Prenatal Diagnosis of Canine and Feline Twins Using Ultrasound: A Retrospective Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
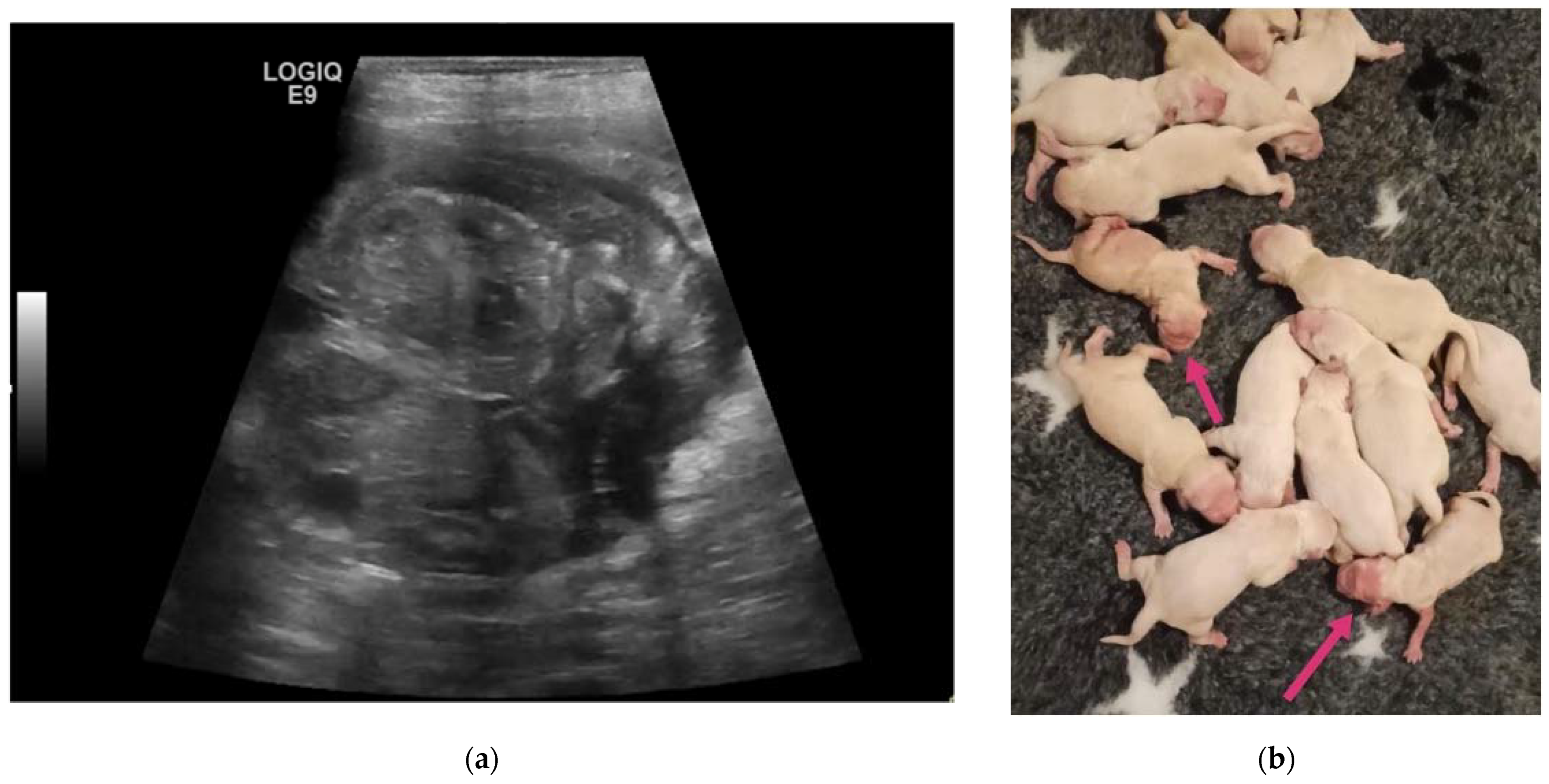
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tabor, A.; Vestergaard, C.H.; Lidegaard, O. Fetal loss rate after chorionic villus sampling and amniocentesis: An 11-year national registry study. Ultrasound Obstet. Gynecol. 2009, 34, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.L.; Hsiao, C.H.; Tseng, H.W.; Lee, T.P. Noninvasive prenatal diagnosis. Taiwan J. Obstet. Gynecol. 2015, 54, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Baharmast, J.; Rad, M.A. Diagnosis of emphysematous fetuses in dogs and cats. Mod. Vet. Pract. 1977, 58, 349–350. [Google Scholar] [PubMed]
- Planellas, M.; Martin, N.; Pons, C.; Font, J.; Cairo, J. Mummified fetus in the thoracic cavity of a domestic short-haired cat. Top. Companion Anim. Med. 2012, 27, 36–37. [Google Scholar] [CrossRef]
- Root Kustritz, M.V. Pregnancy diagnosis and abnormalities of pregnancy in the dog. Theriogenology 2005, 64, 755–765. [Google Scholar] [CrossRef]
- Beccaglia, M.; Alonge, S.; Trovo’, C.; Luvoni, G.C. Determination of gestational time and prediction of parturition in dogs and cats: An update. Reprod. Domest. Anim. 2016, 51, 12–17. [Google Scholar] [CrossRef]
- Fulton, R.M. Focused ultrasound of the fetus, female and male reproductive tracts, pregnancy, and dystocia in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 1249–1265. [Google Scholar] [CrossRef]
- Gil, E.M.; Garcia, D.A.; Giannico, A.T.; Froes, T.R. Use of B-mode ultrasonography for fetal sex determination in dogs. Theriogenology 2015, 84, 875–879. [Google Scholar] [CrossRef]
- Lopate, C. Ultrasonography for the evaluation of pregnancy in the female canine. Reprod. Domest. Anim. 2023, 58 (Suppl. S2), 144–162. [Google Scholar] [CrossRef]
- Zambelli, D.; Prati, F. Ultrasonography for pregnancy diagnosis and evaluation in queens. Theriogenology 2006, 66, 135–144. [Google Scholar] [CrossRef]
- Beccaglia, M.; Luvoni, G.C. Comparison of the accuracy of two ultrasonographic measurements in predicting the parturition date in the bitch. J. Small Anim. Pract. 2006, 47, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Beccaglia, M.; Anastasi, P.; Grimaldi, E.; Rota, A.; Faustini, M.; Luvoni, G.C. Accuracy of the prediction of parturition date through ultrasonographic measurement of fetal parameters in the queen. Vet. Res. Commun. 2008, 32 (Suppl. S1), S99–S101. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.P.; Nyland, T.G.; Tsutsui, T. Pregnancy diagnosis with ultrasound in the domestic cat. Vet. Radiol. 1986, 27, 109–114. [Google Scholar] [CrossRef]
- Lopate, C. Estimation of gestational age and assessment of canine fetal maturation using radiology and ultrasonography: A review. Theriogenology 2008, 70, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Siena, G.; di Nardo, F.; Romagnoli, S.; Mollo, A.; Contiero, B.; Milani, C. Relationship between days before parturition and fetal kidney length, cortical thickness, medullary thickness and their ratio in dogs. Theriogenology 2022, 194, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Siena, G.; Romagnoli, S.; Drigo, M.; Contiero, B.; di Nardo, F.; Milani, C. Ultrasonographic changes in fetal gastrointestinal motility during the last ten days before parturition in dogs. Front. Vet. Sci. 2022, 9, 1000975. [Google Scholar] [CrossRef] [PubMed]
- England, G.C.; Russo, M. Ultrasonographic characteristics of early pregnancy failure in bitches. Theriogenology 2006, 66, 1694–1698. [Google Scholar] [CrossRef]
- Freitas, L.A.; Mota, G.L.; Silva, H.V.; Carvalho, C.F.; Silva, L.D. Can maternal-fetal hemodynamics influence prenatal development in dogs? Anim. Reprod. Sci. 2016, 172, 83–93. [Google Scholar] [CrossRef]
- Mattoon, J.S.; Nyland, T.G. Ultrasonography of the genital system. In Veterinary Diagnostic Ultrasound; Nyland, T.G., Mattoon, J.S., Eds.; W.B. Saunders: Philadelphia, PA, USA, 1995; pp. 146–148. [Google Scholar]
- Urhausen, C.; Wolf, K.; Beineke, A.; Dierks, C.; Schmicke, M.; Einspanier, A.; Günzel-Apel, A.R. Monochorial diamniotic dizygotic twins in a German Shepherd dog: A case report. Reprod. Domest. Anim. 2017, 52, 140–143. [Google Scholar] [CrossRef]
- Pavan, L.; Gasser, B.; Santos, V.J.C.; Maronezi, M.C.; Silva, P.; Assis, A.R.; Garcia, P.H.S.; Martins Junior, R.; Uscategui, R.A.R.; Feliciano, M.A.R. Ultrasonographic diagnosis of twins in two pregnant bitches: Case report. Arq. Bras. Med. Veterinária E Zootec. 2020, 72, 102–106. [Google Scholar] [CrossRef]
- Allen, W.E.; England, G.C.W.; White, K.B. Hydrops fetalis diagnosed by real-time ultrasonography in a bichon frise bitch. J. Small Anim. Pract. 1989, 30, 465–467. [Google Scholar] [CrossRef]
- Cahua, U.J.; Cuesta, T.G. Ultrasound diagnosis of hydrops fetalis in a crossbred bitch. Rev. Investig. Vet. Perú 2021, 32, e20041. [Google Scholar] [CrossRef]
- Cunto, M.; Zambelli, D.; Castagnetti, C.; Linta, N.; Bini, C. Diagnosis and treatment of foetal anasarca in two English bulldog puppies. Pak. Vet. J. 2015, 35, 251–253. [Google Scholar]
- Heng, H.G.; Randall, E.; Kurt, W.; Johnson, C. What is your diagnosis? Hydrops fetalis. J. Am. Vet. Med. Assoc. 2011, 239, 51–52. [Google Scholar] [CrossRef] [PubMed]
- Hopper, B.J.; Richardson, J.L.; Lester, N.V. Spontaneous antenatal resolution of canine hydrops fetalis diagnosed by ultrasound. J. Small Anim. Pract. 2004, 45, 2–8. [Google Scholar] [CrossRef]
- Siena, G.; Corrò, M.; Zanardello, C.; Foiani, G.; Romagnoli, S.; Ferré-Dolcet, L.; Milani, C. A case report of a rapid development of fetal anasarca in a canine pregnancy at term. Vet. Res. Commun. 2022, 46, 597–602. [Google Scholar] [CrossRef]
- Sridevi, P. Ultrasonographic diagnosis and monitoring of pregnancy in the bitch—A review. J. Vet. Anim. Sci. 2013, 44, 1–7. [Google Scholar]
- Cruz Rde, J.; Alvarado, M.S.; Sandoval, J.E.; Vilchez, E. Prenatal sonographic diagnosis of fetal death and hydranencephaly in two Chihuahua fetuses. Vet. Radiol. Ultrasound 2003, 44, 589–592. [Google Scholar] [CrossRef]
- Sananmuang, T.; Mankong, K.; Jeeratanyasakul, P.; Chokeshai-Usaha, K.; Ponglowhapan, S. Prenatal diagnosis of foetal hydrocephalus and suspected X-linked recessive inheritance of cleft lip in a Chihuahua. J. Vet. Med. Sci. 2020, 82, 212–216. [Google Scholar] [CrossRef]
- Saez, D.; Arancibia, C. In utero ultrasonographic diagnosis of a schistosomus reflexus in a cat. In Proceedings of the World Small Animal Veterinary Association (WSAVA), Annual Congress, San Paulo, Brazil, 21–24 July 2009. [Google Scholar]
- Adams, W.H.; Toal, R.L.; Breider, M.A. Ultrasonographic findings in ethylene glycol (antifreeze) poisoning in a pregnant queen and 4 fetal kittens. Vet. Radiol. Ultrasound 1991, 32, 60–62. [Google Scholar] [CrossRef]
- Quintero, R.A.; Mueller, O.T.; Martinez, J.M.; Arroyo, J.; Gilbert-Barness, E.; Hilbelink, D.; Papenhausen, P.; Sutcliffe, M. Twin–twin transfusion syndrome in a dizygotic monochorionic-diamniotic twin pregnancy. J. Matern Fetal Neonatal Med. 2003, 14, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, G.; Fabbro, D.; Demori, E.; Driul, L.; Damante, G.; Xodo, S. Rare spontaneous monochorionic dizygotic twins: A case report and a systematic review. BMC Pregnancy Childbirth 2022, 22, 564. [Google Scholar] [CrossRef] [PubMed]
- Brouillet, S.; Mereuze, S.; Ranisavljevic, N.; Chauveau, C.; Hamamah, S.; Cattin, J.; Verebi, C.; Cabrol, C.; Ishmukhametova, A.; Girardet, A.; et al. Molecular characterization of a rare case of monozygotic dichorionic diamniotic twin pregnancy after single blastocyst transfer in Preimplantation Genetic Testing (PGT). Int. J. Mol. Sci. 2022, 23, 10835. [Google Scholar] [CrossRef] [PubMed]
- Shulman, L.S.; van Vugt, J.M.G. Prenatal Medicine; Taylor & Francis: Washington, DC, USA, 2006; p. 447. [Google Scholar]
- Duke, K.L. Monozygotic twins in the dog. Anat. Rec. 1946, 94, 35–39. [Google Scholar] [CrossRef]
- Joonè, C.J.; Cramer, K.G.M.; Nöthling, J.O. The first case of genetically confirmed monozygotic twinning in the dog. Reprod. Domest. Anim. 2016, 51, 835–839. [Google Scholar] [CrossRef]
- Joonè, C.J.; Cramer, K.G.M.; Nöthling, J.O. Dizygotic monochorionic canine fetuses with blood chimaerism and suspected freemartinism. Reprod. Fertil. Dev. 2017, 29, 368–373. [Google Scholar] [CrossRef]
- Camón, J.; Sabaté, D.; Verdú, J.; Rutllant, J.; López-Plana, C. Morphology of a dicephalic cat. Anat. Embryol. 1992, 185, 45–55. [Google Scholar] [CrossRef]
- Mazzullo, G.; Macrì, F.; Rapisarda, G.; Marino, F. Deradelphous cephalothoracopagus in kittens. Anat. Histol. Embryol. 2009, 38, 327–329. [Google Scholar] [CrossRef]
- Seavers, A.M. Monocephalus dipygus parapagus: A suspected case of complete caudal duplication in a British Blue kitten. J. Feline Med. Surg. 2009, 11, 330–331. [Google Scholar] [CrossRef]
- Grimes, J.A.; Hespel, A.M.; Cole, R.C.; Dillon, A.R. A Case of Parasitic Twinning or Caudal Duplication in a Dog. J. Am. Anim. Hosp. Assoc. 2018, 54, 219–225. [Google Scholar] [CrossRef]
- Moura, E.; Thon, B.; Pimpão, C.T. Canine conjoined twinning: A pathoanatomical study of a Lhasa Apso symmetrical cephalo-thoracopagus. Anat. Histol. Embryol. 2017, 46, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Paquet, M.; El-Warrak, A.O.; Laguë, M.N.; Boerboom, D. Atypical caudal duplication with phenotypic sex reversal in a dog. J. Vet. Diagn. Investig. 2011, 23, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Mazzullo, G.; Monteverde, V.; Macrì, F.; Partanna, S.; Caracappa, S. Incomplete caudal duplication in a puppy: Gross and radio-logical observations. J. Small Anim. Pract. 2007, 48, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Nottidge, H.O.; Omobowale, T.O.; Olopade, J.O.; Oladiran, O.O.; Ajala, O.O. A case of craniothoracopagus (monocephalus thoraco-pagus tetrabrachius) in a dog. Anat. Histol. Embryol. 2007, 36, 179–181. [Google Scholar] [CrossRef]
- Mugnier, A.; Gaillard, V.; Chastant, S. Relative impact of birth weight and early growth on neonatal mortality in puppies. Animals 2023, 13, 1928. [Google Scholar] [CrossRef]
- Münnich, A.; Küchenmeister, U. Dystocia in numbers—Evidence-based parameters for intervention in the dog: Causes for dystocia and treatment recommendations. Reprod. Domest. Anim. 2009, 44 (Suppl. S2), 141–147. [Google Scholar] [CrossRef]
- Azari, O.; Akhtardanesh, B. A clinical report of entangled neonates’ umbilical cord with queen’s fur in Persian cat. Asian Pac. J. Trop. Biomed. 2011, 1, 502–504. [Google Scholar] [CrossRef]
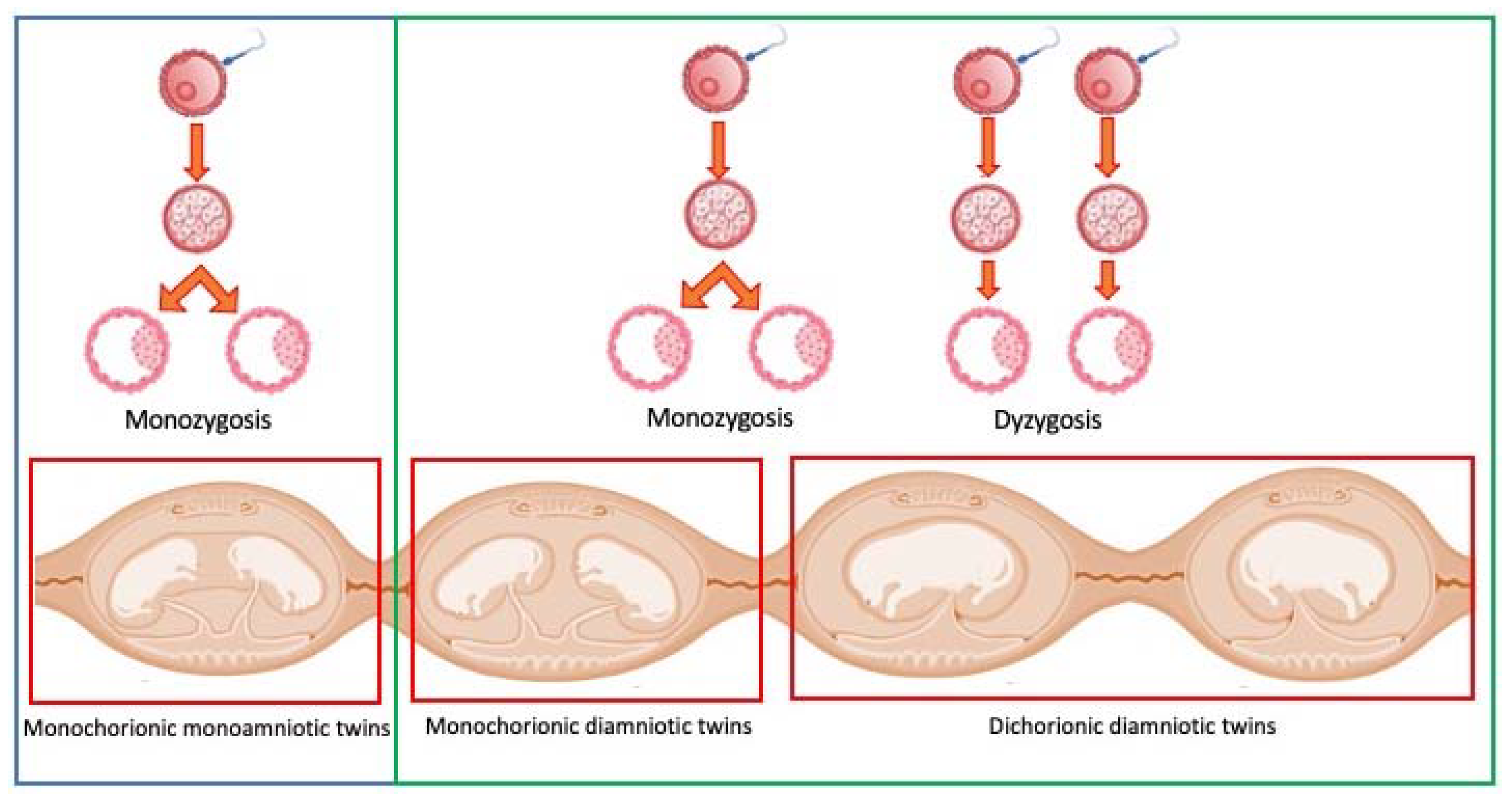

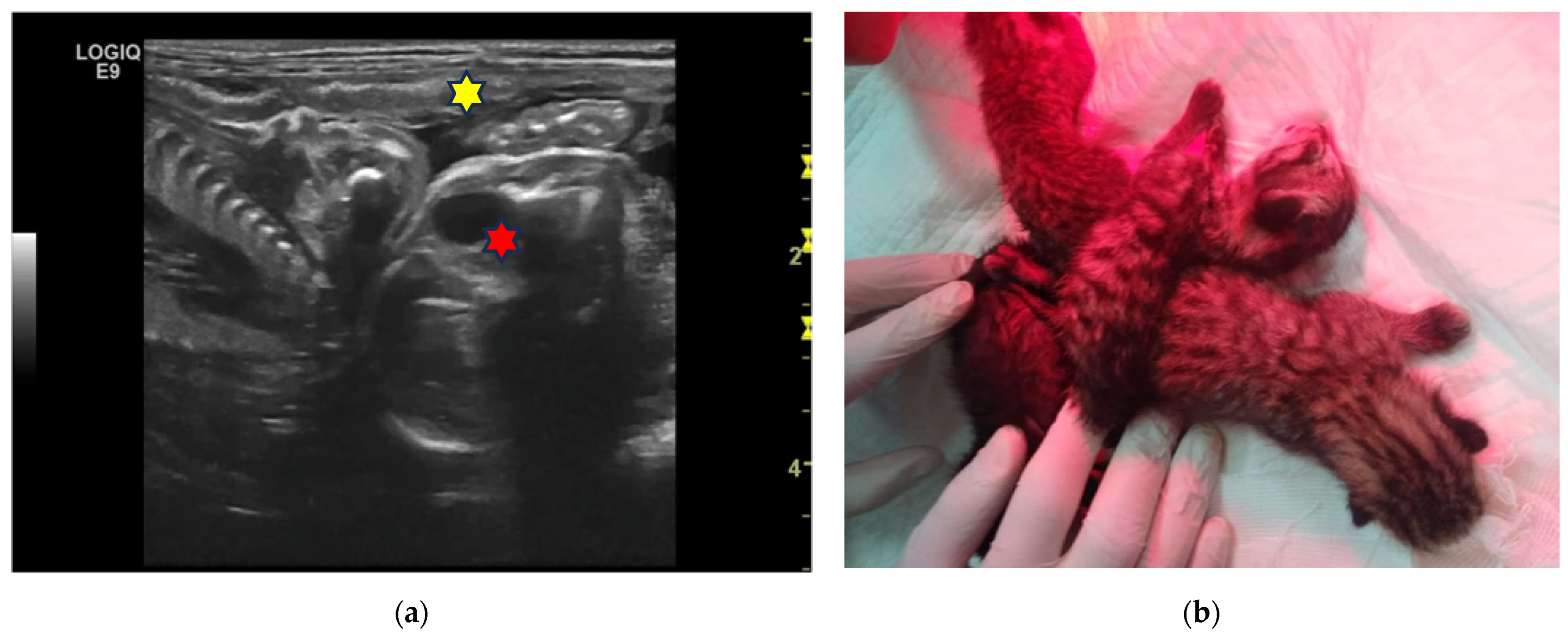
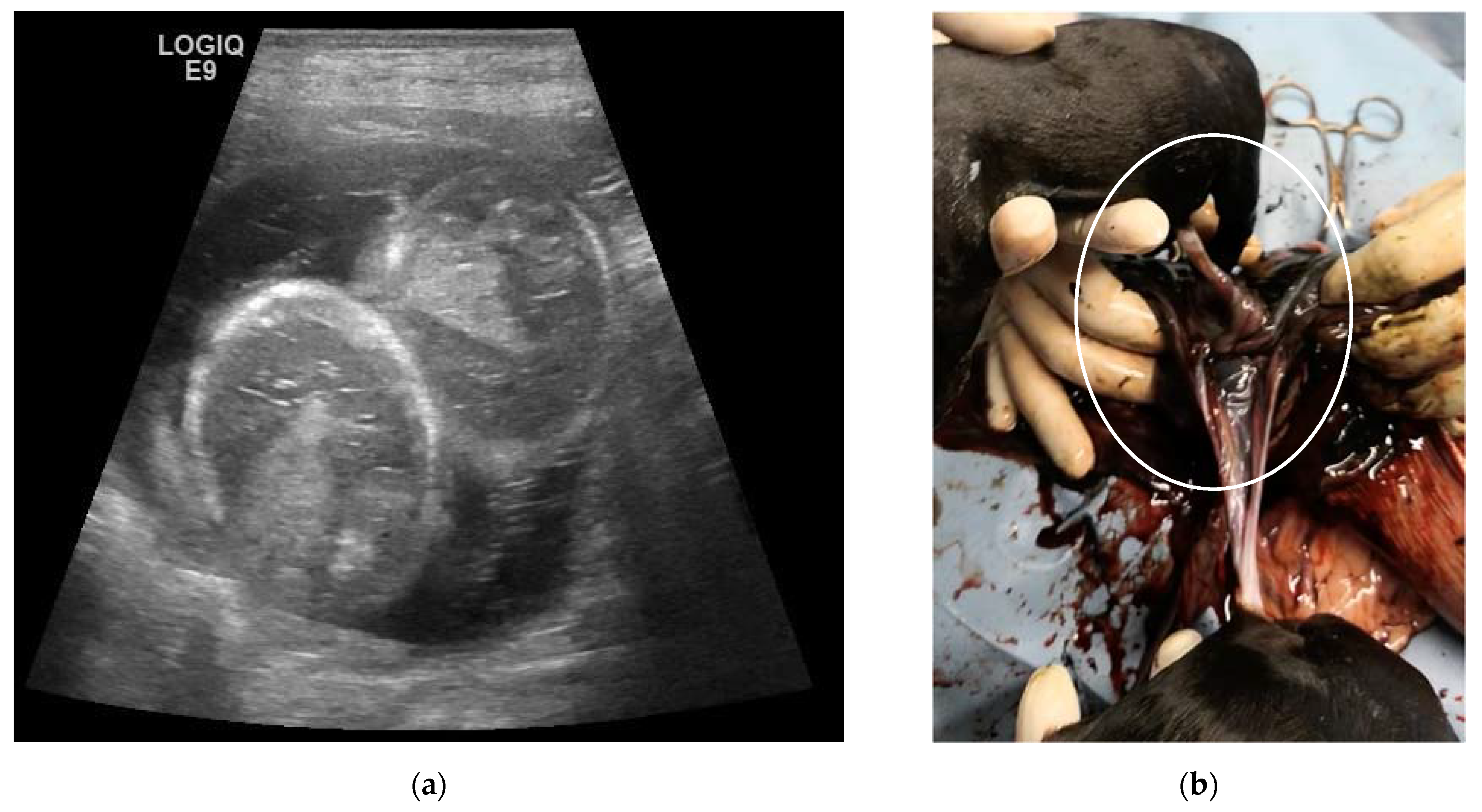
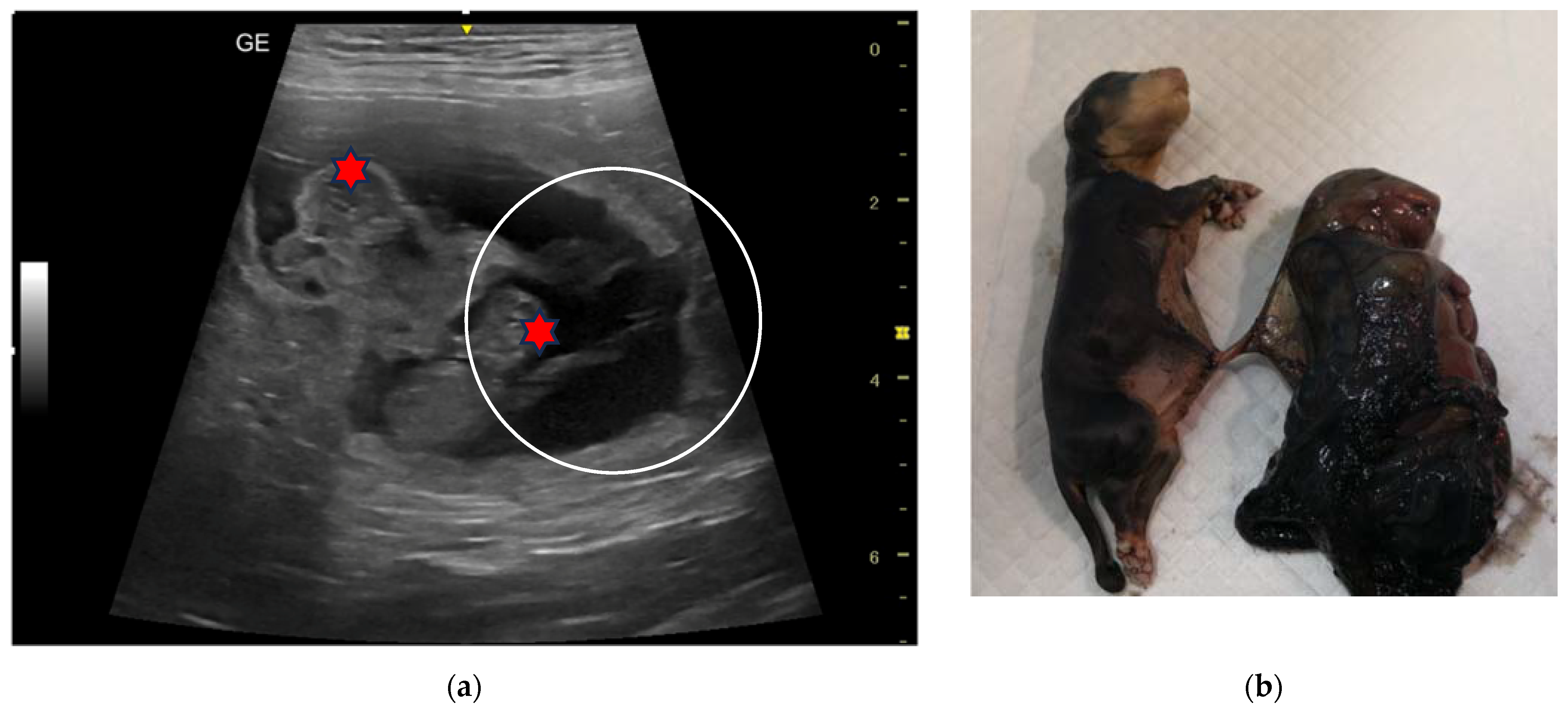

| N | Signalment | Day of Pregnancy | Littermate Numerosity | Ultrasound Evaluation | Term of Pregnancy | Follow-Up |
|---|---|---|---|---|---|---|
| 1F 2F | 6-year-old Birman female cat | 45 and 52 | 5 | Two pairs of monochorionic twins, morphologically comparable to the other foetus. | Natural delivery without assistance, three kitten stillborn. | One kitten dead for sepsis after 7 days. |
| 3F | 4-year-old European female cat | 50 | 4 | Monochorionic twins, morphologically comparable to the other foetuses. | Natural delivery without assistance, entangled neonates’ umbilical cords. | |
| 4C | 5-year-old Labrador Retriever female dog | 25, 32, 39, 46 and 53 | 10 | Monochorionic twins, morphologically comparable to the other embryos/foetuses. | C-section. Twins of different sex, alive, with partial twisting of the umbilical cord. One stillborn, two puppies euthanatized for malformations. | Death of the male twin after 7 days of hypoglycaemia and dehydration. |
| 5C | 3-year-old Australian Shepherd female dog | 23, 30, 37, 44 and 51 | 12 | Monochorionic twins, morphologically comparable to the other embryos/foetuses. | Natural delivery, dystocia, C-section. Ten puppies alive and two dead twins of the opposite sex. | |
| 6C | 5-year-old Rottweiler female dog | 45 and 55 | 4 | Monochorionic twins, morphologically comparable to the other foetuses; foetal death of the twins. | Natural delivery of two puppies, C-section, and extraction of the twins. | |
| 7C | 3-year-old Golden Retriever female dog | 50 | 14 | Monochorionic twins, morphologically comparable to the other foetuses. | C-section. Twins alive and of opposite sex, slightly dysmature. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecchia, F.; Di Giorgio, S.; Sfacteria, A.; Monti, S.; Vullo, C.; Catone, G.; Marino, G. Prenatal Diagnosis of Canine and Feline Twins Using Ultrasound: A Retrospective Study. Animals 2023, 13, 3309. https://doi.org/10.3390/ani13213309
Pecchia F, Di Giorgio S, Sfacteria A, Monti S, Vullo C, Catone G, Marino G. Prenatal Diagnosis of Canine and Feline Twins Using Ultrasound: A Retrospective Study. Animals. 2023; 13(21):3309. https://doi.org/10.3390/ani13213309
Chicago/Turabian StylePecchia, Fabiana, Stefania Di Giorgio, Alessandra Sfacteria, Salvatore Monti, Cecilia Vullo, Giuseppe Catone, and Gabriele Marino. 2023. "Prenatal Diagnosis of Canine and Feline Twins Using Ultrasound: A Retrospective Study" Animals 13, no. 21: 3309. https://doi.org/10.3390/ani13213309
APA StylePecchia, F., Di Giorgio, S., Sfacteria, A., Monti, S., Vullo, C., Catone, G., & Marino, G. (2023). Prenatal Diagnosis of Canine and Feline Twins Using Ultrasound: A Retrospective Study. Animals, 13(21), 3309. https://doi.org/10.3390/ani13213309







