A Scoping Review on Salivary Oxytocin and Vasopressin Measurement in the Dog
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection of the Sources of Evidence
2.2. Data Charting and Synthesis
3. Results
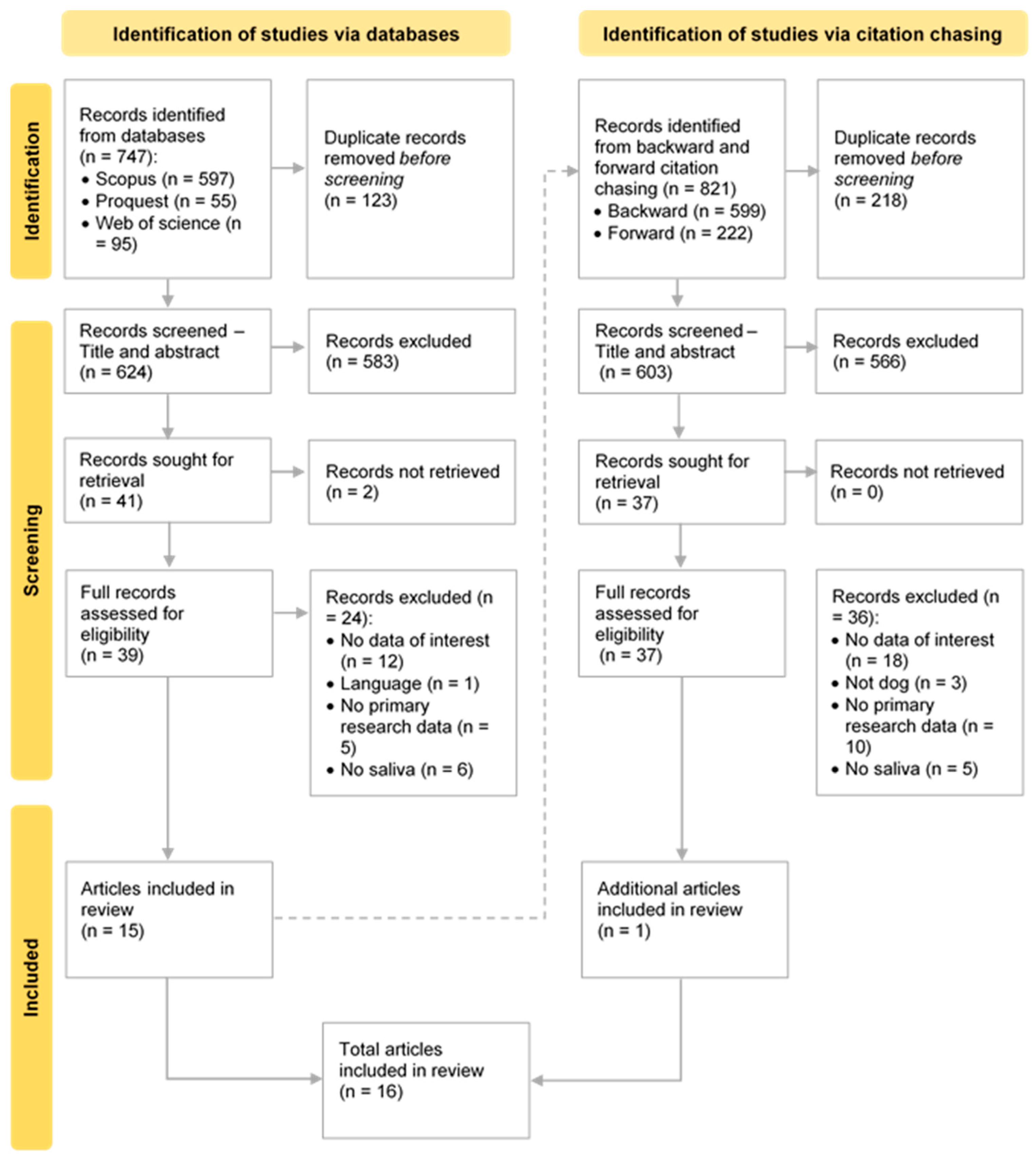
3.1. Selection of Studies
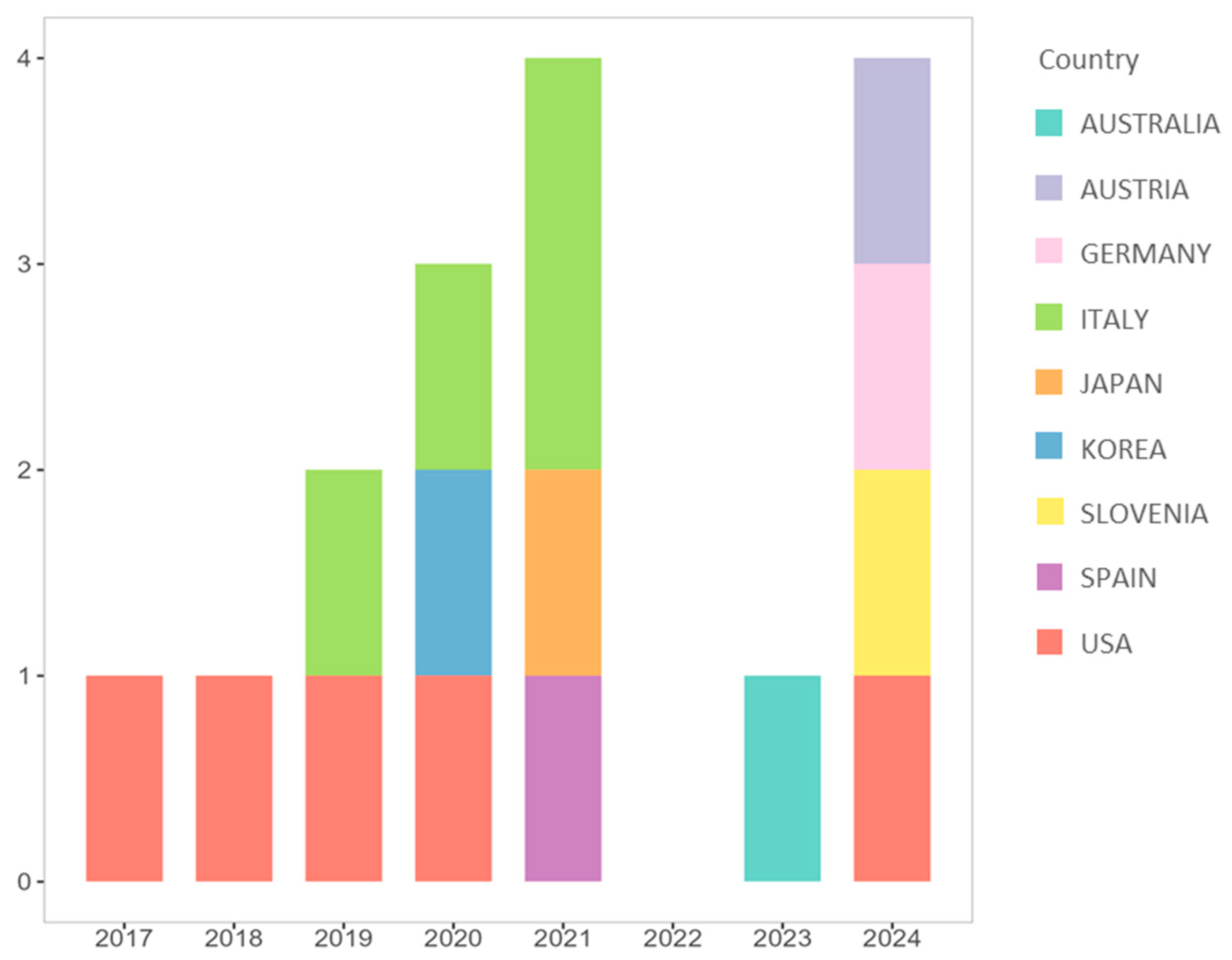
3.2. General Characteristics of the Included Studies
3.3. Development and Validation Studies for Salivary Oxytocin (sOT) and Salivary Vasopressin (sAVP)
3.4. Studies Measuring Salivary Oxytocin (sOT) in Dogs
3.5. Studies Investigating Salivary Vasopressin (sAVP) in Dogs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPA | Hypothalamic-Pituitary-Adrenal |
| OT | Oxytocin |
| AVP | Vasopressin |
| sOT | Salivary Oxytocin |
| sAVP | Salivary Vasopressin |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews |
| WoS | Web of Science |
| ELISA | Enzyme-Linked Immunosorbent Assay; |
| HPLC-MS | High-Performance Liquid Chromatography-Mass Spectrometry |
| LC-MS | Liquid chromatography-mass spectrometry |
| HAI | Human-animal interaction |
| AAIs | Animal-assisted interventions |
| M/DORS | Monash Dog-Owner Relationship |
| UHPLC | Ultra-High Performance Liquid Chromatography |
| SRP | Separation-Related Problems |
References
- Mendl, M.; Neville, V.; Paul, E.S. Bridging the Gap: Human Emotions and Animal Emotions. Affect. Sci. 2022, 3, 703–712. [Google Scholar] [CrossRef]
- Reimert, I.; Webb, L.E.; van Marwijk, M.A.; Bolhuis, J.E. Review: Towards an Integrated Concept of Animal Welfare. Animal 2023, 17, 100838. [Google Scholar] [CrossRef]
- Kremer, L.; Klein Holkenborg, S.E.J.; Reimert, I.; Bolhuis, J.E.; Webb, L.E. The Nuts and Bolts of Animal Emotion. Neurosci. Biobehav. Rev. 2020, 113, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Escribano, D.; Tecles, F. Salivary Biomarkers in Welfare Studies. In Saliva in Health and Disease; Tvarijonaviciute, A., Martinez-Subiela, S., López-Jornet, P., Lamy, E., Eds.; Springer: Cham, Switzerland, 2020; pp. 293–319. [Google Scholar]
- Babington, S.; Tilbrook, A.J.; Maloney, S.K.; Fernandes, J.N.; Crowley, T.M.; Ding, L.; Fox, A.H.; Zhang, S.; Kho, E.A.; Cozzolino, D.; et al. Finding Biomarkers of Experience in Animals. J. Anim. Sci. Biotechnol. 2024, 15, 28. [Google Scholar] [CrossRef]
- Kooriyama, T.; Ogata, N. Salivary Stress Markers in Dogs: Potential Markers of Acute Stress. Res. Vet. Sci. 2021, 141, 48–55. [Google Scholar] [CrossRef]
- Csoltova, E.; Mehinagic, E. Where Do We Stand in the Domestic Dog (Canis familiaris) Positive-Emotion Assessment: A State-of-the-Art Review and Future Directions. Front. Psychol. 2020, 11, 2131. [Google Scholar] [CrossRef] [PubMed]
- Cobb, M.L.; Iskandarani, K.; Chinchilli, V.M.; Dreschel, N.A. A Systematic Review and Meta-Analysis of Salivary Cortisol Measurement in Domestic Canines. Domest. Anim. Endocrinol. 2016, 57, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Chmelíková, E.; Bolechová, P.; Chaloupková, H.; Svobodová, I.; Jovičić, M.; Sedmíková, M. Salivary Cortisol as a Marker of Acute Stress in Dogs: A Review. Domest. Anim. Endocrinol. 2020, 72, 106428. [Google Scholar] [CrossRef]
- Cobb, M.; Jimenez, A.; Dreschel, N. Beyond Cortisol! Physiological Indicators of Welfare for Dogs: Deficits, Misunderstandings and Opportunities. arXiv 2025. [Google Scholar] [CrossRef]
- Carter, C.S.; Kenkel, W.M.; MacLean, E.L.; Wilson, S.R.; Perkeybile, A.M.; Yee, J.R.; Ferris, C.F.; Nazarloo, H.P.; Porges, S.W.; Davis, J.M.; et al. Is Oxytocin “Nature’s Medicine”? Pharmacol. Rev. 2020, 72, 829–861. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K.; Gross, M.M.; Calleja-Agius, J.; Turner, J.D.; Vaudry, H.; Sona, C.; Xiao, L. The Yin and Yang of the Oxytocin and Stress Systems: Opposites, yet Interdependent and Intertwined Determinants of Lifelong Health Trajectories. Front. Endocrinol. 2024, 15, 1272270. [Google Scholar] [CrossRef]
- Caldwell, H.K. The Role of Oxytocin and Vasopressin in the Neural Regulation of Social Behavior. Available online: https://oxfordre.com/neuroscience/display/10.1093/acrefore/9780190264086.001.0001/acrefore-9780190264086-e-10 (accessed on 20 March 2025).
- Beetz, A.; Uvnäs-Moberg, K.; Julius, H.; Kotrschal, K. Psychosocial and Psychophysiological Effects of Human-Animal Interactions: The Possible Role of Oxytocin. Front. Psychol. 2012, 3, 234. [Google Scholar] [CrossRef]
- Nagasawa, M.; Kikusui, T.; Onaka, T.; Ohta, M. Dog’s Gaze at Its Owner Increases Owner’s Urinary Oxytocin during Social Interaction. Horm. Behav. 2009, 55, 434–441. [Google Scholar] [CrossRef]
- MacLean, E.L.; Gesquiere, L.R.; Gruen, M.E.; Sherman, B.L.; Martin, W.L.; Carter, C.S. Endogenous Oxytocin, Vasopressin, and Aggression in Domestic Dogs. Front. Psychol. 2017, 8, 1613. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.L.; Serpell, J.A.; Dalton, K.R.; Waite, K.B.; Morris, D.O.; Redding, L.E.; Dreschel, N.A.; Davis, M.F. The Importance of Evaluating Positive Welfare Characteristics and Temperament in Working Therapy Dogs. Front. Vet. Sci. 2022, 9, 844252. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.L.; van den Munkhof, M.; Buisman-Pijlman, F.T.A. Oxytocin as an Indicator of Psychological and Social Well-Being in Domesticated Animals: A Critical Review. Front. Psychol. 2017, 8, 1521. [Google Scholar] [CrossRef]
- Broom, D.M. Can Positive Welfare Counterbalance Negative and Can Net Welfare Be Assessed? Front. Anim. Sci. 2023, 4, 1101957. [Google Scholar] [CrossRef]
- Mitsui, S.; Yamamoto, M.; Nagasawa, M.; Mogi, K.; Kikusui, T.; Ohtani, N.; Ohta, M. Urinary Oxytocin as a Noninvasive Biomarker of Positive Emotion in Dogs. Horm. Behav. 2011, 60, 239–243. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of Positive Emotions in Animals to Improve Their Welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Mellor, D.J.; Beausoleil, N.J. Extending the ‘Five Domains’ Model for Animal Welfare Assessment to Incorporate Positive Welfare States. Anim. Welf. 2015, 24, 241–253. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Grainger, M.J.; Gray, C.T. Citationchaser: A Tool for Transparent and Efficient Forward and Backward Citation Chasing in Systematic Searching. Res. Synth. Methods 2022, 13, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.D. How Old Is My Dog? Identification of Rational Age Groupings in Pet Dogs Based Upon Normative Age-Linked Processes. Front. Vet. Sci. 2021, 8, 643085. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- MacLean, E.L.; Gesquiere, L.R.; Gee, N.; Levy, K.; Martin, W.L.; Carter, C.S. Validation of Salivary Oxytocin and Vasopressin as Biomarkers in Domestic Dogs. J. Neurosci. Methods 2018, 293, 67–76. [Google Scholar] [CrossRef]
- Wang, L.; Marti, D.W.; Anderson, R.E. Development and Validation of a Simple LC-MS Method for the Quantification of Oxytocin in Dog Saliva. Molecules 2019, 24, 3079. [Google Scholar] [CrossRef]
- López-Arjona, L.-A.M.; Mateo, S.V.; Cerón, C.J.J.; Martínez-Subiela, M.-S.S. Changes in Salivary Oxytocin after Stroking in Dogs: Validation of Two Assays for Its Assessment. Res. Vet. Sci. 2021, 136, 527–534. [Google Scholar] [CrossRef]
- MacLean, E.L.; Gesquiere, L.R.; Gee, N.R.; Levy, K.; Martin, W.L.; Carter, C.S. Effects of Affiliative Human–Animal Interaction on Dog Salivary and Plasma Oxytocin and Vasopressin. Front. Psychol. 2017, 8, 1606. [Google Scholar] [CrossRef]
- Pirrone, F.; Pierantoni, L.; Bossetti, A.; Uccheddu, S.; Albertini, M. Salivary Vasopressin as a Potential Non–Invasive Biomarker of Anxiety in Dogs Diagnosed with Separation–Related Problems. Animals 2019, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Ogi, A.; Mariti, C.; Baragli, P.; Sergi, V.; Gazzano, A. Effects of Stroking on Salivary Oxytocin and Cortisol in Guide Dogs: Preliminary Results. Animals 2020, 10, 708. [Google Scholar] [CrossRef]
- Gnanadesikan, G.E.; King, K.M.; Carranza, E.; Flyer, A.C.; Ossello, G.; Smith, P.G.; Steklis, N.G.; Steklis, H.D.; Carter, C.S.; Connelly, J.J.; et al. Effects of Human-Animal Interaction on Salivary and Urinary Oxytocin in Children and Dogs. Psychoneuroendocrinology 2024, 169, 107147. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.D.; Martin, F.; McGowan, R.T.S.S.; Smidt, J.M.; Anderson, R.; Wang, L.; Turpin, T.; Langenfeld-McCoy, N.; Bauer, B.A.; Mohabbat, A.B. Physiological State of Therapy Dogs during Animal-Assisted Activities in an Outpatient Setting. Animals 2020, 10, 819. [Google Scholar] [CrossRef]
- Hill, J.; Driscoll, C.; Cawdell-Smith, J.; Anderson, S.; Ziviani, J. Investigating Dog Welfare When Interacting with Autistic Children within Canine-Assisted Occupational Therapy Sessions: A Single Case Study. Animals 2023, 13, 1965. [Google Scholar] [CrossRef]
- Akiyama, J.; Ohta, M. Hormonal and Neurological Aspects of Dog Walking for Dog Owners and Pet Dogs. Animals 2021, 11, 2732. [Google Scholar] [CrossRef]
- McGetrick, J.; Fux, L.; Schullern-Schrattenhofen, J.; Rault, J.-L.; Range, F. Do Pet Dogs Reciprocate the Receipt of Food from Familiar and Unfamiliar Conspecifics? Ethology 2024, 130, e13430. [Google Scholar] [CrossRef]
- Peterca, L.; Gobbo, E.; Zupan Šemrov, M. Dog–Owner Relationship and Its Association with Social Cognition in French Bulldogs. Animals 2024, 15, 17. [Google Scholar] [CrossRef]
- Ogi, A.; Mariti, C.; Pirrone, F.; Baragli, P.; Gazzano, A. The Influence of Oxytocin on Maternal Care in Lactating Dogs. Animals 2021, 11, 1130. [Google Scholar] [CrossRef]
- Ogi, A.; Naef, V.; Santorelli, F.M.; Mariti, C.; Gazzano, A. Oxytocin Receptor Gene Polymorphism in Lactating Dogs. Animals 2021, 11, 3099. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-K.; Oh, Y.-I.; Song, K.-H.; Seo, K.W. Evaluation of Salivary Vasopressin as an Acute Stress Biomarker in Healthy Dogs with Stress Due to Noise and Environmental Challenges. BMC Vet. Res. 2020, 16, 331. [Google Scholar] [CrossRef] [PubMed]
- Schroers, M.; Juhasz, A.; Zablotski, Y.; Meyer-Lindenberg, A. Effect of Casozepine Administration on Stress in Dogs during a Veterinary Examination—A Randomized Placebo-Controlled Trial. Vet. J. 2024, 306, 106148. [Google Scholar] [CrossRef]
- Dreschel, N.A.; Granger, D.A. Methods of Collection for Salivary Cortisol Measurement in Dogs. Horm. Behav. 2009, 55, 163–168. [Google Scholar] [CrossRef]
- Gnanadesikan, G.E.; Bray, E.E.; Cook, E.N.; Levy, K.M.; Douglas, L.E.L.C.; Kennedy, B.S.; Tecot, S.R.; MacLean, E.L. Basal Plasma Oxytocin and Fecal Cortisol Concentrations Are Highly Heritable and Associated with Individual Differences in Behavior and Cognition in Dog Puppies. Horm. Behav. 2024, 165, 105612. [Google Scholar] [CrossRef]
- Wirobski, G.; Range, F.; Schaebs, F.S.; Palme, R.; Deschner, T.; Marshall-Pescini, S. Life experience rather than domestication accounts for dogs’ increased oxytocin release during social contact with humans. Sci. Rep. 2021, 11, 14423. [Google Scholar] [CrossRef]
- Franco-Martínez, L.; Castillo-Felipe, C. Saliva as a Non-Invasive Sample: Pros and Cons. In Saliva in Health and Disease; Tvarijonaviciute, A., Martínez-Subiela, S., López-Jornet, P., Lamy, E., Eds.; Springer: Cham, Switzerland, 2020; pp. 49–65. ISBN 978-3-030-37681-9. [Google Scholar]
- Dreschel, N.A.; Granger, D.A. Advancing the Social Neuroscience of Human-Animal Interaction: The Role of Salivary Bioscience. Soc. Neurosci. Hum.-Anim. Interact. 2016, 195–216. [Google Scholar] [CrossRef]
- López-Arjona, M.; Botía, M.; Martínez-Subiela, S.; Cerón, J.J. Oxytocin Measurements in Saliva: An Analytical Perspective. BMC Vet. Res. 2023, 19, 96. [Google Scholar] [CrossRef]
- Ogi, A.; Gazzano, A. Maternal Behavior in Domestic Dogs. Adv. Small Anim. Care 2024, 5, 1–7. [Google Scholar] [CrossRef]
- MacLean, E.L.; Wilson, S.R.; Martin, W.L.; Davis, J.M.; Nazarloo, H.P.; Carter, C.S. Challenges for Measuring Oxytocin: The Blind Men and the Elephant? Psychoneuroendocrinology 2019, 107, 225–231. [Google Scholar] [CrossRef]
- Petersson, M.; Uvnäs-Moberg, K.; Nilsson, A.; Gustafson, L.L.; Hydbring-Sandberg, E.; Handlin, L. Oxytocin and Cortisol Levels in Dog Owners and Their Dogs Are Associated with Behavioral Patterns: An Exploratory Study. Front. Psychol. 2017, 8, 1796. [Google Scholar] [CrossRef]
- Uvnäs-Moberg, K.; Handlin, L.; Petersson, M. Self-Soothing Behaviors with Particular Reference to Oxytocin Release Induced by Non-Noxious Sensory Stimulation. Front. Psychol. 2014, 5, 1529. [Google Scholar] [CrossRef]
- Uvnäs Moberg, K.; Petersson, M. Physiological Effects Induced by Stimulation of Cutaneous Sensory Nerves, with a Focus on Oxytocin. Curr. Opin. Behav. Sci. 2022, 43, 159–166. [Google Scholar] [CrossRef]
- Scandurra, A.; D’Aniello, B.; Pero, M.E.; Pinelli, C.; Di Lucrezia, A.; Tudisco, R.; Iommelli, P.; Mastellone, V.; Lombardi, P. Human social buffer in goats and dogs. Anim. Cogn. 2024, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Petersson, M.; Uvnäs-Moberg, K. Interactions of Oxytocin and Dopamine—Effects on Behavior in Health and Disease. Biomedicines 2024, 12, 2440. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Mitsui, S.; En, S.; Ohtani, N.; Ohta, M.; Sakuma, Y.; Onaka, T.; Mogi, K.; Kikusui, T. Oxytocin-Gaze Positive Loop and the Coevolution of Human-Dog Bonds. Science 2015, 348, 333–336. [Google Scholar] [CrossRef] [PubMed]
| Methods | Details | Number of Studies * | Reference |
|---|---|---|---|
| Saliva collection media | Salimetrics® Children’s swab (Salimetrics LLC, State College, PA, USA) | 1 | [27] |
| Sarstedt Salivette® (Sarstedt AG&Co., Nümbrecht, Germany) | 1 | [27] | |
| VERSISAL® kit Oasis Diagnostics (Vancouver, WA, USA) | 1 | [28] | |
| Sarstedt Salivette® tube containing a sponge (Esponja Marina, La Griega E. Koronis, Madrid, Spain) | 1 | [29] | |
| Salivation stimuli before collection | No/not reported | 3 | [27,28,29] |
| Kibble | 1 | [27] | |
| Citric acid solution | 1 | [27] | |
| Duration of collection | 1 min | 2 | [27,29] |
| 2 min or more | 1 | [27,28] | |
| Sample processing | Extraction | 2 | [27,28] |
| No extraction or reduction/alkylation | 2 | [27,29] | |
| Reduction/alkylation | 1 | [29] | |
| Analysis method/kit sOT | ELISA kit from Cayman Chemical (Ann Arbor, MI, USA) | 2 | [27,29] |
| ELISA kit from Arbor Assays (Ann Arbor, MI, USA) | 1 | [27] | |
| ELISA Enzo Life Sciences (pilot, human sOT) (Farmingdale, NY, USA) | 1 | [27] | |
| HPLC-MS | 1 | [27] | |
| LC-MS | 1 | [28] | |
| AlphaLISA with monoclonal antibody | 1 | [29] | |
| AlphaLISA with polyclonal antibody | 1 | [29] | |
| Analysis method/kit sAVP | ELISA Enzo Life Sciences (Farmingdale, NY, USA) | 1 | [27] |
| HPLC-MS | 1 | [27] |
| Methods | Details | N. | Reference |
|---|---|---|---|
| Saliva collection media | Salimetrics® Children’s swab (Salimetrics LLC, State College, PA, USA) | 5 | [30,31,33,35,37] |
| Sarstedt Salivette® (Sarstedt AG&Co, Nümbrecht, Germany) | 4 | [32,38,39,40] | |
| Sarstedt Salivette® containing a sponge | 1 | [29] | |
| VERSISAL® Oasis Diagnostic (Vancouver, WA, USA) | 1 | [34] | |
| Mentip® hospital cotton swab (JCB Industry Limited, Tokyo, Japan) | 1 | [36] | |
| Salivation stimuli | No/not mentioned | 10 | [29,30,32,34,35,36,37,38,39,40] |
| Odour of food | 2 | [31,33] | |
| Duration of collection | 1 min | 8 | [29,30,31,32,36,38,39,40] |
| 1 to 2 min | 2 | [35,37] | |
| 2 min or more | 2 | [33,34] | |
| Analysis method/kit sOT | ELISA kit from Cayman Chemical | 6 | [30,32,35,37,39,40] |
| ELISA kit from Arbor Assay | 2 | [31,33] | |
| ELISA kit from Enzo Life Sciences (Farmingdale, NY, USA) | 2 | [36,38] | |
| LC-MS with UHPLC | 1 | [34] | |
| AlphaLISA with monoclonal and polyclonal antibody | 1 | [29] | |
| Other measures * | sAVP | 2 | [30,31] |
| Salivary cortisol | 4 | [32,34,35,36] | |
| Salivary alpha-amylase | 1 | [35] | |
| Plasma OT | 1 | [30] | |
| Plasma AVP | 1 | [30] | |
| Urinary OT | 1 | [33] | |
| Tympanic membrane temperature | 1 | [34] | |
| Heart rate and heart rate variability | 1 | [34] | |
| Behaviour | 10 | [29,30,31,32,33,35,37,38,39,40] | |
| Monoamines | 1 | [36] | |
| GABA | 1 | [36] | |
| Demographic and questionnaire data | 2 | [33,38] | |
| Mother-related factors | 1 | [39] | |
| OT receptor gene polymorphism | 1 | [40] |
| Methods | Details | N. | Reference |
|---|---|---|---|
| Saliva collection media | Salimetrics® swab (Salimetrics LLC, State College, PA, USA) | 4 | [30,31,41,42] |
| Salivation stimuli | No/not mentioned | 3 | [30,41,42] |
| Food odour | 1 | [31] | |
| Duration of collection | 1 min | 3 | [30,31,42] |
| 1–3 min | 1 | [41] | |
| Analysis method/kit sAVP | ELISA Enzo Life Sciences (Farmingdale, NY, USA) | 2 | [30,41] |
| ELISA MyBioSource (San Diego, CA, USA) | 1 | [31] | |
| ELISA NordicBioSite (Täby, Sweden) | 1 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Santis, M.; Soncin, M.; Bertazzo, V.; Martelli, L.; Fornasiero, D.; Mutinelli, F.; Contalbrigo, L. A Scoping Review on Salivary Oxytocin and Vasopressin Measurement in the Dog. Animals 2025, 15, 2421. https://doi.org/10.3390/ani15162421
De Santis M, Soncin M, Bertazzo V, Martelli L, Fornasiero D, Mutinelli F, Contalbrigo L. A Scoping Review on Salivary Oxytocin and Vasopressin Measurement in the Dog. Animals. 2025; 15(16):2421. https://doi.org/10.3390/ani15162421
Chicago/Turabian StyleDe Santis, Marta, Margherita Soncin, Valentina Bertazzo, Luca Martelli, Diletta Fornasiero, Franco Mutinelli, and Laura Contalbrigo. 2025. "A Scoping Review on Salivary Oxytocin and Vasopressin Measurement in the Dog" Animals 15, no. 16: 2421. https://doi.org/10.3390/ani15162421
APA StyleDe Santis, M., Soncin, M., Bertazzo, V., Martelli, L., Fornasiero, D., Mutinelli, F., & Contalbrigo, L. (2025). A Scoping Review on Salivary Oxytocin and Vasopressin Measurement in the Dog. Animals, 15(16), 2421. https://doi.org/10.3390/ani15162421






