Simple Summary
Urban biodiversity is often underestimated, yet new discoveries continue to reveal previously unrecognized species within these environments. This study describes a new species of the genus Scincella, Scincella chengduensis sp. nov., from the urban and suburban landscapes of Chengdu, Sichuan Province, China. Integrating detailed morphological comparisons and genetic analyses, this species was determined to be distinct from all known skinks in the region. This discovery underscores the role of Chengdu as a biodiversity reservoir, even amidst rapid urbanization. Furthermore, this study highlights the resilience of species in fragmented and human-altered habitats, emphasizing the importance of urban environments for biodiversity research. The discovery of Scincella chengduensis sp. nov. expands the known diversity of skinks and stresses the urgent need for targeted conservation efforts in urban areas. These findings provide valuable insights for managing urban biodiversity and guiding conservation strategies in cities undergoing rapid development.
Abstract
The genus Scincella Mittleman, 1950, belonging to the family Scincidae, exhibits considerable morphological convergence, complicating species delimitation and resulting in underestimated diversity. Currently, 41 species are formally recognized in this genus, although this figure likely underestimates its true richness. In this study, a new species of the genus Scincella, Scincella chengduensis sp. nov., is described from urban and suburban areas of Chengdu, Sichuan Province, Southwest China. Morphological features and phylogenetic analyses confirmed that the new species is distinct from all previously recognized congeners. The new species can be clearly distinguished by a combination of the following unique characters: (1) slender, medium-sized body, snout-vent length 28.4–43.2 mm; (2) infralabials seven, rarely six; (3) supraciliaries six or seven; (4) tympanum deeply recessed without lobules, tympanum diameters equal to or exceeding palpebral disc diameters; (5) midbody scale-row counts 23; (6) dorsal scales smooth, slightly enlarged, paravertebral scale-row counts 57–60, ventral scale-row counts 42–44, gulars 21–22; (7) upper edge of lateral longitudinal stripes relatively straight, four rows of dorsal scales in middle; (8) enlarged, undivided lamellae beneath finger IV 8–9, enlarged, undivided lamellae beneath toe IV 10–12; (9) ventral surface densely covered with dark spots; (10) grayish-brown, irregular dorsal stripes 2–3, black dorsolateral stripes from posterior corner of eye to lateral side of tail. This discovery underscores the underestimated diversity of Scincella in China and highlights the importance of urban habitats as reservoirs of hidden biodiversity. A diagnostic key to the Scincella species of China is also provided.
1. Introduction
The genus Scincella Mittleman, 1950 represents a highly diverse group within the family Scincidae Gray, 1825, with a broad geographical distribution across North and Central America, as well as South, East, and Southeast Asia [1,2]. In recent decades, various studies have progressed our understanding of the taxonomy and phylogenetic relationships within this genus [1,3,4]. Despite these efforts, the genus remains taxonomically challenging due to its extensive morphological diversity and the presence of cryptic species complexes.
Recent advances in molecular phylogenetic analyses have shed light on the evolutionary dynamics of Scincella, revealing multiple independent radiations and a complex biogeographical history [4,5,6,7,8,9,10,11,12,13,14,15,16]. These analyses have unveiled significant genetic divergence within populations that exhibit morphological similarities, raising critical questions about the true extent of species diversity within the genus. Such findings underscore the importance of integrated approaches that combine morphological and molecular data to refine species delineations and resolve taxonomic ambiguities.
Sichuan Province in China is a recognized hotspot of herpetological diversity, hosting a considerable number of endemic species [17,18,19,20]. The varied topography and climatic conditions of the region create a mosaic of habitats that foster high levels of endemism and speciation. However, the herpetofaunal diversity of Sichuan remains inadequately studied, with extensive areas yet to be systematically surveyed. Preliminary investigations have identified several distinct lineages of Scincella, suggesting the presence of undescribed species. Currently, nine species of Scincella have been documented in Sichuan, including S. doriae (Boulenger, 1887), S. modesta (Günther, 1864), S. monticola (Schmidt, 1925), S. potanini (Günther, 1896), S. reevesii (Gray, 1838), S. schmidti (Barbour, 1927), S. tsinlingensis (Hu and Zhao, 1966), S. liangshanensis Jia, Ren & Wu, 2024, and S. wangyuezhaoi Jia, Gao, Huang, Ren, Jiang & Li, 2023. The latter two, recently described and endemic to Sichuan, underscore the remarkable biodiversity of the region and the untapped potential for further herpetological exploration [2,14,15,17,18,19,20] (Figure 1).
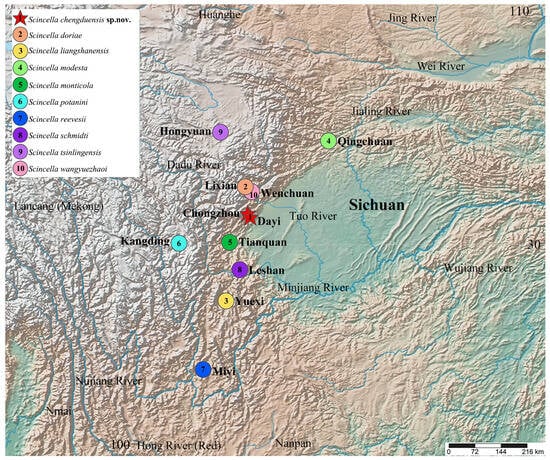
Figure 1.
Geographic distribution of Scincella species in Sichuan Province, China. 1. Scincella chengduensis sp. nov. from Chongzhou and Dayi Counties, Chengdu; 2. S. doriae from Lixian County; 3. S. liangshanensis from Yuexi County; 4. S. modesta from Qingchuan County; 5. S. monticola from Tianquan County; 6. S. potanini from Kangding County; 7. S. reevesii from Miyi County; 8. S. schmidti from Leshan County; 9. S. tsinlingensis from Hongyuan County; 10. S. wangyuezhaoi from Wenchuan County. Base maps were obtained from SimpleMapper (https://www.simplemappr.net (accessed on 20 September 2024)).
Chengdu, the capital of Sichuan, is centrally located within the Chengdu Plain and has emerged as a noteworthy area for biodiversity in western China, with recent surveys uncovering remarkable species richness even in its urbanized areas [21,22,23,24,25]. Qing et al. (2013) [26] reconstructed the phylogenetic tree of the genus Scincella, but their inclusion of an individual identified as ‘S. tsinlingensis’ from the “Sichuan University campus (situated in the urban area of Chengdu)” raises significant questions. Notably, the natural distribution of S. tsinlingensis is confined to the Palearctic region, geographically distant from the Chengdu Plain, casting doubt on the validity of this record.
In an ongoing biodiversity survey across Chengdu, as well as in Chongzhou and Dayi, located northwest of the city, previously undocumented populations of Scincella were discovered. Morphological analyses revealed that these populations shared similarities with S. potanini (Günther, 1896) [15], S. monticola (Schmidt, 1925) [27], and S. liangshanensis Jia, Ren & Wu, 2024 [28] but displayed distinct characteristics that distinguished them from all described members of the genus Scincella. Molecular analyses using both mitochondrial and nuclear DNA sequences confirmed the phylogenetic distinctiveness of this population, supporting its status as a separate species. We herein formally describe this population as a new species within the genus Scincella.
2. Materials and Methods
Sampling. In this study, 90 specimens were examined, including 87 specimens listed in Appendix A and 3 specimens of the newly described species. Due to the limited availability of material, the type series for the new species comprises only 3 specimens. This scarcity is primarily attributed to the species’ restricted distribution and its preference for challenging, inaccessible habitats, making specimen collection difficult. Specimens collected during field surveys were initially preserved in 10% buffered formalin, followed by transfer to 75% ethanol for long-term storage. Tissue samples (liver and muscle) intended for molecular analysis were stored in 95% alcohol at −20 °C to maintain DNA integrity. All specimens were deposited in the Herpetological Museum, Chengdu Institute of Biology (CIB), Chinese Academy of Sciences (CAS), Chengdu, Sichuan Province, China.
DNA extraction, polymerase chain reaction (PCR), and sequencing. Genomic DNA was extracted from liver and muscle tissues with a DNA extraction kit (Sangon Biotech, Shanghai, China). Fragments of four mitochondrial genes (16S rRNA (16S), 12S rRNA (12S), cytochrome b (Cyt b), and cytochrome oxidase I (COI)), alongside one nuclear gene (recombination activating gene 1 (RAG1)), were chose based on protocols outlined in Jia et al. (2023, 2024) [14,15] and preliminary experiments. The genes were amplified using the primers followed by Jia et al. (2024) [15]. Gene amplification was performed using primers and PCR conditions described in Jia et al. (2024) [15]. Newly obtained sequences were submitted to GenBank, with accession numbers provided in Table 1. Homologous DNA sequences of voucher specimens from related species were retrieved from GenBank and incorporated into the phylogenetic analyses.

Table 1.
Localities, voucher information, and GenBank accession numbers for all samples used in this study.
Phylogenetic analyses. Sphenomorphus cryptotis Darevsky, Orlov, and Cuc, 2004 [5] was chosen as the outgroup to root the tree following Pyron et al. (2013) [29] (Table 1). The four mitochondrial genes and one nuclear gene were sequenced using muscle and liver samples collected from seventeen individuals of the species under study. Additionally, 68 sequences representing 48 individuals from 23 Scincella species (including the putative new species) were retrieved from GenBank for comparative analyses, as detailed in Table 1.
Raw nucleotide sequences were manually validated using SeqMan v.7.1.0.44 [30] and subsequently combined with data retrieved from GenBank. Sequence alignment was performed in MEGA X [31] using ClustalW [32] with default parameters to ensure consistency across datasets. The aligned sequences were concatenated with PhyloSuite v.1.2.2 [33]. The optimal partitioning scheme and evolutionary substitution models were determined using PartitionFinder v.2.1.1 [34] with a greedy search algorithm based on the Akaike Information Criterion (AIC).
Phylogenetic trees were constructed using both maximum-likelihood (ML) and Bayesian inference (BI) methods, following the procedures described in Jia et al. (2024) [15]. ML analyses were conducted with RAxML v.8.2.10 [35], while BI analyses were performed in MrBayes v.3.2.6 [36]. Robust node support was considered when Bayesian posterior probability (BPP) was ≥0.95 and ML ultrafast bootstrap support (BS) was ≥70 [37,38]. The phylogenetic trees were visualized with FigTree v.1.4.3 [39]. Uncorrected p-distances for 16S were computed using MEGA X [31].
Morphological analysis. Morphological analyses followed the methodology outlined in Jia et al. (2024) [15].
Morphological abbreviations and measurement standards followed Jia et al. (2024) [15], including: snout-vent length (SVL): distance from tip of snout to posterior edge of vent; tail length (TaL): distance from posterior margin of vent to tip of tail; tail width (TaW): widest section of tail base; tail depth (TaD): ventral to dorsal surface of tail; axilla-groin distance (AGD): distance between posterior edge of forelimb insertion and anterior edge of hindlimb insertion; midbody width (MBW): measured from lateral surface to opposing lateral edge at midpoint of axillagroin region; midbody depth (MBD): measured from ventral surface to dorsal surface at midpoint of axilla-groin region; head length (HL): distance from the tip of the snout to the articulation of jaw; maximum head width (HW): greatest width between the left and right articulations of jaw; head depth (HD): measured from ventral to dorsal surface of head at jaw articulations; eye diameter (ED): maximum horizontal diameter of eye; palpebral disc diameter (PDD): maximum horizontal diameter of palpebral disc; tympanum diameter (TD): ear opening diameter, maximum diameter of ear; eye-narial distance (END): from anterior margin of eye to posterior margin of nare; snout length (SNL): distance from the tip of the snout to the anterior corner of eye; internasal distance (IND): minimum distance between the inner margins of the external nares; interorbital distance (IOD): minimum distance between the inner edges of the upper eyelids; forelimb length (FLL): measured from forelimb insertion to tip of finger IV or longest digit; hind-limb length (HLL): measured from hind-limb insertion to tip of toe IV or longest digit; finger IV length (F4L): measured from the most basal part to tip of finger IV; toe IV length (T4L): measured from the most basal part to tip of toe IV.
The meristic data included the following: midbody scale-row count (MBSR): number of longitudinal scale rows measured around the widest point of midbody; dorsal scale rows between dorsolateral stripes (DBR): number of midbody dorsal scale rows between dark dorsolateral stripes; scale rows covered by dorsolateral stripes (SRB); enlarged, differentiated nuchal count (NU, X pairs or absent); paravertebral scale-row count (PVSR): number of scale rows counted between parietals and the just posterior margin of hindlimbs; ventral scale-row count (VSR): number of scale rows counted between gulars and precloacals; gulars; loreal count (L, left/right): number of scale rows counted between the first scale behind the chin-shields and the middle of the forelimb; axilla-groin scale-row count (AGSR): number of scale rows counted between posterior edge of forelimb insertion and anterior edge of hind-limb insertion; supralabials (SL, left/right); infralabials (IfL, left/right); superciliaries (SC, left/right); supraoculars (SO, left/right); enlarged temporals (TEM, left/right); scale-row on dorsal surface of finger and toe (FTSR, single or paired); number of enlarged, undivided lamellae beneath finger IV (F4S, left/right); number of enlarged, undivided lamellae beneath toe IV (T4S, left/right); maxillary tooth (MT, left only); lower tooth (LT, left only); prefrontals in contact with each other (PF, Yes: in contact/No: not in contact/absent); frontoparietals in contact with each other (FP, Yes: in contact/No: not in contact/absent); parietals in contact with each other (P, Yes: in contact/No: not in contact/absent); chin-shields: paired large scales behind mental or postmentals; and limb posture when adpressed, categorized as toes overlapping, in contact, or not in contact with fingers. Dorsal color patterns were also recorded, including upper margin of lateral longitudinal striation wavy or relatively straight (UMLLS). Ventral color patterns were assessed, including presence or absence of dark-colored large blotches on ventral (DLBV). The raw morphological data for all characters and specimens are presented in Table 2.

Table 2.
Morphometric and meristic traits of Scincella chengduensis sp. nov. (provided in mm). Morphological character abbreviations are detailed in the Materials and Methods section.
Morphological data were derived from published literature [1,2,7,9,12,14,40,41,42,43,44,45,46].
To eliminate the allometric effects of body size, morphometric traits were size corrected by calculating the ratio to SVL. Statistical analyses were performed using Origin 2021 (OriginLab, Northampton, MA, USA). Principal component analysis (PCA) was conducted following the procedures outlined in Jia et al. [15]. Statistical comparisons were conducted between the newly described species and S. potanini, S. monticola, and S. liangshanensis using a Z-score normalized dataset.
3. Results
3.1. Phylogenetic Analyses
The phylogenetic trees were reconstructed using four mitochondrial genes (12S, 360 bp; 16S, 477 bp; Cyt b, 537 bp; COI, 623 bp) and one nuclear gene (RAG1, 1047 bp) from twenty-four species, resulting in a total alignment length of 3044 bp. Both the ML and BI analyses produced highly consistent topologies, providing strong support for the robustness of the phylogenetic inferences (Figure 2).
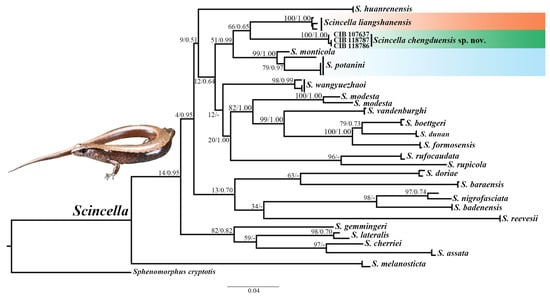
Figure 2.
Phylogenetic tree of relationships within the genus Scincella reconstructed using four mitochondrial fragments and one nuclear gene. BS from ML analyses and BPP from BI analyses are displayed above branches (BS/BPP). Tip labels correspond to ID numbers listed in Table 1.
Specimens from the Chengdu population formed a distinct monophyletic clade within the genus Scincella, displaying substantial genetic divergence from all other congeners. This clade showed the closest relationship to the cluster comprising S. potanini, S. monticola, and S. liangshanensis (BPP = 51; BS = 0.99), although the topology was not fully resolved in the ML analysis. The combination of genetic distances and morphological traits strongly supported the distinctiveness of the Chengdu population.
Uncorrected inter- and intraspecific p-distances are presented in Table 3. Results revealed complete genetic identity (0.0%) among specimens from Chengdu (Chongzhou and Dayi), while displaying considerable genetic divergence from other congeners, ranging from 3.0% to 10.4%. The closest genetic similarity was observed with S. liangshanensis, whereas the greatest divergence was noted with S. reevesii (Gray, 1838) [47].

Table 3.
Uncorrected p-distances (%) for 16S rRNA sequences of Scincella species analyzed in this study.
3.2. Morphological Analyses
Morphologically, the specimens from Chengdu were most similar to S. potanini, S. monticola, and S. liangshanensis. However, detailed morphological comparisons (Table 4) showed significant differences between the Chengdu specimens and these species, as well as all other known congeners, particularly in key characters such as VSR, PVSR, AGSR, and DLBV.

Table 4.
Diagnostic morphometric comparison between Scincella chengduensis sp. nov. and three morphologically similar congeners from Southwest China. Morphological character abbreviations are detailed in the Materials and Methods section.
The PCA results showed that the first two principal components (PCs) explained 28.1% and 15.3% of the variance, respectively, totaling 43.4%. Scatter plots based on PC1 and PC2 clearly separated the Chengdu specimens from other species with similar morphological traits (Figure 3).
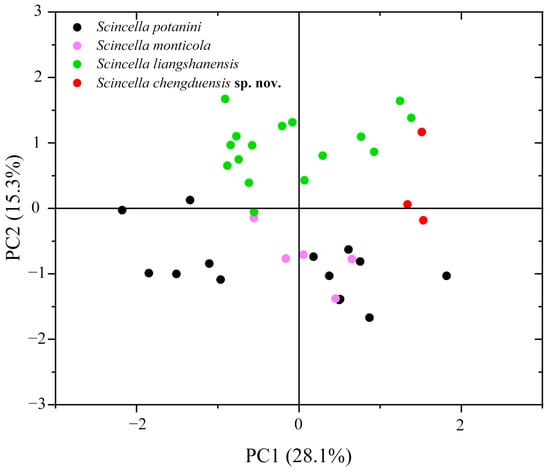
Figure 3.
Scatter plot of PC1 and PC2 from PCA based on morphometric measurements, distinguishing the new species and its closely related species. Red, green, black, and light magenta plots represent Scincella chengduensis sp. nov., S. liangshanensis, S. potanini, and S. monticola, respectively.
Based on comprehensive morphological and phylogenetic analyses, we concluded that the Scincella population from Chengdu, Sichuan, Southwest China, constitutes a distinct new species, described herein.
3.3. Taxonomic Account
Scincella chengduensis sp. nov. Jia, Ren, Jiang, & Li

Figure 4.
Paratype of Scincella chengduensis sp. nov. (CIB 118786) in life. Photo by Jin-Long Ren.
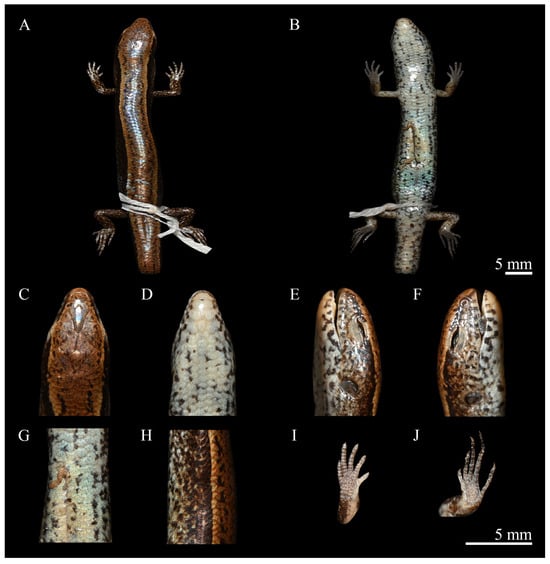
Figure 5.
Holotype of Scincella chengduensis sp. nov. (CIB 118787) in preservative. (A): Dorsal view of body; (B): Ventral view of body; (C): Dorsal view of head; (D): Ventral view of head; (E): Left view of head; (F): Right view of head; (G): Ventral feature of body; (H): Lateral view of body; (I): Ventral view of hand; (J): Ventral view of foot. Scale bar: 5 mm. Photos by Zong-Yuan Gao.
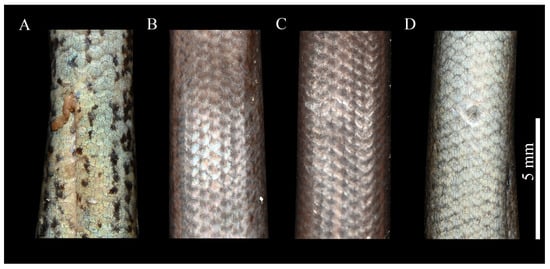
Figure 6.
Comparison of ventral features of Scincella chengduensis sp. nov., Scincella liangshanensis, S. potanini, and S. monticola. (A): Scincella chengduensis sp. nov.; (B): Scincella liangshanensis; (C): S. potanini; (D): S. monticola. Photographs by Zong-Yuan Gao.
Holotype. CIB 118787 (field no. JGS2018016) (Figure 5), adult male, collected from Jiguanshan Forest Park, Chongzhou, Chengdu, Sichuan, China; coordinates 30.77389764° N, 103.22196458° E; elevation 1831 m a.s.l., collected by Ke Jiang, Jin-Long Ren, and Jun Lei on 15 May 2018.
Paratypes. CIB 118786, adult female, collected from the same locality as the holotype. CIB 107637, juvenile, collected from Xiling Snow Mountain Scenic Area, Dayi, Chengdu, Sichuan, China; coordinates 30.68313555° N, 103.28359164° E; elevation 1319 m a.s.l.; collected by Yue-Zhao Wang and Yue-Ying Chen on 30 June 2017.
The holotype and two paratypes are preserved in the Herpetological Museum, CIB, CAS.
Etymology. The specific epithet is derived from the type locality Chengdu, the capital of Sichuan Province and an important urban center in western China known for its high biodiversity. Reflecting its geographic distribution in Chengdu, the proposed common name is “Chengdu ground skink” in English and “Chéng Dū Huá Xī (成都滑蜥)” in Chinese.
Diagnosis. Scincella chengduensis sp. nov. can be clearly distinguished by a combination of the following unique characters: (1) slender, medium-sized body, snout-vent length 28.4–43.2 mm; (2) infralabials seven, rarely six; (3) supraciliaries six or seven; (4) tympanum deeply recessed without lobules, tympanum diameters equal to or exceeding palpebral disc diameters; (5) midbody scale-row counts 23; (6) dorsal scales smooth, slightly enlarged, paravertebral scale-row counts 57–60, ventral scale-row counts 42–44, gulars 21–22; (7) upper edge of lateral longitudinal stripes relatively straight, four rows of dorsal scales in middle; (8) enlarged, undivided lamellae beneath finger IV 8–9, enlarged, undivided lamellae beneath toe IV 10–12; (9) ventral surface densely covered with dark spots; (10) grayish-brown, irregular dorsal stripes 2–3, black dorsolateral stripes from posterior corner of eye to lateral side of tail.
Description of holotype. CIB 118787 (Figure 5): adult male, SVL 37.7 mm; snout short, obtuse; lower eyelid with undivided transparent disc; tympanum recessed, oblique margin prominent; original tail; head elongated, HL 6.8 mm (HL/SVL 0.18), longer than wide, HW 5.1 mm (HW/HL 0.74), lightly flattened, HD 4.2 mm (HD/HL 0.62); neck slender, indistinct from head; scale-row on dorsal surface of fingers and 2nd toe.
Head: Snout circular in profile and dorsal views, SNL 2.6 mm, exceeding twice TD (1.1 mm); ear oval; ED 2.0 mm; PDD 0.7 mm, ear opening to snout breadth and palpebral disc ratio 1.58; END 1.6 mm; IND 1.6 mm, IOD 3.1 mm; snout broad, visible dorsally, contacting 1st SL laterally, nasals, and frontonasal posteriorly; MT 20, LT 20; supranasals absent; frontonasal subtrapezoidal, anterior margin forming nearly straight suture (0.6 mm) with rostral, posterior width 1.7 mm, equaling rostral width, exceeding twice its length (0.8 mm), contacting nasals and 1st loreal laterally, slightly touching frontal posteriorly; two prefrontals not in contact, separated medially by frontal, flanked laterally by two loreals, contacting frontal posteriorly; frontal slender, rhombus-shaped, posterior section longer than anterior, contacting 1st and 2nd supraoculars laterally, frontoparietals posteriorly, anterior edge of frontal lightly separating prefrontals medially, posterior edge of frontal lightly overlapping median seam between frontoparietals; two frontoparietals in contact, diamond-shaped, forming butterfly pattern, contacting 2nd–4th supraoculars laterally, interparietal and parietals posteriorly; interparietal small, rhombus-shaped, posterior section longer than anterior, contacting parietals posteriorly, anterior edge of interparietal acute, intruding slightly into median seam between frontoparietals; parietals large, touching posteriorly, narrowly contacting 4th supraocular and posterior supraciliary, broadly contacting anterior secondary temporal laterally and enlarged nuchals posteriorly. Naris circular, located laterally within nasals; nasals contacting 1st SL ventrally, frontonasal dorsally, 1st loreal posteriorly; loreals two, anterior loreal rhomboidal, touching 2nd SL ventrally, frontonasals and prefrontals dorsally, posterior loreal subtrapezoidal, contacting 2nd and 3rd SL ventrally, preocular and upper presubocular posteriorly, prefrontals and anterior supraciliary dorsally; supraciliaries seven, anterior two largest; supraoculars four, first two touching frontal, 2nd to 3rd contacting frontoparietals; lower eyelid with conspicuous transparent disc (window), bordered by small palpebral scales above; supralabials seven, 1st smallest, 5th situated beneath eye window, 6th largest; infralabials seven (left) and six (right), 1st smallest, 5th largest, rectangular or pentagonal; original temporals two, lower larger, sub-rhomboid, contacting 5th and 6th SL ventrally, touching lower secondary temporal posteriorly, anterior primary temporal sub-rhomboid, contacting secondary temporals posteriorly; secondary temporals two, lower smaller, broadly touching anterior, contacting 7th SL ventrally, anterior secondary temporal twice size of lower, contacting parietals dorsally, nuchals posteriorly; nuchals three, bordering posterior parietal edge, enlarged compared to adjacent posterior scales. Mental rounded, contacting 1st IfL laterally, postmental posteriorly; postmental large, contacting 1st and 2nd IfL laterally, 1st chin shield posteriorly; chin shield pairs three, 1st pair broad, contacting medially, touching 2nd–3rd IfL laterally, 2nd pair separated by sub-triangular gular, contacting 3rd–4th IfL laterally, 3rd pair separated medially by three gulars, contacting 5th–6th IfL laterally, three gulars posteriorly; gulars 21.
Body, limbs, and tail: Body relatively stout, SVL 37.7 mm; axilla-groin distance relatively long, AGD 23.0 mm (AGD/SVL 0.61); MBW 5.1 mm (MBW/SVL 0.13), MBD 4.6 mm (MBD/SVL 0.12); original tail broken during capture, preserved separately in 75% ethanol, original tail relatively long, TaL 60.0 mm, TaL/SVL 1.59; tail width ≈ height: TaW 4.2 mm (TaW/SVL 0.11), TaD 3.9 mm (TaD/SVL 0.10); forelimbs short, FLL 9.6 mm (FLL/SVL 0.26); hindlimbs longer than forelimbs, HLL 12.2 mm (HLL/SVL 0.32).
Body scales smooth, cycloid, imbricate; dorsal scales larger than lateral scales significantly, larger than ventral scales slightly; anterior flank scales between tympanic region and posterior margin of axilla smaller than adjacent dorsal scales; MBSR 23; PVSR 60; VSR 44; AGSR 58; enlarged preanal scale pair one, median scales overlapping outer scales; dorsal scale rows between dorsolateral stripes 4+2 (1/2); limbs pentadactyl, toes not in contact with fingers when limbs adpressed; digits slender, F4L 2.1 mm, T4L 3.8 mm; F4S 9, T4S 12.
Coloration in life: Dorsal surface reddish-brown, marked by two longitudinal stripes formed by contiguous, irregularly shaped black maculations of varying size. Lateral black stripes originate from snout, through supralabials, extend dorsally above eye, continue along flanks above forelimbs and hindlimbs, reaching tail. Axilla-groin stripe black, 1–2 scales wide, with distinct upper boundary. Lateral body surface densely covered with dark spots. Dorsal head brown, with black oval spots. Ventral head and trunk surfaces cream, densely mottled with large, irregular black spots, primarily concentrated along midline. Dorsal limbs brown, ventral surfaces bluish-gray with prominent spotting. Ventral aspect of tail brownish-gray, densely speckled with black spots.
Coloration in preservative: Specimens fixed in 10% formalin and preserved in 75% ethanol exhibit a coloration closely resembling that of live animals. However, cream venter changed to bluish-gray and was no longer transparent (Figure 5).
Variations: The paratypes (Table 2) are similar to the holotype in most morphometric, meristic, and color traits, with the following variations: (1) PVSR and VSR: 57–60 and 42–44, respectively; (2) gulars: 21–22; (3) AGSR: 52–58; (4) four nuchals on left in CIB 107637; (5) six scales on right in CIB 118786; (6) seven superciliaries in CIB 118786; (7) F4S and T4S: 8–9 and 10–12, respectively; (8) MT and LT: 18–20 and 16–20, respectively. Other minor variations are shown in Table 2.
Distribution and habitat: The new species is currently known only from the Jiguanshan Forest Park in Chongzhou, and Dayi, northwestern Chengdu, Sichuan Province, China (Figure 7).

Figure 7.
Habitat of Scincella chengduensis sp. nov. at type locality. Photograph by Jun-Jie Huang.
All collected specimens were found in rocky terrain with decaying leaf litter at elevations between 1319 and 1831 m a.s.l. during both dry and wet seasons. The new species is predominantly diurnal, most frequently seen on rocky areas, on leaf-littered cave floors, and in rocky crevices. Sympatric lizard species include Sphenomorphus indicus [2,17]. Further research is needed to explore the specifics of their ecological interactions.
Comparisons: In terms of pholidosis, Scincella chengduensis sp. nov. is most similar to S. potanini, S. monticola, and S. liangshanensis, sharing the same number of dorsal scale rows between the dorsolateral stripes (DBR = 4), similar range of enlarged, undivided lamellae beneath toe IV (10–15), and a lack of contact between toes and fingers when limbs are adpressed. However, Scincella chengduensis sp. nov. can be distinguished from the previous three species by the presence of dark-colored large blotches on the ventral surface. Moreover, Scincella chengduensis sp. nov. differs from S. potanini by having fewer MBSR (23 vs. 24–27), fewer PVSR and VSR (57–60 vs. 62–80 and 42–44 vs. 45–64, respectively), fewer gulars (21–22 vs. 23–25), and fewer AGSR (52–58 vs. 61–82); from S. monticola by having a greater number of HL/SVL (0.18–0.20 vs. 0.16–0.17), greater number of FIL/SVL and HLL/SVL (0.21–0.26 vs. 0.13–0.19 and 0.23–0.32 vs. 0.20–0.22, respectively), fewer PVSR and VSR (57–60 vs. 69–73 and 42–44 vs. 45–52, respectively), and fewer AGSR (52–58 vs. 65–74).
Scincella chengduensis sp. nov. was recovered as the sister species of S. liangshanensis, with the p-distance between this species pair representing the closest genetic resemblance (3.0%). Nevertheless, Scincella chengduensis sp. nov. can be readily distinguished from the latter species by having fewer PVSR (57–60 vs. 69–80), fewer VSR + gulars (64–65 vs. 68–82), fewer AGSR (52–58 vs. 60–79), and shorter SVL (28.4–43.2 mm [n = 3] vs. 43.1–61.9 mm [n = 16]), as well as the aforementioned differences in ventral color pattern (Figure 3 and Figure 6; Table 4).
For other congeners also showing four dorsal scale rows between dorsolateral stripes, Scincella chengduensis sp. nov. differs from S. tsinlingensis (Hu and Zhao, 1966) and S. huanrenensis Zhao and Huang, 1982 by having fewer MBSR (23 vs. 26–28 and 25–28, respectively), fewer PVSR (57–60 vs. 60–75 and 66–79, respectively), fewer VSR + gulars (64–65 vs. 83–98 and 75–83, respectively), and fewer T4S (10–12 vs. 13–16) [48,49,50]; from S. schmidti (Barbour, 1927) [51] by having a shorter tail, TaL/SVL (1.59 vs. 1.90), fewer MBSR (23 vs. 26), fewer PVSR (57–60 vs. 66), fewer VSR + gulars (64–65 vs. 71), and fewer AGSR (52–58 vs. 60).
For other Chinese congeners displaying six or eight dorsal scale rows between dorsolateral stripes, Scincella chengduensis sp. nov. differs from S. reevesii and S. barbouri (Stejneger, 1925) by having fewer AGD/SVL (0.55–0.61 vs. 0.61–0.66 and 0.61–0.65, respectively), greater FIL/SVL (0.21–0.26 vs. 0.16–0.19 and 0.17–0.20, respectively), fewer MBSR (23 vs. 26–32 and 26–28, respectively), fewer T4S (10–12 vs. 15–18 and 15–17, respectively), and relatively straight UMLLS (vs. wavy) [1,52]; from S. doriae (Boulenger, 1887) by having fewer MBSR (23 vs. 30–32), fewer PVSR (57–60 vs. 66–76), fewer VSR+gulars (64–65 vs. 70–79), fewer T4S (10–12 vs. 15–18), relatively straight UMLLS (vs. wavy), and toes not in contact with fingers when limbs adpressed (vs. overlapping) [3,44,53,54]; from S. formosensis (Van Denburgh, 1912) by having fewer HLL/SVL (0.23–0.32 vs. 0.34–0.39), having fewer MBSR (23 vs. 26–28), more PVSR (69–80 vs. 53–65), fewer T4S (10–12 vs. 14–17), and relatively straight UMLLS (vs. wavy) [40,55]; from S. modesta (Günther, 1864) by having fewer MBSR (23 vs. 26–28), fewer AGSR (52–58 vs. 58–70), fewer T4S (10–12 vs. 13–15), and relatively straight UMLLS (vs. wavy) [56]; from S. przewalskii (Bedriaga, 1912) by having fewer HLL/SVL (0.23–0.32 vs. 0.33), more supraoculars (4 vs. 3) and fewer T4S (10–12 vs. 17) [40]; from S. wangyuezhaoi Jia, Gao, Huang, Ren, Jiang & Li, 2023 by having fewer MBSR (23 vs. 27–30), fewer PVSR (57–60 vs. 60–75), fewer VSR + gulars (64–65 vs. 73–86), and fewer T4S (10–12 vs. 14–15) [14].
Scincella chengduensis sp. nov. can be clearly differentiated from its Asian congeners based on key morphological traits. Notably, the new species contains fewer MBSR compared to most other congeners (23 vs. 24–36), except for S. apraefrontalis Nguyen, Nguyen, Böhme & Ziegler, 2010 (23 vs. 18) [11]. Further distinctions include whether toes are in contact with or overlap fingers when limbs are adpressed. For species in which toes overlap with fingers when limbs are adpressed, Scincella chengduensis sp. nov. can be distinguished by having fewer PVSR (57–60 vs. 63–74), fewer DBR (4 vs. 6–10), and fewer T4S (10–12 vs. 16–22). This pattern is consistent across species such as S. badenensis Nguyen, Nguyen, Nguyen & Murphy, 2019, S. melanosticta (Boulenger, 1887), S. nigrofasciata Neang, Chan & Poyarkov, 2018, S. ouboteri Pham, Pham, Le, Ngoc, Ziegler & Nguyen, 2024, S. rufocaudata (Darevsky and Nguyen, 1983), and S. rupicola (Smith, 1916) [7,12,41,47,53]. For other congeners in which toes are in contact with fingers or not when limbs are adpressed, Scincella chengduensis sp. nov. can be distinguished from S. baraensis Nguyen, Nguyen, Nguyen & Murphy, 2020, S. darevskii Nguyen, Ananjeva, Orlov, Rybaltovsky & Böhme, 2010, S. ochracea (Bourret, 1937), and S. vandenburghi (Schmidt, 1927) by having fewer DBR (4 vs. 6–8) [8,9,54,57]. The new species can also be distinguished from S. boettgeri (Van Denburgh, 1912), S. capitanea Ouboter, 1986, S. devorator (Darevsky, Orlov & Cuc, 2004), S. dunan Koizumi, Ota & Hikida, 2022, S. punctatolineata Boulenger, 1983, and S. victoriana (Shreve, 1940) by having fewer T4S (10–12 vs. 12–20) [1,3,5,13,45,55,58,59].
4. Discussion
Urban cryptic biodiversity: Chengdu is a significant center for economic, scientific, technological, cultural, and transportation activities in southwestern China. Ranking seventh in gross regional product (GDP) nationally and third among sub-provincial cities, Chengdu plays a pivotal role in regional development. Despite this rapid urbanization and economic growth, the city exhibits remarkable biodiversity. The wide altitudinal range, spanning nearly 5000 m (359–5364 m), supports diverse habitats and species. Since 2018, several new taxa have been described, including Gekko cib (Reptilia), Oreolalax longmenmontis (Amphibia), Amolops chaochin (Amphibia), Liobagrus chengduensis (Actinopteri), and Metiochodes tianfuensis (Insecta) [21,22,23,24,25]. These findings highlight the cryptic diversity within Chengdu and emphasize the need for detailed assessments of urban environments where species adapt to fragmented and human-modified habitats.
The discovery of Scincella chengduensis sp. nov. in urban Chengdu also highlights the persistence of cryptic biodiversity within metropolitan environments. Despite the pressures of rapid urbanization, Chengdu functions as a biodiversity reservoir, offering refuge for various species [60]. However, an unresolved record of Scincella tsinlingensis from urban Chengdu raises questions. Qing et al. (2013) [26] reconstructed a phylogenetic tree that included an individual of ‘S. tsinlingensis’, reportedly collected from “Sichuan University campus”, a central urban area of Chengdu. As S. tsinlingensis is a palearctic species unlikely to inhabit the Chengdu Plain, this report may represent a misidentification of Scincella chengduensis sp. nov. Despite extensive field surveys at the campus and surrounding areas, no additional Scincella specimens have been located. As the original data from Qing et al. (2013) cannot be verified (Qing Ning, personal communication), further targeted surveys are needed to investigate the historical presence or potential relict populations of Scincella chengduensis sp. nov. within the same location.
Cryptic biodiversity in urban environments is often overlooked due to several factors. Rapid urbanization and infrastructure development foster the perception that cities are unsuitable for wildlife, reinforced by habitat fragmentation, pollution, and invasive species [61,62]. Infrastructure such as roads and buildings further fragment habitats, reducing the viability of native populations [63]. Species with cryptic behaviors or low population densities, including Scincella chengduensis sp. nov., may evade detection without targeted surveys. Research priorities that focus on pristine environments may exacerbate this issue [64,65]. Conservation efforts frequently neglect urban areas, viewing cities as degraded spaces unsuitable for meaningful wildlife preservation. However, cities like Chengdu demonstrate that urban landscapes can sustain species adapted to human-altered habitats [66,67]. The persistence of biodiversity in such settings underscores the necessity of comprehensive and conservation initiatives targeting urban habitats. Recognizing the ecological value of urban spaces is critical for preserving biodiversity in rapidly urbanizing regions. Conservation strategies must prioritize the remaining natural habitats within urban landscapes to protect these often-overlooked species.
Unique ventral blotches in Scincella. Morphological comparisons revealed that Scincella chengduensis sp. nov. exhibits significant similarity to S. potanini, S. monticola, and S. liangshanensis. However, S. chengduensis can be distinguished from these species by the presence of distinct dark blotches on the ventral surface, a trait absent in other Scincella species (Figure 6). As the functional and evolutionary significance of these blotches remains unresolved, further research is required to explore their role and origin. In reptiles, ventral coloration often plays a pivotal role in species differentiation, particularly within the contexts of sexual selection and interspecific recognition. For instance, in the family Agamidae, male throat coloration is frequently associated with sexual selection, driving pronounced interspecific variation [68,69]. The case of Scincella chengduensis sp. nov. underscores the importance of subtle morphological traits in taxonomic research, especially in closely related species where such traits can provide critical insights into evolutionary divergence and adaptation. While coloration alone may not suffice as the primary basis for species delineation, it can serve as a valuable complementary tool, enhancing the resolution of species boundaries when integrated with other morphological and genetic data. These findings emphasize the importance of comprehensive approaches in reptilian taxonomy, particularly for cryptic or closely related taxa.
Diversity and identification of Scincella species in China. With the addition of the newly described species, the total number of species in the genus Scincella is elevated to 42, with 13 species documented in China [2,70], including Scincella chengduensis sp. nov., S. potanini, S. monticola, S. tsinlingensis, S. liangshanensis, S. modesta, S. huanrenensis, S. reevesii, S. barbouri, S. doriae, S. formosensis, S. przewalskii, and S. schmidti. The identification of Scincella chengduensis sp. nov. highlights the underexplored status of reptile diversity in China, reinforcing the need for systematic field surveys to achieve a comprehensive understanding of the country’s herpetological diversity. This discovery reflects the pivotal role of rigorous taxonomic research in uncovering hidden biodiversity, particularly in regions with high ecological complexity. Recognizing the challenges of species identification in this genus, we provide an updated identification key for the Scincella species in China, based on Wang and Zhao (1986) [40].
Diagnostic key to Scincella species in China
1A Supraoculars 3……………………………………………………………S. przewalskii
1B Supraoculars 4……………………………………………………………….…………..2
2A Toes and fingers overlap when limbs adpressed……………………………S. doriae
2B Toes separated or in contact with fingers when limbs adpressed………………….3
3A Upper margin of lateral longitudinal striation relatively straight………….……….4
3B Upper margin of lateral longitudinal striation wavy……………………………….10
4A Dorsal scale rows between dorsolateral stripes 6……………………………………………………………………………….…S. wangyuezhaoi
4B Dorsal scale rows between dorsolateral stripes 4……………………………………………………………………………………….………5
5A Presence of dark-colored large blotches on ventral…………………………………………………………Scincella chengduensis sp. nov.
5B Absence of dark-colored large blotches on ventral…………………………………..6
6A Number of enlarged, undivided lamellae beneath toe IV 10–13………………………………………………………………………………………………7
6B Number of enlarged, undivided lamellae beneath toe IV 13–16…………………………………………………………………………………….………8
7A Proportion of tympanum diameter/palpebral disc diameter 1.3–2.4………………………………………………………………………….S. liangshanensis
7B Proportion of tympanum diameter/palpebral disc diameter 0.6–1.3……………………………………………………………………………………………8
8A Midbody scale-row count 23–24…………………………………………………………………….………….S. monticola
8B Midbody scale-row count 24–27…………………………………………………………………………….…….S. potanini
9A Infralabials 6……………………………………………………….……S. huanrenensis
9B Infralabials 7–8……………………………………………………………S. tsinlingensis
10A Dorsal scale rows between dorsolateral stripes 4……………………………………………………………………………………S. schmidti
10B Dorsal scale rows between dorsolateral stripes 6 or 8………………………………………………………………………………………..……11
11A Postnasal pairs mostly 1……………………………………………………S. reevesii
11B Postnasals absent………………………………………………………………………12
12A Paravertebral scale-row count 70–79…………………………………………………………………………………S. barbouri
12B Paravertebral scale-row count 51–65……………………………………………………………………………………………13
13A Relative hind-limb length (hind-limb length/snout-vent length) 0.34–0.39……………………………………………………………………………..S. formosensis
13B Relative hind-limb length (hind-limb length/snout-vent length) 0.29–0.33…………………………………………………………………………………S. modesta
5. Conclusions
This study describes a new species of the genus Scincella, Scincella chengduensis sp. nov., based on three specimens collected from urban and suburban areas in Chengdu, Sichuan, China. Detailed morphological and genetic analyses confirm its clear distinction from all known congeners, stressing the remarkable yet underappreciated biodiversity of urbanized landscapes. The discovery of this new species emphasizes the ecological significance of fragmented habitats in supporting cryptic biodiversity, even within rapidly developing metropolitan areas. However, as Chengdu continues to experience extensive urban expansion, there is an urgent need for further research to evaluate its conservation status and identify potential threats.
Author Contributions
Conceptualization, R.-W.J., J.-L.R. and J.-T.L.; methodology, R.-W.J., Z.-Y.G. and D.-H.W.; software, R.-W.J.; validation, all authors.; formal analysis, R.-W.J. and Z.-Y.G.; investigation, all authors; resources, G.-Q.W., G.L. and M.L.; data curation, R.-W.J.; writing—original draft preparation, R.-W.J.; writing—review and editing, R.-W.J., J.-L.R. and K.J.; visualization, R.-W.J. and D.-H.W.; supervision, J.-L.R., K.J., D.-C.J. and J.-T.L.; project administration, J.-T.L.; funding acquisition, J.-T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (32325011, 32200363, 32300370, 32400361); the Second Tibetan Plateau Scientific Expedition and Research Program (STEP) (2019QZKK0501); Biological Resources Programme, Chinese Academy of Sciences (KF-BRP-017-65, KFJ-BRP-017-086); Taxonomist Position, Chinese Academy of Sciences (CAS-TAX-24-051, CAS-TAX-24-052); Chengdu Municipal Park City Construction and Management Bureau.
Institutional Review Board Statement
All animal protocols in this study were reviewed and approved by the Animal Ethical and Welfare Committee of Chengdu Institute of Biology, Chinese Academy of Sciences (permit number: CIBDWLL2021023).
Informed Consent Statement
Not applicable.
Data Availability Statement
All necessary details of the material described, including locations, dates and the name of the collector, are available in this article. Upon reasonable request, the material can be made available by the author.
Acknowledgments
We thank Jun LEI for his help in the field; Jun-Jie Huang (CIB) for photographing; Xiao-Mao Zeng (CIB) and Li Ding (CIB) for providing kind help and giving us access to examine specimens under their care; Ke LV (CIB) for the loan and examination of specimens.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. Specimens Examined (n = 87)
Scincella huanrenensis (n = 8): China: Liaoning, Huanren: CIB 6925–32.
Scincella liangshanensis (n = 16): China: Sichuan, Yuexi: CIB 6977, CIB 6980, CIB 6983, CIB 6986, CIB 6987, CIB 7112, CIB 7144, CIB 7192, CIB 7193, CIB 7197, CIB 7200, CIB 7205, CIB 7210; Meigu: CIB 7026, CIB 119513, CIB 119514.
Scincella modesta (n = 6): China: Zhejiang, Ningpo: CIB 86327–29, CIB 121415, WYF11520; Fujian, Xiamen: CIB 121418.
Scincella monticola (n = 5): China: Yunnan, Lijiang: CIB 8969, Weixi: CIB 6969–71, and Shangri-La: DL-YNJC2020824.
Scincella potanini (n = 14): China: Sichuan, Kangding: CIB 85805–07, CIB 72253–60, DL-KD202109071, DL-KD202109072, DL-KD2018070302.
Scincella reevesii (n = 14): China: Guangdong, Guangzhou: CIB 95481, CIB 7215, CIB7216, CIB 7219; Guangxi: CIB 7218; Hainan, Danzhou: CIB 94929, CIB 94930, CIB 94932, CIB 94933, CIB 7222, Lingshui: CIB 7220, CIB 7221, Ledong: CIB 121416, CIB 121417.
Scincella tsinlingensis (n = 10): China: Shannxi, Zhouzhi: CIB 7226, CIB 7240, CIB 7246, CIB 7249, CIB 7251–53, CIB 7258, CIB 7259, CIB 7261.
Scincella wangyuezhaoi (n = 14): China: Sichuan, Wenchuan: CIB 87244–50; Lixian: CIB 119509, CIB 119510, CIB 119512, CIB 119515, CIB 119516, CIB 119518, CIB 119519.
References
- Ouboter, P.E. A revision of the genus Scincella (Reptilia: Sauria: Scincidae) of Asia, with some notes on its evolution. Zool. Verh. 1986, 229, 1–66. [Google Scholar]
- Zhao, E.; Zhao, K.; Zhou, K. Fauna Sinica Reptila (Vol. 2) Squamata: Lacertilia; Science Press: Beijing, China, 1999; pp. 312–336. (In Chinese) [Google Scholar]
- Smith, M.A. The Fauna of British India, Including Ceylon and Burma. Reptilia and Amphibia. Vol. II. Sauria; Taylor and Francis: London, UK, 1935; p. 440. [Google Scholar]
- Honda, M.; Ota, H.; Köhler, G.; Ineich, I.; Chirio, L.; Chen, S.L.; Hikida, T. Phylogeny of the lizard subfamily Lygosominae (Reptilia: Scincidae), with special reference to the origin of the New World taxa. Genes Genet. Syst. 2003, 78, 71–80. [Google Scholar] [CrossRef][Green Version]
- Darevsky, I.S.; Orlov, N.L.; Ho, T.C. Two new lygosomine skinks of the genus Sphenomorphus Fitzinger, 1843 (Sauria, Scincidae) from northern Vietnam. Russ. J. Herpetol. 2004, 11, 111–120. [Google Scholar]
- García-Vázquez, U.O.; Canseco-Márquez, L.; de Oca, A.N.M. A new species of Scincella (Squamata: Scincidae) from the Cuatro Ciénegas Basin, Coahuila, Mexico. Copeia 2010, 2010, 373–381. [Google Scholar] [CrossRef]
- Nguyen, S.N.; Nguyen, V.D.H.; Nguyen, L.T.; Murphy, R.W. A new skink of the genus Scincella Mittleman, 1950 (Squamata: Scincidae) from Ba Den Mountain, Tay Ninh Province, southern Vietnam. Zootaxa 2019, 4648, 273–286. [Google Scholar] [CrossRef]
- Nguyen, S.N.; Nguyen, V.D.H.; Nguyen, L.T.; Murphy, R.W. A new skink of the genus Scincella Mittleman, 1950 (Squamata: Scincidae) from southern Vietnam. Zootaxa 2020, 4868, 423–434. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Ananjeva, N.B.; Orlov, N.L.; Rybaltovsky, E.; Böhme, W. A new species of the genus Scincella Mittleman, 1950 (Squamata: Scincidae) from Vietnam. Russ. J. Herpetol. 2010, 17, 269–274. [Google Scholar]
- Nguyen, T.Q.; Nguyen, T.T.; Orlov, N.L. New record of the Mountain ground skink Scincella monticola (Schmidt, 1925) (Squamata: Scincidae) from Cao Bang Province, Vietnam. Herpetol. Notes 2010, 3, 201–203. [Google Scholar]
- Nguyen, T.Q.; Nguyen, V.S.; Böhme, W.; Ziegler, T. A new species of Scincella (Squamata: Scincidae) from Vietnam. Folia Zool. 2010, 59, 115–121. [Google Scholar] [CrossRef]
- Neang, T.; Chan, S.; Poyarkov, N.A., Jr. A new species of smooth skink (Squamata: Scincidae: Scincella) from Cambodia. Zool. Res. 2018, 39, 220–240. [Google Scholar] [CrossRef]
- Koizumi, Y.; Ota, H.; Hikida, T. A new species of the genus Scincella (Squamata: Scincidae) from Yonagunijima Island, Southern Ryukyus, Japan. Zootaxa 2022, 5128, 61–83. [Google Scholar] [CrossRef]
- Jia, R.W.; Gao, Z.Y.; Huang, J.J.; Ren, J.L.; Jiang, K.; Li, D.Y.; Li, J.T. A new species of the genus Scincella Mittleman, 1950 (Squamata: Scincidae) from Sichuan Province, Southwest China, with a diagnostic key of Scincella species in China. Asian Herpetol. Res. 2023, 14, 24–40. [Google Scholar]
- Jia, R.W.; Gao, Z.Y.; Wu, D.H.; Ren, J.L.; Jiang, D.C.; Wu, W. A new species of the genus Scincella Mittleman, 1950 (Squamata: Scincidae) from Sichuan Province, Southwest China. Asian Herpetol. Res. 2024, 15, 115–129. [Google Scholar] [CrossRef]
- Pham, A.V.; Pham, C.T.; Le, M.D.; Ngoc, H.N.; Ziegler, T.; Nguyen, T.Q. A new skink of the genus Scincella Mittleman, 1950 (Squamata: Scincidae) from Hoa Binh Province, northern Vietnam. Zootaxa 2024, 5428, 91–106. [Google Scholar] [CrossRef]
- Shi, B.N.; Zhao, E.M. The Fauna of Sichuan Resources; Sichuan People’s Press: Chengdu, China, 1980; Volume 1, p. 13. (In Chinese) [Google Scholar]
- Zhao, E.; Yang, D.T. Preface of “The Series of the Scientific Expedition to the Hengduan Mountains Region of Qinghai-Xizang Plateau”; Science Press: Beijing, China, 1997; p. 175. (In Chinese) [Google Scholar]
- Zhao, E.M. Colored Atlas of Reptiles of Sichuan; Forestry Press: Beijing, China, 2003; pp. 83–84. (In Chinese) [Google Scholar]
- Cai, B.; Lv, K.; Chen, Y.Y.; Li, J.T.; Wang, Y.Z.; Gu, H.J.; Gu, X.D. The distributional list of amphibians and reptiles in Sichuan Province, China. China Sci. Data 2018, 3, 1–29. (In Chinese) [Google Scholar]
- Hou, Y.; Shi, S.; Hu, D.; Deng, Y.; Jiang, J.; Xie, F.; Wang, B. A new species of the toothed toad Oreolalax (Anura, Megophryidae) from Sichuan Province, China. Zookeys 2020, 929, 93–115. [Google Scholar] [CrossRef]
- Jiang, K.; Ren, J.L.; Lyu, Z.T.; Wang, D.; Wang, Z.; Lv, K.; Wu, J.W.; Li, J.T. Taxonomic revision of Amolops chunganensis (Pope, 1929) (Amphibia: Anura) and description of a new species from southwestern China, with discussion on Amolops monticola group and assignment of species groups of the genus Amolops. Zool. Res. 2021, 42, 574–591. [Google Scholar] [CrossRef]
- Li, F.; Liao, T.Y.; Arai, R. Two new species of Rhodeus (Teleostei: Cyprinidae: Acheilognathinae) from the River Yangtze, China. J. Vertebr. Biol. 2020, 69, 19055. [Google Scholar] [CrossRef]
- Lyu, Z.T.; Lin, C.Y.; Ren, J.L.; Jiang, K.; Zhang, Y.P.; Qi, S.; Wang, J. Review of the Gekko (Japonigekko) subpalmatus complex (Squamata, Sauria, Gekkonidae), with description of a new species from China. Zootaxa 2021, 4951, 236–258. [Google Scholar] [CrossRef]
- Wang, R.; Yuan, W.; Zheng, Y.; Gu, J.J.; Ma, L. Pteronemobius yuani Ma & Wang sp. nov. and Metiochodes tianfuensis Ma, Yuan & Gu sp. nov., new species of Trigonidiidae from China (Orthoptera: Trigonidiidae; Trigonidiinae). Zootaxa 2023, 5361, 573–578. [Google Scholar] [PubMed]
- Qing, N.; Lin, H.; Tong, R.; Zhang, X.; Lu, W.; Lazell, J. Biogeographic and phylogenetic relationships of some Scincella (Squamata: Scincidae) from China and North America inferred from 12S rRNA gene sequences of mitochondrial DNA. J. S. China Norm. Univ. (Nat. Sci. Ed.) 2013, 45, 129–139. [Google Scholar]
- Günther, A. Report on the collections of reptiles, batrachians and fishes made by Messrs. Potanin and Berezowski in the Chinese provinces Kansu and Sze-chuen. Ann. Mus. Zool. Acad. Sci. St. Petersbourg 1896, 1, 199–219. [Google Scholar]
- Schmidt, K.P. New reptiles and a new salamander from China. Am. Mus. Nat. Hist. 1925, 157, 1–5. [Google Scholar]
- Pyron, R.A.; Burbrink, F.T.; Wiens, J.J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013, 13, 1–54. [Google Scholar] [CrossRef] [PubMed]
- Skwor, T. The use of DNASTAR Lasergene educational software with molecular techniques to support bacterial identification. Proc. Adv. Biol. Lab. Educ. 2012, 33, 327–334. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Calcott, B.; Ho, S.Y.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Felsenstein, J. Inferring Phylogenies; Sinauer Associates: Sunderland, MA, USA, 2004; p. 580. [Google Scholar]
- Hillis, D.M.; Bull, J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 1993, 42, 182–192. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v.1.4.3. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 1 September 2024).
- Wang, Y.Z.; Zhao, E.M. Studies on Chinese species of Scincella (Scincidae, Sauria). Acta Herpetol. Sin. 1986, 5, 267–277. (In Chinese) [Google Scholar]
- Darevsky, I.; Nguyen, V. New and little known lizard species from Vietnam. Zool. Zhurnal 1983, 62, 1827–1837. [Google Scholar]
- Darevsky, I.S.; Orlov, N.L. A new genus and species of scincid lizards from Vietnam: The first Asiatic skink with double rows of basal subdigital pads. J. Herpetol. 1997, 31, 323–326. [Google Scholar] [CrossRef][Green Version]
- Stuart, B.L.; Emmett, D.A. A collection of amphibians and reptiles from the Cardamom Mountains, southwestern Cambodia. Fieldiana Zool. 2006, 2006, 1–27. [Google Scholar] [CrossRef]
- Stuart, B.L.; Sok, K.; Neang, T. A collection of amphibians and reptiles from hilly eastern Cambodia. Raffles Bull. Zool. 2006, 54, 129–155. [Google Scholar]
- Taylor, E.H. The lizards of Thailand. Univ. Kansas Sci. Bull. 1963, 44, 687–1077. [Google Scholar]
- Gonzalez, M.; Lwin, K.; Vindum, J. New records for Scincella victoriana (Shreve, 1940) from the Chin Hills, Myanmar. Proc. Calif. Acad. Sci. 2005, 56, 391–392. [Google Scholar]
- Gray, J.E. XXXIV.—Catalogue of the slender-tongued Saurians, with descriptions of many new genera and species. Ann. Mag. Nat. Hist. 1838, 2, 287–293. [Google Scholar] [CrossRef]
- Hu, S.C.; Zhao, E.M.; Liu, C.C. A herpetological survey of the Tsinling and Ta-Pa Shan region. Acta Zool. Sin. 1966, 18, 57–89. (In Chinese) [Google Scholar]
- Zhao, E.; Huang, K. A survey of amphibians and reptiles in Liaoning Province. Acta Herpetol. Sin. 1982, 1, 11–12. (In Chinese) [Google Scholar]
- Chen, S.L.; Hikida, T.; Han, S.H.; Shim, J.H.; Oh, H.S.; Ota, H. Taxonomic status of the Korean populations of the genus Scincella (Squamata: Scincidae). J. Herpetol. 2001, 35, 122–129. [Google Scholar] [CrossRef]
- Barbour, T. A new lizard from China. Copeia 1927, 165, 95. [Google Scholar]
- Stejneger, L. Description of a new scincid lizard and a new burrowing frog from China. J. Wash. Acad. Sci. 1925, 15, 150–152. [Google Scholar]
- Boulenger, G.E. An account of the Scincoid lizards collected from Burma for the Genoa Civic Museum by Messrs. G.B. Comotto and L. Fea. Ann. Mus. Civ. Stor. Nat. Genova 1887, 4, 618–624. [Google Scholar]
- Bourret, R. Notes Herpétologiques sur L’indochine Française; Direction de L’instruction Publique: Hanoi, Vietnam, 1937; p. 39. (In French) [Google Scholar]
- Van Denburgh, J. Concerning certain species of reptiles and amphibians from China, Japan, the Loo Choo Islands, and Formosa. Proc. Calif. Acad. Sci. 1912, 3, 187–258. [Google Scholar]
- Günther, A. The Reptiles of British India; Taylor and Francis: London, UK, 1864; pp. 1–452. [Google Scholar]
- Schmidt, K.P. Notes on Chinese reptiles. Bull. Am. Mus. Nat. Hist. 1927, 54, 467–551. [Google Scholar]
- Shreve, B. Reptiles and amphibians from Burma with descriptions of three new skinks. Proc. N. Engl. Zool. 1940, 18, 17–26. [Google Scholar]
- Bourret, R.; Bour, R. Les Lézards de L’indochine; Edition Chimaira: Frankfurt am Main, Germany, 2009; p. 624. (In French) [Google Scholar]
- Liang, K. Chengdu’s Biodiversity Gains Worldwide Notice. China Daily. Available online: https://www.chinadaily.com.cn/ (accessed on 3 August 2023).
- Li, G.D.; Fang, C.L.; Li, Y.; Wang, Z.B.; Sun, S.; He, S.W.; Qi, W.; Bao, C.; Ma, H.T.; Fan, Y.P.; et al. Global impacts of future urban expansion on terrestrial vertebrate diversity. Nat. Commun. 2022, 13, 1628. [Google Scholar] [CrossRef]
- Strokal, M.; Bai, Z.; Franssen, W.; Hofstra, N.; Koelmans, A.A.; Ludwig, F.; Ma, L.; Puijenbroek, P.V.; Spanier, J.E.; Vermeulen, L.C.; et al. Urbanization: An increasing source of multiple pollutants to rivers in the 21st century. Npj Urban Sustain. 2021, 1, 24. [Google Scholar] [CrossRef]
- Wang, D.; Xu, P.Y.; An, B.W.; Guo, Q.P. Urban green infrastructure: Bridging biodiversity conservation and sustainable urban development through adaptive management approach. Front. Ecol. Evol. 2024, 12, 1440477. [Google Scholar] [CrossRef]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Evans, K.L. Cities are hotspots for threatened species. Glob. Ecol. Biogeogr. 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Van Dyke, F.; Lamb, R.L. The Anthropocene: Conservation in a Human-Dominated Nature. In Conservation Biology; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Yuan, L.; Xu, Z.; Xu, N. The Chinese city in mountain and water: Shaping the urban landscape in Chengdu. Landsc. Res. 2024, 49, 176–191. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, W.; Zhang, S.; Peng, L.; Liu, Y. Impacts of urbanization on ecosystem services in the Chengdu-Chongqing Urban Agglomeration: Changes and trade-offs. Ecol. Indic. 2022, 139, 10892. [Google Scholar] [CrossRef]
- Cai, B.; Zhang, M.H.; Li, J.; Du, S.M.; Xie, F.; Hou, M.; Zhou, H.M.; Jiang, J.P. Three new species of Diploderma Hallowell, 1861 (Reptilia: Squamata: Agamidae) from the Shaluli Mountains in western Sichuan, China. Asian Herpetol. Res. 2022, 13, 205–223. [Google Scholar]
- Chen, I.P.; Stuart-Fox, D.; Hugall, A.F.; Symonds, M.R. Sexual selection and the evolution of complex color patterns in dragon lizards. Evolution 2012, 66, 3605–3614. [Google Scholar] [CrossRef] [PubMed]
- Uetz, P.; Freed, P.; Hošek, J. The Reptile Database. Available online: http://www.reptile-database.org (accessed on 5 September 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).