Simple Summary
Few studies have investigated a relationship between t9,c12,c15-C18:3 and ALA, an α-Linolenic acid c9,c12,c15-C18:3 in the rumen. These results indicated that t9,c12,c15-C18:3 was an intermediate of the α-linolenic acid shifted rumen biohydrogenation pathway. This study hypothesized a pathway for α-linolenic acid biohydrogenation in rumen.
Abstract
The t9,c12,c15-C18:3 as an isomer of α-linolenic acid (c9,c12,c15-C18:3; ALA), has been recently detected in milk, but has not been found in the rumen. This study hypothesized that it may be a biohydrogenation product of ALA in rumen and aimed to explore whether it was present in the rumen and help to understand the rumen biohydrogenation mechanisms of ALA. The in vitro experiment included two treatments, a control check (CK group) with 50 µL ethanol added, and ALA group with 50 µL ethanol and 2.6 mg ALA (ALA addition calculated by 1.30% of dry matter base of diet); each sample of fermentation fluid had the composition of C18 fatty acids analyzed at 0, 0.5, 1, 2, 3, 4, 5, and 6 h. The results showed that no t9,c12,c15-C18:3 was detected in the CK group, but ALA addition increased the concentration of t9,c12,c15-C18:3 in fermentation fluid. The content of t9,c12,c15-C18:3 peaked 1 h after fermentation, then declined gradually. At 1 h, no t9c12c15-C18:3 was detected in the fermentation fluid of the CK treatment. The results suggested that ALA converted to the isomer t9,c12,c15-C18:3 through biohydrogenation in the rumen. The addition of ALA can also increase the concentration of t9,c12-C18:2, c9,t11-C18:2, c12-C18:1, t11-C18:1, t9-C18:1, and c6-C18:1 in fermentation fluid. It was concluded using an in vitro experiment that t9,c12,c15-C18:3 was a product of rumen biohydrogenation of ALA.
1. Introduction
The supplementation of α-linolenic acid (c9,c12,c15-C18:3; ALA) in the ruminant diet can increase the concentration of ALA and longer chain ω-3 polyunsaturated fatty acids (n-3 PUFA) in milk [1], which are essential fatty acids for humans. However, ALA biohydrogenation in the rumen is the main limiting factor influencing the efficiency of dietary ALA transport into milk. Therefore, exploring the pathways of ALA biohydrogenation in rumen could help regulate milk ALA and n-3 PUFA. The first step of ALA biohydrogenation in the rumen is isomerization. Pervious research showed that the cis-trans isomerization of ALA could happen in the ortho-position, and ALA could isomerize to t10,c12,c15-C18:3 with c9 to t10 [2], c9,t11,c15-C18:3 with c12 to t11 [3], and c9,t13,c15-C18:3 with c12 to t13 [4]. However, few studies have reported that the isomerization of ALA could happen in an in-situ position such as t9,c12,c15-C18:3 with c9 to t9 in the rumen. The in situ isomerization of ALA has been reported in the processing of food. A previous study reported that ALA could isomerize to t9,c12,c15-C18:3 through frying [5]. Recent studies have found that t9,c12,c15-C18:3 was present in milk [6]. Therefore, it can be hypothesized that ALA may also convert to t9,c12,c15-C18:3 through biohydrogenation in rumen. The purpose of this experiment was to explore whether there was t9,c12,c15-C18:3 in rumen fluid using an in vitro fermentation test and to investigate the relationship between t9,c12,c15-C18:3 and ALA. The t9,c12,c15-C18:3 identified in this study may provide a theoretical basis for the exploration of rumen biohydrogenation.
2. Materials and Methods
2.1. Experimental Design and Animal Management
The ALA pure products were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China) and diluted with ethanol. Rumen fluid was collected from three ruminal cannulated Holstein cows fed a diet that met the feeding standards of dairy cattle in China according to the Ministry of Agriculture of China Feeding Standard of Dairy Cattle (NY/T 34-2004, MOA: Beijing, China, 2004). Rumen contents were withdrawn before the first meal in the morning, pooled in equal proportions into a container with CO2, transferred to the laboratory, and strained through four layers of cheesecloth to obtain rumen fluid.
Eighty fermentation bottles were separated into two groups as a control (CK group), with 50 µL ethanol added and ALA group with 50 µL ethanol and 2.6 mg ALA (ALA addition calculated by 1.30% of dry matter base of diet). The bottles were prewarmed to 39 °C and flushed by CO2, then filled with 200 mg diet (Table S1) and 30 mL oxygen-free buffered rumen fluid, including 10 mL rumen fluid and 20 mL buffer [7]. All the bottles were sealed with rubber stoppers and incubated in the shaking water bath (MRT-60R, shanghai minquan instrument Co., Ltd. Shanghai, China) at 40 r/min and 39 °C for 0, 0.5, 1, 2, 3, 4, 5, and 6 h, respectively. Fermentation fluid was collected in a 5 mL EP tube and stored at −80 °C for further C18 fatty acid analysis.
2.2. C18 Fatty Acid Analysis
The extraction of fatty acids and fatty acid methyl esters (FAMEs) from ruminal fluid was according to a previous protocol [6]. A 2 mL aliquot of each sample was transferred to a 15 mL tube and 4 mL of n-hexane/isopropanol (v/v = 3/2) solution was used to extract the fat. The methyl esterification of the fat was then performed using 10% acetyl chlorocarbinol followed by 2% methanolic NaOH. The FAMES were analyzed using an Agilent 7890A gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) fitted to a 5975C MS mass spectrometer detector (Agilent Technologies, Santa Clara, CA, USA). A sample of 2 μL FAME mixed with hexane was injected through the split injection port in a 10:1 ratio into a capillary column CP-Sil 88 fused silica 100 m × 0.25 mm × 0.20 μm (Agilent, Palo Alto, CA, USA). The injector temperature was set at 250 °C, and oven temperature was initially 120 °C. After holding for 2 min, the temperature was increased by 3 °C per min to 180 °C and then increased by 1.5 °C per min to 200 °C. This temperature was maintained for three min, then increased by 2 °C per min to 220 °C and held for 20 min. The transfer line and MS ion source temperatures were maintained at 250 and 280 °C, respectively, and the ionizing energy was 70 eV.
The FAME peaks were identified using known FAME standards, including linoleic acid methyl ester mix CRM 47791 (t9,t12-C18:2, c9,t12-C18:2, t9,c12-C18:2 and c9,c12-C18:2), α-linolenic acid methyl ester mix CRM 47792 including (t9,t12,t15-C18:3, t9,t12,c15-C18:3, t9,c12,t15-C18:3, c6,c9,c12-C18:3, c9,t12,t15-C18:3, c9,c12,t15-C18:3, c9,t12,c15-C18:3, and t9,c12,c15-C18:3), CLA methyl ester mix Nu-Check prep including c9,t11-C18:2, t10,c12-C18:2 (Elysian, MN, USA), c6,c9,c12-C18:3, t6-C18:1, t9-C18:1, t11-C18:1, c6-C18:1, c9-C18:1, c11-C18:1, and C18:0 FAME standards individually from Nu-Check Prep (Elysian, MN, USA), c8-C18:1 and c12-C18:1 FAME individual standards from Cayman (Ann Arbor, MI, USA), c9-C18:1, c9,c12-C18:2 and c9,c12,c15-C18:3 triacylglycerols (TAGs) individual standards from Nu-Check Prep (Elysian, MN, USA), and c6,c9,c12-C18:3 TAGs from BePure (Beijing, China). The method of Chen et al. [6] was used to calculate fatty acids from FAME data.
2.3. Statistical Analysis
The results of C18 fatty acid were analyzed using ANOVA models of SAS (version 9.4, SAS Inst., Inc., Cary, NC, USA). The following statistical model was used:
where Yij represents the observed dependent variables, μ was the overall mean, Ti was the effect of treatment, and εij was the residual error. Significant and extremely significant levels were set at p < 0.05 and p < 0.01, respectively.
Yij = μ + Ti + εij,
3. Results and Discussion
The composition of C18 fatty acids detected in fermentation in vitro of the two groups is shown in Figure 1. The t9,t12,c15-C18:3, t9,c2,t15-C18:3, c6,c9,c12-C18:3, c9,c12,t15-C18:3 and c9,t12,c15-C18:3 were undetected in this experiment, and t9,t12,t15-C18:3, c9t12t15-C18:3, and ALA were detected in the CK and ALA groups. The t9,c12,c15-C18:3 was found in the ALA group after fermentation. Previous studies reported that t9,c12,c15-C18:3 has been found in the processing of food [5]. Recent studies have found that there are t9,c12,c15-C18:3 in milk [6], but the concentration was low.
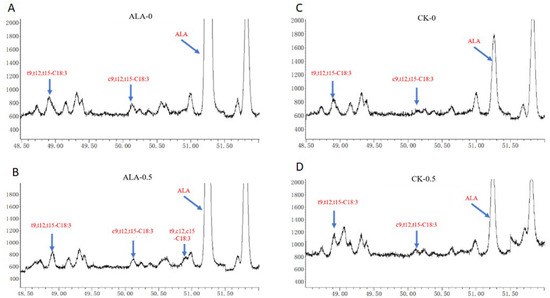
Figure 1.
C18:3 fatty acid from fermentation in vitro. (A): ALA group fermented for 0 h; (B): ALA group fermented for 0.5 h; (C): CK group fermented for 0 h; (D): CK group fermented for 0.5 h.
In this experiment, ALA influenced the content of C18 fatty acids in fermentation, as showed in Figure 2. The addition of ALA in fermentation increased the content of ALA in fluid from 0.256 ng/mL to 1.369 ng/mL (p < 0.01). With the extension of fermentation time, the content of ALA was decreased gradually, and the decline was the fastest in the first 0.5 h of fermentation. The concentration of t9,c12,c15-C18:3 decreased gradually with the fermentation time and the content of t9,c12,c15-C18:3 reached a peak 1 h after fermentation, then declined gradually. The addition of ALA increased the concentration of t9,c12-C18:2, c9,t11-C18:2, c12-C18:1, t11-C18:1, t9-C18:1, and c6-C18:1 in the fermentation fluid (p < 0.01). The change of t9c12-C18:2 showed the same trend, peaking 1 h after fermentation, then declining gradually. In this study, the change in c12-C18:1, t11-C18:1 showed a similar trend, the highest at 3 h after fermentation, then declined gradually and the concentration of c6-C18:1 increased at 3 h after fermentation. Through the biohydrogenation of ALA, the content of ALA decreased and converted to other C18 fatty acids [8]. Baldin et al. [9] reported that the content of ALA in fermentation fluid disappeared rapidly at the initial stages up to 0.5 h, which was like this study. The ALA isomers were further converted to C18:2 fatty acids [2], and the isomer c9,t11-C18:2 was one of the ALA biohydrogenation products present in the rumen. In the in vivo experiment, enhancing the content of ALA in the diet of cows also increased c9,t11-C18:2 in the rumen [10], the same result as observed in the fermentation fluid of the current study. Many studies have shown that the content of t10,c12-C18:2 increased with ALA increase in rumen [11], but few have focused on the content of t9,c12-C18:2. A study reported that ALA supplementation could increase the content of t9,c12-C18:2 in milk fat [12], and that the milk t9,c12-C18:2 might originate from the rumen. A previous study reported that c12-C18:1, t11-C18:1, and t9-C18:1 were products of ALA [2,3,4], and t11-C18:1 was the main product of ALA biohydrogenation in the rumen [4]. This study also found higher concentrations of c12-C18:1, t11-C18:1, and t9-C18:1 in the fermentation fluid when ALA was added to the fluid, where t11-C18:1 increased from 1.804 ng/mL to 3.118 ng/mL at 3 h, c12-C18:1 from 0.322 ng/mL to 0.609 ng/mL at 3 h, and t9-C18:1 increased from 0.256 ng/mL to 0.404 ng/mL at 5 h.
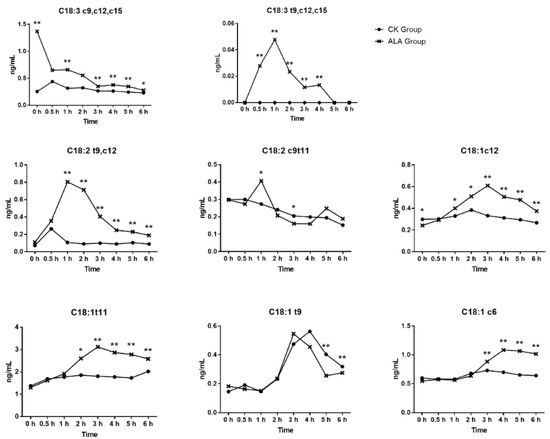
Figure 2.
The C18 fatty acid content in fermentation in vitro at separate times. Significant difference: * p < 0.05, ** p < 0.01.
As shown in Figure 2, with the decreasing concentration of ALA, the content of t9,c12,c15-C18:3 increased in the rumen fermentation fluid, so it was speculated that ALA could convert to t9,c12,c15-C18:3. The concentration of t9,c12,c15-C18:3 was less than 0.048 ng/mL, so the conversion of ALA to t9,c12,c15-C18:3 might not be a major biohydrogenation pathway. A previous study reported that t10,c12,c15-C18:3 could convert to t10,c15-C18:2 in the rumen [13] and the biohydrogenation occurred in a cis double bond. This study showed that ALA supplementation can increase the content of t9,c12,c15-C18:3 and t9,c12-C18:2 in fermentation fluid, so it is likely that t9,c12,c15-C18:3 could convert to t9,c12-C18:2, but t9,c12,c15-C18:3 was not the main source of t9,c12-C18:2, due to the low concentration of t9,c12,c15-C18:3 in fermentation fluid. To the best of our knowledge, no article has reported the next transformation pathway of t9,c12-C18:2. However, t11,c15-C18:2 was one of the isomers of t9,c12-C18:2 reported that t11,c15-C18:2 could covert to c15-C18:1, t11-C18:1, and t16-C18:1 via t11,c15-C18:2 to c15-C18:1, t11,c15-C18:2 to c15-C18:1 and t11,c15-C18:2to t11-C18:1) [2]. The conclusion was that c12-C18:1, t11-C18:1, and t9-C18:1 might also be products of t9c12-C18:2 biohydrogenation, and a higher content of c12-C18:1, t11-C18:1, and t9-C18:1 were also observed in ALA group fermentation fluid. The c12-C18:1, t11-C18:1, and t9-C18:1 were further hydrogenated to C18:0 [4]. On the gas chromatography-mass spectrometry analyses above, t9,c12,c15-C18:3 was the biohydrogenation product of ALA in the rumen and the biohydrogenation pathways may exist in the rumen as t9,c12,c15-C18:3 to t9,c12-C18:2 to c12-C18:1/t11-C18:1/t9-C18:1 to C18:0 as shown by the red line in Figure 3. Numerous studies in vivo and in vitro have reported multiple biohydrogenation pathways for ALA in the rumen [4]. The t9,t11,c15-C18:3 contains two trans configurations, and was previously reported as a biohydrogenation product of ALA, and t9,t11,c15-C18:3 was converted from c9,t11,c15-C18:3 [14]. The t9,c12,c15-C18:3 might also isomerize to t9,t11,c15-C18:3 in the rumen, but due to the lesser standard of t9,t11,c15 C18:3, this study did not detect it. Further analysis could explore whether t9,c12,c15-C18:3 could convert to t9,t11,c15-C18:3.
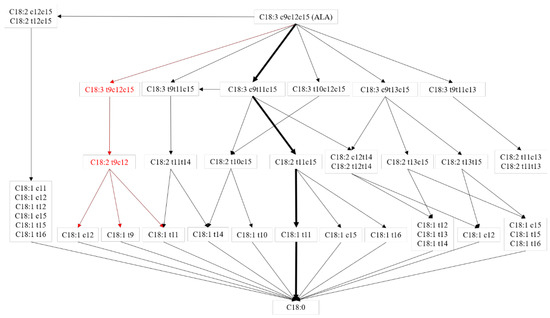
Figure 3.
ALA biohydrogenation pathways in the rumen. Adapt from [4]. Red line pathway: reported in this experiment; black line pathway: reported by previous study; black bold line pathway: main biohydrogenation pathways in the rumen.
4. Conclusions
Overall, this study found that t9,c12,c15-C18:3 was the product of ALA biohydrogenation in the rumen, which may provide a new biohydrogenation pathway in the rumen. The cis-trans isomerization of ALA from c9,c12,c15 C18:3 isomerizes to t9,c12,c15-C18:3 could happen in situ and the ortho-position c9,c12,c15-C18:3 isomerized to t10,c12,c15-C18:3. The enzymes and microorganisms involved in isomerization may be different, and any further study should focus on the manipulation of enzymes and microorganisms involved in the biohydrogenation pathway.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/ani12040502/s1, Table S1: Composition of diets (% DM basis), Table S2: the raw data presented in this study.
Author Contributions
G.H.: Conceptualization, software, data curation, writing the original draft preparation. L.G.: methodology. M.C.: data curation. X.W.: software. W.T.: resources. S.Z.: formal analysis. N.Z.: supervision. Y.Z.: writing and reviewing. J.W.: project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Agricultural Science and Technology Innovation Program (ASTIP-IAS12), the Modern Agro-Industry Technology Research System, China, and the Scientific Research Project for Major Achievements from the Agricultural Science and Technology Innovation Program (CAAS-ZDXT2019004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This research was approved by Experimental Animal Welfare and Ethical of Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing, China. IAS 2021-57. Approved on 16 March 2021.
Data Availability Statement
The raw data presented in this study are available in Supplementary Materials, Supplementary Table S2.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, G.X.; Zhang, Y.D.; Xu, Q.B.; Zheng, N.; Zhao, S.G.; Liu, K.Z.; Qu, X.Y.; Yu, J.; Wang, J.Q. DHA content in milk and biohydrogenation pathway in rumen: A review. PeerJ 2020, 8, e10230. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, A.; Bernard, L.; Meynadier, A.; Malpuech-Brugère, C. Production of trans and conjugated fatty acids in dairy ruminants and their putative effects on human health: A review. Biochimie 2017, 141, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, M.; Ramos-Morales, E.; Wallace, R.J. The role of microbes in rumen lipolysis and biohydrogenation and their manipulation. Animal 2010, 4, 1008–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewanckele, L.; Toral, P.G.; Vlaeminck, B.; Fievez, V. Invited review: Role of rumen biohydrogenation intermediates and rumen microbes in diet-induced milk fat depression: An update. J. Dairy Sci. 2020, 103, 7655–7681. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, Y.; Nie, S.; Xie, M.; Chen, F.; Luo, P.G. Formation of trans fatty acids during the frying of chicken fillet in corn oil. Int. J. Food Sci. Nutr. 2014, 65, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Q.; Zhang, Y.D.; Wang, F.E.; Zheng, N.; Wang, J.Q. Simultaneous determination of C18 fatty acids in milk by GC-MS. Separations 2021, 8, 118. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feeding stuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.X.; Guo, L.Y.; Chang, X.F.; Liu, K.Z.; Tang, W.H.; Zheng, N.; Zhao, S.G.; Zhang, Y.D.; Wang, J.Q. Effect of whole or ground flaxseed supplementation on fatty acid profile, fermentation, and bacterial composition in rumen of dairy cows. Front. Microbiol. 2021, 12, 760528. [Google Scholar] [CrossRef] [PubMed]
- Baldin, M.; Rico, D.E.; Green, M.H.; Harvatine, K.J. Technical note: An in vivo method to determine kinetics of unsaturated fatty acid biohydrogenation in the rumen. J. Dairy Sci. 2018, 101, 5669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pi, Y.; Ma, L.; Pierce, K.M.; Wang, H.R.; Xu, J.C.; Bu, D.P. Rubber seed oil and flaxseed oil supplementation alter digestion, ruminal fermentation and rumen fatty acid profile of dairy cows. Animal 2019, 13, 2811–2820. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.F.A.; Quigley, S.P.; Isherwood, P.; McLennan, S.R.; Sun, X.Q.; Gibbs, S.J.; Poppi, D.P. The inclusion of low quantities of lipids in the diet of ruminants fed low quality forages has little effect on rumen function. Anim. Feed Sci. Tech. 2017, 234, 20–28. [Google Scholar] [CrossRef]
- Collomb, M.; Sollberger, H.; Bütikofer, U.; Sieber, R.; Stoll, W.; Schaeren, W. Impact of a basal diet of hay and fodder beet supplemented with rapeseed, linseed and sunflower seed on the fatty acid composition of milk fat. Int. Dairy J. 2004, 14, 549–559. [Google Scholar] [CrossRef]
- Harfoot, C.G. Lipid metabolism in the rumen. Prog Lipid Res. 1978, 17, 21–54. [Google Scholar] [CrossRef]
- Kishino, S.; Ogawa, J.; Yokozeki, K.; Shimizu, S. Metabolic diversity in biohydrogenation of polyunsaturated fatty acids by lactic acid bacteria involving conjugated fatty acid production. Appl. Microbiol. Biotechnol. 2009, 84, 87–97. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).