Simple Summary
In February 2023, a juvenile humpback whale (Megaptera novaeangliae) became trapped upstream of the Rance Tidal Power Station in Brittany, France. A multidisciplinary team, including the French National Stranding Network, implemented an innovative non-invasive rescue strategy involving water-level adjustments and artificial tidal current generation. The whale’s movements were closely monitored by land-based and boat-based observers, and its body length was estimated between 7 and 10 m. After several attempts to guide the whale out, the rescue team successfully enabled its exit from the estuary on the second day of operations. This case demonstrates the effectiveness of coordinated, adaptive interventions for mitigating anthropogenic impacts on marine mammals in modified coastal habitats.
Abstract
Rescue operations involving baleen whales trapped in dammed environments are difficult to perform successfully, yet increasingly relevant under growing coastal development. We report on a two-day (9–10 February 2023) rescue of a juvenile humpback whale trapped upstream of the Rance Tidal Power Station (TPS) in Brittany, France, providing rare peer-review evidence on response strategies in highly engineered estuaries. A collaborative, non-invasive strategy was implemented by adjusting water levels and creating artificial tidal currents to prevent the whale from stranding and to guide the individual back to open water. Approximately 100 people were mobilized as part of the rescue operation. This paper describes a detailed spatiotemporal account of the whale’s movements and a chronological record of the actions taken by the rescue team. After several attempts to guide it out, rescue efforts enabled its successful exit from the estuary on the second day of operations, and it was not subsequently reported stranded along the French coast. This case demonstrates the value of rapid multidisciplinary coordination between the French National Stranding Network (composed of marine scientists, veterinarians and local correspondents), local organizations, the local marine biology station, international marine mammal experts, national institutions, authorities and a tidal energy operator. Beyond documenting an unusual event, this paper provides operational lessons, highlighting (i) the adaptative management of a TPS as a guidance tool, (ii) the prioritization of animal welfare and responders’ safety, (iii) the effective public and media management and (iv) the involvement of trained volunteers during the rescue, promoting environmentally responsible behavior. These insights are transferable to other cases to inform future baleen whales rescue protocols in anthropogenic environments.
1. Introduction
The humpback whale (Megaptera novaeangliae) is a wide-ranging marine species undertaking one of the most extensive mammalian migrations [1,2]. They migrate annually between high-latitude summer feeding grounds and low-latitude winter breeding grounds in tropical and subtropical waters [3,4,5]. Fourteen Distinct Population Segments (DPS) are currently identified based on photo-identification and genetic information [6,7,8]. The North Atlantic Ocean is home to a single North Atlantic population with regional substructure and distinct migratory connections: the West Indies DPS [6,9,10] and the Cape Verde/West Africa DPS [6,11,12]. While satellite tracking studies provide a detailed description of migratory routes from Northern European feeding grounds to the West Indies breeding grounds [13,14], connections with the Cape Verde/West Africa DPS remain unclear. The English Channel has been suggested as a potential migratory corridor for some individuals, supported by photo-identification studies, and an increased number of sightings and strandings in recent years in the eastern North Atlantic [15,16,17,18,19,20,21].
Like other baleen whales, this species is exposed to various anthropogenic threats and infrastructures, including fishing gear entanglement and vessel strikes [22]. Rescuing large cetaceans (>6 m in length) in distress is logistically complex due to their size and weight, requires specialized equipment, and carries significant risks to both the animal and the rescue team [23,24]. Disentanglement methods at sea are well documented and standardized, contributing to high success rates in animal releases [25,26]. In contrast, live strandings of large cetaceans often leads to natural death, palliative care [27] or euthanasia [28], due to the severe injuries, prolonged suffering and high likelihood of re-stranding post-rescue [23,29,30,31]. However, cases of large cetaceans trapped in anthropogenic environments are poorly documented [32,33]. Different baleen whale species have been observed trapped in areas such as harbors, marinas, dammed rivers or estuaries, including humpback whales in Nova Scotia, Canada [34], and the Sacramento River Delta, California [35], Bryde’s whales in the Manning River, New South Wales [36], and a Minke whale in the old port of Montreal, Canada [37]. Such individuals are commonly described as out of habitat [38], erratic [20] or vagrant [38]. Several factors may lead animals to leave their natural range including climate change, loss of habitat, population growth, underwater noise, disturbance, or morbidity [38]. Understanding these factors is essential, as it may allow the implementation of mitigation measures before animals enter high-risk anthropogenic areas. Nevertheless, preventive measures are not always applied or may prove insufficient, leading some animals to become trapped. In such situations, individuals may experience situations of distress critical for their survival or welfare, often involving pain, suffering, physical injury and/or an inability to escape the situation without assistance [39]. Determining whether human intervention is relevant relies on an ethical and scientific assessment that considers the difficulty of evaluating the animal’s actual level of distress, the potential benefits of intervention and the need to compare the degree of intrusiveness to the likelihood of success to avoid unnecessary stress on the animal [24,39]. Human responses can be classified according to their degree of intrusiveness [39] ranging from the establishment of safety perimeters and indirect guidance (e.g., using acoustic deterrents and surface disturbances [35,37]), to the capture and translocation of the animal [36,38], with variable operational success. In cases where welfare cannot be ensured, palliative care or euthanasia may be required [38].
Describing successful rescue attempts is crucial, as sharing effective methods can improve individual survival rates, operational procedures and outcomes. Yet, such events are rarely documented in peer-reviewed literature, despite the need for transparent and detailed reporting to develop standardized, evidence-based rescue protocols. Publishing in scientific journals also ensures long-term data accessibility, reproducibility, and comparability, supporting best practices, responder training, and management strategies for marine mammal welfare in anthropogenic environments [38,40]. No published studies reporting non-invasive methods to rescue a baleen whale currently exist. Here, we report a rare case of a juvenile humpback whale trapped upstream of the Rance Tidal Power Station (TPS) in Brittany, France, and describe a collaborative, non-invasive rescue operation based on the artificial management of water levels and tidal currents. This paper provides a detailed chronological account of the operational framework, whale movements, and outcomes, offering insight into innovative approaches for marine mammal rescue in habitats altered by human activities.
2. Materials and Methods
2.1. Study Area
The Rance estuary is located on the Brittany coast, along the English Channel, in western France (Figure 1a,b). It is a narrow, steep-sided ria with marine waters entry, spanning approximately 20 km inshore and up to 2.5 km wide at its broadest point [41,42,43,44]. The head of the estuary is located at the Chatelier Lock (Figure 1a), which defines the upstream limit of tidal propagation and restricts the southward progression of seawater [45]. The salinity along the channel shows a clear gradient from brackish to marine waters [45]. Three main zones are observed: (i) within 0–200 m upstream of the Chatelier Lock, where salinity is below 20 g/L; (ii) between 200 and 1800 m, where the channel becomes deeper and salinity rises to 25–30 g/L; and (iii) beyond 1800 m, where salinity reaches about 35 g/L. The estuary has a strong tidal range, reaching up to 13.5 m during spring tides at its mouth (Saint-Servan, Figure 1a) [44,45]. The second largest tidal power station (TPS) in the world harnesses this hyper-tidal regime (Figure 1c). As a bidirectional plant, it generates both flood and ebb, and by maximizing the head between high and low water, it imposes artificial tidal rhythms and alters hydrodynamics [46,47,48]. It is operated and managed by Électricité de France (EDF) and supplies electricity to approximately 225,000 inhabitants [42,49]. Key features of the 750 m-long TPS include a navigational lock connecting the cities of Dinard and Saint-Malo, a 390 m-long and 33 m-wide power station housing 24 turbines and six 15 m-wide, 10 m-high sluice gates and a restricted area extending both upstream and downstream of the TPS, where navigation, nautical-subaquatic activities, and swimming are prohibited [50]. The TPS basin lies within the upstream part of this restricted area. Various maritime activities occur north of the restricted area [51,52,53], including commercial and recreational fishing, recreational boating, watersports, and commercial passenger shipping.
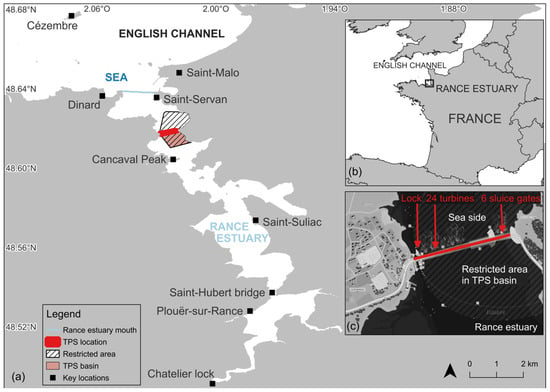
Figure 1.
(a) Study area; (b) Location of the Rance estuary; (c) Tidal Power Station (TPS) © Reproduced with permission from EDF, Mémoguide; published by EDF in 2012 [50]. Data sources: GeoBretagne, Sandre, EDF France.
Given their scale and bidirectional operation, tidal power stations can alter estuarine tidal regimes, with potential effects on ecological continuity between estuary and open sea and on the migration of marine megafauna [46,49,54], including marine mammals. Occasional cases of delphinids and pinnipeds passing through the sluice gates and becoming trapped upstream of the TPS have indeed already been recorded (French National Stranding Network, unpublished data) [55]. As the operator, EDF follows a reporting procedure for every detection of stranded, out of habitat, erratic, or vagrant marine mammals. This procedure complies with legal requirements defined by the French Ministry of the Environment, Energy, and the Sea [56], in accordance with the national transposition of the European Marine Strategy Framework Directive [57]. To reinforce this measure, a partnership has been established since 2020 between the Rance TPS and the non-profit Association AL LARK (hereafter AL LARK), which is dedicated to marine conservation through research, public awareness, and education with a focus on marine mammals. This collaboration has enabled TPS operators (TPSO) to receive training in marine mammal species identification and to implement continuous visual monitoring on both sides of the facility.
2.2. Rescue Coordination
The French National Stranding Network (“Réseau National Échouages”; RNE) is a citizen science network coordinated by the Observatoire Pelagis (UAR 3462, La Rochelle University-CNRS). It relies on ~400 trained local correspondents [18] who take actions as part of their professional duties or as volunteers, following standardized protocols [58].
The trapped humpback whale was first detected by TPS operators (TPSO), who promptly notified AL LARK staff acting as local correspondents of the RNE [18]. As outlined in the RNE guidelines [39], any intervention first requires confirming that the animal is unable to return to its habitat and that action would provide a net benefit without undue risk. Based on video footage and field observations from RNE correspondents, the whale’s situation was assessed as high-risk, with the TPS infrastructure preventing its ability to return to open water under usual TPS conformation. This evaluation justified proceeding with an intervention. The RNE then established a multidisciplinary rescue team (Figure 2) involving local authorities, governmental institutions, the local marine station affiliated with the French National Museum of Natural History (“Muséum national d’Histoire naturelle”; MNHN), local naturalist organizations, a veterinarian and international marine mammal experts. The operation was coordinated through a dual system: the RNE provided guidelines remotely via continuous phone communication, while AL LARK ensured direct operational coordination on-site. Communication with the public and media was jointly managed by AL LARK and RNE employees and volunteers. The strategy was proactive, involving direct engagement with journalists and bystanders on-site to provide clear, consistent information and updates throughout the rescue operation. In total, around one hundred people were mobilized over the two-day operation.
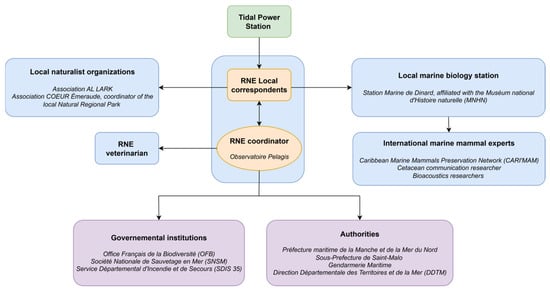
Figure 2.
Schematic overview of the rescue team mobilization. Blue frames represent biologists, orange frames coordinators, green frame the TPS, and purple frames governmental institutions and authorities. All non-English terms refer to the official names of French institutions, organizations, or stakeholder groups and are presented in their original form to ensure accuracy.
2.3. Rescue Strategy
Following the RNE guidelines, the intervention approach followed the precautionary principle, favoring preventive actions and minimizing negative interference with the whale. Once the need for intervention was confirmed, three levels of action were considered: (i) surveillance supported by appropriate monitoring tools to track the whale’s position, condition and signs of distress, (ii) non-intrusive measures aimed at reducing environmental disturbances around the animal and (iii) minimally or non-intrusive operations involving only the least amount of manipulation necessary, such as guidance or relocation without capture or transport. Whenever possible, the least intrusive effective option was selected. The rescue strategy was therefore designed to minimize disturbance to the humpback whale while ensuring operational efficiency and human safety [33,59,60,61]. Direct interactions with the whale were therefore avoided. Vessel approaches were limited to a minimum distance of 100 m, and each approach lasted less than 5 min before vessels returned to standby positions. Engine use and maneuvering were kept to the minimum necessary, with idling or drifting preferred whenever possible. When active deterrence was required, controlled boat maneuvers (i.e., lining up across the river, revving engines, and creating surface disturbances) were used as minimally intrusive physical guidance measures to try to prevent the whale from moving further upstream, toward the southern part of the estuary. No aerial drones were deployed as a precaution due to the whale’s compromised condition, the confined and partially flight-restricted environment (maximum authorized flight altitude of 30 m; Géoportail) [62], and the reduced visibility due to turbidity [63]. Acoustic guidance measures, such as underwater playbacks of conspecific vocalizations [64] and banging pipes, also known as Oikomi pipes [65], were considered but not implemented as the necessary equipment was not available on-site during the operational window for intervention. Moreover, these acoustic tools lack documented effectiveness for baleen whales [35,65]. The main intervention relied on adapting TPS operations. In a normal generation configuration [63], sluice gates can be opened during the flood phase, when turbines operate in inverse turbining (i.e., electricity generation during the rising tide), are turned off or occasionally in pumping mode (i.e., to raise the estuary water level) and they remain closed during direct turbining (i.e., Electricity generation during the falling tide). For the purpose of the operation, turbines and sluice gates were managed to maintain sufficient water level in the estuary to prevent stranding, yet low enough to allow gate opening and facilitate the whale’s movement toward open water. Indeed, the gates cannot be opened when the head difference between the upstream estuary and the downstream sea exceeds 4.5 m. Beyond this threshold, the head difference generates a strong flushing effect and excessive mechanical pressure, which prevent safe gate operations.
2.4. Whale Monitoring
A navigation ban (Arrêté No. 2023/012) was enacted by the Atlantic maritime prefect during the operation, preventing recreational and fishing vessels and allowing access only to rescue boats within the area.
Monitoring was conducted using three 5–7 m rigid-inflatable boats operated by On-Board Observers (OBO), who maintained a minimum distance of 100 m from the whale to minimize stress, in accordance with French environmental regulations (Article L. 334-1, Code de l’Environnement, France). Each boat carried at least three OBOs, each continuously observing their designated sector (port, forward or starboard), and a dedicated pilot responsible for vessel operation and safety. OBO were chosen following their expertise in marine mammal biology, behavior and monitoring, their knowledge of the area and their responsibility for maritime safety. They therefore included authorities, a RNE veterinarian, and marine scientists from the marine station and naturalist organizations. In support, approximately 50 volunteers from RNE and AL LARK land-based citizen science networks were positioned every 500 m from Saint-Servan to Plouër-sur-Rance (Figure 1) as trained Land-Based Observers (LBO). OBO and LBO communicated via instant messaging on cell phones, while communication among OBO teams was carried out through VHF radio.
Whale surfacing events (i.e., blows or behavior in which the whale breaks the water surface [66]) were visually detected by OBOs or LBOs, and whale positions were transmitted to the rescue coordination team in real time. The corresponding GPS coordinates were recorded, and daily sighting maps were produced post-rescue using QGIS v3.34.13. When successive surfacings occurred less than five minutes apart, only the GPS position of the first event was recorded to avoid pseudoreplication and over-representation of short surfacing bouts, and to maintain consistency with the five-minute temporal resolution of hydrodynamic data. A behavioral observation protocol (e.g., scan sampling) was considered to document the whale’s activity state, respiratory rate, swimming speed, and surfacing profile following standard cetacean behavioral sampling methods [67,68,69,70,71], but it could not be implemented due to limited observation conditions.
During selected surfacing events, one OBO boat was positioned 100 m parallel to the whale to visually estimate its size compared to the boat length, visually conduct veterinary assessment, and photo-capture the dorsal fin, fluke and body using a Nikon D7500 equipped with a 150–600 mm telephoto lens (Nikon Corporation, Tokyo, Japan). These photographs were used to complement body condition assessments and to perform photo-identification [72,73], enabling individual identification and potential resightings across geographic areas [12,74,75,76]. The veterinary examination was performed by the RNE veterinarian, initially from the tidal power station platform and later from the rescue boat, to comply with the minimal-disturbance strategy. No physical contact or biopsy sampling was performed to avoid additional stress to the animal. The assessment followed established diagnostic frameworks for external body and skin condition in cetaceans [77,78,79]. Body condition was evaluated visually based on the contour of the epaxial musculature, visibility of spinous processes and depression of the dorsolateral region (“sunken flanks”). Skin condition was assessed from direct observation and high-resolution photographs, focusing on lesion distribution, extent and appearance (e.g., erosions, desquamation, pigmentation changes, and algal deposition) [80].
2.5. TPS Data Analysis
During the rescue, TPSO accessed real-time data on turbine and gates flowrates, TPS operational modes, and water surface elevation (WSE) measurements from tidal gauges at Saint-Suliac (WSE Estuary), immediately downstream of the TPS (WSE Sea), and immediately upstream of the TPS, within the restricted area (WSE TPS) (Figure 1). These data informed adaptive management (e.g., extending gate-opening duration, aligning openings with ebb currents while maintaining sufficient depth) to support the rescue strategy (Figure 3).
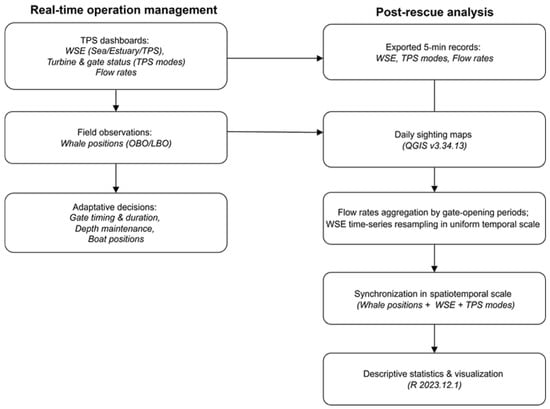
Figure 3.
Data workflow for real-time operation management and post-rescue analysis (TPS = Tidal Power Station, WSE = Water Surface Elevation, OBO = On-board observers, LBO = Land-based observers).
Post-rescue, Électricité de France (EDF) provided flow rate, tidal power station (TPS) operational mode, and water surface elevation (WSE) data recorded at 5 min intervals. WSE values were measured every 5 min, while flow rates were computed at the same temporal resolution using a numerical modelling approach [45], which integrates turbine and sluice gate discharge equations (Figure 3). Flow rates were aggregated by gate-opening periods, and descriptive statistics (mean ± SD) were calculated to compare flow intensity and direction across periods. Flow rates are positive when the current flows in the basin-sea direction, and negative when the current flows in the sea-basin direction. WSE time-series were resampled to a uniform temporal scale using linear interpolation to capture gradual changes and preserve tidal cycle dynamics. Georeferenced whale sightings were projected along the estuary’s longitudinal axis and synchronized with both TPS operational modes and WSE data, allowing integrated spatiotemporal visualization of movements, TPS operational management, and hydrodynamic conditions observed during rescue.
3. Results
3.1. Individual Identification
The animal was identified as a humpback whale (Megaptera novaeangliae) based on its distinctive morphological features, including long [81,82] and completely white flippers (typical of North Atlantic individuals [6,81], and a small dorsal fin located on a dorsal hump [81]. Its estimated body length of 7–10 m indicated that it was a juvenile [3,83].
The whale’s fluke could not be photographed as no tail diving or tail slapping behaviors were observed. However, its dorsal surface and the right side of its dorsal fin were photo-captured and submitted to participatory national (Observatoire Pelagis) and international (Flukebook [84], Happywhale [85]) photo-identification databases (Figure 4). No matches were found, preventing us from confirming from which population it originates.
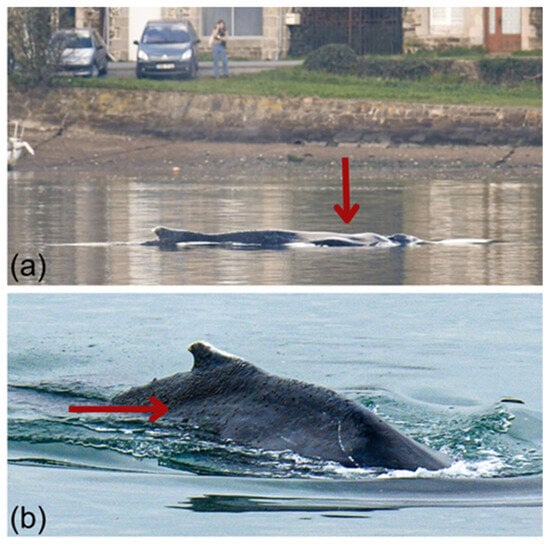
Figure 4.
Photographs of the humpback whale trapped in the Rance estuary. Red arrows indicate external indicators of reduced body condition: (a) Post-nuchal depression, visible as a concave area just posterior to the blowhole, reflecting decreased blubber and epaxial muscle mass; (b) Dorsolateral depression (“sunken flanks”) associated with atrophy of the paravertebral musculature and increased visibility of spinous processes along the thoracolumbar region © AL LARK.
3.2. Whale Condition and Behavior
The visual veterinary assessment indicated poor to moderate body condition, characterized by a visible post-nuchal depression, sunken flanks, atrophy of the paravertebral musculature, and prominence of the spinous processes along the thoracolumbar region (RNE veterinarian, pers. comm.) [86]. These features were consistent with an emaciated body condition as defined by established body condition frameworks [77,79], corresponding to a score of 0–1 [78], where visibility of the dorsal vertebrae and ribcage indicates emaciation.
Skin lesions were observed predominantly along the dorsal midline and dorsal fin. They consisted of multifocal to coalescent erosive and desquamative lesions (Figure 5a), sometimes associated with brownish material adhering to the skin surface, likely composed of algae and/or microorganisms (Figure 5b) [80]. Whitish healed areas were also visible, suggesting prior frictional trauma or cicatrization (Figure 5c), not uncommon in free-ranging humpback whales. Given the absence of biopsy, photogrammetry, or close-range examination, these findings remain qualitative and the temporal stage of the lesions (recent or chronic) could not be determined. Nonetheless, the combination of emaciated body condition and altered skin appearance indicates that the whale’s overall health was likely compromised prior to and possibly aggravated [80] during entrapment.
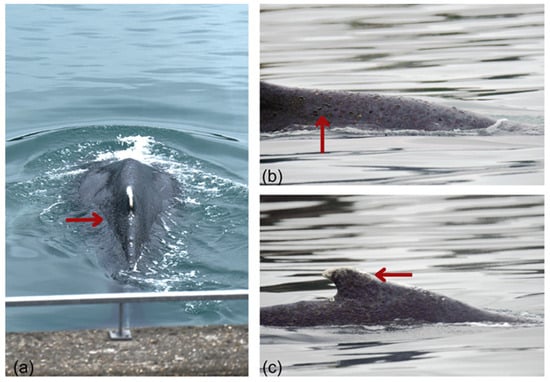
Figure 5.
Skin condition of the humpback whale trapped in the Rance estuary. Red arrows highlight externally visible lesions distributed along the dorsal midline and dorsal fin, including: (a) multifocal erosive and desquamative lesions with adherent brownish material, likely algal or microbial deposits; (b) additional multifocal erosions and surface irregularities consistent with mild to moderate skin compromise; (c) irregular pigmentation and whitish areas compatible with prior frictional trauma or cicatrization. © AL LARK.
Behavioral data could not be quantitatively recorded because the whale’s surfacings were brief, isolated, and irregular. Each surfacing generally consisted of a single blow or a brief dorsal fin appearance followed by a prolonged dive, preventing continuous visual tracking and the systematic application of the planned scan-sampling protocol. As a result, parameters such as respiratory rate, swimming speed, or surfacing profile could not be reliably determined. The data obtained therefore consist primarily of the geographic positions of each surfacing event, as isolated GPS points rather than continuous sequences, which were used to reconstruct the whale’s movements throughout the rescue operations. Nevertheless, behavioral changes were noted over time, including increased lateral movement between the riverbanks, faster swimming, and more frequent surfacing, which were interpreted as signs of stress (RNE veterinarian, pers. comm.) [86]. The whale did not exhibit tail diving or tail slapping behaviors, preventing any photo-documentation of the fluke. Tail diving usually occurs when a whale makes a steep, vertical dive and is uncommon in shallow or confined waters such as the Rance estuary (average WSE = 10.37 ± 1.03 m) [87]. Tail slapping is an energy-demanding behavior associated with social or aggressive signaling [88,89]. It is not depth-dependent and would not be expected in this context unless the whale experienced strong disturbance or threat.
3.3. Rescue Operation & Whale Movement Pattern
3.3.1. First Rescue Day
On 9 February 2023, the juvenile humpback whale entered the Rance estuary, although its exact entry time remains unknown. No whale was reported by TPS operators on the previous day. The gates remained closed overnight and were reopened between 06:00 and 09:40 the morning of 9 February. At that time, tidal and operational conditions corresponded to a rising tide under sea-to-basin turbine operations. Gate water flow was negative (inland direction) with an average discharge of −5770.8 ± 1904.3 m3 s−1. The whale was first sighted by TPSO directly upstream of the TPS (Figure 6a,b; Sighting N° 0) at 10:30, and turbine operations were immediately suspended.
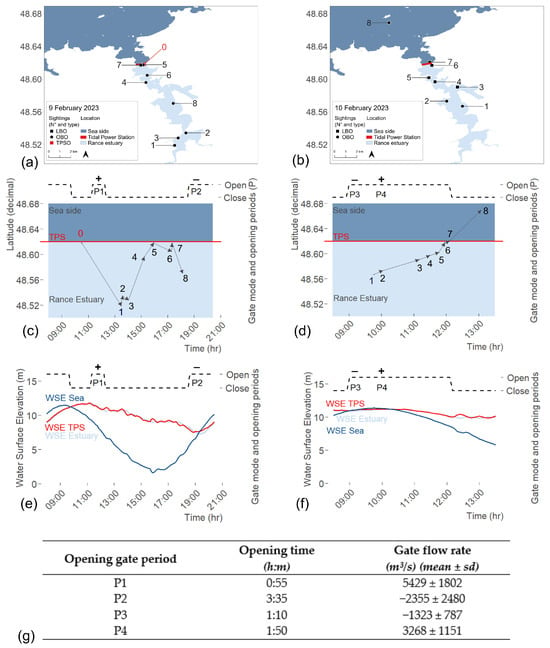
Figure 6.
Summary of the first (a–c) and second (d–f) day of rescue; (a,d) Whale sightings and Sightings observers type (TPSO = TPS operators, OBO = On-board observers, LBO = Land-based observers); (b,e) Whale’s movements through latitudinal axis and time, and Sluice gate opening times; (c,f) Sluice gate opening times and Water Surface Elevation over time at three locations (Sea, TPS, Estuary); (g) Gate opening periods and measurement of flow rate through sluice gates (mean and standard deviation). Positive flows correspond to seaward direction and negative flows to landward direction.
The rescue team was subsequently established and OBO initiated active monitoring at 11:30. The first gate-opening period (11:20–12:15; Figure 6b,c; P1) intended to guide the whale out of the estuary was unsuccessful. During this 55 min attempt, water flow was positive (seaward direction) with an average discharge of 5429 ± 1802 m3/s consistent with the ebb-tide pattern (WSE Sea; Figure 6c, P1), though generated artificially and with reduced intensity.
To reduce stranding risk during the ebb tide, the gates were closed to artificially maintain a higher WSE in the estuary than at sea. At 13:29, estuary WSE was 10.67 m and sea WSE was 4.84 m (Figure 6c). At the same time, the whale was sighted 12 km south of the TPS (Figure 6a,b; sighting N° 1).
From 13:36 to 14:02, the whale swam between the riverbanks at the south of the estuary (Figure 6a,b; sightings N° 2–3) and was then lost from sight. It probably headed north before being resighted at 15:13, 2 km from the TPS and at 15:56 within the TPS basin (Figure 6a,b; sighting N° 4–5). However, the gate-opening process could not be initiated before 17:30 due to the substantial difference in WSE between the TPS basin and the sea (Figure 6c).
Between 15:56 and 17:16, the whale headed south again and was resighted at Cancaval Peak (Figure 6a,b; Sightings N° 5–6). The three rescue boats were used simultaneously during a coordinated attempt to prevent its return to the southern estuary. The boats were aligned across the river and advanced synchronously to create a visual and acoustic barrier. Various maneuvers were performed, including lining up across the river, revving engines, and creating surface disturbances, to encourage the whale to change direction. However, the whale showed no reaction (neither avoidance nor change of direction) and swam beneath the vessels without surfacing, continuing to move south. Consequently, this strategy was abandoned and not used again during the rescue operation. The whale later returned toward the TPS on its own and was sighted at 17:20 within the TPS basin (Figure 6a,b; Sighting N° 7).
Nevertheless, the whale headed south again and was sighted at 18:07 near Saint-Suliac (Figure 6a,b; sighting N° 8). Both on-board and land-based monitoring stopped at 18:30 due to lack of light and safety constraints. The second gate-opening period happened after the tidal change (18:35 to 22:10; Figure 6b,c; P2) but the whale had already been lost from visual contact by that time, and this attempt also failed to guide it out of the estuary. During the night, AL LARK maintained a watch over the water body from the tidal power station operational tower, ensuring readiness to resume coordinated operations early the next morning.
3.3.2. Second Rescue Day
On 10 February 2023, on-board and land-based searching efforts started at 8:00. At 09:46 the whale was first sighted by OBO near Saint-Suliac, 7 km south of the TPS (Figure 6d,e; sighting N° 1). The gates were opened at 9:00 as the tide was rising, creating a flow from the sea to the estuary (Figure 6e,f; P3) and at 10:15, after the tidal change, the gates remained open for three hours, allowing a high average flow of 3268 ± 1151 m3/s toward the sea (Figure 6g; P4).
3.3.3. Back to the Open Sea
The whale was monitored by OBO until it reached Cézembre Island (Figure 6d,e; sighting N° 8), after which it was lost from sight. During the two-day operation, 6% of the sightings were recorded by TPSO, 53% by OBO, and 41% by LBO (Figure 6a,d). No humpback whale strandings were recorded by the RNE along the French coast in the following months, suggesting that the whale could have survived its incursion into and out of the Rance estuary.
3.4. Tidal Power Station Management During Rescue
During the rescue operation, Électricité de France (EDF) suspended normal tidal energy production to allow adaptive gate and turbine management prioritizing the whale’s safety. No formal negotiation phase was required, since animal welfare was pre-identified as the foremost priority guiding all operational decisions. This operational adjustment resulted in an estimated production loss of 1791 MWh compared to forecast values (2230 MWh expected vs. 1578 MWh produced on 9 February; 1771 MWh expected vs. 632 MWh produced on 10 February), corresponding to approximately 0.36% of the annual average output of the Rance Tidal Power Station (500 GWh). Throughout the rescue, the TPS operated in no-production mode for 16% of the time, manual mode for 72%, and power-generation mode for 12%. Power-generation mode was used to lower the WSE Estuary and only when the whale was far from the TPS to avoid any risk of passing through the turbines. Flow rate through the turbine ranged from −2259 to 4758 m3/s and the mean absolute value was 709 ± 1410 m3/s. Through intermittent turbine use, WSE Estuary was regulated and maintained within a suitable range (min = 7.31 m; max = 11.70 m; mean = 10.37 m ± 1.03) that ensured both sufficient depth for the whale’s movement and the prevention of excessive drawdown of the Rance estuary, which could have caused ecological and operational disruptions (Figure 6c,f).
4. Discussion
4.1. Rescue Coordination & Citizen Science Contribution
The success of this operation relied on the rapid response of the rescue team coordinated by the RNE. Marine scientists provided real-time interpretation of the whale’s behavior, movement and stress indicators, allowing the team to adapt interventions to minimize disturbance. The attending veterinarian contributed expertise in welfare assessment and provided immediate advice on the whale’s physical condition and safe interaction distances. The RNE brought extensive operational experience [19,20,39] in managing cetaceans in distress, ensuring the coordination of logistics, safety, and communication among all partners.
RNE and AL LARK trained volunteers played a key role in tracking the whale and maintaining effective communication with OBO to ensure continuous monitoring and adaptation of operations in real-time. Moreover, the involvement of volunteers familiar with marine mammal monitoring and RNE procedures ensured that communication with the public and media remained accurate. This approach not only maintained public support during the rescue but also reduced the risk of misinformation. Indeed, accurate and constructive communication with the public and media is essential to ensure transparency, maintain trust in management decisions, and prevent interference during operations [90,91,92]. Clear information flow also enhances public awareness and supports social acceptance of conservation issues [93]. This case also highlights the value of well-structured citizen science networks (e.g., RNE network and AL LARK’s land-based citizen science program) in supporting marine mammal rescue efforts by raising awareness, promoting responsible public behavior [38], and enhancing operational efficiency [58,94,95,96].
The long-standing partnership between AL LARK and the Rance TPS facilitated early detection of the whale, immediate prioritization of animal welfare over energy production, and coordination of industrial operations for adaptive water-level management. This collaboration also enabled transparent data sharing for post-rescue analysis. Local authorities provided crucial logistical support (e.g., authorizing temporary navigational restrictions, providing safety boats and communication channels) and regulatory supervision. This case demonstrates the value of a multidisciplinary approach [97], where expertise from multiple fields is combined to support better collective decision-making in complex rescue scenarios. Detailed reporting of similar cases would provide valuable insight to guide future interventions.
4.2. Implications for Whale Condition and Behavior
The whale’s presence in the Rance estuary in early February could coincide with the general migration period of North Atlantic humpback whales. During migration, juveniles often show greater variability in their movements and are generally more prone to navigational errors and strandings than adults [9,60,98]. Several European cases similarly involve young individuals entering rivers or shallow coastal systems, suggesting that inexperience or disorientation may contribute to such events [16].
Assessing baleen whale welfare is complex, as it relies on multiple indicators such as skin and body condition, behavior, and environmental factors [78,99]. Methods including visual and photo-based scoring [78,100,101,102], behavioral observations [87,103,104,105,106,107], and tools like drones and high-resolution cameras [108,109,110,111] could therefore improve reproducibility and reliability of real-time welfare assessment. However, the rarity of rescue interventions poses its own challenges to funding and maintaining the investment, maintenance and human resources costs associated with these non-invasive methods and tools.
In this case, welfare assessment and behavioral monitoring were constrained by limited observation opportunities. The whale surfaced infrequently and often at variable distances from observers, which prevented systematic behavioral sampling and reduced the number of high-quality photographs available. These constraints limited a robust, standardized body-condition evaluation and reduced the likelihood of future resightings, due to the absence of a usable fluke photograph. In addition, key behavioral parameters such as activity state, breathing patterns or surfacing frequency could not be quantified, limiting interpretation of stress, weakness or clinical condition progression over time. Although drone imaging could have mitigated some of these limitations by providing a higher vantage point, broader spatial detection, improved photographic and behavioral coverage, it was not used, as the animal’s weakened state called for a cautious approach. The whale’s brief surfacings and the high turbidity of the estuary would also have reduced image quality, since detectability depends on factors such as water clarity and dive behavior [109]. In addition, the operation took place in a confined estuarine area where a legally authorized maximum flight altitude of 30 m applies (Géoportail), which is not consistent with recommended practices from some marine mammal drone studies [62]. These studies note that flights below 30 m may cause avoidance behavior and should be limited to essential tasks, although such responses are documented mainly in odontocetes and should be applied carefully to baleen whales given their different hearing ranges [112]. These trade-offs underscore the challenges of balancing optimal data collection with animal welfare during marine mammal rescue operations. As a result, welfare evaluation relied primarily on qualitative observations and a few opportunistic photo-based assessments, making it difficult to determine whether the rescue operation or the entrapment itself affected the whale’s welfare, especially since the animal likely exhibited poor skin and body condition before entering the estuary. Poor body condition might indeed result from disease, extended migration, prey scarcity, or anthropogenic stressors [113,114,115,116,117]. Nevertheless, prolonged stress experienced during the two-day rescue may have worsened its body condition [118], while freshwater exposure may also have worsened its skin condition [35,78,80]. Future cases would benefit from continuous and more detailed monitoring throughout rescue operations to better evaluate these potential impacts. When conditions allow, the use of aerial drones could support this effort by providing consistent behavioral monitoring, improving body-condition assessment and assisting in the detection of possible injuries or entanglement.
4.3. TPS Management as a Rescue Strategy
Initial attempts to prevent the whale from moving further upstream using noisy vessel maneuvers and surface disturbances proved ineffective. This is consistent with established humpback whale behavior, as individuals often approach or pass beneath vessels rather than avoid them [71,119]. In marine mammal rescue operations, physical guidance techniques such as human or vessel barriers and non-entangling nets can be used for small cetaceans in narrow or shallow areas, but they were unsuitable here due to the whale’s size, its unpredictable diving behavior, and the width of the estuary, which exceeds the operational limits of such devices. Acoustic guidance tools were also considered but not implemented due to the lack of dedicated equipment on-site and the limited evidence supporting their effectiveness for baleen whales. A previous experiment, outside of a rescue operation context, showed that playback of predator sound can induce a rapid horizontal avoidance away from the speaker in humpback whales [120]. However, in a case involving a cow-calf pair of humpback whales observed in the Sacramento River Delta, California, attempts using playbacks of alarm tones, humpback and killer whale sounds, as well as Oikomi pipes, failed to produce downstream movement by the whales [35]. Successful use of such acoustic tools come mainly from killer whale (Orcinus orca) management contexts [33,121], an odontocete species with a different hearing range compared to humpback whales [112].
In the absence of effective physical or acoustic guidance options, given the whale’s lack of reaction to boat-based deterrence and the confined nature of the estuary, the rescue strategy shifted toward a fully non-invasive approach. As the TPS gates formed the only possible entry and exit route, adaptive management of gate openings, turbine operations and hydrodynamic manipulation became the most suitable method to facilitate the whale’s movement downstream.
Results suggest that whale movement may have been influenced by three factors: gate opening duration, whale distance from the TPS, and tidal current velocity and direction. The whale likely entered the estuary during a prolonged gate-opening period at flood tide, when sea-to-basin currents were strongest. Conversely, rescue attempts may have failed (P1, P2, P3) due to the short gate-opening duration (P1), the excessive distance between the whale and the TPS (P2, P3), and flood-tide currents generating adverse inland flow (P2, P3). Hydrodynamic studies of the Rance estuary indicate that TPS operations significantly reshape local flow dynamics [42,45]. Flood currents are strongly modulated by sluice gate operation, which can substantially accelerate flow near the eastern channel upstream of the plant, with effects that remain relatively uniform across the water column. In contrast, ebb currents are more strongly shaped by the morphology of the estuary, with marked intensification in constricted areas such as Saint-Hubert Port [42,45]. During floods, vertical flow variability is limited except at Saint-Hubert Port and Saint-Suliac, where local geometry enhances flow heterogeneity. These combined effects mean that TPS settings can alter the strength, direction and spatial extent of dominant flow pathways within the upper estuary. Although the whale might eventually have exited during a natural ebb tide, this would have required the natural ebb to coincide with TPS operating conditions allowing turbine shutdown or reverse estuary-to-sea flow combined with gate opening, conditions that do not occur systematically under normal production cycles. In contrast, the operational adjustments implemented during P4 (extended gate opening and turbine shutdown) likely reinforced and prolonged a seaward-flow corridor at a moment when the whale was close enough to enter its influence zone. Thus, it appears that the successful exit may have been facilitated by the combination of artificial hydrodynamic reinforcement and spatial proximity of the whale, rather than by tidal phase alone. This hypothesis is consistent with previous descriptions of humpback whale distribution, known to follow predictable tidal cycles in coastal habitats [122,123,124,125]. Other studies in oceanic environments suggest that this species often follows straight migratory tracks [126,127] but may align with high-intensity currents to reduce energetic costs [128]. In a hyper-tidal, confined system like the Rance estuary, artificial modulation of tidal flow therefore likely influenced both inward and outward movements, particularly considering the whale’s compromised condition. These findings underline the importance of increased vigilance during sea-to-basin turbine operations.
4.4. Challenges in Quantifying Whale Responses to TPS Management
In recent years in France, the RNE carried out several interventions to assist marine mammals in distress, including out of habitat cetaceans observed in anthropogenic environments such as estuaries or rivers [18,20]. This case documents the first record of a large cetacean upstream of the Rance TPS, although marine mammals are observed annually by TPSO on both sides of the facility. Occasional cases of delphinids passing through the sluice gates were indeed recorded, including bottlenose, common and Risso’s dolphins, with outcomes ranging from mortalities to successful exits (RNE, unpublished data) [55]. Environmental DNA (eDNA) surveys upstream of the TPS also detected the presence of harbor porpoises, harbor seals, and grey seals [129].
On a global scale, interactions between marine mammals and tidal energy devices remain poorly studied [130,131,132,133,134,135,136,137]. Currently, a dozen TPSs are installed worldwide (located in France, Canada, South Korea, the UK, China, Russia and the Faroe Islands) [138]. However, a recent assessment identified 426 candidate sites with suitable characteristics for tidal stream energy development [139], suggesting that such interactions could become more frequent in the future. To our knowledge, only one technical report describes the response to a large cetacean trapped upstream of a tidal device, documenting a humpback whale confined for at least five days upstream of the Annapolis TPS, Canada, in 2004 [34]. The facility was shut down, navigation was restricted, and the use of whale vocalizations was considered but not applied. While some actions are comparable to the present case (e.g., navigation ban and consideration of acoustic guidance methods), our intervention differed in that TPS operations were modified to manual mode rather than shutdown completely, and artificial flow conditions were created to support the rescue. This lack of comparable cases limited the development of a robust, reproducible framework to assess the juvenile humpback whale response to human-induced hydrodynamic conditions. In this case, data including WSE measured at three tidal gauge locations, TPS modes, and flow rates recorded through turbines and gates provided useful information for real-time adaptive management, but offered limited insight for post-rescue assessment of the whale’s response to the artificial manipulation of tidal currents. Further analysis will include specialized hydrodynamic modeling tools [45,140,141,142,143] to reproduce real-time current velocity measurements along the estuary, flow intensity and direction at sighting locations. These variables will be combined with behavioral observations to test potential current effects on the whale’s movements, to produce a quantitative rather than descriptive study and to ensure full computational reproducibility [40,144,145]. In the absence of these data, we cannot state with certainty that the actions taken to modify the hydrodynamic conditions necessarily influenced the whale’s behavior or directly enabled its exit. Nevertheless, considering the tidal conditions over the two days and the limited possibilities for opening the sluice gates under usual TPS production conditions, without the intervention on these indirect parameters (artificial manipulation of the flows, turbine shutdown, and gate opening), the whale would have had a very low chance of getting out, at least not within a timeframe that would not have put it in danger, given its compromised condition. Therefore, to overcome these limitations and to objectively assess such interactions, future studies should combine high-resolution current data, advanced hydrodynamic modeling, and detailed behavioral assessment to establish standardized methods for evaluating marine mammal interactions with tidal energy devices.
5. Conclusions
The whale’s successful exit coincided with the prolonged opening of the sluice gates, a measure intended to create more favorable conditions for the animal to move toward open water. This operational adjustment was made possible by real-time communication between TPS operators, RNE coordinators, and field observers, who synchronized an adaptive management of the tidal device with the whale’s position and behavior. The effectiveness of this action relied on several enabling factors: the early detection of the whale’s presence, continuous on-site monitoring, and the existence of long-standing partnerships between stakeholders that facilitated rapid, trust-based coordination. Limitations included the whale’s infrequent surfacing, which constrained opportunities for photo-identification, behavior observation and welfare assessments. In addition, the lack of detailed hydrodynamic data and specialized modelling tools restricted the analysis to a descriptive level, thus limiting insights into the whale’s response to the artificial manipulation of tidal currents.
Although some features were context specific, such as the Rance’s hyper-tidal regime, the TPS technical configuration and Électricité de France’s ability to adapt TPS operations, key lessons emerge:
- Establishing structured, multidisciplinary coordination within local emergency protocols, especially in areas where industrial activity overlaps marine mammal habitat, can improve response efficiency, communication, and risk management;
- Adaptive management of hydraulic infrastructure can serve as a non-invasive and effective tool to guide water flow and support animal movement in confined environments;
- Integration of real-time hydrodynamic and environmental data can contextualize observed animal movements, guide management decisions during interventions and improve post-event evaluation of rescue outcomes;
- Systematic, non-invasive monitoring should combine land- and vessel-based observations, drone surveys, photo-identification, behavioral standardized protocols and veterinary assessment;
- Ensuring accurate and constructive communication with the public and media can ensure transparency and prevent misinformation;
- Providing consistent event reporting, including spatiotemporal data, behavioral observations, hydrodynamic conditions, operational decisions and outcomes to facilitate cross-site comparison, can ensure long-term accessibility and support the integration of validated knowledge and best-practice guidelines into national and international rescue frameworks.
This case highlights both generalizable strategies and areas for improvement, offering practical guidance to enhance future marine mammals rescue protocols in anthropogenic environments.
Author Contributions
Conceptualization, O.O. and L.C.; Methodology, O.O. and L.C.; Investigation, J.-L.J., S.L., O.O. and L.C.; Data Provision, F.B.; Formal Analysis, O.O.; Validation, F.B., J.D.W., C.D. and M.D.; Writing—Original Draft Preparation, O.O. and L.C.; Writing—Review & Editing, O.O., L.C., J.D.W., J.-L.J., S.L. and M.D.; Supervision, J.D.W., J.-L.J., C.D. and M.D.; Project Administration, O.O.; APC Funding Acquisition, L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive external research funding. The article processing charge (APC) was covered by Électricité de France (EDF) under a formal sponsorship agreement with Association AL LARK (Convention de Parrainage N°752024), established to support the publication of the case report.
Institutional Review Board Statement
This work did not involve animal experimentation. All actions were emergency response and rescue operations carried out within the official French framework for strictly protected marine mammals, under the Réseau National Échouages (RNE) coordinated by Observatoire Pelagis (UAR 3462, La Rochelle Université/CNRS). Interventions complied with the French Environmental Code provisions on protected species and the national protection order listing marine mammals (Arrêté of 1 July 2011) and followed the national instruction requiring immediate reporting to Observatoire Pelagis and standardized scientific handling of stranded or distressed animals (Ministerial Note of 27 April 2017, NOR DEVL1709454N). Authorizations and mandates to handle, transport, release and sample protected marine mammals are covered by the State authorization issued to La Rochelle Université for Observatoire Pelagis (Arrêté of 17 June 2016) and by the interministerial order of 30 December 2020 (Ministry for the Ecological Transition and Ministry of the Sea) establishing derogations to strict protection for such rescue and scientific operations on marine mammals, in force through 31 December 2026 and implemented via the RNE system for trained responders. All procedures were conducted by trained personnel mandated by the RNE, minimizing stress and ensuring animal welfare. No Institutional Animal Care and Use Committee approval is required in France for such rescue operations; however, all procedures followed national welfare principles (French Rural Code Article L214-1) and minimized stress to the animal.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to EDF data privacy restrictions.
Acknowledgments
We thank the French National Stranding Network (RNE) and all the Observatoire Pelagis team for their leadership and expertise, and all RNE and AL LARK volunteers for their crucial role in detection and monitoring the whale. Special thanks to RNE local correspondents Gaël Gautier, Morgane Perri, Estelle Petiau, and Juliette Biacchi from AL LARK for their field and coordination expertise, data collection and photographic documentation. We are grateful to Matthieu Authier, Jérôme Couvat, CARI’MAM network, Diana Reiss, Charlotte Curé and Olivier Adam for their expert guidance. We also acknowledge RNE veterinarians, the scientists of the Dinard Marine Station of the MNHN, OFB, SNSM and SDIS institutions, the local authorities, TPS operators and COEUR Emeraude naturalists for their collaboration in this rescue.
Conflicts of Interest
Author Florian Boucard is employed by the company ‘EDF R&D (Électricité de France)’. The remaining authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CARI’MAM | Caribbean Marine Mammals Preservation Network |
| DDTM | Direction Départementale des Territoires et de la Mer |
| DPS | Distinct Population Segments |
| EDF | Électricité De France |
| eDNA | Environmental DNA |
| LBO | Land-Based Observers |
| MNHN | Muséum National d’Histoire Naturelle |
| OFB | Office Français de la Biodiversité |
| OBO | On-Board Observers |
| RNE | Réseau National Échouage (National Stranding Network) |
| SNSM | Société Nationale de Sauvetage en Mer |
| SDIS | Service Départemental d’Incendie et de Secours |
| TPS | Tidal Power Station |
| TPSO | Tidal Power Station Operators |
| WSE | Water Surface Elevation |
References
- Dawbin, W.H. The Seasonal Migratory Cycle of Humpback Whales. In Whales, Dolphins, and Porpoises; Norris, K.S., Ed.; University of California Press: Berkeley, CA, USA, 1966; pp. 145–170. ISBN 978-0-520-32137-3. [Google Scholar]
- Stone, G.; Florez-Gonzalez, L.; Katona, S. Whale Migration Record. Nature 1990, 346, 705. [Google Scholar] [CrossRef]
- Chittleborough, R.G. Dynamics of Two Populations of the Humpback Whale, Megaptera novaeangliae (Borowski). Mar. Freshw. Res. 1965, 16, 33–128. [Google Scholar] [CrossRef]
- Katona, S.K.; Beard, J.A. Population Size, Migrations and Feeding Aggregations of the Humpback Whale (Megaptera novaeangliae) in the Western North Atlantic Ocean. Rep. Int. Whal. Comm. 1990, 12, 295–306. [Google Scholar]
- Rasmussen, K.; Palacios, D.; Calambokidis, J.; Saboro, M.; Dalla Rosa, L.; Secchi, E.; Steiger, G.; Allen, J.; Stone, G. Southern Hemisphere Humpback Whales Wintering off Central America: Insights from Water Temperature into the Longest Mammalian Migration. Biol. Lett. 2007, 3, 302–305. [Google Scholar] [CrossRef]
- Bettridge, S.O.M.; Baker, C.S.; Barlow, J.; Clapham, P.; Ford, M.J.; Gouveia, D.; Mattila, D.K.; Pace, R.M.; Rosel, P.E.; Silber, G.K.; et al. Status Review of the Humpback Whale (Megaptera novaeangliae) Under the Endangered Species Act; NOAA: Silver Spring, MD, USA, 2015. Available online: https://repository.library.noaa.gov/view/noaa/4883 (accessed on 14 November 2025).
- Lohman, K. Integrating Genotypes and Photo-Identification Records to Describe Regional Differentiation and Temporal Stability of mtDNA Frequencies from Humpback Whales Feeding in the California Current System. 2022. Available online: https://ir.library.oregonstate.edu/concern/graduate_thesis_or_dissertations/gx41mr88p (accessed on 14 November 2025).
- Llamas-González, M.; Ortega-Ortiz, C.D.; Enríquez-Paredes, L.M.; González-Peral, Ú.A.; Bautista-Guerrero, E.; Martien, K.K. Genetic Data Reveal Wintering Ground Affiliation of Humpback Whales from the Mexican Central Pacific. Mar. Mammal Sci. 2024, 40, 54–72. [Google Scholar] [CrossRef]
- Kettemer, L.E.; Rikardsen, A.H.; Biuw, M.; Broms, F.; Mul, E.; Blanchet, M.-A. Round-Trip Migration and Energy Budget of a Breeding Female Humpback Whale in the Northeast Atlantic. PLoS ONE 2022, 17, e0268355. [Google Scholar] [CrossRef] [PubMed]
- Strevick, P.T.; Bouveret, L.; Gandilhon, N.; Rinaldi, C.; Rinaldi, R.; Broms, F.; Carlson, C.; Kennedy, A.; Ward, N.; Wenzel, F. Migratory Destinations and Timing of Humpback Whales in the Southeastern Caribbean Differ from Those off the Dominican Republic. J. Cetacean Res. Manag. 2018, 18, 127–133. [Google Scholar] [CrossRef]
- Stevick, P.T.; Allen, J.; Clapham, P.J.; Katona, S.K.; Larsen, F.; Lien, J.; Mattila, D.K.; Palsbøll, P.J.; Sears, R.; Sigurjónsson, J.; et al. Population Spatial Structuring on the Feeding Grounds in North Atlantic Humpback Whales (Megaptera novaeangliae). J. Zool. 2006, 270, 244–255. [Google Scholar] [CrossRef]
- Wenzel, F.W.; Broms, F.; López-Suárez, P.; Lopes, K.; Veiga, N.; Yeoman, K.; Rodrigues, M.S.D.; Allen, J.; Fernald, T.W.; Stevick, P.T.; et al. Humpback Whales (Megaptera novaeangliae) in the Cape Verde Islands: Migratory Patterns, Resightings, and Abundance. Aquat. Mamm. 2020, 46, 21–31. [Google Scholar] [CrossRef]
- Kennedy, A.S.; Zerbini, A.N.; Vásquez, O.V.; Gandilhon, N.; Clapham, P.J.; Adam, O. Local and Migratory Movements of Humpback Whales (Megaptera novaeangliae) Satellite-Tracked in the North Atlantic Ocean. Can. J. Zool. 2014, 92, 9–18. [Google Scholar] [CrossRef]
- Whaletrack. UiT—The Arctic University of Norway. Page Administrator: Rikardsen, Audun, Last Updated: 04.03.2024 09:31. 2018. Available online: https://en.uit.no/prosjekter/prosjekt?p_document_id=505966 (accessed on 11 November 2025).
- Ryan, C.; Whooley, P.; Berrow, S.D.; Barnes, C.; Massett, N.; Strietman, W.J.; Broms, F.; Stevick, P.T.; Fernald, T.W.; Schmidt, C. A Longitudinal Study of Humpback Whales in Irish Waters. J. Mar. Biol. Assoc. UK 2016, 96, 877–883. [Google Scholar] [CrossRef]
- Leopold, M.F.; Rotshuizen, E.; Evans, P.G.H. From Nought to 100 in No Time: How Humpback Whales (Megaptera novaeangliae) Came into the Southern North Sea. Lutra 2018, 61, 165–188. Available online: http://www.zoogdiervereniging.nl (accessed on 14 November 2025).
- O’Neil, K.E.; Cunningham, E.G.; Moore, D.M. Sudden Seasonal Occurrence of Humpback Whales Megaptera novaeangliae in the Firth of Forth, Scotland and First Confirmed Movement between High-Latitude Feeding Grounds and United Kingdom Waters. Mar. Biodivers. Rec. 2019, 12, 12. [Google Scholar] [CrossRef]
- Wund, S.; Méheust, E.; Dars, C.; Dabin, W.; Demaret, F.; Guichard, B.; Jauniaux, T.; Labrut, S.; Spitz, J.; Van Canneyt, O.; et al. Strengthening the Health Surveillance of Marine Mammals in the Waters of Metropolitan France by Monitoring Strandings. Front. Mar. Sci. 2023, 10, 1116819. [Google Scholar] [CrossRef]
- Dars, C.; Méheust, E.; Dabin, W.; Doremus, G.; Guichard, B.; Decors, A.; Dixneuf, S.; van Canneyt, O.; Caurant, F. Le Réseau national Échouages: Un outil d’évaluation et de surveillance des populations de mammifères marins. Faune Sauvag. 2020, 325, 32–35. [Google Scholar]
- Meheust, E.; Dars, C.; Caurant, F.; Dabin, W.; Demaret, F.; Genu, M.; Guichard, B.; Fernandez, P.M.; Peltier, H.; Spitz, J.; et al. Les échouages de Mammifères Marins en France en 2023. report; Pelagis (UAR 3462): La Rochelle, France, 2024; p. 58, ⟨mnhn-04826917⟩. [Google Scholar]
- Peltier, H.; Authier, M.; Dabin, W.; Daniel, P.; Dars, C.; Demaret, F.; Meheust, E.; Ridoux, V.; Spitz, J.; Canneyt, O.V. I Sink Therefore I Am: 20 Years of Tagging Small Cetacean Carcasses in the North-East Atlantic for Bycatch Estimation. J. Nat. Conserv. 2025, 87, 127005. [Google Scholar] [CrossRef]
- Fleming, A.; Jackson, J. Global Review of Humpback Whales (Megaptera novaeangliae); NOAA: Silver Spring, MD, USA, 2011. Available online: https://repository.library.noaa.gov/view/noaa/4489 (accessed on 14 November 2025).
- DCCEEW. National Guidelines for Euthanasia of Stranded Large Whales; Department of Climate Change, Energy, the Environment and Water: Canberra, Australia, 2024. Available online: https://www.dcceew.gov.au/sites/default/files/documents/national-guidelines-euthanasia-stranded-large-whales.pdf (accessed on 14 November 2025).
- Alstrup, A.K.O.; Thøstesen, C.B.; Hansen, K.A.; Sonne, C.; Kinze, C.C.; Mikkelsen, L.; Thomsen, A.; Povlsen, P.; Larsen, H.L.; Linder, A.C.; et al. The Self-Stranding Behavior of a Killer Whale (Orcinus Orca) in Inner Danish Waters and Considerations Concerning Human Interference in Live Strandings. Animals 2023, 13, 1948. [Google Scholar] [CrossRef]
- Meÿer, M.; Best, P.; Anderson-Reade, M.; Cliff, G.; Dudley, S.; Kirkman, S. Trends and Interventions in Large Whale Entanglement along the South African Coast. Afr. J. Mar. Sci. 2011, 33, 429–439. [Google Scholar] [CrossRef]
- Frisch-Jordán, A.; López-Arzate, D.C. Large Whale Entanglements in Mexico, a 25-Year Review from 1996 to 2021. Mar. Mammal Sci. 2024, 40, e13106. [Google Scholar] [CrossRef]
- Boys, R.M.; Beausoleil, N.J.; Betty, E.L.; Stockin, K.A. When and How to Say Goodbye: An Analysis of Standard Operating Procedures That Guide End-of-Life Decision-Making for Stranded Cetaceans in Australasia. Mar. Policy 2022, 138, 104949. [Google Scholar] [CrossRef]
- Harms, C.A.; Mclellan, W.A. Chapter 85—Large Whale Euthanasia and Necropsy. In Fowler’s Zoo and Wild Animal Medicine Current Therapy; Miller, E., Lamberski, N., Calle, P., Eds.; W.B. Saunders: New Delhi, India, 2023; Volume 10, pp. 587–594. ISBN 978-0-323-82852-9. [Google Scholar] [CrossRef]
- Daoust, P.Y.; Ortenburger, A.I. Successful Euthanasia of a Juvenile Fin Whale. Can. Vet. J. 2001, 42, 127–129. [Google Scholar] [PubMed] [PubMed Central]
- Kolesnikovas, C.; Groch, K.; Groch, K.; Moraes, A.; Flores, P.; Pretto, D.; Freitas, R.; Gaidzinski, M.; Moreira, L. Euthanasia of an Adult Southern Right Whale (Eubalaena australis) in Brazil. Aquat. Mamm. 2012, 38, 317–321. [Google Scholar] [CrossRef]
- Boys, R.M.; Beausoleil, N.J.; Stockin, K.A. Ethological and Procedural Assessment of Ballistics Euthanasia for Stranded Cetaceans. Appl. Anim. Behav. Sci. 2025, 284, 106537. [Google Scholar] [CrossRef]
- Nunny, L.; Simmonds, M.P. Cetaceans “Out of Habitat”. Wild Animal Welfare. 2020. Available online: https://wildanimalwelfare.com/wp-content/uploads/2021/12/out-of-habitat-marine-mammals-workshop-report-final-1.pdf (accessed on 14 November 2025).
- Jourdain, E.; Barrett-Lennard, L.G.; Ellis, G.M.; Ford, J.K.B.; Karoliussen, R.; Towers, J.R.; Vongraven, D. Natural Entrapments of Killer Whales (Orcinus orca): A Review of Cases and Assessment of Intervention Techniques. Front. Conserv. Sci. 2021, 2, 707616. [Google Scholar] [CrossRef]
- Bowman, M.J.; Gorlov, A.M. Tidal Energy. In Encyclopedia of Ocean Sciences; Cochran, J.K., Bouniewicz, H.J., Yager, P.L., Eds.; Academic Press: Oxford, UK, 2019; Volume 5, pp. 663–673. ISBN 978-0-12-813082-7. [Google Scholar] [CrossRef]
- Gulland, F.; Nutter, F.; Dixon, K.; Calambokidis, J.; Schorr, G.; Barlow, J.; Rowles, T.; Wilkin, S.; Spradlin, T.; Gage, L.; et al. Health Assessment, Antibiotic Treatment, and Behavioral Responses to Herding Efforts of a Cow-Calf Pair of Humpback Whales (Megaptera novaeangliae) in the Sacramento River Delta, California. Aquat. Mamm. 2008, 34, 182–192. [Google Scholar] [CrossRef]
- Priddel, D.; Wheeler, R. Rescue of a Bryde’s Whale Balaenoptera Edeni Entrapped in the Manning River, New South Wales: Unmitigated Success or Unwarranted Intervention? Aust. Zool. 1997, 30, 261–271. [Google Scholar] [CrossRef]
- Anonymous. “Out of Habitat” Marine Mammals II; Second International Workshop Report; OceanCare: Wädenswil, Switzerland, 2023; Available online: https://www.oceancare.org/wp-content/uploads/2023/03/Out-of-Habitat-Report-2-.pdf (accessed on 14 November 2025).
- Nunny, L.; Bossley, M.; Boys, R.M.; Brakes, P.; Genov, T.; Parsons, E.C.M.; Peters, K.J.; Rose, N.A.; Simeone, C.A.; Stockin, K.A.; et al. Out of Habitat Marine Mammals—Identification, Causes, and Management Recommendations. Mar. Policy 2025, 177, 106652. [Google Scholar] [CrossRef]
- Observatoire Pelagis; Office Français de la Biodiversité (OFB). Groupe de Travail National Sur Les Mammifères Marins En Détresse. 2024. Available online: https://www.observatoire-pelagis.cnrs.fr/wp-content/uploads/2024/12/RNE2024_12.GT_National_MM_en_Detresse_PELAGIS_OFB_SWund_BGuichard.pdf (accessed on 14 November 2025).
- Powers, S.M.; Hampton, S.E. Open Science, Reproducibility, and Transparency in Ecology. Ecol. Appl. 2019, 29, e01822. [Google Scholar] [CrossRef]
- Evans, G.; Prego, R. Rias, Estuaries and Incised Valleys: Is a Ria an Estuary? Mar. Geol. 2003, 196, 171–175. [Google Scholar] [CrossRef]
- Rtimi, R.; Sottolichio, A.; Tassi, P. Hydrodynamics of a Hyper-Tidal Estuary Influenced by the World’s Second Largest Tidal Power Station (Rance Estuary, France). Estuar. Coast. Shelf Sci. 2021, 250, 107143. [Google Scholar] [CrossRef]
- Brébant, T.; Sturbois, A.; Robert, A.E.; Desroy, N. Long-Term Changes of Benthic Communities of the Rance Maritime Basin. Estuar. Coast. Shelf Sci. 2025, 321, 109341. [Google Scholar] [CrossRef]
- Bec, C.L.; Legendre, A.; Messiaen, G. Changes in the Annual Harmful Algal Blooms of Alexandrium Minutum: Effects of Environmental Conditions and Drainage Basin Inputs in the Rance Estuary (Brittany, France). Aquat. Living Resour. 2016, 29, 104. [Google Scholar] [CrossRef]
- Rtimi, R.; Sottolichio, A.; Tassi, P.; Bertier, C.; Le Brun, M.; Vandenhove, M.; Parquet, L. Three-Dimensional Hydrodynamic Model of the Rance Estuary (France) Influenced by the World’s Second Largest Tidal Power Plant. LHB 2022, 108, 2016025. [Google Scholar] [CrossRef]
- Trancart, T.; Teichert, N.; Lamoureux, J.; Gharnit, E.; Acou, A.; De Oliveira, E.; Roy, R.; Feunteun, E. A Possible Strong Impact of Tidal Power Plant on Silver Eels’ Migration. Estuar. Coast. Shelf Sci. 2022, 278, 108116. [Google Scholar] [CrossRef]
- Retiere, C. Tidal Power and the Aquatic Environment of La Rance. Biol. J. Linn. Soc. 1994, 51, 25–36. [Google Scholar] [CrossRef]
- Brébant, T.; Robert, A.; Sturbois, A.; Desroy, N.; Sturbois, A.; Desroy, N. Functional Evolution of Benthic Communities in the Rance Basin: Analysis of Biological Traits and Functional Trajectories. Preprint 2024. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wang, Z.W. A Review on Tidal Power Utilization and Operation Optimization. IOP Conf. Ser. Earth Environ. Sci. 2019, 240, 052015. [Google Scholar] [CrossRef]
- EDF. The Rance Tidal Power Station; Memoguide. 2012. Available online: https://www.edf.fr/sites/groupe/files/2024-12/memoguide-la-rance-en.pdf (accessed on 14 November 2025).
- Cabral, P.; Levrel, H.; Schoenn, J.; Thiébaut, E.; Le Mao, P.; Mongruel, R.; Rollet, C.; Dedieu, K.; Carrier, S.; Morisseau, F.; et al. Marine Habitats Ecosystem Service Potential: A Vulnerability Approach in the Normand-Breton (Saint Malo) Gulf, France. Ecosyst. Serv. 2015, 16, 306–318. [Google Scholar] [CrossRef]
- Martin, J.-C.; Mongruel, R.; Levrel, H. Integrating Cultural Ecosystem Services in an Ecosystem Satellite Account: A Case Study in the Gulf of Saint-Malo (France). Ecol. Econ. 2018, 143, 141–152. [Google Scholar] [CrossRef]
- Berkenbosch, K.; Stoffelen, A.; Bono, F. More-Than-Human Geopolitics: How Fishing Shapes Cross-Border Relations in the Gulf of Saint-Malo. Geopolitics 2025, 1–25. [Google Scholar] [CrossRef]
- Desroy, N.; Retière, C. Using Benthos as a Tool for Coastal Management: The Impact of the Tidal Power Station on Benthic Communities of the Rance Basin. Aquat. Ecosyst. Health Manag. 2004, 7, 59–72. [Google Scholar] [CrossRef]
- Observatoire Pelagis. UAR 3462, La Rochelle Université-/CNRS, 5 Allées de l’Océan, 17000 La Rochelle, France. Unpublished work, 2025. [Google Scholar]
- Ministère de L’environnement, de L’énergie et de la mer Note Relative Au Signalement Des Mammifères Marins Échoués Ou à La Dérive, Morts Ou En Détresse, Pour Leur Exploitation Scientifique (NOR: DEVL1709454N). 2017. Available online: https://www.legifrance.gouv.fr/circulaire/id/42149 (accessed on 14 November 2025).
- 2008/56/EC Directive. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). ELI. Available online: http://data.europa.eu/eli/dir/2008/56/oj (accessed on 14 November 2025).
- Peltier, H.; Beaufils, A.; Cesarini, C.; Dabin, W.; Dars, C.; Demaret, F.; Dhermain, F.; Doremus, G.; Labach, H.; Van Canneyt, O.; et al. Monitoring of Marine Mammal Strandings Along French Coasts Reveals the Importance of Ship Strikes on Large Cetaceans: A Challenge for the European Marine Strategy Framework Directive. Front. Mar. Sci. 2019, 6, 486. [Google Scholar] [CrossRef]
- Hunt, T.; Ziccardi, M.; Gulland, F.; Yochem, P.; Hird, D.; Rowles, T.; Mazet, J. Health Risks for Marine Mammal Workers. Dis. Aquat. Organ. 2008, 81, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Meynecke, J.-O.; Meager, J.J. Understanding Strandings: 25 Years of Humpback Whale (Megaptera novaeangliae) Strandings in Queensland, Australia. J. Coast. Res. 2016, 75, 897–901. [Google Scholar] [CrossRef]
- Moore, K.M.; Simeone, C.A.; Brownell, R.L. Strandings. In Encyclopedia of Marine Mammals (Third Edition 14 November 2025); Würsig, B., Thewissen, J.G.M., Kovacs, K.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 945–951. ISBN 978-0-12-804327-1. [Google Scholar] [CrossRef]
- Afridi, S.; Laporte-Devylder, L.; Maalouf, G.; Kline, J.M.; Penny, S.G.; Hlebowicz, K.; Cawthorne, D.; Lundquist, U.P.S. Impact of Drone Disturbances on Wildlife: A Review. Drones 2025, 9, 311. [Google Scholar] [CrossRef]
- Rtimi, R. Hydro-Sedimentary Dynamics of the Rance Estuary: Processes, Evolution and Management. Ph.D. Thesis, Université de Bordeaux, Bordeaux, France, 2022. Available online: https://theses.hal.science/tel-04011162/document (accessed on 14 November 2025).
- Mobley, J.R.; Herman, L.M.; Frankel, A.S. Responses of Wintering Humpback Whales (Megaptera novaeangliae) to Playback of Recordings of Winter and Summer Vocalizations and of Synthetic Sound. Behav. Ecol. Sociobiol. 1988, 23, 211–223. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration (NOAA). Live and Dead Large Whale Emergency Response Best Practices; NOAA: Silver Spring, MD, USA, 2022. [CrossRef]
- Helm, J.E.; Gende, S.M.; Lukacs, P.M. Quantifying Behavior and Collision Risk of Humpback Whales Surfacing near Large Ships: Implications for Detection and Avoidance. Endanger. Species Res. 2023, 51, 115–126. [Google Scholar] [CrossRef]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef]
- Mann, J. Behavioral Sampling Methods for Cetaceans: A Review and Critique. Mar. Mammal Sci. 1999, 15, 102–122. [Google Scholar] [CrossRef]
- Nowacek, D.P.; Christiansen, F.; Bejder, L.; Goldbogen, J.A.; Friedlaender, A.S. Studying Cetacean Behaviour: New Technological Approaches and Conservation Applications. Anim. Behav. 2016, 120, 235–244. [Google Scholar] [CrossRef]
- Godwin, E.M.; Noad, M.J.; Kniest, E.; Dunlop, R.A. Comparing Multiple Sampling Platforms for Measuring the Behavior of Humpback Whales (Megaptera novaeangliae). Mar. Mammal Sci. 2016, 32, 268–286. [Google Scholar] [CrossRef]
- Stamation, K.A.; Croft, D.B.; Shaughnessy, P.D.; Waples, K.A.; Briggs, S.V. Behavioral Responses of Humpback Whales (Megaptera novaeangliae) to Whale-Watching Vessels on the Southeastern Coast of Australia. Mar. Mammal Sci. 2010, 26, 98–122. [Google Scholar] [CrossRef]
- Katona, S.; Baxter, B.; Brazier, O.; Kraus, S.; Perkins, J.; Whitehead, H. Identification of Humpback Whales by Fluke Photographs. In Behavior of Marine Animals; Winn, H.E., Olla, B.L., Eds.; Springer: Boston, MA, USA, 1979; pp. 33–44. ISBN 978-1-4684-2987-9. [Google Scholar] [CrossRef]
- Katona, S.K.; Whitehead, H.P. Identifying Humpback Whales Using Their Natural Markings. Polar Rec. 1981, 20, 439–444. [Google Scholar] [CrossRef]
- McMillan, C. Migratory Patterns of Humpback Whales in Canadian Pacific Waters; Canadian Technical Report of Fisheries and Aquatic Sciences, 1488-5379; 3519; Department of Fisheries and Oceans: Nanaimo, BC, Canada, 2023; Available online: https://coilink.org/20.500.12592/f0b8q6 (accessed on 27 November 2025).
- Cheeseman, T.; Southerland, K.; Acebes, J.M.; Audley, K.; Barlow, J.; Bejder, L.; Birdsall, C.; Bradford, A.L.; Byington, J.K.; Calambokidis, J.; et al. A Collaborative and Near-Comprehensive North Pacific Humpback Whale Photo-ID Dataset. Sci. Rep. 2023, 13, 10237. [Google Scholar] [CrossRef]
- Blázquez, M.; Massett, N.; Whooley, P.; O’Brien, J.; Wenzel, F.; O’Connor, I.; Berrow, S. Abundance Estimates of Humpback Whales (Megaptera novaeangliae) in Irish Coastal Waters Using Mark-Recapture and Citizen Science. J. Cetacean Res. Manag. 2023, 24, 209–225. [Google Scholar] [CrossRef]
- Gulland, F.M.D.; Dierauf, L.A.; Whitman, K.L. (Eds.) CRC Handbook of Marine Mammal Medicine, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-315-14493-1. [Google Scholar] [CrossRef]
- Boileau, A.; Blais, J.; Van Bressem, M.-F.; Hunt, K.E.; Ahloy-Dallaire, J. Physical Measures of Welfare in Fin (Balaenoptera Physalus) and Humpback Whales (Megaptera novangliae) Found in an Anthropized Environment: Validation of a First Animal-Based Indicator in Mysticetes. Animals 2024, 14, 3519. [Google Scholar] [CrossRef]
- Castrillon, J.; Bengtson Nash, S. Evaluating Cetacean Body Condition; a Review of Traditional Approaches and New Developments. Ecol. Evol. 2020, 10, 6144–6162. [Google Scholar] [CrossRef]
- Raverty, S.; Leger, J.S.; Noren, D.P.; Huntington, K.B.; Rotstein, D.S.; Gulland, F.M.D.; Ford, J.K.B.; Hanson, M.B.; Lambourn, D.M.; Huggins, J.; et al. Pathology Findings and Correlation with Body Condition Index in Stranded Killer Whales (Orcinus Orca) in the Northeastern Pacific and Hawaii from 2004 to 2013. PLoS ONE 2020, 15, e0242505. [Google Scholar] [CrossRef]
- Clapham, P.J. Humpback Whale: Megaptera novaeangliae. In Encyclopedia of Marine Mammals (Second Edition 14 November 2025); Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: London, UK, 2009; pp. 582–585. ISBN 978-0-12-373553-9. [Google Scholar] [CrossRef]
- Cooper, L.N. Forelimb Anatomy. In Encyclopedia of Marine Mammals (Second Edition 14 November 2025); Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: London, UK, 2009; pp. 449–452. ISBN 978-0-12-373553-9. [Google Scholar] [CrossRef]
- Russel, G. Assessing the Body Condition and Migration Timing of Humpback and Pygmy Blue Whales in Australia. Ph.D. Thesis, Southern Cross University, Lismore, Australia, 2023. [Google Scholar] [CrossRef]
- Blount, D.; Gero, S.; Van Oast, J.; Parham, J.; Kingen, C.; Scheiner, B.; Stere, T.; Fisher, M.; Minton, G.; Khan, C.; et al. Flukebook: An Open-Source AI Platform for Cetacean Photo Identification. Mamm. Biol. 2022, 102, 1005–1023. [Google Scholar] [CrossRef]
- Cheeseman, T.; Johnson, T.; Southerland, K.; Muldavin, N. Happywhale: Globalizing Marine Mammal Photo Identification via a Citizen Science Web Platform. Report to International Whaling Commission SC/67A/PH/02. 2017. Available online: https://cabowhaletrek.com/wp-content/uploads/2017/06/Cheeseman-et-al-2017-Happywhale-for-IWC-SC_67A_PH_02.pdf (accessed on 14 November 2025).
- Labrut, S. LABOCEA, 7 rue du Sabot, CS 30054, Zoopole, 22440 Ploufragan, France. Personal communication, 2025. [Google Scholar]
- Dolphin, W.F. Ventilation and Dive Patterns of Humpback Whales, Megaptera novaeangliae, on Their Alaskan Feeding Grounds. Can. J. Zool. 1987, 65, 83–90. [Google Scholar] [CrossRef]
- Fabietti, Y.; Spadaro, C.; Tigani, A.; Giglio, G.; Barbuto, G.; Romano, V.; Fedele, G.; Leonetti, F.L.; Venanzi, E.; Barba, C.; et al. Surface Behaviours of Humpback Whale Megaptera novaeangliae at Nosy Be (Madagascar). Biology 2024, 13, 996. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, R.A.; Noad, M.J.; Cato, D.H.; Kniest, E.; Miller, P.J.O.; Smith, J.N.; Stokes, M.D. Multivariate Analysis of Behavioural Response Experiments in Humpback Whales (Megaptera novaeangliae). J. Exp. Biol. 2013, 216, 759–770. [Google Scholar] [CrossRef]
- Geraci, J.R.; Lounsbury, V.J. Marine Mammals Ashore: A Field Guide for Strandings; National Aquarium in Baltimore: Baltimore, MD, USA, 2005; ISBN 0-9774609-0-8. [Google Scholar] [CrossRef]
- Kelly, R.; Fleming, A.; Pecl, G.T.; von Gönner, J.; Bonn, A. Citizen Science and Marine Conservation: A Global Review. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190461. [Google Scholar] [CrossRef]
- Kelly, R.; Fleming, A.; Pecl, G.; Richter, A.; Bonn, A. Social License through Citizen Science: A Tool for Marine Conservation. Ecol. Soc. 2019, 24, 16. [Google Scholar] [CrossRef]
- Meyer, R.; Korabik, A.; Harwell, T.; Petersen, N.; Ballard, H. Examining the Role of Community and Citizen Science in Marine Protected Area Implementation. eScholarship. 2022. Available online: https://escholarship.org/uc/item/4f89v0b5 (accessed on 12 November 2025).
- Ogle, M. Managing the Welfare of Marine Mammals at Mass Strandings in Golden Bay, New Zealand. In Marine Mammal Welfare: Human Induced Change in the Marine Environment and its Impacts on Marine Mammal Welfare; Butterworth, A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 137–146. ISBN 978-3-319-46994-2. [Google Scholar] [CrossRef]
- Kurniawati, A.; Hidayat, J.W. The Role of the Coastal Communities as First Responders of Stranded Marine Mammals in East Java. E3S Web Conf. 2018, 73, 02006. [Google Scholar] [CrossRef]
- Gómez-Hernández, G.; Norzagaray-López, O.; Lubinsky-Jinich, D.; Heckel, G.; Schramm, Y.; Seingier, G. From Beach Users to First Responders: The Role of Civil Society in Response Actions to Marine Mammal Stranding Events. Ocean Coast. Manag. 2022, 219, 106073. [Google Scholar] [CrossRef]
- Oliveira, B.S.S.P.; Santos, R.G.; Santos, B.A. Improving the Knowledge Management of Marine Megafauna Strandings. J. Environ. Manag. 2024, 351, 119815. [Google Scholar] [CrossRef] [PubMed]
- Warlick, A.J.; Huggins, J.L.; Lambourn, D.M.; Duffield, D.A.; D’Alessandro, D.N.; Rice, J.M.; Calambokidis, J.; Hanson, M.B.; Gaydos, J.K.; Jeffries, S.J.; et al. Cetacean Strandings in the US Pacific Northwest 2000–2019 Reveal Potential Linkages to Oceanographic Variability. Front. Mar. Sci. 2022, 9, 758812. [Google Scholar] [CrossRef]
- Nicol, C.; Bejder, L.; Green, L.; Johnson, C.; Keeling, L.; Noren, D.; Van Der Hoop, J.; Simmonds, M. Anthropogenic Threats to Wild Cetacean Welfare and a Tool to Inform Policy in This Area. Front. Vet. Sci. 2020, 7, 57. [Google Scholar] [CrossRef]
- Bradford, A.L.; Weller, D.W.; Punt, A.E.; Ivashchenko, Y.V.; Burdin, A.M.; VanBlaricom, G.R.; Brownell, R.L. Leaner Leviathans: Body Condition Variation in a Critically Endangered Whale Population. J. Mammal. 2012, 93, 251–266. [Google Scholar] [CrossRef]
- Hermosilla, C.; Silva, L.M.R.; Prieto, R.; Kleinertz, S.; Taubert, A.; Silva, M.A. Endo- and Ectoparasites of Large Whales (Cetartiodactyla: Balaenopteridae, Physeteridae): Overcoming Difficulties in Obtaining Appropriate Samples by Non- and Minimally-Invasive Methods. Int. J. Parasitol. Parasites Wildl. 2015, 4, 414–420. [Google Scholar] [CrossRef]
- Hunt, K.E.; Moore, M.J.; Rolland, R.M.; Kellar, N.M.; Hall, A.J.; Kershaw, J.; Raverty, S.A.; Davis, C.E.; Yeates, L.C.; Fauquier, D.A.; et al. Overcoming the Challenges of Studying Conservation Physiology in Large Whales: A Review of Available Methods. Conserv. Physiol. 2013, 1, cot006. [Google Scholar] [CrossRef] [PubMed]
- Watters, J.V.; Krebs, B.L.; Eschmann, C.L. Assessing Animal Welfare with Behavior: Onward with Caution. J. Zool. Bot. Gard. 2021, 2, 75–87. [Google Scholar] [CrossRef]
- Schuler, A.R.; Piwetz, S.; Di Clemente, J.; Steckler, D.; Mueter, F.; Pearson, H.C. Humpback Whale Movements and Behavior in Response to Whale-Watching Vessels in Juneau, AK. Front. Mar. Sci. 2019, 6, 710. [Google Scholar] [CrossRef]
- De Weerdt, J.; Calambokidis, J.; Pouplard, E.; Pouey-Santalou, V.; Patulny, C.; Vanschoenwinkel, B.; Kochzius, M.; Clapham, P. Abundance, Distribution and Behaviour of Humpback Whales (Megaptera novaeangliae) along the Pacific coast of Nicaragua, Central America. Mar. Freshw. Res. 2022, 73, 1041–1055. [Google Scholar] [CrossRef]
- Di Clemente, J.; Christiansen, F.; Pirotta, E.; Steckler, D.; Wahlberg, M.; Pearson, H.C. Effects of Whale Watching on the Activity Budgets of Humpback Whales, Megaptera novaeangliae (Borowski, 1781), on a Feeding Ground. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 810–820. [Google Scholar] [CrossRef]
- Gunnufsen, R. Dive Behaviour and Respiration Rates of Humpback Whales (Megaptera novaeangliae) During Foraging off Northern Norway, with Implications for Metabolic Rate Estimates. Master’s Thesis, UiT Norges Arktiske Universitet, Tromsø, Norway, 2022. Available online: https://hdl.handle.net/10037/25553 (accessed on 14 November 2025).
- Horton, T.W.; Hauser, N.; Cassel, S.; Klaus, K.F.; Fettermann, T.; Key, N. Doctor Drone: Non-Invasive Measurement of Humpback Whale Vital Signs Using Unoccupied Aerial System Infrared Thermography. Front. Mar. Sci. 2019, 6, 466. [Google Scholar] [CrossRef]
- Álvarez-González, M.; Suarez-Bregua, P.; Pierce, G.J.; Saavedra, C. Unmanned Aerial Vehicles (UAVs) in Marine Mammal Research: A Review of Current Applications and Challenges. Drones 2023, 7, 667. [Google Scholar] [CrossRef]
- Napoli, C.; Hirtle, N.; Stepanuk, J.; Christiansen, F.; Heywood, E.I.; Grove, T.J.; Stoller, A.; Dodds, F.; Glarou, M.; Rasmussen, M.H.; et al. Drone-Based Photogrammetry Reveals Differences in Humpback Whale Body Condition and Mass across North Atlantic Foraging Grounds. Front. Mar. Sci. 2024, 11, 1336455. [Google Scholar] [CrossRef]
- Stepanuk, E.F.; Heywood, E.I.; Lopez, J.F.; DiGiovanni, R.A.; Thorne, L.H. Age-Specific Behavior and Habitat Use in Humpback Whales: Implications for Vessel Strike. Mar. Ecol. Prog. Ser. 2021, 663, 209–222. [Google Scholar] [CrossRef]
- Nummela, S. Hearing. In Encyclopedia of Marine Mammals (Second Edition 14 November 2025); Perrin, W.F., Würsig, B., Thewissen, J.G.M., Eds.; Academic Press: London, UK, 2009; pp. 553–562. ISBN 978-0-12-373553-9. [Google Scholar] [CrossRef]
- Fleming, A.H.; Clark, C.T.; Calambokidis, J.; Barlow, J. Humpback Whale Diets Respond to Variance in Ocean Climate and Ecosystem Conditions in the California Current. Glob. Change Biol. 2016, 22, 1214–1224. [Google Scholar] [CrossRef]
- Braithwaite, J.E.; Meeuwig, J.J.; Letessier, T.B.; Jenner, K.C.S.; Brierley, A.S. From Sea Ice to Blubber: Linking Whale Condition to Krill Abundance Using Historical Whaling Records. Polar Biol. 2015, 38, 1195–1202. [Google Scholar] [CrossRef]
- Groch, K.R.; Díaz-Delgado, J.; Marcondes, M.C.C.; Colosio, A.C.; Santos-Neto, E.B.; Carvalho, V.L.; Boos, G.S.; Oliveira De Meirelles, A.C.; Ramos, H.G.D.C.; Guimarães, J.P.; et al. Pathology and Causes of Death in Stranded Humpback Whales (Megaptera novaeangliae) from Brazil. PLoS ONE 2018, 13, e0194872. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, F.; Rodríguez-González, F.; Martínez-Aguilar, S.; Urbán, J.; Swartz, S.; Warick, H.; Vivier, F.; Bejder, L. Poor Body Condition Associated with an Unusual Mortality Event in Gray Whales. Mar. Ecol. Prog. Ser. 2021, 658, 237–252. [Google Scholar] [CrossRef]
- Meynecke, J.-O.; de Bie, J.; Barraqueta, J.-L.M.; Seyboth, E.; Dey, S.P.; Lee, S.B.; Samanta, S.; Vichi, M.; Findlay, K.; Roychoudhury, A.; et al. The Role of Environmental Drivers in Humpback Whale Distribution, Movement and Behavior: A Review. Front. Mar. Sci. 2021, 8, 720774. [Google Scholar] [CrossRef]
- Lemos, L.S.; Olsen, A.; Smith, A.; Burnett, J.D.; Chandler, T.E.; Larson, S.; Hunt, K.E.; Torres, L.G. Stressed and Slim or Relaxed and Chubby? A Simultaneous Assessment of Gray Whale Body Condition and Hormone Variability. Mar. Mammal Sci. 2021, 38, 801–811. [Google Scholar] [CrossRef]
- Gulesserian, M.; Slip, D.; Heller, G.; Harcourt, R. Modelling the Behaviour State of Humpback Whales Megaptera novaeangliae in Response to Vessel Presence off Sydney, Australia. Endanger. Species Res. 2011, 15, 255–264. [Google Scholar] [CrossRef]
- Curé, C.; Sivle, L.; Visser, F.; Wensveen, P.; Isojunno, S.; Harris, C.; Kvadsheim, P.; Lam, F.; Miller, P. Predator Sound Playbacks Reveal Strong Avoidance Responses in a Fight Strategist Baleen Whale. Mar. Ecol. Prog. Ser. 2015, 526, 267–282. [Google Scholar] [CrossRef]
- Robertson, F.C.; Giles, D.; Frayne, A.; Noviello, D.; Dykens, B. NWAC Southern Resident Killer Whale Deterrence Task Force Final Report for Puget Sound Partnership and the Northwest Area Committee Regional Response Team 10 Whale Deterrence Task Force. 2024, pp. 43 + Appendices. Available online: https://nrt.org/sites/175/files/NWAC-RRT10-SRKW%20Deterrence%20Task%20Force%20Final%20Report-with%20appendices.pdf (accessed on 14 November 2025).
- Hazen, E.; Friedlaender, A.; Thompson, M.; Ware, C.; Weinrich, M.; Halpin, P.; Wiley, D. Fine-Scale Prey Aggregations and Foraging Ecology of Humpback Whales Megaptera novaeangliae. Mar. Ecol. Prog. Ser. 2009, 395, 75–89. [Google Scholar] [CrossRef]
- Cotté, C.; Simard, Y. Formation of Dense Krill Patches under Tidal Forcing at Whale Feeding Hot Spots in the St. Lawrence Estuary. Mar. Ecol. Prog. Ser. 2005, 288, 199–210. [Google Scholar] [CrossRef]
- Benjamins, S.; Dale, A.C.; Hastie, G.; Waggitt, J.J.; Lea, M.-A.; Scott, B.; Wilson, B. Confusion Reigns? A Review of Marine Megafauna Interactions with Tidal-Stream Environments. Oceanogr. Mar. Biol. Annu. Rev. 2015, 53, 1–54. [Google Scholar] [CrossRef]
- Markowitz, T.M.; Keener, W.; Webber, M.A.; Payne, A.R.; Lane, R.S.; Fahlbusch, J.A.; Calambokidis, J. New Urban Habitat for Endangered Humpback Whales: San Francisco Bay. Aquat. Conserv. Mar. Freshw. Ecosyst. 2024, 34, e4107. [Google Scholar] [CrossRef]
- Horton, T.W.; Holdaway, R.N.; Zerbini, A.N.; Hauser, N.; Garrigue, C.; Andriolo, A.; Clapham, P.J. Straight as an Arrow: Humpback Whales Swim Constant Course Tracks during Long-Distance Migration. Biol. Lett. 2011, 7, 674–679. [Google Scholar] [CrossRef]
- Horton, T.W.; Zerbini, A.N.; Andriolo, A.; Danilewicz, D.; Sucunza, F. Multi-Decadal Humpback Whale Migratory Route Fidelity despite Oceanographic and Geomagnetic Change. Front. Mar. Sci. 2020, 7, 414. [Google Scholar] [CrossRef]
- Trudelle, L.; Cerchio, S.; Zerbini, A.N.; Geyer, Y.; Mayer, F.-X.; Jung, J.-L.; Hervé, M.R.; Pous, S.; Sallée, J.-B.; Rosenbaum, H.C. Influence of Environmental Parameters on Movements and Habitat Utilization of Humpback Whales (Megaptera novaeangliae) in the Madagascar Breeding Ground. R. Soc. Open Sci. 2016, 3, 160616. [Google Scholar] [CrossRef]
- Haderlé, R.; Alexandre, C.; Gaël, K.; Anne, L.; Nils, T.; Visotheary, U.; Jean-Luc, J. A Multi-Taxa Approach to Estuarine Biomonitoring: Assessing Vertebrate Biodiversity and Ecological Continuity Using Environmental DNA Metabarcoding in the Rance River (Brittany, France). bioRxiv 2025. submitted. [Google Scholar] [CrossRef]
- Dadswell, M.J.; Rulifson, R.A. Macrotidal Estuaries: A Region of Collision between Migratory Marine Animals and Tidal Power Development. Biol. J. Linn. Soc. 1994, 51, 93–113. [Google Scholar] [CrossRef]
- Carlson, T.; Jepsen, R.; Copping, A. Potential Effects of the Interaction between Marine Mammals and Tidal Turbines—An Engineering and Biomechanical Analysis. In Proceedings of the 10th European Wave and Tidal Energy Conference (EWTEC 2013), Aalborg, Denmark, 2–5 September 2013. [Google Scholar]
- Garavelli, L. 2020 State of the Science Report, Chapter 8: Encounters of Marine Animals with Marine Renewable Energy Device Mooring Systems and Subsea Cables. 2020, p. PNNL-29976CHPT8, 1633184. Available online: https://www.osti.gov/servlets/purl/1633184/ (accessed on 14 November 2025).
- Benjamins, D.S. Understanding the Potential for Marine Megafauna Entanglement Risk from Marine Renewable Energy Developments; Commissioned Report No. 791; Scottish Natural Heritage: Edinburgh, UK, 2014; ISBN 1-78391-189-1. [Google Scholar]
- Sparling, C.; Lonergan, M.; McConnell, B. Harbour Seals (Phoca vitulina) around an Operational Tidal Turbine in Strangford Narrows: No Barrier Effect but Small Changes in Transit Behaviour. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 194–204. [Google Scholar] [CrossRef]
- Copping, A.; Grear, M.; Jepsen, R.; Chartrand, C.; Gorton, A. Understanding the Potential Risk to Marine Mammals from Collision with Tidal Turbines. Int. J. Mar. Energy 2017, 19, 110–123. [Google Scholar] [CrossRef]
- Simmonds, M.P.; Brown, V.C. Is There a Conflict between Cetacean Conservation and Marine Renewable-Energy Developments? Wildl. Res. 2010, 37, 688–694. [Google Scholar] [CrossRef]
- Grear, M. Characterization of Marine Mammal Biomechanics to Evaluate Tidal Turbine Collision Impact. Ph.D. Dissertation, University of Washington, Washington, DC, USA, 2018. [Google Scholar]
- Dalal-Clayton, B.; Scott-Brown, M. Improving Decision-Making for the Energy Transition: Guidance for Using Strategic Environmental Assessment. Report by International Association for Impact Assessment (IAIA). Report for Asian Development Bank (ADB). 2024. Available online: https://training.iaia.org/guidance-for-using-strategic-environmental-assessment-sea/ (accessed on 12 November 2025).
- Coles, D.; Adcock, T.; Cornett, A.; Haas, K.; Jo, C.H.; Liu, H.; Miles, J.; Garcia Novo, P.; Thiébot, J. A Review of Global Tidal Stream Energy Resources. Proc. R. Soc. Math. Phys. Eng. Sci. 2025. [Google Scholar] [CrossRef]
- Le Mouel, M.; Matte, P.; Hammouti, A.; Pham Van Bang, D. Investigation of 3D Circulation and Secondary Flows in the St. Lawrence Fluvial Estuary at a Tidal Junction. Estuar. Coast. Shelf Sci. 2025, 313, 109058. [Google Scholar] [CrossRef]
- Benito, I.; Aerts, J.C.J.H.; Ward, P.J.; Eilander, D.; Muis, S. A Multiscale Modelling Framework of Coastal Flooding Events for Global to Local Flood Hazard Assessments. Nat. Hazards Earth Syst. Sci. 2025, 25, 2287–2315. [Google Scholar] [CrossRef]
- Niemi, F.M.; Andersson, A.G.; Hellström, J.G.I.; Hajiesmaeili, M.; Aldvén, D. Investigating Steady-State Interpolation and Transient Hydraulic Modelling to Evaluate European Grayling Habitat in a Hydropeaking River. Water 2025, 17, 1083. [Google Scholar] [CrossRef]
- Bentivoglio, R.; Isufi, E.; Jonkman, S.N.; Taormina, R. Rapid Spatio-Temporal Flood Modelling via Hydraulics-Based Graph Neural Networks. Hydrol. Earth Syst. Sci. 2023, 27, 4227–4246. [Google Scholar] [CrossRef]
- Cohen-Boulakia, S.; Belhajjame, K.; Collin, O.; Chopard, J.; Froidevaux, C.; Gaignard, A.; Hinsen, K.; Larmande, P.; Bras, Y.L.; Lemoine, F.; et al. Scientific Workflows for Computational Reproducibility in the Life Sciences: Status, Challenges and Opportunities. Future Gener. Comput. Syst. 2017, 75, 284–298. [Google Scholar] [CrossRef]
- Jenkins, G.B.; Beckerman, A.P.; Bellard, C.; Benítez-López, A.; Ellison, A.M.; Foote, C.G.; Hufton, A.L.; Lashley, M.A.; Lortie, C.J.; Ma, Z.; et al. Reproducibility in Ecology and Evolution: Minimum Standards for Data and Code. Ecol. Evol. 2023, 13, e9961. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).