, or lyophilized form, has been shown to increase milk production by 10–12% [22,68]. In addition to milk yield, this dose can enhance DMI by 13% and neutral detergent fiber digestibility by 5% [22]. Furthermore, CLV interacts synergistically with copper sulfate, leading to a 9% increase in NDF digestibility when combined [22,68]. When combined with Nigella sativa in pregnant does, CLV has been shown to increase milk yield by 4% and serum antioxidant levels by 22% [69]. Additionally, 5 g/day of CLV with vitamin C improved serum antioxidant levels by 10% in breeding bucks [70].
CLV supplementation has also demonstrated a positive impact on reproductive parameters by improving sperm quality and reducing sperm abnormalities in bucks [70]. A higher dose of 20 g/day of CLV can improve embryo quality and mitochondrial functionality, while 10 g/day supports ovarian follicular growth [71,72]. Moreover, CLV supplementation can improve thermoregulation in lactating goats, reducing rectal temperature and pulse rate under heat stress conditions [73]. In terms of milk composition, CLV supplementation, especially at 10 g/day, has been found to increase monounsaturated fatty acids and conjugated linoleic acid, while reducing saturated fatty acids [29,68,74]. CLV can also increase the milk’s antioxidant levels; for example, an increase in superoxide dismutase by 68% [50]. Table 8 summarizes various studies which confirm the benefits of CLV supplementation in goats, such as increased milk yield and serum antioxidant levels, improved dry matter intake, and enhanced digestibility. However, some studies have shown a reduction in milk yield and an increase in omega-6 [74]. Generally, CLV supplementation at various doses has been shown to increase polyunsaturated fatty acids and omega-3 in milk [29].

Table 8.
Summary of studies on Chlorella vulgaris supplementation in goats.
Table 8.
Summary of studies on Chlorella vulgaris supplementation in goats.
| Studies | CLV, g DM/d | Stage | Dose | Production | Ruminal Fermentation | Digestibility | Anti- Oxidant a | Milk FA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMI | ADG | MY | VFA | A:P | NH3-N | DM | NDF | ||||||
| [69] | 10 g (and/or 5 g N. sativa) | Pregnant | 10 g with 5 g N. sativa | - | - | +4% ↑ | - | +7% ↑ | - | +22% ↑ | |||
| [70] | 5 g (and/or 2 g Vit C) | Breeding buck | 5 g with 2 g Vit C | +27% ↑ | +10% ↑ | ||||||||
| [22] | 5 g and 10 g | Lactating | 10 g | +13% ↑ | +12% ↑ | ↑ | ↓ | ↓ | +3% ↑ | +5% ↑ | PUFA ↑ | ||
| [68] | 5 g, 10 g and 15 g (with CuSO4) | Lactating | 10 g with CuSO4 | - | +10% ↑ | ↑ | - | - | +10% ↑ | +9% ↑ | MUFA ↑ | ||
| [74] | 10 g | Lactating | - | ↓ | ω-6 ↑ | ||||||||
| [29] | 5 g and 10 g (dried) | Lactating | 10 g (Linear response) | PUFA ↑ ω-3 ↑ | |||||||||
a serum antioxidant level; “-”, “↑”, “↓” indicates no difference, increase, and decrease, respectively (p < 0.05). DMI = dry matter intake; ADG = average dairy gain; MY = milk yield; VFA = volatile fatty acid; A:P = acetate: propionate; NH3-N = ammonia nitrogen; DM = dry matter; NDF = neutral detergent fiber; FA = fatty acid; PUFA = polyunsaturated fatty acid; MUFA = mono-unsaturated fatty acid; ω = omega.
9. The Effects of Chlorella vulgaris in Broiler Diets
The dietary supplementation of broiler feed with CLV has been extensively researched for its potential to enhance growth performance, health biomarkers, and meat quality (Table 9). Studies have consistently shown that broilers fed CLV at various concentrations and durations exhibit higher growth rates and body weights compared to the control groups. For instance, broilers receiving 1 g of CLV/kg of diet for 32 days showed increased final body weight and weight gain [58], and similar results were observed with 2.5% dried CLV for 8 weeks [75], and with 0.8% CLV for 35 days [21]. Despite the variable incorporation levels, CLV improved broiler growth rate, even at a lower rate of 0.8%. Moreover, improvements in weight gain have been observed with different forms of CLV, including powder, liquid, and Chlorella growth factor [34,76,77]. Interestingly, the inclusion of 1.0% E. coli fermented liquor with Chlorella not only increased weight gain but also improved the feed conversion ratio [78]. However, research indicates that higher inclusion rates of CLV (10–20%) can lead to reduced feed intake and, in some cases, lower body weight and weight gain, suggesting that there may be an optimal level for CLV inclusion to maximize benefits [26,51]. While some studies have shown similar feed intake and feed conversion ratios regardless of CLV inclusion [58,77,79], others have shown improved FCR [21,78], indicating that the effect of CLV on feed efficiency may depend on the concentration and context of its inclusion.
Beyond growth performance, CLV has shown the ability to enhance metabolic health indicators and reduce inflammatory responses in broiler chickens (Table 9). Studies have shown that broilers fed CLV had decreased levels of total lipids in their serum, and lower levels of haptoglobin and interleukin-13, indicating its anti-inflammatory effects [21,34]. Further, CLV supplementation has been associated with improved immunity, as evidenced by increased serum IgG and IgM levels, as well as enhanced gut barrier function through the improved distribution of immune cells and integrity of the intestinal barrier [34,36]. Moreover, CLV is recognized for its ability to regulate the gut microbiota, increasing beneficial bacteria while inhibiting harmful ones, which is believed to contribute to improvements in growth performance and feed efficiency [37,80,81]. Specifically, CLV has been shown to increase Lactobacillus populations, and other beneficial bacterial taxa, such as Clostridium ASF356 and Coriobacteriaceae CHKCI002, enhancing the microbial diversity of the gut [76,82]. The use of CLV as a natural feed additive in broiler diets could contribute to sustainable poultry production, as it promotes healthier birds and safer products for consumers (Table 9).

Table 9.
Key Findings of Chlorella vulgaris supplementation on broiler chicken performance and health.
Table 9.
Key Findings of Chlorella vulgaris supplementation on broiler chicken performance and health.
| Summary of Main Findings | References |
|---|---|
| Broilers fed 1 g of CLV/kg diet for 32 d showed an increase in final body weight and weight gain as compared to control groups | [58] |
| Broilers fed 2.5% dried Chlorella vulgaris for 8 weeks had higher body weight in comparison to the control | [75] |
| Broilers fed with 0.8% CLV showed better final weight and feed conversion after 35 d than the control group | [21] |
| Birds fed Chlorella, in powder, Chlorella growth factor, or liquid form, gained more weight by 5 weeks of age than the control group, without affecting feed intake or efficiency | [76] |
| Chicks fed 0.05–0.5% Chlorella for 35 d had greater weight gains than the control group | [34] |
| Broilers fed CLV over 5 weeks had significantly higher body weight gain as compared to the control group | [77] |
| Broilers fed 1.0% E. coli fermented liquor with Chlorella showed a 2.6% higher weight gain and a 2.8% improved FCR than the control group | [78] |
| Broilers fed CLV over 5 weeks had similar feed intake and FCR | [77] |
| Broilers fed 10% CLV for 14 d showed similar growth and feed conversion rates, with or without added CAZymes | [79] |
| Broilers given 0, 10, or 20 g/kg of CLV had lower feed intake, yet their weight gain and FCR were similar | [26] |
| Birds on 15% and 20% CLV diets had reduced body weight, weight gain, and feed intake, unlike those on CLV10%, which were comparable to the control group | [51] |
| Birds fed with 15% and 20% CLV diets showed similar feed conversion ratios to the control group | [51] |
| Broilers receiving 1 g of CLV/kg of diet had similar feed intake and FCR as compared to the control groups | [58] |
| Adding 10% CLV and CAZymes to broiler diets did not significantly affect weight gain or feed efficiency | [83] |
| Chicks fed with 0.15% or 0.5% Chlorella or Chlorella growth factor showed increased serum IgG and IgM levels compared to the control group | [34] |
| Chicks fed 0.5% dried Chlorella powder had lower serum total lipid concentrations compared to the control | [34] |
| Broilers fed 0.8% CLV for 35 d showed reduced haptoglobin and interleukin-13 levels compared to the control | [21] |
| Supplementing broiler diets with 10 g/kg of Chlorella by-product enhances health, immunity, antioxidant capacity, and gut morphology | [26] |
CLV = Chlorella vulgaris; FCR = Feed conversion ratio; d = day/s; CAZymes = carbohydrate-active enzymes.
10. Impact of Chlorella vulgaris on Meat Quality Parameters of Broiler Chickens
The inclusion of CLV in broiler chicken diets has been shown to enhance meat quality through several mechanisms, including improved nutrient absorption via maintaining the structure of the jejunum [21], and increased breast muscle yield [51], which is associated with higher concentrations of CLV in the diet (Table 10). Furthermore, CLV increases the levels of beneficial fatty acids such as DHA, EPA, and omega-3, leading to a healthier omega-6 to omega-3 ratio, while also boosting levels of carotenoids and antioxidants in the meat, and consequently improving meat color and shelf life [51,79,83]. Additionally, a 20% CLV diet has been found to improve the meat’s water-holding capacity, reduce cooking loss, and make the meat more tender and juicier [34,51,58,83], while also contributing to its oxidative stability through the reduction of harmful compounds, lowered bacterial counts, and increased superoxide dismutase (SOD) activity [58]. Studies have also revealed that meat from chickens fed with 10% CLV had higher consumer acceptance ratings, which suggests that CLV can be included without negatively impacting the taste or overall quality and that it also leads to a reduction in HDL-cholesterol levels in broiler meat [51,79].

Table 10.
Summary of studies on the Effects of Chlorella vulgaris on broiler chicken meat quality.
11. Impact on Laying Hen Performance and Egg Quality
Studies have explored the benefits of supplementing laying hen diets with CLV (Table 11), with research indicating that a 75 g/kg inclusion of CLV can improve both feed intake and egg production [84]. However, other studies have shown that lower levels of CLV, such as 5 g/kg, do not significantly impact the laying performance but can increase egg weight and production [36,85]. The addition of CLV can also improve gut health by increasing the diversity of beneficial bacteria in the gut, which can enhance nutrient absorption and immunity [25]. Further research revealed that CLV can positively affect liver fat content and gut bacteria profiles [27], while fermented CLV is associated with increased egg production, improved yolk color, and enhanced egg freshness [27,36]. Moreover, CLV supplementation has been shown to improve the color parameters of both fresh and boiled eggs, which makes them more appealing to consumers (Table 11). This is because CLV enhances the fatty acid content and ß-carotene concentration in eggs, which can improve the nutritional profile of the eggs [85]. Importantly, studies have found that CLV supplementation does not alter the total cholesterol content in egg yolks [86], which is good news for health-conscious consumers. Furthermore, CLV supplementation has been found to change the fatty acid composition of egg yolks by increasing palmitic and linoleic acids and decreasing docosatetraenoic acid levels [86]. While some studies reported that 5 g of CLV per kg of diet did not affect egg freshness or eggshell quality [36], other studies show that fermented CLV and dietary CLV significantly influenced egg yolk color [85]. Most studies focus on broilers or layers, not breeders. The long-term effects of CLV on reproductive cycles including embryo development, chick vitality, or growth remain understudied.

Table 11.
Summary of studies on the effects of Chlorella vulgaris on laying hen performance and egg quality.
12. Impact of Chlorella vulgaris on Pig
Studies on pigs showed that adding CLV to their diet can have varied effects, with most studies indicating that it does not significantly change growth parameters like average daily gain, average daily feed intake, and feed conversion ratio (Table 12). For instance, a 5% CLV diet led to increased feed intake in weaned piglets, but it did not affect final weight or growth rate [3,87], and similarly, other studies using 1% and 5% CLV found no differences in growth performance [45,88,89]. However, fermented CLV at 0.1% has been shown to improve ADG and dry matter digestibility, suggesting potential probiotic benefits [90]. CLV has also demonstrated some immune and metabolic impacts, including an increase in immunoglobulin G levels and a decrease in immunoglobulin M levels, but it can also increase total cholesterol, LDL, and VLDL cholesterol while decreasing HDL cholesterol [3,47]. Although CLV does not significantly impact meat quality [47], it can increase antioxidant pigments and omega-3 fatty acids in pork fat, along with decreasing the ratio of omega-6 to omega-3 fatty acids in the liver and pork [3,47,87,89]. Overall, CLV supplementation can improve fatty acid profiles and increase beneficial pigments; however, it is important to carefully manage its levels in pig diets due to potential negative effects on the immune system (Table 12).

Table 12.
Summary of studies on the effects of Chlorella vulgaris on pig growth performance and health.
13. Chlorella vulgaris in Rabbit Diets
Research on supplementing rabbit diets with CLV has shown varied results regarding growth and feed efficiency, with some studies reporting improved growth and feed-to-gain ratios at CLV levels of 200–500 mg/kg of body weight, while others found no significant differences in these parameters (Table 13). Specifically, Sikiru et al. [91] found that rabbits fed 300 and 500 mg/kg of CLV showed higher final weight and weight gain, while another indicated that 500 mg/kg of CLV led to decreased feed consumption [92]. Additionally, CLV supplementation has been associated with reduced oxidative stress, indicated by lower levels of malondialdehyde and protein carbonyl concentrations, and increased antioxidant levels [91,93]. Moreover, rabbits given CLV exhibited improved immune health, with higher levels of immunoglobulins (IgG and IgM) increased glutathione activity, and better lipid profiles, with reduced serum triglycerides and low-density lipoprotein levels, thus showing potential cardiovascular benefits in rabbits [91,93]. However, CLV did not significantly affect nutrient digestibility or dressing percentages [94].

Table 13.
Effects of dietary Chlorella vulgaris on growth performance and health parameters in rabbits.
14. Impact of Chlorella vulgaris on Fish
Chlorella vulgaris is a promising functional feed additive for aquaculture due to its rich nutritional profile and bioactive compounds, which have been shown to improve growth, feed utilization, and overall health in various fish species (Table 14). Studies have shown that dietary inclusion of CLV enhances feed palatability, likely due to amino acids, peptides, and nucleotides that act as flavor enhancers, which leads to increased feed intake and improved nutrient digestibility [95,96]. Several studies have explored the effects of different levels of CLV inclusion on fish diets, with optimal levels varying depending on the species [97,98,99]; for instance, 5% CLV in Nile tilapia diets has been shown to improve growth and feed efficiency [100], while levels up to 15 g/kg have also shown positive effects [96]. CLV has been shown to significantly improve growth rates and feed conversion ratio in several species, including tilapia [96,100], seabass [33], and common carp [101], as well as other aquatic species (Table 14), with some studies even observing a 70% increase in final body weight in juvenile seabass [33]. Additionally, CLV has demonstrated positive impacts on immunity and disease resistance, with lipopolysaccharides and carotenoids stimulating immune responses and providing protection against bacterial pathogens in several species [102,103]. Furthermore, CLV’s antioxidant properties and ability to manage stress have been observed, as it modulates key antioxidant enzymes such as Superoxide Dismutase, Catalase, and Glutathione Peroxidase, and mitigates the negative effects of stress [39,104,105]. However, it’s important to note that while CLV generally enhances growth and health, some studies have reported varied results, such as similar growth, feed conversion, and protein efficiency in seabreams when using CLV as a fishmeal substitute [106]. Others reported that a 5% CLV diet resulted in similar SGR, FCR, and PER values as the control group in one case [100], and some studies have shown that high levels (75%) of CLV in the diet can lead to decreased feed consumption [107]. Our findings demonstrate that CLV exhibits dual prebiotic and phytogenic effects, enhancing the growth and viability of probiotic bacteria while showing potential inhibitory activity against intestinal pathogens. These results suggest that CLV could serve as a multifunctional feed supplement, leveraging its prebiotic properties to support beneficial gut microbiota and its phytogenic properties to mitigate pathogenic infections. However, the precise mechanisms underlying these effects remain incompletely understood. Further research is warranted to elucidate the specific pathways through which CLV functions as a prebiotic, probiotic, and/or phytogenic agent across diverse animal species. Such investigations will be critical for optimizing its application in animal nutrition and for validating its potential to improve gut health and overall productivity in livestock.

Table 14.
Studies on the impact of Chlorella vulgaris on fish performance and health.
15. Conclusions
Chlorella vulgaris shows promise as a beneficial ingredient in animal diets, with studies indicating improvements in growth performance, health outcomes, and product quality when used at appropriate inclusion rates. However, due to variability in responses across species and inclusion levels, species-specific dietary formulations are necessary. Further research is needed to optimize CLV inclusion rates, maximizing benefits while minimizing potential drawbacks. Therefore, CLV represents a valuable alternative to traditional feed ingredients, which could significantly contribute to more environmentally friendly and resilient livestock production systems.
Author Contributions
I.U.G., Conceptualization, Data Extraction, Table Visualization, Writing Original Draft, Project Management, Coordination, Major Revision, Graphical Abstract, Writing—Review & Editing, Final Proofreading Before Publication; S.R., Conceptualization, Resources, Writing—Review & Editing; R.M., Methodology, Funding acquisition, Critical Evaluation of the Content, Writing—Review & Editing; I.M.M., Writing—Review & Editing; T.G., Writing—Review & Editing; M.A.R., Critical Evaluation of the Content, Writing—Review & Editing; M.R.A.R., Writing—Review & Editing; M.F.R., Writing—Review & Editing; A.E.K., Critical Evaluation of the Content, Writing—Review & Editing; M.S., Writing—Review & Editing, Specifically, Critical Review of the Manuscript; A.F.B., Critical Evaluation of the Content, Constructive Feedback, Writing—Review & Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This article was made possible through funding by the German Federal Ministry of Education and Research (BMBF), grant agreement 031B0751 BioKum—Cumulative effects of bioeconomic strategies for a more sustainable agriculture.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Acknowledgments
The authors are grateful to their respective organizations. The graphical abstract was created using BioRender.com.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO. The Future of Food and Agriculture: Trends and Challenges; FAO: Rome, Italy, 2017; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/2e90c833-8e84-46f2-a675-ea2d7afa4e24/content (accessed on 10 December 2024).
- Gadzama, I.U.; Mugweru, I.M.; Makombe, W.S.; Madungwe, C.; Hina, Q.; Omofunmilola, E.O.; Panuel, P.; Olanrewaju, T.J.; Ray, S. Improving poultry production with black soldier fly larvae. Acta Sci. Agric. 2025, 9, 60–77. Available online: https://actascientific.com/ASAG/pdf/ASAG-08-1450.pdf (accessed on 16 March 2025).
- Martins, C.F.; Trevisi, P.; Coelho, D.F.; Correa, F.; Ribeiro, D.M.; Alfaia, C.M.; Pinho, M.; Pestana, J.M.; Mourato, M.P.; Almeida, A.M.; et al. Influence of Chlorella vulgaris on growth, digestibility and gut morphology and microbiota of weaned piglet. Sci. Rep. 2022, 12, 6012. [Google Scholar] [CrossRef] [PubMed]
- Spínola, M.P.; Costa, M.M.; Prates, J.A.M. Effect of Selected Mechanical/Physical Pre-Treatments on Chlorella vulgaris Protein Solubility. Agriculture 2023, 13, 1309. [Google Scholar] [CrossRef]
- Gadzama, I.U.; Hoffman, L.C.; Holman, B.; Chaves, A.V.; Meale, S.J. Effects of supplementing a feedlot diet with microalgae (Chlorella vulgaris) on the performance, carcass traits and meat quality of lambs. Livest. Sci. 2024, 288, 105552. [Google Scholar] [CrossRef]
- Gadzama, I.U.; Meale, S.J.; Chaves, A.V. Effect of microalgae on in vitro rumen fermentation, gas and methane production. In Proceedings of the Recent Advances in Animal Nutrition—Australia (RAAN-A) Conference, Gold Coast, QLD, Australia, 27–28 July 2023; Available online: https://www.researchgate.net/publication/383180460_Effect_of_microalgae_on_in_vitro_rumen_fermentation_gas_and_methane_production (accessed on 5 January 2025).
- Little, H.; Brown, R.M. The culture of Chlorella vulgaris in synthetic media of varying composition with special reference to heterotrophic growth on organic compounds. Ann. Bot. 1953, 17, 433–454. [Google Scholar]
- Montoya, J.D.; Weathers, P.J.; McCabe, J.G. Engineering challenges in high-density algal production: A critical review of existing methods and future needs. J. Appl. Phycol. 2014, 26, 1519–1538. [Google Scholar]
- Liu, J.; Chen, F. Biology and Industrial Applications of Chlorella: Advances and Prospects. Adv. Biochem. Eng. Biotechnol. 2016, 153, 1–35. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; MacPherson, T.; McGinn, P.J.; Fredeen, A.H. In vitro digestion of microalgal biomass from freshwater species isolated in Alberta, Canada for monogastric and ruminant animal feed applications. Algal Res. 2016, 19, 324–332. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Saadaoui, I.; Rasheed, R.; Aguilar, A.; Cherif, M.; Al Jabri, H.; Sayadi, S.; Manning, S.R. Microalgal-based feed: Promising alternative feedstocks for livestock and poultry production. J. Anim. Sci. Biotechnol. 2021, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from microalgae- a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Panahi, Y.; Yari Khosroushahi, A.; Sahebkar, A.; Heidari, H.R. Impact of Cultivation Condition and Media Content on Chlorella vulgaris Composition. Adv. Pharm. Bull. 2019, 9, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Guccione, A.; Biondi, N.; Sampietro, G.; Rodolfi, L.; Bassi, N.; Tredici, M.R. Chlorella for protein and biofuels: From strain selection to outdoor cultivation in a Green Wall Panel photobioreactor. Biotechnol. Biofuels 2014, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.A.; Elbaily, Z.I. A review: Importance of chlorella and different applications. Alex. J. Vet. Sci. 2020, 65, 16. [Google Scholar] [CrossRef]
- Roques, S.; Koopmans, S.-J.; Mens, A.; van Harn, J.; van Krimpen, M.; Kar, S.K. Effect of Feeding 0.8% Dried Powdered Chlorella vulgaris Biomass on Growth Performance, Immune Response, and Intestinal Morphology during Grower Phase in Broiler Chickens. Animals 2022, 12, 1114. [Google Scholar] [CrossRef]
- Kholif, A.E.; Elghandour, M.M.Y.; Salem, A.Z.M.; Barbabosa, A.; Márquez, O.; Odongo, N.E. The effects of three total mixed rations with different concentrate to maize silage ratios and different levels of microalgae Chlorella vulgaris on in vitro total gas, methane and carbon dioxide production. J. Agric. Sci. 2017, 115, 494–507. [Google Scholar] [CrossRef]
- Kholif, A.E.; Morsy, T.A.; Matloup, O.H.; Anele, U.Y.; Mohamed, A.G.; El-Sayed, A.B. Dietary Chlorella vulgaris microalgae improves feed utilization, milk production and concentrations of conjugated linoleic acids in the milk of Damascus goats. J. Agric. Sci. 2017, 155, 508–518. [Google Scholar] [CrossRef]
- Ciliberti, M.G.; Albenzio, M.; Francavilla, M.; Neglia, G.; Esposito, L.; Caroprese, M. Extracts from Microalga Chlorella sorokiniana Exert an Anti-Proliferative Effect and Modulate Cytokines in Sheep Peripheral Blood Mononuclear Cells. Animals 2019, 9, 45. [Google Scholar] [CrossRef]
- Janczyk, P.; Halle, B.; Souffrant, W.B. Microbial community composition of the crop and ceca contents of laying hens fed diets supplemented with Chlorella vulgaris. Poult. Sci. 2009, 88, 2324–2332. [Google Scholar] [CrossRef]
- Mirzaie, S.; Sharifi, S.D.; Zirak-Khattab, F. The effect of a Chlorella by-product dietary supplement on immune response, antioxidant status, and intestinal mucosal morphology of broiler chickens. J. Appl. Phycol. 2020, 32, 1771–1777. [Google Scholar] [CrossRef]
- Zheng, L.; Oh, S.T.; Jeon, J.Y.; Moon, B.H.; Kwon, H.S.; Lim, S.U.; An, B.K.; Kang, C.W. The Dietary Effects of Fermented Chlorella vulgaris (CBT(®)) on Production Performance, Liver Lipids and Intestinal Microflora in Laying Hens. Asian-Australas. J. Anim. Sci. 2012, 25, 261–266. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Abdullah, M.A.M.; Mavrommatis, A.; Chatzikonstantinou, M.; Skliros, D.; Sotirakoglou, K.; Flemetakis, E.; Labrou, N.E.; Zervas, G. The effect of dietary Chlorella vulgaris inclusion on goat’s milk chemical composition, fatty acids profile and enzymes activities related to oxidation. J. Anim. Physiol. Anim. Nutr. 2018, 102, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kouřimská, L.; Vondráčková, E.; Fantová, M.; Nový, P.; Nohejlová, L.; Michnová, K. Effect of Feeding with Algae on Fatty Acid Profile of Goat’S Milk. Sci. Agric. Bohem. 2014, 45, 162–169. [Google Scholar] [CrossRef]
- Saleh, H.A.; Gaber, H.S.; El-Khayat, H.M.M.; Abdel-Motleb, A.; Mohammed, W.A.-A.; Okasha, H. Influences of Dietary Supplementation of Chlorella vulgaris and Spirulina platensis on Growth-Related Genes Expression and Antioxidant Enzymes in Oreochromis niloticus Fish Exposed to Heavy Metals. Aquac. Stud. 2021, 22, 445–456. [Google Scholar] [CrossRef]
- Xi, L.; Lu, Q.; Liu, Y.; Su, J.; Chen, W.; Gong, Y.; Han, D.; Yang, Y.; Zhang, Z.; Jin, J.; et al. Effects of fish meal replacement with Chlorella meal on growth performance, pigmentation, and liver health of largemouth bass (Micropterus salmoides). Anim. Nutr. 2022, 10, 26–40. [Google Scholar] [CrossRef]
- Pradhan, J.; Sahu, S.; Das, B.K. Protective Effects of Chlorella vulgaris Supplemented Diet on Antibacterial Activity and Immune Responses in Rohu Fingerlings, Labeo rohita (Hamilton), Subjected to Aeromonas hydrophila Infection. Life 2023, 13, 1028. [Google Scholar] [CrossRef]
- Mota, C.S.C.; Pinto, O.; Sá, T.; Ferreira, M.; Delerue-Matos, C.; Cabrita, A.R.J.; Almeida, A.; Abreu, H.; Silva, J.; Fonseca, A.J.M.; et al. A commercial blend of macroalgae and microalgae promotes digestibility, growth performance, and muscle nutritional value of European seabass (Dicentrarchus labrax L.) juveniles. Front. Nutr. 2023, 10, 1165343. [Google Scholar] [CrossRef] [PubMed]
- An, B.-K.; Kim, K.-E.; Jeon, J.-Y.; Lee, K.W. Effect of dried Chlorella vulgaris and Chlorella growth factor on growth performance, meat qualities and humoral immune responses in broiler chickens. Springerplus 2016, 5, 718. [Google Scholar] [CrossRef]
- Ahmad, M.T.; Shariff, M.; Goh, Y.M.; Banerjee, S.; Yusoff, F.M. Interaction of low-level dietary supplementation of Chlorella vulgaris Beijerinck, 1890, and feeding duration on growth hormone, growth performance and serum biochemistry of red hybrid tilapia (Oreochromis mossambicus × Oreochromis niloticus). J. Fish Biol. 2023, 103, 715–726. [Google Scholar] [CrossRef]
- Kim, Y.-B.; Park, J.; Heo, Y.-J.; Lee, H.-G.; Kwon, B.-Y.; Joo, S.S.; Joo, S.Y.; Kim, M.; Kim, Z.-H.; Lee, K.-W. Effect of Dietary Chlorella vulgaris or Tetradesmus obliquus on Laying Performance and Intestinal Immune Cell Parameters. Animals 2023, 13, 1589. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gao, J.; Peng, C.; Song, J.; Xie, Z.; Jia, J.; Li, H.; Zhao, S.; Liang, Y.; Gong, B. The Effect of the Microalgae Chlorella vulgaris on the Gut Microbiota of Juvenile Nile Tilapia (Oreochromis niloticus) Is Feeding-Time Dependent. Microorganisms 2023, 11, 1002. [Google Scholar] [CrossRef] [PubMed]
- González-Arceo, M.; Trepiana, J.; Aguirre, L.; Ibarruri, J.; Martínez-Sanz, M.; Cebrián, M.; Recio, I.; Portillo, M.P.; Gómez-Zorita, S. Anti-Steatotic Effects of Chlorella vulgaris, Nannochloropsis gaditana and Gracilaria vermiculophylla Algae Extracts in AML-12 Hepatocytes. Nutrients 2023, 15, 1960. [Google Scholar] [CrossRef]
- Edrees, A.; Shaban, N.S.; Hassan, N.E.-H.Y.; Abdel-Daim, A.S.A.; Sobh, M.S.; Ibrahim, R.E. Acrylamide exposure induces growth retardation, neurotoxicity, stress, and immune/antioxidant disruption in Nile tilapia (Oreochromis niloticus): The alleviative effects of Chlorella vulgaris diets. Fish Shellfish Immunol. 2024, 146, 109411. [Google Scholar] [CrossRef]
- Ibrahim, D.; Rahman, M.M.I.A.; Abd El-Ghany, A.M.; Hassanen, E.A.; Al-Jabr, O.A.; Abd El-Wahab, R.A.; Zayed, S.; Abd El Khalek Salem, M.; Nabil El Tahawy, S.; Youssef, W.; et al. Chlorella vulgaris extract conjugated magnetic iron nanoparticles in nile tilapia (Oreochromis niloticus): Growth promoting, immunostimulant and antioxidant role and combating against the synergistic infection with Ichthyophthirius multifiliis and Aeromonashydrophila. Fish Shellfish Immunol. 2024, 145, 109352. [Google Scholar] [CrossRef]
- Han, K.J.; McCormick, M.E. Evaluation of nutritive value and in vitro rumen fermentation gas accumulation of de-oiled algal residues. J. Anim. Sci. Biotechnol. 2014, 5, 31. [Google Scholar] [CrossRef]
- Wild, K.J.; Trautmann, A.; Katzenmeyer, M.; Steingaß, H.; Posten, C.; Rodehutscord, M. Chemical composition and nutritional characteristics for ruminants of the microalgae Chlorella vulgaris obtained using different cultivation conditions. Algal Res. 2019, 38, 101385. [Google Scholar] [CrossRef]
- Vargas, J.d.J.; Tarnonsky, F.; Maderal, A.; Fernández-Marenchino, I.; Podversich, F.; Schulmeister, T.M.; DiLorenzo, N. Increasing levels of Chlorella spp. on in vitro fermentation and methane production in a corn silage-base diet. Rev. Colomb. Cienc. Pecu. 2023, 37, 42–51. [Google Scholar] [CrossRef]
- Meehan, D.J.; Cabrita, A.R.; Silva, J.L.; Fonseca, A.J.; Maia, M.R. Effects of Chlorella vulgaris, Nannochloropsis oceanica and Tetraselmis sp. supplementation levels on in vitro rumen fermentation. Algal Res. 2021, 56, 102284. [Google Scholar] [CrossRef]
- Furbeyre, H.; van Milgen, J.; Mener, T.; Gloaguen, M.; Labussière, E. Effects of dietary supplementation with freshwater microalgae on growth performance, nutrient digestibility and gut health in weaned piglets. Animal 2017, 11, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Furbeyre, H.; van Milgen, J.; Mener, T.; Gloaguen, M.; Labussière, E. Effects of oral supplementation with Spirulina and Chlorella on growth and digestive health in piglets around weaning. Animal 2018, 12, 2264–2273. [Google Scholar] [CrossRef]
- Coelho, D.; Alfaia, C.M.; Lopes, P.A.; Pestana, J.M.; Costa, M.M.; Pinto, R.M.A.; Almeida, J.M.; Moreira, O.; Fontes, C.M.G.A.; Prates, J.A.M. Impact of Chlorella vulgaris as feed ingredient and carbohydrases on the health status and hepatic lipid metabolism of finishing pigs. Res. Vet. Sci. 2022, 144, 44–53. [Google Scholar] [CrossRef]
- Sucu, E. Effects of Microalgae Species on In Vitro Rumen Fermentation Pattern and Methane Production. Ann. Anim. Sci. 2020, 20, 207–218. [Google Scholar] [CrossRef]
- Carullo, D.; Abera, B.D.; Scognamiglio, M.; Donsì, F.; Ferrari, G.; Pataro, G. Application of Pulsed Electric Fields and High-Pressure Homogenization in Biorefinery Cascade of C. vulgaris Microalgae. Foods 2022, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Tsiplakou, E.; Abdullah, M.A.M.; Skliros, D.; Chatzikonstantinou, M.; Flemetakis, E.; Labrou, N.; Zervas, G. The effect of dietary Chlorella vulgaris supplementation on micro-organism community, enzyme activities and fatty acid profile in the rumen liquid of goats. J. Anim. Physiol. Anim. Nutr. 2017, 101, 275–283. [Google Scholar] [CrossRef]
- Cabrol, M.B.; Martins, J.C.; Malhão, L.P.; Alves, S.P.; Bessa, R.J.B.; Almeida, A.M.; Raymundo, A.; Lordelo, M.M.; Boskovic Cabrol, M.; Martins, J.C.; et al. Partial replacement of soybean meal with Chlorella vulgaris in broiler diets influences performance and improves breast meat quality and fatty acid composition. Poult. Sci. 2022, 101, 101955. [Google Scholar] [CrossRef]
- Kholif, A.E.; Gouda, G.A.; Abu Elella, A.A.; Patra, A.K. Replacing the concentrate feed mixture with Moringa oleifera leaves silage and Chlorella vulgaris microalgae mixture in diets of Damascus goats: Lactation performance, nutrient utilization, and ruminal fermentation. Animals 2022, 12, 1589. [Google Scholar] [CrossRef]
- Halmemies-Beauchet-Filleau, A.; Rinne, M.; Lamminen, M.; Mapato, C.; Ampapon, T.; Wanapat, M.; Vanhatalo, A. Alternative and novel feeds for ruminants: Nutritive value, product quality and environmental aspects. Animal 2018, 12, 295–309. [Google Scholar] [CrossRef]
- Jeon, J.-Y.; Park, K.-K.; Lee, K.-W.; Jang, S.-W.; Moon, B.-H.; An, B.-K. Dietary effects of lutein-fortified chlorella on milk components of Holstein cows. Springerplus 2016, 5, 908. [Google Scholar] [CrossRef]
- Tokuşoglu, Ö.; Üunal, M.K. Biomass Nutrient Profiles of Three Microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J. Food Sci. 2003, 68, 1144–1148. [Google Scholar] [CrossRef]
- Gadzama, I.U. Evaluation of Fresh Microalgae in Ruminant Nutrition: Impact on Rumen Fermentation, Productive Performance and Meat Quality. Master’s Thesis, School of Agriculture and Food Sustainability, The University of Queensland, Gatton Campus, Gatton, QLD, Australia, 2024. [Google Scholar] [CrossRef]
- Gadzama, I.U. Microalgae Supplementation in Sheep Nutrition: Impact on Wool Production and Quality; Wikifarmer: Athens, Greece, 2024; Available online: https://www.researchgate.net/publication/384767611_Microalgae_Supplementation_in_Sheep_Nutrition_Impact_on_Wool_Production_and_Quality (accessed on 29 December 2024).
- El-Bahr, S.; Shousha, S.; Shehab, A.; Khattab, W.; Ahmed-Farid, O.; Sabike, I.; El-Garhy, O.; Albokhadaim, I.; Albosadah, K. Effect of Dietary Microalgae on Growth Performance, Profiles of Amino and Fatty Acids, Antioxidant Status, and Meat Quality of Broiler Chickens. Animals 2020, 10, 761. [Google Scholar] [CrossRef]
- Ötleş, S.; Pire, R. Fatty acid composition of Chlorella and Spirulina microalgae species. J. AOAC Int. 2001, 84, 1708–1714. [Google Scholar] [CrossRef]
- Kholif, A.E.; Gouda, G.A.; Morsy, T.A.; Matloup, O.H.; Sallam, S.M.; Patra, A.K. Associative effects between Chlorella vulgaris microalgae and Moringa oleifera leaf silage used at different levels decreased in vitro ruminal greenhouse gas production and altered ruminal fermentation. Environ. Sci. Pollut. Res. Int. 2023, 30, 6001–6020. [Google Scholar] [CrossRef]
- Luzzi, S.C.; Gardner, R.D.; Heins, B.J. Taste preference of Chlorella sp. algae from dairy wastewater by weaned dairy calves. JDS Commun. 2020, 1, 41–44. [Google Scholar] [CrossRef]
- Shams, A.; Elsadany, A.; Abou-Aiana, R. Effects of Orally Chlorella vulgaris Algae Additive on Productive and Reproductive Performance of Lactating Friesian Cows. J. Anim. Poult. Prod. 2020, 11, 125–131. [Google Scholar] [CrossRef]
- Akhmedkhanova, R.; Dzhambulatov, Z.; Gadzhaeva, Z.; Shabanov, G.; Alieva, S. The influence of chlorella suspension on the quality of milk and its processing products. E3S Web Conf. 2020, 222, 2021. [Google Scholar] [CrossRef]
- Malyugina, S.; Černohous, M.; Látal, O. Characterization of Microalgae-Based Feed Supplement and Their Possible Influence on Cattle Rumen Microbial Ecosystem. Rural. Dev. 2020, 2019, 40–45. [Google Scholar] [CrossRef]
- Kuzmaitė, I.; Oberauskas, V.; Kantautaitė, J.; Žymantienė, J.; Želvytė, R.; Monkevičienė, I.; Sederevičius, A.; Bakutis, B. The effect of Chlorella vulgaris IFR-111 on microflora of the digestive system of neonate calves. Vet. Ir Zootech. 2009, 47, 44–49. [Google Scholar]
- Rabee, A.E.; Younan, B.R.; Kewan, K.Z.; Sabra, E.A.; Lamara, M. Modulation of rumen bacterial community and feed utilization in camel and sheep using combined supplementation of live yeast and microalgae. Sci. Rep. 2022, 12, 12990. [Google Scholar] [CrossRef]
- Slyusarenko, I.; Kitayeva, A.; Susol, R. Effect of Chlorella Microalgae Suspension on Dairy Productivity of Sheep Mothers and Growth Intensity of Lambs. Acta Biol. Univ. Daugavp. 2021, 21, 117–126. [Google Scholar]
- Kholif, A.E.; Kassab, A.Y.; Hamdon, H.A. Chlorella vulgaris Microalgae and Copper Mixture Supplementation Enhanced the Nutrient Digestibility and Milk Attributes in Lactating Boer Goats. Ann. Anim. Sci. 2021, 21, 939–957. [Google Scholar] [CrossRef]
- Ali, A.M.; Alshaheen, T.; Senosy, W.; Mohammed, A.E.N.; Kassab, A. Effects of feeding green microalgae and Nigella sativa on productive performance and metabolic profile of Boer goats during peripartum period in subtropics. Fresenius Environ. Bull. 2021, 30, 8203–8212. [Google Scholar]
- Abdel-Khalek, A.E.; El-Maghraby, M.M.; Elbialy, Z.I.; Al Wakeel, R.A.; Almadaly, E.A.; Shukry, M.; El-Badawy, A.A.; Zaghloul, H.K.; Assar, D.H. Mitigation of endogenous oxidative stress and improving growth, hemato-biochemical parameters, and reproductive performance of Zaraibi goat bucks by dietary supplementation with Chlorella vulgaris or/and vitamin C. Trop. Anim. Health Prod. 2023, 55, 267. [Google Scholar] [CrossRef]
- Silva, M.R.L.; Alves, J.P.M.; Fernandes, C.C.L.; Cavalcanti, C.M.; Conde, A.J.H.; Bezerra, A.F.; Soares, A.C.S.; Teixeira, D.Í.A.; do Rego, A.C.; Rondina, D. Effect of short-term nutritional supplementation of green microalgae on some reproductive indicators of Anglo-Nubian crossbred goats. Vet. World 2023, 16, 464–473. [Google Scholar] [CrossRef]
- Silva, M.R.L.; Alves, J.P.M.; Fernandes, C.C.L.; Cavalcanti, C.M.; Conde, A.J.H.; Bezerra, A.F.; Soares, A.C.S.; Tetaping, G.M.; de Sá, N.A.R.; Teixeira, D.Í.A.; et al. Use of green microalgae Chlorella as a nutritional supplement to support oocyte and embryo production in goats. Anim. Reprod. Sci. 2023, 256, 107296. [Google Scholar] [CrossRef]
- Kassab, A.; Hamdon, H.; Senosy, W.; Wardy, E.M. Impact of Chlorella vulgaris Microalgae as Anti Stress on Hematological Parameters and Thermoregulation of Boer Goats in Arid Subtropical Regions. New Val. J. Agric. Sci. 2023, 3, 21–27. [Google Scholar]
- Novotná, K.; Fantová, M.; Nohejlová, L.; Borková, M.; Stádník, L.; Ducháček, J. Effect of Chlorella vulgaris and Japonochytrium sp. Microalgae Supplementation on Composition and Fatty Acid Profile of Goat Milk. Acta Univ. Agric. Silvic. Mendelianae Brun. 2017, 65, 1585–1593. [Google Scholar] [CrossRef]
- Swati; Waidha, K.; Negam, S.; Parveen, N.; Chuskit, D.; Mayarngam, K.; Chaurasia, O.P. Effect of dietary supplementation of microalgae Spirulina and Chlorella on growth performance and blood profile of broiler chicken at high altitude. Indian J. Anim. Sci. 2022, 92, 995–998. [Google Scholar] [CrossRef]
- Kang, H.K.; Salim, H.M.; Akter, N.; Kim, D.W.; Kim, J.H.; Bang, H.T.; Kim, M.J.; Na, J.C.; Hwangbo, J.; Choi, H.C.; et al. Effect of various forms of dietary Chlorella supplementation on growth performance, immune characteristics, and intestinal microflora population of broiler chickens. J. Appl. Poult. Res. 2013, 22, 100–108. [Google Scholar] [CrossRef]
- Salim, H.M.; Kang, H.K.; Kim, D.W.; Choi, H.C.; Amin, M.R.; Khaleduzzaman, A.; Beg, M. Effect of Chlorella supplementation on growth performance, immune characteristics, and gut microbiota of broiler chickens. J. Appl. Poult. Res. 2019, 22, 100–108. [Google Scholar]
- Choi, H.; Jung, S.K.; Kim, J.S.; Kim, K.-W.; Oh, K.B.; Lee, P.-Y.; Byun, S.J. Effects of dietary recombinant chlorella supplementation on growth performance, meat quality, blood characteristics, excreta microflora, and nutrient digestibility in broilers. Poult. Sci. 2017, 96, 710–716. [Google Scholar] [CrossRef]
- Coelho, D.F.M.; Alfaia, C.M.R.P.M.; Assunção, J.M.P.; Costa, M.; Pinto, R.M.A.; de Andrade Fontes, C.M.G.; Lordelo, M.M.; Prates, J.A.M. Impact of dietary Chlorella vulgaris and carbohydrate-active enzymes incorporation on plasma metabolites and liver lipid composition of broilers. BMC Vet. Res. 2021, 17, 229. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Nomaguchi, T.; Mori, Y.; Ito, M.; Nakamura, Y.; Fujishima, M.; Fukuda, S. The nutritional efficacy of Chlorella supplementation depends on the individual gut environment: A randomised control study. Front. Nutr. 2021, 8, 648073. [Google Scholar] [CrossRef]
- Velankanni, P.; Go, S.H.; Jin, J.B.; Park, J.S.; Park, S.; Lee, S.B.; Lee, C.G. Chlorella vulgaris Modulates Gut Microbiota and Induces Regulatory T Cells to Alleviate Colitis in Mice. Nutrients 2023, 15, 3293. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yoon, J.H.; An, S.H.; Cho, I.H.; Lee, C.W.; Jeon, Y.J.; Kong, C. Intestinal Immune Cell Populations, Barrier Function, and Microbiomes in Broilers Fed a Diet Supplemented with Chlorella vulgaris. Animals 2023, 13, 2380. [Google Scholar] [CrossRef]
- Alfaia, C.M.; Pestana, J.M.; Rodrigues, M.; Coelho, D.; Aires, M.J.; Ribeiro, D.M.; Major, V.T.; Martins, C.F.; Santos, H.; Lopes, P.A.; et al. Influence of dietary Chlorella vulgaris and carbohydrate-active enzymes on growth performance, meat quality and lipid composition of broiler chickens. Poult. Sci. 2021, 100, 926–937. [Google Scholar] [CrossRef]
- Kim, C.H.; Kang, H.K. Effect of dietary supplementation with a chlorella by-product on the performance, immune response and metabolic function in laying hens. Europ. Poult. Sci. 2015, 79. [Google Scholar] [CrossRef]
- Panaite, T.D.; Cornescu, G.M.; Predescu, N.C.; Cismileanu, A.; Turcu, R.P.; Saracila, M.; Soica, C. Microalgae (Chlorella vulgaris and Spirulina platensis) as a Protein Alternative and Their Effects on Productive Performances, Blood Parameters, Protein Digestibility, and Nutritional Value of Laying Hens’ Egg. Appl. Sci. 2023, 13, 10451. [Google Scholar] [CrossRef]
- Grigorova, S.; Surdjiiska, S.; Banskalieva, V.; Dimitrov, G. The effect of biomass from green algae of Chlorella genus on the biochemical characteristics of table eggs. J. Cent. Eur. Agric. 2006, 7, 111–116. [Google Scholar]
- Martins, C.F.; Pestana, J.M.; Alfaia, C.M.; Costa, M.; Ribeiro, D.M.; Coelho, D.; Lopes, P.A.; Almeida, A.M.; Freire, J.P.B.; Prates, J.A.M. Effects of Chlorella vulgaris as a Feed Ingredient on the Quality and Nutritional Value of Weaned Piglets’ Meat. Foods 2021, 10, 1155. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Marin, D.E.; Untea, A.; Janczyk, P.; Motiu, M.; Criste, R.D.; Souffrant, W.B. Effect of dietary natural supplements on immune response and mineral bioavailability in piglets after weaning. Czech J. Anim. Sci. 2012, 57, 332–343. [Google Scholar] [CrossRef]
- Coelho, D.; Pestana, J.; Almeida, J.M.; Alfaia, C.M.; Fontes, C.M.G.A.; Moreira, O.; Prates, J.A.M. A High Dietary Incorporation Level of Chlorella vulgaris Improves the Nutritional Value of Pork Fat without Impairing the Performance of Finishing Pigs. Animals 2020, 10, 2384. [Google Scholar] [CrossRef]
- Yan, L.; Lim, S.U.; Kim, I.H. Effect of fermented chlorella supplementation on growth performance, nutrient digestibility, blood characteristics, fecal microbial and fecal noxious gas content in growing pigs. Asian-Australas. J. Anim. Sci. 2012, 25, 1742–1747. [Google Scholar] [CrossRef]
- Sikiru, A.B.; Arangasamy, A.; Alemede, I.C.; Egena, S.S.A.; Bhatta, R. Dietary supplementation effects of Chlorella vulgaris on performances, oxidative stress status and antioxidant enzymes activities of prepubertal New Zealand White rabbits. Bull. Natl. Res. Cent. 2019, 43, 162. [Google Scholar] [CrossRef]
- El Basuini, M.F.; Khattab, A.A.A.; Hafsa, S.H.A.; Teiba, I.I.; Elkassas, N.E.M.; El-Bilawy, E.H.; Dawood, M.A.O.; Atia, S.E.S. Impacts of algae supplements (Arthrospira & Chlorella) on growth, nutrient variables, intestinal efficacy, and antioxidants in New Zealand white rabbits. Sci. Rep. 2023, 13, 7891. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Sheiha, A.M.; Taha, A.E.; Swelum, A.A.; Alarifi, S.; Alkahtani, S.; Ali, D.; AlBasher, G.; Almeer, R.; Falodah, F.; et al. Impacts of Enriching Growing Rabbit Diets with Chlorella vulgaris Microalgae on Growth, Blood Variables, Carcass Traits, Immunological and Antioxidant Indices. Animals 2019, 9, 788. [Google Scholar] [CrossRef]
- Hassanein, H.; Arafa, M.M.; Abo Warda, M.A.; Abd–Elall, A. Effect of Using Spirulina Platensis and Chlorella Vulgaris As Feed Additives on Growing Rabbit Performance. Egypt. J. Rabbit. Sci. 2014, 24, 413–431. [Google Scholar] [CrossRef]
- Ahmad, M.T.; Shariff, M.; Yusoff, F.; Goh, Y.M.; Banerjee, S. Applications of microalga Chlorella vulgaris in aquaculture. Rev. Aquac. 2020, 12, 328–346. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Mousa, M.A.; Mamoon, A.; Abdelghany, M.F.; Abdel-Hamid, E.A.; Abdel-Razek, N.; Ali, F.S.; Shady, S.H.; Gewida, A.G. Dietary Chlorella vulgaris modulates the performance, antioxidant capacity, innate immunity, and disease resistance capability of Nile tilapia fingerlings fed on plant-based diets. Anim. Feed. Sci. Technol. 2022, 283, 115181. [Google Scholar] [CrossRef]
- Maliwat, G.C.; Velasquez, S.; Robil, J.L.; Chan, M.; Traifalgar, R.F.; Tayamen, M.; Ragaza, J.A. Growth and immune response of giant freshwater prawn Macrobrachium rosenbergii (De Man) postlarvae fed diets containing Chlorella vulgaris (Beijerinck). Aquac. Res. 2017, 48, 1666–1676. [Google Scholar] [CrossRef]
- Shi, X.; Luo, Z.; Chen, F.; Wei, C.C.; Wu, K.; Zhu, X.M.; Liu, X. Effect of fish meal replacement by Chlorella meal with dietary cellulase addition on growth performance, digestive enzymatic activities, histology and myogenic genes’ expression for crucian carp Carassius auratus. Aquac. Res. 2017, 48, 3244–3256. [Google Scholar] [CrossRef]
- Safari, O.; Paolucci, M.; Motlagh, H.A. Dietary supplementation of Chlorella vulgaris improved growth performance, immunity, intestinal microbiota and stress resistance of juvenile narrow clawed crayfish, Pontastacus leptodactylus Eschscholtz. Aquaculture 2022, 1823, 738138. [Google Scholar] [CrossRef]
- Abdelhamid, F.M.; Elshopakey, G.E.; Aziza, A.E. Ameliorative effects of dietary Chlorella vulgaris and β-glucan against diazinon-induced toxicity in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 96, 213–222. [Google Scholar] [CrossRef]
- Ramírez-Coronel, A.A.; Jasim, S.A.; Zadeh, A.H.A.; Jawad, M.A.; Al-Awsi, G.R.L.; Adhab, A.H.; Kodirov, G.; Soltanifar, Z.; Mustafa, Y.F.; Norbakhsh, M. Dietary Chlorella vulgaris mitigated the adverse effects of Imidacloprid on the growth performance, antioxidant, and immune responses of common carp (Cyprinus carpio). Ann. Anim. Sci. 2023, 23, 845–857. [Google Scholar] [CrossRef]
- Amar, E.C.; Kiron, V.; Satoh, S.; Watanabe, T. Enhancement of innate immunity in rainbow trout (Oncorhynchus mykiss Walbaum) associated with dietary intake of carotenoids from natural products. Fish Shellfish Immunol. 2004, 16, 527–537. [Google Scholar] [CrossRef]
- Quico, C.A.; Astocondor, M.M.; Ortega, R.A. ary supplementation with Chlorella peruviana improve the growth and innate immune response of rainbow trout Oncorhynchus mykiss fingerlings. Aquaculture 2021, 533, 736117. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Park, H.G.; Lee, S.M. Effects of dietary inclusion of Chlorella vulgaris on growth, blood biochemical parameters and antioxidant enzymes activity in olive flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2016, 53, 106. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; de Brabanter, J.; de Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Metsoviti, M.N.; Gkalogianni, E.Z.; Psofakis, P.; Asimaki, A.; Katsoulas, N.; Papapolymerou, G.; Zarkadas, I. The effects of replacing fishmeal by Chlorella vulgaris and fish oil by Schizochytrium sp. and Microchloropsis gaditana blend on growth performance, feed efficiency, muscle fatty acid composition and liver histology of gilthead seabream (Sparus aurata). Aquaculture 2022, 561, 738709. [Google Scholar] [CrossRef]
- Raji, A.A.; Alaba, P.A.; Yusuf, H.; Bakar, N.H.A.; Taufek, N.M.; Muin, H.; Razak, S.A. Fishmeal replacement with Spirulina platensis and Chlorella vulgaris in African catfish (Clarias gariepinus) diet: Effect on antioxidant enzyme activities and haematological parameters. Res. Vet. Sci. 2018, 119, 67–75. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Lee, S.-M.; Park, H.-G.; Choi, J. Effects of Dietary Inclusion of Chlorella vulgaris on Growth, Blood Biochemical Parameters, and Antioxidant Enzyme Activity in Olive Flounder, Paralichthys olivaceus. J. World Aquac. Soc. 2017, 48, 103–112. [Google Scholar] [CrossRef]
- Gouveia, L.; Gomes, E.; Empis, J. Use of Chlorella vulgaris in Rainbow Trout, Oncorhynchus mykiss, Diets to Enhance Muscle Pigmentation. J. Appl. Aquac. 1997, 7, 61–70. [Google Scholar] [CrossRef]
- Sleman, H.; Abdulrahman, N.M.; Hassan, N.; HamaSalih, H. Evaluation of blood, biochemical and biological effects of microalgae Chlorella and germinated barley powder as a source of prebiotic on common carp Cyprinus carpio L. Iraqi J. Vet. Sci. 2021, 35, 271–277. [Google Scholar] [CrossRef]
- Mahmoud, E.A.; El-Sayed, B.M.; Mahsoub, Y.H.; El-Murr, A.E.I.; Neamat-Allah, A.N.F. Effect of Chlorella vulgaris enriched diet on growth performance, hemato-immunological responses, antioxidant and transcriptomics profile disorders caused by deltamethrin toxicity in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 102, 422–429. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
