Simple Summary
This study aimed to evaluate the effects of a biofloc-based aquaponics system (FLOCponics) on the growth performance of two economically important fish species in Korea—Far Eastern catfish (Silurus asotus) and tropical eel (Anguilla bicolor)—applied with crop productivity using caipira lettuce (Lactuca sativa). Three systems were compared: a conventional flow-through system (FTS), a biofloc system (BFT), and a biofloc-based aquaponics system (BAPs). The BAPs improved fish growth and feed efficiency compared to the FTS, while also maintaining stable water quality. Additionally, crops grown in BAPs-cat showed comparable growth to those in hydroponics, suggesting strong synergy between fish and plant production. Although some root degradation occurred in BAPs-eel due to water characteristics, the system still supported healthy fish growth. These findings highlight FLOCponics as a promising sustainable method for indoor aquaculture and crop production, offering environmental and economic benefits.
Abstract
In this study, we sought to improve the productivity of Far Eastern catfish (Silurus asotus) and tropical eel (Anguilla bicolor), which are high-value fish species in the Republic of Korea, as well as that of associated crops by applying biofloc technology (BFT)-based aquaponics systems. The following three systems were used: the flow-through system (FTS), BFT, and BFT aquaponics system (BAPs). Caipira lettuce (Lactuca sativa) was utilized and hydroponics (HP) was implemented to compare crop productivity. After 42 days of treatment, the BAPs and BFT systems improved fish productivity, with weight gain rates of 134.47 ± 1.80% in BAPs-cat, 130.38 ± 0.95% in BFT, and 114.21 ± 6.62% in FTS for S. asotus, and 70.61 ± 3.26% in BAPs-eel, 62.37 ± 7.04% in BFT, and 47.83 ± 1.09% in FTS for A. bicolor. During the experiment, the total ammonia nitrogen and NO2−-N concentrations were stable in all plots. In the case of NO3−-N, BFT showed an increasing tendency while both BAPs showed a decrease compared with that of the BFT. BAPs-cat (total weight: 224.1 ± 6.37 g) and HP (220.3 ± 7.17 g) resulted in similar growth. However, in BAPs-eel was 187.7 ± 3.46 g due to root degradation. Water content analysis showed that BAPs-cat and BAPs-eel contained sufficient K, Ca, P, and S, which are important for crop growth. Overall, the effect of BAPs on fish growth was higher than that of FTS. This study reveals that integrating BFT with aquaponics improves productivity for high-value fish and associated crops while maintaining stable water quality. This method offers sustainable, efficient production, reduces environmental impact, and provides insights for future research in sustainable aquaculture practices.
1. Introduction
The aquaculture industry is influenced by the natural environment and uses large amounts of water []. Aquaculture farms are affected by environmental problems such as sewage, poor water quality, infections, and antibiotics overuse. Therefore, the demand for sustainable aquaculture systems is high. In particular, biofloc technology (BFT) and recirculating aquaculture systems (RASs) can reduce sewage by reusing rearing water [,,]. Furthermore, BFT and RAS can decrease energy wastage and environmental problems caused by closed and indoor farming by removing nitrogen components (ammonia and nitrite) using a biological filtering system [].
In a BFT, microorganisms (bioflocs) can reduce ammonia via heterotrophic bacteria dominance by controlling the carbon/nitrogen (C/N) ratio for ammonification []. Bioflocs can be used as additional feed for culturing species [], thereby improving their immune system and growth [,,]. However, low water exchange cannot reduce nitrate (NO3−), and floc overproduction has undesirable consequences, such as respiratory depression and rapid pH degradation, for species culturation [,,] Recently, these problems have been addressed using aquaponics [,].
Aquaponic systems combine aquaculture and hydroponic systems []. Integrating the production of fish and plants is a currently expected practice. Aquaponics is an eco-friendly technology that primarily uses RAS combined with hydroponic systems and nutrients generated during fish rearing to grow crops [,,,]. Aquaponic systems have a substantial potential for producing proteins and crops for future industries. However, aquaponic systems have some limitations, including deficiencies in Ca, K, and Fe, which should be supplemented artificially []. Typically, 1 kg of fish can produce 20 individuals. Aquaponics primarily emphasizes plant rather than fish production due to the rapid harvesting of plants [,].
Generally, aquaponic systems operate as RAS. RAS with a coupled system has been shown to result in low nutrient concentrations [] and crop productivity. To increase nutrient concentrations, decoupled aquaponic systems is recommended. However, decoupled aquaponic systems require more equipment and space for sump installation.
Recently, aquaponics based on BFT, also known as FLOCponics, has been developed and recommended to overcome the above issues [,]. BFT can effectively and quickly remove nitrogen compounds from feed debris and excrement using heterotrophic microorganisms with a high carbon/nitrogen (C/N) ratio to add carbon sources with low water exchange [,,]. BFT also saves warming energy and blocks the inflow of diseases and parasites due to minimal rearing water exchange []. Carp, catfish, tilapia, and eel have been reportedly cultured in BFT []. BFT rearing water provides a source of nutrients for growing plants []. Electrical conductivity (EC) and nitrate (NO3−) are important indicators of plant growth due to the abundant organic matter in plants [,,]. BFT is expected to serve as an additional biofilter in aquaponics because it provides high levels of EC and NO3− for crop production [,,].
This study aimed to determine the effect of applying BFT-based aquaponics to two types of fish, the Far Eastern catfish (Silurus asotus) and tropical eel (Anguilla bicolor), along with hydroponically grown caipira lettuce (Lactuca sativa). It also analyzes the elemental composition of rearing water to demonstrate the superiority of BFT water.
2. Materials and Methods
2.1. Systems
The experiment ran for 86 days (inoculation = 30 days, seedlings = 14 days, = fish and crop rearing = 42 days). Indoor catfish (cat)- and eel-rearing systems were implemented and classified into three types (Figure 1C,D): BFT, BFT with aquaponic systems (BAPs), and flow-through systems [FTSs (control): two rotations of water change/day (at 11:30 and 17:30)]. Two major types of aquaponic systems are available: deep water culture (DWC) and the nutrient film technique []. In this study, crop cultivation systems were combined with a DWC-coupled aquaponics system, which also included a hydroponics (HP) system as a control plot for crops produced using BAPs. The experiments consisted of 18 fiber-reinforced plastic (FRP) tanks (diameter 1.2 m × height 1.0 m) (in triplicate each for BFT-cat, BFT-eel, BAPs-cat, BAPs-eel, FTS-cat, and FTS-eel) for rearing fish and 9 crop FRP beds (width 2.0 m × length 2.0 m × height 0.8 m) (in triplicate each for BAPs and HP) for crop cultivation (Figure 1A,B).
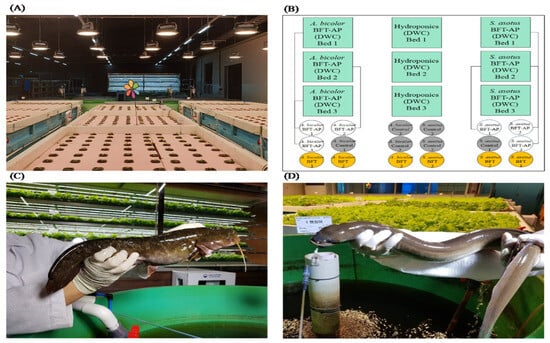
Figure 1.
Preview and system view of experiments for Far Eastern catfish (Silurus asotus) and tropical eel (Anguilla bicolor). (A) Overview of experimental fish tank with crop beds; (B) ichnography of the BFT-based aquaponics system, Green: Plants bed, White: BAPs-, Yellow: BFT, Grey: Control; (C) Far Eastern catfish (S. asotus); (D) tropical eel (A. bicolor).
2.2. Rearing and Water Management
BFT rearing was performed as described by former research []. Briefly, 20 t of water was inoculated by adding BFT-ST (Bacteria seed, EcoTech Service, Gimpo-si, Republic of Korea) at 100 ppm, along with commercial feed (crude protein: 44%, Sajo Dong-A One, Seoul, Republic of Korea) as a nitrogen source, at a rate of 5 kg/day for approximately 1 month. By maintaining a C/N ratio of 15:1, refs. [,] calculated the carbon demand by adding molasses (Carbon: 50.08%). Water temperature was maintained at approximately 25.0 °C using a 1 kW heater (OKE-HE-100, SEWON OKE, Suwon, Republic of Korea). At the end of inoculation, the TAN was 0.482 mg/L, NO2−-N was 0.683, NO3−-N was 88 mg/L, and the Imhoff cone measurement was 15.5 mL/L (Figure 2). After inoculation (when the TAN and NO2−-N reached the maximum values and decreased to approximately 1 mg/L), the fish were acclimated by feeding for 10 days to start the experiment with 20% of rearing freshwater.
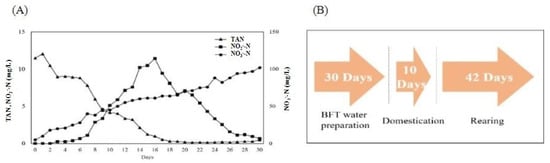
Figure 2.
Nitrogen component variations of the BFT inoculating period (A) and total experimental process and days (Arrow indicate the order of the experiment. First, 30 days; BFT water preparation, Second, 10 days; Domestication, Final, 42 days; Rearing) (B).
After acclimation, the fish were grouped, and water was distributed to each fish tank and plant bed. During the rearing period, the C/N ratio of the BFT and BAPs was maintained at 15:1. The BFT and BAPs were strongly aerated to prevent the settlement of bioflocs using an air blower (Hi-blow HP-80, Techno Takatsuki Co., Ltd., Osaka, Japan) and a water pump (25 W; Hyupsin, Seoul, Republic of Korea). An oxygen supply machine (KMOS-40Rl, Kumho Marine, Busan, Republic of Korea) was used to supply sufficient oxygen for FTS. Each crop bed was weakly aerated to prevent floc deposition on crop roots. Additionally, the BAPs were installed with drainage pipes to provide water to the crop beds, and the water quantity was set to two cycles (input volume from the fish tank) per day. No water changes were made in the BFT and BAPs.
2.3. FLOCponics and Hydroponics
The crop cultivation systems were divided into three types (BAPs-catfish, BAPs-eel, and HP). Each type included triplicate setups. All crop beds were subjected to DWC and comprised 90 pots per bed to cultivate the crops and a coupled system, as described by []. Artificial lighting was installed for indoor crop cultivation, and the 12 L:12 D conditions adopted >6000 lx (daily average) for photosynthesis. HP was conducted as a control for crop cultivation and commercial artificial nutrient solutions (Liquid A: N 2%, K 3.5%, Ca 2%, Fe 0.05%; Liquid B: 1.3%, P 1.5%, K, 5%, Mg 0.7%, B 0.05%, Mn 0.01%, Zn 0.002%) (Mulfuresiriz, Daeyu Business Limited, Seoul, Republic of Korea) were used at 1000 ppm by manual.
Caipira lettuce was selected for crop cultivation. Lettuce is typically grown in cool environments. Caipira lettuce seeds (Enza Zaden, Enkhuizen, The Netherlands) grafted onto terra-plugs (Smithers-Oasis Co., Ltd., Kent, OH, USA) were germinated in a germination chamber (indoor temperature; 24 °C) at the Advanced Aquaculture Research Center. After 14 days of germination, the seedlings reached the true-leaf stage (4 days post-germination), and 90 seedlings (2.0 ± 0.47 g) were transplanted per bed.
2.4. Fish
A total density of 5.75 ± 0.005 kg (5 kg/m2) for Far Eastern catfish (mean body weight: 61.6 ± 5.91 g) and tropical eel (mean body weight: 83.8 ± 6.89 g) was used for the experiment. The fish were acclimated and cultured at the Advanced Aquaculture Research Center, Changwon, Republic of Korea, until maximum crop growth was reached (approximately 30 days after transplanting) [].
The commercial feed (extruded pellets) used was obtained from Sajo Dong-A One and contained 44% crude protein (CP), crude lipid, 1% calcium, 14% moisture, 1.8% phosphate, 5% crude fiber, and 17% ash. Generally, eels consume high-protein feed (over 50% CP), but both fish species were given the same feed to ensure the analysis of the breeding water contents was not influenced. Feed was provided to full satiety for Far Eastern catfish (approximately 5% of total weight/day) and tropical eels (approximately 3% of total weight/day) thrice daily (09:30, 13:00, and 16:30) at the concentration of each biomass for 6 weeks. Molasses (carbon source) was added to the final feeding after 1 h at 17:30. Water temperature was maintained at approximately 25.0 °C using a 1-kW heater (OKE-HE-100, Sewon OKE, Suwon, Republic of Korea).
2.5. Growth Performance and Production of Fish and Crops
At the conclusion of the 4th week of the experiment, the growth performance and production metrics of each experimental group of fish and crops were evaluated.
The water conditions in the tanks were assessed using a YSI-650 Multiparameter Display System (YSI Incorporated, Yellow Springs, OH, USA) for the following parameters: temperature (°C), dissolved oxygen (DO, mg/L), pH, EC (dS/cm), and total dissolved solids (TDS, mg/L). The breeding water in all plots was sampled daily at AM 09:30 before feeding. The pH was controlled using sodium bicarbonate when the pH was lower than 6.5. The nitrogen components, including total ammonia nitrogen (TAN), nitrite nitrogen (NO2−-N), and nitrate nitrogen (NO3−-N), were measured using an analysis kit (Merck KGaA, Darmstadt, Germany) and an absorptiometer (Merck KGaA), employing the three dust spot methods. At the end of the experiment, rearing water from Far Eastern catfish, tropical eel, and the HP system was preserved at −80 °C. Subsequently, 500 mL of rearing water was analyzed (mixed with each plot bed). The analysis included the total nitrogen measurement using a Kjeltec 2300 nitrogen analyzer (FOSS Tecator, Hillerød, Denmark), the total phosphate using a UV2450 spectrophotometer (Shimadzu, Kyoto, Japan), and K, Ca, Mg, Fe, Cu, Zn, and Si concentrations using an Optima 8300 inductively coupled plasma optical emission spectrometer (PerkinElmer. Waltham, MA, USA). Cl and SO4 were analyzed using ion chromatography (930 Compact IC Flex; Metrohm Co., Herisan, Switzerland).
The growth performance and production of each experimental fish and crop group were measured at the end of the study period. Thirty fishes were randomly sampled from each tank and anesthetized using 100 ppm MS-222 (Sigma Aldrich, St. Louis, MO, USA) to measure their body weight and total length. Growth performance factors were calculated using the following equations:
Weight gain rate (WGR, %) = (Final weight − Initial weight)/(Initial weight) × 100
Specific growth rate (SGR, %/day) = [ln (final body weight) − ln (initial body weight)]/day × 100
Feed conversion ratio (FCR) = (supplied feed)/(final weight − initial weight)
Survival rate (%) = (Final individuals/initial individuals) × 100
After 6 weeks, 10 crops from each bed were randomly sampled from each experimental group, and the total weight and length, shoot weight and length, leaf length and width, and number of leaves were recorded.
An electronic scale (MW-200; CAS, Seoul, Republic of Korea) was used to measure body weight to an accuracy of 0.01 g, and Vernier calipers (Mitutoyo Electronic, Kawasaki, Japan) were used to measure the total length with a precision of 0.1 mm.
2.6. Stress Parameters
Serum stress parameters indicate the health of freshwater fish []. Fish were randomly sampled from each experimental group, anesthetized using 100 ppm MS-222 (Sigma Aldrich), and blood was drawn from the caudal vein using 20 IU/mL heparin (Sterile Solution HEPARIN Inj.; Choongwae Pharma Corporation, Seoul, Republic of Korea)-treated syringes (3 mL; Dong Shin Medical Instruments Co., Miryang, Republic of Korea). Thereafter, the samples were centrifuged at 2339× g and 4 °C for 15 min (Smart R17 Plus; Hanil Co., Dangjin, Republic of Korea) to obtain plasma, which was stored at −80 °C until use. Subsequently, plasma glucose (GLU), glutamate–pyruvate transaminase (GPT), and glutamate–oxaloacetate transaminase (GOT) levels were analyzed using an automatic dry chemistry analyzer (Fuji Dri-Chem NX600V, Fujifilm Corporation, Tokyo, Japan), and cortisol levels were determined using an ELISA kit (Mybiosource, San Diego, CA, USA).
2.7. Statistical Analysis
Fish growth performance and crop production were analyzed using one-way analysis of variance in SPSS (version 22.0; SPSS Inc., Chicago, IL, USA), and differences between treatment groups were assessed via Tukey’s test. p < 0.05 was considered statistically significant.
3. Results
3.1. Water Quality
The water conditions in each fish tank were consistently maintained throughout the experiment. The water parameters for the BAPs-cat, BFT-cat, and FTS experimental groups were as follows: temperature (°C) = 25.19 ± 0.53, 25.70 ± 0.42, and 25.76 ± 0.42; DO (mg/L) = 11.44 ± 1.51, 11.16 ± 1.39, and 11.12 ± 0.64 mg/L; pH = 7.15 ± 0.20, 7.25 ± 0.30, and 7.09 ± 0.27; EC (dS/cm) = 0.78 ± 0.05, 1.30 ± 0.21, and 0.20 ± 0.04; TDS (mg/L) = 0.39 ± 0.02, 0.66 ± 0.11, and 0.10 ± 0.01, respectively. The BAPs-eel, BFT-eel, and FTS experimental groups were as follows: temperature (°C) = 25.55 ± 0.45, 25.67 ± 0.43, and 25.21 ± 1.91; DO (mg/L) = 11.29 ± 1.86, 11.36 ± 1.09, and 11.13 ± 0.85 mg/L; pH = 7.04 ± 0.25, 6.48 ± 0.84, and 7.19 ± 0.11; EC (dS/cm) = 1.53 ± 0.02, 1.86 ± 0.26, and 0.25 ± 0.02; TDS (mg/L) = 0.77 ± 0.01, 0.94 ± 0.13, and 0.12 ± 0.02, respectively (Table 1).

Table 1.
Water condition of S. asotus and A. bicolor in BAPs, BFT, and FTS for 6 weeks.
The nitrogen parameters for the BAPs-cat, BFT-cat, and FTS experimental groups were as follows: TAN = 0.75 ± 0.353, 1.26 ± 0.970, and 0.17 ± 0.011 mg/L and NO2−-N = 0.50 ± 0.160, 0.44 ± 0.254, 0.13 ± 0.042 mg/L, respectively. The parameters were stabilized at an average of approximately 1 mg/L during the experiment (Figure 3A,C). The NO3−-N levels were highest in BFT (day 28, 100.10 ± 8.480 mg/L), followed by BAPs (day 6, 63.12 ± 5.500 mg/L) and the control (day 16, 7.11 ± 1.220 mg/L). In BAPs, the NO3−-N levels decreased after day 6 (Figure 3E). The BAPs-eel, BFT-eel, and FTS experimental groups were as follows: TAN = 0.58 ± 0.259, 0.57 ± 0.286, and 0.20 ± 0.049 mg/L and NO2−-N = 0.39 ± 0.184, 0.36 ± 0.175, 0.06 ± 0.017 mg/L, respectively. The parameters were stabilized at an average below 1 mg/L during the experiment (Figure 3B,D). NO3−-N levels were highest in BFT (day 28, 139.48 ± 8.480 mg/L), followed by BAPs (day 6, 66.54 ± 5.540 mg/L) and the control (day 16, 7.66 ± 1.220 mg/L) (Figure 3F). In BAPs, NO3−-N levels decreased after day 6 but increased in BFT, similar to catfish.
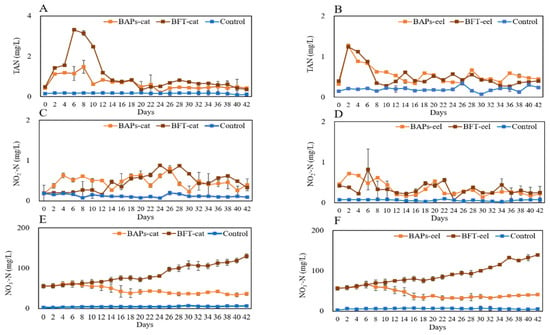
Figure 3.
Water quality variations of BAPs, BFT, and FTS systems containing catfish and eel for 6 weeks ((A,C,E) for catfish, (B,D,F) for eel).
3.2. Fish Growth
Both catfish and eels showed higher growth in BAPs and BFT than in FTS. The growth performance of catfish is presented in Table 2. The tank final weight, WGR, FCR, and SGR were higher in the BAPs group (13.48 ± 0.10 kg, 83.83% ± 1.55%, 1.04 ± 0.01, and 2.95 ± 0.12%/day, respectively), followed by the BFT (13.25 ± 0.06 kg, 78.66 ± 0.84%, 1.07 ± 0.01, and 2.94 ± 0.02%/day, respectively) and control (12.32 ± 0.38 kg, 72.72 ± 5.03%, 0.23 ± 0.07, 2.78 ± 0.14%/day) groups (Tukey’s test, p < 0.05).

Table 2.
Growth performance of S. asotus and A. bicolor in BAPs, BFT, and FTS for 6 weeks.
The growth performance of the eels showed a similar tendency. The tank final weight, WGR, FCR, and SGR were higher in the BAPs group (9.81 ± 0.19 kg, 70.61% ± 3.26%, 1.33 ± 0.06, and 1.82 ± 0.03%/day, respectively), followed by the BFT (9.34 ± 0.41 kg, 62.38 ± 7.04%, 1.52 ± 0.18, and 1.62 ± 0.06%/day, respectively) and control (8.50 ± 0.06 kg, 47.83 ± 1.09%, 1.96 ± 0.04, 1.46 ± 0.10%/day) groups (Tukey’s test, p < 0.05). The 100% survival rate is shown in each plot (Table 2).
3.3. Crop Growth
Figure 4 presents an overview at the conclusion of the rearing experiment. The total length (557.03 ± 8.33 mm) and weight (187.73 ± 3.63 g) of caipira lettuce in BAPs-eel were lower than those in the other two systems due to root degradation (BAPs-cat, 594.07 ± 19.53 mm, 224.11 ± 6.37 g; HP, 596.15 ± 52.62 mm, 220.28 ± 7.17 g, respectively).

Figure 4.
Overview of caipira lettuce in BAPs-cat, BAPs-eel, and HP (Control) beds 6 weeks after planting seedlings (A) and crop view of BAPs-cat, BAPs-eel, and HP (Control) (B).
However, the other parameters were similar for all three systems, as follows: shoot length: BAPs-cat, 219.97 ± 6.20 mm; BAPs-eel, 219.48 ± 10.20 mm; HP, 215.48 ± 11.33 mm; shoot weight: BAPs-cat, 186.28 ± 6.02 g; BAPs-eel, 182.12 ± 3.33 g; HP, 186.13 ± 7.24 g; leaf width: BAPs-cat, 211.88 ± 9.89 mm, BAPs-eel, 212.08 ± 6.44 mm, HP, 209.22 ± 12.53 mm; leaf length: BAPs-cat, 157.45 ± 12.42 mm, BAPs-eel, 156.09 ± 15.27 mm, HP, 155.01 ± 21.83 mm; number of leaves: BAPs-cat, 26 ± 1; BAPs-eel, 27 ± 2; HP, 26 ± 1 (p ≥ 0.05) (Table 3). However, except for the total weight and length in BAPs-eel, these parameters showed that the caipira lettuce production in the two BAPs systems was similar to that in the HP system.

Table 3.
Comparison of caipira lettuce (L. sativa) in BAPs-cat, BAPs-eel, and hydroponics (HP) for 6 weeks.
3.4. Stress Analysis
The hematologic responses of catfish and eel to each method are presented in Table 4. Hematologic and serum parameters indicate the health and nutritional status of organisms []. The AST/GOT, ALT/GPT, and GLU levels did not significantly differ between the systems. Additionally, catfish showed higher AST/GOT and ALT/GPT ratios than eels but higher GLU than eels.

Table 4.
Plasma chemistry parameters of S. asotus and A. bicolor in BAPs, BFT, and FTS for 6 weeks.
3.5. Rearing Water Content Composition
K, Ca, P, and S are the most important mineral components for plant growth []. K was highest in HP (61.01 mg/L), followed by BFT-eel (30.75 mg/L), BFT-catfish (20.48 mg/L), BAPs-eel (14.11 mg/L), BAPs-cat (13.43 mg/L), FTS-cat (2.43 mg/L), and FTS-eel (2.32 mg/L). Ca was highest in BFT-eel (121.85 mg/L), followed by BFT-cat (117.33 mg/L), HP (114.85 mg/L), BAPs-eel (51.04 mg/L), BAPs-cat (47.33 mg/L), FTS-eel (31.26 mg/L), and FTS-cat (24.54 mg/L). P was highest in HP (28.13 mg/L), followed by BFT-eel (25.93 mg/L), BAPs-eel (18.13 mg/L), BFT-cat (15.21 mg/L), BAPs-cat (8.07 mg/L), FTS-eel (4.40 mg/L), and FTS-cat (3.47 mg/L). S was highest in BFT-eel (131.19 mg/L), followed by HP (124.11 mg/L), BFT-cat (111.45 mg/L), BAPs-eel (75.16 mg/L), BAPs-cat (51.45 mg/L), FTS-cat (12.05 mg/L), and FTS-eel (11.64 mg/L) (Table 5).

Table 5.
Water content analysis of BAPs-cat, BAPs-eel, and hydroponics for 6 weeks.
Notably, Na was higher in BFT-eel (108.05 mg/L) and BAPs-eel (75.95 mg/L) than in the other systems. Other minerals were highest in HP, followed by BFT, BAPs, and FTS.
4. Discussion
Water can be used to create suitable growth environments for fish and vegetable cultivation []. While a C/N ratio of 15:1 or more in BFT water can facilitate the dominance of heterotrophic bacteria (Bacillus sp., Cellulomonas sp.), a C/N ratio under 15:1 could facilitate the dominance of autotrophic bacteria (Nitrosomonas sp. Nitrobacter sp.) [,].
Over the 30-day inoculation period, fluctuations were observed with increases and decreases in TAN and NO2−-N, along with an increase in NO3−-N. These concentration levels indicated that feeds could sufficiently sustain bacterial growth and activity.
Ammonia decomposition occurs through three processes: autotrophic decomposition by phytoplankton, nitrification by autotrophic bacteria, and ammonification by heterotrophic bacteria [,,]. Over the 42-day rearing period, the TAN levels in both BFT and BAPs increased during the first week but gradually diminished and stabilized under 1 mg/L by the end of the experiment.
Heterotrophic bacteria dominance in the BAPs and BFT increased, and flocs were formed in the early phase, indicating that in the first week, bacteria were less abundant than the input from fish. The feces of rearing fish were released into BFT water, enabling heterotrophic bacteria proliferation with a decrease in TAN []. The input of feed and molasses to maintain the C/N ratio could provide adequate conditions for fish rearing. Rearing water with a stable quality results in low fish mortality []. When the pH is low, high NO2−-N concentrations damage the fish, resulting in high mortality. However, in the present study, high NO2−-N concentrations were not observed (<1 mg/L). In the case of NO3−-N, FTS showed low concentrations while BFT showed increased concentrations until the end of the experiment. In contrast, in both BAPs-cat and BAPs-eel, the NO3−-N concentrations decreased after 1 week of rearing. NO3− is a source of nutrients for crops []. Therefore, the decreasing tendency of NO3−-N in BAPs than in BFT indicates that BFT has high nutrient concentrations. This suggests that crops can be used as biological filter tools for nitrogen [].
The growth performance of catfish and eel in both BAPs and BFT was superior to that in FTS. According to previous studies, BFT positively affects feed efficiency in African catfish (Clarias gariepinus) [], European eel (A. anguilla) [], and other species []. Biofloc provides an additional source of nutrients for fish [] by decomposing ammonia into amino acids using carbon sources (ammonification) and partially decomposing nitrogen components through nitrification []. The current study showed that 1 kg of feed was conserved by BAPs and BFT compared with that in FTS, and the catfish culture in BAPs and BFT saved more energy than did the eel culture []. All tanks showed > 90% survival rates, with most catfish being of similar size. However, some abnormally growing fish appeared, leading to incidents of cannibalism. No cannibalism was observed in the eel plots. Reducing cannibalism in catfish culture in BAPs and BFT could result in better productivity relative to FTS.
At the end of the experiment, similar growth was observed in BAPs-cat, BAPs-eel, and caipira lettuce HP. The number of edible portions (shoots, i.e., leaves and stems) was similar in each plot. However, BAPs-eel had the lowest total plant weight and length among the three systems. Compared with those in the other two plots, no damaged individuals were observed in the BAPs-catfish and HP plots, but the roots were damaged and shortened in the BAPs-eel plot. Roots allow plants to absorb minerals and nutrients, with their absorbance capacity depending on the root surface area []. In aquaponics based on BFT, root degradation is caused by the amounts of flocs and solids [].
In this study, decoupled FLOCponics with solid removal and flow rate control is recommended in BAPs-eel, and reducing the flow rate can reduce the high floc and solid concentrations in the plant bed. However, lower flow rates cause clogging of the pipes by flocs and solids. Our results showed that only one system cannot be applied to all fish species, and it would be better to supplement the characterized system to increase economic feasibility depending on the fish species.
The causes of stress are distinguished based on the physical, chemical, and biological effects of external and internal factors []. GOT, GPT, and GLU are functional health and metabolic biomarkers in aquatic animals []. In the present study, the concentrations of AST/GOT, ALT/GPT, GLU, and cortisol in all fish and systems did not significantly differ (p > 0.05). Cortisol concentration is related to many stress processes, metabolism, osmoregulation, and feeding behavior [,].
Regardless of how well designed current systems are, they should cause less stress and provide better welfare for cultured species than the old methods. A previous study showed that ALT/GPT (U/L) was 6.3 ± 0.5, AST/GPT (U/L) was 153.7 ± 16.2, and GLU (mg/dL) was 67.7 ± 29.0 in S. asotus []. Japanese eel (A. japonica) showed ALT/GPT (U/L), AST/GOT (U/L), and GLU (mg/dL) of <10 (U/L), 105.6 ± 38.3 (U/L) and 164.8 ± 45.7 (mg/dL), respectively, healthy A. japonica in freshwater culture [].
The stress parameters and survival rates showed that BAPs and BFT provided adequate and suitable conditions for rearing S. asotus and A. bicolor. However, as the rearing period increased, the size of the fish gradually increased, and the food supply increased accordingly. This can lead to higher nutrient concentrations in BFT water over time, resulting in an inappropriate environment for fish and crops. Therefore, as the rearing period increases, modifying and maintaining a high producible density and continuously monitoring water quality and mineral component variations are important.
Generally, RAS-based aquaponics produces adequate amounts of NO3− to meet plant requirements. Nevertheless, most aquaponic systems undergo a lack of K, Ca, P, and S. Additionally, RAS-based aquaponics requires high concentrations of artificial nutrient solutions, resulting in eutrophication due to sewage from aquaponic beds []. In a BFT system, the addition of organic carbon sources is vital for maintaining the C/N ratio. BFT water can be produced using feed and molasses; other carbon sources, such as glucose and glycerol, are more expensive than molasses. Minerals in molasses comprise K (1.960%), Na (0.120%), Ca (0.52%), Mg (0.20%), P (0.78%), and Fe (0.02%). This enriched nutrient concentration in BFT water could replace artificial nutrient solutions [,].
According to Yang and Kim, 2020 [], the important element K, P, and S were found to be higher compared to this study, with concentrations of 75.2 mg/L, 30.1 mg/L, and 242.3 mg/L, respectively, whereas Ca was lower at 18.4 mg/L. The deficiency of K, Ca, P, and S in plantation systems results in poor root and leaf growth, chlorosis, and increased susceptibility to diseases []. In the present study, the water content in BAPs-eel was adequate and higher than that in BAPs-cat. Nevertheless, the lettuce in the BAPs-eel system showed damaged and shortened roots compared with those in the other systems.
During the rearing period, the pH in BAPs-eel and BFT-eel decreased faster than that in BAPs-cat and BFT-cat. The low pH in eel systems requires more sodium bicarbonate to increase the pH. This leads to high Na concentrations in BAPs-eel and BFT-eel. The lettuce in BAPs-eel was influenced by this Na concentration, resulting in shortened roots [,].
Therefore, in eel aquaponics, conducting further research on optimal stocking density and water quality management is necessary to reduce the use of sodium bicarbonate to control Na concentrations. Despite the lower content of BAPs-cat compared to BAPs-eel, S. asotus is more adaptable than A. bicolor for crop growth in BAPs with this experimental design. These results indicate that combining fish species and crops can influence BAPs productivity. The most important aspects of aquaponics are its high productivity, lack of fish and crop deterioration, and system sustainability.
For example, fish cultured in BFT with high mineral concentrations would be well suited for nutrient-demanding crops such as basil (Ocinum basilicum) [] and cherry tomato (Solanum lycopersicum) []. BAPs-eel resulted in a higher concentration and degradation of roots than BAPs-cat. To enhance the productivity and economic feasibility of A. bicolor, reforms in BAPs-eel should include increasing the number of crops and crop beds, lowering eel density, or adopting decoupled FLOCponics to improve the mineral absorbing capacity and decrease root degradation.
Water pollution and scarcity and agricultural land degradation have increased []. Aquaponic systems can be applied to several crops and fish species to solve water availability problems caused by radical changes in the global environment and climate. Although our results show that BAPs could give adequate for fish and crop growth, further studies are necessary to refine the operations. Achieving sustainable culturing will be possible by determining the optimal combination of composition, density, and environment through studies of crop and fish species, as well as gut and water microbiota.
5. Conclusions
FLOCponics, which integrates biofloc technology with aquaponics, significantly enhanced the growth of fish and the productivity of lettuce. Compared to traditional systems, it provided superior water quality, nutrient availability, and feed conversion efficiency. While the BAPs-cat system was successful, root degradation in the BAPs-eel system indicated that species-specific adjustments are necessary. The study concludes that FLOCponics is a viable and eco-friendly alternative for sustainable food production, recommending future research to focus on system refinement and species compatibility.
Author Contributions
Conceptualization, J.-a.H.; Methodology, J.S.P. and H.S.J.; Validation H.S.J.; Formal Analysis, J.S.P. and H.S.J.; Investigation, J.-a.H.; Resources, J.-a.H.; Writing—Original Draft Preparation, J.-a.H. and J.S.P.; Writing—Review and Editing, J.-a.H. and J.S.P.; Visualization, J.S.P.; Supervision, J.-a.H.; Project administration, J.-a.H. and J.-h.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Institute of Fisheries Science, Ministry of Oceans and Fisheries, Republic of Korea (grant number R2025033).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee (IACUC, NIFS-2022-5).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge the financial support that was generously provided by National Institute of Fisheries Science, Ministry of Oceans and Fisheries, Republic of Korea.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Buhmann, A.K.; Waller, U.; Wecker, B.; Papenbrock, J. Optimization of culturing conditions and selection of species for the use of halophytes as biofilter for nutrient-rich saline water. Agric. Water Manag. 2015, 149, 102–114. [Google Scholar] [CrossRef]
- van Rijn, J. The potential for integrated biological treatment systems in recirculating fish culture—A review. Aquaculture 1996, 139, 181–201. [Google Scholar] [CrossRef]
- Crab, R.; Kochva, M.; Verstraete, W.; Avnimelech, Y. Bio-flocs technology application in over-wintering of tilapia. Aquac. Eng. 2009, 40, 105–112. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology—A Practical Guide Book, 3rd ed.; The World Aquaculture Society: Baton Rouge, LA, USA, 2012. [Google Scholar]
- Avnimelech, Y. Bio-filters: The need for a new comprehensive approach. Aquac. Eng. 2006, 34, 172–178. [Google Scholar] [CrossRef]
- Avnimelech, Y. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 1999, 176, 227–235. [Google Scholar] [CrossRef]
- Hargreaves, J.A. Photosynthetic suspended-growth systems in aquaculture. Aquac. Eng. 2006, 34, 344–363. [Google Scholar] [CrossRef]
- Kim, S.K.; Pang, Z.; Seo, H.C.; Cho, Y.R.; Samocha, T.; Jang, I.K. Effect of bioflocs on growth and immune activity of Pacific white shrimp, Litopenaeus vannamei postlarvae. Aquacul. Res. 2014, 45, 362–371. [Google Scholar] [CrossRef]
- Longo, S.B.; Clark, B.; York, R.; Jorgenson, A.K. Aquaculture and displacement of fisheries captures. Conserv. Biol. 2019, 33, 832–841. [Google Scholar] [CrossRef]
- Tong, R.; Chen, W.; Pan, L.; Zhang, K. Effects of feeding level and C/N ratio on water quality, growth performance, immune and antioxidant status of Litopenaeus vannamei in zero-water exchange bioflocs-based outdoor soil culture ponds. Fish Shellfish Immunol. 2020, 101, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Crab, R.; Defoirdt, T.; Bossier, P.; Verstraete, W. Biofloc technology in aquaculture: Beneficial effects and future challenges. Aquaculture. 2012, 356, 351–356. [Google Scholar] [CrossRef]
- Bossier, P.; Ekasari, J. Biofloc technology application in aquaculture to support sustainable development goals. Microb. Biotechnol. 2017, 10, 1012–1016. [Google Scholar] [CrossRef]
- Knaus, U.; Pribbernow, M.; Xu, L.; Appelbaum, S.; Palm, H.W. Basil (Ocimum basilicum) Cultivation in Decoupled Aquaponics with Three Hydro-Components (Grow Pipes, Raft, Gravel) and African Catfish (Clarias gariepinus) Production in Northern Germany. Sustainability 2020, 12, 8745. [Google Scholar] [CrossRef]
- Pinho, S.M.; de Lima, J.P.; David, L.H.; Emerenciano, M.G.C.; Goddek, S.; Verdegem, M.C.J.; Keesman, K.J.; Portella, M.C. FLOCponics: The integration of biofloc technology with plant production. Rev. Aquac. 2021, 14, 647–675. [Google Scholar] [CrossRef]
- Rakocy, J.E. Ten guidelines for aquaponics systems. Aquaponics J. 2007, 46, 14–17. [Google Scholar]
- Knaus, U.; Palm, H.W. Effects of the fish species choice on vegetables in aquaponics under spring summer conditions in northern Germany (Mecklenburg Western Pomerania). Aquaculture 2017, 473, 62–73. [Google Scholar] [CrossRef]
- Farrant, D.N.; Frank, K.L.; Larsen, A.E. Reuse and recycle: Integrating aquaculture and agricultural systems to increase production and reduce nutrient pollution. Sci. Total Envrion. 2021, 785, 146859. [Google Scholar] [CrossRef]
- David, L.H.; Pinho, S.M.; Agostinho, F.; Costa, J.I.; Portella, M.C.; Keesman, K.J.; Garcia, F. Sustainability of urban aquaponics farms: An energy point of view. J. Clean. Prod. 2022, 331, 129896. [Google Scholar] [CrossRef]
- dos Santos, M.J.P.L. Smart cities and urban areas-Aquaponics as innovative urban agriculture. Urban For. Urban Green. 2016, 20, 402–406. [Google Scholar] [CrossRef]
- Greenfeld, A.; Becker, N.; McIlwain, J.; Fotedar, R.; Bornman, J. Economically viable aquaponics? Identifying the gap between potential and current uncertainties. Rev. Aquac. 2019, 11, 848–862. [Google Scholar] [CrossRef]
- Aslanidou, M.; Elvanidi, A.; Mourantian, A.; Levizou, E.; Mente, E.; Katsoulas, N. Evaluation of productivity and efficiency of a large-scale coupled or decoupled aquaponic system. Sci. Hortic. 2024, 337, 113552. [Google Scholar] [CrossRef]
- Emerenciano, M.G.C.; Gaxiola, G.; Cuzon, G. Biofloc Technology (BFT): A Review for Aquaculture Application and Animal Food Industry. Biomass Now-Cultiv. Util. 2013, 12, 301–328. [Google Scholar]
- Vyas, A. Chapter 3—Biofloc systems in aquaculture: Global status and trends. In New and Future Developments in Microbial Biotechnology and Bioengineering Trends of Microbial Biotechnology for Sustainable Agriculture and Biomedicine Systems: Perspectives for Human Health; Elsevier: Amsterdam, The Netherlands, 2020; pp. 31–42. [Google Scholar]
- Pinho, S.M.; de Lima, J.P.; Tarigan, N.B.; David, L.H.; Portella, M.C.; Keesman, K.J. Modelling FLOCponics systems: Towards improved water and nitrogen use efficiency in biofloc-based fish culture. Biosyst. Eng. 2023, 229, 96–115. [Google Scholar] [CrossRef]
- Kafkafi, U. Root Temperature, Concentration and the Ratio NO3−/NH4+ Effect on Plant Development. J. Plant Nutr. 1990, 13, 1291–1306. [Google Scholar] [CrossRef]
- Macduff, J.H.; Jarvis, S.C.; Larsson, C.M.; Oscarson, P. Plant Growth in Relation to the Supply and Uptake of NO3−: A Comparison Between Relative Addition Rate and External Concentration as Driving Variables. J. Exp. Biol. 1993, 44, 1475–1484. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Status and Trends; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2012; pp. 1–100. [Google Scholar]
- Pinho, S.M.; Molinari, D.; de Mello, G.L.; Fitzsimmons, K.M.; Emerenciano, M.G.C. Effluent from a biofloc technology (BFT) tilapia culture on the aquaponics production of different lettuce varieties. Ecol. Eng. 2017, 103, 146–153. [Google Scholar] [CrossRef]
- Hwang, J.A.; Lee, J.H.; Park, J.S.; Choe, J.R.; Lee, D.; Kim, H. Effect on Eel Anguilla japonica and Crop Growth by the Development of a Biofloc Technology (BFT) Aquaponics System. Kor. J. Fish. Aquat. Sci. 2021, 54, 418–425. [Google Scholar]
- Saseendran, S.; Dube, K.; Chandrakant, M.H.; Rani, A.M.B. Enhanced growth response and stress mitigation of genetically improved farmed Tilapia in a biofloc integrated aquaponic system with bell pepper. Aquaculture 2021, 533, 736200. [Google Scholar] [CrossRef]
- Palm, H.W.; Knaus, U.; Appelbaum, S.; Strauch, S.M.; Kotzen, B. Coupled Aquaponic Systems. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Park, J.S.; Hwang, J.A.; Choe, J.R.; Lee, D.; Kim, H. Enhancing Indoor Culture of Weather Loach (Misgurnus anguillicaudatus) and Caipira Lettuce (Lactuca sativa) in a Decoupled FLOCponics System. Fishes 2024, 9, 150. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.J. Comparisons of nitrogen and phosphorus mass balance for tomato-basil-, and lettuce-based aquaponic and hydroponic systems. J. Clean. Prod. 2020, 274, 122619. [Google Scholar] [CrossRef]
- Blaxhall, P.C. The haematological assessment of the health of freshwater fish. A review of selected literature. J. Fish Biol. 1972, 4, 593–604. [Google Scholar] [CrossRef]
- Sadoul, B.; Vijayan, M.M. Stress and growth. In Biology of Stress in Fish; Fish Physiology; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 167–205. [Google Scholar]
- Jones, B.J., Jr. Complete Guide for Growing Plants Hydroponically, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Wei, Y.; Li, W.; Daoliang, L.; Jiao, Y.; Wei, Q. Equipment and Intelligent Control System in Aquaponics: A Review. IEEE Access 2019, 7, 169306–1693267. [Google Scholar] [CrossRef]
- Padeniya, U.; Davis, D.A.; Wells, D.E.; Bruce, T.J. Microbial Interactions, Growth and Health of Aquatic Species in Biofloc Systems. Water 2022, 14, 4019. [Google Scholar] [CrossRef]
- Suttle, C.A.; Fuhrman, J.A.; Capone, D.G. Rapid ammonium cycling and concentration-dependent partitioning of ammonium and phosphate: Implications for carbon transfer in plankton communities. Limnol. Oceanogr. 1990, 35, 424–433. [Google Scholar] [CrossRef]
- Strock, J.S. Ammonification. In Encyclopedia of Ecology; Academic Press: Cambridge, MA, USA, 2008; pp. 162–165. [Google Scholar]
- Ward, B.B.; Bouskill, N.J. The Utility of Functional Gene Arrays for Assessing Community Composition, Relative Abundance, and Distribution of Ammonia-Oxidizing Bacteria and Archaea. Methods Enzymol. 2011, 496, 373–396. [Google Scholar]
- Kirchman, D.L. The Uptake of Inorganic Nutrients by Heterotrophic Bacteria. Microb. Ecol. 1994, 28, 255–271. [Google Scholar] [CrossRef]
- Jana, B.B.; Sarkar, D. Water quality in aquaculture-Impact and Management: A Review. Indian J. Anim. Sci. 2005, 75, 1354–1361. [Google Scholar]
- Gent, M.P. Solution electrical conductivity and ratio of nitrate to other nutrients affect accumulation of nitrate in hydroponic lettuce. HortScience 2003, 38, 222–227. [Google Scholar] [CrossRef]
- Gebrai, Y.; Ghebremichael, K.; Mihelcic, J.R. A Systems approach to analyzing food, energy and water uses of a multifunctional crop: A review. Sci. Total Environ. 2021, 791, 148254. [Google Scholar] [CrossRef] [PubMed]
- Alinsangao, A.M.; Igano, L.B.; Flores, P.A.M. Efficiency of biofloc system on the growth and survival of African catfish (Clarias gariepinus) fingerlings. J. Agric. Res. Dev. Extens. Technol. 2019, 1, 10–20. [Google Scholar]
- Vinatea, L.; Carbo, R.; Andree, K.B.; Gisbert, E.; Estevez, A. Rearing European Eel (Anguilla anguilla) Elvers in a Biofloc system. Animals 2023, 13, 3234. [Google Scholar] [CrossRef]
- Yu, Y.B.; Lee, J.H.; Choi, J.H.; Choi, Y.J.; Jo, A.H.; Choi, C.Y.; Kang, J.C.; Kim, J.H. The application and future of biofloc technology (BFT) in aquaculture industry: A review. J. Environ. Manag. 2023, 342, 118237. [Google Scholar] [CrossRef]
- Browdy, C.L.; Ray, A.J.; Leffler, J.W.; Avnimelech, Y. Biofloc-based aquaculture systems. In Aquaculture Production Systems; Tidwell, J.H., Ed.; John Wiley & Sons, Inc.: Ames, IA, USA; Oxford, UK, 2012; pp. 278–307. [Google Scholar]
- Baiyin, B.; Tagawa, K.; Yamada, M.; Wang, X.; Yamada, S.; Shao, Y.; An, P.; Yamamoto, S.; Ibraki, Y. Effect of Nutrient Solution Flow-Rate on Hydroponic Plant Growth and Root Morphology. Plants 2021, 10, 1840. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. The Stress of Life; Longmans Green and Co.: Toronto, ON, Canada, 1958; pp. 1–50. [Google Scholar]
- Ramesh, M.; Anitha, S.; Poolpal, R.K.; Shobana, C. Evaluation of acute and sublethal effects of chloroquine (C18H26CIN3) on certain enzymological and histophathological biomarker responses of a freshwater fish Cyprinus carpio. Toxicol. Rep. 2018, 5, 18–27. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.D.; Bradshaw, D. Hormonal control of salt and water balance in vertebrates. Gen. Comp. Endocrinol. 2006, 147, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Jerez-Cepa, I.; Gorissen, M.; Mancera, J.M.; Ruiz-Jarabo, I. What can we learn from glucocorticoid administration in fish? Effects of cortisol and dexamethasone on intermediary metabolism of gilthead seabream (Sparus aurata L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 231, 1–10. [Google Scholar] [CrossRef]
- Amoah, Y.T.; Moniruzzaman, M.; Lee, S.; Bae, J.; Won, S.; Seong, M.; Bai, S.C. Evaluation of different dietary additives based on growth performance, innate immunity and disease resistance in juvenile Amur catfish, Silurus asotus. Int. Aquat. Res. 2017, 9, 351–360. [Google Scholar] [CrossRef]
- Jung, S.H.; Seo, J.S.; Kim, J.D.; Choi, H.S.; Park, M.A. Application of automatic dry chemistry analyzer (FUJI DRI-CHEM 3000) used to hematological analysis of cultured freshwater fish in low temperature season. J. Fish Pathol. 2011, 24, 247–254. [Google Scholar] [CrossRef]
- Mainston, C.P.; Parr, W. Phosphorus in rivers-ecology and management. Sci. Total Environ. 2002, 282–283, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Aslanidou, M.; Elvanidi, A.; Mourantian, A.; Levizou, E.; Mente, E.; Katsoulas, N. Nutrients Use Efficiency in Coupled and Decoupled Aquaponic Systems. Horticulturae 2023, 9, 1077. [Google Scholar] [CrossRef]
- Peterhans, H. Aquaponic Nutrient Model. Master’s Thesis, Biobased Chemistry and Technology, Wageningen University & Research, Wageningen, The Netherlands, 2015. [Google Scholar]
- Goddek, S.; Vermeulen, T. Comparison of Lactuca sativa growth performance in conventional RAS-based hydroponic systems. Aquac. Int. 2018, 26, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Vanacore, L.; El-Nakhel, C.; Modarelli, G.C.; Rouphael, Y.; Pannico, A.; Langellotti, A.L.; Masi, P.; Cirillo, C.; De Pascale, S. Growth, Ecophysiological responses, and Leaf Mineral Composition of Lettuce and Curly Endive in Hydroponic and Aquaponic Systems. Plants 2024, 13, 2852. [Google Scholar] [CrossRef] [PubMed]
- Bordignon, F.; Birolo, M.; Fanizza, C.; Trocino, A.; Zardinoni, G.; Stevanato, P.; Nicoletto, C.; Xiccato, G. Effects of water salinity in an aquaponic system with rainbow trout (Oncorhynchus mykiss), black bullhead catfish (Ameiurus melas), Swiss chard (Beta vulgaris), and cherry tomato (Solanum lycopersicum). Aquaculture 2024, 584, 740634. [Google Scholar] [CrossRef]
- Solh, M.; van Ginkel, M.; Ortiz, R. Innovative Agriculture for Food Security Be Smart, Be Systematic; International Center for Agricultural Research in the Dry Areas: Beirut, Lebanon, 2013; p. 13. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).