Investigating Owner Use of Dietary Supplements in Dogs with Canine Cognitive Dysfunction
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Clinical History
3.2. Cognitive Dysfunction Data
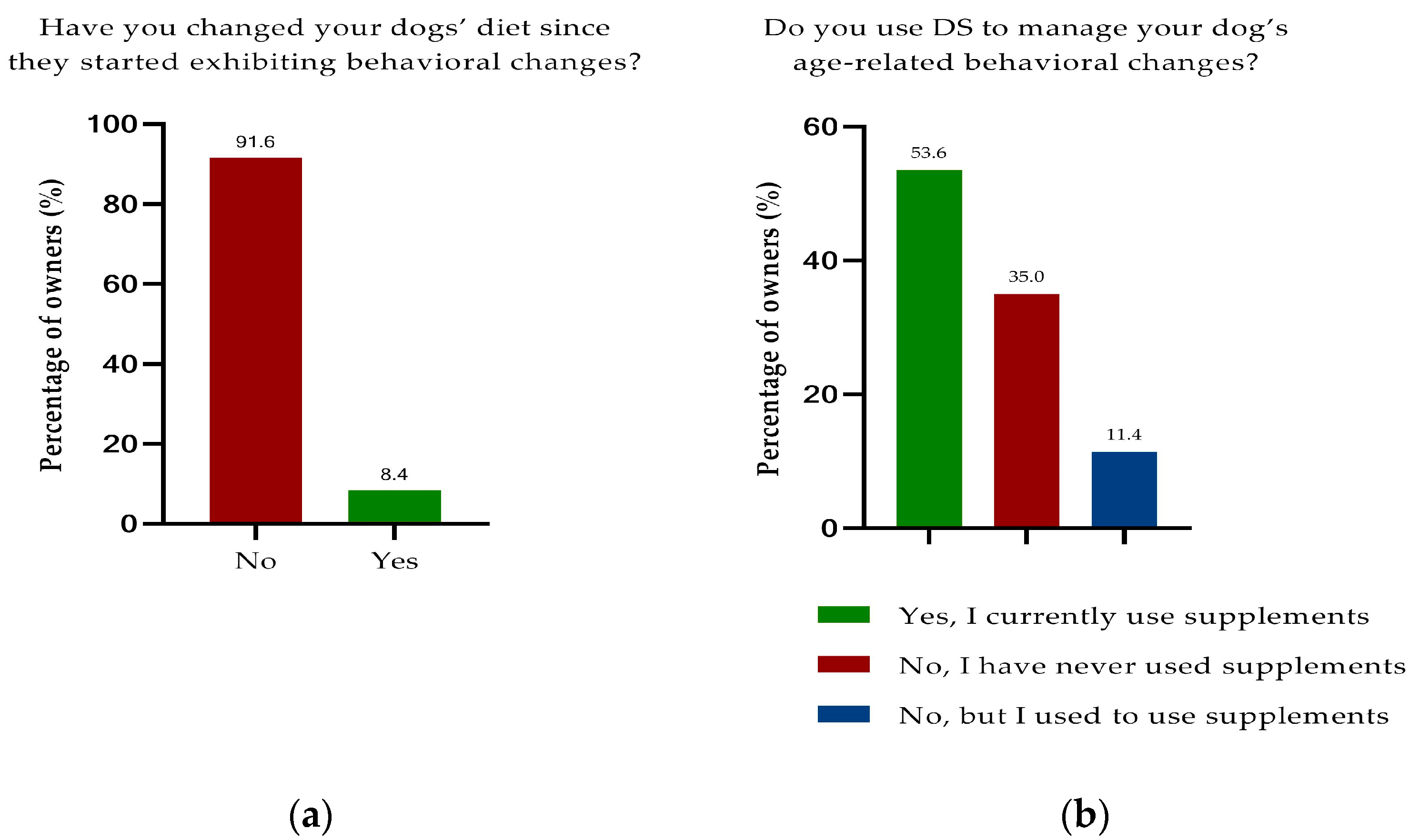
3.3. Nutrition
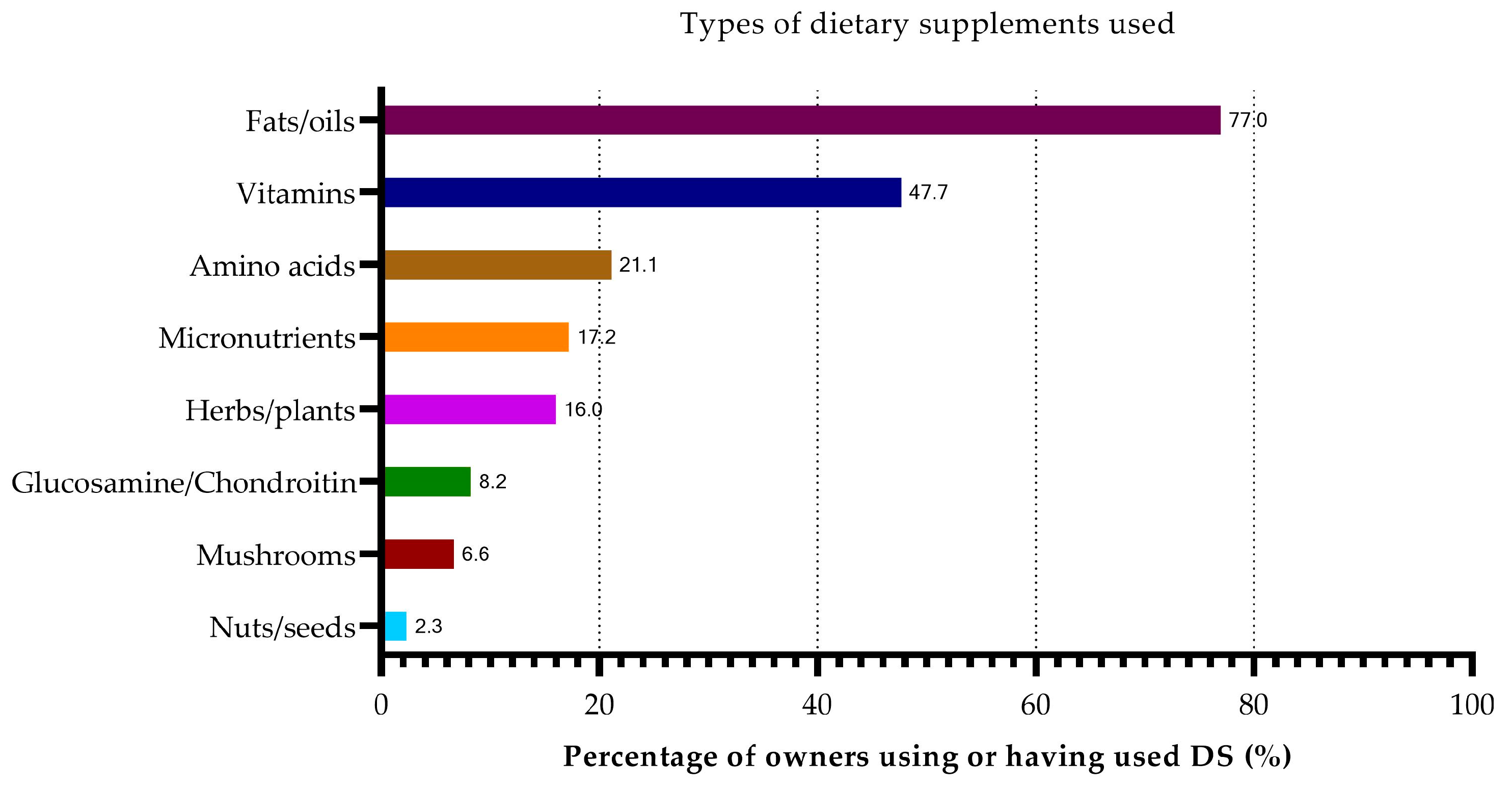
3.4. Dietary Supplementation
3.4.1. Overall Study Population
3.4.2. Dogs Diagnosed with CCD
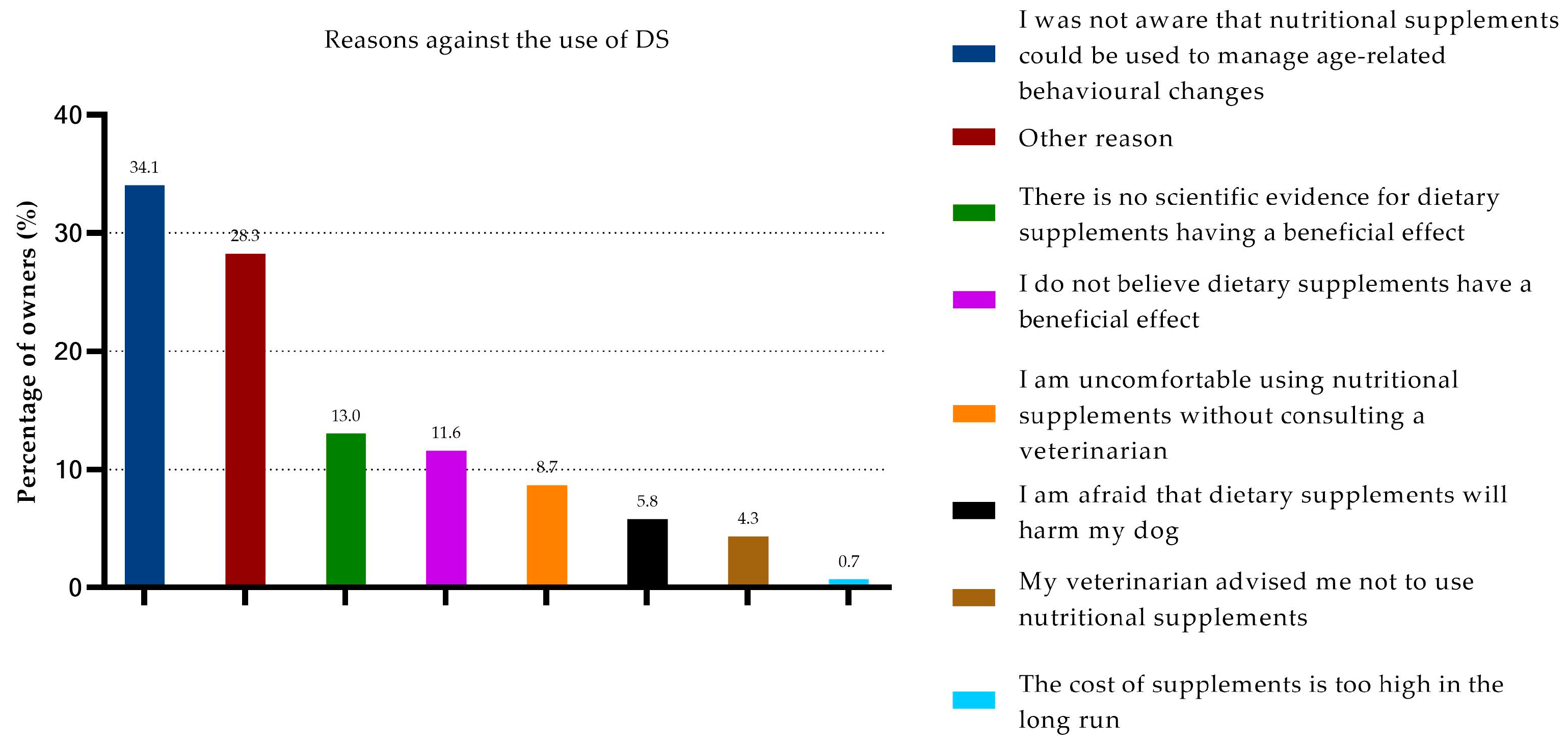
3.5. Non-Use of DSs
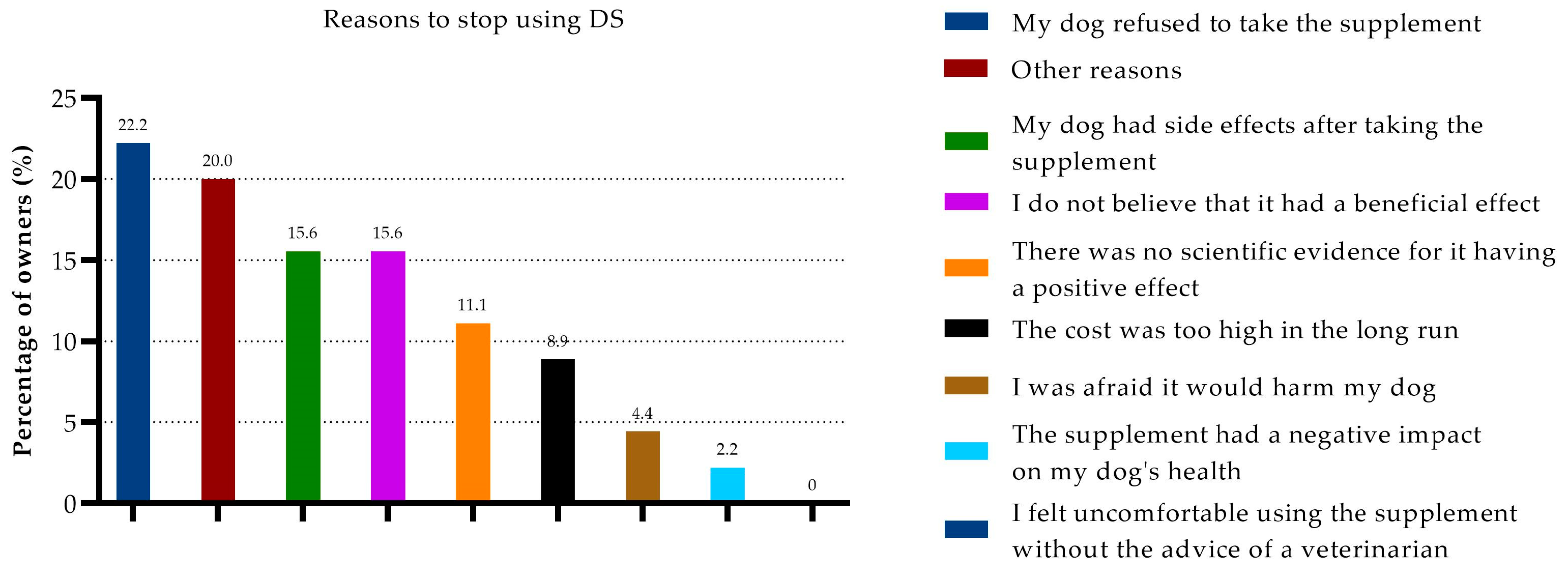
3.6. Cessation of DSs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Veterinary Medical Association. US Pet Ownership & Demographics Sourcebook; American Veterinary Medical Association: Schaumburg, IL, USA, 2012. [Google Scholar]
- Salvin, H.E.; McGreevy, P.D.; Sachdev, P.S.; Valenzuela, M.J. Under diagnosis of canine cognitive dysfunction: A cross-sectional survey of older companion dogs. Vet. J. 2010, 184, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Neilson, J.C.; Hart, B.L.; Cliff, K.D.; Ruehl, W.W. Prevalence of behavioral changes associated with age-related cognitive impairment in dogs. J. Am. Vet. Med. Assoc. 2001, 218, 1787–1791. [Google Scholar] [CrossRef] [PubMed]
- Azkona, G.; García-Belenguer, S.; Chacón, G.; Rosado, B.; León, M.; Palacio, J. Prevalence and risk factors of behavioural changes associated with age-related cognitive impairment in geriatric dogs. J. Small Anim. Pract. 2009, 50, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.J.; Head, E.; Ruehl, W.; Milgram, N.W.; Cotman, C.W. The canine as an animal model of human aging and dementia. Neurobiol. Aging 1996, 17, 259–268. [Google Scholar] [CrossRef]
- Milgram, N.W.; Head, E.; Weiner, E.; Thomas, E. Cognitive functions and aging in the dog: Acquisition of nonspatial visual tasks. Behav. Neurosci. 1994, 108, 57–68. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Nichol, J.; Araujo, J.A. Cognitive dysfunction syndrome: A disease of canine and feline brain aging. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 749–768. [Google Scholar] [CrossRef]
- Ruehl, W.W.; Bruyette, D.S.; DePaoli, A.; Cotman, C.W.; Head, E.; Milgram, N.W.; Cummings, B.J. Canine cognitive dysfunction as a model for human age-related cognitive decline, dementia and Alzheimer’s disease: Clinical presentation, cognitive testing, pathology and response to 1-deprenyl therapy. In Progress in Brain Research; Yu, P.M., Tipton, K.F., Boulton, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; Volume 106, pp. 217–225. [Google Scholar]
- Schmidt, F.; Boltze, J.; Jäger, C.; Hofmann, S.; Willems, N.; Seeger, J.; Härtig, W.; Stolzing, A. Detection and quantification of β-amyloid, pyroglutamyl Aβ, and tau in aged canines. J. Neuropathol. Exp. Neurol. 2015, 74, 912–923. [Google Scholar] [CrossRef]
- Tynes, V.V.; Landsberg, G.M. Nutritional management of behavior and brain disorders in dogs and cats. Vet. Clin. Small Anim. Pract. 2021, 51, 711–727. [Google Scholar] [CrossRef]
- Han, F.Y.; Conboy-Schmidt, L.; Rybachuk, G.; Volk, H.A.; Zanghi, B.; Pan, Y.; Borges, K. Dietary medium chain triglycerides for management of epilepsy: New data from human, dog, and rodent studies. Epilepsia 2021, 62, 1790–1806. [Google Scholar] [CrossRef]
- May, K.A.; Laflamme, D.P. Nutrition and the aging brain of dogs and cats. J. Am. Vet. Med. Assoc. 2019, 255, 1245–1254. [Google Scholar] [CrossRef]
- Pimentel-Coelho, P.M.; Rivest, S. The early contribution of cerebrovascular factors to the pathogenesis of Alzheimer’s disease. Eur. J. Neurosci. 2012, 35, 1917–1937. [Google Scholar] [CrossRef]
- Cummings, B.J.; Head, E.; Afagh, A.J.; Milgram, N.W.; Cotman, C.W. β-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol. Learn. Mem. 1996, 66, 11–23. [Google Scholar] [CrossRef]
- Yu, C.-H.; Song, G.-S.; Yhee, J.-Y.; Kim, J.-H.; Im, K.-S.; Nho, W.-G.; Lee, J.-H.; Sur, J.-H. Histopathological and immunohistochemical comparison of the brain of human patients with Alzheimer’s disease and the brain of aged dogs with cognitive dysfunction. J. Comp. Pathol. 2011, 145, 45–58. [Google Scholar] [CrossRef]
- Siwak-Tapp, C.T.; Head, E.; Muggenburg, B.A.; Milgram, N.W.; Cotman, C.W. Region specific neuron loss in the aged canine hippocampus is reduced by enrichment. Neurobiol. Aging 2008, 29, 39–50. [Google Scholar] [CrossRef]
- Head, E. Oxidative damage and cognitive dysfunction: Antioxidant treatments to promote healthy brain aging. Neurochem. Res. 2009, 34, 670–678. [Google Scholar] [CrossRef]
- Hoyer, S. The young-adult and normally aged brain. Its blood flow and oxidative metabolism. A review-part I. Arch. Gerontol. Geriat. 1982, 1, 101–116. [Google Scholar] [CrossRef]
- Reger, M.A.; Henderson, S.T.; Hale, C.; Cholerton, B.; Baker, L.D.; Watson, G.S.; Hyde, K.; Chapman, D.; Craft, S. Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging 2004, 25, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Shidler, M.D.; Dangelo, K.; Couch, S.C.; Benoit, S.C.; Clegg, D.J. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol. Aging 2012, 33, 425.e419–425.e427. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Egan, J.M.; Mattson, M.P.; Kapogiannis, D. Medium chain triglycerides induce mild ketosis and may improve cognition in Alzheimer’s disease. A systematic review and meta-analysis of human studies. Ageing Res. Rev. 2020, 58, 101001. [Google Scholar] [CrossRef]
- Hunt, A.; Schönknecht, P.; Henze, M.; Seidl, U.; Haberkorn, U.; Schröder, J. Reduced cerebral glucose metabolism in patients at risk for Alzheimer’s disease. Psychiatry Res. Neuroimaging 2007, 155, 147–154. [Google Scholar] [CrossRef]
- Romanucci, M.; Della Salda, L. Oxidative stress and protein quality control systems in the aged canine brain as a model for human neurodegenerative disorders. Oxid. Med. Cell. Longev. 2015, 2015, 940131. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y. Nutrients, cognitive function, and brain aging: What we have learned from dogs. Med. Sci. 2021, 9, 72. [Google Scholar] [CrossRef]
- Landsberg, G. Therapeutic agents for the treatment of cognitive dysfunction syndrome in senior dogs. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 471–479. [Google Scholar] [CrossRef]
- Pan, Y.; Kennedy, A.D.; Jönsson, T.J.; Milgram, N.W. Cognitive enhancement in old dogs from dietary supplementation with a nutrient blend containing arginine, antioxidants, B vitamins and fish oil. Br. J. Nutr. 2018, 119, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Milgram, N.W.; Ivy, G.O.; Head, E.; Murphy, M.P.; Wu, P.H.; Ruehl, W.W.; Yu, P.H.; Durden, D.A.; Davis, B.A.; Paterson, I.A.; et al. The effect ofl-deprenyl on behavior, cognitive function, and biogenic amines in the dog. Neurochem. Res. 1993, 18, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.W. Encephalopathies: Disorders of the brain. Pract. Guide Canine Feline Neurol. 2008, 2, 115–220. [Google Scholar]
- Evans, J.G.; Wilcock, G.; Birks, J. Evidence-based pharmacotherapy of Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2004, 7, 351–369. [Google Scholar] [CrossRef]
- Ebadi, M.; Brown-Borg, H.; Ren, J.; Sharma, S.; Shavali, S.; El ReFaey, H.; Carlson, E.C. Therapeutic efficacy of selegiline in neurodegenerative disorders and neurological diseases. Curr. Drug Targets 2006, 7, 1513–1529. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.W.; Davies, E.S.; Xie, H.; Wakshlag, J.J. Canine cognitive dysfunction: Pathophysiology, diagnosis, and treatment. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 477–499. [Google Scholar] [CrossRef] [PubMed]
- Frampton, M.A.; Harvey, R.J.; Kirchner, V. Propentofylline for dementia. Cochrane Database Syst. Rev. 2003, 2, 1–55. [Google Scholar] [CrossRef]
- Milgram, N.W.; Landsberg, G.; Merrick, D.; Underwood, M.Y. A novel mechanism for cognitive enhancement in aged dogs with the use of a calcium-buffering protein. J. Vet. Behav. 2015, 10, 217–222. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, X.; Cao, Y.; Mak, S.H.; Zha, L.; Li, N.; Su, Z.; Han, Y.; Wang, Y.; Man Hoi, M.P. Therapeutic efficacy of novel memantine nitrate MN-08 in animal models of Alzheimer’s disease. Aging Cell 2021, 20, e13371. [Google Scholar] [CrossRef] [PubMed]
- Zakošek Pipan, M.; Prpar Mihevc, S.; Štrbenc, M.; Košak, U.; German Ilić, I.; Trontelj, J.; Žakelj, S.; Gobec, S.; Pavlin, D.; Majdič, G. Treatment of canine cognitive dysfunction with novel butyrylcholinesterase inhibitor. Sci. Rep. 2021, 11, 18098. [Google Scholar] [CrossRef] [PubMed]
- Milgram, N.W.; Zicker, S.C.; Head, E.; Muggenburg, B.A.; Murphey, H.; Ikeda-Douglas, C.J.; Cotman, C.W. Dietary enrichment counteracts age-associated cognitive dysfunction in canines. Neurobiol. Aging 2002, 23, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Milgram, N.W.; Head, E.; Zicker, S.C.; Ikeda-Douglas, C.; Murphey, H.; Muggenberg, B.A.; Siwak, C.T.; Dwight Tapp, P.; Lowry, S.R.; Cotman, C.W. Long-term treatment with antioxidants and a program of behavioral enrichment reduces age-dependent impairment in discrimination and reversal learning in beagle dogs. Exp. Gerontol. 2004, 39, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Head, E.; Thornton, P.L.; Tong, L.; Cotman, C.W. Initiation and propagation of molecular cascades in human brain aging: Insight from the canine model to promote successful aging. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2000, 24, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Larson, B.; Araujo, J.A.; Lau, W.; de Rivera, C.; Santana, R.; Gore, A.; Milgram, N.W. Dietary supplementation with medium-chain TAG has long-lasting cognition-enhancing effects in aged dogs. Br. J. Nutr. 2010, 103, 1746–1754. [Google Scholar] [CrossRef]
- Pan, Y.; Landsberg, G.; Mougeot, I.; Kelly, S.; Xu, H.; Bhatnagar, S.; Gardner, C.L.; Milgram, N.W. Efficacy of a therapeutic diet on dogs with signs of cognitive dysfunction syndrome (CDS): A prospective double blinded placebo controlled clinical study. Front. Nutr. 2018, 5, 127. [Google Scholar] [CrossRef]
- Rebello, C.J.; Keller, J.N.; Liu, A.G.; Johnson, W.D.; Greenway, F.L. Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: A randomized controlled trial. BBA Clin. 2015, 3, 123–125. [Google Scholar] [CrossRef]
- Henderson, S.T. High carbohydrate diets and Alzheimer’s disease. Med. Hypotheses 2004, 62, 689–700. [Google Scholar] [CrossRef]
- Cotman, C.W.; Head, E.; Muggenburg, B.A.; Zicker, S.; Milgram, N.W. Brain aging in the canine: A diet enriched in antioxidants reduces cognitive dysfunction. Neurobiol. Aging 2002, 23, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Bottiglieri, T. S-Adenosyl-l-methionine (SAMe): From the bench to the bedside—Molecular basis of a pleiotrophic molecule. Am. J. Clin. Nutr. 2002, 76, 1151S–1157S. [Google Scholar] [CrossRef] [PubMed]
- Traystman, R.J.; Kirsch, J.R.; Koehler, R.C. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J. Appl. Physiol. 1991, 71, 1185–1195. [Google Scholar] [CrossRef]
- Gonzalez-Correa, J.A.; De La Cruz, J.P.; Martin-Aurioles, E.; Lopez-Egea, M.A.; Ortiz, P.; De La Cuesta, F.S. Effects of S-adenosyl-L-methionine on hepatic and renal oxidative stress in an experimental model of acute biliary obstruction in rats. Hepatology 1997, 26, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Faubert, M.L.; Brooks, M.L.; Landsberg, G.M.; Lobprise, H. NOVIFIT® (NoviSAMe®) tablets improve executive function in aged dogs and cats: Implications for treatment of cognitive dysfunction syndrome. Intern. J. Appl. Res. Vet. Med. 2012, 10, 90–98. [Google Scholar]
- De La Cruz, J.P.; Pavia, J.; Gonzalez-Correa, J.A.; Ortiz, P.; Sánchez De La Cuesta, F. Effects of chronic administration of S-adenosyl-L-methionine on brain oxidative stress in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000, 361, 47–52. [Google Scholar] [CrossRef]
- Rème, C.A.; Dramard, V.; Kern, L.; Hofmans, J.; Halsberghe, C.; Mombiela, D.V. Effect of S-adenosylmethionine tablets on the reduction of age-related mental decline in dogs: A double-blinded, placebo-controlled trial. Vet. Ther. 2008, 9, 69–82. [Google Scholar]
- Panza, F.; Frisardi, V.; Capurso, C.; D’Introno, A.; Colacicco, A.M.; Di Palo, A.; Imbimbo, B.P.; Vendemiale, G.; Capurso, A.; Solfrizzi, V. Polyunsaturated fatty acid and S-adenosylmethionine supplementation in predementia syndromes and Alzheimer’s disease: A review. Sci. World J. 2009, 9, 209876. [Google Scholar] [CrossRef]
- Selhub, J.; Bagley, L.C.; Miller, J.; Rosenberg, I.H. B vitamins, homocysteine, and neurocognitive function in the elderly. Am. J. Clin. Nutr. 2000, 71, 614S–620S. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003, 60, 940–946. [Google Scholar] [CrossRef]
- Cole, G.M.; Ma, Q.-L.; Frautschy, S.A. Omega-3 fatty acids and dementia. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Landsberg, G.M.; Milgram, N.W.; Miolo, A. Improvement of short-term memory performance in aged beagles by a nutraceutical supplement containing phosphatidylserine, ginkgo biloba, vitamin E, and pyridoxine. Can. Vet. J. 2008, 49, 379–385. [Google Scholar] [PubMed]
- Cena, F.; Fassola, F.; Furlanello, T. Effect of a combination of phosphatidylserine, ginkgo biloba, vitamin E and pyridoxine on clinical signs of canine brain aging: A pilot multicentric study. Vet. Ital. 2005, 4, 13–18. [Google Scholar]
- Osella, M.C.; Re, G.; Odore, R.; Girardi, C.; Badino, P.; Barbero, R.; Bergamasco, L. Canine cognitive dysfunction syndrome: Prevalence, clinical signs and treatment with a neuroprotective nutraceutical. Appl. Anim. Behav. Sci. 2007, 105, 297–310. [Google Scholar] [CrossRef]
- Heath, S.E.; Barabas, S.; Craze, P.G. Nutritional supplementation in cases of canine cognitive dysfunction—A clinical trial. Appl. Anim. Behav. Sci. 2007, 105, 284–296. [Google Scholar] [CrossRef]
- Berk, B.A.; Packer, R.M.A.; Law, T.H.; Volk, H.A. Investigating owner use of dietary supplements in dogs with idiopathic epilepsy. Res. Vet. Sci. 2018, 119, 276–284. [Google Scholar] [CrossRef]
- Larsen, J.A.; Farcas, A. Nutrition of aging dogs. Vet. Clin. Small Anim. Pract. 2014, 44, 741–759. [Google Scholar] [CrossRef]
- Bartges, J.W. Chronic kidney disease in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 669–692. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Elliott, J.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Chronic kidney disease in dogs in UK veterinary practices: Prevalence, risk factors, and survival. J. Vet. Intern. Med. 2013, 27, 814–821. [Google Scholar] [CrossRef]
- Polzin, D.J.; Osborne, C.A.; Adams, L.D.; O’Brien, T.D. Dietary management of canine and feline chronic renal failure. Vet. Clin. N. Am. Small Anim. Pract. 1989, 19, 539–560. [Google Scholar] [CrossRef]
- Bronson, R.T. Variation in age at death of dogs of different sexes and breeds. Am. J. Vet. Res. 1982, 43, 2057–2059. [Google Scholar] [PubMed]
- Dewey, C.W.; Rishniw, M. Periodontal disease is associated with cognitive dysfunction in aging dogs: A blinded prospective comparison of visual periodontal and cognitive questionnaire scores. Open Vet. J. 2021, 11, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, G.; Pan, Y.G.; Mougeot, I.; Kelly, S.; Xu, H.U.I.; Bhatnagar, S.; Milgram, N.W. Efficacy of a therapeutic diet on dogs with signs of cognitive dysfunction syndrome. In Proceedings of the 11th International Veterinary Behaviour Meeting, Samorin, Slovakia, 14–16 September 2017; pp. 114–115. [Google Scholar]
- Katina, S.; Farbakova, J.; Madari, A.; Novak, M.; Zilka, N. Risk factors for canine cognitive dysfunction syndrome in Slovakia. Acta Vet. Scand. 2015, 58, 17. [Google Scholar] [CrossRef]
- Bain, M.J.; Hart, B.L.; Cliff, K.D.; Ruehl, W.W. Predicting behavioral changes associated with age-related cognitive impairment in dogs. J. Am. Vet. Med. Assoc. 2001, 218, 1792–1795. [Google Scholar] [CrossRef] [PubMed]
- Goodnight, S.H., Jr.; Harris, W.S.; Connor, W.E.; Illingworth, D.R. Polyunsaturated fatty acids, hyperlipidemia, and thrombosis. Arterioscler. Off. J. Am. Heart Assoc. Inc. 1982, 2, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-h.; Shi, Y.; Wang, L.-s.; Yang, Z.-r. The influence of orally administered docosahexaenoic acid on cognitive ability in aged mice. J. Nutr. Biochem. 2009, 20, 735–741. [Google Scholar] [CrossRef]
- Heinemann, K.M.; Bauer, J.E. Docosahexaenoic acid and neurologic development in animals. J. Am. Vet. Med. Assoc. 2006, 228, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S.; Shimada, T.; Sugioka, K.; Yamasaki, H.; Fujii, Y.; Ishibashi, Y.; Oka, J.I.; Shido, O. Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J. Neurochem. 2002, 81, 1084–1091. [Google Scholar] [CrossRef]
- Catalan, J.; Toru, M.; Slotnick, B.; Murthy, M.; Greiner, R.S.; Salem, N., Jr. Cognitive deficits in docosahexaenoic acid-deficient rats. Behav. Neurosci. 2002, 116, 1022. [Google Scholar] [CrossRef]
- Suzuki, H.; Morikawa, Y.; Takahashi, H. Effect of DHA oil supplementation on intelligence and visual acuity in the elderly. In Fatty Acids and Lipids-New Findings; Karger Publishers: Basel, Switzerland, 2001; Volume 88, pp. 68–71. [Google Scholar]
- Neuringer, M.; Connor, W.E.; Lin, D.S.; Barstad, L.; Luck, S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc. Natl. Acad. Sci. USA 1986, 83, 4021–4025. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hossain, S.; Tanabe, Y.; Kawashima, A.; Harada, T.; Yano, T.; Mizuguchi, K.; Shido, O. The protective effect of dietary eicosapentaenoic acid against impairment of spatial cognition learning ability in rats infused with amyloid β (1–40). J. Nutr. Biochem. 2009, 20, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560s–569s. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E.; Kang, J.X.; Leaf, A. Prevention of sudden cardiac death by dietary pure ω-3 polyunsaturated fatty acids in dogs. Circulation 1999, 99, 2452–2457. [Google Scholar] [CrossRef]
- Blaskovic, M.; Rosenkrantz, W.; Neuber, A.; Sauter-Louis, C.; Mueller, R.S. The effect of a spot-on formulation containing polyunsaturated fatty acids and essential oils on dogs with atopic dermatitis. Vet. J. 2014, 199, 39–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, S.A.; Brown, C.A.; Crowell, W.A.; Barsanti, J.A.; Kang, C.-W.; Allen, T.; Cowell, C.; Finco, D.R. Effects of dietary polyunsaturated fatty acid supplementation in early renal insufficiency in dogs. J. Lab. Clin. Med. 2000, 135, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, D.P. Nutritional care for aging cats and dogs. Vet. Clin. N. Am. Small Anim. Pract. 2012, 42, 769–791. [Google Scholar] [CrossRef]
- Floyd, R.A.; West, M.; Hensley, K. Oxidative biochemical markers; clues to understanding aging in long-lived species. Exp. Gerontol. 2001, 36, 619–640. [Google Scholar] [CrossRef]
- Liu, J.; Mori, A. Stress, aging, and brain oxidative damage. Neurochem. Res. 1999, 24, 1479–1497. [Google Scholar] [CrossRef]
- Head, E.; Liu, J.; Hagen, T.M.; Muggenburg, B.A.; Milgram, N.W.; Ames, B.N.; Cotman, C.W. Oxidative damage increases with age in a canine model of human brain aging. J. Neurochem. 2002, 82, 375–381. [Google Scholar] [CrossRef]
- Cotman, C.W.; Head, E. The canine (dog) model of human aging and disease: Dietary, environmental and immunotherapy approaches. J. Alzheimer’s Dis. 2008, 15, 685–707. [Google Scholar] [CrossRef]
- Rosenberg, I.H.; Miller, J.W. Nutritional factors in physical and cognitive functions of elderly people. Am. J. Clin. Nutr. 1992, 55, 1237S–1243S. [Google Scholar] [CrossRef] [PubMed]
- Oulhaj, A.; Jernerén, F.; Refsum, H.; Smith, A.D.; De Jager, C.A. Omega-3 fatty acid status enhances the prevention of cognitive decline by B vitamins in mild cognitive impairment. J. Alzheimer’s Dis. 2016, 50, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Bottiglieri, T.; Crellin, R.; Reynolds, E.H.; Bailey, L.B. Folate and neuropsychiatry. In Folate in Health and Disease; Marcel Dekker: New York, NY, USA, 1995; pp. 435–462. [Google Scholar]
- Selhub, J.; Miller, J.W. The pathogenesis of homocysteinemia: Interruption of the coordinate regulation by S-adenosylmethionine of the remethylation and transsulfuration of homocysteine. Am. J. Clin. Nutr. 1992, 55, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Boushey, C.J.; Beresford, S.A.A.; Omenn, G.S.; Motulsky, A.G. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease: Probable benefits of increasing folic acid intakes. JAMA 1995, 274, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.R.; Edman, J.S.; Selhub, J.; Morrow, F.D.; Marby, D.W.; Kayne, H.L.; Cole, J.O. Plasma homocysteine in vascular disease and in nonvascular dementia of depressed elderly people. Acta Psychiatr. Scand. 1992, 86, 386–390. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H. Homocysteine, B vitamins, and cognitive impairment. Annu. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Smith, S.M.; De Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef]
- Adams, R.; Hunt, M.; Clark, J.H. Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp. I. J. Am. Chem. Soc. 1940, 62, 196–200. [Google Scholar] [CrossRef]
- Klein, B.D.; Jacobson, C.A.; Metcalf, C.S.; Smith, M.D.; Wilcox, K.S.; Hampson, A.J.; Kehne, J.H. Evaluation of cannabidiol in animal seizure models by the epilepsy therapy screening program (ETSP). Neurochem. Res. 2017, 42, 1939–1948. [Google Scholar] [CrossRef]
- Rosenberg, E.C.; Patra, P.H.; Whalley, B.J. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav. 2017, 70, 319–327. [Google Scholar] [CrossRef]
- Johannessen Landmark, C.; Potschka, H.; Auvin, S.; Wilmshurst, J.M.; Johannessen, S.I.; Kasteleijn-Nolst Trenité, D.; Wirrell, E.C. The role of new medical treatments for the management of developmental and epileptic encephalopathies: Novel concepts and results. Epilepsia 2021, 62, 857–873. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.; Bartner, L.R.; Rao, S.; Packer, R.A.; Gustafson, D.L. Randomized blinded controlled clinical trial to assess the effect of oral cannabidiol administration in addition to conventional antiepileptic treatment on seizure frequency in dogs with intractable idiopathic epilepsy. J. Am. Vet. Med. Assoc. 2019, 254, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.; Kulpa, J.; Paulionis, L. Preliminary investigation of the safety of escalating cannabinoid doses in healthy dogs. Front. Vet. Sci. 2020, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.M.; Paulionis, L.J.; Kulpa, J.E. Randomized, placebo-controlled, 28-day safety and pharmacokinetics evaluation of repeated oral cannabidiol administration in healthy dogs. Am. J. Vet. Res. 2021, 82, 405–416. [Google Scholar] [CrossRef]
- Potschka, H.; Bhatti, S.F.M.; Tipold, A.; McGrath, S. Cannabidiol in canine epilepsy. Vet. J. 2022, 290, 105913. [Google Scholar] [CrossRef] [PubMed]
- Gaston, T.E.; Martin, R.C.; Szaflarski, J.P. Cannabidiol (CBD) and cognition in epilepsy. Epilepsy Behav. 2021, 124, 108316. [Google Scholar] [CrossRef]
- de Mello Schier, A.R.; de Oliveira Ribeiro, N.P.; Coutinho, D.S.; Machado, S.; Arias-Carrión, O.; Crippa, J.A.; Zuardi, A.W.; Nardi, A.E.; Silva, A.C. Antidepressant-like and anxiolytic-like effects of cannabidiol: A chemical compound of Cannabis sativa. CNS Neurol. Disord. Drug Targets 2014, 13, 953–960. [Google Scholar] [CrossRef]
- Melas, P.A.; Scherma, M.; Fratta, W.; Cifani, C.; Fadda, P. Cannabidiol as a potential treatment for anxiety and mood disorders: Molecular targets and epigenetic insights from preclinical research. Int. J. Mol. Sci. 2021, 22, 1863. [Google Scholar] [CrossRef]
- Brien, S.; Lewith, G.T.; McGregor, G. Devil’s claw (Harpagophytum procumbens) as a treatment for osteoarthritis: A review of efficacy and safety. J. Altern. Complement. Med. 2006, 12, 981–993. [Google Scholar] [CrossRef]
- Chrubasik, S.; Wink, M. Zur pharmakologischen Wirkung der Teufelskralle (Harpagophytum procumbens). Complement. Med. Res. 1995, 2, 323–325. [Google Scholar] [CrossRef]
- Mastellone, V.; Vassalotti, G.; Musco, N.; Grossi, M.; Calabró, S.; Grazioli, R.; Pero, M.E.; Cutrignelli, M.I. Effects on oxidative status and metabolism of curcuma longa alone or associated with krill oil and ribes nigrum in healthy dogs. J. Nutr. Ecol. Food Res. 2014, 2, 348–352. [Google Scholar] [CrossRef]
- Innes, J.F.; Fuller, C.J.; Grover, E.R.; Kelly, A.L.; Burn, J.F. Randomised, double-blind, placebocontrolled parallel group study of P54FP for the treatment of dogs with osteoarthritis. Vet. Rec. 2003, 152, 457–460. [Google Scholar] [CrossRef]
- Sharma, S.S.; Kochupillai, V.; Gupta, S.K.; Seth, S.D.; Gupta, Y.K. Antiemetic efficacy of ginger (Zingiber officinale) against cisplatin-induced emesis in dogs. J. Ethnopharmacol. 1997, 57, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Frautschy, S.A.; Cole, G.M.; Masterman, D.L.; Cummings, J.L. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr. Alzheimer Res. 2005, 2, 131–136. [Google Scholar] [CrossRef]
- Hackett, E.S.; Twedt, D.C.; Gustafson, D.L. Milk thistle and its derivative compounds: A review of opportunities for treatment of liver disease. J. Vet. Intern. Med. 2013, 27, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Mosier, J.E. Caring for the aging dog in today’s practice. Vet. Med. 1990, 85, 460–471. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haake, J.; Meyerhoff, N.; Meller, S.; Twele, F.; Charalambous, M.; Wilke, V.; Volk, H. Investigating Owner Use of Dietary Supplements in Dogs with Canine Cognitive Dysfunction. Animals 2023, 13, 3056. https://doi.org/10.3390/ani13193056
Haake J, Meyerhoff N, Meller S, Twele F, Charalambous M, Wilke V, Volk H. Investigating Owner Use of Dietary Supplements in Dogs with Canine Cognitive Dysfunction. Animals. 2023; 13(19):3056. https://doi.org/10.3390/ani13193056
Chicago/Turabian StyleHaake, Julia, Nina Meyerhoff, Sebastian Meller, Friederike Twele, Marios Charalambous, Volker Wilke, and Holger Volk. 2023. "Investigating Owner Use of Dietary Supplements in Dogs with Canine Cognitive Dysfunction" Animals 13, no. 19: 3056. https://doi.org/10.3390/ani13193056
APA StyleHaake, J., Meyerhoff, N., Meller, S., Twele, F., Charalambous, M., Wilke, V., & Volk, H. (2023). Investigating Owner Use of Dietary Supplements in Dogs with Canine Cognitive Dysfunction. Animals, 13(19), 3056. https://doi.org/10.3390/ani13193056




