Characterization of the Complete Mitogenomes of Four Dacinae Species (Diptera: Tephritidae) with Phylogenetic Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Isolation
2.2. Mitogenome Sequencing, Assembly and Annotation
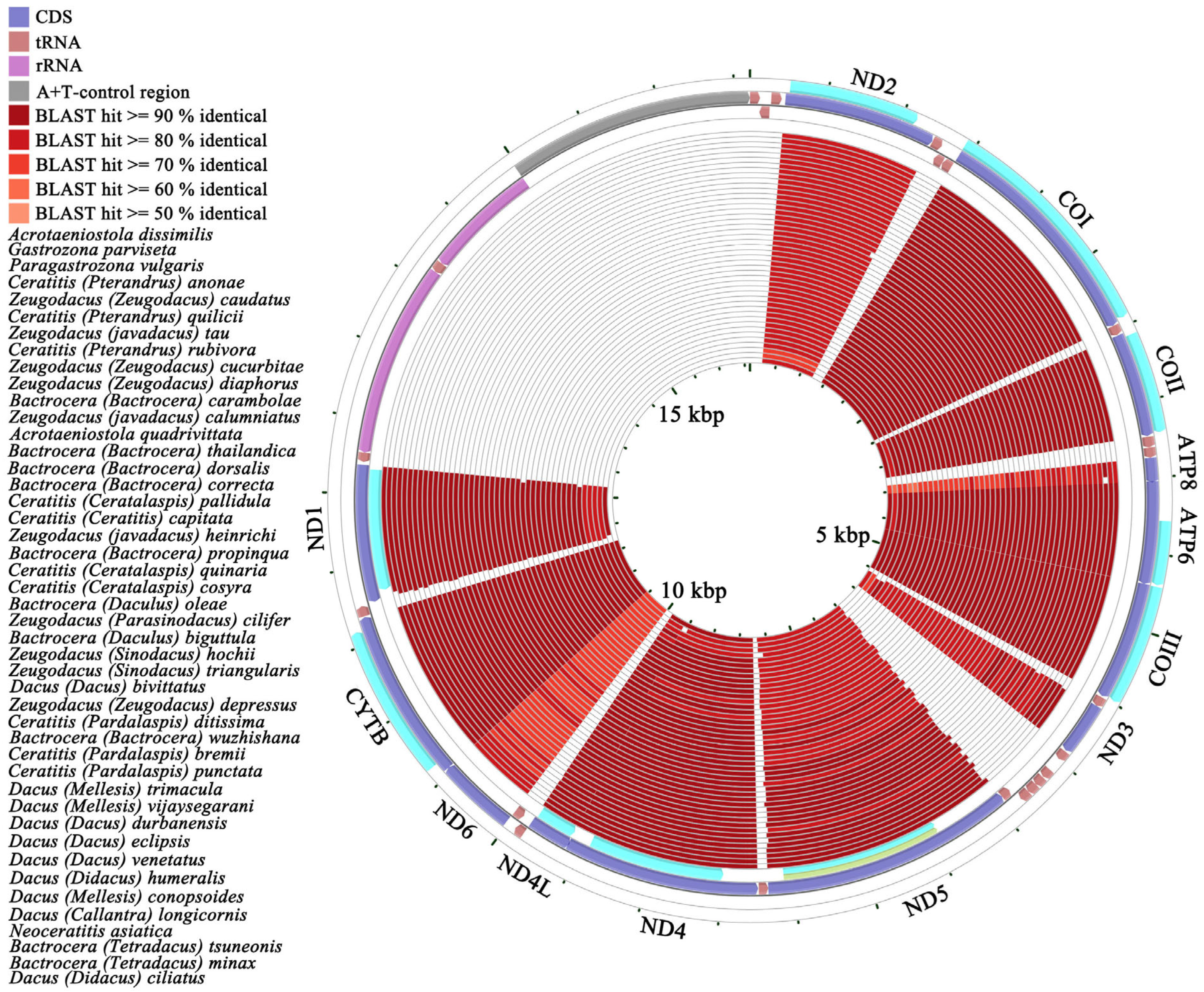
2.3. Comparative Mitochondrial Genomic Analysis
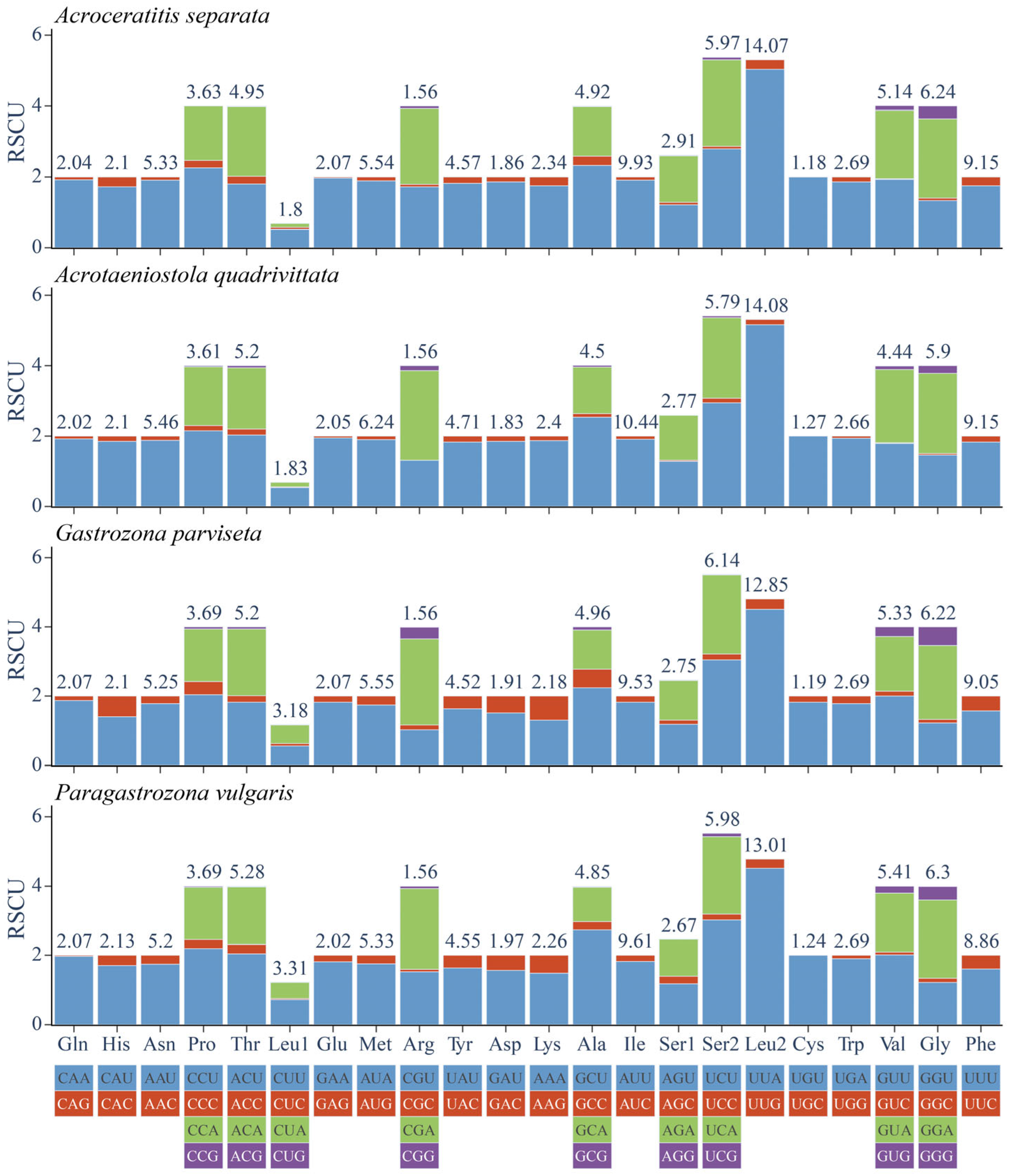
2.4. Indices of Codon Usage Bias
2.5. Sequence Alignment and Analysis of Sequence Heterogeneity
| Subfamily | Tribe | Species | Accession Number | Reference |
|---|---|---|---|---|
| Dacinae | Ceratitidini | Ceratitis (Ceratalaspis) cosyra | MT036784 | [47] |
| Dacinae | Ceratitidini | Ceratitis (Ceratalaspis) pallidula | MT036775 | [47] |
| Dacinae | Ceratitidini | Ceratitis (Ceratalaspis) quinaria | MT036788 | [47] |
| Dacinae | Ceratitidini | Ceratitis (Ceratitis) capitata | NC_000857 | [48] |
| Dacinae | Ceratitidini | Ceratitis (Pardalaspis) bremii | MT036781 | [47] |
| Dacinae | Ceratitidini | Ceratitis (Pardalaspis) ditissima | MT036785 | [47] |
| Dacinae | Ceratitidini | Ceratitis (Pardalaspis) punctata | MT036786 | [47] |
| Dacinae | Ceratitidini | Ceratitis (Pterandrus) anonae | MT036768 | [47] |
| Dacinae | Ceratitidini | Ceratitis (Pterandrus) quilicii | MT036780 | [47] |
| Dacinae | Ceratitidini | Ceratitis (Pterandrus) rubivora | MT036789 | [47] |
| Dacinae | Ceratitidini | Neoceratitis asiatica | MF434829 | [31] |
| Dacinae | Dacini | Bactrocera (Bactrocera) carambolae | MT121283 | [49] |
| Dacinae | Dacini | Bactrocera (Bactrocera) correcta | NC_018787 | [50] |
| Dacinae | Dacini | Bactrocera (Bactrocera) dorsalis | NC_008748 | [51] |
| Dacinae | Dacini | Bactrocera (Bactrocera) propinqua | OR085850 | [52] |
| Dacinae | Dacini | Bactrocera (Bactrocera) thailandica | MT121271 | [49] |
| Dacinae | Dacini | Bactrocera (Bactrocera) wuzhishana | NC_071744 | [49] |
| Dacinae | Dacini | Bactrocera (Daculus) biguttula | NC_042712 | [53] |
| Dacinae | Dacini | Bactrocera (Daculus) oleae | PQ801851 | [54] |
| Dacinae | Dacini | Bactrocera (Tetradacus) minax | NC_014402 | [55] |
| Dacinae | Dacini | Bactrocera (Tetradacus) tsuneonis | NC_038164 | [56] |
| Dacinae | Dacini | Dacus (Callantra) longicornis | NC_032690 | [57] |
| Dacinae | Dacini | Dacus (Dacus) bivittatus | NC_046468 | [58] |
| Dacinae | Dacini | Dacus (Dacus) durbanensis | NC_071727 | [49] |
| Dacinae | Dacini | Dacus (Dacus) eclipsis | NC_071728 | [49] |
| Dacinae | Dacini | Dacus (Dacus) venetatus | NC_071730 | [49] |
| Dacinae | Dacini | Dacus (Didacus) ciliatus | MG962405 | [58] |
| Dacinae | Dacini | Dacus (Didacus) humeralis | NC_071729 | [49] |
| Dacinae | Dacini | Dacus (Mellesis) conopsoides | NC_043843 | [33] |
| Dacinae | Dacini | Dacus (Mellesis) trimacula | MK940811 | [59] |
| Dacinae | Dacini | Dacus (Mellesis) vijaysegarani | MW429439 | [34] |
| Dacinae | Dacini | Zeugodacus (Parasinodacus) cilifer | NC_052852 | Direct submission |
| Dacinae | Dacini | Zeugodacus (Sinodacus) hochii | NC_071734 | [49] |
| Dacinae | Dacini | Zeugodacus (Sinodacus) triangularis | NC_071736 | [49] |
| Dacinae | Dacini | Zeugodacus (Zeugodacus) caudatus | NC_062801 | [60] |
| Dacinae | Dacini | Zeugodacus (Zeugodacus) cucurbitae | OQ158899 | Direct submission |
| Dacinae | Dacini | Zeugodacus (Zeugodacus) depressus | MT477832 | Direct submission |
| Dacinae | Dacini | Zeugodacus (Zeugodacus) diaphorus | NC_028347 | [30] |
| Dacinae | Dacini | Zeugodacus (javadacus) calumniatus | NC_079944 | [35] |
| Dacinae | Dacini | Zeugodacus (javadacus) heinrichi | NC_079945 | [35] |
| Dacinae | Dacini | Zeugodacus (javadacus) tau | MH900081 | [61] |
| Dacinae | Gastrozonini | Acroceratitis separata | PV920620 | This study |
| Dacinae | Gastrozonini | Acrotaeniostola dissimilis | MH900079 | [36] |
| Dacinae | Gastrozonini | Acrotaeniostola quadrivittata | PV920621 | This study |
| Dacinae | Gastrozonini | Gastrozona parviseta | PV920622 | This study |
| Dacinae | Gastrozonini | Paragastrozona vulgaris | PV920623 | This study |
| Trypetinae | Carpomyini | Carpomya vesuviana | NC_071721 | [49] |
| Tachiniscinae | Ortalotrypetini | Cyaforma shenonica | PV866838 | Unpublished |
| Phytalmiinae | Acanthonevrini | Felderimyia fuscipennis | NC_052851 | Direct submission |
| Trypetinae | Carpomyini | Myiopardalis pardalina | NC_087865 | Direct submission |
| Tephritinae | Cecidocharini | Procecidochares utilis | NC_020463 | Direct submission |
| Tephritinae | Tephritini | Tephritis femoralis | NC_047184 | [62] |
2.6. Phylogenetic Analysis
3. Results
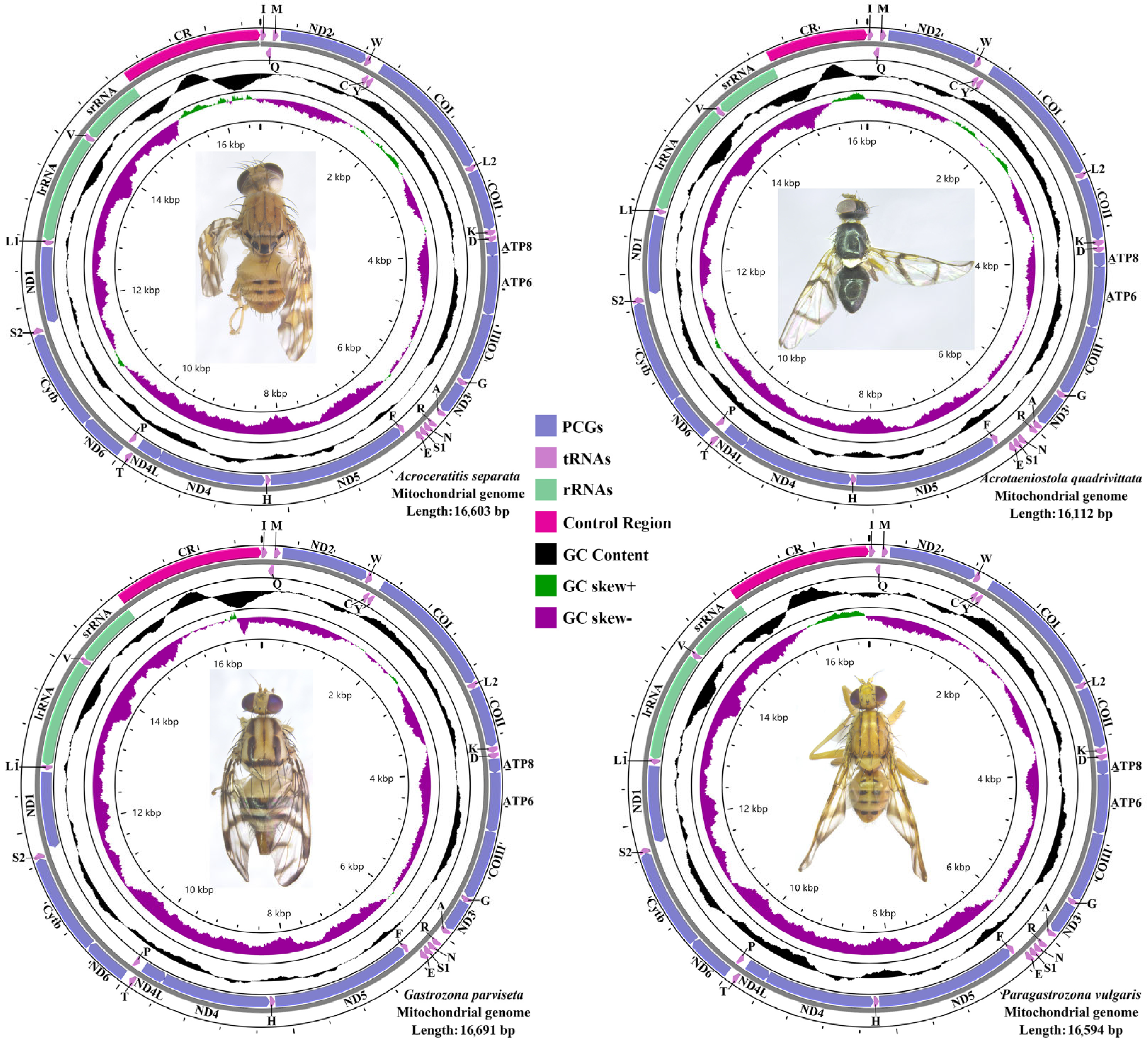
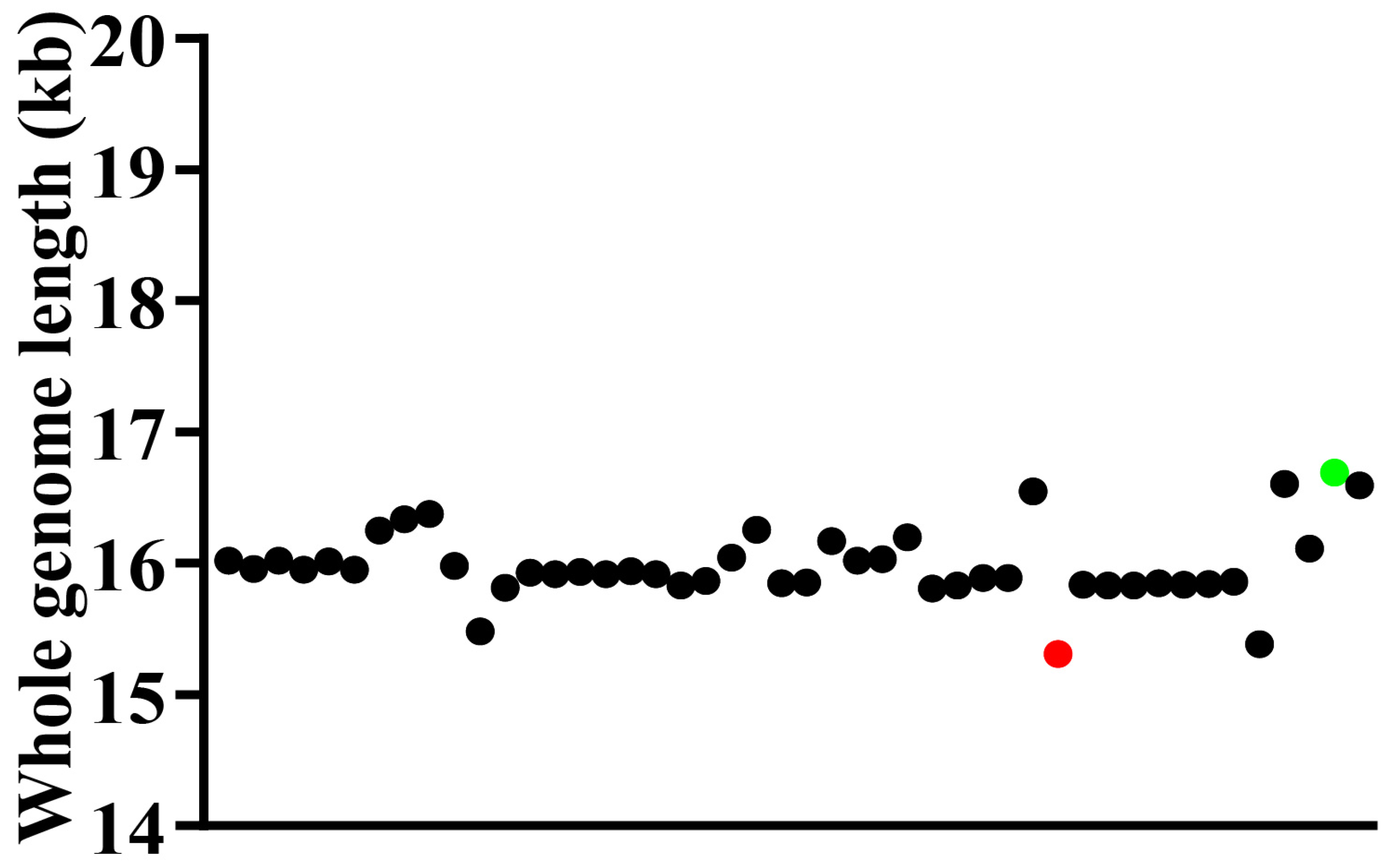
3.1. The General Features of Mitogenome Organization
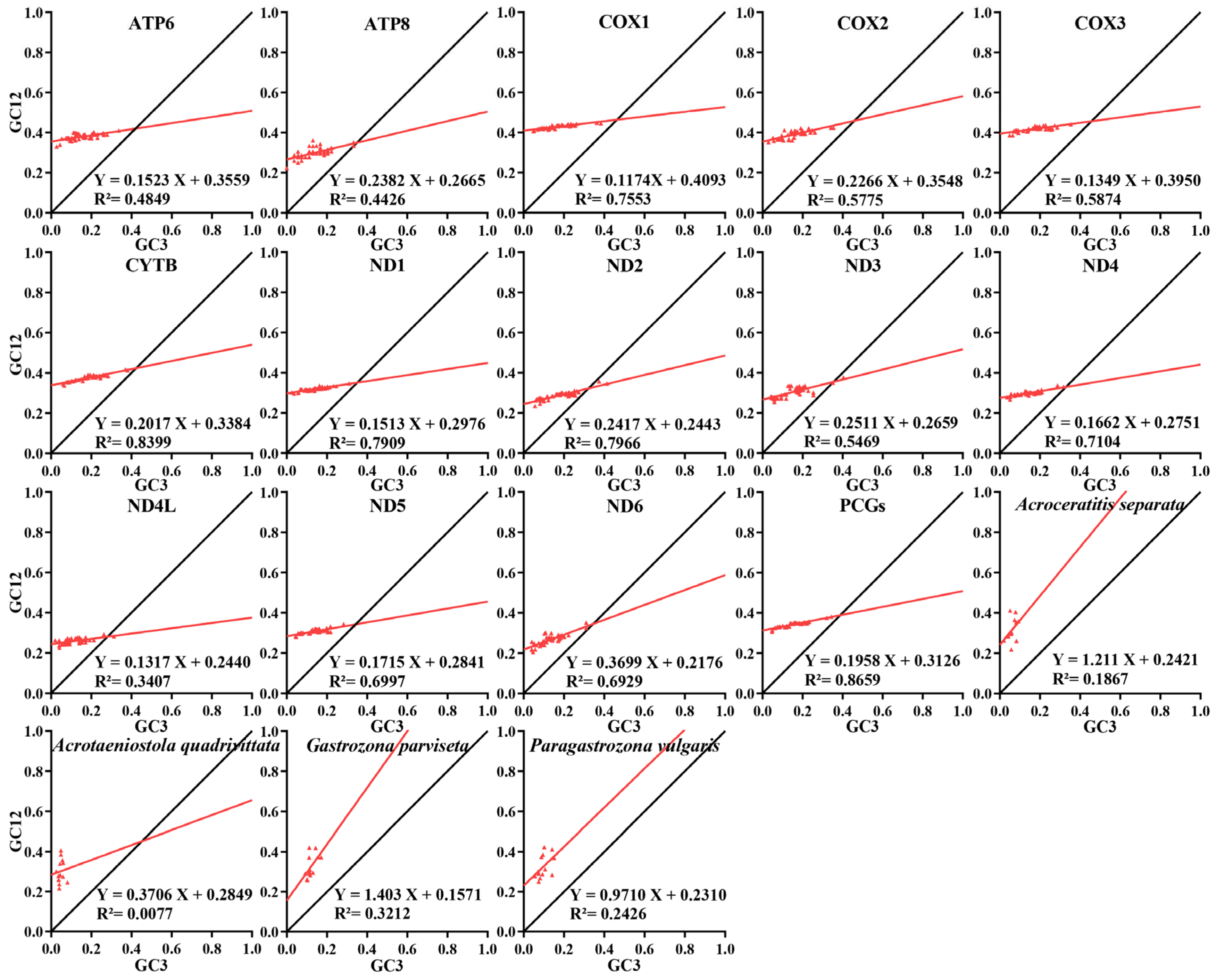
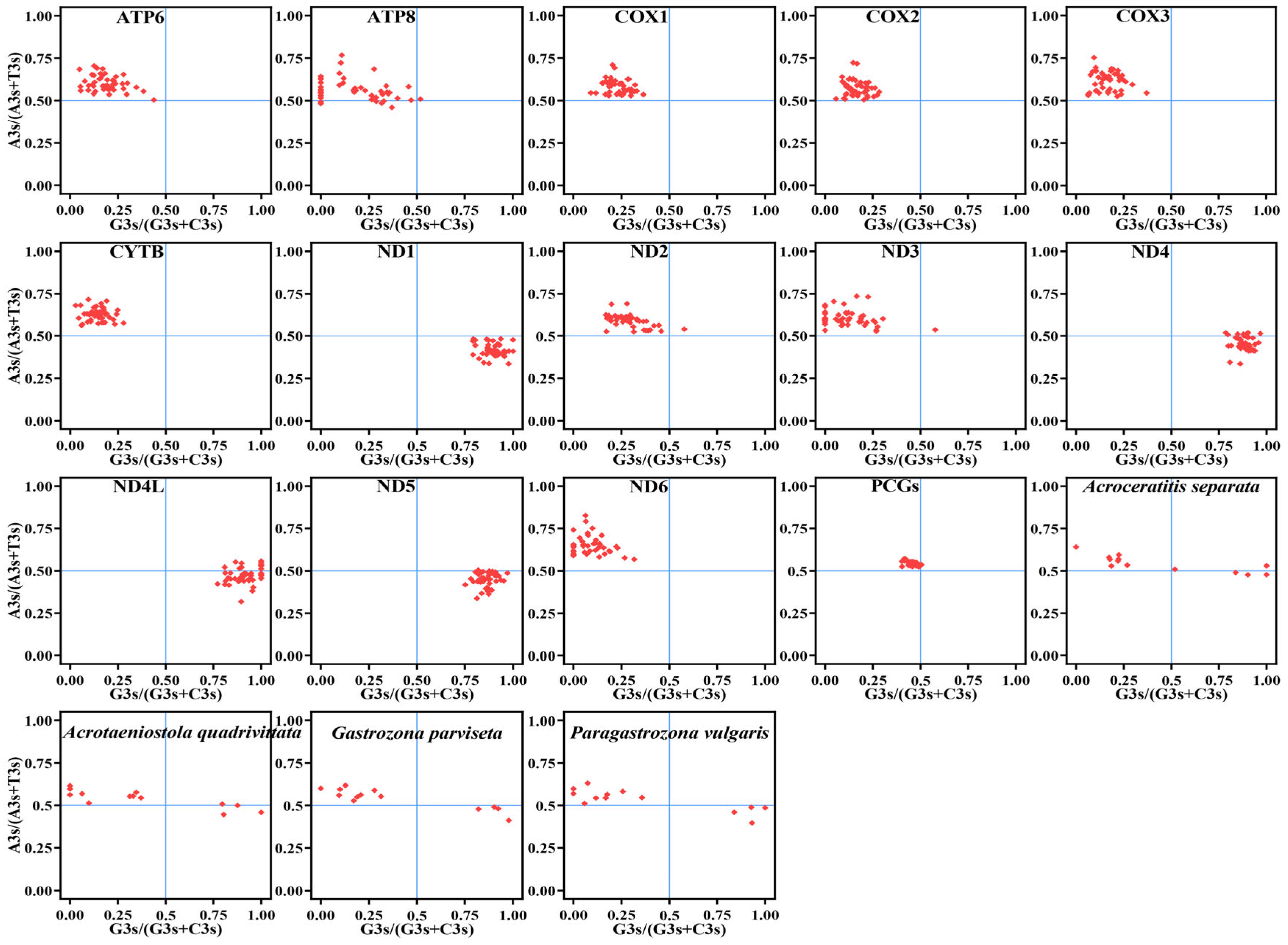
3.2. Comparative Analysis of Nucleotide Composition
3.3. PCGs and CCT Analysis
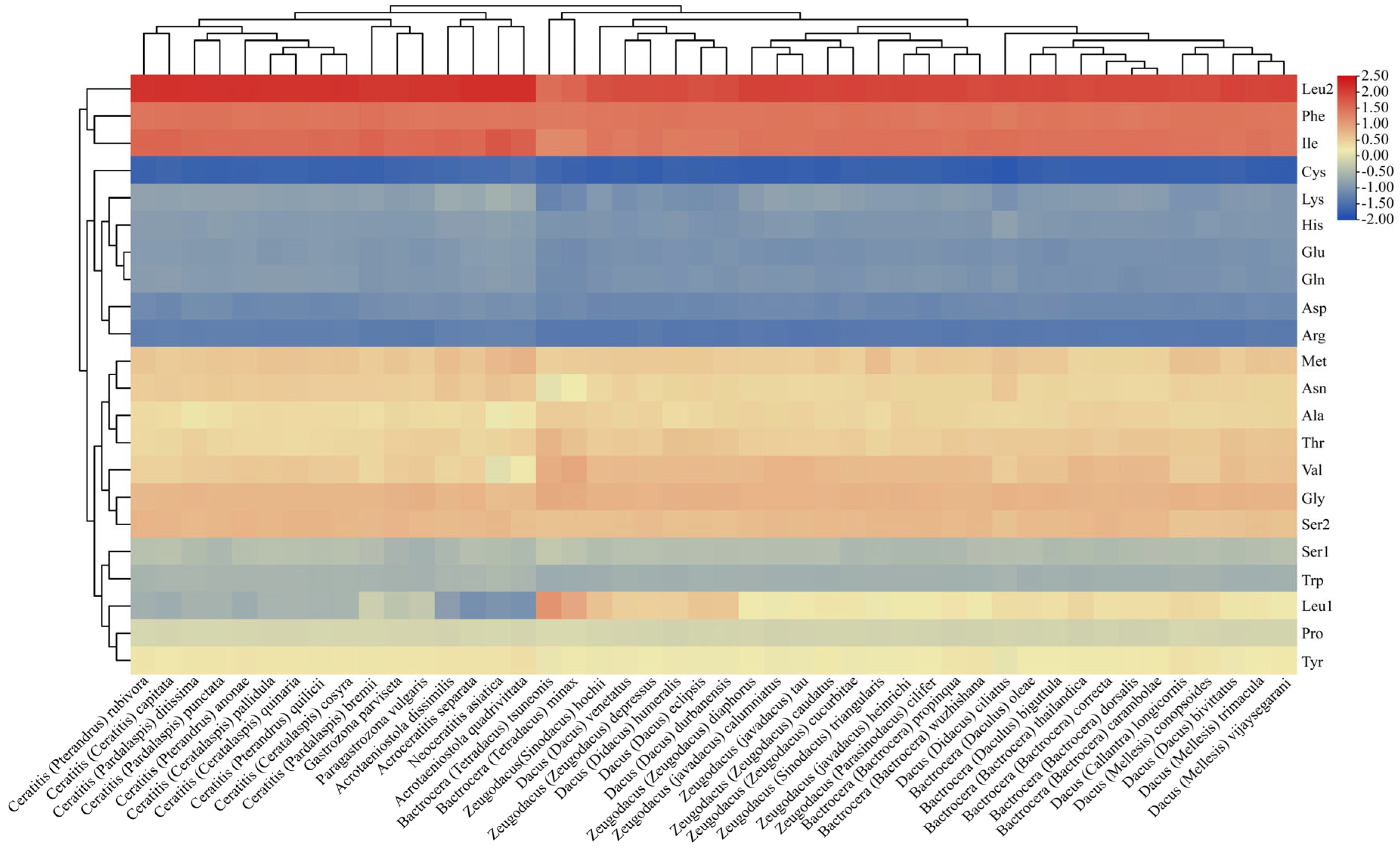
3.4. Comparative Analysis of CUB
3.5. Gene Overlap and Intergenic Regions
3.6. RNA Genes
3.7. A+T-Control Region
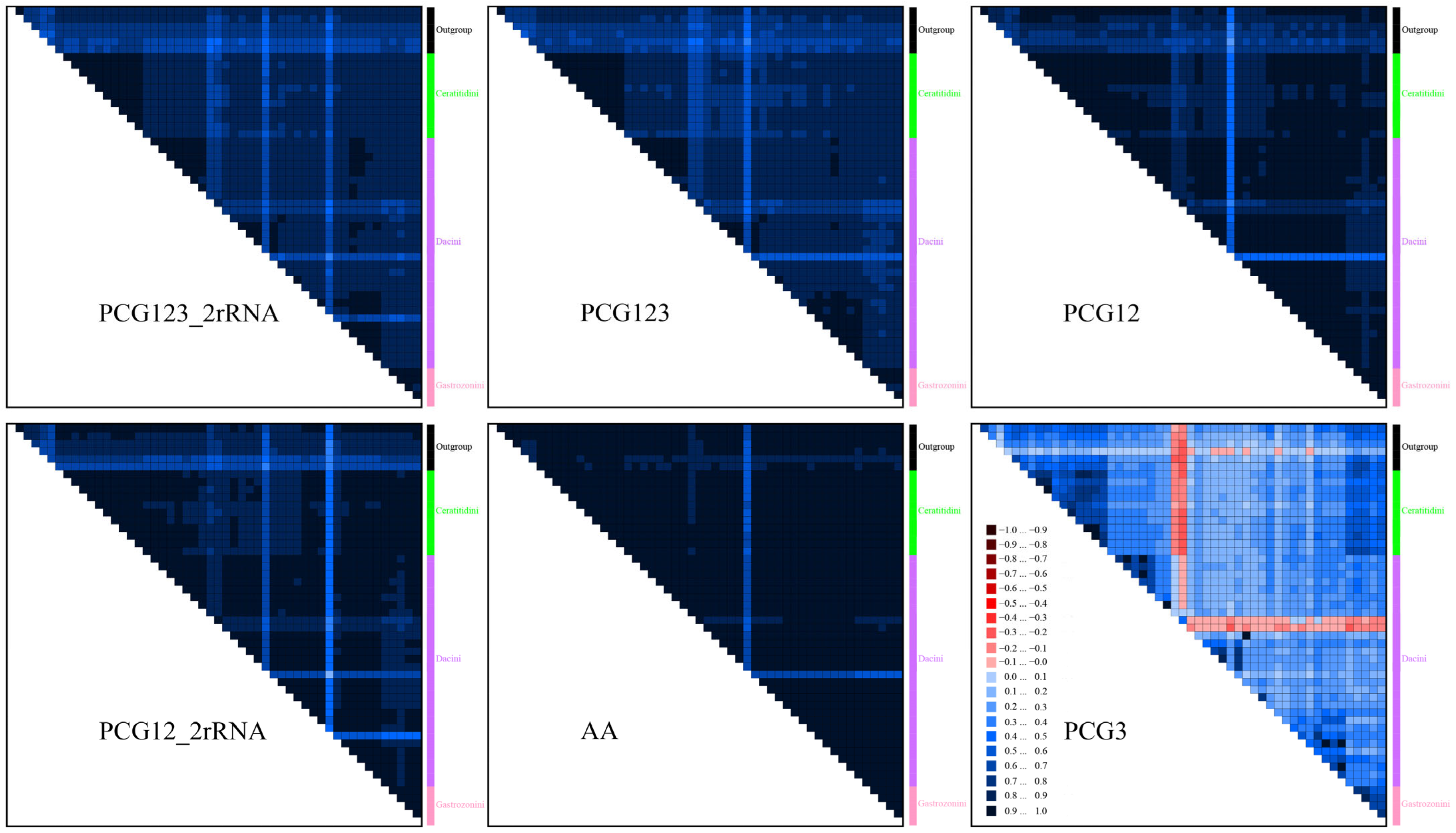
3.8. Heterogeneity of Base Composition
3.9. Phylogenetic Reconstruction Using Site-Homogenous Models
3.10. Phylogenetic Reconstruction Using Site-Heterogeneous Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korneyev, V.A. Phylogenetic relationships among higher groups of Tephritidae. In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 73–113. [Google Scholar]
- Kovac, D.; Dohm, P.; Freidberg, A.; Norrbom, A.L. Catalog and revised classification of the Gastrozonini (Diptera: Tephritidae: Dacinae). Isr. J. Entomol. 2006, 35, 163–196. [Google Scholar]
- David, K.J.; Sachin, K.; Hancock, D.L. A new species of Acrotaeniostola Hendel and new records of Gastrozonini and Ceratitidini (Diptera: Tephritidae) from India. Zootaxa 2020, 4731, 425–432. [Google Scholar] [CrossRef]
- Doorenweerd, C.; Leblanc, L.; Norrbom, A.L.; Jose, M.S.; Rubinoff, D. A global checklist of the 932 fruit fly species in the tribe Dacini (Diptera: Tephritidae). ZooKeys 2018, 730, 17–54. [Google Scholar] [CrossRef]
- Drew, R.A.I.; Romig, M.C. The Fruit Fly Fauna (Diptera: Tephritidae: Dacinae) of Papua New Guinea, Indonesian Papua, Associated Islands and Bougainville. CABI: Wallingford, UK, 2022; pp. 1–152. [Google Scholar] [CrossRef]
- Hancock, D.L.; Freidberg, A.; Friedman, A.-L.-L. Tephritidae. Chapter 71 (True fruit flies). In Manual of Afrotropical Diptera. Vol. 3. Brachycera: Cyclorrhapha, Excluding Calyptratae; Kirk-Spriggs, A.H., Sinclair, B.J., Eds.; Suricata; South African National Biodiversity Institute: Pretoria, South Africa, 2021; Volume 8, pp. 1669–1734. [Google Scholar]
- Sapkota, R.; Dahal, K.C.; KC, G.B.; Bajracharya, A.S.R.; Thapa, R.B. Fruit flies (Diptera: Tephritidae: Dacinae: Dacini) species diversity in Nepal: A review. SAARC J. Agric. 2024, 22, 1–15. [Google Scholar] [CrossRef]
- Hancock, D.L. Notes on some African Ceratitinae (Diptera: Tephritidae), with special reference to the Zimbabwean fauna. Trans. Zimb. Sci. Assoc. 1987, 63, 47–57. [Google Scholar]
- White, I.M.; Elson-Harris, M.M. Fruit Flies of Economic Significance: Their Identification and Bionomics; CAB International: Wallingford, UK, 1992; pp. 1–601. [Google Scholar]
- Permkam, S.; Hancock, D.L. Australian Trypetinae (Diptera: Tephritidae). Invertebr. Taxon. 1995, 9, 1047–1209. [Google Scholar] [CrossRef]
- Hardy, D.E. Fruit flies of the subtribe Gastrozonina of Indonesia, New Guinea and the Bismarck and Solomon islands (Diptera, Tephritidae, Trypetinae, Acanthonevrini). Zool. Scr. 1988, 17, 77–121. [Google Scholar] [CrossRef]
- Hancock, D.L.; Drew, R.A.I. Observations on the genus Acanthonevra Macquart in Thailand and Malaysia (Diptera: Tephritidae: Trypetinae). Entomologist 1995, 114, 99–103. [Google Scholar]
- Dohm, P.; Kovac, D.; Freidberg, A.; Rull, J.; Aluja, M. Basic biology and host use patterns of Tephritid flies (Phytalmiinae: Acanthonevrini, Dacinae: Gastrozonini) breeding in bamboo (Poaceae: Bambusoidea). Ann. Entomol. Soc. Am. 2014, 107, 184–203. [Google Scholar] [CrossRef]
- Hancock, D.L. New species and records of African Dacinae (Diptera: Tephritidae). Arnoldia Zimb. 1985, 9, 299–314. [Google Scholar]
- Zhang, N.N. Study on the morphological classification and phylogenesis of subfamily Dacinae (Diptera: Tephritidae) of China. Doctoral Dissertation, Fujian Agriculture and Forestry University, Fuzhou, China, 2012; pp. 1–208. [Google Scholar]
- David, K.J.; Hancock, D.L.; Gracy, R.G.; Sachin, K. A new genus of fruit fly in subfamily Dacinae (Diptera: Tephritidae) from India. Zootaxa 2022, 5195, 585–597. [Google Scholar] [CrossRef]
- Norrbom, A.L.; Carroll, L.E.; Freidberg, A. Status of knowledge. In Fruit Fly Expert Identification System and Systematic Information Database; Thompson, F.C., Ed.; Myia; Backhuys Publishers: Oegstgeest, The Netherlands, 1999; pp. 9–47. [Google Scholar]
- Hancock, D.L. Grass-breeding fruit flies and their allies of Africa and Asia (Diptera: Tephritidae: Ceratitidinae). J. Nat. Hist. 1999, 33, 911–948. [Google Scholar] [CrossRef]
- Hancock, D.L.; Drew, R.A.I. Bamboo-shoot fruit flies of Asia (Diptera: Tephritidae: Ceratitidinae). J. Nat. Hist. 1999, 33, 633–775. [Google Scholar] [CrossRef]
- Chua, T.H. New bamboo-shoot flies from Peninsular Malaysia (Diptera: Tephritidae: Ceratitidinae). J. Nat. Hist. 2003, 37, 463–472. [Google Scholar] [CrossRef]
- White, I.M. Morphological features of the tribe Dacini (Dacinae): Their significance to behavior and classification. In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 505–533. [Google Scholar]
- Han, H.Y.; McPheron, B.A. Phylogenetic study of selected tephritid flies (Insecta: Diptera: Tephritidae) using partial sequences of the nuclear 18S ribosomal DNA. Biochem. Syst. Ecol. 1994, 22, 447–457. [Google Scholar] [CrossRef]
- Han, H.Y.; McPheron, B.A. Molecular phylogenetic study of Tephritidae (Insecta: Diptera) using partial sequences of the mitochondrial 16S ribosomal DNA. Mol. Phylogenet. Evol. 1997, 7, 17–32. [Google Scholar] [CrossRef]
- Han, H.Y.; Ro, K.E. Molecular phylogeny of the family Tephritidae (Insecta: Diptera): New insight from combined analysis of the mitochondrial 12S, 16S, and COII genes. Mol. Cells 2009, 27, 55–66. [Google Scholar] [CrossRef]
- He, R.; Wang, S.P.; Li, Q.; Wang, Z.Q.; Mei, Y.; Li, F. Phylogenomic analysis and molecular identification of true fruit flies. Front. Genet. 2024, 15, 1414074. [Google Scholar] [CrossRef]
- Wolstenholme, D.R. Animal mitochondrial DNA: Structure and evolution. Int. Rev. Cytol. 1992, 141, 173–216. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.L.; Miller, K.B.; D’Haese, C.A.; Whiting, M.F.; Barker, S.C. Mitochondrial genome data alone are not enough to unambiguously resolve the relationships of Entognatha, Insecta and Crustacea sensu lato (Arthropoda). Cladistics 2004, 20, 534–557. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Buckley, T.R.; Frati, F.; Stewart, J.B.; Beckenbach, A.T. Incorporating molecular evolution into phylogenetic analysis, and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 545–579. [Google Scholar] [CrossRef]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef]
- Zhang, K.J.; Liu, L.; Xia, R.; Zhang, G.H.; Liu, H.; Liu, Y.H. The complete mitochondrial genome of Bactrocera diaphora (Diptera: Tephtitidae). Mitochondrial DNA 2015, 27, 4314–4315. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Y.; Feng, S.Q.; He, J.; Zhao, Z.H.; Bai, Z.Z.; Liu, L.J.; Zhang, R.; Li, Z.H. The mitochondrial genome of the wolfberry fruit fly, Neoceratitis asiatica (Becker) (Diptera: Tephritidae) and the phylogeny of Neoceratitis Hendel genus. Sci. Rep. 2017, 7, 16612. [Google Scholar] [CrossRef]
- Song, S.L.; Yong, H.S.; Suana, I.W.; Lim, P.E. Complete mitochondrial genome of Bactrocera ritsemai (Insecta: Tephritidae) and phylogenetic relationship with its congeners and related tephritid taxa. J. Asia-Pac. Entomol. 2018, 21, 252–257. [Google Scholar] [CrossRef]
- Song, S.L.; Yong, H.S.; Suana, I.W.; Lim, P.E.; Eamsobhana, P. Complete mitochondrial genome of Dacus conopsoides (Insecta: Tephritidae) with tRNA gene duplication and molecular phylogeny of Dacini tribe. J. Asia-Pac. Entomol. 2019, 22, 997–1003. [Google Scholar] [CrossRef]
- Yong, H.S.; Chua, K.O.; Song, S.L.; Liew, Y.J.M.; Eamsobhana, P.; Chan, K.G. Complete mitochondrial genome of Dacus vijaysegarani and phylogenetic relationships with congeners and other tephritid fruit flies (Insecta: Diptera). Mol. Biol. Rep. 2021, 48, 6047–6056. [Google Scholar] [CrossRef]
- Yong, H.S.; Song, S.L.; Chua, K.O.; Liew, Y.J.M.; Suana, I.W.; Lim, P.E.; Chan, K.G.; Eamsobhana, P. Comparative analysis of the complete mitochondrial genomes of three Zeugodacus species (Insecta: Tephritidae: Dacinae) and their phylogenetic relationships with other congeners. Arthropod Syst. Phylo. 2023, 81, 747–759. [Google Scholar] [CrossRef]
- Jia, P.F.; Liu, J.H.; Dan, W.L. Complete mitochondrial genome of the bamboo-shoot fruit fly, Acrotaeniostola dissimilis (Diptera: Tephritidae) and its phylogenetic relationship within family Tephritidae. Mitochondrial DNA Part B 2020, 5, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Donath, A.; Jühling, F.; Al-Arab, M.; Bernhart, S.H.; Reinhardt, F.; Stadler, P.F.; Middendorf, M.; Bernt, M. Improved annotation of protein-coding genes boundaries in metazoan mitochondrial genomes. Nucleic Acids Res. 2019, 47, 10543–10552. [Google Scholar] [CrossRef]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.Y.; Graham, M.; Domselaar, G.V.; Stothard, P. Proksee: In-depth characterization and visualization of bacterial genomes. Nucleic Acids Res. 2023, 51, 484–492. [Google Scholar] [CrossRef]
- Xiang, C.Y.; Gao, F.L.; Jakovlić, I.; Lei, H.P.; Hu, Y.; Zhang, H.; Zou, H.; Wang, G.T.; Zhang, D. Using PhyloSuite for molecular phylogeny and tree-based analyses. iMeta 2023, 2, e87. [Google Scholar] [CrossRef]
- Grant, J.R.; Arantes, A.S.; Stothard, P. Comparing thousands of circular genomes using the CGView comparison tool. BMC Genom. 2012, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Wu, Y.; Li, J.W.; Wang, X.; Zeng, Z.H.; Xu, J.; Liu, Y.L.; Feng, J.T.; Chen, H.; He, Y.H.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant. 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Wright, F. The ‘effective number of codons’ used in a gene. Gene 1990, 87, 23–29. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef]
- Kück, P.; Meid, S.A.; Groß, C.; Wagele, J.W.; Misof, B. AliGROOVE–visualization of heterogeneous sequence divergence within multiple sequence alignments and detection of inflated branch support. BMC Bioinform. 2014, 15, 294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meyer, M.D.; Virgilio, M.; Feng, S.Q.; Badji, K.; Li, Z.H. Phylogenomic resolution of the Ceratitis FARQ complex (Diptera: Tephritidae). Mol. Phylogenet. Evol. 2021, 161, 107160. [Google Scholar] [CrossRef] [PubMed]
- Spanos, L.; Koutroumbas, G.; Kotsyfakis, M.; Louis, C. The mitochondrial genome of the Mediterranean fruit fly, Ceratitis capitata. Insect Mol. Biol. 2000, 9, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Feng, S.Q.; Qin, Y.J.; Meyer, M.D.; Virgilio, M.; Singh, S.; Jiang, F.; Kawi, A.P.; Susanto, A.; et al. Mitochondrial phylogenomics reveals the evolutionary and biogeographical history of fruit flies (Diptera: Tephritidae). Entomol. Gen. 2022, 43, 359–368. [Google Scholar] [CrossRef]
- Liu, J.H.; Xu, J.; Li, Y.H.; Dan, W.L.; Pan, Y.Z. Complete mitochondrial genome of the guava fruit fly, Bactrocera correcta (Diptera: Tephritidae). Mitochondrial DNA 2015, 27, 4553–4554. [Google Scholar] [CrossRef]
- Yu, D.J.; Xu, L.; Nardi, F.; Li, J.G.; Zhang, R.J. The complete nucleotide sequence of the mitochondrial genome of the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Gene 2007, 396, 66–74. [Google Scholar] [CrossRef]
- Yong, H.S.; Song, S.L.; Chua, K.O.; Liew, Y.J.M.; Chan, K.G.; Lim, P.E.; Eamsobhana, P. Complete mitochondrial genomes of Bactrocera (Bulladacus) cinnabaria and B. (Bactrocera) propinqua (Diptera: Tephritidae) and their phylogenetic relationships with other congeners. Arthropod Syst. Phylogeny 2024, 82, 515–526. [Google Scholar] [CrossRef]
- Teixeira da Costa, L.; Powell, C.; van Noort, S.; Costa, C.; Sinno, M.; Caleca, V.; Rhode, C.; Kennedy, R.J.; van Staden, M.; van Asch, B. The complete mitochondrial genome of Bactrocera biguttula (Bezzi) (Diptera: Tephritidae) and phylogenetic relationships with other Dacini. Int. J. Biol. Macromol. 2019, 126, 130–140. [Google Scholar] [CrossRef]
- Teixeira da Costa, L.; Bon, M.C.; van Asch, B. Revisiting the history and biogeography of Bactrocera oleae and other olive-feeding fruit flies in Africa and Asia. Insects 2024, 16, 30. [Google Scholar] [CrossRef]
- Zhang, B.; Nardi, F.; Hull-Sanders, H.; Wan, X.; Liu, Y. The complete nucleotide sequence of the mitochondrial genome of Bactrocera minax (Diptera: Tephritidae). PLoS ONE 2014, 9, e100558. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, S.Q.; Zeng, Y.Y.; Ning, H.; Liu, L.J.; Zhao, Z.H.; Jiang, F.; Li, Z.H. The first complete mitochondrial genome of Bactrocera tsuneonis (Miyake) (Diptera: Tephritidae) by next-generation sequencing and its phylogenetic implications. Int. J. Biol. Macromol. 2018, 118, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Pan, X.B.; Li, X.K.; Yu, Y.X.; Zhang, J.H.; Jiang, H.S.; Dou, L.D.; Zhu, S.F. The first complete mitochondrial genome of Dacus longicornis (Diptera: Tephritidae) using next-generation sequencing and mitochondrial genome phylogeny of Dacini tribe. Sci. Rep. 2016, 6, 36426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, S.Q.; Fekrat, L.; Jiang, F.; Khathutshelo, M.; Li, Z.H. The first two complete mitochondrial genome of Dacus bivittatus and Dacus ciliatus (Diptera: Tephritidae) by next-generation sequencing and implications for the higher phylogeny of Tephritidae. Int. J. Biol. Macromol. 2019, 140, 469–476. [Google Scholar] [CrossRef]
- Ren, Y.L.; Wang, T.; Dai, R.H.; Wu, Z.Y.; Wang, J. Complete mitochondrial genome of Dacus trimacula (Diptera: Tephritidae) using next-generation sequencing from China. Mitochondrial DNA Part B 2019, 4, 2355–2356. [Google Scholar] [CrossRef]
- Wang, T.; Cai, B.; Wu, Z.Y.; Ren, Y.L.; He, L.Y. Phylogenetic relationship and characterization of the complete mitochondrial genome of Bactrocera caudata (Diptera: Tephritidae) from China. Mitochondrial DNA Part B 2020, 5, 1828–1829. [Google Scholar] [CrossRef]
- Liu, J.H.; Jia, P.F.; Dan, W.L.; Zhou, X.H.; Yang, M.J. Characterization of mitogenome for pumpkin fruit fly, Zeugodacus tau (Walker) (Diptera: Tephritidae) from Kunming, Southwest China and the phylogeny within subfamily Dacinae. Mitochondrial DNA Part B 2019, 4, 470–471. [Google Scholar] [CrossRef]
- Liu, D.F.; Dong, Y.R.; Xi, X.Q.; Sun, S.C. The complete mitochondrial genome of the Tephritid fly Tephritis femoralis (Diptera: Tephritidae). Mitochondrial DNA Part B 2020, 5, 1813–1814. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Lartillot, N.; Rodrigue, N.; Stubbs, D.; Richer, J. PhyloBayes MPI: Phylogenetic reconstruction with infinite mixtures of profiles in a parallel environment. Syst. Biol. 2013, 62, 611–615. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, 78–82. [Google Scholar] [CrossRef]
- Ojala, D.; Montoya, J.; Attardi, G. tRNA punctuation model of RNA processing in human mitochondria. Nature 1981, 290, 470–474. [Google Scholar] [CrossRef]
- Xing, K.; Kang, C.; Zhao, F. The first complete mitochondrial genome sequences for Ulidiidae and phylogenetic analysis of Diptera. Mol. Biol. Rep. 2023, 50, 2501–2510. [Google Scholar] [CrossRef]
- Garey, J.R.; Wolstenholme, D.R. Platyhelminth mitochondrial DNA: Evidence for early evolutionary origin of a tRNAser AGN that contains a dihydrouridine arm replacement loop, and of Serine-specifying AGA and AGG codons. J. Mol. Evol. 1989, 28, 374–387. [Google Scholar] [CrossRef]
- Clayton, D.A. Transcription and replication of animal mitochondrial DNAs. Int. Rev. Cytol. 1992, 141, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Lartillot, N.; Lepage, T.; Blanquart, S. PhyloBayes 3: A Bayesian software package for phylogenetic reconstruction and molecular dating. Bioinformatics 2009, 25, 2286–2288. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.B.; Chen, J.H.; Song, N.; Zhang, F.M. The complete mitochondrial genome of Sphaeniscus atilius (Walker, 1849) (Diptera: Tephritidae) and implication for the phylogeny of Tephritidae. Pak. J. Zool. 2024, 56, 2929–2942. [Google Scholar] [CrossRef]
- Jose, M.S.; Doorenweerd, C.; Leblanc, L.; Barr, N.; Geib, S.; Rubinoff, D. Incongruence between molecules and morphology: A seven-gene phylogeny of Dacini fruit flies paves the way for reclassification (Diptera: Tephritidae). Mol. Phylogenet. Evol. 2018, 121, 139–149. [Google Scholar] [CrossRef]
- Krosch, M.N.; Schutze, M.K.; Armstrong, K.F.; Graham, G.C.; Yeates, D.K.; Clarke, A.R. A molecular phylogeny for the Tribe Dacini (Diptera: Tephritidae): Systematic and biogeographic implications. Mol. Phylogenet. Evol. 2012, 64, 513–523. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Ding, S.; Zeng, X.; Du, L. Characterization of the Complete Mitogenomes of Four Dacinae Species (Diptera: Tephritidae) with Phylogenetic Analysis. Animals 2025, 15, 3301. https://doi.org/10.3390/ani15223301
Xu D, Ding S, Zeng X, Du L. Characterization of the Complete Mitogenomes of Four Dacinae Species (Diptera: Tephritidae) with Phylogenetic Analysis. Animals. 2025; 15(22):3301. https://doi.org/10.3390/ani15223301
Chicago/Turabian StyleXu, Deliang, Shuangmei Ding, Xiaojie Zeng, and Lele Du. 2025. "Characterization of the Complete Mitogenomes of Four Dacinae Species (Diptera: Tephritidae) with Phylogenetic Analysis" Animals 15, no. 22: 3301. https://doi.org/10.3390/ani15223301
APA StyleXu, D., Ding, S., Zeng, X., & Du, L. (2025). Characterization of the Complete Mitogenomes of Four Dacinae Species (Diptera: Tephritidae) with Phylogenetic Analysis. Animals, 15(22), 3301. https://doi.org/10.3390/ani15223301





