Lipidomics Reveals Differences in Lipid Composition Between Lipid Droplets and Milk Fat Globules in Dairy Goat Mammary Tissue
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Experimental Design and Management
2.3. Oil Red O Staining
2.4. Staining of Breast Tissue with Nile Red
2.5. Lipid Droplet Extraction
2.6. Lipidomics Analysis
2.7. Statistical Analysis
3. Results
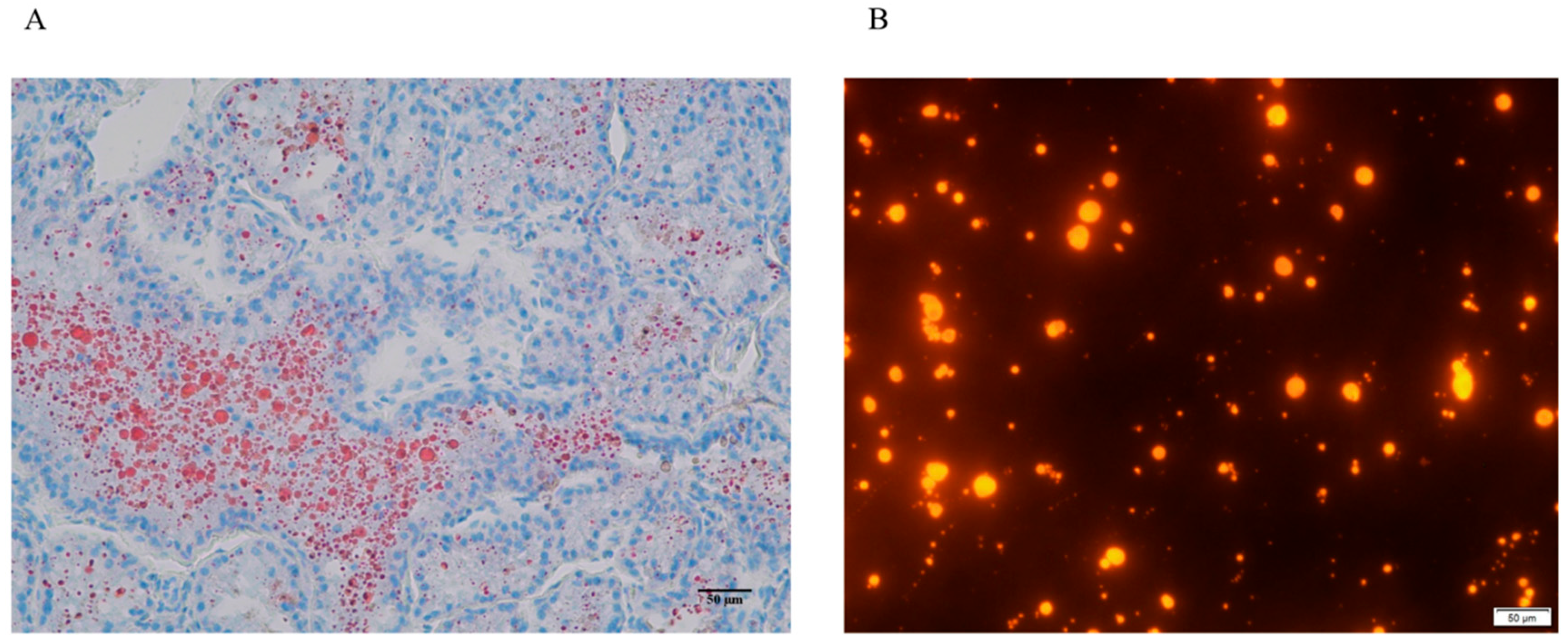
3.1. LDs in Dairy Goat Mammary Tissue
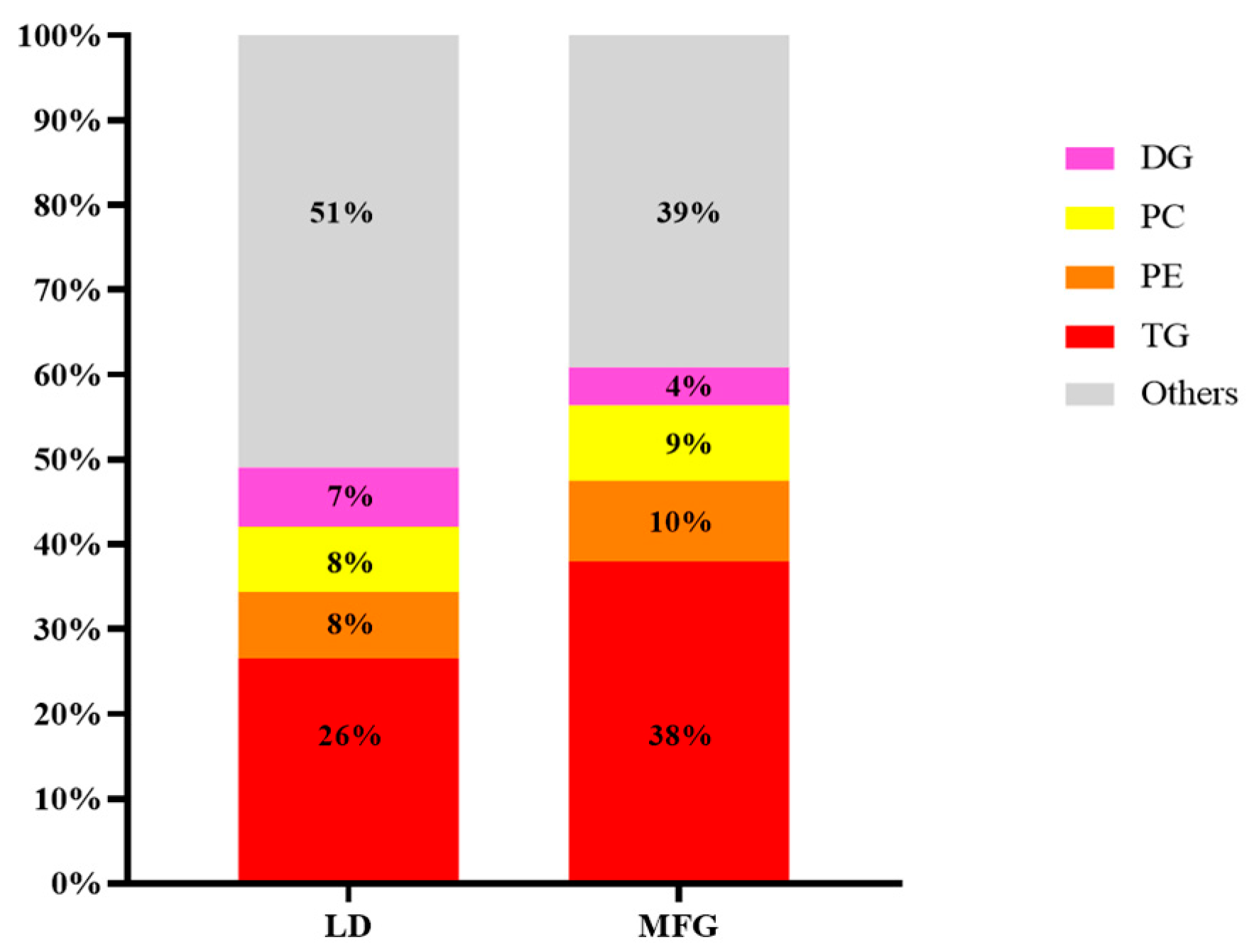
3.2. Comparative Analysis of Lipidomics Between LDs and MFGs
3.3. Diversity of LDs in Mammary Tissue with MFGs
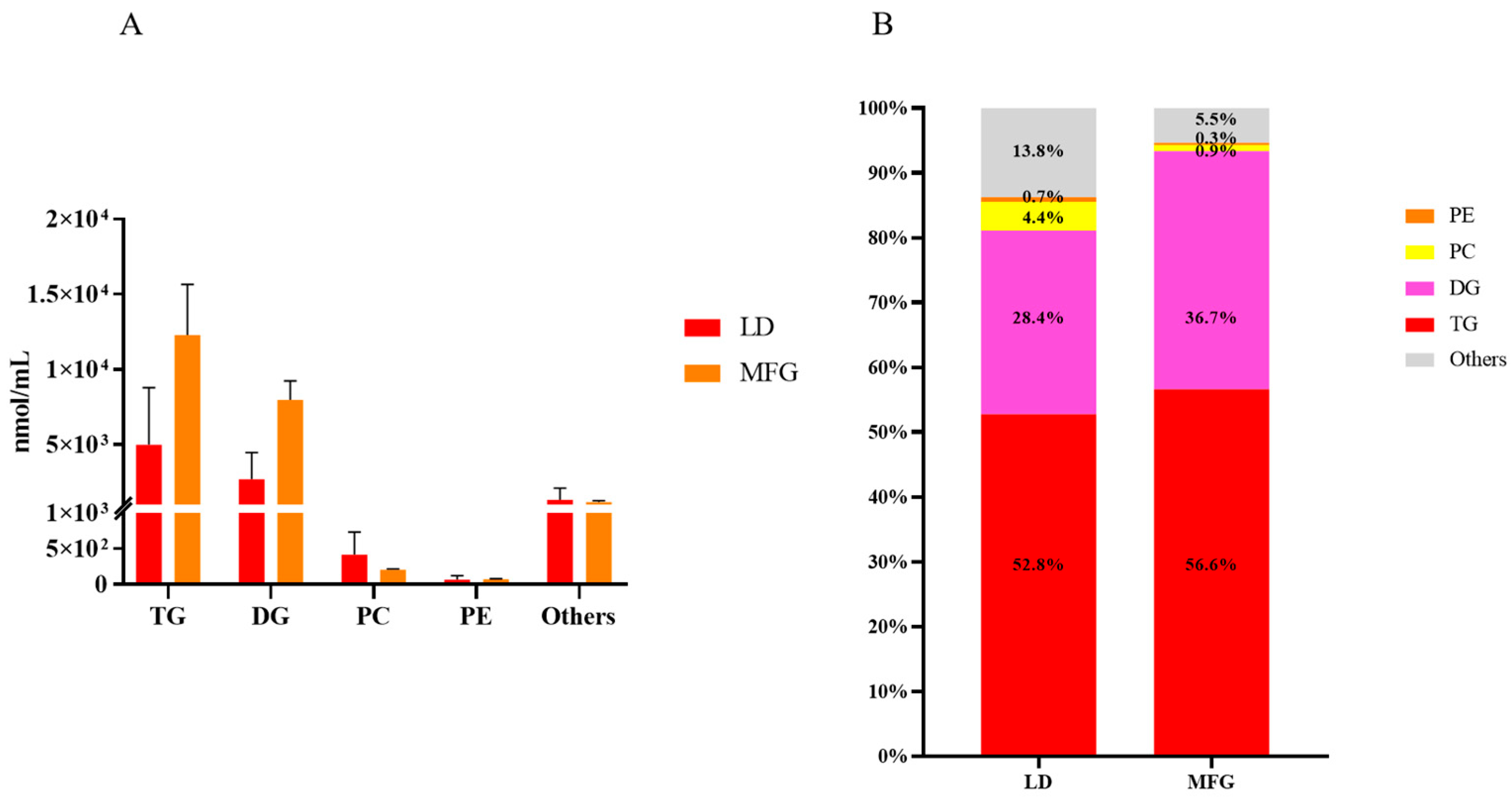
3.4. The Quantity of and Differences Between Each Lipid Subclass
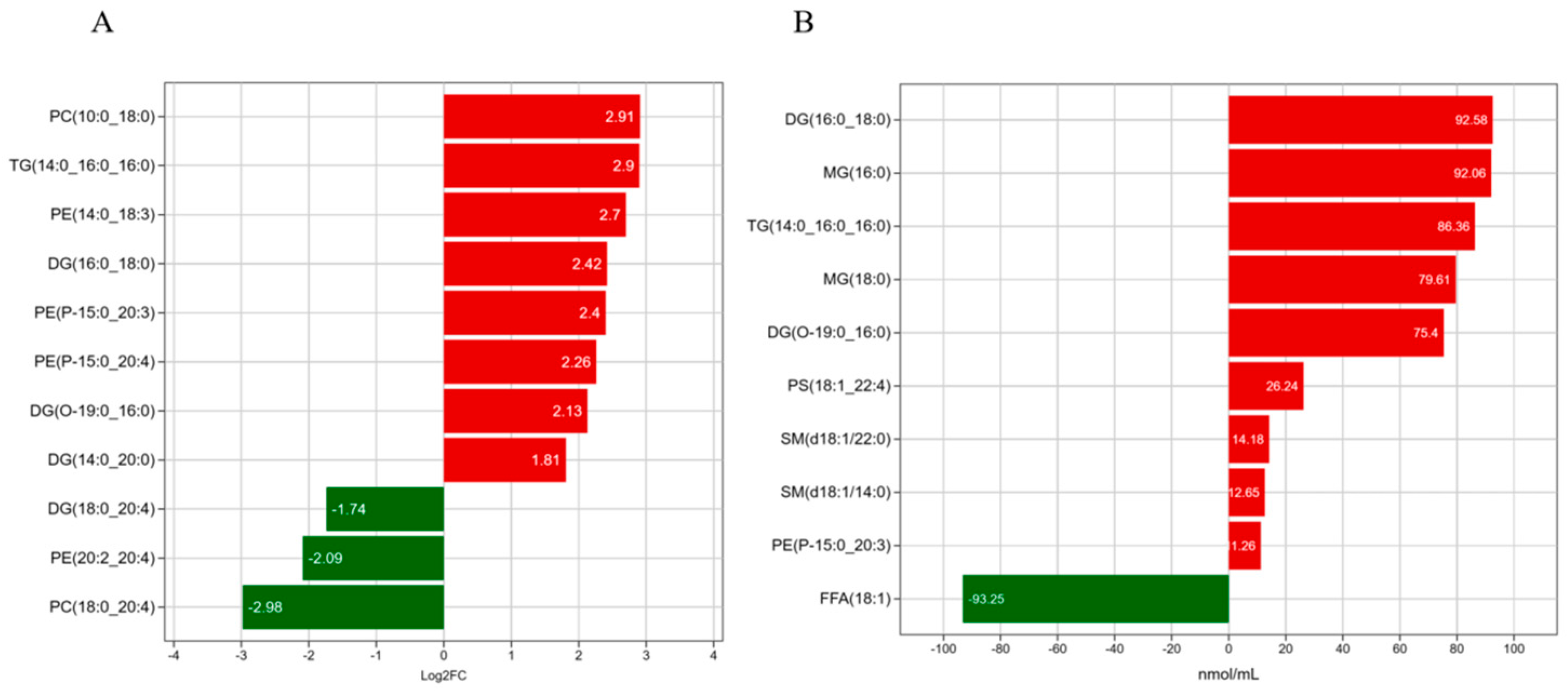
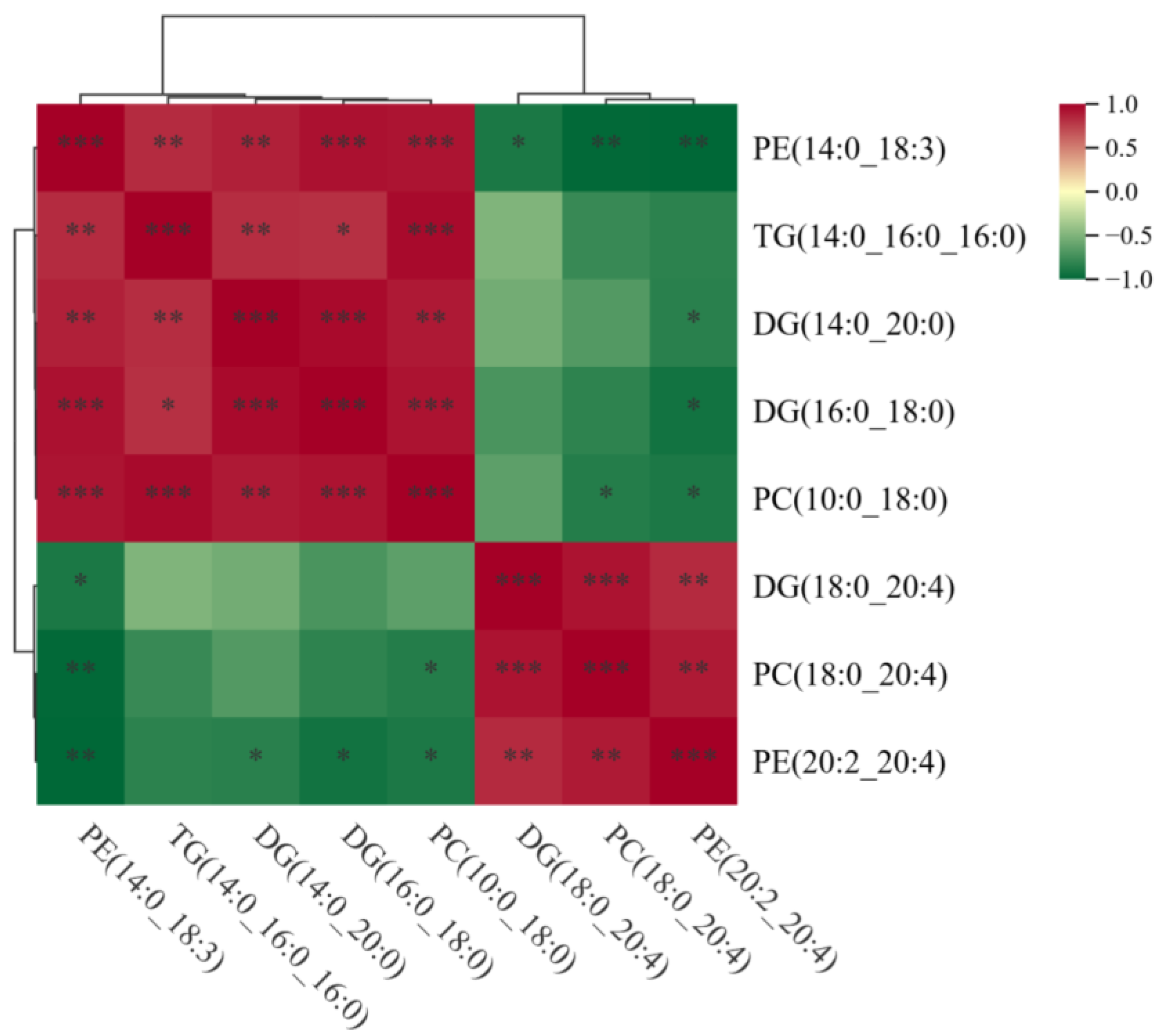
3.5. Analysis of Significant Differences Between LDs and MFGs in Breast Tissue
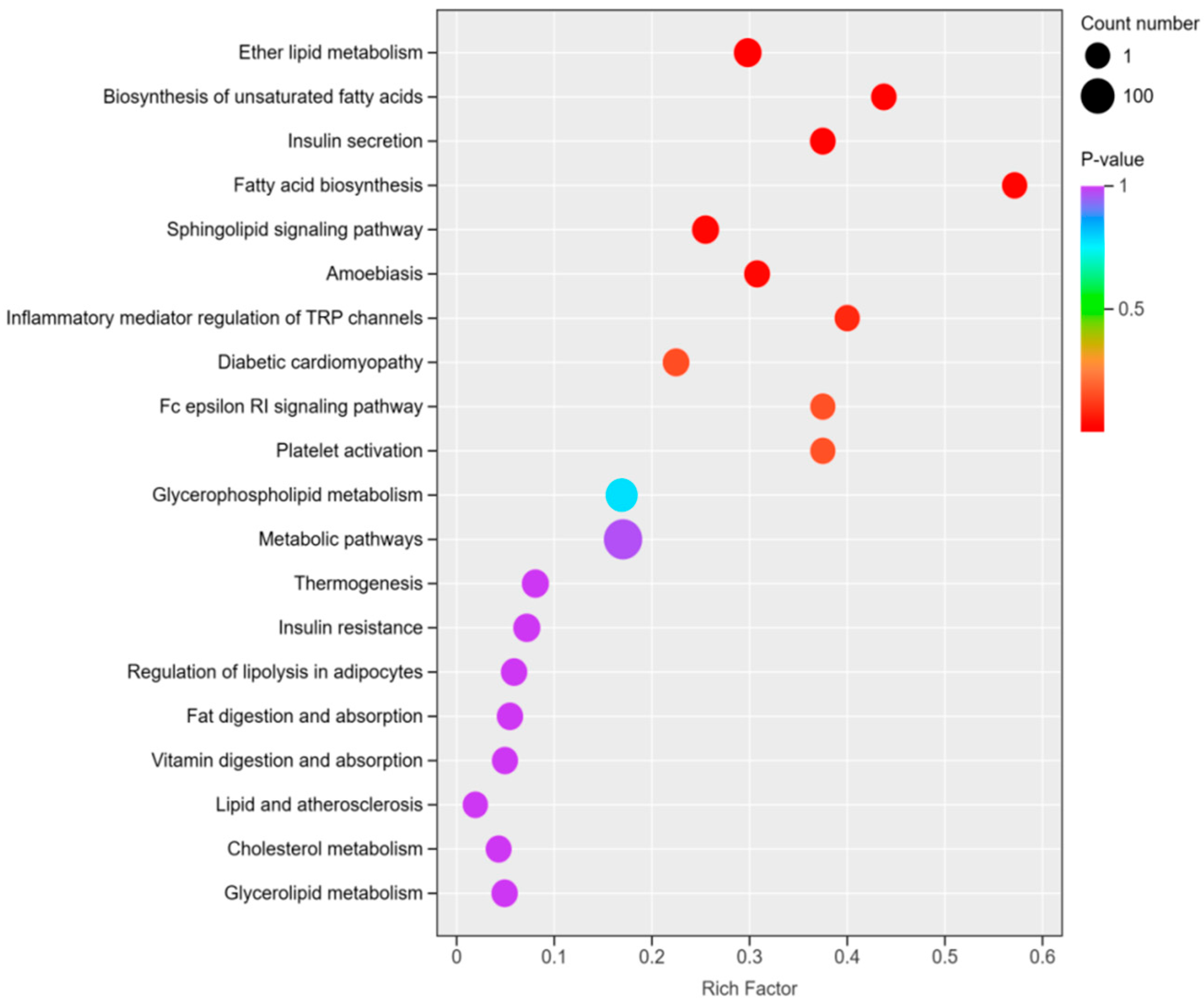
3.6. KEGG Pathway Analysis of Significantly Different Lipids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.L.; Liu, Z.T.; Wu, K.X.; Zhang, C.K.; Fu, T.; Sun, Y.; Gao, T.Y.; Han, L.Q. The ruminal microbiome alterations associated with diet-induced milk fat depression and milk fat globule size reduction in dairy goats. Animals 2024, 14, 2614. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.; Wang, S.; Li, P.; Yao, Z.; Li, G. Evaluation of carcass traits, meat quality and the expression of lipid metabolism-related genes in different slaughter ages and muscles of Taihang black goats. Anim. Biosci. 2024, 37, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Tzirkel-Hancock, N.; Sharabi, L.; Argov-Argaman, N. Milk fat globule size: Unraveling the intricate relationship between metabolism, homeostasis, and stress signaling. Biochimie 2023, 215, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Fu, T.; Huang, Q.; Xing, Z.; Yang, J.; Lu, W.; Hu, M.; Han, L.Q.; Loor, J.J.; Gao, T.Y. Size, number and phospholipid composition of milk fat globules are affected by dietary conjugated linoleic acid. J. Anim. Physiol. Anim. Nutr. 2023, 107, 995–1005. [Google Scholar] [CrossRef]
- Zadoorian, A.; Du, X.; Yang, H. Lipid droplet biogenesis and functions in health and disease. Nat. Rev. Endocrinol. 2023, 19, 443–459. [Google Scholar] [CrossRef]
- Mohan, M.S.; O’Callaghan, T.F.; Kelly, P.; Hogan, S.A. Milk fat: Opportunities, challenges and innovation. Crit. Rev. Food Sci. 2021, 61, 2411–2443. [Google Scholar] [CrossRef]
- Teng, Z.; Wang, L.; Du, H.; Yang, G.; Fu, T.; Lian, H.; Sun, Y.; Liu, S.; Zhang, L.; Gao, T.Y. Metabolomic and lipidomic approaches to evaluate the effects of eucommia ulmoides leaves on milk quality and biochemical properties. Front. Vet. Sci. 2021, 8, 644967. [Google Scholar] [CrossRef]
- Cohen, S. Lipid droplets as organelles. Int. Rev. Cell Mol. Biol. 2018, 337, 83–110. [Google Scholar]
- Bartz, R.; Li, W.H.; Venables, B.; Zehmer, J.K.; Roth, M.R.; Welti, R.; Anderson, R.G.; Liu, P.; Chapman, K.D. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res. 2007, 48, 837–847. [Google Scholar] [CrossRef]
- Liu, K.; Chen, M.; Huang, G.; Su, C.; Tang, W.; Li, N.; Yang, J.; Wu, X.; Si, B.; Zhao, S.; et al. Variations in the milk lipidomic profile of lactating dairy cows fed the diets containing alfalfa hay versus alfalfa silage. Anim. Nutr. 2024, 19, 261–271. [Google Scholar] [CrossRef]
- Jumabay, M.; Abdmaulen, R.; Ly, A.; Cubberly, M.R.; Shahmirian, L.J.; Heydarkhan-Hagvall, S.; Dumesic, D.A.; Yao, Y.; Boström, K.I. Pluripotent stem cells derived from mouse and human white mature adipocytes. Stem Cell Transl. Med. 2014, 3, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Luo, J.; Tian, H.; Li, J.; Zhang, X.; Chen, Z.; Li, M.; Loor, J.J. Rapid communication: Lipid metabolic gene expression and triacylglycerol accumulation in goat mammary epithelial cells are decreased by inhibition of SREBP-1. J. Anim. Sci. 2018, 96, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Bersuker, K.; Peterson, C.W.H.; To, M.; Sahl, S.J.; Savikhin, V.; Grossman, E.A.; Nomura, D.K.; Olzmann, J.A. A proximity labeling strategy provides insights into the composition and dynamics of lipid droplet proteomes. Dev. Cell 2018, 44, 97–112.e117. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.; Van Camp, J.; Rombaut, R.; van Leeckwyck, F.; Dewettinck, K. Effect of washing conditions on the recovery of milk fat globule membrane proteins during the isolation of milk fat globule membrane from milk. J. Dairy Sci. 2009, 92, 3592–3603. [Google Scholar] [CrossRef]
- Guerin, J.; Burgain, J.; Gomand, F.; Scher, J.; Gaiani, C. Milk fat globule membrane glycoproteins: Valuable ingredients for lactic acid bacteria encapsulation? Crit. Rev. Food Sci. 2019, 59, 639–651. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Yang, P.; Han, H.; Zhang, G.; Xu, H.; Quan, K. Overexpression of ATGL impairs lipid droplet accumulation by accelerating lipolysis in goat mammary epithelial cells. Anim. Biotechnol. 2023, 34, 3126–3134. [Google Scholar] [CrossRef]
- Nie, C.; Zhao, Y.; Wang, X.; Li, Y.; Fang, B.; Wang, R.; Wang, X.; Liao, H.; Li, G.; Wang, P. Structure, biological functions, separation, properties, and potential applications of milk fat globule membrane (MFGM): A review. Nutrients 2024, 16, 587. [Google Scholar] [CrossRef]
- Cavaletto, M.; Givonetti, A.; Cattaneo, C. The immunological role of milk fat globule membrane. Nutrients 2022, 14, 4574. [Google Scholar] [CrossRef]
- Brink, L.R.; Lönnerdal, B. Milk fat globule membrane: The role of its various components in infant health and development. J. Nutr. Biochem. 2020, 85, 108465. [Google Scholar] [CrossRef]
- Yao, D.; Ranadheera, C.S.; Shen, C.; Wei, W.; Cheong, L.Z. Milk fat globule membrane: Composition, production and its potential as encapsulant for bioactives and probiotics. Crit. Rev. Food Sci. 2024, 64, 12336–12351. [Google Scholar] [CrossRef]
- Jin, Q.; Qi, D.; Zhang, M.; Qu, H.; Dong, Y.; Sun, M.; Quan, C. CLDN6 inhibits breast cancer growth and metastasis through SREBP1-mediated RAS palmitoylation. Cell. Mol. Biol. Lett. 2024, 29, 112. [Google Scholar] [CrossRef]
- Brettschneider, J.; Correnti, J.M.; Lin, C.; Williams, B.; Oranu, A.; Kuriakose, A.; McIver-Jenkins, D.; Haba, A.; Kaneza, I.; Jeon, S.; et al. Rapid Lipid Droplet Isolation Protocol Using a Well-established Organelle Isolation Kit. J. Vis. Exp. 2019, 19, e59290. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Hao, J.; Jiang, Y.; Sun, X.; Cheng, J. Lipidomics of Sannen goat milk subjected to pasteurization and spray drying based on LC-ESI-MS/MS. Food Res Int. 2023, 169, 112841. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yang, J.; Hu, M.; Zhong, K.; Wang, Y.; Yang, Y.; Loor, J.J.; Yang, G.; Han, L. Effects of choline deficiency and supplementation on lipid droplet accumulation in bovine primary liver cells in vitro. J. Dairy Sci. 2023, 106, 9868–9878. [Google Scholar] [CrossRef] [PubMed]
- Singh, H. Symposium review: Fat globules in milk and their structural modifications during gastrointestinal digestion. J. Dairy Sci. 2019, 102, 2749–2759. [Google Scholar] [CrossRef]
- Tian, L.M.; He, Z.H.; Wang, G.; Zhang, S.H.; Di, T.G.; Chang, M.H.; Han, W.; Gao, J.Y.; Li, M.; Wang, Z.Y.; et al. Decoding the function of FGFBP1 in sheep adipocyte proliferation and differentiation. Animals 2025, 15, 1456. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Fan, H.; Tan, Y. Lipid droplet-mitochondria contacts in health and disease. Int. J. Mol. Sci. 2024, 25, 6878. [Google Scholar] [CrossRef]
- Garcia, E.J.; Liao, P.C.; Tan, G.; Vevea, J.D.; Sing, C.N.; Tsang, C.A.; McCaffery, J.M.; Boldogh, I.R.; Pon, L.A. Membrane dynamics and protein targets of lipid droplet microautophagy during ER stress-induced proteostasis in the budding yeast, Saccharomyces cerevisiae. Autophagy 2021, 17, 2363–2383. [Google Scholar] [CrossRef]
- Bailey, A.P.; Koster, G.; Guillermier, C.; Hirst, E.M.; MacRae, J.I.; Lechene, C.P.; Postle, A.D.; Gould, A.P. Antioxidant role for lipid droplets in a stem cell niche of drosophila. Cell 2015, 163, 340–353. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, W.; Zeng, L.Q.; Bai, H.; Li, J.; Zhou, J.; Zhou, G.Y.; Fang, C.W.; Wang, F.; Qin, X.J. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. 2020, 36, 101635. [Google Scholar] [CrossRef]
- Walther, T.C.; Kim, S.; Arlt, H.; Voth, G.A.; Farese, R.V., Jr. Structure and function of lipid droplet assembly complexes. Curr. Opin. Struct. Biol. 2023, 80, 102606. [Google Scholar] [CrossRef]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Deeney, J.T.; Valivullah, H.M.; Dapper, C.H.; Dylewski, D.P.; Keenan, T.W. Microlipid droplets in milk secreting mammary epithelial cells: Evidence that they originate from endoplasmic reticulum and are precursors of milk lipid globules. Eur. J. Cell Biol. 1985, 38, 16–26. [Google Scholar] [PubMed]
- Ding, Y.F.; Zhang, S.Y.; Yang, L.; Na, H.M.; Zhang, P.; Zhang, H.N.; Wang, Y.; Chen, Y.; Yu, J.H.; Huo, C.X.; et al. Isolating lipid droplets from multiple species. Nat. Protoc. 2023, 8, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Corbo, J.H.; Chung, J. Mechanisms of lipid droplet degradation. Curr. Opin. Cell Biol. 2024, 90, 102402. [Google Scholar] [CrossRef]
- Kanno, C.; Kim, H.D. A simple procedure for the preparation of bovine milk fat globule membrane and a comparison of its composition, enzymatic activities, and electrophoretic properties with those prepared by other methods. Agric. Biol. Chem. 2014, 54, 2845–2854. [Google Scholar]
- Vesper, H.; Schmelz, E.M.; Nikolova-Karakashian, M.N. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J. Nutr. 1999, 129, 1239–1250. [Google Scholar] [CrossRef]
- Lopez, C. Milk fat globules enveloped by their biological membrane: Unique colloidal assemblies with a specific composition and structure. Curr. Opin. Colloid Interface Sci. 2017, 16, 391–404. [Google Scholar] [CrossRef]

| Lipid Subclass | LD | MFG |
|---|---|---|
| Triacylglycerol (TG) | 259 | 236 |
| Phosphatidylethanolamine (PE) | 76 | 59 |
| Phosphatidylcholine (PC) | 75 | 55 |
| Diacylglycerol (DG) | 68 | 28 |
| Ceramide (Cer-NS) | 65 | 16 |
| Phosphatidylinositol (PI) | 57 | 43 |
| Phosphatidylglycerol (PG) | 50 | 27 |
| Free fatty acid (FFA) | 44 | 22 |
| Lysophosphatidylcholine (LPC) | 38 | 14 |
| Phosphatidylserine (PS) | 31 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.; Yang, J.; Yang, Y.; Wang, H.; Fan, Y.; Yang, Y.; Liu, Y.; Han, L. Lipidomics Reveals Differences in Lipid Composition Between Lipid Droplets and Milk Fat Globules in Dairy Goat Mammary Tissue. Animals 2025, 15, 3303. https://doi.org/10.3390/ani15223303
Wu K, Yang J, Yang Y, Wang H, Fan Y, Yang Y, Liu Y, Han L. Lipidomics Reveals Differences in Lipid Composition Between Lipid Droplets and Milk Fat Globules in Dairy Goat Mammary Tissue. Animals. 2025; 15(22):3303. https://doi.org/10.3390/ani15223303
Chicago/Turabian StyleWu, Kuixian, Jingna Yang, Yu Yang, Haohan Wang, Yuxin Fan, Yanbin Yang, Yang Liu, and Liqiang Han. 2025. "Lipidomics Reveals Differences in Lipid Composition Between Lipid Droplets and Milk Fat Globules in Dairy Goat Mammary Tissue" Animals 15, no. 22: 3303. https://doi.org/10.3390/ani15223303
APA StyleWu, K., Yang, J., Yang, Y., Wang, H., Fan, Y., Yang, Y., Liu, Y., & Han, L. (2025). Lipidomics Reveals Differences in Lipid Composition Between Lipid Droplets and Milk Fat Globules in Dairy Goat Mammary Tissue. Animals, 15(22), 3303. https://doi.org/10.3390/ani15223303





