Simple Summary
Enterocytozoon bieneusi is a fungus-like protist that is distributed worldwide and can cause malabsorption and diarrhea in sheep, other animals, and humans, threatening the development of animal husbandry and public health. However, little is known of the prevalence and genotypes of E. bieneusi in sheep in Shanxi Province, North China. Thus, 492 fecal samples were collected from sheep in three representative counties in northern, central, and southern Shanxi Province, and nested PCR amplification targeting the internal transcribed spacer (ITS) region of the rRNA gene was carried out to detect the prevalence and identify the genotypes of E. bieneusi. The overall E. bieneusi prevalence in the examined sheep in Shanxi Province was 34.2% (168/492). Four known genotypes (BEB6, COS-I, CHS7, and CHC8) and one novel genotype (SY-1) were detected, and the prevalent genotype was BEB6. The five genotypes observed in this study belong to the host-adapted Group 2. This is the first report on the occurrence and genotypes of E. bieneusi in sheep in Shanxi Province, which enhances an understanding of the global distribution and genetic diversity of E. bieneusi and has implications for the better control of E. bieneusi in animals and humans.
Abstract
Enterocytozoon bieneusi is a fungus-like protist that can cause malabsorption and diarrhea in sheep, other animals, and humans, threatening the development of animal husbandry and public health. To date, there are no data about the prevalence and genotypes of E. bieneusi in sheep in Shanxi Province, North China. In this study, 492 fecal samples were collected from sheep in three representative counties in northern, central, and southern Shanxi Province. Nested PCR amplification was performed to detect the prevalence and identify the genotypes of E. bieneusi based on the internal transcribed spacer (ITS) region of the rRNA gene. Overall, 168 of 492 examined samples were E. bieneusi-positive, with a prevalence of 34.2% (168/492). Significant differences in the prevalence of E. bieneusi were observed among the three sampled regions (χ2 = 95.859, df = 2, p < 0.001), but the differences in E. bieneusi prevalence were not statistically significant between different genders and age groups (p > 0.05). Sequence analysis showed that four known genotypes (BEB6, COS-I, CHS7, and CHC8) and one novel genotype (named SY-1) were identified. BEB6 was the prevalent genotype found within the three counties. Phylogenetic analysis revealed that the five genotypes observed in this study belong to Group 2. The present study reported the presence and genotypes of E. bieneusi infection in sheep in Shanxi Province for the first time, which enriches the knowledge of the genetic diversity of E. bieneusi and provides baseline data for the prevention and control of E. bieneusi infection in animals and humans.
1. Introduction
Microsporidia are a class of obligate intracellular parasites that have been detected in most invertebrates and vertebrate hosts. Over 220 genera and approximately 1700 species have now been described [1]. Among them, 17 species have been frequently reported in humans [2], of which the most common and important species is E. bieneusi [3]. E. bieneusi was first isolated and identified in the intestinal epithelial cells of AIDS patients in 1985 [4]. E. bieneusi can cause symptomatic and asymptomatic intestinal infections. It can also cause acute diarrhea, poor absorption, respiratory inflammation, non-calculus cholecystitis, and even life-threatening diarrhea in individuals with immunodeficiency [5]. Moreover, E. bieneusi could lead to asymptomatic infections in people with normal immune status and transmit from infected individuals to susceptible humans or animals via a fecal-oral route [1]. Although animals and water are the potential reservoirs of E. bieneusi transmission, its public health risks have always been neglected due to its scarce incidence rate in humans.
Up to now, over 500 genotypes of E. bieneusi have been identified based on sequences of the internal transcribed spacer (ITS) region of the ribosomal DNA [6], and all genotypes can be allocated into 11 distinct phylogenetic groups (Groups 1 to 11). Group 1 is the largest group that comprises the majority of genotypes isolated from various hosts, including humans, and Group 2 was previously considered to consist of genotypes found in ruminants, but some of these genotypes are also found in humans [7], such as genotype I, J, BEB4, and BEB6 [8]. Groups 3–11 are host adaptation groups and might be present in specific hosts and wastewater [9]. At present, all the genotypes of E. bieneusi detected in sheep belonged to Groups 1, 2, and 7 [7].
Sheep are among the important food-producing animals, and over 8 million sheep are being raised in Shanxi Province, North China, annually. However, little is known of the prevalence, genetic diversity, and population genetic structure of E. bieneusi in sheep in Shanxi Province. Thus, the objectives of the present study were to investigate the prevalence and identify the genotypes of E. bieneusi in sheep in Shanxi Province, North China, which will enhance an understanding of the global genetic diversity of E. bieneusi and provide baseline data for executing measures for the prevention and control of E. bieneusi infection.
2. Materials and Methods
2.1. Specimen Collection
In November 2020, a total of 492 fecal samples were randomly collected from sheep in Qi County (n = 97), Shanyin County (n = 135), and Jishan County (n = 260) in central, northern, and southern Shanxi Province, respectively. All the sheep were intensively raised on farms and fed with grass. All fecal samples were separately packed in sterile tubes and loaded into foam boxes with ice packs. Then, these samples were transferred to the laboratory and stored in freezer at −20 °C until extraction of genomic DNA. Information of all samples was recorded, such as number, region, and age.
2.2. DNA Extraction and PCR Amplification
The genomic DNA of each fecal sample was extracted using the E.Z.N.A.® Stool DNA Kit (Omega Bio-Tek Inc., Norcross, GA, USA) according to the manufacturer’s protocols. Then, the genomic DNA was stored at −20 °C prior to PCR amplification. The E. bieneusi positivity in sheep was determined by nested PCR amplification of ~389 bp fragment of the ITS rRNA gene. The outer primers for the primary amplification were F1 (5′-GGTTAGTAGTAGAGAGAGAG-3′) and R1 (5′-TTCGAGTTCTCGCGCTC-3′), and the inner primers for the second PCR amplification were F2 (5′-GCTCTATATCTATGGCT-3′) and R2 (5′-ATCGCGGAGAGACAAGTG-3′), as reported in a previous study [10]. The PCR reaction mixture (25 μL) consisted of 14.875 μL of ddH2O, 2 μL of dNTP Mix (2.5 mM each), 2.5 μL of 10× PCR Buffer, 2 mM of MgCl2 (25 mM), 0.625 U of r-Taq (TaKaRa Bio Inc., Tokyo, Japan), 0.25 μM of each primer, and approximately 150 ng of the genomic DNA. The first PCR product was used as the template for secondary PCR amplification. The PCR conditions were as follows: initial denaturation at 94 °C for 5 min, 35 cycles of denaturation at 94 °C for 45 s, annealing at 55 °C (primary PCR and secondary PCR) for 45 s, and extension at 72 °C for 1 min; this was followed by a final extension at 72 °C for 10 min. The secondary PCR products were analyzed by electrophoresis in 1.5% agarose gels containing ethidium bromide, and the secondary positive products were sent to Sangon Biotech Co. Ltd. (Shanghai, China) for bidirectional sequencing.
2.3. Sequencing and Phylogenetic Analysis
The obtained sequences were revised and aligned by Chromas V2.6 and Basic Local Alignment Search Tool (BLAST), respectively, to identify the genotypes of E. bieneusi. The phylogenetic tree was constructed using neighbor-joining (NJ) method with 1000 bootstraps in MEGA7 software. The novel genotypes were identified according to the established nomenclature system [11]. All obtained representative sequences in this study were deposited in GenBank with the accession numbers OL604153 to OL604157.
2.4. Statistical Analysis
All statistical analyses in this study were performed using the software SPSS 26.0 (IBM, Chicago, IL, USA) for data processing. Chi-square (χ2) test was used to calculate the significant differences in E. bieneusi among different regions, ages, and genders. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were used to assess the reliability of results among prevalence and test conditions. Results were considered statistically significant when p value < 0.05.
3. Results
3.1. Prevalence of E. bieneusi in Sheep
In this study, 168 samples tested E. bieneusi-positive, and the overall prevalence of E. bieneusi in sheep in Shanxi Province was 34.15% (168/492). The E. bieneusi infection existed in each examined county and all age groups (Table 1), and there were significant differences in E. bieneusi prevalence among the different counties (p < 0.001). Moreover, the highest prevalence of E. bieneusi was detected in Qi County (74.2%), followed by Jishan County (29.6%) and Shanyin County (14.1%). Between the two age groups, the prevalence of E. bieneusi in lamb (<6 month) was 36.0%, which was higher than that in older sheep (>6 months) (32.7%), but the difference was not statistically significant (p = 0.448).

Table 1.
Factors associated with prevalence of Enterocytozoon bieneusi in sheep in Shanxi Province.
3.2. Genotype Distribution of E. bieneusi in Sheep
Among the 168 E. bieneusi-positive samples, 4 known genotypes (BEB6, COS-I, CHS7, and CHC8) and 1 novel genotype (designated SY-1) were identified (Table 2). Of these genotypes, BEB6 sequences (n = 135), COS-I sequences (n = 23), CHS7 sequences (n = 2), and a CHC8 sequence (n = 1) showed a 100% similarity to MN728943 (BEB6), KX383622 (COS-I), KP262392 (CHS7), and MK139946 (CHC8), respectively. Genotype BEB6 was the dominant genotype distributed in Shanyin County (52.63%, 10/19), Jishan County (68.83%, 53/77), and Qi County (100%, 72/72). The phylogenetic tree revealed that all five genotypes were clustered into the host-adapted Group 2 (Figure 1).

Table 2.
Prevalence and genotypes of Enterocytozoon bieneusi in sheep in Shanxi Province.
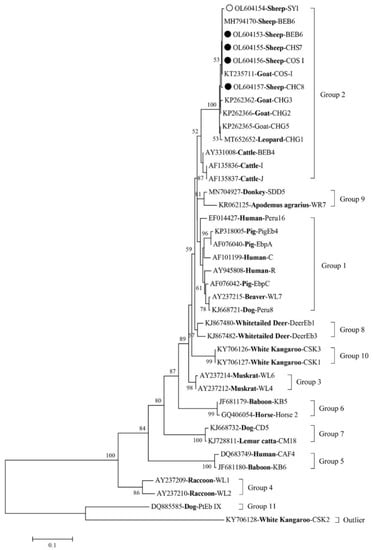
Figure 1.
Phylogenetic relationships of four known genotypes (marked with black circle) and one novel genotype (marked with unfilled circle) of Enterocytozoon bieneusi, along with other genotypes identified in this study and previous reports. Bootstrap value higher than 50% is shown.
4. Discussion
Prior to the present study, little was known regarding the prevalence and genotypes of E. bieneusi in sheep in Shanxi Province, North China. The present study revealed that the overall prevalence of E. bieneusi in sheep in Shanxi Province was 34.15% (168/492), which was higher than that reported in sheep in many other Chinese counties and provinces but lower than that compared with some other counties and provinces in China (Table 3). There are many factors influencing the prevalence reported in these studies, such as herd size, sheep breed, sample collection area, age, feeding habits, and hygiene conditions. Measures need to be taken to prevent and control the infection of E. bieneusi in sheep. For example, animals infected with parasites should be kept separately from healthy animals and treated with albendazole or fumagillin [12], disinfected regularly, and ventilated properly [13].

Table 3.
Prevalence of Enterocytozoon bieneusi in sheep worldwide.
Shanxi Province has a temperate continental monsoon climate with a mountain area of 80%, and basins and valleys are the principal locations for residents [33]. Among the three investigation regions considered in the present study, the difference in the prevalence of E. bieneusi was statistically significant (p < 0.001). As shown in Table 2, the highest E. bieneusi prevalence was observed in Farm 1 to Farm 4 in Qi County. Qi County is close to the city of Taiyuan, which is the trade center and transportation hub in Shanxi Province. Thus, we reasoned that the specific geographical location and the high mobilization of persons might be directly or indirectly responsible for the higher E. bieneusi prevalence in Qi County; thus, more samples should be examined in the future to further clarify the possible reasons explaining E. bieneusi prevalence in these regions.
The incomplete immunity of young sheep and living in a susceptible environment may be the causes of the high risk of E. bieneusi infection [13], which may also be the reasons why the higher prevalence was observed in young sheep in this study.
In this study, four known E. bieneusi genotypes (BEB6, COS-I, CHS7, and CHC8) and one novel genotype (SY-1) were identified. Among these genotypes, BEB6, COS-I, CHS7, and CHC8 have commonly been identified in ruminants worldwide [14,25,27,28]. The BEB6 (135/170, 79.4%) genotype was the predominant genotype found within the three study regions, which is consistent with what was found in sheep in the Inner Mongolia Autonomous Region [24]. BEB6 (n = 72) was also the only genotype found in sheep in Qi County, which suggests its importance and zoonotic potential in this local area. With its host range expansion, BEB6 is currently considered to be a human genotype with extensive geographical distribution [22]. In addition, the BEB6 genotype has been identified in deer [34], goats [13], wild and domestic animals, and humans [7]. Moreover, the COS-I (synonym: CM7) genotype has been identified in humans, NHPs, and domestic and wild animals [5,7]; and the genotypes CHS7 and CHC8 have been identified in domestic animals, such as cattle, sheep, and goats in China [5]. Phylogenetic analysis showed that all five genotypes identified in the present study were clustered into the host-adapted Group 2, indicating that sheep may be a source of E. bieneusi infection for humans and other animals.
5. Conclusions
The present study revealed an E. bieneusi prevalence of 34.15% in sheep in Shanxi Province, North China. Significant differences in the prevalence of E. bieneusi were found among the different study regions. ITS sequencing identified four known genotypes (BEB6, COS-I, CHS7, and CHC8) and one novel genotype (SY-1) in the sheep, with BEB6 being the zoonotic genotype distributed in all three examined counties. This is the first documentation of E. bieneusi prevalence and genotypes in sheep in Shanxi Province, which enriched the knowledge of the geographical distribution and genetic diversity of E. bieneusi and provided baseline data for the better control of E. bieneusi in animals and humans.
Author Contributions
X.-Q.Z. and S.-C.X. conceived and designed the study. R.-L.Q. performed the experiments, analyzed the data, and drafted the manuscript. Y.-Y.L., J.-J.M., Z.-H.Z., W.-B.Z., Q.L. and W.-W.G. participated in the implementation of the study. X.-Q.Z., S.-C.X. and Y.Z. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Shanxi Province Excellent Doctoral Work Award-Scientific Research Project (Grant No. SXBYKY2021019), the Fund for Shanxi “1331 Project” (Grant No. 20211331-13), the Special Research Fund of Shanxi Agricultural University for High-level Talents (Grant No. 2021XG001), and the Yunnan Expert Workstation (Grant No. 202005AF150041). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data sets supporting the results of this article have been submitted to the GenBank and the accession number is shown in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Han, B.; Pan, G.; Weiss, L.M. Microsporidiosis in humans. Clin. Microbiol. Rev. 2021, 34, e0001020. [Google Scholar] [CrossRef]
- Deng, L.; Chai, Y.; Xiang, L.; Wang, W.; Zhou, Z.; Liu, H.; Zhong, Z.; Fu, H.; Peng, G. First identification and genotyping of Enterocytozoon bieneusi and Encephalitozoon spp. in pet rabbits in China. BMC Vet. Res. 2020, 16, 212. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Fayer, R. Microsporidiosis: Enterocytozoon bieneusi in domesticated and wild animals. Res. Vet. Sci. 2011, 90, 363–371. [Google Scholar] [CrossRef]
- Desportes, I.; Le Charpentier, Y.; Galian, A.; Bernard, F.; Cochand-Priollet, B.; Lavergne, A.; Ravisse, P.; Modigliani, R. Occurrence of a new microsporidan: Enterocytozoon bieneusi n.g., n. sp., in the enterocytes of a human patient with AIDS. J. Protozool. 1985, 32, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Wang, R.J.; Fan, X.C.; Liu, T.L.; Zhang, L.X.; Zhao, G.H. Prevalence and genotypes of Enterocytozoon bieneusi in China. Acta Trop. 2018, 183, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Y.; Wang, W.; Chao, L.; Jia, Y.; Yuan, Y.; Wang, J.; Qiu, J.; Qi, M. Molecular identification and genotyping of Enterocytozoon bieneusi in experimental rats in China. Exp. Parasitol. 2020, 210, 107850. [Google Scholar] [CrossRef]
- Li, W.; Feng, Y.; Santin, M. Host specificity of Enterocytozoon bieneusi and public health implications. Trends Parasitol. 2019, 35, 436–451. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Su, Y.; Liang, X.; Sun, X.; Peng, S.; Lu, H.; Jiang, N.; Yin, J.; Xiang, M.; et al. Identification and genotyping of Enterocytozoon bieneusi in China. J. Clin. Microbiol. 2011, 49, 2006–2008. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Luo, N.; Wang, C.; Qi, M.; Cao, J.; Cui, Z.; Huang, J.; Wang, R.; Zhang, L. Occurrence, molecular characterization and predominant genotypes of Enterocytozoon bieneusi in dairy cattle in Henan and Ningxia, China. Parasit. Vectors 2016, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, I.M.; Fayer, R.; Lal, A.A.; Trout, J.M.; Schaefer, F.W., 3rd; Xiao, L. Molecular characterization of microsporidia indicates that wild mammals Harbor host-adapted Enterocytozoon spp. as well as human-pathogenic Enterocytozoon bieneusi. Appl. Environ. Microbiol. 2003, 69, 4495–4501. [Google Scholar] [CrossRef] [Green Version]
- Santin, M.; Fayer, R. Enterocytozoon bieneusi genotype nomenclature based on the internal transcribed spacer sequence: A consensus. J. Eukaryot. Microbiol. 2009, 56, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Didier, E.S.; Weiss, L.M. Microsporidiosis: Not just in AIDS patients. Curr. Opin. Infect. Dis. 2011, 24, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, S.S.; Zou, Y.; Li, Z.; Xie, S.C.; Shi, L.Q.; Zou, F.C.; Zhu, X.Q.; Yang, J.F.; Zhao, G.H. Prevalence and multi-locus genotypes of Enterocytozoon bieneusi in black-boned sheep and goats in Yunnan Province, southwestern China. Infect. Genet. Evol. 2018, 65, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Beser, J.; Ljungstrom, B.; Troell, K.; Lebbad, M. Low host-specific Enterocytozoon bieneusi genotype BEB6 is common in Swedish lambs. Vet. Parasitol. 2014, 205, 371–374. [Google Scholar] [CrossRef]
- Askari, Z.; Mirjalali, H.; Mohebali, M.; Zarei, Z.; Shojaei, S.; Rezaeian, T.; Rezaeian, M. Molecular detection and identification of zoonotic microsporidia spore in fecal samples of some animals with close-contact to human. Iran J. Parasitol. 2015, 10, 381–388. [Google Scholar]
- Fiuza, V.; Lopes, C.W.G.; Cosendey, R.I.J.; de Oliveira, F.C.R.; Fayer, R.; Santin, M. Zoonotic Enterocytozoon bieneusi genotypes found in brazilian sheep. Res. Vet. Sci. 2016, 107, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Al-Herrawy, A.Z.; Gad, M.A. Microsporidial spores in fecal samples of some domesticated animals living in Giza, Egypt. Iran J. Parasitol. 2016, 11, 195–203. [Google Scholar]
- Valencakova, A.; Danisova, O. Molecular characterization of new genotypes Enterocytozoon bieneusi in Slovakia. Acta Trop. 2019, 191, 217–220. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Ai, S.; Wang, X.; Zhang, R.; Duan, Z. Cryptosporidium spp., Enterocytozoon bieneusi, and Giardia duodenalis from animal sources in the Qinghai-Tibetan Plateau Area (QTPA) in China. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101346. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, J.; Li, P.; Wang, L.; Guo, Y.; Li, C.; Lei, M.; Feng, Y.; Xiao, L. Enterocytozoon bieneusi genotypes in Tibetan sheep and yaks. Parasitol. Res. 2018, 117, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mi, R.; Cheng, L.; Huang, Y.; An, R.; Zhang, Y.; Jia, H.; Zhang, X.; Wang, X.; Han, X.; et al. Prevalence and genetic diversity of Enterocytozoon bieneusi in sheep in China. Parasit. Vectors 2018, 11, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.C.; Wang, K.; Gu, Y.F. Detection and genotyping study of Enterocytozoon bieneusi in sheep and goats in East-central China. Acta Parasitol. 2019, 64, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zhang, Z.; Zhao, A.; Jing, B.; Guan, G.; Luo, J.; Zhang, L. Distribution and molecular characterization of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi amongst grazing adult sheep in Xinjiang, China. Parasitol. Int. 2019, 71, 80–86. [Google Scholar] [CrossRef]
- Ye, J.; Xiao, L.; Wang, Y.; Guo, Y.; Roellig, D.M.; Feng, Y. Dominance of Giardia duodenalis assemblage A and Enterocytozoon bieneusi genotype BEB6 in sheep in Inner Mongolia, China. Vet. Parasitol. 2015, 210, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mi, R.; Yang, J.; Wang, J.; Gong, H.; Huang, Y.; Wang, X.; Han, X.; Zhou, H.; Chen, Z. Enterocytozoon bieneusi genotypes in farmed goats and sheep in Ningxia, China. Infect. Genet. Evol. 2020, 85, 104559. [Google Scholar] [CrossRef]
- Peng, J.J.; Zou, Y.; Li, Z.X.; Liang, Q.L.; Song, H.Y.; Li, T.S.; Ma, Y.Y.; Zhu, X.Q.; Zhou, D.H. Occurrence of Enterocytozoon bieneusi in Chinese Tan sheep in the Ningxia Hui Autonomous Region, China. Parasitol. Res. 2019, 118, 2729–2734. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, Y.; Wu, Y.; Niu, Z.; Li, J.; Zhang, S.; Wang, R.; Jian, F.; Ning, C.; Zhang, L. Molecular characterization of Giardia duodenalis and Enterocytozoon bieneusi isolated from Tibetan sheep and Tibetan goats under natural grazing conditions in Tibet. J. Eukaryot. Microbiol. 2020, 67, 100–106. [Google Scholar] [CrossRef]
- Shi, K.; Li, M.; Wang, X.; Li, J.; Karim, M.R.; Wang, R.; Zhang, L.; Jian, F.; Ning, C. Molecular survey of Enterocytozoon bieneusi in sheep and goats in China. Parasit. Vectors 2016, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Zhang, W.; Yang, D.; Zhang, L.; Wang, R.; Liu, A. Prevalence of Enterocytozoon bieneusi and genetic diversity of ITS genotypes in sheep and goats in China. Infect. Genet. Evol. 2015, 32, 265–270. [Google Scholar] [CrossRef]
- Jiang, Y.; Tao, W.; Wan, Q.; Li, Q.; Yang, Y.; Lin, Y.; Zhang, S.; Li, W. Zoonotic and potentially host-adapted Enterocytozoon bieneusi genotypes in sheep and cattle in northeast China and an increasing concern about the zoonotic importance of previously considered ruminant-adapted genotypes. Appl. Environ. Microbiol. 2015, 81, 3326–3335. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Li, Y.; Li, W.; Yang, J.; Song, M.; Diao, R.; Jia, H.; Lu, Y.; Zheng, J.; Zhang, X.; et al. Genotypes of Enterocytozoon bieneusi in livestock in China: High prevalence and zoonotic potential. PLoS ONE 2014, 9, e97623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Chang, Y.; Chen, Y.; Zhang, X.; Li, D.; Zheng, S.; Wang, L.; Li, J.; Ning, C.; Zhang, L. Occurrence and molecular characterization of Cryptosporidium spp., Giardia duodenalis, and Enterocytozoon bieneusi from Tibetan sheep in Gansu, China. Infect. Genet. Evol. 2018, 64, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhuo, H.M.; Fu, S.Z.; Ren, L.J. Air pollution characteristics, health risks, and source analysis in Shanxi Province, China. Environ. Geochem. Health 2021, 43, 391–405. [Google Scholar] [CrossRef]
- Zhang, X.X.; Cong, W.; Liu, G.H.; Ni, X.T.; Ma, J.G.; Zheng, W.B.; Zhao, Q.; Zhu, X.Q. Prevalence and genotypes of Enterocytozoon bieneusi in sika deer in Jilin province, Northeastern China. Acta Parasitol. 2016, 61, 382–388. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).